Microcystis Sp. Co-Producing Microcystin and Saxitoxin from Songkhla Lake Basin, Thailand
Abstract
:1. Introduction
2. Results
2.1. General Water Quality Parameters and Bacterial Isolation
2.2. Microcystin Genes and a Saxitoxin Gene Analysis
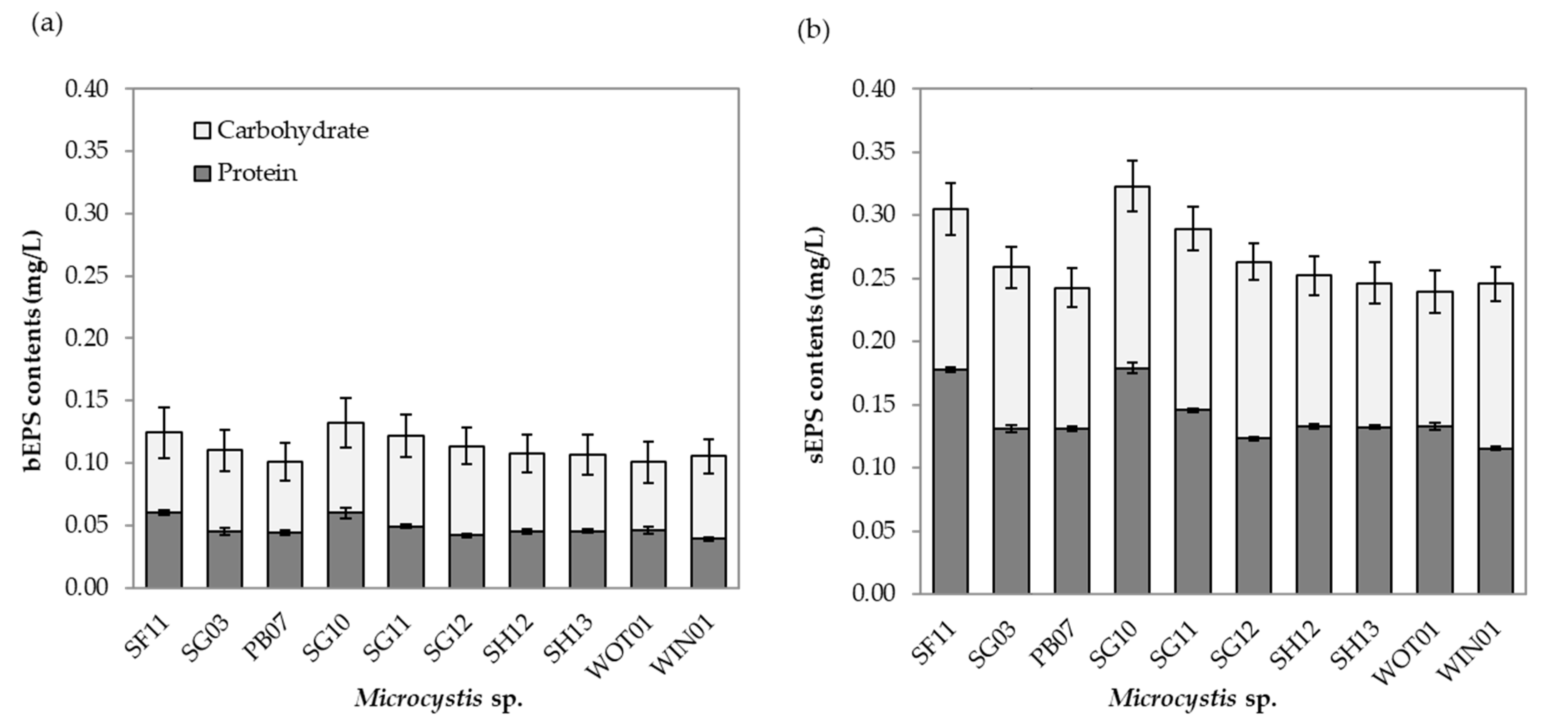
2.3. Growth and EPS Composition
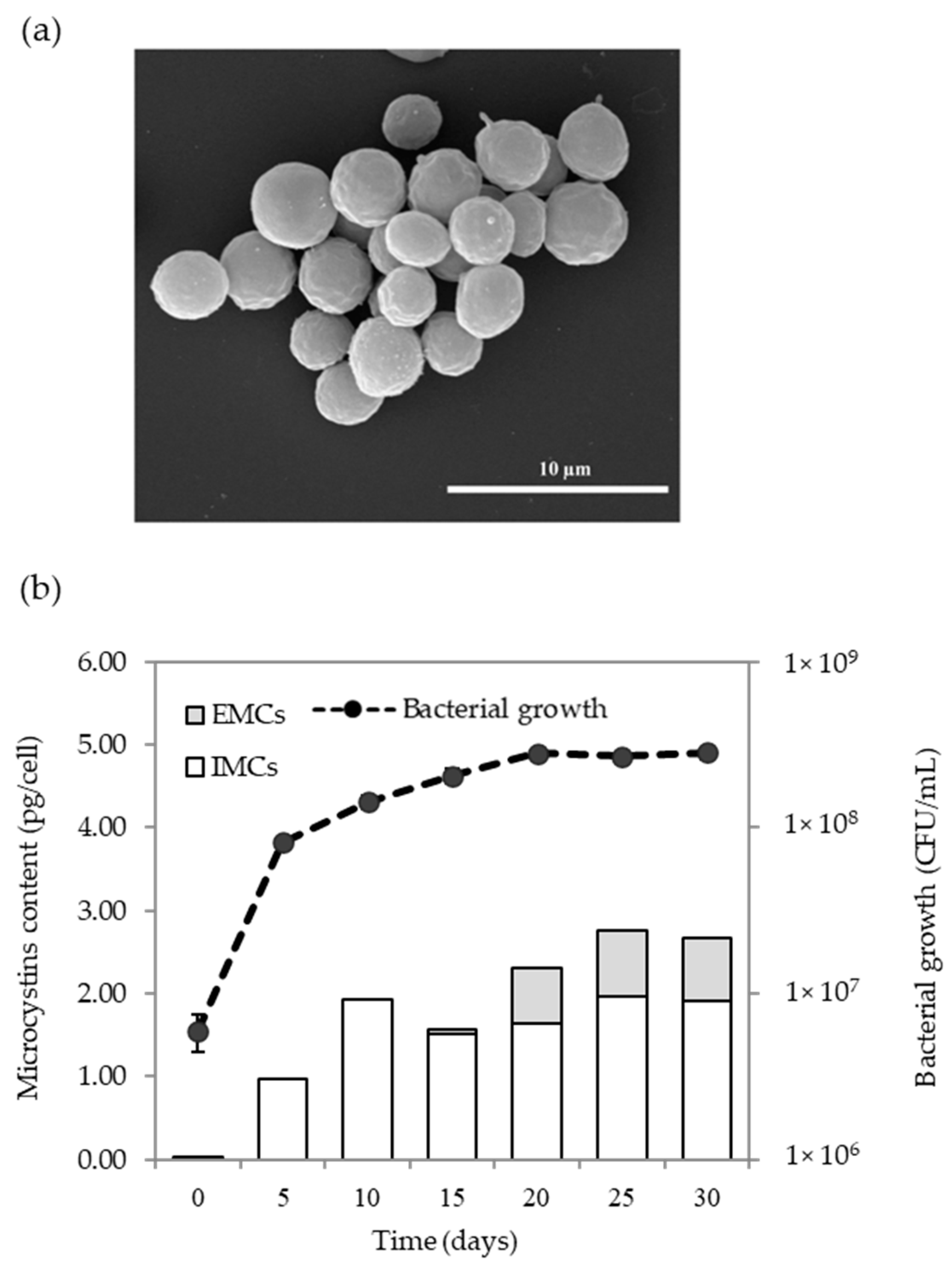
2.4. Microcystins Production of Microcystis Sp.
2.5. Kinetics of MCs Production Based on Microcystis Growth
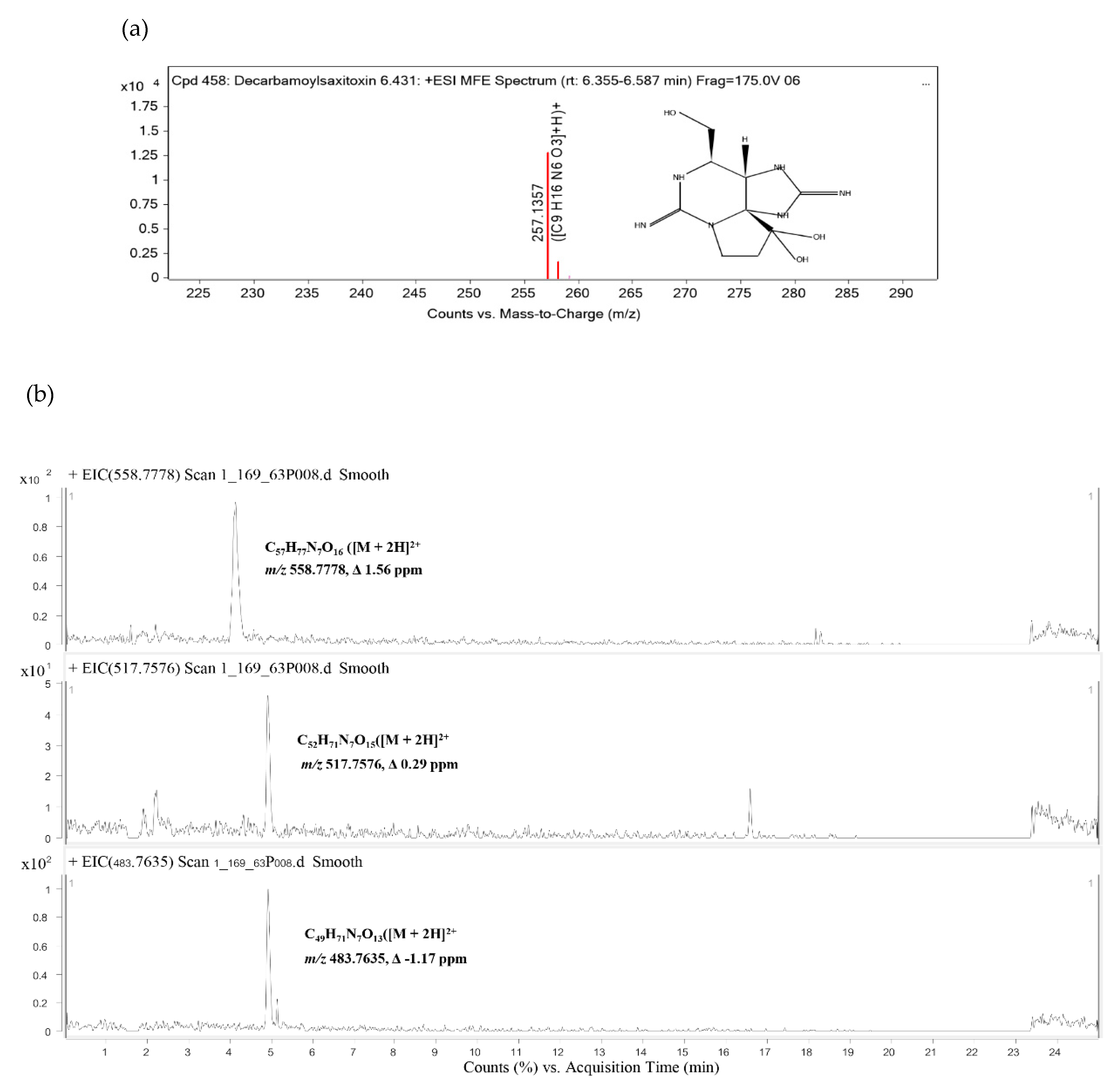
2.6. Identification of Cyanotoxins
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Water Quality Characterization
5.2. Microcystis Spp. Isolation
5.3. Cyanobacterial DNA Extraction
5.4. Detection of Microcystin Genes, Saxitoxin Gene and 16S rDNA by PCR
5.5. Measurements of Specific Growth Rate and Chlorophyll a (Chl-a)
5.6. Microcystins Production
5.7. Extracellular Polymeric Substances Determination
5.8. Scum Formation
5.9. Dynamic of MCs Production and Growth
5.10. Analyses of Cyanotoxins
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, M.; Willis, A.; Burford, M.A.; Li, M. A meta-analysis comparing cell-division and cell-adhesion in Microcystis colony formation. Harmful Algae 2017, 67, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbiol. 2015, 6, 1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Sant, C.L.; De Carvalho, L.R.; Fiore, M.F.; Silva-Stenico, M.E.; Lorenzi, A.S.; Rios, F.R.; Konno, K.; Garcia, C.; Lagos, N. Highly toxic Microcystis aeruginosa strain, isolated from São Paulo—Brazil, produce hepatotoxins and paralytic shellfish poison neurotoxins. Neurotox. Res. 2011, 19, 389–402. [Google Scholar]
- Podduturi, R.; Schlüter, L.; Liu, T.; Osti, J.A.S.; Moraes, M.d.A.B.; Jørgensen, N.O. Monitoring of saxitoxin production in lakes in Denmark by molecular, chromatographic and microscopic approaches. Harmful Algae 2021, 101, 101966. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Z.; Zhang, S.-F.; Zhang, Y.; Lin, L. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: A molecular overview. J. Proteom. 2016, 135, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.J.; Wilson, K.; Veitch, M.G. An outbreak of paralytic shellfish poisoning in Tasmania. Commun. Dis. Intell. 2018, 42, S2209–S6051. [Google Scholar]
- Suleiman, M.; Jelip, J.; Rundi, C.; Chua, T.H. Case report: Paralytic shellfish poisoning in Sabah, Malaysia. Am. J. Trop. Med. Hyg. 2017, 97, 1731–1736. [Google Scholar] [CrossRef] [Green Version]
- WHO. Cyanobacterial Toxins: Microcystin-Lr Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- WHO. Guidelines for Safe Recreational Water Environments: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2003; Volume 1. [Google Scholar]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef] [Green Version]
- Drugă, B.; Buda, D.-M.; Szekeres, E.; Chiş, C.; Chiş, I.; Sicora, C. The impact of cation concentration on Microcystis (cyanobacteria) scum formation. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563. [Google Scholar] [CrossRef]
- Naveed, S.; Li, C.; Lu, X.; Chen, S.; Yin, B.; Zhang, C.; Ge, Y. Microalgal extracellular polymeric substances and their interactions with metal (loid) s: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1769–1802. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human-and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef]
- Metcalf, J.; Banack, S.; Wessel, R.; Lester, M.; Pim, J.; Cassani, J.; Cox, P. Toxin analysis of freshwater cyanobacterial and marine harmful algal blooms on the west coast of Florida and implications for estuarine environments. Neurotox. Res. 2020, 39, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Hodoki, Y.; Sano, T.; Tada, K.; Watanabe, M.M. Adaptation of the freshwater bloom-forming cyanobacterium Microcystis aeruginosa to brackish water is driven by recent horizontal transfer of sucrose genes. Front. Microbiol. 2018, 9, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, S.W.; Bullerjahn, G.S.; McKay, R.M.L. The complicated and confusing ecology of Microcystis blooms. Mbio 2020, 11, e00529-20. [Google Scholar] [CrossRef]
- Donald, D.B.; Bogard, M.J.; Finlay, K.; Leavitt, P.R. Comparative effects of urea, ammonium, and nitrate on phytoplankton abundance, community composition, and toxicity in hypereutrophic freshwaters. Limnol. Oceanogr. 2011, 56, 2161–2175. [Google Scholar] [CrossRef]
- Posch, T.; Köster, O.; Salcher, M.M.; Pernthaler, J. Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat. Clim. Chang. 2012, 2, 809–813. [Google Scholar] [CrossRef] [Green Version]
- Wagner, N.D.; Quach, E.; Buscho, S.; Ricciardelli, A.; Kannan, A.; Naung, S.W.; Phillip, G.; Sheppard, B.; Ferguson, L.; Allen, A. Nitrogen form, concentration, and micronutrient availability affect microcystin production in cyanobacterial blooms. Harmful Algae 2021, 103, 102002. [Google Scholar] [CrossRef]
- Sa-Nguansil, S.; Lheknim, V. The occurrence and reproductive status of Yucatan molly Poecilia velifera (Regan, 1914) (Poeciliidae; Cyprinodontiformes): An alien fish invading the Songkhla Lake Basin, Thailand. Aquat. Invasions 2010, 5, 423–430. [Google Scholar] [CrossRef]
- Sakai, K.; Lheknim, V. Two new species of the genera Neocallichirus and Wolffogebia (Decapoda, Pleocyemata) from Thale Sap Songkhla, Songkhla Lagoon System, Songkhla Province, Thailand. Crustaceana 2014, 87, 91–100. [Google Scholar] [CrossRef]
- Rattanama, K.; Pattaratumrong, M.S.; Towatana, P.; Wongkamhaeng, K. Three new records of gammarid a mphipod in Songkhla Lake, Thailand. Trop. Life Sci. Res. 2016, 27, 53. [Google Scholar] [CrossRef]
- Chesoh, S.; Lim, A.; Tongkumchum, P. Trend of water quality and model for forecasting eutrophication occurrence in Songkhla Lake, Thailand. In Proceedings of the Taal 2007: The 12th World Lake Conference, Jaipur, India, 28 October–2 November 2007; pp. 834–839. [Google Scholar]
- Suwanidcharoen, S.; Liengcharernsit, W. Development of phytoplankton model with application to Songkhla Lake, Thailand. Lowl. Technol. Int. 2012, 14, 50–59. [Google Scholar]
- Pham, T.-L.; Tran, T.H.Y.; Shimizu, K.; Li, Q.; Utsumi, M. Toxic cyanobacteria and microcystin dynamics in a tropical reservoir: Assessing the influence of environmental variables. Environ. Sci. Pollut. Res. 2020, 1–14. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Massey, I.Y.; Al Osman, M.; Yang, F. An overview on cyanobacterial blooms and toxins production: Their occurrence and influencing factors. Toxin Rev. 2020, 1–21. [Google Scholar] [CrossRef]
- Tillett, D.; Dittmann, E.; Erhard, M.; Von Döhren, H.; Börner, T.; Neilan, B.A. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: An integrated peptide–polyketide synthetase system. Chem. Biol. 2000, 7, 753–764. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Clara, T.; Huang, F.; Wei, J.; Yang, F. Identification and characterization of the dominant Microcystis sp. cyanobacteria detected in Lake Dong Ting, China. J. Toxicol. Environ. Health Part A 2019, 82, 1143–1150. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Fatka, M.; Swanner, E.; Ikuma, K.; Liang, X.; Leung, T.; Howe, A. Improved detection of mcyA genes and their phylogenetic origins in harmful algal blooms. Water Res. 2020, 176, 115730. [Google Scholar] [CrossRef]
- Hu, C.; Rea, C.; Yu, Z.; Lee, J. Relative importance of Microcystis abundance and diversity in determining microcystin dynamics in Lake Erie coastal wetland and downstream beach water. J. Appl. Microbiol. 2016, 120, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Hisbergues, M.; Christiansen, G.; Rouhiainen, L.; Sivonen, K.; Börner, T. PCR-based identification of microcystin-producing genotypes of different cyanobacterial genera. Arch. Microbiol. 2003, 180, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Filatova, D.; Nunez, O.; Farré, M. Recent advances in the detection of natural toxins in freshwater environments. TrAC Trends Anal. Chem. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Christiansen, G.; Molitor, C.; Philmus, B.; Kurmayer, R. Nontoxic strains of cyanobacteria are the result of major gene deletion events induced by a transposable element. Mol. Biol. Evol. 2008, 25, 1695–1704. [Google Scholar] [CrossRef]
- Gkelis, S.; Zaoutsos, N. Cyanotoxin occurrence and potentially toxin producing cyanobacteria in freshwaters of Greece: A multi-disciplinary approach. Toxicon 2014, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, R.; Mihali, T.K.; Jeon, Y.J.; Pickford, R.; Pomati, F.; Neilan, B.A. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008, 74, 4044–4053. [Google Scholar] [CrossRef] [Green Version]
- Wiese, M.; D’agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, M.A.; Mohd Yusof, N.Y.; Tahir, N.I.; Ahmad, A.; Usup, G.; Sahrani, F.K.; Bunawan, H. Biosynthesis of saxitoxin in marine dinoflagellates: An omics perspective. Mar. Drugs 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereyra, J.P.; D’Agostino, P.M.; Mazmouz, R.; Woodhouse, J.N.; Pickford, R.; Jameson, I.; Neilan, B.A. Molecular and morphological survey of saxitoxin-producing cyanobacterium Dolichospermum circinale (Anabaena circinalis) isolated from geographically distinct regions of Australia. Toxicon 2017, 138, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; CRC Press: London, UK, 2021. [Google Scholar]
- Yang, Y.; Hou, J.; Wang, P.; Wang, C.; Wang, X.; You, G. Influence of extracellular polymeric substances on cell-NPs heteroaggregation process and toxicity of cerium dioxide NPs to Microcystis aeruginosa. Environ. Pollut. 2018, 242, 1206–1216. [Google Scholar] [CrossRef]
- Blanco, Y.; Rivas, L.A.; González-Toril, E.; Ruiz-Bermejo, M.; Moreno-Paz, M.; Parro, V.; Palacín, A.; Aguilera, Á.; Puente-Sánchez, F. Environmental parameters, and not phylogeny, determine the composition of extracellular polymeric substances in microbial mats from extreme environments. Sci. Total Environ. 2019, 650, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, M.; Duan, P.; Qu, Z.; Wu, H. Insights into the relationship between colony formation and extracellular polymeric substances (EPS) composition of the cyanobacterium Microcystis spp. Harmful Algae 2019, 83, 34–41. [Google Scholar] [CrossRef]
- Chen, M.; Tian, L.-L.; Ren, C.-Y.; Xu, C.-Y.; Wang, Y.-Y.; Li, L. Extracellular polysaccharide synthesis in a bloom-forming strain of Microcystis aeruginosa: Implications for colonization and buoyancy. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Zhang, H.; Qian, A.; Yu, H.; Xu, C.; Pan, R.; Shi, Y. The influence of extracellular polymeric substances on the coagulation process of cyanobacteria. Sci. Total Environ. 2020, 720, 137573. [Google Scholar] [CrossRef]
- Holland, A.; Kinnear, S. Interpreting the possible ecological role (s) of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Qin, B.; Zhang, Y.; Zhu, G.; Gao, G.; Huang, Q.; Yao, X. Extraction and characterization of bound extracellular polymeric substances from cultured pure cyanobacterium (Microcystis wesenbergii). J. Environ. Sci. 2014, 26, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, K.; Ren, J.; Wang, X.; Mu, Q.; Fan, W. Characterisation of extracellular polymeric substances from different cyanobacterial species and their influence on biocalcification processes. Environ. Chem. 2017, 14, 254–265. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shiah, F.-K.; Chen, Y.-L. Importance of large colony formation in bloom-forming cyanobacteria to dominate in eutrophic ponds. Ann. Limnol. Int. J. Limnol. 2011, 47, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Dervaux, J.; Mejean, A.; Brunet, P. Irreversible collective migration of cyanobacteria in eutrophic conditions. PLoS ONE 2015, 10, e0120906. [Google Scholar] [CrossRef] [PubMed]
- Medrano, E.A.; Uittenbogaard, R.; Van De Wiel, B.; Pires, L.D.; Clercx, H. An alternative explanation for cyanobacterial scum formation and persistence by oxygenic photosynthesis. Harmful Algae 2016, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Lee, A.K.; Yates, R.S.; Liang, S.; Rochelle, P.A. Analysis of microcystins in drinking water by ELISA and LC/MS/MS. J. Am. Water Work. Assoc. 2017, 109, 13–25. [Google Scholar] [CrossRef]
- Botha, C.J.; Laver, P.; Singo, A.; Venter, E.; Ferreira, G.C.H.; Rösemann, M.; Myburgh, J.G. Evaluation of a Norwegian-developed ELISA to determine microcystin concentrations in fresh water. Water Supply 2019, 19, 743–752. [Google Scholar] [CrossRef]
- Fischer, W.J.; Garthwaite, I.; Miles, C.O.; Ross, K.M.; Aggen, J.B.; Chamberlin, A.R.; Towers, N.R.; Dietrich, D.R. Congener-independent immunoassay for microcystins and nodularins. Environ. Sci. Technol. 2001, 35, 4849–4856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Sano, T.; Li, R.; Watanabe, M.M.; Liu, Y.; Kaya, K. Microcystin production of Microcystis viridis (cyanobacteria) under different culture conditions. Phycol. Res. 1998, 46, 19–23. [Google Scholar] [CrossRef]
- Long, B.M.; Jones, G.J.; Orr, P.T. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 2001, 67, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Makower, A.K.; Schuurmans, J.M.; Groth, D.; Zilliges, Y.; Matthijs, H.C.; Dittmann, E. Transcriptomics-aided dissection of the intracellular and extracellular roles of microcystin in Microcystis aeruginosa PCC 7806. Appl. Environ. Microbiol. 2015, 81, 544–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beversdorf, L.J.; Miller, T.R.; McMahon, K.D. Long-term monitoring reveals carbon–nitrogen metabolism key to microcystin production in eutrophic lakes. Front. Microbiol. 2015, 6, 456. [Google Scholar] [CrossRef] [Green Version]
- Miles, C. Toxinmasslist COM v15b [Data set]. Unpublished Work, 2018; Volume 604. Available online: https://www.researchgate.net/publication/324039408_Toxinmasslist_COM_v15b?channel=doi&linkId=5aba5f4caca2728f4fa3ed38&showFulltext=true (accessed on 29 July 2021).
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Liu, Y.; Ge, J.; Wang, W.; Chen, Y.; Montagnes, D. Aggregate formation and polysaccharide content of Chlorella pyrenoidosa Chick (Chlorophyta) in response to simulated nutrient stress. Bioresour. Technol. 2010, 101, 8336–8341. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Pan, R.; Qian, A.; Cong, H.; Lamas-Samanamud, G. Consideration on the Water Contamination of Cyanobacterial Extracellular Polymeric Substances (EPS). J. Environ. Sci. Allied Res. 2017, 1, 19–24. [Google Scholar]
- Pham, T.-L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Wiedner, C.; Visser, P.M.; Fastner, J.; Metcalf, J.S.; Codd, G.A.; Mur, L.R. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003, 69, 1475–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Li, X.; Xia, Q.; Dai, R. Transcriptomic survey on the microcystins production and growth of Microcystis aeruginosa under nitrogen starvation. Sci. Total Environ. 2020, 700, 134501. [Google Scholar] [CrossRef]
- Peng, G.; Lin, S.; Fan, Z.; Wang, X. Transcriptional and physiological responses to nutrient loading on toxin formation and photosynthesis in Microcystis aeruginosa FACHB-905. Toxins 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jüttner, F.; Lüthi, H. Topology and enhanced toxicity of bound microcystins in Microcystis PCC 7806. Toxicon 2008, 51, 388–397. [Google Scholar] [CrossRef]
- Yeung, A.C.; D’Agostino, P.M.; Poljak, A.; McDonald, J.; Bligh, M.W.; Waite, T.D.; Neilan, B.A. Physiological and proteomic responses of continuous cultures of Microcystis aeruginosa PCC 7806 to changes in iron bioavailability and growth rate. Appl. Environ. Microbiol. 2016, 82, 5918–5929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foss, A.J.; Miles, C.O.; Wilkins, A.L.; Rise, F.; Trovik, K.W.; Cieslik, K.; Aubel, M.T. Analysis of total microcystins and nodularins by oxidative cleavage of their ADMAdda, DMAdda, and Adda moieties. Anal. Chim. Acta X 2020, 6, 100060. [Google Scholar]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Cary, S.C.; Hamilton, D.P.; Holland, P.T. Further characterization of glycine-containing microcystins from the McMurdo dry valleys of Antarctica. Toxins 2015, 7, 493–515. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Namikoshi, M.; Evans, W.R.; Fardig, M.; Carmichael, W.W.; Rinehart, K.L. Three new microcystins, cyclic heptapeptide hepatotoxins, from Nostoc sp. strain 152. Chem. Res. Toxicol. 1992, 5, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Namikoshi, M.; Sun, F.; Choi, B.W.; Rinehart, K.L.; Carmichael, W.W.; Evans, W.R.; Beasley, V.R. Seven more microcystins from homer lake cells: Application of the general method for structure assignment of peptides containing Alpha, Beta-dehydroamino acid unit (s). J. Org. Chem. 1995, 60, 3671–3679. [Google Scholar] [CrossRef]
- Bouhaddada, R.; Nélieu, S.; Nasri, H.; Delarue, G.; Bouaïcha, N. High diversity of microcystins in a Microcystis bloom from an Algerian lake. Environ. Pollut. 2016, 216, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.-I.; Murata, H.; Qiang, Z.; Suzuki, M.; Kondo, F. Mass spectrometric screening method for microcystins in cyanobacteria. Toxicon 1996, 34, 701–710. [Google Scholar] [CrossRef]
- Borges, H.; Branco, L.; Martins, M.; Lima, C.; Barbosa, P.; Lira, G.; Bittencourt-Oliveira, M.; Molica, R. Cyanotoxin production and phylogeny of benthic cyanobacterial strains isolated from the northeast of Brazil. Harmful Algae 2015, 43, 46–57. [Google Scholar] [CrossRef]
- Harland, F.; Wood, S.A.; Broady, P.; Williamson, W.; Gaw, S. Changes in saxitoxin-production through growth phases in the metaphytic cyanobacterium Scytonema cf. crispum. Toxicon 2015, 103, 74–79. [Google Scholar] [CrossRef]
- Mesquita, M.C.; Lürling, M.; Dorr, F.; Pinto, E.; Marinho, M.M. Combined effect of light and temperature on the production of saxitoxins in Cylindrospermopsis raciborskii strains. Toxins 2019, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Moustaka-Gouni, M.; Hiskia, A.; Genitsaris, S.; Katsiapi, M.; Manolidi, K.; Zervou, S.-K.; Christophoridis, C.; Triantis, T.M.; Kaloudis, T.; Orfanidis, S. First report of Aphanizomenon favaloroi occurrence in Europe associated with saxitoxins and a massive fish kill in Lake Vistonis, Greece. Mar. Freshw. Res. 2017, 68, 793–800. [Google Scholar] [CrossRef]
- Smith, Z.J.; Martin, R.M.; Wei, B.; Wilhelm, S.W.; Boyer, G.L. Spatial and temporal variation in paralytic shellfish toxin production by benthic Microseira (Lyngbya) wollei in a freshwater New York lake. Toxins 2019, 11, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trainer, V.L.; Hardy, F.J. Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef]
- Yunes, J.S.; De La Rocha, S.; Giroldo, D.; Silveira, S.B.d.; Comin, R.; Bicho, M.d.S.; Melcher, S.S.; Sant’anna, C.L.; Vieira, A.A.H. Release of carbohydrates and proteins by a subtropical strain of Raphidiopsis brookii (cyanobacteria) able to produce saxitoxin at three nitrate concentrations. J. Phycol. 2009, 45, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.I.; Gomes, M.T.S.; Botelho, M.J.; Rudnitskaya, A. Paralytic shellfish toxins (PST)-transforming enzymes: A review. Toxins 2020, 12, 344. [Google Scholar] [CrossRef]
- Garcıa, C.; del Carmen Bravo, M.; Lagos, M.; Lagos, N. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef]
- Christensen, V.G.; Maki, R.P.; Stelzer, E.A.; Norland, J.E.; Khan, E. Phytoplankton community and algal toxicity at a recurring bloom in Sullivan Bay, Kabetogama Lake, Minnesota, USA. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Prommana, R.; Peerapornpisal, Y.; Whangchai, N.; Morrison, L.F.; Metcalf, J.S.; Ruangyuttikarn, W.; Towprom, A.; Codd, G.A. Microcystins in cyanobacterial blooms from two freshwater prawn (Macrobrachium rosenbergii) ponds in Northern Thailand. Sci. Asia 2006, 32, 365–370. [Google Scholar] [CrossRef]
- Ruangrit, K.; Whangchai, N.; Pekkoh, J.; Ruangyuttikarn, W.; Peerapornpisal, Y. First report on microcystins contamination in giant freshwater prawn (Macrobrachium rosenbergii) and nile tilapia (Tilapia nilotica) cultured in earthen ponds. Int. J. Agric. Biol. 2011, 13, 1–4. [Google Scholar]
- Whangchai, N.; Wanno, S.; Gutierrez, R.; Kannika, K.; Promna, R.; Iwami, N.; Itayama, T. Accumulation of microcystins in water and economic fish in Phayao Lake, and fish ponds along the Ing River tributary in Chiang Rai, Thailand. Agric. Sci. 2013, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Ruangsomboon, S.; Yongmanitchai, W.; Taveekijakarn, P.; Ganmanee, M. Cyanobacterial composition and microcystin accumulation in catfish pond. Chiang Mai J. Sci. 2014, 41, 27–38. [Google Scholar]
- Somdee, T.; Kaewkhiaw, K.; Somdee, A. Detection of toxic cyanobacteria and quantifi cation of microcystins in four recreational water reservoirs in Khon Kaen, Thailand. Asia-Pac. J. Sci. Technol. 2013, 18, 1–8. [Google Scholar]
- Pongswat, S.; Suphan, S. Toxic Algae as a Component of Phytoplankton in Irrigation Canals (Thailand). Chiang Mai J. Sci. 2015, 42, 560–577. [Google Scholar]
- Kungsuwan, A.; Arakawa, O.; Promdet, M.; Onoue, Y. Occurrence of paralytic shellfish poisons in Thai freshwater puffers. Toxicon 1997, 35, 1341–1346. [Google Scholar] [CrossRef]
- Sato, S.; Kodama, M.; Ogata, T.; Saitanu, K.; Furuya, M.; Hirayama, K.; Kakinuma, K. Saxitoxin as a toxic principle of a freshwater puffer, Tetraodon fangi, in Thailand. Toxicon 1997, 35, 137–140. [Google Scholar] [CrossRef]
- Belcher, H.; Swale, E. Culturing Algae: A Guide for Schools and Colleges; Institute of Terrestrial Ecology: Cambridge, UK, 1982. [Google Scholar]
- Ballot, A.; Fastner, J.; Wiedner, C. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany. Appl. Environ. Microbiol. 2010, 76, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungblut, A.D.; Hawes, I.; Mountfort, D.; Hitzfeld, B.; Dietrich, D.R.; Burns, B.P.; Neilan, B.A. Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ. Microbiol. 2005, 7, 519–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvola, L. Spectrophotometric determination of chlorophyll a and phaeopigments in ethanol extractions. Ann. Bot. Fenn. 1981, 18, 221–227. [Google Scholar]
- Dai, R.; Liu, H.; Qu, J.; Zhao, X.; Hou, Y. Effects of amino acids on microcystin production of the Microcystis aeruginosa. J. Hazard. Mater. 2009, 161, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Gkelis, S.; Tussy, P.F.; Zaoutsos, N. Isolation and preliminary characterization of cyanobacteria strains from freshwaters of Greece. Open Life Sci. 2015, 10, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Jittawuttipoka, T.; Planchon, M.; Spalla, O.; Benzerara, K.; Guyot, F.; Cassier-Chauvat, C.; Chauvat, F. Multidisciplinary evidences that Synechocystis PCC6803 exopolysaccharides operate in cell sedimentation and protection against salt and metal stresses. PLoS ONE 2013, 8, e55564. [Google Scholar]
- Yang, Z.; Kong, F.; Shi, X.; Zhang, M.; Xing, P.; Cao, H. Changes in the morphology and polysaccharide content of Microcystis aeruginosa (Cyanobacteria) during flagellate grazing. J. Phycol. 2008, 44, 716–720. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Dos Anjos, F.M.; do Carmo Bittencourt-Oliveira, M.; Zajac, M.P.; Hiller, S.; Christian, B.; Erler, K.; Luckas, B.; Pinto, E. Detection of harmful cyanobacteria and their toxins by both PCR amplification and LC-MS during a bloom event. Toxicon 2006, 48, 239–245. [Google Scholar] [CrossRef] [PubMed]


| Water Characteristics | Songkhla Lake, Songkhla, Thailand | Thale Noi, Pattalung, Thailand | Hat Yai Municipal Wastewater Treatment Plant (HMWTP) | ||
|---|---|---|---|---|---|
| BOD (mg/L) | 4.62 | 4.62 | Below LOD * | 3.63 | 5.4 |
| COD (mg/L) | 46 | 50 | 28 | 35 | 37.6 |
| TP (mg/L) | 0.11 | 0.32 | 0.08 | 0.11 | 0.24 |
| TKN (mg/L) | 1.15 | 1.1 | 0.78 | 0.69 | 0.58 |
| DO (mg/L) | 4.37 | 1.82 | 4.58 | 7.02 | 5.8 |
| Temperature (°C) | 32 | 32 | 31 | 29 | 30 |
| pH | 8.34 | 8.1 | 7.23 | 7.38 | 6.7 |
| Salinity (g/L) | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 |
| Coordinates | 7°47′335.9″ N 100°15′28.0″ E | 7°4610.0″ N 100°18′08.8″ E | 7°41′11.0″ N 100°12′31.1″ E | 7°46′40.6″ N 100°07′22.1″ E | 7°46′40.6″ N 100°07′22.1″ E |
| Isolates | SF11 | SG03, SG10, SG11, SG12 | SH12, SH13 | PB07 | WOT01, WIN01 |
| Microcystis Sp. | Genotypes | Phenotypes | ||||
|---|---|---|---|---|---|---|
| mcyAa | mcyAb | stxtA | The Specific Growth Rate *, µ (Day−1) | Chlorophyll a (Day 15, µg/mL) | MCs Production (Day 15, µg/mL) | |
| SF11 | - | + | - | 0.291 | 3.386 | 194 |
| SG03 | + | + | + | 0.312 | 4.139 | 314 |
| PB07 | - | + | - | 0.332 | 3.514 | 188 |
| SG10 | + | + | - | 0.312 | 4.182 | 210 |
| SG11 | + | - | + | 0.312 | 4.024 | 306 |
| SG12 | + | + | + | 0.329 | 4.37 | 281 |
| SH12 | + | + | - | 0.211 | 3.889 | 43 |
| SH13 | - | + | - | 0.209 | 4.173 | 142 |
| WOT01 | - | + | + | 0.271 | 3.982 | 279 |
| WIN01 | - | + | + | 0.261 | 4 | 273 |
| Compound Name | Neutral Formular | Confidence | Rt (min) | m/z | Mass | Diff. (ppm) | |
|---|---|---|---|---|---|---|---|
| 1 | Decarbamoylsaxitoxin | C9H16N6O3 | Confirmed | 6.431 | 257.136 | 256.1286 | −0.63 |
| 2 | [Gly1,D-Asp3,(EtOH)Mdhb7]MC-Y(H2)Y(OMe) | C57H77N7O16 | Tentative | 4.132 | 558.778 | 1115.5409 | 1.56 |
| 3 | [L-Ser1,D-Asp3]MC-LY(OMe) | C52H71N7O15 | Tentative | 4.926 | 517.7576 | 1033.5005 | 0.29 |
| 4 | [DMAdda5,Mglu6,Mala7]MC-Y(H4)A | C49H71N7O13 | Tentative | 4.929 | 483.764 | 965.5125 | −1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naknaen, A.; Ratsameepakai, W.; Suttinun, O.; Sukpondma, Y.; Khan, E.; Pomwised, R. Microcystis Sp. Co-Producing Microcystin and Saxitoxin from Songkhla Lake Basin, Thailand. Toxins 2021, 13, 631. https://doi.org/10.3390/toxins13090631
Naknaen A, Ratsameepakai W, Suttinun O, Sukpondma Y, Khan E, Pomwised R. Microcystis Sp. Co-Producing Microcystin and Saxitoxin from Songkhla Lake Basin, Thailand. Toxins. 2021; 13(9):631. https://doi.org/10.3390/toxins13090631
Chicago/Turabian StyleNaknaen, Ampapan, Waraporn Ratsameepakai, Oramas Suttinun, Yaowapa Sukpondma, Eakalak Khan, and Rattanaruji Pomwised. 2021. "Microcystis Sp. Co-Producing Microcystin and Saxitoxin from Songkhla Lake Basin, Thailand" Toxins 13, no. 9: 631. https://doi.org/10.3390/toxins13090631
APA StyleNaknaen, A., Ratsameepakai, W., Suttinun, O., Sukpondma, Y., Khan, E., & Pomwised, R. (2021). Microcystis Sp. Co-Producing Microcystin and Saxitoxin from Songkhla Lake Basin, Thailand. Toxins, 13(9), 631. https://doi.org/10.3390/toxins13090631





