Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review
Abstract
1. Introduction
2. Results
- Idiopathic hyperhidrosis
- Chromhidrosis
- Bromhidrosis
- Epidermolysis bullosa simplex, Weber–Cockayne type
- Darier disease
- Pachyonychia congenita
- Alopecia areata
- Androgenetic alopecia
2.1. Sweat Gland Disorders
2.1.1. Idiopathic Hyperhidrosis
2.1.2. Chromhidrosis
2.1.3. Bromhidrosis
2.2. Facial Erythema and Flushing
2.3. Raynaud Phenomenon
2.4. Pompholyx
2.5. Eccrine Nevus
2.6. Postherpetic Neuralgia
2.7. Oily Skin
2.8. Notalgia Paresthetica
2.9. Hailey–Hailey Disease
2.10. Genodermatoses
2.10.1. Epidermolysis Bullosa Simplex, Weber–Cockayne Type
2.10.2. Darier Disease
2.10.3. Pachyonychia Congenita
2.11. Hidradenitis Suppurativa
2.12. Aquagenic Keratoderma
2.13. Alopecia
2.13.1. Alopecia Areata
2.13.2. Androgenetic Alopecia
2.14. Psoriasis
3. Discussion
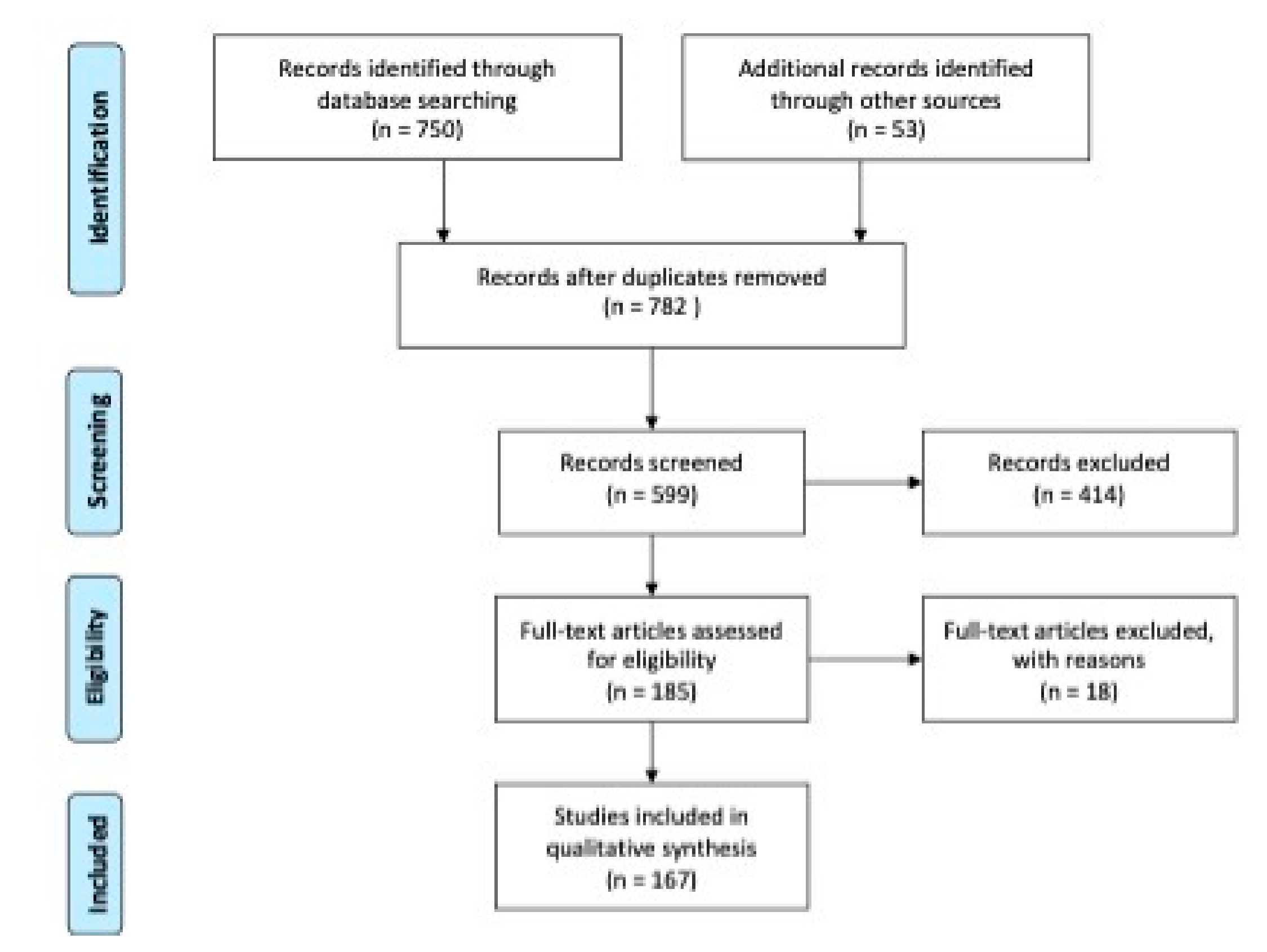
4. Materials and Methods
4.1. Literature Searches and Selection
4.2. Data Extraction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lowe, N.; Campanati, A.; Bodokh, I.; Cliff, S.; Jaen, P.; Kreyden, O.; Naumann, M.; Offidani, A.; Vadoud, J.; Hamm, H. The place of botulinum toxin type A in the treatment of focal hyperhidrosis. Br. J. Dermatol. 2004, 151, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Giuliodori, K.; Martina, E.; Giuliano, A.; Ganzetti, G.; Offidani, A. Onabotulinumtoxin type A (Botox(®)) versus Incobotulinumtoxin type A (Xeomin(®)) in the treatment of focal idiopathic palmar hyperhidrosis: Results of a comparative double-blind clinical trial. J. Neural Transm. 2014, 121, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Sandroni, L.; Gesuita, R.; Giuliano, A.; Giuliodori, K.; Marconi, B.; Ganzetti, G.; Offidani, A. Treatment of focal idiopathic hyperhidrosis with Botulinum Toxin Type A: Clinical predictive factors of relapse-free survival. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Bernardini, M.L.; Gesuita, R.; Offidani, A. Plantar focal idiopathic hyperhidrosis and botulinum toxin: A pilot study. Eur. J. Dermatol. 2007, 17, 52–54. [Google Scholar]
- Campanati, A.; Lagalla, G.; Penna, L.; Gesuita, R.; Offidani, A. Local neural block at the wrist for treatment of palmar hyperhidrosis with botulinum toxin: Technical improvements. J. Am. Acad. Dermatol. 2004, 51, 345–348. [Google Scholar] [CrossRef]
- Campanati, A.; Giuliodori, K.; Giuliano, A.; Martina, E.; Ganzetti, G.; Marconi, B.; Chiarici, A.; Offidani, A. Treatment of palmar hyperhidrosis with botulinum toxin type A: Results of a pilot study based on a novel injective approach. Arch. Dermatol. Res. 2013, 305, 691–697. [Google Scholar] [CrossRef]
- Campanati, A.; Penna, L.; Guzzo, T.; Menotta, L.; Silvestri, B.; Lagalla, G.; Gesuita, R.; Offidani, A. Quality-of-life assessment in patients with hyperhidrosis before and after treatment with botulinum toxin: Results of an open-label study. Clin. Ther. 2003, 25, 298–308. [Google Scholar] [CrossRef]
- Campanati, A.; Martina, E.; Giuliodori, K.; Consales, V.; Bobyr, I.; Offidani, A. Botulinum Toxin Off-Label Use in Dermatology: A Review. Ski. Appendage Disord. 2017, 3, 39–56. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Lakraj, A.A.; Moghimi, N.; Jabbari, B. Hyperhidrosis: Anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins 2013, 5, 821–840. [Google Scholar] [CrossRef]
- Mirkovic, S.E.; Rystedt, A.; Balling, M.; Swartling, C. Hyperhidrosis Substantially Reduces Quality of Life in Children: A Retrospective Study Describing Symptoms, Consequences and Treatment with Botulinum Toxin. Acta Derm. Venereol. 2018, 98, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.M.; Cantor, R.M.; Lange, K.L.; Ahn, S.S. Palmar hyperhidrosis: Evidence of genetic transmission. J. Vasc. Surg. 2002, 35, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, F.; Brancati, F.; De Joanna, G.; Valente, E.M.; Lauria, G.; Albanese, A. Primary focal hyperhidrosis in a new family not linked to known loci. Dermatology 2011, 223, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, J.; Grimes, K.; Naumann, M.; Glaser, D.A.; Lowe, N.J.; Naver, H.; Ahn, S.; Stolman, L.P. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J. Am. Acad. Dermatol. 2004, 51, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, S.; Cha, J. Botulinum toxin: Pharmacology and injectable administration for the treatment of primary hyperhidrosis. J. Am. Acad. Dermatol. 2020, 82, 969–979. [Google Scholar] [CrossRef]
- Nawrocki, S.; Cha, J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Therapeutic options. J. Am. Acad. Dermatol. 2019, 81, 669–680. [Google Scholar] [CrossRef]
- Pariser, D.M.; Ballard, A. Topical therapies in hyperhidrosis care. Dermatol. Clin. 2014, 32, 485–490. [Google Scholar] [CrossRef]
- Bushara, K.O.; Park, D.M.; Jones, J.C.; Schutta, H.S. Botulinum toxin—A possible new treatment for axillary hyperhidrosis. Clin. Exp. Dermatol. 1996, 21, 276–278. [Google Scholar] [CrossRef]
- Hoorens, I.; Ongenae, K. Primary focal hyperhidrosis: Current treatment options and a step-by-step approach. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1–8. [Google Scholar] [CrossRef]
- Rosell, K.; Hymnelius, K.; Swartling, C. Botulinum toxin type A and B improve quality of life in patients with axillary and palmar hyperhidrosis. Acta Derm. Venereol. 2013, 93, 335–339. [Google Scholar] [CrossRef]
- Skiveren, J.; Nordahl Larsen, H.; Kjaerby, E.; Larsen, R. The influence of needle size on pain perception in patients treated with botulinum toxin A injections for axillary hyperhidrosis. Acta Derm. Venereol. 2011, 91, 72–74. [Google Scholar] [CrossRef]
- Iannitti, T.; Palmieri, B.; Aspiro, A.; Di Cerbo, A. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des. Dev. Ther. 2014, 8, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Del Boz, J.; Padilla-España, L.; Segura-Palacios, J.M. Botulinum toxin injection technique for axillary hyperhidrosis. Actas Dermosifiliogr. 2014, 105, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Marcella, S.; Goodman, G.; Cumming, S.; Foley, P.; Morgan, V. Thirty-five units of botulinum toxin type A for treatment of axillary hyperhidrosis in female patients. Australas. J. Dermatol. 2011, 52, 123–126. [Google Scholar] [CrossRef]
- Scamoni, S.; Valdatta, L.; Frigo, C.; Maggiulli, F.; Cherubino, M. Treatment of primary axillary hyperhidrosis with botulinum toxin type a: Our experience in 50 patients from 2007 to 2010. ISRN Dermatol. 2012, 2012, 702714. [Google Scholar] [CrossRef] [PubMed]
- D′Epiro, S.; Macaluso, L.; Salvi, M.; Luci, C.; Mattozzi, C.; Marzocca, F.; Salvo, V.; Scarnò, M.; Calvieri, S.; Richetta, A.G. Safety and prolonged efficacy of Botulin Toxin A in primary hyperhidrosis. Clin. Ther. 2014, 165, e395–e400. [Google Scholar]
- Lecouflet, M.; Leux, C.; Fenot, M.; Célerier, P.; Maillard, H. Duration of efficacy increases with the repetition of botulinum toxin A injections in primary axillary hyperhidrosis: A study in 83 patients. J. Am. Acad. Dermatol. 2013, 69, 960–964. [Google Scholar] [CrossRef]
- Lecouflet, M.; Leux, C.; Fenot, M.; Célerier, P.; Maillard, H. Duration of efficacy increases with the repetition of botulinum toxin A injections in primary palmar hyperhidrosis: A study of 28 patients. J. Am. Acad. Dermatol. 2014, 70, 1083–1087. [Google Scholar] [CrossRef]
- Berthin, C.; Maillard, H. Duration of Efficacy Increases with the Repetition of Botulinum Toxin A Injections in Primary Axillary Hyperhidrosis: A 15-year Study in 117 Patients. Acta Derm. Venereol. 2019, 99, 1237–1240. [Google Scholar] [CrossRef]
- Budamakuntla, L.; Loganathan, E.; George, A.; Revanth, B.N.; Sankeerth, V.; Sarvjnamurthy, S.A. Comparative Study of Efficacy and Safety of Botulinum Toxin a Injections and Subcutaneous Curettage in the Treatment of Axillary Hyperhidrosis. J. Cutan. Aesthet. Surg. 2017, 10, 33–39. [Google Scholar]
- Ibrahim, O.; Kakar, R.; Bolotin, D.; Nodzenski, M.; Disphanurat, W.; Pace, N.; Becker, L.; West, D.P.; Poon, E.; Veledar, E.; et al. The comparative effectiveness of suction-curettage and onabotulinumtoxin-A injections for the treatment of primary focal axillary hyperhidrosis: A randomized control trial. J. Am. Acad. Dermatol. 2013, 69, 88–95. [Google Scholar] [CrossRef]
- Iwase, S.; Ikeda, T.; Kitazawa, H.; Hakusui, S.; Sugenoya, J.; Mano, T. Altered response in cutaneous sympathetic outflow to mental and thermal stimuli in primary palmoplantar hyperhidrosis. J. Auton. Nerv. Syst. 1997, 64, 65–73. [Google Scholar] [CrossRef]
- Rajagopal, R.; Mallya, N.B. Comparative evaluation of botulinum toxin versus iontophoresis with topical aluminium chloride hexahydrate in treatment of palmar hyperhidrosis. Med. J. Armed Forces India 2014, 70, 247–252. [Google Scholar] [CrossRef]
- Campanati, A.; Gregoriou, S.; Kontochristopoulos, G.; Offidani, A. Oxybutynin for the Treatment of Primary Hyperhidrosis: Current State of the Art. Ski. Appendage Disord. 2015, 1, 6–13. [Google Scholar] [CrossRef]
- Teivelis, M.P.; Wolosker, N.; Krutman, M.; Milanez de Campos, J.R.; Kauffman, P.; Puech-Leão, P. Compensatory hyperhidrosis: Results of pharmacologic treatment with oxybutynin. Ann. Thorac. Surg. 2014, 98, 1797–1802. [Google Scholar] [CrossRef]
- Wolosker, N.; Teivelis, M.P.; Krutman, M.; de Paula, R.P.; Kauffman, P.; de Campos, J.R.; Puech-Leão, P. Long-term results of the use of oxybutynin for the treatment of axillary hyperhidrosis. Ann. Vasc. Surg. 2014, 28, 1106–1112. [Google Scholar] [CrossRef]
- Wolosker, N.; de Campos, J.R.; Kauffman, P.; Puech-Leão, P. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J. Vasc. Surg. 2012, 55, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, N.; de Campos, J.R.; Kauffman, P.; Yazbek, G.; Neves, S.; Puech-Leao, P. Use of oxybutynin for treating plantar hyperhidrosis. Int. J. Dermatol. 2013, 52, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Gregoriou, S.; Consales, V.; Rizzetto, G.; Bobyr, I.; Diotallevi, F.; Martina, E.; Kontochristopoulos, G.; Platsidaki, E.; Offidani, A. Combined treatment of palmar hyperhidrosis with botulinum toxin type A and oxybutynin chloride: Results of a clinical, multicenter, prospective study. Dermatol. Ther. 2020, 33, e14039. [Google Scholar] [CrossRef]
- Yuncu, G.; Turk, F.; Ozturk, G.; Atinkaya, C. Comparison of only T3 and T3–T4 sympathectomy for axillary hyperhidrosis regarding treatment effect and compensatory sweating. Interact Cardiovasc. Thorac. Surg. 2013, 17, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, A.R.; Del Grande, A.; Petracca, M.; Ialongo, T.; Ricciardi, L. Clinical differences between botulinum neurotoxin type A and B. Toxicon 2015, 107, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Karlqvist, M.; Rosell, K.; Rystedt, A.; Hymnelius, K.; Swartling, C. Botulinum toxin B in the treatment of craniofacial hyperhidrosis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1313–1317. [Google Scholar] [CrossRef]
- Basciani, M.; Di Rienzo, F.; Bizzarrini, M.; Zanchi, M.; Copetti, M.; Intiso, D. Efficacy of botulinum toxin type B for the treatment of primary palmar hyperhidrosis: A prospective, open, single-blind, multi-centre study. Arch. Dermatol. Res. 2014, 306, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural Transm. 2013, 120, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Kouris, A.; Agiasofitou, E.; Gregoriou, S.; Sofouri, E.; Kontochristopoulos, G.; Panagopoulos, G. Generalized neurological symptoms following treatment of focal hyperhidrosis with botulinum toxin A. Int. J. Dermatol. 2014, 53, e544–e547. [Google Scholar] [CrossRef]
- Schnider, P.; Binder, M.; Auff, E.; Kittler, H.; Berger, T.; Wolff, K. Double-blind trial of botulinum A toxin for the treatment of focal hyperhidrosis of the palms. Br. J. Dermatol. 1997, 136, 548–552. [Google Scholar] [CrossRef]
- Kouris, A.; Vavouli, C.; Markantoni, V.; Kontochristopoulos, G. Muscle weakness in treatment of palmar hyperhidrosis with botulinum toxin type a: Can it be prevented? J. Drugs Dermatol. 2014, 13, 1315–1316. [Google Scholar]
- Weinberg, T.; Solish, N.; Murray, C. Botulinum neurotoxin treatment of palmar and plantar hyperhidrosis. Dermatol. Clin. 2014, 32, 505–515. [Google Scholar] [CrossRef]
- Shah, A.; Tsianou, Z.; Suchak, R.; Mann, J. Apocrine Chromhidrosis. Am. J. Dermatopathol. 2020, 42, e147–e148. [Google Scholar] [CrossRef]
- Wu, J.M.; Mamelak, A.J.; Nussbaum, R.; McElgunn, P.S. Botulinum toxin a in the treatment of chromhidrosis. Dermatol. Surg. 2005, 31, 963–965. [Google Scholar] [CrossRef]
- Matarasso, S.L. Treatment of facial chromhidrosis with botulinum toxin type A. J. Am. Acad. Dermatol. 2005, 52, 89–91. [Google Scholar] [CrossRef]
- Pérez Tato, B.; Zamora Martínez, E.; Sánchez Albisua, B.; Pérez González, Y.C.; Polimón Olabarrieta, I.; Marinero Escobedo, S.; Fernández López, P. Facial and axillary apocrine chromhidrosis. Dermatol. Online J. 2012, 18, 13. [Google Scholar]
- He, J.; Wang, T.; Dong, J. Effectiveness of botulinum toxin A injection for the treatment of secondary axillary bromhidrosis. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Chang, C.K.; Wang, C.Y.; Liao, Y.S.; Chen, S.G. Efficacy and Safety of Botulinum Toxin A in Axillary Bromhidrosis and Associated Histological Changes in Sweat Glands: A Prospective Randomized Double-Blind Side-by-Side Comparison Clinical Study. Dermatol. Surg. 2019, 45, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dong, J.; He, J. Long-Term Safety and Efficacy of Botulinum Toxin A Treatment in Adolescent Patients with Axillary Bromhidrosis. Aesthet. Plast. Surg. 2018, 42, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, B.S.; Kim, M.B.; Oh, C.K.; Jang, H.S.; Kwon, K.S. A case of foul genital odor treated with botulinum toxin A. Dermatol. Surg. 2004, 30, 1233–1235. [Google Scholar] [PubMed]
- Pérez-Pérez, L.; García-Gavín, J.; Allegue, F.; Caeiro, J.L.; Fabeiro, J.M.; Zulaica, A. Notalgia paresthetica: Treatment using intradermal botulinum toxin A. Actas Dermosifiliogr. 2014, 105, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Sterodimas, A.; Nicolaou, M.; Paes, T.R. Successful use of Botulinum toxin—A for the treatment of neck and anterior chest wall flushing. Clin. Exp. Dermatol. 2003, 28, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.T.; Herne, K.; Dayan, S.H.; Woodward, J.A. Facial blanching due to neurotoxins: Proposed mechanisms. Dermatol. Surg. 2013, 39, 24–29. [Google Scholar] [CrossRef]
- Yuraitis, M.; Jacob, C.I. Botulinum toxin for the treatment of facial flushing. Dermatol. Surg. 2004, 30, 102–104. [Google Scholar]
- Alexandroff, A.B.; Sinclair, S.A.; Langtry, J.A. Successful use of botulinum toxin a for the treatment of neck and anterior chest wall flushing. Dermatol. Surg. 2006, 32, 1536. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, S.K.; Ferris, L.K.; Obagi, S. Re: Botulinum toxin for the treatment of facial flushing. Dermatol. Surg. 2005, 31, 491. [Google Scholar] [CrossRef] [PubMed]
- Odo, M.E.; Odo, L.M.; Farias, R.V.; Primavera, R.A.; Leão, L.; Cucé, L.C.; Juliano, Y. Botulinum toxin for the treatment of menopausal hot flushes: A pilot study. Dermatol. Surg. 2011, 37, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Geddoa, E.; Matar, H.E.; Paes, T.R. The use of botulinum toxin-A in the management of neck and anterior chest wall flushing: Pilot study. Int. J. Dermatol. 2013, 52, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Hyun, M.Y.; Jeong, S.Y.; Kim, B.J.; Kim, M.N.; Hong, C.K. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology 2015, 230, 299–301. [Google Scholar] [CrossRef]
- Abokwidir, M.; Feldman, S.R. Rosacea Management. Ski. Appendage Disord. 2016, 2, 26–34. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Glagoleva, E.; Araviiskaia, E. Pulsed dye laser followed by intradermal botulinum toxin type-A in the treatment of rosacea-associated erythema and flushing. Dermatol. Ther. 2020, e13976. [Google Scholar] [CrossRef] [PubMed]
- Friedman, O.; Koren, A.; Niv, R.; Mehrabi, J.N.; Artzi, O. The toxic edge-A novel treatment for refractory erythema and flushing of rosacea. Lasers Surg. Med. 2019, 51, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Bloom, B.S.; Payongayong, L.; Mourin, A.; Goldberg, D.J. Impact of intradermal abobotulinumtoxinA on facial erythema of rosacea. Dermatol. Surg. 2015, 41, S9–S16. [Google Scholar] [CrossRef]
- Oh, Y.J.; Lee, N.Y.; Suh, D.H.; Koh, J.S.; Lee, S.J.; Shin, M.K. A split-face study using botulinum toxin type B to decrease facial erythema index. J. Cosmet. Laser Ther. 2011, 13, 243–248. [Google Scholar] [CrossRef]
- Shah, A.R. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J. Drugs Dermatol. 2008, 7, 847–850. [Google Scholar]
- Herrick, A.L. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat. Rev. Rheumatol. 2012, 8, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Herrick, A.L.; Wigley, F.M. Raynaud′s phenomenon. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101474. [Google Scholar] [CrossRef] [PubMed]
- Levien, T.L. Advances in the treatment of Raynaud′s phenomenon. Vasc. Health Risk Manag. 2010, 6, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Hao, Y.; Feng, Y.; Pan, L.; Liu, W.; Li, B.; Xiao, L.; Jin, L.; Nie, Z. The mechanism of botulinum A on Raynaud syndrome. Drug Des. Dev. Ther. 2018, 12, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Sycha, T.; Graninger, M.; Auff, E.; Schnider, P. Botulinum toxin in the treatment of Raynaud′s phenomenon: A pilot study. Eur. J. Clin. Investig. 2004, 34, 312–313. [Google Scholar] [CrossRef]
- Van Beek, A.L.; Lim, P.K.; Gear, A.J.; Pritzker, M.R. Management of vasospastic disorders with botulinum toxin A. Plast. Reconstr. Surg. 2007, 119, 217–226. [Google Scholar] [CrossRef]
- Neumeister, M.W.; Chambers, C.B.; Herron, M.S.; Webb, K.; Wietfeldt, J.; Gillespie, J.N.; Bueno, R.A., Jr.; Cooney, C.M. Botox therapy for ischemic digits. Plast. Reconstr. Surg. 2009, 124, 191–201. [Google Scholar] [CrossRef]
- Medina, S.; Gómez-Zubiaur, A.; Valdeolivas-Casillas, N.; Polo-Rodríguez, I.; Ruíz, L.; Izquierdo, C.; Guirado, C.; Cabrera, A.; Trasobares, L. Botulinum toxin type A in the treatment of Raynaud′s phenomenon: A three-year follow-up study. Eur. J. Rheumatol. 2018, 5, 224–229. [Google Scholar] [CrossRef]
- Zhao, H.; Lian, Y. Clinical and image improvement of Raynaud′s phenomenon after botulinum toxin type A treatment. Australas. J. Dermatol. 2015, 56, 202–205. [Google Scholar] [CrossRef]
- Dhaliwal, K.; Griffin, M.; Denton, C.P.; Butler, P.E.M. The novel use of botulinum toxin A for the treatment of Raynaud′s phenomenon in the toes. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Berk-Krauss, J.; Christman, M.P.; Franks, A.; Sicco, K.L.; Liebman, T.N. Botulinum toxin for treatment of Raynaud phenomenon in CREST syndrome. Dermatol. Online J. 2018, 24, 13030. [Google Scholar]
- Winter, A.R.; Camargo Macias, K.; Kim, S.; Sami, N.; Weinstein, D. The Effect of Abobotulinum Toxin A on the Symptoms of Raynaud′s Phenomenon: A Case Series. Cureus 2020, 12, e8235. [Google Scholar]
- Quintana Castanedo, L.; Feito Rodríguez, M.; Nieto Rodríguez, D.; Maseda Pedrero, R.; Chiloeches Fernández, C.; de Lucas Laguna, R. Botulinum Toxin A Treatment for Primary and Secondary Raynaud′s Phenomenon in Teenagers. Dermatol. Surg. 2021, 47, 61–64. [Google Scholar] [CrossRef]
- Jenkins, S.N.; Neyman, K.M.; Veledar, E.; Chen, S.C. A pilot study evaluating the efficacy of botulinum toxin A in the treatment of Raynaud phenomenon. J. Am. Acad. Dermatol. 2013, 69, 834–835. [Google Scholar] [CrossRef]
- Bello, R.J.; Cooney, C.M.; Melamed, E.; Follmar, K.; Yenokyan, G.; Leatherman, G.; Shah, A.A.; Wigley, F.M.; Hummers, L.K.; Lifchez, S.D. The Therapeutic Efficacy of Botulinum Toxin in Treating Scleroderma-Associated Raynaud′s Phenomenon: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arthritis Rheumatol. 2017, 69, 1661–1669. [Google Scholar] [CrossRef]
- Fregene, A.; Ditmars, D.; Siddiqui, A. Botulinum toxin type A: A treatment option for digital ischemia in patients with Raynaud′s phenomenon. J. Hand Surg. Am. 2009, 34, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Castanedo, L.; Feito-Rodríguez, M.; De Lucas-Laguna, R. Interdigital injection of botulinum toxin for patients with Raynaud phenomenon. J. Am. Acad. Dermatol. 2020, 83, e399. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, K.; Griffin, M.F.; Salinas, S.; Howell, K.; Denton, C.P.; Butler, P.E.M. Optimisation of botulinum toxin type a treatment for the management of Raynaud′s phenomenon using a dorsal approach: A prospective case series. Clin. Rheumatol. 2019, 38, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- Motegi, S.; Yamada, K.; Toki, S.; Uchiyama, A.; Kubota, Y.; Nakamura, T.; Ishikawa, O. Beneficial effect of botulinum toxin A on Raynaud′s phenomenon in Japanese patients with systemic sclerosis: A prospective, case series study. J. Dermatol. 2016, 43, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Żebryk, P.; Puszczewicz, M.J. Botulinum toxin A in the treatment of Raynaud′s phenomenon: A systematic review. Arch. Med. Sci. 2016, 12, 864–870. [Google Scholar] [CrossRef]
- Uppal, L.; Dhaliwal, K.; Butler, P.E. A prospective study of the use of botulinum toxin injections in the treatment of Raynaud′s syndrome associated with scleroderma. J. Hand Surg. Eur. 2014, 39, 876–880. [Google Scholar] [CrossRef]
- Kossintseva, I.; Barankin, B. Improvement in both Raynaud disease and hyperhidrosis in response to botulinum toxin type A treatment. J. Cutan. Med. Surg. 2008, 12, 189–193. [Google Scholar] [CrossRef]
- Motegi, S.I.; Uehara, A.; Yamada, K.; Sekiguchi, A.; Fujiwara, C.; Toki, S.; Date, Y.; Nakamura, T.; Ishikawa, O. Efficacy of Botulinum Toxin B Injection for Raynaud′s Phenomenon and Digital Ulcers in Patients with Systemic Sclerosis. Acta Derm. Venereol. 2017, 97, 843–850. [Google Scholar] [CrossRef]
- Todberg, T.; Zachariae, C.; Bregnhøj, A.; Hedelund, L.; Bonefeld, K.K.; Nielsen, K.; Iversen, L.; Skov, L. The effect of botulinum neurotoxin A in patients with plaque psoriasis—an exploratory trial. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, M.W. Botulinum toxin type A in the treatment of Raynaud′s phenomenon. J. Hand Surg. Am. 2010, 35, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Swartling, C.; Naver, H.; Lindberg, M.; Anveden, I. Treatment of dyshidrotic hand dermatitis with intradermal botulinum toxin. J. Am. Acad. Dermatol. 2002, 47, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U. Pompholyx: A review of clinical features, differential diagnosis, and management. Am. J. Clin. Dermatol. 2010, 11, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Veien, N.K.; Menne, T. Treatment of hand eczema. Ski. Ther. Lett. 2003, 8, 4–7. [Google Scholar]
- Klein, A.W. Treatment of dyshidrotic hand dermatitis with intradermal botulinum toxin. J. Am. Acad. Dermatol. 2004, 50, 153–154. [Google Scholar] [CrossRef]
- Wollina, U.; Karamfilov, T. Adjuvant botulinum toxin A in dyshidrotic hand eczema: A controlled prospective pilot study with left-right comparison. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 40–42. [Google Scholar] [CrossRef]
- Kontochristopoulos, G.; Gregoriou, S.; Agiasofitou, E.; Nikolakis, G.; Rigopoulos, D.; Katsambas, A. Letter: Regression of relapsing dyshidrotic eczema after treatment of concomitant hyperhidrosis with botulinum toxin-A. Dermatol. Surg. 2007, 33, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; El-Kholy, S.; Farid, C. Botulinum toxin type A in chronic non-dyshidrotic palmar eczema: A side-by-side comparative study. J. Dermatol. 2020, 47, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Lera, M.; España, A.; Idoate, M. Focal hyperhidrosis secondary to eccrine naevus successfully treated with botulinum toxin type A. Clin. Exp. Dermatol. 2015, 40, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Kawaoka, J.C.; Gray, J.; Schappell, D.; Robinson-Bostom, L. Eccrine nevus. J. Am. Acad. Dermatol. 2004, 51, 301–304. [Google Scholar] [CrossRef]
- Honeyman, J.F.; Valdés, R.; Rojas, H.; Gaete, M. Efficacy of botulinum toxin for a congenital eccrine naevus. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1275–1276. [Google Scholar] [CrossRef]
- Sonntag, M.; Rauch, L.; Ruzicka, T.; Bruch-Gerharz, D. Botulinum toxin treatment of eccrine sweat gland nevus. Hautarzt 2005, 56, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Dalager, S.; Spaun, E.; Hedelund, L. Large eccrine angiomatous hamartoma: A novel clinical presentation of disease. J. Dermatol. Case Rep. 2015, 9, 58–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sotiriou, E.; Apalla, Z.; Panagiotidou, D.; Ioannidis, D. Severe post-herpetic neuralgia successfully treated with botulinum toxin A: Three case reports. Acta Derm. Venereol. 2009, 89, 214–215. [Google Scholar]
- Xiao, L.; Mackey, S.; Hui, H.; Xong, D.; Zhang, Q.; Zhang, D. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med. 2010, 11, 1827–1833. [Google Scholar] [CrossRef]
- Li, D.; Xiao, L. Combining Botulinum Toxin (A) Injection With Peripheral Nerve Stimulation in a Patient for Intractable Ophthalmic Postherpetic Neuralgia. Neuromodulation 2015, 18, 769–771. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, X.; Yang, G.; Shen, J.; Xie, P.; Zuo, X.; Xia, L.; Han, Q.; Zhao, Y. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: A meta-analysis of randomized controlled trials. Brain Behav. 2019, 9, e01409. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Jain, M.; Jain, S. Subcutaneous Injection of Botulinum Toxin-A in Postherpetic Neuralgia During Pregnancy. Ann. Indian Acad. Neurol. 2017, 20, 430. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.D.; Zhong, J.; Liu, Y.P.; Chen, H.X. Botulinum as a Toxin for Treating Post-herpetic Neuralgia. Iran. J. Public Health 2017, 46, 608–611. [Google Scholar]
- Emad, M.R.; Emad, M.; Taheri, P. The efficacy of intradermal injection of botulinum toxin in patients with post-herpetic neuralgia. Iran. Red Crescent Med. J. 2011, 13, 323–327. [Google Scholar] [PubMed]
- Apalla, Z.; Sotiriou, E.; Lallas, A.; Lazaridou, E.; Ioannides, D. Botulinum toxin A in postherpetic neuralgia: A parallel, randomized, double-blind, single-dose, placebo-controlled trial. Clin. J. Pain 2013, 29, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Tsai, S.K.; Kao, M.C.; Hu, J.S. Botulinum toxin A relieved neuropathic pain in a case of post-herpetic neuralgia. Pain Med. 2006, 7, 89–91. [Google Scholar] [CrossRef]
- BoTN-A: The efficacy of botulinum toxin for the treatment of trigeminal and postherpetic neuralgia: A systematic review with meta-analyses. Br. Dent. J. 2016, 221, 475. [CrossRef]
- Moon, Y.E.; Choi, J.H.; Park, H.J.; Park, J.H.; Kim, J.H. Ultrasound-Guided Nerve Block with Botulinum Toxin Type A for Intractable Neuropathic Pain. Toxins 2016, 8, 18. [Google Scholar] [CrossRef]
- Rose, A.E.; Goldberg, D.J. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol. Surg. 2013, 39, 443–448. [Google Scholar] [CrossRef]
- Li, Z.J.; Park, S.B.; Sohn, K.C.; Lee, Y.; Seo, Y.J.; Kim, C.D.; Kim, Y.S.; Lee, J.H.; Im, M. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. J. Dermatol. Sci. 2013, 72, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Endly, D.C.; Miller, R.A. Oily Skin: A review of Treatment Options. J. Clin. Aesthet. Dermatol. 2017, 10, 49–55. [Google Scholar] [PubMed]
- Shuo, L.; Ting, Y.; KeLun, W.; Rui, Z.; Rui, Z.; Hang, W. Efficacy and possible mechanisms of botulinum toxin treatment of oily skin. J. Cosmet. Dermatol. 2019, 18, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Raison-Peyron, N.; Meunier, L.; Acevedo, M.; Meynadier, J. Notalgia paresthetica: Clinical, physiopathological and therapeutic aspects. A study of 12 cases. J. Eur. Acad. Dermatol. Venereol. 1999, 12, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Weinfeld, P.K. Successful treatment of notalgia paresthetica with botulinum toxin type A. Arch. Dermatol. 2007, 143, 980–982. [Google Scholar] [CrossRef]
- Wallengren, J.; Bartosik, J. Botulinum toxin type A for neuropathic itch. Br. J. Dermatol. 2010, 163, 424–426. [Google Scholar] [CrossRef]
- Maari, C.; Marchessault, P.; Bissonnette, R. Treatment of notalgia paresthetica with botulinum toxin A: A double-blind randomized controlled trial. J. Am. Acad. Dermatol. 2014, 70, 1139–1141. [Google Scholar] [CrossRef]
- Ansari, A.; Weinstein, D.; Sami, N. Notalgia paresthetica: Treatment review and algorithmic approach. J. Dermatol. Treat. 2020, 31, 424–432. [Google Scholar] [CrossRef]
- Bagherani, N.; Smoller, B.R. The efficacy of botulinum toxin type A in the treatment of Hailey-Hailey disease. Dermatol. Ther. 2016, 29, 394–395. [Google Scholar] [CrossRef]
- Kang, N.G.; Yoon, T.J.; Kim, T.H. Botulinum toxin type A as an effective adjuvant therapy for Hailey-Hailey disease. Dermatol. Surg. 2002, 28, 543. [Google Scholar] [CrossRef]
- Garayar Cantero, M.; Canseco Martín, M.; Aguado García, Á.; Ruiz-Sánchez, D.; Valtueña, J.; Manchado López, P. Use of low-dose naltrexone in the treatment of severe Hailey-Hailey disease: One case report. Dermatol. Ther. 2019, 32, e12892. [Google Scholar] [CrossRef] [PubMed]
- Lapiere, J.C.; Hirsh, A.; Gordon, K.B.; Cook, B.; Montalvo, A. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol. Surg. 2000, 26, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Konrad, H.; Karamfilov, T.; Wollina, U. Intracutaneous botulinum toxin A versus ablative therapy of Hailey-Hailey disease—a case report. J. Cosmet. Laser Ther. 2001, 3, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Koeyers, W.J.; Van Der Geer, S.; Krekels, G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J. Dermatol. Treat. 2008, 19, 251–254. [Google Scholar] [CrossRef]
- López-Ferrer, A.; Alomar, A. Botulinum toxin A for the treatment of familial benign pemphigus. Actas Dermosifiliogr. 2012, 103, 532–535. [Google Scholar] [CrossRef]
- Charlton, O.A.; Stewart, T.J.; Rosen, R.H. Treatment of Hailey-Hailey disease with botulinum toxin. Australas. J. Dermatol. 2018, 59, 229–231. [Google Scholar] [CrossRef]
- Kothapalli, A.; Caccetta, T. Botulinum toxin type A for the first-line treatment of Hailey-Hailey disease. Australas. J. Dermatol. 2019, 60, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Swartling, C.; Karlqvist, M.; Hymnelius, K.; Weis, J.; Vahlquist, A. Botulinum toxin in the treatment of sweat-worsened foot problems in patients with epidermolysis bullosa simplex and pachyonychia congenita. Br. J. Dermatol. 2010, 163, 1072–1076. [Google Scholar] [CrossRef]
- Langan, S.M.; Williams, H.C. A systematic review of randomized controlled trials of treatments for inherited forms of epidermolysis bullosa. Clin. Exp. Dermatol. 2009, 34, 20–25. [Google Scholar] [CrossRef]
- Holahan, H.M.; Farah, R.S.; Ferguson, N.N.; Paller, A.S.; Legler, A.A. Treatment of symptomatic epidermolysis bullosa simplex with botulinum toxin in a pediatric patient. JAAD Case Rep. 2016, 2, 259–260. [Google Scholar] [CrossRef][Green Version]
- Kontochristopoulos, G.; Katsavou, A.N.; Kalogirou, O.; Agelidis, S.; Zakopoulou, N. Letter: Botulinum toxin type A: An alternative symptomatic management of Darier′s disease. Dermatol. Surg. 2007, 33, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Santiago-et-Sánchez-Mateos, J.L.; Beà, S.; Fernández, M.; Pérez, B.; Harto, A.; Jaén, P. Botulinum toxin type A for the preventive treatment of intertrigo in a patient with Darier′s disease and inguinal hyperhidrosis. Dermatol. Surg. 2008, 34, 1733–1737. [Google Scholar] [PubMed]
- Grando, S.A.; Zachary, C.B. The non-neuronal and nonmuscular effects of botulinum toxin: An opportunity for a deadly molecule to treat disease in the skin and beyond. Br. J. Dermatol. 2018, 178, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.R.; Sahota, A. Pachyonychia congenita and botulinum toxin. Br. J. Dermatol. 2020, 182, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Swartling, C.; Vahlquist, A. Treatment of pachyonychia congenita with plantar injections of botulinum toxin. Br. J. Dermatol. 2006, 154, 763–765. [Google Scholar] [CrossRef]
- González-Ramos, J.; Sendagorta-Cudós, E.; González-López, G.; Mayor-Ibarguren, A.; Feltes-Ochoa, R.; Herranz-Pinto, P. Efficacy of botulinum toxin in pachyonychia congenita type 1: Report of two new cases. Dermatol. Ther. 2016, 29, 32–36. [Google Scholar] [CrossRef]
- Koren, A.; Sprecher, E.; Reider, E.; Artzi, O. A treatment protocol for botulinum toxin injections in the treatment of pachyonychia congenita-associated keratoderma. Br. J. Dermatol. 2020, 182, 671–677. [Google Scholar] [CrossRef]
- Scheinfeld, N. Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol. Online J. 2013, 19, 1. [Google Scholar]
- Hua, V.J.; Kuo, K.Y.; Cho, H.G.; Sarin, K.Y. Hyperhidrosis affects quality of life in hidradenitis suppurativa: A prospective analysis. J. Am. Acad. Dermatol. 2020, 82, 753–754. [Google Scholar] [CrossRef]
- Martina, E.; Campanati, A.; Giuliodori, K.; Offidani, A. Hidradenitis suppurativa in Crohn′s disease during adalimumab therapy: A paradox? Acta Dermatovenerol. Alp. Pannonica Adriat. 2017, 26, 21–23. [Google Scholar]
- O′Reilly, D.J.; Pleat, J.M.; Richards, A.M. Treatment of hidradenitis suppurativa with botulinum toxin A. Plast. Reconstr. Surg. 2005, 116, 1575–1576. [Google Scholar] [CrossRef] [PubMed]
- Feito-Rodríguez, M.; Sendagorta-Cudós, E.; Herranz-Pinto, P.; de Lucas-Laguna, R. Prepubertal hidradenitis suppurativa successfully treated with botulinum toxin A. Dermatol. Surg. 2009, 35, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.B.; Burova, E.P. Hidradenitis suppurativa treated with Clostridium botulinum toxin A. Clin. Exp. Dermatol. 2014, 39, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Schultz, S.; Strouse, A.; Gater, D.R. Successful treatment of stage III hidradenitis suppurativa with botulinum toxin A. BMJ Case Rep. 2019, 12, e226064. [Google Scholar] [CrossRef]
- Campanati, A.; Martina, E.; Giuliodori, K.; Bobyr, I.; Consales, V.; Offidani, A. Two cases of Hidradenitis suppurativa and botulinum toxin type a therapy: A novel approach for a pathology that is still difficult to manage. Dermatol. Ther. 2019, 32, e12841. [Google Scholar] [CrossRef]
- Grimstad, Ø.; Kvammen, B.; Swartling, C. Botulinum Toxin Type B for Hidradenitis Suppurativa: A Randomised, Double-Blind, Placebo-Controlled Pilot Study. Am. J. Clin. Dermatol. 2020, 21, 741–748. [Google Scholar] [CrossRef]
- Rodríguez-Villa Lario, A.; Vega-Díez, D.; González-Cañete, M.; Gómez-Zubiaur, A.; Vélez-Velázquez, M.D.; Polo-Rodríguez, I.; Medina-Montalvo, S.; Trasobares-Marugán, L. Aquagenic keratoderma with dorsal involvement treated with botulinum toxin. Case report and review of literature. Dermatol. Ther. 2020, 33, e14347. [Google Scholar] [CrossRef]
- Diba, V.C.; Cormack, G.C.; Burrows, N.P. Botulinum toxin is helpful in aquagenic palmoplantar keratoderma. Br. J. Dermatol. 2005, 152, 394–395. [Google Scholar] [CrossRef]
- Houle, M.C.; Al Dhaybi, R.; Benohanian, A. Unilateral aquagenic keratoderma treated with botulinum toxin A. J. Dermatol. Case Rep. 2010, 4, 1–5. [Google Scholar] [CrossRef]
- Bagazgoitia, L.; Pérez-Carmona, L.; Salgüero, I.; Harto, A.; Jaén, P. Letter: Aquagenic keratoderma: Successful treatment with botulinum toxin. Dermatol. Surg. 2010, 36, 434–436. [Google Scholar] [CrossRef]
- Montoya, C.; Arias, L.M.; Salazar, M.; Restrepo, R. Water-induced dermatosis: Aquagenic keratoderma. A case report. Biomedica 2019, 39, 247–251. [Google Scholar] [CrossRef]
- Garayar Cantero, M.; Delgado Mucientes, C.; Muñoz Fernández-Lomana, C. Use of botulinum toxin in the treatment of aquagenic keratoderma: One case report. Dermatol. Ther. 2018, 31, e12689. [Google Scholar] [CrossRef]
- Rossi, R.; Del Bianco, E.; Isolani, D.; Baccari, M.C.; Cappugi, P. Possible involvement of neuropeptidergic sensory nerves in alopecia areata. Neuroreport 1997, 8, 1135–1138. [Google Scholar] [CrossRef]
- Paus, R.; Heinzelmann, T.; Schultz, K.D.; Furkert, J.; Fechner, K.; Czarnetzki, B.M. Hair growth induction by substance P. Lab. Investig. 1994, 71, 134–140. [Google Scholar]
- Cutrer, F.M.; Sandroni, P.; Wendelschafer-Crabb, G. Botulinum toxin treatment of cephalalgia alopecia increases substance P and calcitonin gene-related peptide-containing cutaneous nerves in scalp. Cephalalgia 2010, 30, 1000–1006. [Google Scholar] [CrossRef]
- Cutrer, F.M.; Pittelkow, M.R. Cephalalgic alopecia areata: A syndrome of neuralgiform head pain and hair loss responsive to botulinum A toxin injection. Cephalalgia 2006, 26, 747–751. [Google Scholar] [CrossRef]
- Irimia, P.; Palma, J.A.; Idoate, M.A.; España, A.; Riverol, M.; Martinez-Vila, E. Cephalalgia alopecia or nummular headache with trophic changes? A new case with prolonged follow-up. Headache 2013, 53, 994–997. [Google Scholar] [CrossRef]
- Cho, H.R.; Lew, B.L.; Lew, H.; Sim, W.Y. Treatment effects of intradermal botulinum toxin type A injection on alopecia areata. Dermatol. Surg. 2010, 36, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Carloni, R.; Pechevy, L.; Postel, F.; Zielinski, M.; Gandolfi, S. Is there a therapeutic effect of botulinum toxin on scalp alopecia? Physiopathology and reported cases: A systematic review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Freund, B.J.; Schwartz, M. Treatment of male pattern baldness with botulinum toxin: A pilot study. Plast. Reconstr. Surg. 2010, 126, 246e–248e. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Neema, S.; Vasudevan, B. A Pilot Study to Evaluate Effectiveness of Botulinum Toxin in Treatment of Androgenetic Alopecia in Males. J. Cutan. Aesthet. Surg. 2017, 10, 163–167. [Google Scholar]
- Zhang, L.; Yu, Q.; Wang, Y.; Ma, Y.; Shi, Y.; Li, X. A small dose of botulinum toxin A is effective for treating androgenetic alopecia in Chinese patients. Dermatol. Ther. 2019, 32, e12785. [Google Scholar] [CrossRef]
- Goldman, B.E.; Fisher, D.M.; Ringler, S.L. Transcutaneous PO2 of the scalp in male pattern baldness: A new piece to the puzzle. Plast. Reconstr. Surg. 1996, 97, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Shon, U.; Kim, M.H.; Lee, D.Y.; Kim, S.H.; Park, B.C. The effect of intradermal botulinum toxin on androgenetic alopecia and its possible mechanism. J. Am. Acad. Dermatol. 2020, 83, 1838–1839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, S.; Zhao, J.; Feng, X.; Zhang, M.; Zhao, Z. Effectiveness and Safety of Botulinum Toxin Type A in the Treatment of Androgenetic Alopecia. Biomed. Res. Int. 2020, 2020, 1501893. [Google Scholar] [CrossRef]
- Di Pietro, A.; Piraccini, B.M. Frontal Alopecia after Repeated Botulinum Toxin Type A Injections for Forehead Wrinkles: An Underestimated Entity? Ski. Appendage Disord. 2016, 2, 67–69. [Google Scholar] [CrossRef]
- Giannoni, M.; Consales, V.; Campanati, A.; Ganzetti, G.; Giuliodori, K.; Postacchini, V.; Liberati, G.; Azzaretto, L.; Vichi, S.; Guanciarossa, F.; et al. Homocysteine plasma levels in psoriasis patients: Our experience and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1781–1785. [Google Scholar] [CrossRef]
- Campanati, A.; Moroncini, G.; Ganzetti, G.; Pozniak, K.N.; Goteri, G.; Giuliano, A.; Martina, E.; Liberati, G.; Ricotti, F.; Gabrielli, A.; et al. Adalimumab Modulates Angiogenesis in Psoriatic Skin. Eur. J. Inflamm. 2013, 11, 489–498. [Google Scholar] [CrossRef]
- Campanati, A.; Paolinelli, M.; Diotallevi, F.; Martina, E.; Molinelli, E.; Offidani, A. Pharmacodynamics OF TNF α inhibitors for the treatment of psoriasis. Expert Opin. Drug Metab. Toxicol. 2019, 15, 913–925. [Google Scholar] [CrossRef]
- Radi, G.; Campanati, A.; Diotallevi, F.; Molinelli, E.; Offidani, A. Skin involvement in patients with psoriatic arthritis: Preliminary results of treatment with apremilast in real world setting. G. Ital. Dermatol. Venereol. 2019, 154, 166–169. [Google Scholar] [CrossRef]
- Ward, N.L.; Kavlick, K.D.; Diaconu, D.; Dawes, S.M.; Michaels, K.A.; Gilbert, E. Botulinum neurotoxin A decreases infiltrating cutaneous lymphocytes and improves acanthosis in the KC-Tie2 mouse model. J. Investig. Dermatol. 2012, 132, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, M.; Favot, F.; Bizzarini, M.; Piai, M.; Donini, M.; Sedona, P. Botulinum toxin type-A for the treatment of inverse psoriasis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 431–436. [Google Scholar] [CrossRef]
- Aschenbeck, K.A.; Hordinsky, M.K.; Kennedy, W.R.; Wendelschafer-Crabb, G.; Ericson, M.E.; Kavand, S.; Bertin, A.; Dykstra, D.D.; Panoutsopoulou, I.G. Neuromodulatory treatment of recalcitrant plaque psoriasis with onabotulinumtoxinA. J. Am. Acad. Dermatol. 2018, 79, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Chroni, E.; Monastirli, A.; Tsambaos, D. Botulinum toxin for inverse psoriasis? J. Eur. Acad. Dermatol. Venereol. 2009, 23, 955. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Brassard, D.; Benohanian, A. Inverse psoriasis and hyperhidrosis of the axillae responding to botulinum toxin type A. Arch. Dermatol. 2011, 147, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.; Ward, N.L. Efficacy of botulinum neurotoxin type A for treating recalcitrant plaque psoriasis. J. Drugs Dermatol. 2014, 13, 1407–1408. [Google Scholar]
- Bessa, G.R.; Grazziotin, T.C.; Manzoni, A.P.; Weber, M.B.; Bonamigo, R.R. Hailey-Hailey disease treatment with Botulinum toxin type A. An. Bras. Dermatol. 2010, 85, 717–722. [Google Scholar] [CrossRef]
- Ho, D.; Jagdeo, J. Successful botulinum toxin (onabotulinumtoxinA) treatment of Hailey-Hailey disease. J. Drugs Dermatol. 2015, 14, 68–70. [Google Scholar]
- González, C.; Franco, M.; Londoño, A.; Valenzuela, F. Breaking paradigms in the treatment of psoriasis: Use of botulinum toxin for the treatment of plaque psoriasis. Dermatol. Ther. 2020, 33, e14319. [Google Scholar] [CrossRef]
- Bagherani, N.; Smoller, B.R. The efficacy of botulinum neurotoxin A in the treatment of plaque psoriasis. Dermatol. Ther. 2018, 31, e12587. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
| First Author [Ref.], year | Type of Study | n (site) | BoNT-A Doses | Retreatment | Follow-Up | Results |
|---|---|---|---|---|---|---|
| Wu [54], 2019 | Prospective | 19 (axilla) | NA | NA | 3 months | mean degree of malodor and mean sweat production in the BoNT-A-treated axilla were significantly lower than those in the control axilla at 3 months after therapy. |
| Wang [55], 2018 | Prospective | 62 adolescents (axilla) | 50 U BoNT-a/axilla | Yes | The average follow-up was 2.64 years | 82% of patients (51/62) ranked the BoNT-A treatment to be very good or good. |
| He [53], 2017 | Prospective | 53 (secondary bromhidrosis, axillae) | 50 U BoNT-a/axilla | NA | 12months | 48 patients ranked the satisfaction with BoNT-A treatment as “very good” or “good” |
| Lee [56], 2004 | Cae report | 1 (genitalia) | 40 different sites (2.5 mU/0.1 mL per site) | NA | 9 months | odorless and anhydrous response in the genital region, |
| First Author [Ref.], year | Type of Study | n | BoNT Doses | Follow-Up | Results |
|---|---|---|---|---|---|
| Al-Niaimi F 2020 [67] | Prospective | 20 | In a 5 ml dilution of 500 units using typically 20–50 units per cheek or onabotulinum (BotoxTM, Allergan, Irvine, CA, USA) at 2.5 mL dilution in 100 units with doses ranging from 10 to 20 units per cheek. | 3,9 months | All patients experienced improvement of erythema (documented by a 3D Antera camera) |
| Friedman 2019 [68] | Retrospective | 16 | 100 U of abobotulinumtoxin after Tixel treament | 1,3,6 months | flushing and erythema improvement (photographic assessment) |
| Park, 2015 [65] | Case report | 2 | 3 U in chin and the eyebrow area were injected; after 1 week, 5 U in each cheek and 2 U in chin and the eyebrow area were additionally injected (patient 1) 40, 15 U in the first treatment and 5 U in the second treatment for each cheek (patient 2) | 1 week to 3 months | Good improvement (photographic assessment) |
| Bloom, 2015 [69] | Prospective | 25 | 15, 45 U of intradermal injections of abobotulinum toxin A into the nasal tip, nasal bridge, and nasal alae | 3 months | The treatment resulted in statistically significant improvement in erythema grade at 1, 2, and 3 months after treatment when compared with baseline (3-grade scale of erythema severity on photographic assessment) |
| Geddoa, 2013 [64] | Pilot prospective | 22 | 2 U per injection point with maximum dose of 100 U (neck and/or chest) | 4 weeks | Twenty patients (90.9%) reported immediate improvement, and the remaining 2 patients had a second treatment session to achieve similar responses; at 4 weeks follow-up significant improvement in quality of life was measured with DLQI score |
| Odo, 2011 [63] | RCT | 60 | 500 U abobotulinum toxin A, 6.2 U injection at each selected point in the skin (40 injection points of face, chest, neck, scalp); for the control group, saline solution was used at the same volume of 0.04 mL per injection point | 6 months | The symptoms were less severe than before treatment; in the control group, there was no significant difference in mean intensity of sweating or in the mean number of hot flashes |
| Oh, 2011 [70] | RCT | 15 | BoNT-B doses NA; one side of the face was treated with BoNT-B, the other side with saline | 8 weeks | Ineffective; mexameter demonstrated significant improvement of erythema at 8 weeks after injections on both sides; the BoNT-B injection side did not show a significant decrease in objective erythema, compared with the control side; subjective satisfaction did not differ between the treated side and the control side |
| Alexandroff, 2006 [61] | Case report | 2 | 10 U spaced/hemifacial 1 cm between injections | 6 weeks | No improvement was noted 6 weeks after treatment |
| Kranendonk 2005 [62] | Case report | 1 | 2 U in midcheek region | Not reported | Paralysis of the zygomaticus major; no improvement after 1 week |
| Yuraitis, 2004 [60] | Case report | 1 | A total of 10 U were distributed at 1-cm increments to each cheek in the areas of the most prominent erythema | 2 weeks | Marked improvement and high satisfaction |
| First Author [Ref.], year | Type of Study | n | BoNT-A Doses/Hand | Follow-Up | Retreatment | Results |
|---|---|---|---|---|---|---|
| Quintana Castanedo [84], 2020 | Prospective | 8 | _ | _ | No | Reduction in pain and in the frequency of RP episodes (7 patients) any changes (1 patient) |
| Winter [83], 2020 | Case series | 4 | 40–300 | 3–21 | Yes (50%) | Improved up to one year after treatment. |
| Dhaliwal [89], 2019 | Prospective | 40 | 100 across both hands reconstituted with 2 mL of normal saline by a single surgeon | 6–12 | no | Improved (Colour change and pain, swelling reduction) |
| Berk-Krauss [82], 2018 | Case report | 1 | 20 | 1, 3, 6 weeks | no | Improved pain Ulceration healed |
| Medina [79], 2018 | Retrospective | 15 | 100 botulinum toxin units type A in 5 mL of saline serum to 0.9% (dilution: 20 IU/mL). | 1 week 1, 3, 6, 12 months | no | Improved pain Ulceration healed (70%) |
| Dhaliwal [81], 2018 | Case reports | 3 | 10 | 6 weeks | no | Improved (pain, colour changes and cold intolerance) Thermographic imaging assessed |
| Bello [86], 2017 | RCT doubleblind; placebo | 40 | 50 units in 2.5 mL | 1, 4 months | no | Improved (pain) No changes in blod flow. Moor LDI2-IR scanner assessed) |
| Motegi [94], 2017 | Randomized trial single-blind no placebo | 45 | 250, 1000 or 2000 (U) of BoNT-B | 16 weeks | No | Improved (pain, DU) |
| Motegi [90], 2016 | Prospective, case series | 10 | 10 U/finger | 16 weeks | No | Improved (pain, DU, skin temperature) |
| Zhao [80], 2015 | Case series | 2 | 200–280 | 1 week, 5 months | No | Improved (pain, colour change, skin temperature) |
| Uppal [92], 2014 | Prospective | 20 | 100 U | 6 months | No | Improved (pain with VAS, DU) |
| Jenkins [85], 2013 | RCT pilot; doubleblind; placebo | 8 | 40 U | – | No | Increase in digital pulp temperature |
| Todberg [95], 2018 | Case report | 1 | 100 U | – | No | Patient reported improvement in pain and DU |
| Neumeister [96], 2010 Neumeister [78], 2009 | Retrospective case series | 33 | 50 U | 1–6 years | Yes (21%) | 100% of DU healed, relief in 85% of patients |
| Fregene [87], 2009 | Retrospective case series | 26 | 20, 100 U | 18 months | Yes (20%) | 48% of DU healed, 35% pain reduction (VAS) |
| Kossintseva [93], 2008 | Case report | 1 | 100 U | 12 months | No | Pain decreased, DU not reported |
| Van Beek [77], 2007 | Retrospective case series | 11 | 50, 200 U | 9.6 months | Yes (45%) | 100% decrease in pain (VAS), 82% of DU healed |
| Sycha [76], 2004 | Pilot to RCT, case report | 2 | 12, 300 U | – | No | 37% pain reduced in 1 patient (VAS), other unknown |
| First Author [Ref.], year | Type of Study | n | BoNT-A Doses/Hand | Follow-Up | Retreatment | Results |
|---|---|---|---|---|---|---|
| Ismail [102], 2020 | Prospective non-randomized side-by-side comparative study | 40 | 100 | 1, 4, 6 months | No | Improvement |
| Kontochristopoulos [101], 2007 | Case reports | 2 | 4.0 mL saline in 100 U BoNT-A; 100 U/hand | 8 weeks | No | Improvement |
| Swartling [96], 2002 | Prospective | 10 | 1.0 mL saline in 100 U BoNT-A; mean of 162 U/hand | 28–59 days | No | 7 of 10 patients reported a good result; improving in VAS for itching and disease activity score |
| Wollina [100], 2002 | Prospective; side-by-side monotherapy with topical steroid vs. adjuvant BoNT-A injection | 6 | 2.0 mL saline in 100 U BoNT-A | 8 weeks | No | Six of 6 hands treated with topical steroids in combination with BoNT-A showed improvement; BoNT-A showed a more rapid release from itching than steroids alone |
| First Author [Ref.], year | Type of Study | n | BoNT-A Doses | Follow-Up | Retreatment | Results |
|---|---|---|---|---|---|---|
| Sonntag [106], 2005 | Case report | 200 mU Dysport | 36 | Yes | Improvement | |
| Honeyman [105], 2008 | Case report | 1 | BoNT-A, dilution in 4 mL of saline, 5 U per injection; total amount not specified | 1 year | Not reported | Improvement |
| Lera [103], 2015 | Case report | 1 | 100 U BoNT-A in 2.5 mL of saline, 2 U per injection | 9 months | Yes | Improvement |
| Nygaard [107], 2015 | Case report | 1 | 100 U BoNT-A (dilution not specified) | 1 year | Not reported | Improvement |
| First Author [Ref.] | Type of Study | n | BoNT-A Doses | Follow-Up | Retreatment | Results |
|---|---|---|---|---|---|---|
| Ding [113], 2017 | Prospective | 58 | 50 to 100 | 2 weeks 1, 3, 6 months | No | 75% of patients improved (Variable VAS, NPS reduction) |
| Jain [112], 2017 | Case report | 2 | 500 units Dysport diluted with 5 mL of normal saline, making a concentration of 100 units/mL | 1, 2, 4, 8, 12, 16 weeks | No | VAS for pain decreased from 9, 10 to 1 |
| Moon [118], 2016 | Case report | 2 | 50 U BoNT-A and bupivacaine 0.1% injected under ultrasound guide in brachial plexus | 5 months | No | VAS for pain decreased from 8 to 2, 3 |
| Li [110], 2015 | Case report (ophthalmic) | 1 | 100 U of BoNT-A in the orbital region (subcutaneous) | 6 months | No | VAS for pain decreased from 8–9 to 2–3 |
| Apalla [115], 2013 | Randomized, double-blind, placebo-controlled trial | 29 (4 postherpetic) | 20, 190 U of BoNT-A intradermally | 16 weeks | No | VAS decreasing |
| Emad [114], 2011 | interventional study | 15U per 10 cm2 (The amount of toxin was different for every patient: not reported) intradermally | 2, 4 weeks | No | VAS decreasing | |
| Xiao [109], 2010 | Randomized, double-blind, placebo-controlled trial | 60 | 5 U/mL of BoNT-A vs. 0.5% of lidocaine vs. 0.9% of saline | 3 months | No | Decrease in VAS score and improving in sleep hours superior to control group |
| Sotiriou [108], 2009 | Case reports | 3 | 100 U of BoNT-A in 4 mL of saline; subcutaneous in chessboard pattern | 12 weeks | No | Decrease in VAS score |
| Liu [116], 2006 | Case report | 1 | 100 U of BoNT-A injected in a fanning pattern | 9 months | No | VAS pain reduction from 10 to 1 |
| First Author [Ref.], year | Type of Study | n | BoNT-A Doses | Retreatment | Follow-Up | Results |
|---|---|---|---|---|---|---|
| Maari [126], 2014 | RCT vs. placebo double-blind | 20 | max 200 U | No | 12 weeks, then placebo arm shifted to BoNT-A; total 24 weeks | No significant difference for pruritus (VAS) and hyperpigmentation |
| Pèrez-Pèrez [57], 2014 | Retrospective, case series | 5 | 48–56 U | No | 18 months | 2 worsening pruritus, little improvement in other 3 but for only 1 month |
| Wallengren [125], 2010 | Prospective | 6 | 18–100 U | No | 18 months | 5/6 patients a mean reduction of VAS by 28% at week 6; at 18 months 1 patient had a VAS of 45%, another one was still free from itch |
| Weinfeld [124], 2007 | Case report | 2 | 16–24 U | Yes, 18 months later with 48 U (only 1 patient) | 18 months | Improvement (patient self-assessment) |
| First Author [Ref.], year | n | Sites | BoNT-A Doses | Follow-Up | Results |
|---|---|---|---|---|---|
| Lapiere [131], 2000 | 1 | Axillae | 25 U, 50 U of per axilla after 6 months | 4 months at time of publication | Complete remission |
| Kang [129], 2002 | 1 | Groin, axillae | 100 U for each inguinal fold | 6 months | Improvement |
| Lopez-Ferrer [134], 2012 | 3 | Axillae groin breast, axilla, axillae and groin | 80 U/axilla 200 U total | 5 months | All patients improved but needed at least one retreatment after 1–3 months |
| Charlton [135], 2017 | 1 | Axillae and groin | 50 U for axillae 50 U for groin Once a year | 2 years | This therapy has restricted his disease activity to 1–2 episodes per year |
| Kothapalli [136], 2018 | NA | Axillae and groin | 50 units per axilla or groin | NA | Improvement |
| First Author [Ref.], year | Type of Study | n | BoNT-A Doses | Follow-Up | Retreatment | Results |
|---|---|---|---|---|---|---|
| O’Reilly [150], 2005 | Case report | 1 | 250 U Dysport/axilla | 10 months | No | Complete remission |
| Feito-Rodriguez [151], 2009 | Case report | 1 | 40 U total dose (inguinal folds) | 6 months | Yes | Complete remission |
| Khoo [152], 2014 | Case report | 3, but only 1 described | 50 U/axilla | 3 years | Yes (3 other times) | Complete remission |
| Shi [153], 2019 | Case report | 1 | 100 units for each area (bilateral axillary, inframammary and groin) | NA | Yes (5 total injections) | Resolution of inflammation and healing of draining sinuses |
| Campanati [154], 2019 | Case report | 2 | 50 U per axilla 100 U for each side of groin | 1 year | Yes (for patient 1, after 10 months after the first injection) | Real improvement |
| Grimstad [156], 2020 | Randomised, Double-Blind, Placebo-Controlled Pilot Study | 20 | 150 U/armpit, 200 U/groin, and 600 U in the perianal/perigenital areas | 6 months | Yes (3 months after the first injection) | Clear improvement of the quality of life |
| First Author [Ref.], year | Type of Study | n | BoNT Doses | Follow-Up | Retreatment | Results |
|---|---|---|---|---|---|---|
| Freund [168], 2010 | Open-label pilot study | 50 male | 150 U | 60 weeks | Two treatment cycles of 24 weeks each | Treatment response rate was 75 percent |
| Singh [169], 2017 | Open-label pilot study | 10 male | 150 U | 6 months | No | Of 10 patients, 8 had good to excellent response on photographic assessment |
| Zhang [170], 2019 | Open-label pilot study | 24 male | 50 U | 6 months | No | Variable results |
| Shon [172], 2020 | Open-label pilot study | 18 male | 30 U | 6 months | Yes (every 4 weeks for 24 weeks). | Air density was significantly improved after 6 months but not after 3 months. |
| Zhou [173], 2020 | Opena label, randomized study (BoNT-A vs. BoNT-A+Finasteride) | 63 male and 1 woman | 100 U | 1 year | Yes (every 3 months) | BTA combined with FNS presents excellent results |
| First Author [Ref.], year | Type of Study | n (Type of Psoriasis) | BoNT-A Doses | Retreatment | Follow-Up | Results |
|---|---|---|---|---|---|---|
| Zanchi [178], 2008 | Observational, no RCT | 15 (inverse psoriasis) | 50-100 U | Not reported | 12 weeks | Improvement in VAS scale score; photographic assessment with improvement of erythema, infiltration (87%) |
| Saber [181], 2011 | Case report | 1 (inverse psoriasis and hyperhidrosis) | 100 U per axilla | No | 4 weeks | Greatly improved (photographic documentation) |
| Gilbert [182], 2014 | Case report | 1 (plaque psoriasis) | 30 U for a single plaque | No | 8 months | Complete remission but recurrence after 8 months. |
| Todberg [186], 2018 | Exploratory, multicenter, randomized double-blinded trial (BoNT-A vs. vehicle). | 8 (plaque psoriasis) | 36 U for a single plaque | No | 8 weeks | No clinical or histological differences from the vehicle |
| Aschenbeck [179], 2018 | Open-label pilot study | 8 (plaque psoriasis) | Variable number of units per plaque (average units, 53; range, 25–92) | No | 10 weeks | PASI and BSA reduction |
| González [185], 2018 | Descriptive cross-sectional study | 8 (plaque psoriasis) | Variable number of units per plaque (maximum 50 U) | No | 4 weeks | All parameters evaluated (desquamation, erythema and infiltration) for TCS score showed improvement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martina, E.; Diotallevi, F.; Radi, G.; Campanati, A.; Offidani, A. Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review. Toxins 2021, 13, 120. https://doi.org/10.3390/toxins13020120
Martina E, Diotallevi F, Radi G, Campanati A, Offidani A. Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review. Toxins. 2021; 13(2):120. https://doi.org/10.3390/toxins13020120
Chicago/Turabian StyleMartina, Emanuela, Federico Diotallevi, Giulia Radi, Anna Campanati, and Annamaria Offidani. 2021. "Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review" Toxins 13, no. 2: 120. https://doi.org/10.3390/toxins13020120
APA StyleMartina, E., Diotallevi, F., Radi, G., Campanati, A., & Offidani, A. (2021). Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review. Toxins, 13(2), 120. https://doi.org/10.3390/toxins13020120






