Abstract
Background/Objectives: Vitamin D is classically associated with calcium and phosphate homeostasis, but recent research has expanded its role to include several new roles such as immune regulation, inflammation, and potential anti-cancer properties. The vitamin D receptor (VDR) is expressed in over 400 tissues, including those of the head and neck, implying a potential link between vitamin D and head and neck cancers (HNCs). Given the need for newer and better therapeutic approaches, this systematic review aims to synthesize existing clinical evidence on the relationship between vitamin D status and clinical outcomes in HNC patients. Methods and Results: A comprehensive literature search, across multiple databases including PubMed, Google Scholar and Science Direct, identified 187,642 studies related to vitamin D and cancer, from which 16 studies met the inclusion criteria. The inclusion criteria were English-language, full-text original research (2015–2025) on vitamin D’s role in HNC progression and treatment, focusing on human studies. The findings indicate that vitamin D deficiency is highly prevalent among HNC patients, with rates ranging from 47% to 95%, particularly in advanced-stage cancers and those undergoing intensive treatment. Inverse association between vitamin D levels and HNC risk was reported, with higher serum 25(OH)D levels linked to a 30–32% reduction in cancer risk. Additionally, higher vitamin D levels correlated with improved survival rates and reduced recurrence, though some findings lacked statistical significance. Deficiencies were associated with higher rates of malnutrition and postoperative complications, reinforcing vitamin D’s role in nutritional stability and surgical recovery. Conclusions: This systematic review highlights how common and significant vitamin D deficiency is among head and neck cancer (HNC) patients, exploring its possible role in cancer risk, prognosis, survival, treatment-related side effects, malnutrition, and post-surgical complications. The evidence suggests that while higher vitamin D levels are linked to better survival and fewer treatment-related issues, the benefits seem to level off beyond a certain point, indicating a more complex relationship. Additionally, vitamin D supplementation appears to help reduce chemoradiation side effects like mucositis, skin toxicity, dysphagia, and pain, ultimately improving patients’ quality of life during treatment.
1. Introduction
Vitamin D, a fat-soluble secosteroid, has long been recognized for its pivotal role in maintaining calcium and phosphate homeostasis, which are essential for bone mineralization and skeletal integrity [1,2]. It is synthesized in the skin via ultraviolet B (UVB) exposure to 7-dehydrocholesterol, which turns into pre-vitamin D that spontaneously converts to cholecalciferol (commonly known as vitamin D3), which can also be ingested through diet and supplements. Cholecalciferol undergoes two hydroxylation steps, the first in the liver to form calcidiol (25-hydroxyvitamin D, 25(OH)D), which is the primary circulating form and marker of vitamin D status, and the second in the kidneys to produce calcitriol (1,25-dihydroxyvitamin D, 1,25(OH)2D), its biologically active metabolite [3,4,5]. Transported by vitamin D-binding protein (DBP) in plasma, calcitriol exerts its effects by binding to the vitamin D receptor (VDR), a nuclear transcription factor expressed in numerous tissues [6]. Beyond skeletal health, recent decades have unveiled vitamin D’s broader physiological roles, including immune modulation, inflammation regulation, and cellular differentiation, sparking interest in its potential as an anti-cancer agent [7,8,9,10]. These discoveries stem from VDR’s presence in over 36 organs and hundreds of cell types, ranging from immune cells like lymphocytes to epithelial tissues, suggesting a systemic influence far beyond its classical domain.
This expanded understanding has fueled research into vitamin D’s association with cancer, a disease marked by uncontrolled cellular proliferation and immune evasion. Epidemiological studies have linked low serum 25(OH)D levels to increased risks of colorectal, breast, and prostate cancers, with meta-analyses reporting risk reductions of 15–30% with higher vitamin D status [7,8,9]. Mechanistically, calcitriol inhibits tumor growth by promoting apoptosis, suppressing angiogenesis, and modulating the tumor microenvironment through VDR-mediated gene regulation [9,10]. In vitro and animal models further support these anti-cancer properties, showing reduced proliferation and metastasis in cancer cell lines exposed to 1,25(OH)2D [11]. However, clinical evidence remains mixed; while some trials suggest protective effects, others find no causal link, highlighting variability across cancer types, populations, and study designs [12]. This inconsistency raises a critical question: could vitamin D’s role extend to less-studied malignancies, such as those of the head and neck, where its receptor is also widely expressed [13]?
Head and neck cancers (HNCs) encompass a heterogeneous group of malignancies originating in the oral cavity, pharynx, larynx, nasal cavity, and salivary glands, with squamous cell carcinoma (SCC) predominating, particularly in the oral cavity [14,15]. GLOBOCAN 2022 estimates rank lip and oral cavity cancers 16th globally by incidence (377,713 cases), but combining nasopharyngeal, oropharyngeal, hypopharyngeal, and salivary gland cancers elevates HNC to approximately 8th, with over 650,000 cases annually [16,17]. This burden is rising, driven by multifactorial etiologies; tobacco and excessive alcohol use synergistically increase risk by up to five-fold, while environmental carcinogens and viral infections—human papillomavirus (HPV) in oropharyngeal cancers and Epstein–Barr virus (EBV) in nasopharyngeal cases—further complicate the landscape [18]. HPV-related oropharyngeal cancers are surging in Western countries, shifting HNC’s demographic profile [19]. In developing nations, incidence is climbing due to lifestyle changes and limited prevention, amplifying its public health toll [20]. Despite advances in surgery, radiotherapy, chemotherapy, and immune checkpoint inhibitors, survival remains stubbornly stagnant—global deaths dropped only modestly from 404,000 in 2012 to 378,785 in 2022 [21]. This persistent challenge underscores an urgent need to explore modifiable biological factors beyond traditional risk profiles.
Within this context, vitamin D emerges as a candidate warranting scrutiny in HNC. Its receptor’s expression in head and neck tissues—the salivary glands, tongue, and lymphoid cells—mirrors sites of HNC origin, hinting at a potential role in pathogenesis and prognosis [22]. Yet, evidence remains fragmented; while some studies [23] report no causal link between 25(OH)D and oral/oropharyngeal cancer risk, others [24] suggest associations with reduced incidence and improved outcomes, particularly in specific subtypes. This ambiguity, coupled with HNC’s rising burden and static survival rates, justifies a deeper dive into vitamin D’s clinical impact. Our systematic review aims to synthesize recent evidence from epidemiological studies, clinical trials, and patient-centered research, complemented by key in vitro and animal findings, to evaluate whether vitamin D status influences HNC risk, prognosis, treatment response, and complications. By addressing this gap, we seek to clarify vitamin D’s potential as a modifiable factor in HNC management, offering insights for future research and therapeutic strategies.
2. Materials and Methods
2.1. Search Strategy
This systematic review utilized standard operating principles as outlined in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guidelines [25] (Figure 1).
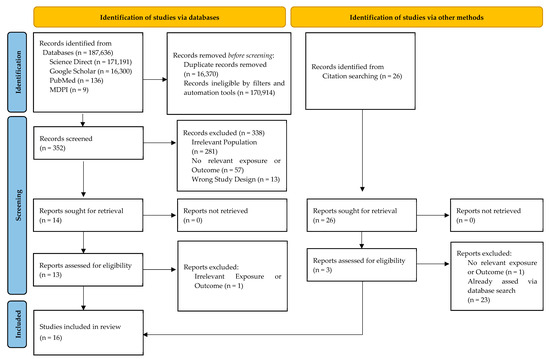
Figure 1.
PRISMA flowchart of study selection process.
Databases included PubMed, Google Scholar, and ScienceDirect, supplemented by searches in MDPI journals, to overcome query limitations like ScienceDirect’s Boolean operator cap.
The search strategy involved the use of specific keywords and phrases, utilizing the MeSH feature of PubMed, such as “head and neck cancer”, “Oral Cancer”, “Vitamin D”, “25(OH)D3” “Cholecalciferol”, “Calcitriol”, “Squamous Cell Carcinoma of Head and Neck”, “Oropharyngeal Neoplasms”, “Laryngeal Neoplasms”, “Survival Rate”, “Treatment Outcome”, “Prognosis”, and “VDR”, and Boolean Operators such as AND, OR, and NOT were used to refine search results (e.g., (“Vitamin D”[MeSH] OR “Cholecalciferol”[MeSH]) AND (“Head and Neck Neoplasms”[MeSH] OR “Squamous Cell Carcinoma of Head and Neck”[MeSH])).
Articles available in the English language were used, and the rest were excluded. Abstracts were reviewed to select studies that met the inclusion criteria. Only articles that had the full text available were utilized for review and citation. Any duplicates from different databases and various search queries were then removed. When possible, the search was narrowed down by excluding paper categories other than research articles, such as book chapters, correspondence, editorials, mini reviews, etc.
We employed a snowballing citation search method by systematically examining the reference lists of key articles identified during the initial search. By tracing previous works’ notable citations, we aimed to identify highly relevant publications that may not have been captured in our primary search strategy and to thus yield a more complete literature review. Using a few key articles as “seed” papers and expanding the search based on their citations and indexing terms also helped in broadening the literature review.
Our review included publications from the last 10 years to ensure that the most current and relevant research was considered. However, through the snowballing method, highly cited papers were sought, with the aim of consulting older yet fundamental papers for the topic in question. This review was not prospectively registered in any database.
2.2. Study Selection and Data Analysis
To ensure the quality and reliability of the included sources, two authors independently assessed the publications. Any disagreements were resolved through discussion or, when necessary, by consulting a third author. For the screening process, two independent reviewers (A.A. and C.P.B.) evaluated all records for eligibility. The inter-rater reliability, measured by Cohen’s Kappa, was 0.81, indicating a high level of agreement. Discrepancies were addressed through consensus or, if unresolved, by involving a third reviewer (B.H).
The inclusion criteria were (1) studies with abstracts relevant to the potential role of vitamin D in the evolution of and response to the treatment of head and neck cancers; (2) full-text articles published in English from 1 January 2015 to 1 March 2025; and (3) original research articles with a given study design (human studies (observational cohort studies and controlled trials) with consideration given to animal and in vitro studies in the Introduction and Discussion sections).
The exclusion criteria were (1) studies focusing on topics irrelevant to our research objective; (2) articles published in a language other than English, with no translation available; (3) studies not published in a peer-reviewed journal; (4) research resources without available full-text (abstract only); and (5) inappropriate study designs (e.g., letters, case reports, conference abstracts, etc.).
After the final list of selected studies was agreed upon, two separate reviewers (B.H. and E.C.) independently extracted the relevant data from the included studies and systematically organized it into a table, ensuring a methodological approach for our analysis. The following data were extracted: study ID (first author and year of publication), study design, sample size, sample type, analysis methodology, response to treatment, and statistical significance. Any gaps in the data presented in selected studies were addressed as weaknesses when discussing said studies.
When presenting results, the statistical measures used to report outcomes were as follows: odds ratio (OR) used to assess the association between vitamin D levels and HNC risk; hazard ratio (HR) used for survival analysis, evaluating mortality risk, and overall survival; mean and median differences used to compare vitamin D levels between HNC patients and controls; and p-values used to assess statistical significance. Studies selected for each synthesis were based on whether the data in question were present or not. Due to heterogeneity in data across studies, missing data were not handled in any specific manner.
3. Results
3.1. Overview of Selected Studies
The initial search, using keyword variants in advanced searches, identified 187,642 records addressing vitamin D, head and neck cancer (HNC), other cancers, and diverse populations. This broad yield stemmed from ScienceDirect’s eight-operator limit, necessitating multiple queries, while PubMed, Google Scholar, and MDPI journals allowed refined searches. A 10-year timeframe (2015–2025) ensured inclusion of current research, reflecting data up to March 2025. Before screening, 170,914 records were excluded, primarily via filters (e.g., language, study type) and automation tools targeting relevant samples. After consolidating results across databases and queries, 16,370 duplicates were removed, leaving 352 papers for abstract screening. Of these, 338 were excluded, and 14 underwent full-text review, with 13 deemed eligible. Via snowballing citation searching, 3 additional papers were added, resulting in 16 studies included. This selection process is detailed in Figure 1.
Participant ages varied, from a mean of 42.67 ± 10.83 years [26] to a median of 69 years (range 60–78) [27], with most studies targeting middle-aged adults (40–60 years). Several included matched controls, who were primarily healthy individuals, for serum 25(OH)D comparisons [26,28,29,30]. The studies spanned diverse geographic regions and ethnic groups, with higher deficiency suggested in non-Caucasian and lower socioeconomic groups in some contexts [30,31]. HNC patients consistently showed lower 25(OH)D levels than controls, supporting a potential link to cancer incidence and progression [26,28,29,30]. Deficiency prevalence ranged from 47% to 95%, varying by population and subtype [30,32], with severe deficiencies noted in advanced-stage, malnourished, or intensively treated patients [29,31,32,33].
Table 1 provides a detailed overview of the studies included in this review, highlighting study designs, populations with HNC, clinical outcomes, and key findings.

Table 1.
Overview of the included studies: HC—healthy controls; NM—not mentioned, HNC—head and neck cancer; CI—confidence interval; OR—odds ratio; HR— hazard ratio; QOL—quality of life; NA—not applicable OS—overall survival, PFS—progression-free survival; OPC—oropharyngeal cancer; * median age; 1 no stratification by type of cancer for demographic characteristics, only the whole study group.
3.2. Prevalence of Vitamin D Deficiency in Head and Neck Cancer Patients
Among the included studies, six reported a high prevalence of vitamin D deficiency in patients with head and neck cancer [28,29,30,31,33,40]. The prevalence of vitamin D deficiency in these studies is summarized in Table 2. Both Fanidi et al. and Ulaganathan et al. reported some high rates of vitamin D deficiency in HNC patients [28,30]. Approximately 75% of cases had vitamin D levels below 20 ng/mL, and 95% of cases showed vitamin D deficiency, suggesting that low vitamin D levels may be a common risk factor for nasopharyngeal carcinoma (NPC) [28]. Similarly, Bochen et al. reported that 47% of patients had vitamin D deficiency [29], a finding that was mirrored by Radivojevic et al., who observed that approximately 47% of their study population had deficient levels [33]. Additionally, Kapala et al. found that 66.8% of cancer patients were deficient in vitamin D, even among those receiving supplementation, suggesting that standard vitamin D supplementation may not be sufficient for these patients [30]. Bhanu et al. further reinforced this issue, reporting that 71.42% of patients had suboptimal vitamin D levels [40].

Table 2.
Overview of vitamin D deficiency among the included studies.
3.3. Influence of Demographic Characteristics and Season
Among the included studies, some did not address the variations between 25(OH)D levels and demographic characteristics [26,27,30,32,33,34,39,40,42]. This is not to be confused with the fact that the included studies did not contain the demographic characteristics of the study groups, but no stratification by 25(OH)D status was performed, rather stratifications by existing condition (cancer/cancer-free), progression, intervention, survival, and/or other correlations with demographic characteristics were performed instead.
Pu et al. suggests a possible negative correlation between age and vitamin D, but in no conclusive manner due to possible bias, since HNC is highly prevalent around 50 years old [36]. This finding is supported, however, by the work of Kapała et al., which shows increased vitamin D deficiency with age. Their findings also include a significantly higher occurrence of vitamin D deficiency in males (p < 0.001) [31].
Fanidi et al. observed that current smokers had 7% lower 25(OH)D concentrations on average (95% CI: −14% to 0%), while former smokers had 9% higher 25(OH)D concentrations (95% CI: 2–16%) and seasonal variation, with the lowest levels among patients tested around February and March and the highest levels among patients tested in August. BMI was inversely associated with 25(OH)D3 concentrations [28].
Bochen et al., however, concluded that vitamin D deficiency is no indicator of a general malnutrition status, as neither BMI nor serum albumin level were associated with 25(OH)D levels [29].
Both Westmark et al. and Fanidi et al. showed no differences in 25(OH)D level in relation to alcohol intake [28,37].
3.4. Impact of Vitamin D on Cancer Risk and Development
Four studies showed a significant association between low vitamin D levels and an increased risk of HNC. Both Fanidi et al. and Vaughan-Shaw et al. demonstrated a significant inverse association between vitamin D levels and HNC risk [28,34]. Fanidi et al. reported that a doubling of 25(OH)D levels was associated with a 30% lower risk of HNC (OR = 0.70, 95% CI: 0.56–0.88, p-trend = 0.001), with even stronger associations observed in specific subtypes such as laryngeal and hypopharyngeal cancer (OR = 0.55, 95% CI: 0.39–0.78). Vaughan-Shaw et al. supported these findings, showing that higher vitamin D levels were associated with a 32% reduction in HNC risk (HR = 0.74, 95% CI: 0.66–0.82). Similarly, Pu et al. and Ulaganathan et al. found that higher vitamin D intake and serum levels were protective against HNC [30,36].
Pu et al. conducted a large-scale analysis of 81,908 participants and found that higher vitamin D intake was linked to a significantly lower incidence of HNC (OR = 0.68, 95% CI: 0.59–0.78). Ulaganathan et al. focused on nasopharyngeal carcinoma (NPC) and reported that lower vitamin D levels were associated with an increased NPC risk (AOR = 0.73, 95% CI: 0.57–0.94, p = 0.016), further supporting the hypothesis that vitamin D deficiency may contribute to cancer development. These findings are illustrated in Table 3.

Table 3.
Vitamin D and cancer risk.
A noteworthy consideration when analyzing these results summarized in Table 3 that two studies (Vaughan-Shaw et al., and Pu et al.) are meta-analyses, and they present condensed comparisons of previous research. The results are in accordance with the more recent findings of Ulaganathan et al., 2024, as presented.
3.5. Vitamin D and Prognostic Outcomes
Among the nine studies that examined clinical outcomes [27,28,29,33,34,35,36,37,40], eight found a positive association between higher vitamin D levels and improved survival, though statistical significance was not always reached, while three studies examined its role in recurrence and disease progression [29,35,36]. Their findings are summarized in Table 4. Fanidi et al. found that each doubling of 25(OH)D levels reduced mortality risk by 27% (HR = 0.73, 95% CI 0.55–0.97). Patients with vitamin D levels of 10 ng/mL had a 1.72-fold higher risk of death compared to those with 20 ng/mL, but no additional survival benefits were observed above this threshold [28]. Similarly, Vaughan-Shaw et al. reported that higher vitamin D levels were associated with better overall survival (HR = 0.74, 95% CI: 0.66–0.82) and progression-free survival (HR = 0.84, 95% CI: 0.77–0.91) [34].

Table 4.
Vitamin D and clinical outcomes: HNSCC—head and neck squamous cell carcinoma; OS—overall survival; PFS—progression-free survival; HNC—head and neck cancer; * A conversion from nmol/L to ng/mL was performed.
In a cohort of 231 HNSCC patients, Bochen et al. found that low vitamin D levels were linked to shorter overall survival, with 42.6% of patients in the vitamin D-deficient group dying compared to 30.3% in the sufficient group (p = 0.0085). The study also found that vitamin D levels significantly influenced survival in HPV-negative patients (p = 0.018) but had no effect in HPV-positive cases (p = 0.98) [29]. Similarly, Pu et al. demonstrated that higher vitamin D levels correlated with lower HNC mortality (HR = 0.75, 95% CI 0.60–0.94) over an 8–12-year follow-up [36]. Further analyses showed that patients with higher circulating vitamin D had significantly better survival over 4–5 years (HR = 1.13, 95% CI 1.05–1.22).
Beyond survival, three studies investigated vitamin D’s role in recurrence and disease progression. Yokosawa et al. highlighted a significant inverse association between vitamin D intake and cancer recurrence (HR = 0.47, 95% CI = 0.20–1.10, p-trend = 0.048), with the strongest effect observed in stage 4 patients. However, no association was found between vitamin D intake and overall or HNC-specific mortality [35]. Similarly, Radivojevic et al. reported that vitamin D deficiency was linked to a 2-year disease-free survival (DFS) rate of 57%, compared to 60% in the insufficient group and 64% in the sufficient group (p = 0.497). For OS, rates were 60% in the deficient group, 75% in the insufficient group, and 71% in the sufficient group (p = 0.577), though none of these differences reached statistical significance [33].
3.6. Vitamin D and Treatment-Related Toxicity
Four studies examined the relationship between vitamin D levels and treatment-related toxicity [26,32,39,40]. Two focused on the benefits of vitamin D supplementation [26,39], while two highlighted the association between low vitamin D levels and increased toxicity rates [32,40]. Table 5 summarizes their findings with regard to treatment toxicity.

Table 5.
Association between vitamin D levels, supplementation and treatment-related toxicity.
Both Anand et al. and Abdelaziz et al. demonstrated that vitamin D supplementation significantly reduced treatment-related side effects. Anand et al. found that supplementation improved chemoradiation-induced toxicity, leading to significant reductions in mucositis, pain, and swallowing difficulties (p < 0.001), alongside an overall enhancement in quality of life [26]. Similarly, Abdelaziz et al. reported that supplementation was associated with lower rates of oral mucositis, skin toxicity, taste changes, and dysphagia (p < 0.001 for mucositis), suggesting a protective effect of vitamin D in mitigating treatment-related side effects [39].
On the other hand, Nejatinamini et al. and Bhanu et al. linked low vitamin D levels to greater treatment toxicity [32,40]. Nejatinamini et al. observed that 52% of patients developed moderate to severe mucositis, and those affected had significantly lower plasma vitamin D levels compared to patients without mucositis (p < 0.02) [32]. Bhanu et al. further supported these findings, reporting that patients with optimal vitamin D levels had significantly lower rates of radiation-induced dermatitis and mucositis (p = 0.0011) [40].
3.7. Vitamin D, Malnutrition, and Postoperative Complications
Four studies examined the relationship between vitamin D levels, malnutrition, and postoperative complications [31,32,33,38]. Two studies focused on the impact of vitamin D deficiency on malnutrition and muscle loss [31,32], while two highlighted its predictive role in postoperative complications [33,38]. Table 6 presents the key findings of these articles.

Table 6.
Malnutrition and postoperative complications in relation to vitamin D levels.
Both Nejatinamini et al. and Kapala et al. demonstrated a strong association between low vitamin D levels and malnutrition [31,32]. Kapala et al. found that weight loss was significantly associated with vitamin D deficiency (p = 0.002), suggesting that maintaining adequate vitamin D levels may be essential for nutritional stability in cancer patients. Nejatinamini et al. found that patients with vitamin D deficiency had a higher risk of malnutrition (OR = 1.76, 95% CI: 1.02–3.04) and that low vitamin D levels were significantly associated with muscle loss (p = 0.031), suggesting a detrimental effect on nutritional status and physical function.
On the other hand, Nett et al. and Radivojevic et al. linked low vitamin D levels to increased postoperative complications [33,38]. Nett et al. found that patients with vitamin D deficiency experienced greater weight loss (pT2 = 0.0031, pT4 = 0.0424) and were at higher risk of digestive issues and muscular complaints (p = 0.0062 and p = 0.0448, respectively), reinforcing the role of vitamin D in post-surgical nutritional recovery. Radivojevic et al. further expanded on this by showing that vitamin D deficiency was a strong predictor of postoperative complications (OR = 2.4, 95% CI: 1.30–4.42, p = 0.011) and that patients with high malnutrition risk had significantly lower 2-year survival rates (30% vs. 62% and 83%, p = 0.010). Multivariate analysis confirmed both vitamin D deficiency and malnutrition as independent risk factors (p < 0.05), highlighting the clinical importance of vitamin D in surgical outcomes.
4. Discussion
Vitamin D’s role in head and neck cancer (HNC) weaves a complex narrative, one that our systematic review of 16 studies (2015–2025) sought to unravel with a rigorous, systematic approach. We found a striking prevalence of vitamin D deficiency (47–95%) among HNC patients, especially those with advanced-stage disease or those under intensive treatment, alongside a 26–32% reduced HNC risk with higher 25(OH)D levels (e.g., OR 0.68–0.74). Several studies within this review [27,28,29,33,34,36,37,41] suggest that elevated 25(OH)D levels may enhance survival outcomes in HNC patients. Particular attention has been drawn to vitamin D’s capacity to regulate immune checkpoint pathways and influence the tumor microenvironment, notably in HPV-positive HNCs, where immune modulation significantly governs disease progression.
4.1. Vitamin D Supplementation
The relationship between vitamin D status and clinical outcomes in HNCs is complex and influenced by multiple factors, including genetic variability in vitamin D receptor (VDR) expression, tumor heterogeneity, and patient-specific metabolic differences, all these serving as weak points in clinical studies [42,43]. Emerging evidence indicates that VDR polymorphisms may modulate treatment efficacy and prognostic trajectories [34,44,45,46], underscoring the potential for personalized therapeutic strategies involving vitamin D as an adjunct. Specifically, variants such as FokI and TaqI can alter VDR’s binding affinity to calcitriol, disrupting downstream gene regulation of apoptosis and immune response pathways critical to tumor control [46,47]. These polymorphisms may also influence VDR expression levels in HNC tissues, potentially amplifying or dampening the anti-tumor effects of vitamin D supplementation, thus shaping individual responses to therapy and survival outcomes.
Previous studies on vitamin D supplementation in head and neck cancer (HNC) offer a critical lens for contextualizing our systematic review’s findings, revealing both alignment and divergence. Our observed reductions in treatment-related toxicities, such as mucositis and dysphagia (p < 0.001) with supplementation [26,39], align with Ruggiero et al. (2006), who reported a 35% lower mucositis incidence (p = 0.03) in HNC patients given 2000 IU/day during radiotherapy [48]. However, our mixed survival outcomes—i.e., benefits in some studies (e.g., HR 0.73–0.84) [28,34,36] and none in others [26,35]—mirror the work of Wactawski-Wende et al. (2006), where 1000 IU/day provided no survival advantage across cancers (HR 0.98, 95% CI 0.91–1.05) [49]. Zarrati et al. found that high-dose vitamin D supplementation (>4000 IU/day) significantly reduced treatment-induced pain in cancer patients (SMD −0.49, p = 0.005) [50], corroborating our observed reductions in mucositis and dysphagia (p < 0.001) [26,39], suggesting a shared anti-inflammatory mechanism enhancing patient tolerance. Martinez-Alonso et al. reported that 25(OH)D levels below 20 ng/mL increased cancer mortality (HR 1.42, 95% CI 1.18–1.71) with benefits plateauing above 40 ng/mL [51], aligning with our high deficiency prevalence (47–95%) and non-linear survival trends and reinforcing the prognostic relevance of baseline vitamin D status in HNC. This high prevalence of deficiency is further corroborated by Alexandru et al., who reported that 50–90% of pediatric cancer patients exhibited 25(OH)D levels below 20 ng/mL during chemotherapy, highlighting a consistent vulnerability across cancer populations [9].
While confounding variables such as BMI, diet, alcohol consumption, tobacco exposure, race, education and economic status might seem to play a significant role at first, among the presented studies, evidence is scarce and the conclusion leans towards little to no impact of 25(OH)D levels in HNC patients [28,37]. For example, Fanidi et al. showed that even when accounting for most confounding variables (education, alcohol consumption, circulating cotinine, tobacco exposure, and BMI) existing correlations suffer little change [28].
The divergence in findings across vitamin D supplementation studies likely stems from genetic variability in the vitamin D receptor, as evidenced by polymorphisms such as FokI and TaqI, which modulate treatment efficacy and prognostic outcomes in HNC. FokI variants can impair VDR’s calcitriol-binding affinity, altering regulation of apoptosis and immune pathways [47,52], while TaqI affects mRNA stability, potentially reducing VDR expression and responsiveness to supplementation [53], thus contributing to inconsistent survival benefits. These parallels with prior work affirm vitamin D’s potential in HNC management yet underscore the need for tailored strategies to unlock consistent benefits. We recommend that supplementation be administered only after categorizing patients by deficiency status, thereby ensuring safety and maximizing therapeutic impact, rather than as a universal practice. Additionally, stratifying patient populations by VDR polymorphisms, HPV status, and baseline 25(OH)D levels could identify responders versus non-responders, refining vitamin D’s role as an adjunct in personalized HNC care.
Clinical evidence suggests that vitamin D supplementation may potentiate responses to radiotherapy and chemotherapy in head and neck cancer (HNC), yet its interactions with other treatments warrant cautious consideration. Studies like that by Abdelaziz et al. [39] within our review demonstrate that adequate 25(OH)D levels (>30 ng/mL) enhance radiotherapy efficacy, reducing the severity of oral mucositis (p < 0.001) by upregulating DNA repair genes (e.g., GADD45) and dampening inflammation via NF-κB suppression. These findings are based on previous work by Feldman et al., who noted calcitriol’s potentiation of cisplatin cytotoxicity in HNSCC cells [54]. Conversely, the National Cancer Institute cautions that dietary supplements like vitamin D can alter cancer treatment efficacy and safety, as evidenced by Christakos et al., who describe high-dose vitamin D inducing CYP3A4 via VDR/PXR pathways, potentially accelerating the metabolism of drugs like imatinib and increasing toxicity risk [55]. Similarly, Schwartz et al. found that 800 IU/day vitamin D2 inhibited CYP3A4, raising atorvastatin levels by 17% (p = 0.04) in non-cancer patients [56], suggesting bidirectional pharmacokinetic shifts that could elevate HNC drug levels (e.g., cisplatin) or reduce efficacy. These mechanisms—VDR-driven gene regulation and CYP enzyme modulation—highlight supplementation’s dual potential to amplify therapeutic benefits while posing interaction risks, urging tailored monitoring in HNC care.
4.2. Limitations and Future Research Suggestions
This systematic review acknowledges several limitations inherent in the synthesized literature, including inconsistencies across study designs, the scarcity of large-scale randomized controlled trials (RCTs), and potential confounding factors such as lifestyle variables, nutritional status, and comorbidities, all of which may obscure the true impact of vitamin D on HNC outcomes. Our decision to include only studies from the past decade (2015–2025), while ensuring relevance and alignment with current clinical and research standards, may have overlooked valuable older investigations; however, this temporal constraint also minimized noise from outdated findings, with key foundational studies retained to anchor the analysis. The exclusion of non-English-language studies introduced a regional bias, potentially underrepresenting data from high-prevalence HNC areas, while the systematic selection process, though rigorous, remains susceptible to human judgment errors. Variability in study designs, patient cohorts, and vitamin D assessment methods—ranging from serum thresholds to timing—fostered heterogeneity, challenging the generalizability of our conclusions. Additionally, the limited availability of data on specific clinical endpoints—such as infection rates, recurrence beyond two years, or quality of life (QoL) metrics—constrained our ability to link vitamin D levels directly to tangible patient outcomes, potentially diminishing real-world applicability. Furthermore, the absence of uniform supplementation protocols across studies complicates direct comparisons, and our reliance on observational data over interventional evidence limits causal inference, leaving gaps in understanding optimal therapeutic strategies.
To address these shortcomings, well-designed, large-scale randomized controlled trials are imperative to rigorously evaluate vitamin D supplementation’s efficacy in improving HNC outcomes, with meticulous monitoring to manage potential toxicity risks and drug interactions. Standardizing supplementation protocols, establishing consistent deficiency thresholds (e.g., <20 ng/mL), and defining precise measurement timepoints represent critical initial steps to enhance the quality of future studies. It is also worth comparing the efficacy of dietary changes versus supplementation, as current evidence is insufficient.
Moreover, investigations should explore the interplay between vitamin D supplementation and VDR polymorphisms, assessing their combined influence on survival, recurrence, and QoL across treatment stages and chemotherapeutic agents. Incorporating additional biomarkers—such as parathyroid hormone, vitamin D-binding protein, oxidative stress markers, or interleukins—could deepen insights into vitamin D’s mechanistic role, while its potential as a preventive intervention also merits attention. Examining geographic and ethnic variations in deficiency prevalence, alongside long-term effects on survivors’ bone health, immune function, and QoL, will pave the way for more personalized HNC treatment approaches, tailoring interventions to individual patient profiles and disease contexts.
5. Conclusions
This systematic review elucidates the multifaceted role of vitamin D status in head and neck cancer (HNC), synthesizing evidence from 16 studies (2015–2025) to highlight its impact on risk, prognosis, treatment outcomes, and complications. We identified a high prevalence of vitamin D deficiency (47–95%) among HNC patients, which was particularly pronounced in advanced-stage disease and intensive treatment settings, alongside a consistent 26–32% reduction in HNC risk with higher 25(OH)D levels (OR 0.68–0.74). Adequate vitamin D levels correlated with improved survival in several studies (HR 0.73–0.84), though benefits plateaued beyond a threshold (~20 ng/mL), suggesting a non-linear relationship, while supplementation significantly mitigated treatment-related toxicities, such as mucositis and dysphagia (p < 0.001), thus enhancing patient tolerance. Conversely, deficiencies exacerbated malnutrition and postoperative complications (OR 2.4), underscoring vitamin D’s broader clinical relevance. These findings align with prior research affirming vitamin D’s therapeutic potential, but its variability—potentially driven by VDR polymorphisms (e.g., FokI, TaqI)—and interactions with treatments like imatinib highlight the need for precision. We advocate for routine 25(OH)D screening to identify deficient HNC patients (<20 ng/mL) for targeted supplementation, coupled with stratification by VDR genotypes, HPV status, and baseline levels in order to optimize efficacy and safety. While these insights position vitamin D as a modifiable factor in HNC management, the heterogeneity and observational nature of the evidence necessitate large-scale RCTs to confirm causality and refine strategies, paving the way for personalized interventions that could meaningfully alleviate HNC’s burden.
Author Contributions
Conceptualization, C.I.M. and R.G.; methodology, B.H.; validation, C.I.M., B.H. and R.G.; formal analysis, N.-I.V.; investigation, N.-I.V. and E.C.; resources, A.A. and E.C.; data curation, A.A. and C.P.-B.; writing—original draft preparation, A.A. and C.P.-B.; writing—review and editing, D.I.H. and N.C.B.; visualization, D.I.H.; supervision, C.I.M.; project administration, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the “Victor Babes” University of Medicine and Pharmacy Timisoara.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to acknowledge the “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania, for their support in covering the costs of publication for this research paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the existing affiliation information. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| HNC | Head and neck cancer |
| VDR | Vitamin D receptor |
References
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef]
- Acar, S.; Özkan, B. Vitamin D Metabolism. In Vitamin D; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. Vitamin D and Vitamin D-Binding Protein in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef]
- Orlov, I.; Rochel, N.; Moras, D.; Klaholz, B.P. Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. EMBO J. 2012, 31, 291–300. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Vitamin D Receptor (VDR)—Tissue Expression. Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000111424-VDR/tissue#top (accessed on 1 March 2025).
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef]
- Jenab, M.; Bueno-De-Mesquita, H.B.; Ferrari, P.; van Duijnhoven, F.J.B.; Norat, T.; Pischon, T.; Jansen, E.H.J.M.; Slimani, N.; Byrnes, G.; Rinaldi, S.; et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ 2010, 340, b5500. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, A.; Ivan, C.-S.; Tanasescu, S.; Oprisoni, L.A.; Dragomir, T.-L.; Varga, N.-I.; Mateescu, D.; Diaconu, M.; Margan, M.-M.; Boeriu, E. Are Pediatric Cancer Patients a Risk Group for Vitamin D Deficiency? A Systematic Review. Cancers 2024, 16, 4201. [Google Scholar] [CrossRef]
- Sluyter, J.D.; E Manson, J.; Scragg, R. Vitamin D and Clinical Cancer Outcomes: A Review of Meta-Analyses. JBMR Plus 2021, 5, e10420. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Shintani, T.; Higaki, M.; Rosli, S.N.Z.; Okamoto, T. Potential treatment of squamous cell carcinoma by targeting heparin-binding protein 17/fibroblast growth factor-binding protein 1 with vitamin D3 or eldecalcitol. Vitr. Cell. Dev. Biol. Anim. 2024, 60, 583–589. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). GLOBOCAN 2024: World Fact Sheet. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 1 March 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Brennan, P.; Chuang, S.-C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored By Am. Soc. Prev. Oncol. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Gupta, B.; Johnson, N.W.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Koll, L.; Gül, D.; Elnouaem, M.I.; Raslan, H.; Ramadan, O.R.; Knauer, S.K.; Strieth, S.; Hagemann, J.; Stauber, R.H.; Khamis, A. Exploiting Vitamin D Receptor and Its Ligands to Target Squamous Cell Carcinomas of the Head and Neck. Int. J. Mol. Sci. 2023, 24, 4675. [Google Scholar] [CrossRef]
- Arem, H.; Weinstein, S.J.; Horst, R.L.; Virtamo, J.; Yu, K.; Albanes, D.; Abnet, C.C. Serum 25-hydroxyvitamin D and risk of oropharynx and larynx cancers in Finnish men: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1178–1184. [Google Scholar] [CrossRef]
- Mäkitie, A.; Tuokkola, I.; Laurell, G.; Mäkitie, O.; Olsen, K.; Takes, R.P.; Florek, E.; Szyfter, K.; Sier, C.F.M.; Ferlito, A. Vitamin D in Head and Neck Cancer: A Systematic Review. Curr. Oncol. Rep. 2021, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Singh, S.; Sonkar, A.A.; Husain, N.; Singh, K.R.; Singh, S.; Kushwaha, J.K. Expression of Vitamin D receptor and Vitamin D status in patients with oral neoplasms and effect of Vitamin D supplementation on quality of life in advanced cancer treatment. Wspolczesna Onkol. Oncol. 2017, 2, 145–151. [Google Scholar] [CrossRef]
- Weinstein, S.J.; Mondul, A.M.; Yu, K.; Layne, T.M.; Abnet, C.C.; Freedman, N.D.; Stolzenberg-Solomon, R.Z.; Lim, U.; Gail, M.H.; Albanes, D. Circulating 25-hydroxyvitamin D up to 3 decades prior to diagnosis in relation to overall and organ-specific cancer survival. Eur. J. Epidemiol. 2018, 33, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Fanidi, A.; Muller, D.C.; Midttun, Ø.; Ueland, P.M.; Vollset, S.E.; Relton, C.; Vineis, P.; Weiderpass, E.; Skeie, G.; Brustad, M.; et al. Circulating Vitamin D in relation to cancer incidence and survival of the head and neck and oesophagus in the EPIC cohort. Sci. Rep. 2016, 6, 36017. [Google Scholar] [CrossRef]
- Bochen, F.; Balensiefer, B.; Körner, S.; Bittenbring, J.T.; Neumann, F.; Koch, A.; Bumm, K.; Marx, A.; Wemmert, S.; Papaspyrou, G.; et al. Vitamin D deficiency in head and neck cancer patients—Prevalence, prognostic value and impact on immune function. OncoImmunology 2018, 7, e1476817. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Lye, M.S.; Loh, S.P.; Yap, Y.Y.; Kandiah, M.; Augundhooa, D.; Bhattacharya, T.; Al-Olayan, E.; Wang, C. Serum 25-Hydroxyvitamin D Is Inversely Associated with Nasopharyngeal Carcinoma: A Hospital-Based Matched Case–Control Study in Malaysia. Nutrients 2024, 16, 397. [Google Scholar] [CrossRef]
- Kapała, A.; Szlendak, M.; Grochowska, E. Cross-sectional observational study—Investigation of vitamin D concentration in Caucasian cancer patients. what is the adequate dose of vitamin D for these patients? Clin. Nutr. 2021, 40, 3852–3858. [Google Scholar] [CrossRef]
- Radivojevic, N.; Grujicic, S.S.; Suljagic, V.; Stojkovic, S.; Arsovic, K.; Jakovljevic, S.; Bukurov, B.; Arsovic, N. Prognostic value of serum 25-hydroxyvitamin D levels and malnutrition status on postoperative complications in patients following laryngectomy with neck dissection. Eur. Arch. Oto-Rhino-Laryngol. 2024, 282, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Nejatinamini, S.; Debenham, B.J.; Clugston, R.D.; Mawani, A.; Parliament, M.; Wismer, W.V.; Mazurak, V.C. Poor vitamin status is associated with skeletal muscle loss and mucositis in head and neck cancer patients. Nutrients 2018, 10, 1236. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; O’Sullivan, F.; Farrington, S.M.; Theodoratou, E.; Campbell, H.; Dunlop, M.G.; Zgaga, L. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyVitamin D on cancer outcome: Systematic review and meta-Analysis. Br. J. Cancer 2017, 116, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Yokosawa, E.B.; Arthur, A.E.; Rentschler, K.M.; Wolf, G.T.; Rozek, L.S.; Mondul, A.M. Vitamin D intake and survival and recurrence in head and neck cancer patients. Laryngoscope 2018, 128, E371–E376. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhu, G.; Xu, Y.; Zheng, S.; Tang, B.; Huang, H.; Wu, I.X.Y.; Huang, D.; Liu, Y.; Zhang, X. Association Between Vitamin D Exposure and Head and Neck Cancer: A Systematic Review with Meta-Analysis. Front. Immunol. 2021, 12, 627226. [Google Scholar] [CrossRef]
- Westmark, N.L.W.; Sroussi, H.; Tamayo, I.; Villa, A. Vitamin D status in patients with oropharyngeal cancer: Association with HPV status and prognosis. Oral Dis. 2023, 29, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Nett, H.; Steegmann, J.; Tollkühn-Prott, B.; Hölzle, F.; Modabber, A. A prospective randomized comparative trial evaluating postoperative nutritional intervention in patients with oral cancer. Sci. Rep. 2022, 12, 14213. [Google Scholar] [CrossRef]
- Abdelaziz, R.A.; Elkhouly, E.; Hegazy, A.; Kamal, L.; Abd, A.; Ghany, E. Impact of Vitamin D Supplementation on Head and Neck Cancer Patients Receiving Radiotherapy. Egypt. J. Hosp. Med. 2022, 95, 1568–1575. [Google Scholar]
- Bhanu, A.; Waghmare, C.M.; Jain, V.S.; Pawar, H.J. Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: Potential in cancer therapeutics. Indian J. Cancer 2024, 61, 403–407. [Google Scholar] [CrossRef]
- Brust, L.A.; Vorschel, M.; Körner, S.; Knebel, M.; Kühn, J.P.; Wemmert, S.; Smola, S.; Wagner, M.; Schick, B.; Linxweiler, M. Impact of T Cell Exhaustion and Stroma Senescence on Tumor Cell Biology and Clinical Outcome of Head and Neck Squamous Cell Carcinomas. Int. J. Mol. Sci. 2024, 25, 13490. [Google Scholar] [CrossRef]
- Raimondi, S.; Johansson, H.; Maisonneuve, P.; Gandini, S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 2009, 30, 1170–1180. [Google Scholar] [CrossRef]
- Azad, A.K.; Bairati, I.; Qiu, X.; Huang, H.; Cheng, D.; Liu, G.; Meyer, F.; Adjei, A.; Xu, W. Genetic sequence variants in vitamin D metabolism pathway genes, serum vitamin D level and outcome in head and neck cancer patients. Int. J. Cancer 2013, 132, 2520–2527. [Google Scholar] [CrossRef]
- Hama, T.; Norizoe, C.; Suga, H.; Mimura, T.; Kato, T.; Moriyama, H.; Urashima, M. Prognostic Significance of Vitamin D Receptor Polymorphisms in Head and Neck Squamous Cell Carcinoma. PLoS ONE 2011, 6, e29634. [Google Scholar] [CrossRef]
- Starska-Kowarska, K. Role of Vitamin D in Head and Neck Cancer—Immune Function, Anti-Tumour Effect, and Its Impact on Patient Prognosis. Nutrients 2023, 15, 2592. [Google Scholar] [CrossRef]
- Liu, Z.; Calderon, J.I.; Zhang, Z.; Sturgis, E.M.; Spitz, M.R.; Wei, Q. Polymorphisms of vitamin D receptor gene protect against the risk of head and neck cancer. Pharmacogenet. Genom. 2005, 15, 159–165. [Google Scholar] [CrossRef]
- Zeljic, K.; Supic, G.; Radak, M.S.; Jovic, N.; Kozomara, R.; Magic, Z. Vitamin D receptor, CYP27B1 and CYP24A1 genes polymorphisms association with oral cancer risk and survival. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2012, 41, 779–787. [Google Scholar] [CrossRef]
- Ruggiero, S.; Gralow, J.; Marx, R.E.; Hoff, A.O.; Schubert, M.M.; Huryn, J.M.; Toth, B.; Damato, K.; Valero, V. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J. Oncol. Pract. 2006, 2, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wactawski-Wende, J.; Kotchen, J.M.; Anderson, G.L.; Assaf, A.R.; Brunner, R.L.; O’Sullivan, M.J.; Margolis, K.L.; Ockene, J.K.; Phillips, L.; Pottern, L.; et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 2006, 354, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Zarrati, M.; Sohouli, M.H.; Aleayyub, S.; Keshavarz, N.; Razmpoosh, E.; Găman, M.-A.; Fatahi, S.; Heydari, H. The Effect of Vitamin D Supplementation on Treatment-Induced Pain in Cancer Patients: A Systematic Review. Pain Manag. Nurs. Off. J. Am. Soc. Pain Manag. Nurses 2022, 23, 458–466. [Google Scholar] [CrossRef]
- Martinez-Alonso, M.; Dusso, A.; Ariza, G.; Nabal, M. The effect on quality of life of vitamin D administration for advanced cancer treatment (VIDAFACT study): Protocol of a randomised controlled trial. BMJ Open 2014, 4, e006128. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.; Verlinden, L.; Giulietti, A.; Ramos-Lopez, E.; Branisteanu, D.D.; Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Bouillon, R.; Roep, B.O.; et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007, 37, 395–405. [Google Scholar] [CrossRef]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.; Pols, H.A.; van Leeuwen, J.P. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Effects of vitamin D supplementation in atorvastatin-treated patients: A new drug interaction with an unexpected consequence. Clin. Pharmacol. Ther. 2009, 85, 198–203. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).