BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway
Abstract
1. Introduction
2. Results
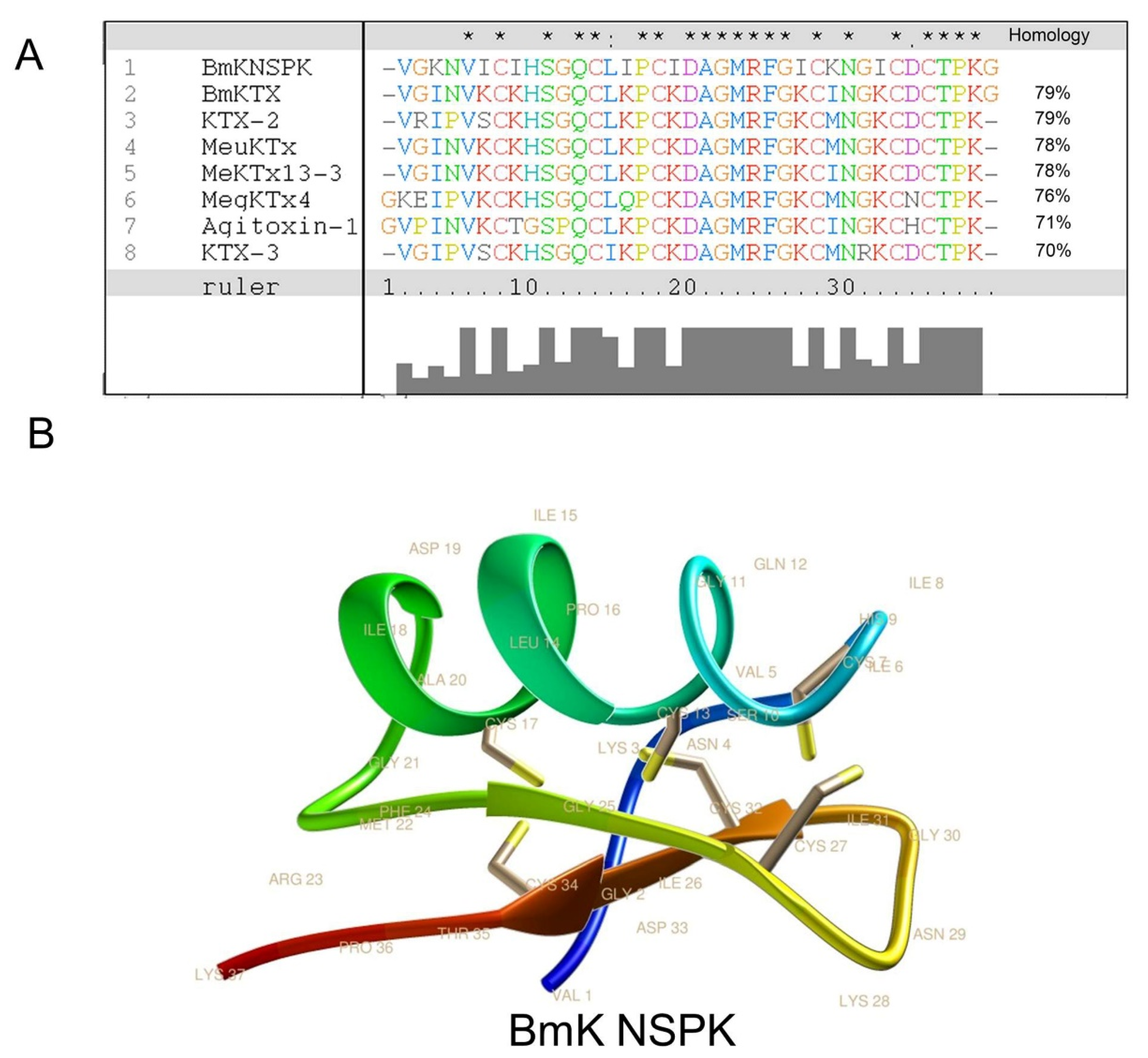
2.1. BmK NSPK Purification and Structure Determination
2.2. BmK NSPK Altered Calcium Dynamics and Depolarized Membrane in Primary Cultured SCNs
2.3. BmK NSPK Directly Inhibits Kv Channels in Spinal Cord Neurons
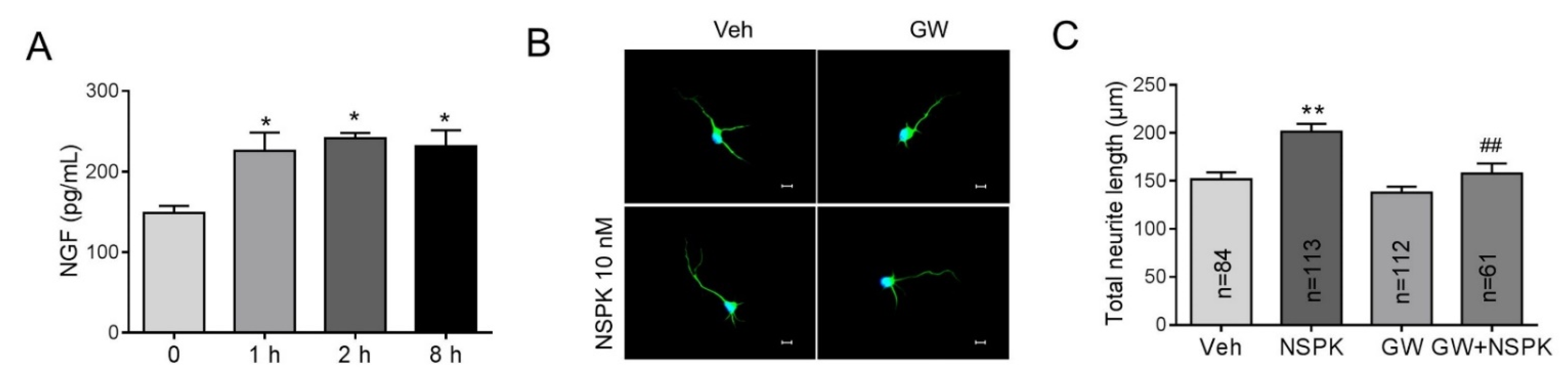
2.4. BmK NSPK Enhances Neurite Outgrowth in Cultured SCNs
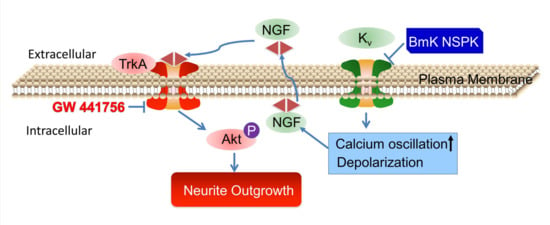
2.5. BmK NSPK-Induced Neurite Outgrowth is Dependent on NGF/TrkA Signaling Pathway
3. Discussions
4. Materials and Methods
4.1. Animal Care
4.2. Materials
4.3. BmK NSPK Purification
4.4. Mass Spectrometry
4.5. Edman Degradation
4.6. Sequence Alignment and Molecular Modeling
4.7. Primary Culture of Spinal Cord Neurons (SCNs)
4.8. Intracellular Calcium Concentration Measurement
4.9. Whole-Cell Patch-Clamp Recording
4.10. Immunocytochemistry
4.11. Enzyme-Linked Immunosorbent Assay (ELISA)
4.12. Western Blot
4.13. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavon, O.; Bentur, Y. Poison exposures in young Israeli military personnel: A National Poison Center Data analysis. Clin. Toxicol. 2017, 55, 322–325. [Google Scholar] [CrossRef]
- Kang, A.M.; Brooks, D.E. Geographic distribution of scorpion exposures in the United States, 2010–2015. Am. J. Public Health 2017, 107, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, Y.; Zou, X.; Tang, Q.; Zhao, F.; Cao, Z. BmK AEP, an anti-epileptic peptide distinctly affects the gating of brain subtypes of voltage-gated sodium channels. Int. J. Mol. Sci. 2019, 20, 729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, W.; Shao, Z.; Gao, B.; Li, J.; Ma, J.; Li, J.; Che, H.; Zhang, W. Eukaryotic expression and purification of anti-epilepsy peptide of Buthus martensii Karsch and its protein interactions. Mol. Cell. Biochem. 2009, 330, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. The biochemical research on scorpion venoms and their application in therapy. Progr. Biochem. Biophys. 1984, 56, 20–29. [Google Scholar] [CrossRef]
- Mao, Q.; Ruan, J.; Cai, X.; Lu, W.; Ye, J.; Yang, J.; Yang, Y.; Sun, X.; Cao, J.; Cao, P. Antinociceptive effects of analgesic-antitumor peptide (AGAP), a neurotoxin from the scorpion Buthus martensii Karsch, on formalin-induced inflammatory pain through a mitogen-activated protein kinases–dependent mechanism in mice. PLoS ONE 2013, 8, e78239. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wang, Y.; Yu, Y.; He, J.; Zhao, F.; Xi, C.; Zhang, C.; Cao, Z. BmK NSP, a new sodium channel activator from Buthus martensii Karsch, promotes neurite outgrowth in primary cultured spinal cord neurons. Toxicon 2020. [Google Scholar] [CrossRef] [PubMed]
- Luna-Ramírez, K.; Bartok, A.; Restano-Cassulini, R.; Quintero-Hernández, V.; Coronas, F.I.; Christensen, J.; Wright, C.E.; Panyi, G.; Possani, L.D. Structure, molecular modeling, and function of the novel potassium channel blocker urotoxin isolated from the venom of the Australian scorpion Urodacus yaschenkoi. Mol. Pharmacol. 2014, 86, 28–41. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zou, X.; Li, X.; Chen, J.; Jin, L.; Zhang, F.; Yu, B.; Cao, Z. Activation of sodium channels by α-scorpion toxin, BmK NT1, produced neurotoxicity in cerebellar granule cells: An association with intracellular Ca2+ overloading. Arch. Toxicol. 2017, 91, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Chuang, R.S.; Jaffe, H.; Cribbs, L.; Perez-Reyes, E.; Swartz, K.J. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat. Neurosci. 1998, 1, 668–674. [Google Scholar] [CrossRef]
- Dardevet, L.; Rani, D.; Aziz, T.A.E.; Bazin, I.; Sabatier, J.-M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Jaimes, L.; Xiao, L.; Zhang, J.; Possani, L.D.; Valdivia, H.H.; Quintero-Hernández, V. Recombinant expression of Intrepicalcin from the scorpion Vaejovis intrepidus and its effect on skeletal ryanodine receptors. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Wang, B.; Cao, Z.; Li, W.; Wu, Y. A single conserved basic residue in the potassium channel filter region controls KCNQ1 insensitivity toward scorpion toxins. Biochem. Biophys. Rep. 2015, 3, 62–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dueñas-Cuellar, R.A.; Santana, C.J.C.; Magalhães, A.C.M.; Pires, O.R.; Fontes, W.; Castro, M.S. Scorpion Toxins and Ion Channels: Potential Applications in Cancer Therapy. Toxins 2020, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Cornet, B.; Bonmatin, J.-M.; Hetru, C.; Hoffmann, J.A.; Ptak, M.; Vovelle, F. Refined three-dimensional solution structure of insect defensin A. Structure 1995, 3, 435–448. [Google Scholar] [CrossRef]
- Van Ooyen, A.; Van Pelt, J. Activity-dependent neurite outgrowth and neural network development. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1994; Volume 102, pp. 245–259. [Google Scholar]
- Zheng, J.Q.; Poo, M.-M. Calcium signaling in neuronal motility. Annu. Rev. Cell Dev. Biol. 2007, 23. [Google Scholar] [CrossRef]
- Cao, Z.; Cui, Y.; Busse, E.; Mehrotra, S.; Rainier, J.D.; Murray, T.F. Gambierol inhibition of voltage-gated potassium channels augments spontaneous Ca2+ oscillations in cerebrocortical neurons. J. Pharmacol. Exp. Ther. 2014, 350, 615–623. [Google Scholar] [CrossRef]
- George, J.; Baden, D.G.; Gerwick, W.H.; Murray, T.F. Bidirectional influence of sodium channel activation on NMDA receptor–dependent cerebrocortical neuron structural plasticity. Proc. Natl. Acad. Sci. USA 2012, 109, 19840–19845. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.-W.; Zhang, Y.; Wu, X.-F.; Peng, Y.; Cao, Z.; Ge, B.-Y.; Wang, X.; Wu, Q.; Lin, J.-T. Scorpion venom heat-resistant peptide (SVHRP) enhances neurogenesis and neurite outgrowth of immature neurons in adult mice by up-regulating brain-derived neurotrophic factor (BDNF). PLoS ONE 2014, 9, e109977. [Google Scholar] [CrossRef]
- Zou, X.; Wu, Y.; Chen, J.; Zhao, F.; Zhang, F.; Yu, B.; Cao, Z. Activation of sodium channel by a novel α-scorpion toxin, BmK NT2, stimulates ERK1/2 and CERB phosphorylation through a Ca2+ dependent pathway in neocortical neurons. Int. J. Biol. Macromol. 2017, 104, 70–77. [Google Scholar] [CrossRef]
- Calabrese, E.J. Enhancing and regulating neurite outgrowth. Crit. Rev. Toxicol. 2008, 38, 391–418. [Google Scholar] [CrossRef]
- Renisio, J.G.; Romi-Lebrun, R.; Blanc, E.; Bornet, O.; Nakajima, T.; Darbon, H. Solution structure of BmKTX, a K+ blocker toxin from the Chinese scorpion Buthus Martensi. Proteins Struct. Funct. Bioinform. 2000, 38, 70–78. [Google Scholar] [CrossRef]
- De La Vega, R.C.R.G.; Possani, L.D. Current views on scorpion toxins specific for K+-channels. Toxicon 2004, 43, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Peigneur, S.; Tytgat, J.; Zhu, S. A potent potassium channel blocker from Mesobuthus eupeus scorpion venom. Biochimie 2010, 92, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Gigolaev, A.M.; Kuzmenkov, A.I.; Peigneur, S.; Tabakmakher, V.M.; Pinheiro-Junior, E.L.; Chugunov, A.O.; Efremov, R.G.; Tytgat, J.; Vassilevski, A.A. Tuning Scorpion Toxin Selectivity: Switching From KV1. 1 to KV1. 3. Front. Pharmacol. 2020, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.S.; Chandy, K.G. Venom-derived peptide inhibitors of voltage-gated potassium channels. Neuropharmacology 2017, 127, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Gruenstein, E.I. Mechanism of synchronized Ca2+ oscillations in cortical neurons. Brain Res. 1997, 767, 239–249. [Google Scholar] [CrossRef]
- Czarnecki, A.; Magloire, V.; Streit, J. Local oscillations of spiking activity in organotypic spinal cord slice cultures. Eur. J. Neurosci. 2008, 27, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Hammock, B.D.; McCoy, M.; Rogawski, M.A.; Lein, P.J.; Pessah, I.N. Tetramethylenedisulfotetramine alters Ca2+ dynamics in cultured hippocampal neurons: Mitigation by NMDA receptor blockade and GABAA receptor-positive modulation. Toxicol. Sci. 2012, 130, 362–372. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Huang, Q.-L.; Chen, J.; Zhang, W.-J.; Jin, H.-X.; Wang, H.-B.; Naman, C.B.; Cao, Z.-Y. Phthalideisoquinoline Hemiacetal Alkaloids from Corydalis decumbens That Inhibit Spontaneous Calcium Oscillations, Including Alkyl Derivatives of (+)-Egenine That Are Strikingly Levorotatory. J. Nat. Prod. 2019, 82, 2713–2720. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, Y.; Feng, W.; Li, J.; Liu, J.; Zhang, C.; Dong, Y.; Pessah, I.N.; Cao, Z. Influence of nanomolar deltamethrin on the hallmarks of primary cultured cortical neuronal network and the role of ryanodine receptors. Environ. Health Perspect. 2019, 127, 067003. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Cui, Y.; Nguyen, H.M.; Jenkins, D.P.; Wulff, H.; Pessah, I.N. Nanomolar bifenthrin alters synchronous Ca2+ oscillations and cortical neuron development independent of sodium channel activity. Mol. Pharmacol. 2014, 85, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Shen, W.Q.; Pan, Y.P.; Xiao, X.; Liu, X.M.; Wang, X.L.; Liang, X.T.; Yu, D.Q. Purification, characterization of two peptides from Buthus martensi Karch. Chem. Biol. Drug Des. 2010, 62, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Nakahira, K.; Shibasaki, K.; Wakazono, Y.; Imoto, K.; Ikenaka, K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J. Neurosci. 2000, 20, 4145–4155. [Google Scholar] [CrossRef] [PubMed]
- Dodson, P.D.; Forsythe, I.D. Presynaptic K+ channels: Electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004, 27, 210–217. [Google Scholar] [CrossRef]
- Breton, J.D.; Poisbeau, P.; Darbon, P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol. Pain 2009, 5, 1744–8069. [Google Scholar] [CrossRef]
- Howe, C.L. Depolarization of PC12 cells induces neurite outgrowth and enhances nerve growth factor-induced neurite outgrowth in rats. Neurosci. Lett. 2003, 351, 41–45. [Google Scholar] [CrossRef]
- Castillo, X.; Melo, Z.; Varela-Echavarría, A.; Tamariz, E.; Aroña, R.M.; Arnold, E.; Clapp, C.; de la Escalera, G.M. Vasoinhibin suppresses the neurotrophic effects of VEGF and NGF in newborn rat primary sensory neurons. Neuroendocrinology 2018, 106, 221–233. [Google Scholar] [CrossRef]
- Song, E.-J.; Yoo, Y.-S. Nerve growth factor-induced neurite outgrowth is potentiated by stabilization of TrkA receptors. BMB Rep. 2011, 44, 182–186. [Google Scholar] [CrossRef]
- Xie, Y.; Tisi, M.A.; Yeo, T.T.; Longo, F.M. Nerve growth factor (NGF) loop 4 dimeric mimetics activate ERK and AKT and promote NGF-like neurotrophic effects. J. Biol. Chem. 2000, 275, 29868–29874. [Google Scholar] [CrossRef]
- Bergeron, Z.L.; Bingham, J.-P. Scorpion toxins specific for potassium (K+) channels: A historical overview of peptide bioengineering. Toxins 2012, 4, 1082–1119. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Possani, L.D. Scorpion toxins to unravel the conundrum of ion channel structure and functioning. Toxicon 2018, 150, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Romi-Lebrun, R.; Lebrun, B.; Martin-Eauclaire, M.-F.; Ishiguro, M.; Escoubas, P.; Wu, F.Q.; Hisada, M.; Pongs, O.; Nakajima, T. Purification, characterization, and synthesis of three novel toxins from the Chinese scorpion Buthus martensi, which act on K+ channels. Biochemistry 1997, 36, 13473–13482. [Google Scholar] [CrossRef] [PubMed]
- Costantin, J.L.; Charles, A.C. Modulation of Ca2+ Signaling by K+ Channels in a Hypothalamic Neuronal Cell Line (GT1–1). J. Neurophysiol. 2001, 85, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kelamangalath, L.; Dravid, S.M.; George, J.; Aldrich, J.V.; Murray, T.F. κ-Opioid receptor inhibition of calcium oscillations in spinal cord neurons. Mol. Pharmacol. 2011, 79, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Dravid, S.M.; Murray, T.F. Spontaneous synchronized calcium oscillations in neocortical neurons in the presence of physiological [Mg2+]: Involvement of AMPA/kainate and metabotropic glutamate receptors. Brain Res. 2004, 1006, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, Q.; Crook, R.J.; O’Neil, R.G.; Walters, E.T. TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. PAIN 2013, 154, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- McAlexander, M.A.; Undem, B.J. Potassium channel blockade induces action potential generation in guinea-pig airway vagal afferent neurones. J. Auton. Nerv. Syst. 2000, 78, 158–164. [Google Scholar] [CrossRef]
- Wilson, J.R.; Duncan, N.A.; Giles, W.R.; Clark, R.B. A voltage-dependent K+ current contributes to membrane potential of acutely isolated canine articular chondrocytes. J. Physiol. 2004, 557, 93–104. [Google Scholar] [CrossRef]
- Konur, S.; Ghosh, A. Calcium signaling and the control of dendritic development. Neuron 2005, 46, 401–405. [Google Scholar] [CrossRef]
- George, J.; Dravid, S.M.; Prakash, A.; Xie, J.; Peterson, J.; Jabba, S.V.; Baden, D.G.; Murray, T.F. Sodium channel activation augments NMDA receptor function and promotes neurite outgrowth in immature cerebrocortical neurons. J. Neurosci. 2009, 29, 3288–3301. [Google Scholar] [CrossRef] [PubMed]
- Reber, B.; Porzig, H.; Becker, C.; Reuter, H. Depolarization-induced changes of free intracellular Ca2+ concentration and of [3H] dopamine release in undifferentiated and differentiated PC12 cells. Neurochem. Int. 1990, 17, 197–203. [Google Scholar] [CrossRef]
- Wu, Y.; Krüttgen, A.; Möller, J.; Shine, D.; Chan, J.; Shooter, E.; Cosgaya, J. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 are sorted to dense-core vesicles and released via the regulated pathway in primary rat cortical neurons. J. Neurosci. Res. 2004, 75, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.-G.; Zhang, Q.; Liu, X.-J.; Liu, X.; Jiao, L.; Zhu, W.; Zhang, Z.-H.; Zhao, X.-L.; He, C. Interaction of Mint2 with TrkA is involved in regulation of nerve growth factor-induced neurite outgrowth. J. Biol. Chem. 2009, 284, 12469–12479. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Tanaka, H.; Temporin, K.; Okamoto, M.; Kuroda, Y.; Moritomo, H.; Murase, T.; Yoshikawa, H. Akt/mammalian target of rapamycin signaling pathway regulates neurite outgrowth in cerebellar granule neurons stimulated by methylcobalamin. Neurosci. Lett. 2011, 495, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 2009, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Gadagkar, S.R.; Call, G.B. Computational tools for fitting the Hill equation to dose–response curves. J. Pharmacol. Toxicol. Methods 2015, 71, 68–76. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Zou, X.; Li, S.; He, J.; Xi, C.; Tang, Q.; Wang, Y.; Cao, Z. BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway. Toxins 2021, 13, 33. https://doi.org/10.3390/toxins13010033
Zhao F, Zou X, Li S, He J, Xi C, Tang Q, Wang Y, Cao Z. BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway. Toxins. 2021; 13(1):33. https://doi.org/10.3390/toxins13010033
Chicago/Turabian StyleZhao, Fang, Xiaohan Zou, Shaoheng Li, Jing He, Chuchu Xi, Qinglian Tang, Yujing Wang, and Zhengyu Cao. 2021. "BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway" Toxins 13, no. 1: 33. https://doi.org/10.3390/toxins13010033
APA StyleZhao, F., Zou, X., Li, S., He, J., Xi, C., Tang, Q., Wang, Y., & Cao, Z. (2021). BmK NSPK, a Potent Potassium Channel Inhibitor from Scorpion Buthus martensii Karsch, Promotes Neurite Outgrowth via NGF/TrkA Signaling Pathway. Toxins, 13(1), 33. https://doi.org/10.3390/toxins13010033