Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review
Abstract
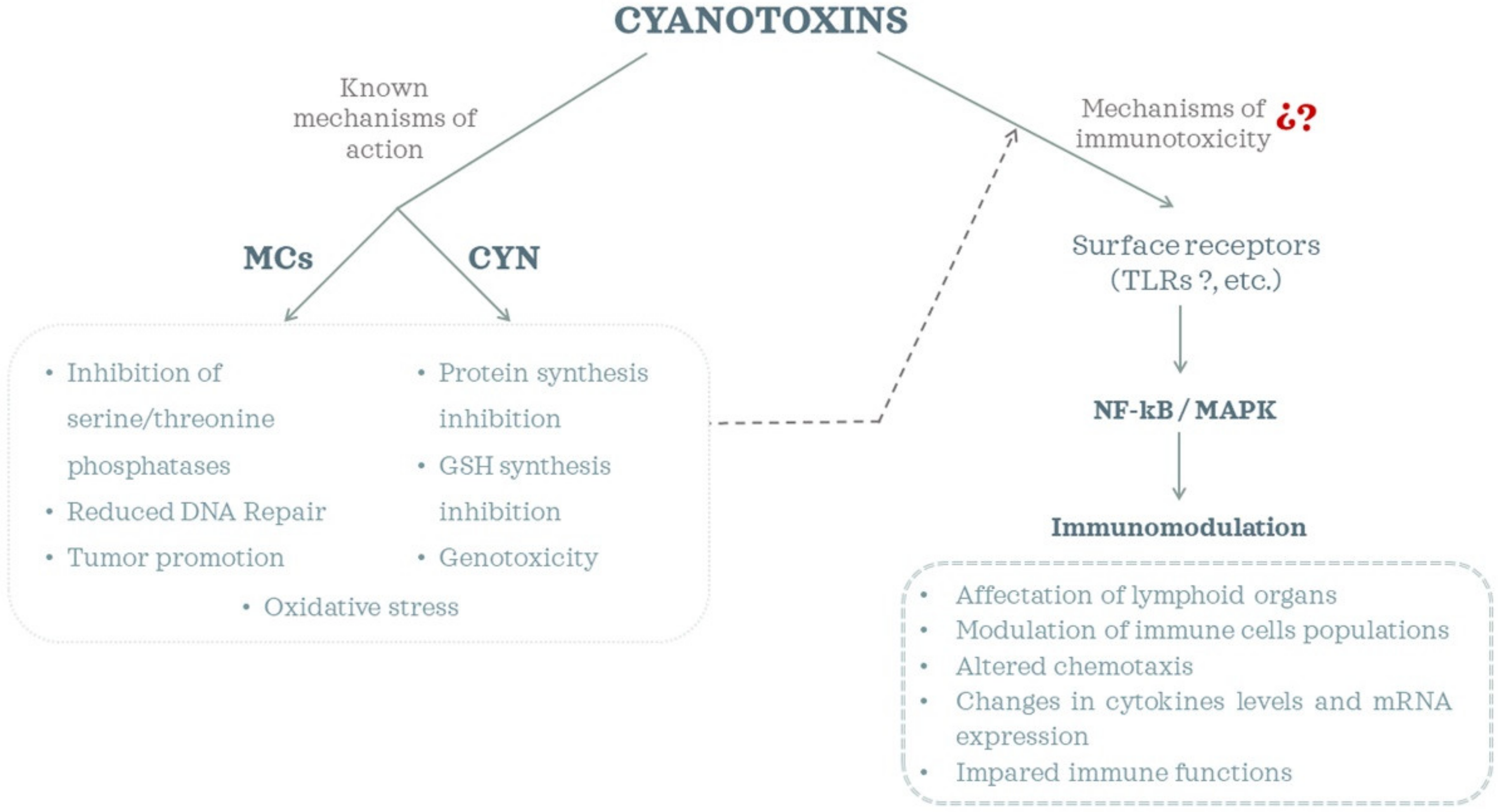
1. Introduction
2. Microcystins
2.1. In Vitro Studies
2.2. In Vivo Studies
2.2.1. Aquatic Organisms
2.2.2. Mammals
3. Cylindrospermopsin
4. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, I.-S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and Biology. Harmful Algae 2019, 83, 42–94. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Bormans, M.; Amzil, Z.; Mineaud, E.; Brient, L.; Savar, V.; Robert, E.; Lance, E. Demonstrated transfer of cyanobacteria and cyanotoxins along a freshwater-marine continuum in France. Harmful Algae 2019, 87, 101639. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, M.; Scardala, S.; Stefanelli, M.; Palazzo, F.; Funari, E.; Vichi, S.; Buratti, F.M.; Testai, E. Emerging health issues of cyanobacterial blooms. Ann. Ist. Super. Sanita. 2012, 48, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, A.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016, 13, 998E. [Google Scholar] [CrossRef]
- De la Cruz, A.A.; Chernoff, N.; Sinclair, J.L.; Hill, D.; Diggs, D.L.; Lynch, A.T. Introduction to Cyanobacteria and Cyanotoxins. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2020; pp. 1–35. ISBN 978-1-118-92861-5. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Pichardo, S.; Jos, Á.; Zurita, J.L.; Salguero, M.; Cameán, A.M.; Repetto, G. Acute and subacute toxic effects produced by microcystin-YR on the fish cell lines RTG-2 and PLHC-1. Toxicol. Vitr. 2007, 21, 1460–1467. [Google Scholar] [CrossRef]
- Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. Comparison of the toxicity induced by microcystin-RR and microcystin-YR in differentiated and undifferentiated Caco-2 cells. Toxicon 2009, 54, 161–169. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Puerto, M.; Gutiérrez-Praena, D.; Llana-Ruiz-Cabello, M.; Jos, Á.; Cameán, A.M. Microcystin-RR: Occurrence, content in water and food and toxicological studies. A review. Environ. Res. 2019, 168, 467–489. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, Á.; Cameán, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, P.; Chen, J.; Liang, G. Distribution of microcystins in various organs (heart, liver, intestine, gonad, brain, kidney and lung) of Wistar rat via intravenous injection. Toxicon 2008, 52, 721–727. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, W.; Liu, C.; Li, L.; Yu, L.; Zhao, S.; Li, G. Microcystin-LR exposure induces developmental neurotoxicity in zebrafish embryo. Environ. Pollut. 2016, 213, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yan, W.; Wu, Q.; Liu, C.; Gong, X.; Hung, T.-C.; Li, G. Parental exposure to microcystin-LR induced thyroid endocrine disruption in zebrafish offspring, a transgenerational toxicity. Environ. Pollut. 2017, 230, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Meng, F.; Yao, L. Hepatotoxicity and immunotoxicity of MC-LR on silver carp. Ecotoxicol. Environ. Saf. 2019, 169, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, G.; Wu, Q.; Liu, C.; Shen, J.Z.; Yan, W. Microcystin-LR exposure induced nephrotoxicity by triggering apoptosis in female zebrafish. Chemosphere 2019, 214, 598–605. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Gutiérrez-Praena, D.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, Á.; Cameán, A.M. Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Sci. Total Environ. 2019, 668, 547–565. [Google Scholar] [CrossRef]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T.C. Cylindrospermopsin, a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 1992, 114, 7941–7942. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef]
- Runnegar, M.T.; Kong, S.-M.; Zhong, Y.-Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Runnegar, M.T.C.; Jackson, A.R.B.; Falconer, I.R. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 1985, 50, 1292–1295. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin cylindrospermopsin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 472, 155–161. [Google Scholar] [CrossRef]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the Chemistry, Toxicology and Genetics of the Cyanobacterial Toxins, Microcystin, Nodularin, Saxitoxin and Cylindrospermosin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef]
- Pichardo, S.; Cameán, A.M.; Jos, Á. In vitro toxicological assessment of cylindropermopsin: A review. Toxins 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Prieto, A.I.; Maisanaba, S.; Gutiérrez-Praena, D.; Mellado-García, P.; Jos, Á.; Cameán, A.M. Mutagenic and genotoxic potential of pure Cylindrospermopsin by a battery of in vitro test. Food Chem. Toxicol. 2018, 121, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quijada, L.; Llana-Ruiz-Cabello, M.; Cătunescu, G.M.; Puerto, M.; Moyano, R.; Jos, Á.; Cameán, A.M. In vivo genotoxicity evaluation of cylindrospermopsin in rats using a combined micronucleus and comet assay. Food Chem. Toxicol. 2019, 132, 110664. [Google Scholar] [CrossRef] [PubMed]
- Puerto, M.; Pichardo, S.; Jos, Á.; Prieto, A.I.; Sevilla, E.; Frías, J.E.; Cameán, A.M. Differential oxidative stress responses to pure Microcystin-LR and Microcystin-containing and non-containing cyanobacterial crude extracts on Caco-2 cells. Toxicon 2010, 55, 514–522. [Google Scholar] [CrossRef]
- Puerto, M.; Jos, Á.; Pichardo, S.; Gutiérrez-Praena, D.; Cameán, A.M. Acute effects of pure cylindrospermopsin on the activity and transcription of antioxidant enzymes in tilapia (Oreochromis niloticus) exposed by gavage. Ecotoxicology 2011, 20, 1852–1860. [Google Scholar] [CrossRef]
- Puerto, M.; Jos, Á.; Pichardo, S.; Moyano, R.; Blanco, A.; Cameán, A.M. Acute exposure to pure cylindrospermopsin results in oxidative stress and pathological alterations in tilapia (Oreochromis niloticus). Environ. Toxicol. 2014, 29, 371–385. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, Y.; Wang, F.; Wu, Z.; Zhao, S. Microcystin-LR induced oxidative stress, inflammation, and apoptosis in alveolar type II epithelial cells of ICR mice in vitro. Toxicon 2020, 174, 19–25. [Google Scholar] [CrossRef]
- Sierosławska, A. Immunotoxic, genotoxic and carcinogenic effects of cyanotoxins. Centr. Eur. J. Immunol. 2010, 35, 105–110. [Google Scholar]
- Hinton, D.M. US FDA “Redbook II” Immunotoxicity Testing Guidelines and Research in Immunotoxicity Evaluations of Food Chemicals and New Food Proteins. Toxicol. Pathol. 2000, 28, 467–478. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A. Cell Biology of the Immune System. In Goodman’s Medical Cell Biology, 4th ed.; Goodman, S.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 337–360. ISBN 9780128179277. [Google Scholar] [CrossRef]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Soma, G.-I. ROS and Innate Immunity. Anticancer Res. 2009, 29, 817–821. [Google Scholar]
- Jou, I.-M.; Lin, C.-F.; Tsai, K.-J.; Wei, S.-J. Macrophage-Mediated Inflammatory Disorders. Mediators Inflamm. 2013, 2013, 316482. [Google Scholar] [CrossRef]
- Moosova, Z.; Pekarova, M.; Sindlerova, L.S.; Vasicek, O.; Kubala, L.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of cyanobacterial toxin cylindrospermopsin on innate immune cells. Chemosphere 2019, 226, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Goeddel, D.V.G. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef]
- Flajnik, M.F.; Kasahara, M. Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat. Rev. Genet. 2010, 11, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Ruehl-Fehlert, C.; Elmore, S.A.; Parker, G.A. Immune System. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume III, pp. 1795–1862. ISBN 978-0-12-415759-0. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Adaptive Immune System. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; ISBN 10:0-8153-4072-9. [Google Scholar]
- Nakano, M.; Nakano, Y.; Sato-Taki, T.; Mori, N.; Kojima, M.; Ohtake, A.; Shirai, M. Toxicity of Microcystis aeruginosa K-139 strain. Microbiol. Immunol. 1989, 33, 787–792. [Google Scholar] [CrossRef]
- Pahan, K.; Sheikh, F.G.; Namboodiri, A.M.S.; Singh, I. Inhibitors of protein phosphatase 1 and 2A differentially regulate the expression of inducible nitric-oxide synthase in rat astrocytes and macrophages. J. Bio. Chem. 1998, 273, 12219–12226. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.F.G.; Sidrim, J.J.C.; Soares, A.M.; Jiménez, G.C.; Guerrant, R.L.; Ribeiro, R.A.; Lima, A.A.M. Supernatants from Macrophages Stimulated with Microcystin-LR Induce Electrogenic Intestinal Response in Rabbit Ileum. Pharmacol. Toxicol. 2000, 87, 46–51. [Google Scholar] [CrossRef]
- Repavich, W.M.; Sonzogni, W.C.; Standridge, J.H.; Wedepohl, R.E.; Meisner, L.F. Cyanobacteria (blue-green algae) in wisconsin waters: Acute and chronic toxicity. Water Res. 1990, 24, 225–231. [Google Scholar] [CrossRef]
- Mankiewicz, J.; Tarczynska, M.; Fladmark, K.E.; Doskeland, S.O.; Walter, Z.; Zalewski, M. Apoptotic effect of cyanobacterial extract on rat hepatocytes and human lymphocytes. Environ. Toxicol. 2001, 16, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Mankiewicz, J.; Walter, Z.; Tarczynska, M.; Palyvoda, O.; Wojtysiak-Staniaszczyk, M.; Zalewski, M. Genotoxicity of cyanobacterial extracts containing microcystins from Polish water reservoirs as determined by SOS chromotest and comet assay. Environ. Toxicol. 2002, 17, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.F.; Harada, K.I.; Ito, E.; Watanabe, M. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon 1994, 32, 833–843. [Google Scholar] [CrossRef]
- Seawright, A.A.; Nolan, C.C.; Shaw, G.R.; Chiswell, R.K.; Norris, R.L.; Moore, M.R.; Smith, M.J. The oral toxicity for mice of the tropical cyanobacterium Cylindrospermopsis raciborskii (Wolonszynska). Environ. Toxicol. 1999, 14, 135–142. [Google Scholar] [CrossRef]
- Shaw, G.R.; Seawright, A.A.; Moore, M.R.; Lam, P.K.S. Cylindrospermopsin, a cyanobacterial alkaloid: Evaluation of its toxicological activity. Ther. Drug Monit. 2000, 22, 89–92. [Google Scholar] [CrossRef]
- Žegura, B.; Gajski, G.; Štraser, A.; Garaj-Vrhovac, V. Cylindrospermopsin induced DNA damage and alteration in the expression of genes involved in the response to DNA damage, apoptosis and oxidative stress. Toxicon 2011, 58, 471–479. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. First report of cylindrospermopsin effect on human peripheral blood lymphocytes proliferation in vitro. Cent. Eur. J. Immunol. 2012, 37, 314–317. [Google Scholar] [CrossRef]
- Shen, P.P.; Zhao, S.W.; Zheng, W.J.; Hua, Z.C.; Shi, Q.; Liu, Z.T. Effects of cyanobacteria bloom extract on some parameters of immune function in mice. Toxicol. Lett. 2003, 143, 27–36. [Google Scholar] [CrossRef]
- Kujbida, P.; Hatanaka, E.; Campa, A.; Curi, R.; Farsky, S.H.P.; Pinto, E. Analysis of chemokines and reactive oxygen species formation by rat and human neutrophils induced by microcystin-LA, -YR and -LR. Toxicon 2008, 51, 1274–1280. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. Toxicity of cylindrospermopsin in human lymphocytes: Proliferation, viability and cell cycle studies. Toxicol. Vitr. 2014, 28, 968–974. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. Cylindrospermopsin decreases the oxidative burst capacity of human neutrophils. Toxicon 2014, 87, 113–119. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Kokociński, M.; Karczewski, J. Toxic potencies of metabolite(s) of non-cylindrospermopsin producing Cylindrospermopsis raciborskii isolated from temperate zone in human white cells. Chemosphere 2015, 120, 608–614. [Google Scholar] [CrossRef]
- Lin, W.; Hou, J.; Guo, H.; Qiu, Y.; Li, L.; Li, D.; Tang, R. Dualistic immunomodulation of sub-chronic microcystin-LR exposure on the innate-immune defense system in male zebrafish. Chemosphere 2017, 183, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Chen, X.; Li, D.; Han, X. Microcystin-leucine arginine mediates apoptosis and engulfment of Leydig cell by testicular macrophages resulting in reduced serum testosterone levels. Aquat. Toxicol. 2018, 199, 116–126. [Google Scholar] [CrossRef]
- Dar, H.Y.; Lone, Y.; Koiri, R.K.; Mishra, P.K.; Srivastava, R.K. Microcystin-leucine arginine (MC-LR) induces bone loss and impairs bone micro-architecture by modulating host immunity in mice: Implications for bone health. Environ. Pollut. 2018, 238, 792–802. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, X.; Liu, Y.; Shi, Q.; Hua, Z.; Shen, P. Analysis of immunomodulating nitric oxide, iNOS and cytokines mRNA in mouse macrophages induced by microcystin-LR. Toxicology 2004, 197, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kujbida, P.; Hatanaka, E.; Campa, A.; Colepicolo, P.; Pinto, E. Effects of microcystins on human polymorphonuclear leukocytes. Biochem. Biophys. Res. Commun. 2006, 341, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A.; Adaszek, L. Effects of cylindrospermopsin on the phagocytic cells of the common carp (Cyprinus carpio L.). J. Appl. Toxicol. 2015, 35, 1406–1414. [Google Scholar] [CrossRef]
- Shi, Q.; Cui, J.; Zhang, J.; Kong, F.X.; Hua, Z.C.; Shen, P.P. Expression modulation of multiple cytokines in vivo by cyanobacteria blooms extract from taihu lake, China. Toxicon 2004, 44, 871–879. [Google Scholar] [CrossRef]
- Chen, T.; Shen, P.; Zhang, J.; Hua, Z. Effects of Microcystin-LR on Patterns of iNOS and Cytokine mRNA Expression in Macrophages In vitro. Environ. Toxicol. 2005, 20, 85–91. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, M.; Liu, G.; Lu, T.; Sun, L.; Pan, X. Effects of different concentrations of Microcystis aeruginosa on the intestinal microbiota and immunity of zebrafish (Danio rerio). Chemosphere 2019, 214, 579–586. [Google Scholar] [CrossRef]
- Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Qiu, Y.; Yang, L.; Li, D.; Tang, R. Parental Transfer of Microcystin-LR-Induced Innate Immune Dysfunction of Zebrafish: A Cross-Generational Study. Environ. Sci. Technol. 2020, 54, 1014–1023. [Google Scholar] [CrossRef]
- Adegoke, E.O.; Adeniran, S.O.; Zeng, Y.; Wang, X.; Wang, H.; Wang, C.; Zhang, H.; Zheng, P.; Zhang, G. Pharmacological inhibition of TLR4/NF-κB with TLR4-IN-C34 attenuated microcystin-leucine arginine toxicity in bovine Sertoli cells. J. Appl. Toxicol. 2019, 39, 832–843. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wang, L.; Li, J.; Chen, Y.; Jin, J.; Kawan, A.; Zhang, X. Pathological damage and immunomodulatory effects of zebrafish exposed to microcystin-LR. Toxicon 2016, 118, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Zhang, Q.; Xiang, Z.; Li, D.; Han, X. Microcystin-leucine arginine exhibits immunomodulatory roles in testicular cells resulting in orchitis. Environ. Pollut. 2017, 229, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Guo, H.; Wang, L.; Zhang, D.; Wu, X.; Li, L.; Qiu, Y.; Yang, L.; Li, D.; Tang, R. Waterborne microcystin-LR exposure induced chronic inflammatory response via MyD88-dependent toll-like receptor signaling pathway in male zebrafish. Sci. Total Environ. 2020, 702, 134969. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Carmichael, W.W.; Grasman, K.A.; Yuan, M. The uptake kinetics and immunotoxic effects of microcystin-LR in human and chicken peripheral blood lymphocytes in vitro. Toxicology 2004, 204, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Mankiewicz-Boczek, J.; Palus, J.; Gagala, I.; Izydorczyk, K.; Jurczak, T.; Dziubałtowska, E.; Stepnik, M.; Arkusz, J.; Komorowska, M.; Skowron, A.; et al. Effects of microcystins-containing cyanobacteria from a temperate ecosystem on human lymphocytes culture and their potential for adverse human health effects. Harmful Algae 2011, 10, 356–365. [Google Scholar] [CrossRef]
- Moosova, Z.; Hrouzek, P.; Kapuscik, A.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of selected cyanobacterial peptides in vitro. Toxicon 2018, 149, 20–25. [Google Scholar] [CrossRef]
- Teneva, I.; Mladenov, R.; Popov, N.; Dzhambazov, B. Cytotoxicity and Apoptotic Effects of Microcystin-LR and Anatoxin-a in Mouse Lymphocytes. Folia Biol. 2005, 51, 62–67. [Google Scholar]
- Zhang, J.; Zhang, H.; Chen, Y. Sensitive apoptosis induced by microcystins in the crucian carp (Carassius auratus) lymphocytes in vitro. Toxicol. Vitr. 2006, 20, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Yea, S.S.; Kim, H.M.; Oh, H.-M.; Paik, K.-H.; Yang, K.-H. Microcystin-induced down-regulation of lymphocyte functions through reduced IL-2 mRNA stability. Toxicol. Lett. 2001, 122, 21–31. [Google Scholar] [CrossRef]
- Christen, V.; Meili, N.; Fent, K. Microcystin-LR induces endoplasmatic reticulum stress and leads to induction of NFκB, interferon-alpha, and tumor necrosis factor-alpha. Environ. Sci. Technol. 2013, 47, 3378–3385. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Xia, Z. Microcystin-LR exhibits immunomodulatory role in mouse primary hepatocytes through activation of the NF-κB and MAPK signaling pathways. Toxicol. Sci. 2013, 136, 86–96. [Google Scholar] [CrossRef]
- Adamovsky, O.; Moosova, Z.; Pekarova, M.; Basu, A.; Babica, P.; Sindlerova, L.S.; Kubala, L.; Blaha, L. Immunomodulatory potency of microcystin, an important water-polluting cyanobacterial toxin. Environ. Sci. Technol. 2015, 49, 12457–12464. [Google Scholar] [CrossRef]
- Hernández, M.; Macia, M.; Padilla, C.; Del Campo, F.F. Modulation of Human Polymorphonuclear Leukocyte Adherence by Cyanopeptide Toxins. Environ. Res. 2000, 84, 64–68. [Google Scholar] [CrossRef]
- Adegoke, E.O.; Chen, W.; Machebe, N.S.; Xue, W.; Hao, W.; Adeniran, S.O.; Han, Z.; Peng, Z.; Guixue, Z. Microcystin-leucine arginine (MC-LR) induced inflammatory response in bovine sertoli cell via TLR4/NF-κB signaling pathway. Environ. Toxicol. Pharmacol. 2018, 63, 115–126. [Google Scholar] [CrossRef]
- Hansen, J.D.; Loftin, K.A.; Laughrey, Z.; Adamovsky, O. Neither microcystin, nor nodularin, nor cylindrospermopsin directly interact with human toll-like receptors. Chemosphere 2021, 274, 129623. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Guzmán-Guillén, R.; Pichardo, S.; Moreno, F.J.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Cytotoxic and morphological effects of microcystin-LR, cylindrospermopsin, and their combinations on the human hepatic cell line HepG2. Environ. Toxicol. 2018, 34, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, M.G.; Prieto, A.I.; Gutiérrez-Praena, D.; Moreno, F.J.; Cameán, A.M.; Jos, Á. Neurotoxic assessment of Microcystin-LR, cylindrospermopsin and their combination on the human neuroblastoma SH-SY5Y cell line. Chemosphere 2019, 224, 751–764. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Prieto, A.I.; Puerto, M.; Jos, Á.; Cameán, A.M. In vitro Mutagenic and Genotoxic Assessment of a Mixture of the Cyanotoxins Microcystin-LR and Cylindrospermopsin. Toxins 2019, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quijada, L.; Hercog, K.; Štampar, M.; Filipič, M.; Cameán, A.M.; Jos, Á.; Žegura, B. Genotoxic Effects of Cylindrospermopsin, Microcystin-LR and Their Binary Mixture in Human Hepatocellular Carcinoma (HepG2) Cell Line. Toxins 2020, 12, 778. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Medrano-Padial, C.; Llana-Ruiz-Cabello, M.; Cătunescu, G.M.; Moyano, R.; Risalde, M.A.; Cameán, A.M.; Jos, Á. Cylindrospermopsin-Microcystin-LR Combinations May Induce Genotoxic and Histopathological Damage in Rats. Toxins 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Rymuszka, A.; Sieroslawska, A.; Bownik, A.; Skowronski, T. In vitro effects of pure microcystin-LR on the lymphocyte proliferation in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2007, 22, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Sieroslawska, A.; Rymuszka, A.; Bownik, A.; Skowronski, T. The influence of microcystin-LR on fish phagocytic cells. Hum. Exp. Toxicol. 2007, 26, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Rymuszka, A.; Sieroslawska, A.; Bownik, A.; Skowronski, T. Microcystin-LR modulates selected immune parameters and induces necrosis/apoptosis of carp leucocytes. Environ. Toxicol. Chem. 2010, 29, 569–574. [Google Scholar] [CrossRef]
- Rymuszka, A. Microcystin-LR induces cytotoxicity and affects carp immune cells by impairment of their phagocytosis and the organization of the cytoskeleton. J. Appl. Toxicol. 2013, 33, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Rymuszka, A.; Adaszek, L. Pro- and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress—An in vitro study. Fish Shellfish Immunol. 2012, 33, 382–388. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Fang, W.; Wang, D. Regulatory effect of quercetin on hazardous microcystin-LR-induced apoptosis of Carassius auratus lymphocytes in vitro. Fish Shellfish Immunol. 2014, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Guo, G.-L.; Sun, L.; Yang, Q.-S.; Wang, G.-Q.; Zhang, D.-M. Modulatory role of L-carnitine against microcystin-LR-induced immunotoxicity and oxidative stress in common carp. Fish Physiol. Biochem. 2017, 43, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Rymuszka, A.; Adaszek, L. Cytotoxic effects and changes in cytokine gene expression induced by microcystin-containing extract in fish immune cells- An in vitro and in vivo study. Fish Shellfish Immunol. 2013, 34, 1524–1532. [Google Scholar] [CrossRef]
- Rymuszka, A.; Sieroslawska, A.; Bownik, A.; Skowronski, T. Immunotoxic potential of cyanotoxins on the immune system of fish. Centr. Eur. J. Immunol. 2008, 33, 150–152. [Google Scholar]
- Smutná, M.; Babica, P.; Jarque, S.; Hilscherová, K.; Maršálek, B.; Haeba, M.; Bláha, L. Acute, chronic and reproductive toxicity of complex cyanobacterial blooms in Daphnia magna and the role of microcystins. Toxicon 2014, 79, 11–18. [Google Scholar] [CrossRef]
- Ujvárosi, A.Z.; Hercog, K.; Riba, M.; Gonda, S.; Filipič, M.; Vasas, G.; Žegura, B. The cyanobacterial oligopeptides microginins induce DNA damage in the human hepatocellular carcinoma (HepG2) cell line. Chemosphere 2020, 240, 124880. [Google Scholar] [CrossRef]
- Falconer, I.R. Cyanobacterial toxins present in Microcystis aeruginosa extracts—More than microcystins! Toxicon 2007, 50, 585–588. [Google Scholar] [CrossRef]
- Qiao, Q.; Liang, H.; Zhang, X. Effect of cyanobacteria on immune function of crucian carp (Carassius auratus) via chronic exposure in diet. Chemosphere 2013, 90, 1167–1176. [Google Scholar] [CrossRef]
- Wei, L.L.; Liu, Y.; Zhong, S.; Wu, H.; Ruan, J.; Liu, M.; Zhou, Q.; Zhong, Q. Transcriptome analysis of grass carp provides insights into the immune-related genes and pathways in response to MC-LR induction. Aquaculture 2018, 488, 207–216. [Google Scholar] [CrossRef]
- Xia, H.; Song, T.; Wang, L.; Jiang, L.; Zhou, Q.; Wang, W.; Liu, L.; Yang, P.; Zhang, X. Effects of dietary toxic cyanobacteria and ammonia exposure on immune function of blunt snout bream (Megalabrama amblycephala). Fish Shellfish Immunol. 2018, 78, 383–391. [Google Scholar] [CrossRef]
- Wei, L.L.; Sun, B.J.; Nie, P. Ultrastructural alteration of lymphocytes in spleen and pronephros of grass carp (Ctenopharyngodon idella) experimentally exposed to microcystin-LR. Aquaculture 2008, 280, 270–275. [Google Scholar] [CrossRef]
- Wei, L.; Sun, B.; Chang, M.X.; Liu, Y.; Nie, P. Effects of cyanobacterial toxin microcystin-LR on the transcription levels of immune-related genes in grass carp Ctenopharyngodon Idella. Environ. Biol. Fish 2009, 85, 231–238. [Google Scholar] [CrossRef]
- Li, H.; Cai, Y.; Xie, P.; Li, G.; Hao, L.; Xiong, Q. Identification and Expression Profiles of IL-8 in Bighead Carp (Aristichthys nobilis) in Response to Microcystin-LR. Arch. Environ. Contam. Toxicol. 2013, 65, 537–545. [Google Scholar] [CrossRef]
- Gao, J.; Zuo, H.; Yang, L.; He, J.-H.; Niu, S.; Weng, S.; He, J.; Xu, X. Long-term influence of cyanobacterial bloom on the immune system of Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 61, 79–85. [Google Scholar] [CrossRef]
- Guzmán Guillén, R.; Prieto, A.I.; Vazquez, C.M.; Vasconcelos, V.; Cameán, A.M. The Protective Role of L-Carnitine against Cylindrospermopsin-Induced Oxidative Stress in Tilapia Fish (Orechromis Niloticus). Aquat. Toxicol. 2013, 132–133, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Guzmán Guillén, R.; Prieto Ortega, A.I.; Moyano, R.; Blanco, A.; Vasconcelos, V.; Cameán, A.M. Dietary l-carnitine prevents histopathological changes in tilapia (Oreochromis Niloticus) exposed to cylindrospermopsin. Environ. Toxicol. 2017, 32, 241–254. [Google Scholar] [CrossRef]
- Puerto, M.; Prieto, A.I.; Pichardo, S.; Moreno, I.; Jos, Á.; Moyano, R.; Cameán, A.M. Effects of dietary N-Acetylcysteine on the oxidative stress induced in Tilapia (Oreochromis Niloticus) exposed to a microcystin-producing cyanobacterial water bloom. Environ. Toxicol. Chem. 2009, 28, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.I.; Jos, Á.; Pichardo, S.; Moreno, I.; de Sotomayor, M.Á.; Moyano, R.; Blanco, A.; Cameán, A.M. Time-dependent protective efficacy of Trolox (vitamin E analog) against microcystin-induced toxicity in tilapia (Oreochromis niloticus). Environ. Toxicol. 2009, 24, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zheng, C.; Shi, X. Effect of paternal exposure to microcystin-LR on testicular dysfunction, reproduction, and offspring immune response in the oriental river prawn (Macrobrachium nipponense). Aquaculture 2021, 534, 736332. [Google Scholar] [CrossRef]
- Li, Y.; Sun, B.; Wu, H.; Nie, P. Effects of pure microcystin-LR on the transcription of immune related genes and heat shock proteins in larval stage of zebrafish (Danio rerio). Aquaculture 2009, 289, 154–160. [Google Scholar] [CrossRef]
- Yuan, G.; Xie, P.; Zhang, X.; Tang, R.; Gao, Y.; Li, D.; Li, L. In vivo Studies on the Immunotoxic Effects of Microcystins on Rabbit. Environ. Toxicol. 2010, 27, 83–89. [Google Scholar] [CrossRef]
- Palikova, M.; Ondrackova, P.; Mares, J.; Adamovsky, O.; Pikula, J.; Kohoutek, J.; Navratil, S.; Blaha, L.; Kopp, R. In vivo effects of microcystins and complex cyanobacterial biomass on rats (Rattus norvegicus var. alba): Changes in immunological and haematological parameters. Toxicon 2013, 73, 1–8. [Google Scholar] [CrossRef]
- Li, G.; Yan, W.; Qiao, Q.; Chen, J.; Cai, F.; He, Y.; Zhang, X. Global effects of subchronic treatment of microcystin-LR on rat splenetic protein levels. J. Proteom. 2012, 77, 383–393. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Xiao, W.; Ye, X.; Zhong, Q.; Gu, K. Comparison of response indices to toxic microcystin-LR in blood of mice. Chemosphere 2013, 92, 563–569. [Google Scholar] [CrossRef]
- Cao, L.; Huang, F.; Massey, I.Y.; Wen, C.; Zheng, S.; Xu, S.; Yang, F. Effects of Microcystin-LR on the microstructure and inflammation-related factors of jejunum in mice. Toxins 2019, 11, 482. [Google Scholar] [CrossRef]
- World Health Organization. Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/338066 (accessed on 7 September 2021).
- Lone, Y.; Bhide, M.; Koiri, R.K. Microcystin-LR Induced Immunotoxicity in Mammals. J. Toxicol. 2016, 2016, 8048125. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Chen, Y.; Jia, N.; Li, R. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2021, 406, 124653. [Google Scholar] [CrossRef] [PubMed]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. Vitr. 2015, 29, 926–932. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A. Effects of cylindrospermopsin on a common carp leucocyte cell line. J. Appl. Toxicol. 2015, 35, 83–89. [Google Scholar] [CrossRef]
- Zhang, X.; Young, H.A. PPAR and immune system--what do we know? Int. Immunopharmacol. 2002, 2, 1029–1044. [Google Scholar] [CrossRef]
- Ito, A.; Yamaguchi, K.; Tomita, H.; Suzuki, T.; Onogawa, T.; Sato, T.; Mizutamari, H.; Mikkaichi, T.; Nishio, T.; Suzuki, T.; et al. Distribution of Rat Organic Anion Transporting Polypeptide-E (oatp-E) in the Rat Eye. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4877–4884. [Google Scholar] [CrossRef][Green Version]
- Moreau, A.; Le Vee, M.; Jouan, E.; Parmentier, Y.; Fardel, O. Drug transporter expression in human macrophages. Fundam. Clin. Pharmacol. 2011, 25, 743–752. [Google Scholar] [CrossRef]
- Janneh, O.; Hartkoorn, R.C.; Jones, E.; Owen, A.; Ward, S.A.; Davey, R.; Back, D.J.; Khoo, S.H. Cultured CD4T cells and primary human lymphocytes express hOATPs: Intracellular accumulation of saquinavir and lopinavir. Br. J. Pharmacol. 2008, 155, 875–883. [Google Scholar] [CrossRef]
| Cyanotoxin Congener | Experimental Model | Assays | Exposure Conditions | Effects | Reference |
|---|---|---|---|---|---|
| MC-LR | Rat astrocytes and macrophages. C6 glial cells | LPS and cytokine-induced production of NO. Expression of iNOS (protein and mRNA level) | 2 nM MC-LR for 24 h | It stimulated the LPS- and cytokine-mediated expression of iNOS and production of NO. However, it inhibited the LPS- and cytokine-mediated expression of iNOS and production of NO in rat resident macrophages and RAW 264.7 cells. | [44] |
| MC-LR | Supernatants from macrophages isolated from Wistar rats | IL-1β and TNF-α levels | 0.1, 0.3 and 1.0 µg/mL MC-LR at 0, 40, 50 and 60 min | MC-LR induced the release of IL-1β and TNF-α by peritoneal macrophages in vitro and the supernatants from these macrophages induced electrogenic secretion in rabbit ileal mucosa. | [45] |
| MC-LR | Human peripheral polymorphonuclear leukocytes | Adherence assays: spontaneous and fMLP stimulated | 10−11, 10−10, 10−9 M MC-LR, 8, 25 min | It significantly enhanced the early spontaneous adherence of PMNs but not the late spontaneous adherence or the early or late PMN-stimulated adherence. | [84] |
| Cyanobacterial bloom samples (containing MC-LR, MC-RR and MC-YR) | Human lymphocytes | Light microscopy, Flow cytometry and analysis of DNA | Lymphocytes were treated with cyanobacterial extract containing MC-LR eq. at 250, 500, 750 or 1000 nM for 24 or 48 h | Morphological changes (apoptosis) and clastogenic effects were observed. | [47] |
| MC-LR MC-YR | B6C3F1 mouse splenocytes and thymocytes; C57BL/6 mouse thymoma EL-4 cells. | Mitogen-induced lymphoproliferation (LPS and ConA) in splenocytes. IL-2 mRNA expression by RT-PCR, and IL-2 mRNA stability in splenocytes, thymocytes and EL-4 cells | Splenocytes were exposed to 0.1, 1, 10 or 50 μM of each MC-congener for 30 min. Splenocytes, thymocytes and EL-4 cells were exposed to 1 μM of each cyanotoxin for 30 min | Concentration-dependent inhibition of in vitro polyclonal antibody response and lymphoproliferation to LPS were reported. MC-YR decreased Con-A-induced lymphoproliferation. Each MC suppressed IL-2 mRNA expression in splenocytes and thymocytes induced by PMA, but not in EL-4 mouse thymoma cells. MC down-regulated lymphocyte functions and immunosuppression was mediated in part by decreased IL-2 mRNA stability. | [80] |
| MC-LR | BALB/c mice peritoneal macrophages | NO production, mRNA abundance of iNOS and diverse cytokines using RT-PCR | Stimulation of macrophages with LPS (100 µg/L) and exposure to MC-LR at 1–1000 nmol/L for 24 h | NO production, mRNA levels of iNOS, IL-1β, TNF-α were concentration-dependently decreased, and mRNA levels of GM-CSF and IFN-γ in an independent manner. | [62] |
| MC-LR | Chicken and human peripheral blood lymphocytes | Lymphocyte proliferation assay, cytokine production (IL-2 and IL-6), and detection of apoptosis | 1, 10 and 25 µg/mL MC-LR for 12, 24, 48 and 72 h | At 25 µg/mL MC-LR decreased T-cell proliferation; all concentrations of the toxin inhibited B-cell proliferation. The frequency of apoptotic and necrotic cells increased in a concentration and time-dependent manner. Increased production of IL-6 and decreased production of IL-2. | [75] |
| MC-LR | BALB/c mice peritoneal macrophages | Expression of mRNA for iNOS and several cytokines (IL-1β, TNF-α, GM-CSF and IFN-γ) by RT-PCR | Induced macrophages with LPS at 1, 10, 100 and 1000 nmol/L C-LR for 6 h | mRNA expression of iNOS, IL-1β, TNG-α, GM-CSF and IFN-γ decreased in comparison to positive control. | [68] |
| MC-LR | Mouse lymphocytes | Cytotoxicity. Apoptosis by flow cytometry | 7.5 µg/mL MC-LR for 4 and 24 h | Decreased the cell viability after 4 and 24 h. Induced apoptosis in mouse B cells, while the T cells were not affected. | [78] |
| MC-LR and [Asp3]-MC-LR were isolated from a Microcystis panniformis strain | Human neutrophils (PMN) | Effects on PMN migration, production of ROS, and phagocytosis and killing assays | PMN migration assay: 0.01–1000 nM of each MC for 60 min. ROS production: 10, 100 nM, for 5 min. Phagocytosis and killing assays: 1 and 1000 nM, for 10 and 15 min | Both MCs induced the production of ROS and increased phagocytosis of C. albicans. MC-LR also increased C. albicans killing. | [63] |
| MC-LR MC-RR | Crucian carp (Carassius auratus) lymphocytes | In vitro apoptosis assays, using confocal and transmission electron microscopy (TEM), agarose gel electrophoresis and flow cytometry analysis (FC) | Confocal microscopy: 1 nM, 2 h Agarose gel electrophoresis: 1,5,10 nM 2 h TEM: 1 nM 4 h FC: 1,5,10 nM 2 h and 1 nM for 2, 4, 6 and 8 h | Apoptosis even at a low concentration (1 nM) was detected after exposure to both MC-congeners, in a concentration and time-dependent manner. Agarose gel electrophoresis revealed DNA fragmentation caused by apoptosis. | [79] |
| MC-LR | Rainbow trout (Oncorrhynchus mykiss) lymphocytes | Lymphocyte cell viability and proliferation activity assay | Exposure to 1, 5, 10, 20, 40 mg/mL of cell suspension | Concentration-dependent effects of MC-LR on the lymphocyte viability and lymphocyte proliferation. | [92] |
| MC-LR | Rainbow trout (O. mykiss) phagocytic cells | Cell viability assay, leukocyte phagocytosis assay and metabolic activity assay | Cells exposed to 1, 5, 10, 20 µg/mL MC-LR after 2, 4, and 24 h | MC-LR induced time- and concentration-dependent viability decrease. The phagocytic ability was elevated at 5 µg/mL, and MC-LR has also modulatory influence on respiratory burst activity. | [93] |
| MC-LR MC-LA MC-YR | Rat neutrophils (male wistar rats) | Cell viability and DNA fragmentation assays. Lucigenin-enhanced chemiluminescence assay (ROS release). Cytokine assays by ELISA: IL-8 (human), CINC-2αβ (rat) and TNF-α (human and rat) | 1–1000 nM of MCs congeners, for 24 h | Changes in percentage of cells with fragmented DNA after exposure to MC-LA and MC-LR. Increased CINC-2αβ after exposure to MC-LR, and increased extracellular ROS for the three MCs. No significant changes in TNF-α levels and in intracellular ROS levels. | [55] |
| Neutrophils from the blood of healthy volunteers | Increased human neutrophils viability after treatment with MC-LR and MC-LA. Increased IL-8 production and extracellular ROS levels. | ||||
| MC-LR | Common carp (Cyprinus carpio L.) leucocytes | Cytotoxicity studies, respiratory burst activity (RBA test), lymphoproliferation studies and determination of necrotic/apoptotic cells | MC-LR at concentrations of 0.01, 0.1, 0.5 and 1.0 µg/mL | The RBA of phagocytes was increased at 0.01 µg/mL, but it decreased at higher concentrations. MC-LR did not have influence on the T-cell proliferation but decreased the proliferation of B lymphocytes. The toxin induced necrosis to a higher degree than apoptosis. | [94] |
| Crude MC-containing cyanobacterial extracts; purified MC-containing and no-MC containing extracts | Human lymphocytes | Cytotoxicity by XTT re-duction test, genotoxicity by alkaline comet assay and micronucleus test | Cytotoxicity: incubation for 24h with extracts containing 0-10 µg/mL in culture. Genotoxicity: cells exposed to cyanobacterial extracts with MC between 0-4 µg/mL for 3 and 6h | The highest cytotoxicity and genotoxicity were induced by the purified extract containing MCs (MC-LR, MC-RR and MC-YR). No clear effect with crude extracts. The influence of other compoundsapart from MCs was indated. | [76] |
| MC-LR | Lymphocytes and phagocytes isolated from the blood of carp (Cyprinus carpio L.) | Cytotoxicity, GSH level, DNA fragmentation, caspase-3/7 activities, phagocytosis, effects on the actin and tubulin in the phagocytes | 0.01–0.1 µg/mL MC-LR for 2, 6 and 24 h | Pure MC-LR was cytotoxic and induced apoptosis or necrosis in lymphocytes in a time- and concentration-dependent manner. GSH levels did not change in lymphocytes at 6 and 24 h and in phagocytes at 2 h. The toxin induced significant re-organization on the actin and tubulin in phagocytic cells. | [95] |
| MC-LR | Leucocytes from blood and head kidney from carp (Cyprinus carpio L.) | RT-PCR for cytokine gene expression (IL-1β, TNF-α, IL-10, and TGF-β) | 0.01–0.1 µg/mL MC-LR for 4 h | MC-LR increased the expression of IL-1β in leukocytes at 0.01 µg/mL, but decreased in head kidney cells at 0.1 µg/mL. The expression of TNF-α mRNA was induced at lower concentrations; in contrast, it was suppressed in blood and head kidney at the highest concentrations. MC-LR, at the highest concentration, increased IL-10 expression, and TGF-β expression was only increased in head kidney cells. | [96] |
| MC-LR | Human hepatoma cells Huh7 | Cytotoxicity, NF-κB activation, levels of TNF-α and IFN-α by ELISA. Gene expression involved in apoptosis, PP2A mRNA by RT-PCR, and protein levels by Western blot. Expression of several INF-α genes by RT-PCR | Cells were exposed to 0.5, 5, 25, 50 µM MC-LR for 6, 24, 48, and 72 h | The expression of NF-κB, IFN-α and several INF-α-stimulated genes was strongly activated. The cytokine TNF-α was also induced. MC-LR induced all endoplasmic reticulum (ER) stress response pathways. | [81] |
| An extract from a cyanobacterial bloom containing MCs | Lymphocytes and phagocytes from carp blood and head kidney (Cyprinus carpio L.). | In vitro: cytotoxicity, apoptosis/necrosis, ROS, lymphocyte proliferation In vivo: Table 2 | 0.01, 0.1, 0.5 and 1 µg/mL for 24 h | Cytotoxicity at 0.5 and 1 µg/mL. 3-fold higher levels of apoptosis in lymphocytes from 0.5 µg/mL and necrosis at 1 µg/mL. In phagocytes, apoptosis from 0.1 and necrosis from 0.5 µg/mL. ROS changes. Lymphocyte proliferation inhibition in both cell types. | [101] |
| MC-LR | HepG2 cells and primary mouse hepatocytes (PMHs) | Cytotoxicity, protein content and Western blotting analysis, NF-κB and MAPK, IL-6 production, and ultrastructural changes by transmission electron microscopy | Cytotoxicity: 0–1000 nM of MC-LR for 24 h Protein content: 0–100 nM for 24 h for C-dependent assays and to 25 nM for 3–24 h for time-course assays IL-6: 1–50 nM for 24 h or 20 nM for 6–48 h TEM: 0–100 nMfor 24 h | At noncytotoxic concentrations of MC-LR a proinflammatory effect on hepatocytes was demonstrated by inducing the activation of the NF-κB and MAPK pathways and IL-6 expression in a concentration-dependent manner. Cytotoxic concentrations of MC-LR induced a dysfunction of the NF-κB and MAPK signaling pathways, disruption of mitochondria, and even cell death. PMHs were more sensitive to MC-LR induced cytotoxicity than HepG2. | [82] |
| MC-LR | Carp (C. auratus) lymphocytes | Cytotoxicity assay, oxidative stress parameters (ROS, MDA, GSH, SOD, CAT) and apoptosis | Lymphocytes were incubated with MC-LR (1 µg/L) and different concentrations of quercetin (QE) (0–1000 µg/L), 24 h | Cytotoxicity and ROS formation were suppressed by QE in a concentration-dependent manner. It also enhanced the endogenous antioxidant defense system and the Bax/Bcl-2 ratio. The percentage of apoptosis at the highest QE concentration was lower by nearly half compared with the only MC-LR exposed group, and this concentration inhibited the expression of caspase-3 protein. | [97] |
| MC-LR | RAW 264.7 murine macrophages | NO production, release of TNFα and IL-6, PP activities, Western-Blot analysis of MC-LR, MAPKs, NF-κB and iNOS | 1–1000 nM MC-LR at different times (30 min to 24 h), various combinations of treatments including the activation of cells by LPS | MC-LR-dependent activation of NF-κB and ERK1/2, as well as the production of TNFα can be associated with the stimulation of toll-like receptors (TLRs). | [83] |
| MC-LR | Bovine Sertoli cells | Morphological changes, cell viability, gene expression by RT-PCR of cytokines and TLR4, TNF-α, NF-κB, etc. | Cells were exposed to 0, 20, 40, 60, and 80 µg MC-LR/L | Nuclear morphological changes and downregulation of the blood-testis barrier constituent proteins within 48 h after treatment. The TLR4 and NF-κB were activated and upregulated, and the proinflammatory cytokines were upregulated within 48 h. At 72 h, upregulation of cytokines and expression of blood-testis barrier constituents proteins were found. | [85] |
| MC-LR | Bovine Sertoli cells | Mitophagy, mitochondria membrane assessment, gene expression of diverse cytokines and DNA replication related genes by RT-PCR and Western blot analysis | Cells were pretreated with TLR4-IN-C34 (C34) factor for 1 h and exposed to 80 µg MC-LR/L for 24 h | TLR4-IN-C34 attenuated the effects of MC-LR: inhibited mitochondria membrane damage, mitophagy and downregulation of blood-testis barrier constituent proteins via TLR4/NF-κB and mitochondria-mediated apoptosis signaling pathway blockage. Downregulation of the mitochondria electro transport chain, energy production and DNA replication related genes, and upregulation of inflammatory cytokines were modulated by TLR4-IN-C34. | [71] |
| MC-RR MC-YR | Murine macrophage cell line RAW264.7 | Cell viability, NO production and release of TNF-α and IL-6 | Cells were preactivated for 2 h by LPS and/or (co)exposed with MC-RR, MC-YR (1–1000 nM) for 24 h | At non-cytotoxic concentrations, both MCs congeners did not have significant effects on macrophage production of pro-inflammatory cytokines (TNF-α and IL-6) neither alone nor with co-exposure with LPS. They had no effect on production of NO. | [77] |
| MC-LR, MC-RR, MC-LA | Engineered HEK293 cells that utilize an NF-κB-inducible SEAP | Immunomodulatory Compound screening method based on engineered HEK293-hTLR NF-κB assay, to identify activators or inhibitors of specific TLRs | 100 nM of each MC | Cells expressing TLR2-9 are not activated by the different MCs, and none of the toxins blocked the ability of TLR2-9 to interact with specific TLR agonists and they are not considered as TLR antagonists. Moreover, TLR negative cells were not stimulated by the assayed toxins. None of the assayed toxins directly interacted with human TLRs in either an agonistic or antagonistic manner. | [86] |
| Cyanotoxin Congener | Experimental Model | Assays | Exposure Conditions | Effects | Reference |
|---|---|---|---|---|---|
| MC-LR | Grass carp (Ctenopharyngodon idella) | Pathological changes by transmission electron microscopy | Intraperitoneal (i.p.) injection of 50 µg/kg MC-LR body weight (b.w.) Spleen and pronephros were dissected at 1, 2, 7, 14 and 21 days post injection. | Apoptosis was detected only in lymphocytes in the spleen, and pathological changes were also observed in lymphocytes from pronephros (i.e., edematous mitochondria). The recovery occurred at 21 days post injection. | [109] |
| MC-LR | Zebra fish larvae (Danio rerio) | Transcription of essential genes for early lymphoid development (Rag1, Rag2. Ikaros, GATA1, LcK, TCRα) and heat shock proteins (HSP90, HSP70, HSP60, HSP27) by RT-PCR | Larval zebrafish were exposed in glass wares containing 200 or 800 µg/L MC-LR after 72 h post fertilization and fish were sampled at 12, 24, 48, 96 and 168 h after exposure. | Transcription of Rag1, Rag2. Ikaros, GATA1, LcK, TCRα were upregulated at the highest MC-LR concentration assayed. Increased transcription of HSPs. | [118] |
| MC-LR | Grass carp (Ctenopharyngodon idella) | Changes of 6 immune -related genes (TNF-α, IL-1β, Type I IFN, PGRP-L, IgM and MHC-1) by RT-PCR in spleen and head kidney. | i.p. injection of 50 µg/kg MC-LR b.w. and fish were examined at 1, 2, 4, 7, 14 and 21 days post-injection. | The transcription levels of TNF-α, IL-1β, type I IFN, and PGRP-L in both organs were low at all time points, and those of IL-1β were low in the head kidney at different points. IgM and MHC-1 transcription levels were low in both organs studied only at 21 days post injection. MC-LR inhibited immune function at the transcription level. | [110] |
| MC-LR isolated from cyanobacterial blooms (mainly M. aeruginosa) | Bighead carp (Aristichthys nobilis) | Expression profile of IL-8 in carps by RT-PCR | Carps were injected i.p. with 50, 200 and 500 µg MC-LR/kg b.w. and sampling times points were 3 and 24 h after injection. | The expression of IL-8 gene in different tissues (liver, kidney, intestine, etc.) was upregulated by MC-LR exposure in a temporal and dose-dependent manner. | [111] |
| Lyophilized cyanobacteria containing MCs | Carp (Carassius auratus) | Morphological and immunological parameters. | Two diets containing 20% and 40% of cyanobacteria lyophilized powder and a control group. 30 days of exposure. The MCs content were: 1.41 mg MCs/g d.w., among which MC-RR, -LR, and –YR were 0.84, 0.50 and 0.07 mg/g dry weight (d.w.) | Significant increases in head kidney and spleen indexes in the high dose group. Marked hemorrhage and hyperemia in kidney and spleen in high dose group. Edematous mitochondria, nucleus deformation and compaction of chromatin in lymphocytes of head kidney and spleen in both exposed groups. Lysozyme activity increased in low dose group but decreased sharply in high dose group. Significant increase of macrophage bactericidal activity in the low dose group. | [106] |
| MC-LR standard and an extract from a cyanobacterial bloom containing MCs | Lymphocytes and phagocytes from carp blood and head kidney (Cyprinus carpio L.). | In vitro: Table 1. In vivo: ROS production by phagocytes, mitogen-stimulated proliferation of lymphocytes and cytokine gene expression. | Control fish and fish exposed by immersion to pure MC-LR (25 µg/L), or to an extract containing 25 µg/L MCs. Fish were sacrificed after 1, 3, and 5 days of exposure. | In vivo, the extract containing MCs had greater suppressive effects on immune cells in comparison to MC-LR standard. After exposure to the extract an up-regulation of IL-1β, TNF-α and IL-10 expression was reported, and there was no impact of the expression of TGF-β. This cytokine was up-regulated in head kidney cells only after 24 h exposure to the extract. The pure toxin only increased IL-1β levels in leucocytes isolated from exposed fish, although to a lower extend than the extract. | [101] |
| MC-LR | Zebra fish adult (Danio rerio) | Cytokines gene expression by RT-PCR, and histology of spleen, gut and gills. | 0, 1, 5 or 20 µg/L MC-LR for 30 days. | The transcriptional levels of IFN-1 and IL-8 in spleen were up-regulated at 20 µg/L, and IL-1β and TNF-α increased at 1 µg/L. The mRNA levels of IFN-1, IL-1β, IL-8, TGF-β and TNF-α were increased in intestine and gills in all MC-LR treated fish. Pathological changes were observed in the three organs studied. | [72] |
| MC-LR | Common carp (C. carpio) | Antioxidant enzyme activities, LPO, serum complement C3, lysozyme, bactericidal activity, and gene expression of inflammatory cytokines (IL-1β, TNF-α and IFN I) and heat shock proteins (HSP70 and HSP90). | Four treatment diets: Group I and II fed with control diet; groups III, IV and V fed with 0.5, 1.0 and 2.0 g/kg L-carnitine, respectively. After 4 weeks of feeding, the II-V groups were injected i.p. with MC-LR at dose of 150 µg/kg, and control group with saline solution. Sampling points were set at 0, 12, 24, 48 and 96 h. | MC-LR alone led to a significant downregulation in immune response, including serum complement (C3), lysozyme and bactericidal activity, and increased oxidative stress response. L-carnitine pretreated groups caused elevation in immune response and gene expression of inflammatory cytokines, including heat shock proteins. Antioxidant activities and LPO returned to background levels after L-carnitine pretreatment. | [98] |
| Cyanobacterial Bloom | Shrimps (Litopenaeus vannamei) | Expression profiles of immune genes (RT-PCR), phagocytic activity of hemocytes | Shrimp were sampled at 15, 30, 50 and 70 days post the cyanobacterial bloom occurrence in a shrimp farm (China) | Many antimicrobial peptides genes were down-regulated, whereas the expression of C-type lectins was up-regulated. The concentration of hemocytes in hemolymph was decreased, but their phagocytic activity was increased. | [112] |
| MC-LR | Male zebrafish (D. rerio) | Immunological gene expression, serum immune splenic inflammatory changes and pathology | 0–30 µg/L MC-LR for 30 days | At 0.3–3 µg/L MC-LR splenic inflammatory changes were described, including increased serum C3 levels and upregulated expression of innate immune related genes. At 10–30 µg/L MC-LR, degeneration of splenic lymphocytes and macrophages, down-regulation of immune-related genes, and decreased level of serum C3. | [59] |
| MC-LR | Grass carp | Transcriptome analysis: 457 differentially expressed genes (DEGs) were identified using RNA-Seq. Histopathological study | i.p. injection of 25, 75 and 100 µg MC-LR/kg b.w., and control group. Fish were sacrificed 96 h after injection. | 61, 203 and 129 genes immune-related genes were regulated at 25, 75 and 100 µg/kg MC-LR, respectively, indicating a disruption of the immune system. Liver damage induced by MC-LR was also observed. | [107] |
| Cyanobacterial cells containing MC-LR, MC-RR and MC-YR | Blunt snout bream (Megalobrama amblycephala) | Accumulation of MCs in the organs, macrophage phagocytosis and respiratory burst activities, pathological changes in lymphocytes | Fish exposed to NH3-N (0, 0.06, 0.12 mg/L) and fed with diets containing 15 and 30% of toxic cyanobacteria lyophilized powder for 30 days. | NH3-N could promote the accumulation of MC-LR and MC-RR in immune organs of fish (head kidney and spleen). Morphological changes were also detected in head kidney lymphocytes. Significant interaction between cyanobacteria and ammonia exposure on head kidney macrophage phagocytosis activity, respiratory burst activity, number of white blood cells and the transcriptional levels of some immunoglobulins. | [108] |
| MC-LR | Silver carp (Hypophthalmichthys molitrix) | ALT, AST, white blood cells, C3 and lysozyme activity in serum, IgM level, and TNF-α, IL-1β, IFN-γ contents | i.p. injection of ½ and 1/5 of 24 h LD50 (261.1 and 104.9 µg/kg, respectively) and sacrificed after 6, 9, 12, 24, 72, 168 h. | Increased ALT and AST activities in serum, and the number of leukocytes, complement C3 level, lysozyme activity (9 h) and contents of cytokines. Increased IgM levels were also observed. | [16] |
| Microcystis aeruginosa cells | Zebrafish (D. rerio) | Histological analysis, levels of inter-leukines (IL-1α, IL-1β, IFN-α, and TNF-α). Gene transcription analyses. | Zebrafish were exposed by immersion for 96 h to different concentrations of M. aeruginosa cells (low and high densities) | At high concentrations significant increases of cytokine levels were reported, and transcription of inflammatory genes were restrained. Low concentrations promoted the transcription. In intestines, increased goblet cell proliferation, and intestinal desquamation were detected. | [69] |
| Cyanobacterial Bloom | Shrimps (Litopenaeus vannamei) | Expression profiles of immune genes (RT-PCR), phagocytic activity of hemocytes | Shrimp were sampled at 15, 30, 50 and 70 days post the cyanobacterial bloom occurrence in a shrimp farm (China) | Many antimicrobial peptides genes were down-regulated, whereas the expression of C-type lectins was up-regulated. The concentration of hemocytes in hemolymph was decreased, but their phagocytic activity was increased. | [112] |
| MC-LR | Male zebrafish (D. rerio) | Immunological gene expression, serum immune splenic inflammatory changes and pathology | 0–30 µg/L MC-LR for 30 days | At 0.3–3 µg/L MC-LR splenic inflammatory changes were described, including increased serum C3 levels and upregulated expression of innate immune related genes. At 10–30 µg/L MC-LR, degeneration of splenic lymphocytes and macrophages, down-regulation of immune-related genes, and decreased level of serum C3. | [59] |
| MC-LR | Grass carp | Transcriptome analysis: 457 differentially expressed genes (DEGs) were identified using RNA-Seq. Histopathological study | i.p. injection of 25, 75 and 100 µg MC-LR/kg b.w., and control group. Fish were sacrificed 96 h after injection. | 61, 203 and 129 genes immune-related genes were regulated at 25, 75 and 100 µg/kg MC-LR, respectively, indicating a disruption of the immune system. Liver damage induced by MC-LR was also observed. | [107] |
| Cyanobacterial cells containing MC-LR, MC-RR and MC-YR | Blunt snout bream (Megalobrama amblycephala) | Accumulation of MCs in the organs, macrophage phagocytosis and respiratory burst activities, pathological changes in lymphocytes | Fish exposed to NH3-N (0, 0.06, 0.12 mg/L) and fed with diets containing 15 and 30% of toxic cyanobacteria lyophilized powder for 30 days. | NH3-N could promote the accumulation of MC-LR and MC-RR in immune organs of fish (head kidney and spleen). Morphological changes were also detected in head kidney lymphocytes. Significant interaction between cyanobacteria and ammonia exposure on head kidney macrophage phagocytosis activity, respiratory burst activity, number of white blood cells and the transcriptional levels of some immunoglobulins. | [108] |
| MC-LR | Silver carp (Hypophthalmichthys molitrix) | ALT, AST, white blood cells, C3 and lysozyme activity in serum, IgM level, and TNF-α, IL-1β, IFN-γ contents | i.p. injection of ½ and 1/5 of 24 h LD50 (261.1 and 104.9 µg/kg, respectively) and sacrificed after 6, 9, 12, 24, 72, 168 h. | Increased ALT and AST activities in serum, and the number of leukocytes, complement C3 level, lysozyme activity (9 h) and contents of cytokines. Increased IgM levels were also observed. | [16] |
| Microcystis aeruginosa cells | Zebrafish (D. rerio) | Histological analysis, levels of inter-leukines (IL-1α, IL-1β, IFN-α, and TNF-α). Gene transcription analyses. | Zebrafish were exposed by immersion for 96 h to different concentrations of M. aeruginosa cells (low and high densities) | At high concentrations significant increases of cytokine levels were reported, and transcription of inflammatory genes were restrained. Low concentrations promoted the transcription. In intestines, increased goblet cell proliferation, and intestinal desquamation were detected. | [69] |
| MC-LR | Male zebrafish (D. rerio) | Histology, serum immune parameters: IL-1β, TNF-α, complement C3. Transcriptions of genes representing the TLR/MyS88 signaling pathway by RT-PCR and western blot assay to measure MyD88 protein levels. Tunnel staining for apoptosis detection. | 0, 0.4, 2 and 10 μg/L MC-LR, for 30 days | MC-LR induced increases of serum TNF-α and IL-1β, and a significant upregulated expression of TLR/Myd88 signaling pathway genes. The immunohistochemical and western blot results validated that MC-LR enhanced the MyD88 signal. Significant decreases of serum C3 at the highest concentration was also reported. Pathological changes in spleen, and increased spleen index. | [74] |
| MC-LR | Zebrafish (D. rerio) | Quantification of MC-LR in gonads, eggs, water samples, histological examination, plasma sex hormone measurement, cytokines contents in F1 larvae. Gene transcription by RT-PCR; western blot to measure MyD88 protein levels | Adult zebra pairs were exposed to 0, 0.4, 2 and 10 μg/L for 60 days and the embryos (F1) were hatched without or with continued MC-LR exposure at the same concentrations until 5 days postfertilization (dpf). | Upregulation of innate immune-related genes and increased proinflammation cytokine contents (IL-1β, IL-6, TNF-α) were observed in F1 offspring with/without continued MC-LR exposure. | [70] |
| MC-LR | Male oriental river prawns (Macrobrachium nipponense) | Hormone level analysis, testicular histology examination, sperm quality and testicular antioxidant ability. Gene transcription by RT-PCR and MC-LR levels determination in water samples, embryos and testis. F1 larvae development assay. | 0, 0.5, and 5 μg/L MC-LR, for 1, 2 and 4 weeks. F1 embryos did or did not receive continued MC-LR treatment after hatching. The rates of survival, hatching, and malformation in the larvae of the F1 generation prawn after 4 days of exposure to microcystin-LR were determined. | The F1 offspring showed downregulation of immunity molecules (lysozyme, lectin3) and antioxidant molecules, and increased expression of innate immune-related factors (TLR3, MyD88), despite not being treated with MC-LR. | [117] |
| Cyanotoxin Congener | Experimental Model | Assays | Exposure Conditions | Effects | References |
|---|---|---|---|---|---|
| Microcystin extract from a bloom | BALB/c Mice | Uptake capacity of peripheral phagocytes. lymphocyte proliferation. Antibody response. | Intraperitoneal (i.p.) injection for 14 days of three doses:16, 32 and 64 mg lyophilized algae cells/kg body weight (b.w.), containing 4.97, 9.94, and 19.88 μg MCs equiv/kg b.w. | Decreased body weight. Spleen and thymus body ratios were altered. A reduction of phagocytosis was reported using phagocytic index of peritoneal phagocytes. Inhibition of LPS-induced lymphoproliferation and a dose-dependent decrease of the numbers of antibody-forming cells were observed. However, no effects on conA-induced T cell proliferation were detected. | [54] |
| MC extract from cyanobacterial blooms | BALB/c female mice | Expression of multiple cytokines: proinflammatory (IL-1β, TNF-α, and IL-6) and Th1/Th2-related cytokines (IL-2, IL-4 and IL-10) by RT-PCR | Injection of doses of 23, 38, 77, 115 mg lyophilized algae cells/kg b.w., containing 7, 12, 24 and 36 µg MC/kg b.w., respectively. | mRNA levels of some cytokines decreased after injection of all doses, while IL-6 level was unaffected. The level of IL-10 mRNA was transiently up regulated at the lowest dose. | [67] |
| Blooms containing MC-LR and MC-RR and MC-YR | Rabbits | White blood cell (WBC) numbers and cytokine production (IL-3, IL-4, IL-6, TNF-α, IFN-γ) | Injection (i.p.) of 12.5, 50 µg/kg b.w. Sera were collected from the hearts of rabbits at different times: 0-3 h post treatment in the group of the highest dose, and between 0–168 h post treatment in the group with the lowest dose. | At the high dose WBC number increased but Th1 (TNF-α, IFN-γ) and Th2 (IL-3, IL-4. IL-6) production decreased. In the low dose group the number of WBC and some cytokines production increased in first 12 h, and dropped after 24 h. Some positive correlations between cytokines production were also found. | [119] |
| MC-LR | Wistar rats | Toxin accumulation. Lysozyme activity, spleen index, histopathological examination and proteome analysis | i.p. injection of MC-LR (1 or 10 µg/kg/day) for 50 days, and control rats. | Significant accumulation in spleen. Decreased lysozyme activity and decreased spleen indexes at the high dose group. Severe damage in spleen and impaired immune functions. 48 splenetic protein levels were modified, mainly involved in immune response, oxidative stress, energetic metabolism and the cytoskeleton assembly. | [121] |
| MC-LR | Mice (serum, macrophages, leukocytes) | Phagocytosis and ROS of leukocytes, cytokines (IL-6, IL-10 and TNF-α) and DNA-protein crosslink detection (DPC formation) | i.p. injection of 0–25.000 µg/kg/day for 7 days | The level of IL-6 increased with the dose, while TNF-α decreased, and IL-10 had no relationship with the MC-LR exposure. No significant induction of DNA-protein crosslink was detected. The toxin caused a significant dose-dependent counter-effect of phagocytosis for macrophages, but not on the leukocytes. | [122] |
| Cyanobacterial bloom biomass containing MCs | Peripheral blood, spleen and thymus from Wistar rats | Hematological parameters. Histopathology of spleen and thymus, and lymphocyte populations in peripheral blood, spleen and thymus. | Rats with a diet containing fish meat with and without MCs and complex toxic biomass, for 28 days. Groups D,E: rats fed with diet with 25% of fish (no-MCs) enriched with 700 or 5000 µg total MCs/kg wet weight (w.w.); F: rats fed with diet with 25% of fish from the locality with heavy cyanobacterial bloom. | No histological changes were observed in spleen or thymus. NK cells and γδ+ T lymphocytes were increased in peripheral blood in the group exposed to isolated MCs in the food (group E). Significant changes in the ratio CD4+ and CD8+ cells (increase of CD4+ and a drop in CD8+) were found in the group F. The greatest changes in lymphoid organs were observed in groups E and F. There was an increase of spleen subpopulations of γδ+ T lymphocytes in group E as well as of IgM+ lymphocytes (B) and CD8+ T lymphocytes. | [120] |
| MC-LR | Male specific pathogen free (SPF) BALB/c mice | Immune responses in Leydig (LC), Sertoli (SC) and germ cells (GC) were studied, cytokines and chemo-quines in testicular cells, immunohistochemical analysis, sperm counts, q-PCR and western blotting techniques. Spermatogenesis. | Histopathology, Elisa, and western blotting: Mice were i.p. exposed to 20 μg/kg b.w. daily for 7 days. Spermatogenesis: Mice were given drinking water containing 1, 10, 20, or 30 mg/L MC-LR for 90 consecutive days. | MC-LR induced innate immune responses in SC, GC and LC cells. In SC and GC the effects were via the PP2A-dependent phosphatidylinositol 3-kinase (PI3K(AKT/NF-κB) signaling pathway. In LC cells, MC-LR dependent activation of NF-κB and production of proinflammatory cytokines and chemokines may be mediated by Toll-like receptor 2 (TLR2). PI3K(AKT/NF-κB) were also activated in SC, GC, and LC in vivo, with induction of cytokines and chemokines. After chronic exposure decreased sperm counts and abnormal sperm morphology were also reported. | [73] |
| MC-LR | Male SPF BALB/c mice | Flow cytometry, q-RT- PCR, western blotting, coimmunoprecipitation, Elisa analyses, immuno-histo-chemical analyses, immunofluorescence staining | Mice were given water containing 1–30 μg MC-LR/L for 180 consecutive days. | No apoptosis in Leydig cells (LC) alone. MC-LR can activate macrophages to produce TNF-α and GAS6 in testes, and secreted TNF-α induced apoptosis of surrounding LC. Activated macrophages could engulf apoptotic LC via the Axl-GAS6-PtdSer axis. Reduced serum testosterone levels may be associated with decrease of LCs. | [60] |
| MC-LR | Male BALB/c mice | SEM, AFM, μ-CT and FTIR in femur bones. Flow cytometry in a suspension of T and B cells from bone marrow, spleen, lymph node and thymus. Levels of cytokines in blood serum (IL-6, IL-10, IL-17A, IFN-γ, and TNF-α) | 10 μg/kg b.w. MC-LR day, i.p. for 15 days, and control group. Animals were sacrificed, and bones, lymphoid organs and serum were collected. | MC-LR induced bone loss, impaired lumbar vertebral, tibial and femoral bone micro-architecture, and decreased the mineral density and heterogeneity of bones in mice. MC-LR modulated the population of CD4+T, CD8+T and B cells, and the toxin increased levels of osteoclastogenic cytokines (IL-6, IL-17A, TNF-α, and decreased the levels of osteoprotective cytokines (IFN-γ, IL-10). | [61] |
| MC-LR | C57BL/6J mice | Histopathologic study and mRNA expression levels of inflammation factors in jejunum (IL-1β, IL-8, TNF-α, IL-10, TGF- β1) | Oral exposure to MC-LR from drinking water at several concentrations (1, 30, 60, 90, 120 μg/L) for 6 months. | Microstructure of jejunum was destroyed and expression levels of IL-1β, IL-8, TNF-α, IL-10, TGF- β1 were altered. | [123] |
| Cyanotoxin | Experimental Model | Assays | Exposure Conditions | Effects | Reference |
|---|---|---|---|---|---|
| C. raciborskii culture containing CYN | Male mice | Acute oral toxicity assay, including histopathological study | Single oral dose of CYN from a C. raciborskii culture containing 0.2% CYN | The median lethal dose was in the range of 4.4-6.9 mg/kg equiv CYN. Death occurred at 2–6 days. The most remarkable changes were observed in the liver, with periacinal coagulative necrosis. Acute renal tubular necrosis, atrophy of the thymic cortex and the lymphoid follicles in the spleen, myocardial hemorrhages, and ulcerations in esophagus and gastric mucosa were also observed. | [50] |
| CYN | Human peripheral blood lymphocytes | Proliferation lymphocytes | 0.01, 0.1,1 µg/mL CYN for 24 h | Significant inhibition of lymphocytes proliferation at the highest concentration assayed. | [53] |
| CYN | Human peripheral blood lymphocytes | Proliferation assay, cell viability and cell cycle assays | 0.01, 0.1, 1 µg/mL CYN after 0, 6, 24, 48 h of the 72 h culture | Cell proliferation was inhibited at 1 µg/mL. CYN affected the viability of human T-lymphocytes in a concentration and exposure time dependent manner. Exposure to 1 µg/mL CYN at the beginning of activation and after 6 h decreased the number of cells entering G2/M phase, and increased number of cells blocked in G0/G1 or prolonged S phase. | [56] |
| CYN purified from C. raciborskii | Human peripheral blood neutrophils | ROS production, phagocytic activity, cell number and viability | 0.01, 0.1, 1 µg/mL CYN during 1 h exposure | CYN decreased the level of ROS production in stimulated neutrophils, and in unstimulated cells. CYN did not affect the percentage of phagocytic cells or the number of engulfed bacteria. The toxin did not induce apoptosis or necrosis. | [57] |
| CYN purified from C. raciborskii and non-CYN producing C. raciborskii | Human neutrophils and lymphocytes | Viability assays of lymphocytes and neutrophils, and lymphocyte proliferation | Cells were exposed to 0.01, 0.1 and 1 µg/mL of purified CYN. Cells were exposed to C. raciborskii cell-free extract | Short term extract treatments altered viability of cells, but CYN did not induce similar effects. Lymphocytes in general were more resistant to cell-free extracts than neutrophils. Differences between lymphocyte responses in isolated and whole-blood cultures were observed. Significant antiproliferative properties were found for the lowest CYN concentration in whole-blood culture. Both extracts exhibited immunomodulatory potencies, so unknown metabolites with a toxic pattern different to that of CYN could be involved. | [58] |
| Purified CYN | Human lymphocytes | ROS production, cell counts, SOD, GPx, CAT activities, LPO | 0.01, 0.1 and 1 µg/mL of purified CYN. ROS evaluated at 0.5, 1, 1.5, 3, 6, 24 and 48 h. Cell counts and oxidative stress biomarkers after 3 and 6 h | Concentration-dependent increase in H2O2 from 0.5 h with the highest values after 3 and 6 h. At both times SOD and CAT activities decreased and GPx and LPO increased. | [127] |
| CYN | Leucocyte cell line Cyprinus carpio (CLC) | Cell viability, proliferation, apoptosis/necrosis, cell morphology and phagocytic activity | 0.1, 1, 10 µg/mL CYN for up to 48 h | 1, 10 µg/mL CYN were cytotoxic and altered all parameters. CYN impaired the function of phagocytic cells, their ability to engulf bacteria at the lowest and not cytotoxic concentration. | [128] |
| CYN | Phagocytic cells from the common carp (C. carpio) | Phagocytosis, ROS and nitrogen species production, cytokine expression | 0.05, 0.1, 0.5, 1 µg/mL CYN for up to 24 h | Cytotoxicity at 1 µg/mL CYN. Decreased phagocytic activity and changes in actin cytoskeletal structures at 0.5–1 µg/mL Increased ROS and nitrogen species at all tested concentrations. Increased expression in the mRNA level of IL-1β and TNF-α. Expression of the TGF-β gene was elevated at the lowest CYN concentration, whereas the toxin did not induce increases at higher concentrations. No effects on IL-10. | [66] |
| CYN | Murine macrophage RAW 264.7 cells | Cell viability, NO and ROS production, TNF-α and IL-6, western blot analysis of MAPKs, NF-κB, and iNOS | 0.001–1 µM CYN alone or together with cyanobacterial lipopolysaccharide (LPS) for 20, 60 min and 24 h | No effects on viability. CYN alone increased the production of TNF-α which correlated with its effects on ROS production, but it had no effect on either expression of iNOS or several pro-inflammatory mediators (NO and IL-6). Moreover, CYN potentiated the effect of cyanobacterial LPS by induction TNF-α, IL-6, and ROS production, including mitogen-activated protein kinase p38 and expression of iNOS. | [37] |
| CYN | Engineered HEK293 cells that utilize an NF- κB-inducible SEAP (secreted embryonic alkaline phosphatase) reporter | Immunomodulatory Compound screening method based on engineered HEK293-hTLR NF-κB reporter assay, to identify activators or inhibitors of specific TLRs | 100 nM CYN | Cells expressing TLR2-9 were not activated by CYN, and the toxin did not block the ability of TLR2-9 to interact with specific TLR agonists, and thus, the toxin is not considered as TLR antagonist. TLR negative cells (HEK293 parental cells) were not stimulated by CYN. | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez-Quijada, L.; Benítez-González, M.d.M.; Puerto, M.; Jos, A.; Cameán, A.M. Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review. Toxins 2021, 13, 711. https://doi.org/10.3390/toxins13100711
Diez-Quijada L, Benítez-González MdM, Puerto M, Jos A, Cameán AM. Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review. Toxins. 2021; 13(10):711. https://doi.org/10.3390/toxins13100711
Chicago/Turabian StyleDiez-Quijada, Leticia, Maria del Monte Benítez-González, María Puerto, Angeles Jos, and Ana M. Cameán. 2021. "Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review" Toxins 13, no. 10: 711. https://doi.org/10.3390/toxins13100711
APA StyleDiez-Quijada, L., Benítez-González, M. d. M., Puerto, M., Jos, A., & Cameán, A. M. (2021). Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review. Toxins, 13(10), 711. https://doi.org/10.3390/toxins13100711







