A Pharmacokinetic Study Comparing the Clearance of Vancomycin during Haemodialysis Using Medium Cut-Off Membrane (Theranova) and High-Flux Membranes (Revaclear)
Abstract
1. Introduction
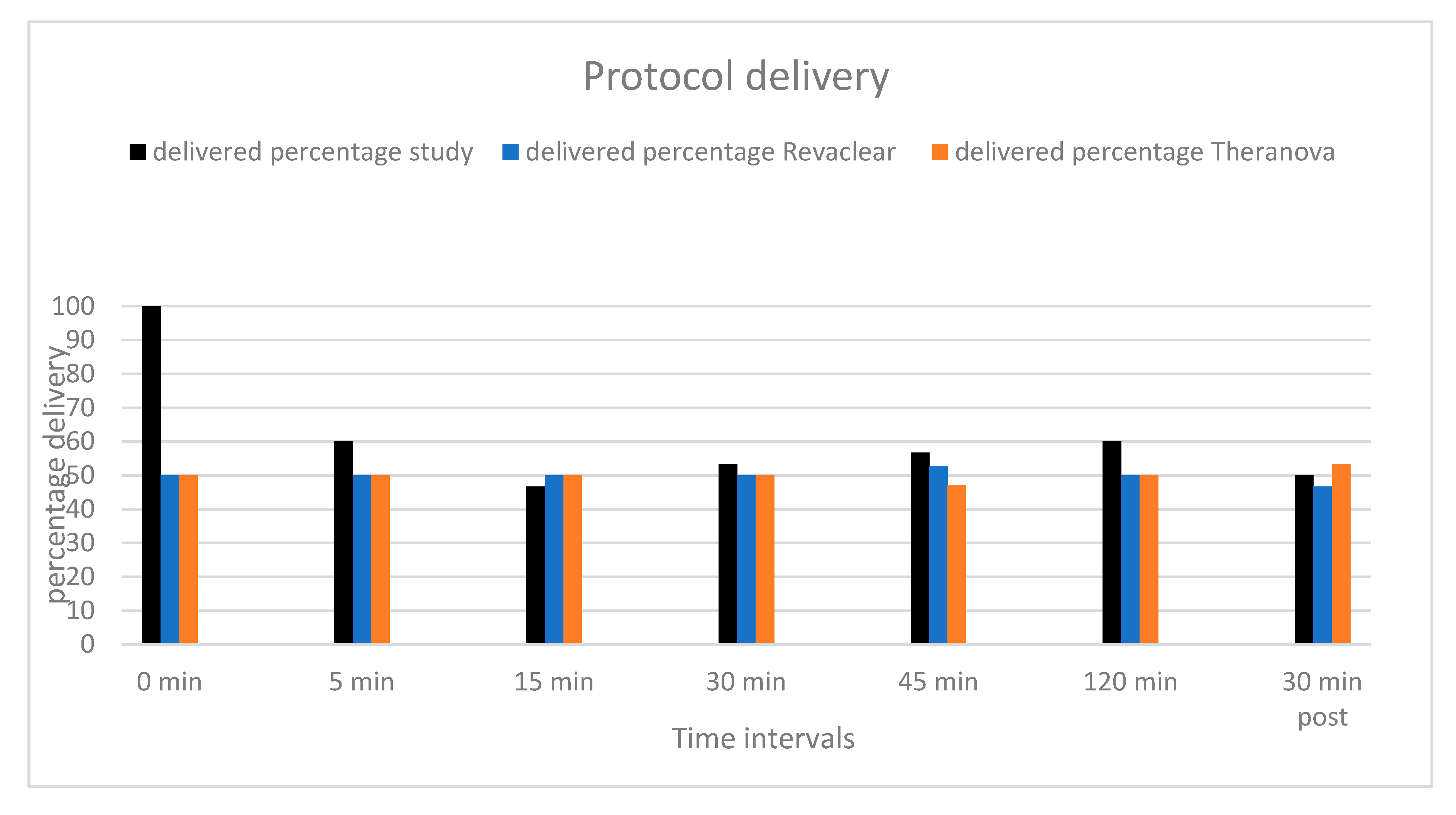
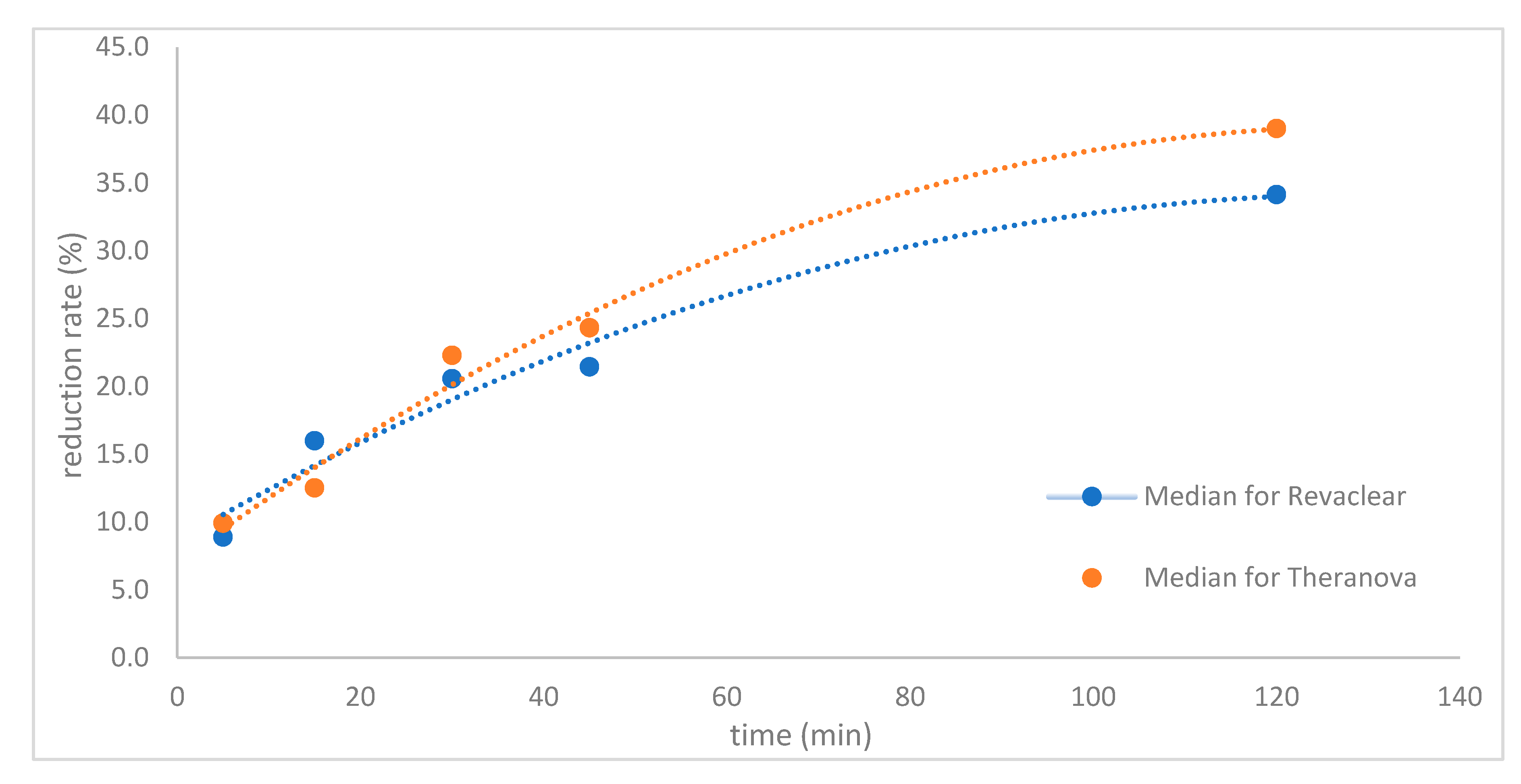
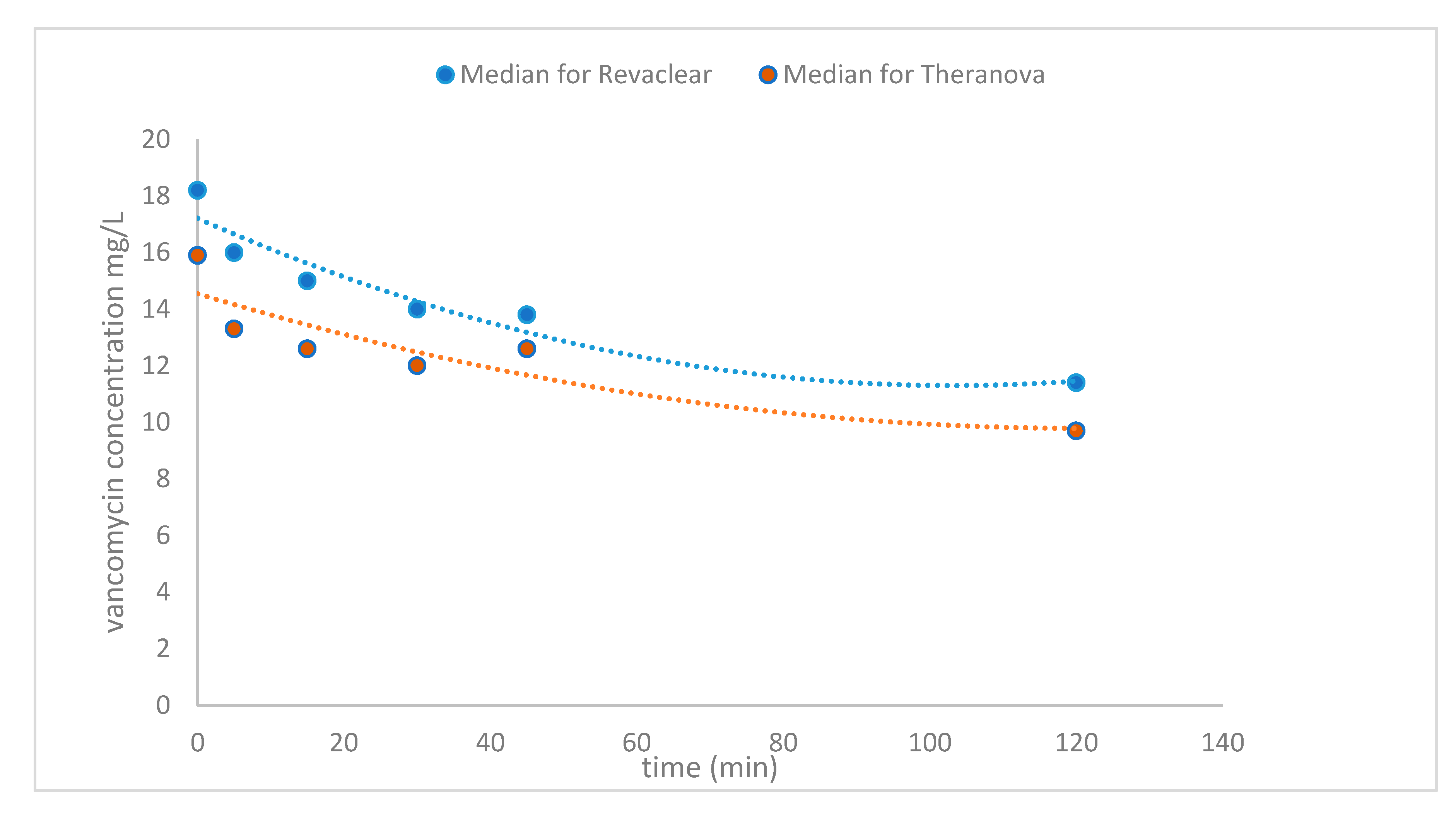
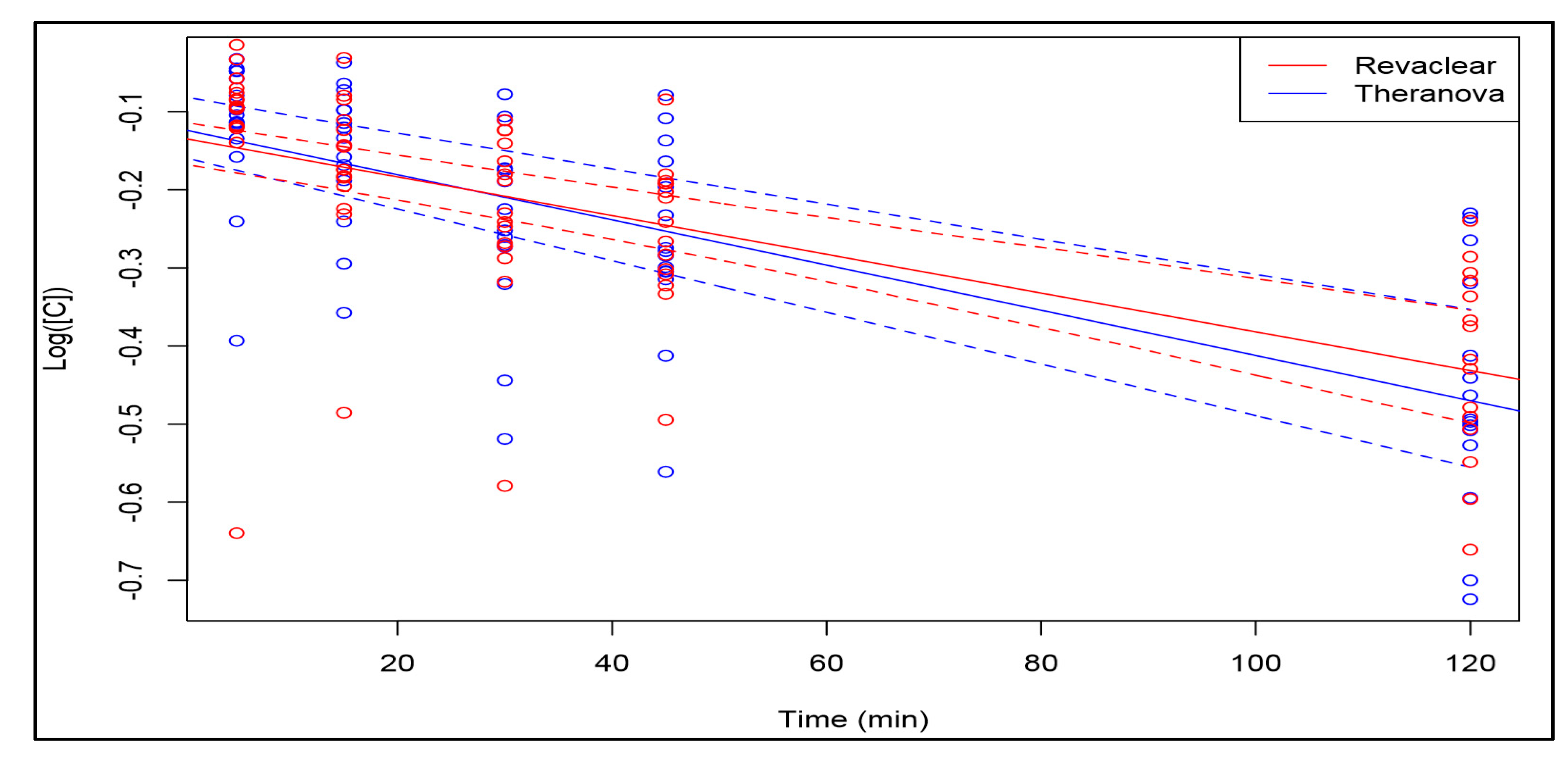
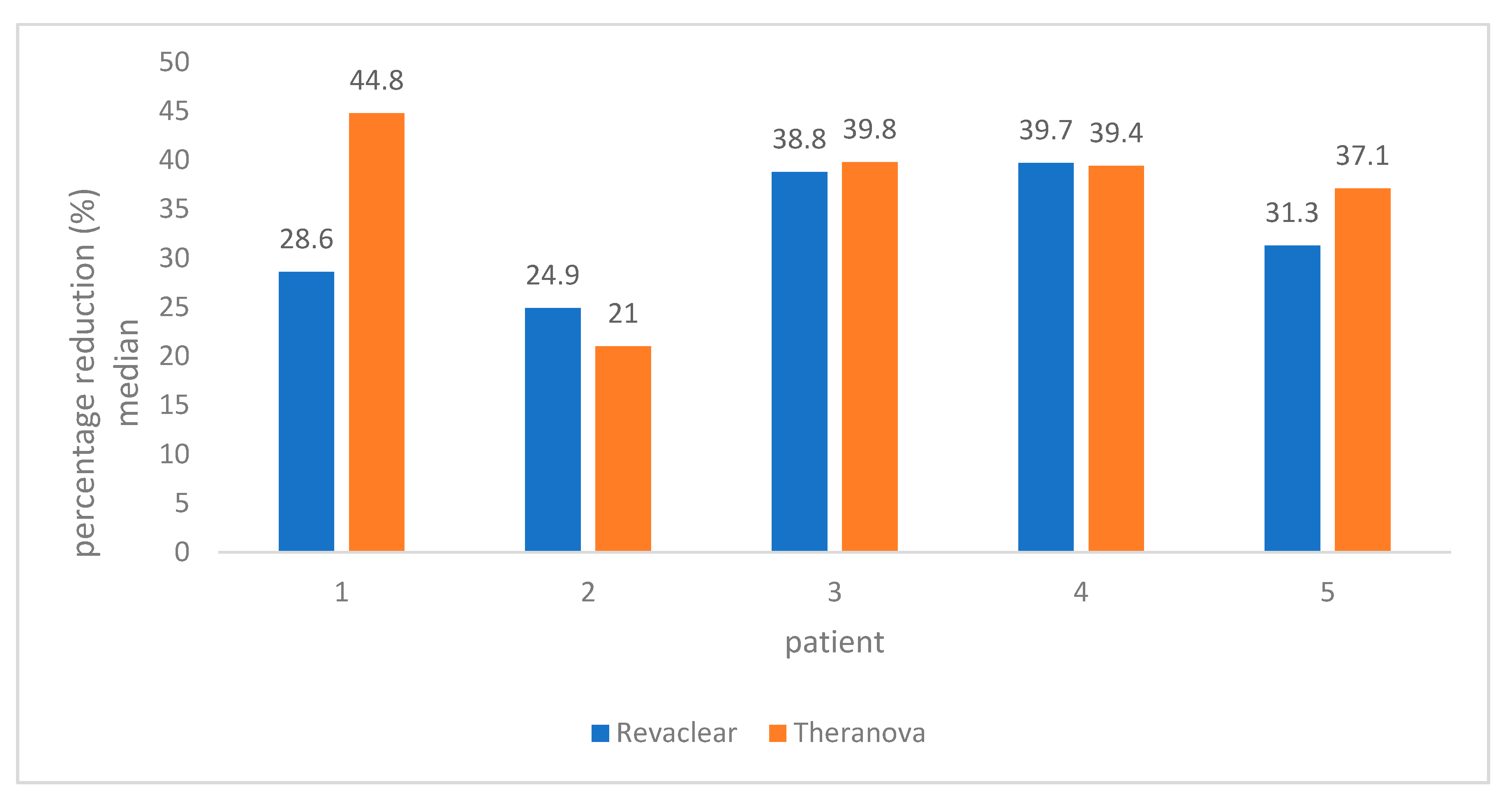
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Subjects
5.2. Study Procedure
5.3. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Day | Membrane | Patient | Pre-HD con | 5 min con | % Reduction at 5 min | 15 min con | % Reduction at 15 min | 30 min con | % Reduction at 30 min | 45 min con | % Reduction at 45 min | 120 min con | % Reduction at 120 min |
| 1 | MCO | 1 | 16.3 | 11 | 32.5 | 11.4 | 30.1 | 9.7 | 40.5 | 9.3 | 42.9 | 7.9 | 51.5 |
| 2 | HF | 1 | 18.2 | 9.6 | 47.3 | 11.2 | 38.5 | 10.2 | 44.0 | 11.1 | 39.0 | 9.4 | 48.4 |
| 3 | MCO | 1 | 18.3 | 17.5 | 4.4 | 16.6 | 9.3 | 15.4 | 15.8 | 13.5 | 26.2 | 10.1 | 44.8 |
| 4 | HF | 1 | 21 | 20.7 | 1.4 | 19.3 | 8.1 | 18.8 | 10.5 | 19.3 | 8.1 | 15 | 28.6 |
| 5 | MCO | 1 | 10.6 | 10.1 | 4.7 | 9.4 | 11.3 | 8.9 | 16.0 | 9 | 15.1 | 7.7 | 27.4 |
| 6 | HF | 1 | 16.3 | 15.2 | 6.7 | 15.8 | 3.1 | 14.4 | 11.7 | 13.5 | 17.2 | 12 | 26.4 |
| 1 | MCO | 2 | 10.7 | 10.1 | 5.6 | 9.7 | 9.3 | 9.9 | 7.5 | 9.6 | 10.3 | 8.5 | 20.6 |
| 2 | HF | 2 | 18.8 | 17.1 | 9.0 | 16.3 | 13.3 | 15.7 | 16.5 | 15.7 | 16.5 | 13.7 | 27.1 |
| 3 | MCO | 2 | 12.9 | 12.3 | 4.7 | 12 | 7.0 | 11.6 | 10.1 | 10.6 | 17.8 | 9.9 | 23.3 |
| 4 | HF | 2 | 18.3 | 16.8 | 8.2 | 16.9 | 7.7 | 15.9 | 13.1 | 15.1 | 17.5 | 14.4 | 21.3 |
| 5 | MCO | 2 | 21.9 | 21.2 | 3.2 | 21.1 | 3.7 | 19.6 | 10.5 | 19.1 | 12.8 | 17.3 | 21.0 |
| 6 | HF | 2 | 16.9 | 15 | 11.2 | 14.2 | 16.0 | 14 | 17.2 | 13.8 | 18.3 | 12.7 | 24.9 |
| 1 | MCO | 3 | 22.6 | 20.5 | 9.3 | 19.1 | 15.5 | 17.2 | 23.9 | 16.5 | 27.0 | 13.6 | 39.8 |
| 2 | HF | 3 | 12.6 | 11.9 | 5.6 | 10.9 | 13.5 | 10.7 | 15.1 | 10.4 | 17.5 | 8.3 | 34.1 |
| 3 | MCO | 3 | 20.2 | 18.2 | 9.9 | 17.5 | 13.4 | 15.7 | 22.3 | 15.2 | 24.8 | 13 | 35.6 |
| 4 | HF | 3 | 21.4 | 19.5 | 8.9 | 17.1 | 20.1 | 16.3 | 23.8 | 15.5 | 27.6 | 13.1 | 38.8 |
| 5 | MCO | 3 | 14.5 | 11.4 | 21.4 | 10.8 | 25.5 | 9.3 | 35.9 | 9.6 | 33.8 | 7.2 | 50.3 |
| 6 | HF | 3 | 18 | 16 | 11.1 | 15 | 16.7 | 13.1 | 27.2 | 12.9 | 28.3 | 10.4 | 42.2 |
| 1 | MCO | 4 | 19.4 | 17.3 | 10.8 | 17.3 | 10.8 | 14.8 | 23.7 | 14.3 | 26.3 | 11.8 | 39.2 |
| 2 | HF | 4 | 21.9 | 20.3 | 7.3 | 18.4 | 16.0 | 17.4 | 20.5 | 16.5 | 24.7 | 13.2 | 39.7 |
| 3 | MCO | 4 | 14.4 | 13.3 | 7.6 | 12.6 | 12.5 | 11.1 | 22.9 | 10.9 | 24.3 | 8.5 | 41.0 |
| 4 | HF | 4 | 16.9 | 14.7 | 13.0 | 13.9 | 17.8 | 13.2 | 21.9 | 12.5 | 26.0 | 11 | 34.9 |
| 5 | MCO | 4 | 17.5 | 15.3 | 12.6 | 14.5 | 17.1 | 12.7 | 27.4 | 13.3 | 24.0 | 10.6 | 39.4 |
| 6 | HF | 4 | 19.6 | 17.8 | 9.2 | 16.3 | 16.8 | 15.4 | 21.4 | 14.4 | 26.5 | 10.8 | 44.9 |
| 1 | MCO | 5 | 8.9 | 7.6 | 14.6 | 7.6 | 14.6 | 1.7 | 80.9 | 6.6 | 25.8 | 5.6 | 37.1 |
| 2 | HF | 5 | 11.2 | 10.3 | 8.0 | 9.9 | 11.6 | 9.9 | 11.6 | 8.8 | 21.4 | 7.7 | 31.3 |
| 3 | MCO | 5 | 14.5 | 12.9 | 11.0 | 13.6 | 6.2 | 12 | 17.2 | 13.4 | 7.6 | 9.6 | 33.8 |
| 4 | HF | 5 | 15.3 | 14.8 | 3.3 | 13.7 | 10.5 | 11.7 | 23.5 | 12.4 | 19.0 | 10.6 | 30.7 |
| 5 | MCO | 5 | 15.9 | 14.2 | 10.7 | 12.5 | 21.4 | 12.7 | 20.1 | 12.6 | 20.8 | 9.7 | 39.0 |
| 6 | HF | 5 | 18.4 | 16.3 | 11.4 | 14.6 | 20.7 | 13.8 | 25.0 | 14.1 | 23.4 | 11.4 | 38.0 |
References
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Trotman, R.L.; Williamson, J.C.; Shoemaker, D.M.; Salzer, W.L. Antibiotics dosing in critically ill adult patients receiving continue renal replacement therapy. Clin. Infect. Dis. 2005, 41, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Lanese, D.M.; Alfrey, P.S.; Molitoris, B.A. Markedly increased clearance of vancomycin during hemodialysis using polysulfone dialyzers. Kidney Int. 1989, 35, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Torlas, J.; Cao, C.; Rivas, M.C.; Cano, M.; Fernandez, E.; Montoliu, J. Pharmacokinetics of vancomycin in patients undergoing hemodialysis with polyacrylonitrile. Clin. Nephrol. 1991, 36, 35–41. [Google Scholar]
- De Bock, V.; Verbeelen, D.; Maes, V.; Sennesael, J. Pharmacokinetics of vancomycin in patients undergoing hemodialysis and hemofiltration. Nephrol. Dial. Transplant. 1989, 4, 635–639. [Google Scholar] [PubMed]
- Stryjewski, M.E.; Szczech, L.A.; Benjamin, D.K., Jr.; Inrig, J.K.; Kanafani, Z.A.; Engemann, J.J.; Chu, V.H.; Joyce, M.J.; Reller, L.B.; Corey, R.; et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependant patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2007, 44, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Wong, T.; Romney, M.; Leung, V. Comparative effectiveness of β-lactam versus vancomycin empiric therapy in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 27. [Google Scholar] [CrossRef]
- Mason, N.A.; Neudeck, B.L.; Welage, L.S.; Patel, J.A.; Swartz, R.D. Comparison of 3 vancomycin dosage regimens during hemodialysis with cellulose triacetate dialyzers: Post-Dialysis versus intradialytic administration. Clin. Nephrol. 2003, 60, 96–104. [Google Scholar] [CrossRef]
- Kirsch, A.H.; Nilsson, L.G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2017, 32, 165–172. [Google Scholar] [CrossRef]
- Ronco, C.; Marchionna, N.; Brendolan, A.; Neri, M.; Lorenzin, A.; Rueda, A. Expanded haemodialysis: From operational mechanism to clinical results. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii41–iii47. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Wolley, M. The rationale for expanded hemodialysis therapy (HDx). Contrib. Nephrol. 2017, 191, 142–152. [Google Scholar] [PubMed]
- García-Prieto, A.; Vega, A.; Linares, T.; Abad, S.; Macías, N.; Aragoncillo, I.; Torres, E.; Hernández, A.; Barbieri, D.; Luño, J. Evaluation of the efficacy of a medium cut-off dialyser and comparison with other high-flux dialysers in conventional haemodialysis and online haemodiafiltration. Clin. Kidney J. 2018, 11, 742–746. [Google Scholar] [CrossRef]
- Boschetti-de-Fierro, A.; Voigt, M.; Storr, M.; Krause, B. MCO membranes: Enhanced selectivity in high-flux class. Sci. Rep. 2015, 5, 18448. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Hoen, B.; Mayeux, D.; Hestin, D.; Fontenaille, C. Bacteremia in patients on chronic hemodialysis. A multicenter prospective survey. Nephron 1993, 64, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Villalon, N.; Farzan, N.; Freeman, K. Rate of bacteremia in the hemodialysis patient presenting to the emergency department with fever: A retrospective chart review. Int. J. Emerg. Med. 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Czock, D.; Schöpke, T.; Hafer, C.; Bode-Böger, S.M.; Kuse, E.; Keller, F.; Fliser, D. Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis. Crit. Care Med. 2006, 34, 51–56. [Google Scholar] [CrossRef] [PubMed]
- El Nekidy, W.S.; El-Masri, M.M.; Umstead, G.S.; Dehoorne-Smith, M. Factors influencing vancomycin loading dose for hospitalized hemodialysis patients: Prospective observational cohort study. Can. J. Hosp. Pharm. 2012, 65, 436–442. [Google Scholar] [CrossRef]
- Vandecasteele, S.J.; De Bacquer, D.; De Vriese, A.S. Implementation of a dose calculator for vancomycin to achieve target pre-HD concentrations of 15–20 microg/mL in persons undergoing hemodialysis. Clin. Infect. Dis. 2011, 53, 124–129. [Google Scholar] [CrossRef]
- Gibson, T.P. Problems in designing hemodialysis drug studies. Pharmacotherapy 1985, 5, 23–29. [Google Scholar] [CrossRef]
- Jamal, J.A.; Udy, A.A.; Lipman, J.; Roberts, J.A. The impact of variation in renal replacement therapy settings on piperacillin, meropenem, and vancomycin drug clearance in the critically ill: An analysis of published literature and dosing regimens. Crit. Care Med. 2014, 42, 1640–1650. [Google Scholar] [CrossRef]
- Vandecasteele, S.J.; De Vriese, A.S. The rebound of vancomycin serum concentrations occurs following dialysis with highly permeable membranes. Kidney Int. 2010, 77, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, L.B.; Chaabouni, Y.; Ghozzi, H.; Feriani, H.; Hakim, A.; Kharrat, M.; Marrakchi, C.; Sahnoun, Z.; Jmaa, M.B.; Zeghal, K.; et al. Optimization of therapeutic drug monitoring of vancomycin in patients with chronic hemodialysis. Clin. Nephrol. 2017, 88, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Bassetti, M.; François, B.; Karam, G.; Chastre, J.; Torres, A.; Roberts, J.A.; Taccone, F.S.; Rello, J.; Calandra, T.; et al. Advances in antibiotic therapy in the critically ill. Crit. Care 2016, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, S.; Khalili, H. Vancomycin dosing nomograms targeting high serum trough levels in different populations: Pros and cons. Eur. J. Clin. Pharmacol. 2016, 72, 777. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Castillo, P.M.; Sánchez, A.; Santisteban, M.A.; Gallego, S.; Marigliano, N. Vancomycin dosing in hemodialysis patients. Nefrología 2008, 28, 607–612. [Google Scholar]
| Characteristic | Number of Patient (%) |
|---|---|
| Sex | |
| Female | 4 (80) |
| Age | |
| ≤65 | 3 (60) |
| Ethnicity | |
| Maori | 4 (80) |
| Caucasian | 1 (20) |
| BMI | |
| ≤30 | 2 (40) |
| Access used | |
| Fistula/Graft | 2 (40) |
| Central lines tunneled or temporary | 5 (60) |
| Bloods Flow (pump velocity) mL/min, per session | |
| ≤250 | 16 sessions (53.3) |
| >250 | 14 sessions (46.7) |
| Use of loop diuretics | 3 (60) |
| Use of ACEi or ARB | 1 (20) |
| Patients with vancomycin trough ≥15 mg/L on day 1 | 3 (60) |
| Number of iHD per week = 3 | 5 (100) |
| Duration of HD per session | |
| ≤240min | 1 (20) |
| >240min | 4 (80) |
| Patient | Indication for Treatment |
|---|---|
| 1 | Dialysis Tunnelled central line sepsis |
| 2 | Peritoneal Dialysis catheter exit site infection with MRSA |
| 3 | Dialysis Tunnelled central line sepsis |
| 4 | Coagulase negative staphylococcus species bacteraemia |
| 5 | Cellulitis/Infected synthetic Arteriovenous Graft |
| Patient Variable | Median Study Percentage Reduction (%) | Median High-Flux Membrane Percentage Reduction (%) | Median Medium Cut-Off Membrane Percentage Reduction (%) | |
|---|---|---|---|---|
| BMI (kg/m2) | <30 | 39.3 | 36.5 | 42.5 |
| ≥30 | 34.4 | 31.3 | 37.1 | |
| Qb (mL/min) | ≤250 | 31.3 | 31.3 | 31.3 |
| >250 | 38.5 | 34.9 | 39.2 | |
| Vancomycin concentration at start of HD (mg/L) | <15 | 33.8 | 32.7 | 33.8 |
| ≥15 | 38.8 | 34.9 | 39.3 | |
| Vancomycin concentration at start of HD (mg/L) | <18 | 34 | 31 | 38 |
| ≥18 | 39 | 38.8 | 39.2 | |
| Loop diuretics | Yes | 38.5 | 34.9 | 39.2 |
| No | 30.6 | 30.6 | 29.4 | |
| ACEi/ARB | Yes | 36.7 | 28.6 | 44.8 |
| No | 36.4 | 34.5 | 38 | |
| Interval between HD session (Hours) | ≤48 | 37.6 | 34.9 | 39.2 |
| >48 (i.e., 72hs) | 34.9 | 32.7 | 37.3 | |
| Interval between doses (Hours) | ≤48 | 36 | 34.9 | 37.1 |
| >48 (i.e., 72hs) | 37.2 | 32.7 | 39.2 | |
| Duration on HD (minutes) | ≤240 | 37.1 | 31.3 | 38 |
| >240 | 35.6 | 34.5 | 39.2 |
| Variable | Estimate | Concentration Ratio |
|---|---|---|
| (Intercept or Baseline) | −0.43 | NA |
| Medium cut-off membrane | −0.04 | 0.96 |
| Use of loop diuretic | 0.05 | 1.05 |
| Blood flow of dialysis (per mL/min) | −0.001 | 1.00 |
| BMI (per kg/m2) | 0.01 | 1.01 |
| Loading Dose | Infusion Duration |
|---|---|
| 750 mg | 60 min |
| 1000 mg | 60 min |
| 1250 mg | 90 min |
| 1500 mg | 90 min |
| 1750 mg | 120 min |
| 2000 mg | 120 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allawati, H.; Dallas, L.; Nair, S.; Palmer, J.; Thaikandy, S.; Hutchison, C. A Pharmacokinetic Study Comparing the Clearance of Vancomycin during Haemodialysis Using Medium Cut-Off Membrane (Theranova) and High-Flux Membranes (Revaclear). Toxins 2020, 12, 317. https://doi.org/10.3390/toxins12050317
Allawati H, Dallas L, Nair S, Palmer J, Thaikandy S, Hutchison C. A Pharmacokinetic Study Comparing the Clearance of Vancomycin during Haemodialysis Using Medium Cut-Off Membrane (Theranova) and High-Flux Membranes (Revaclear). Toxins. 2020; 12(5):317. https://doi.org/10.3390/toxins12050317
Chicago/Turabian StyleAllawati, Hussain, Linda Dallas, Sreejith Nair, Janine Palmer, Shaiju Thaikandy, and Colin Hutchison. 2020. "A Pharmacokinetic Study Comparing the Clearance of Vancomycin during Haemodialysis Using Medium Cut-Off Membrane (Theranova) and High-Flux Membranes (Revaclear)" Toxins 12, no. 5: 317. https://doi.org/10.3390/toxins12050317
APA StyleAllawati, H., Dallas, L., Nair, S., Palmer, J., Thaikandy, S., & Hutchison, C. (2020). A Pharmacokinetic Study Comparing the Clearance of Vancomycin during Haemodialysis Using Medium Cut-Off Membrane (Theranova) and High-Flux Membranes (Revaclear). Toxins, 12(5), 317. https://doi.org/10.3390/toxins12050317





