Abstract
Food allergies are rising globally, posing a multifactorial public health challenge driven by complex interactions among diet, immune development, and environmental exposures. This review highlights emerging insights into the cellular and molecular mechanisms by which specific dietary components, particularly vitamin A, fibre, indole compounds, and proteins, promote intestinal homeostasis. These nutrients act through both microbiota-dependent and -independent pathways, primarily in the small intestine, enhancing epithelial barrier integrity and supporting tolerogenic immune responses. Two key signalling axes, mediated by retinoic acid (RA) and aryl hydrocarbon receptor (AhR) ligands, converge to regulate RORγt-expressing immune cells, including group 3 innate lymphoid cells, TCRγδ+CD8αα+ intraepithelial lymphocytes (IELs), and Th17 cells, which are essential for secondary lymphoid organ development and barrier reinforcement. RA and AhR also guide the homing and specialization of diverse regulatory T cell subsets and CD4+ IELs, which collectively sustain peripheral tolerance to dietary antigens. Recent findings implicate RORγt+ antigen-presenting cells in the induction of peripheral Tregs during early life, particularly at weaning, underscoring a critical window for tolerance establishment. Microbial metabolites and commensal-derived signals further shape these immune pathways, reflecting the intricate interplay between host, diet, and microbiota in the regulation of oral tolerance.
1. Introduction
The global adoption of the Western diet, characterized by high intake of fat, protein, and ultra-processed foods but low fibre consumption, has paralleled the rise in non-communicable chronic diseases, which have now reached pandemic proportions []. Among them, the global prevalence of IgE-mediated food allergies has markedly increased to become a health burden worldwide and particularly in high-income, urbanized regions []. While genetic predisposition plays a role, this trend is attributed to a multifactorial interplay of early-life immune system development, dietary patterns, and environmental exposures [,].
The “hygiene hypothesis” proposed that reduced microbial exposure, due to improved sanitation and decreased contact with natural environments, impairs immune system maturation, triggering allergic disease []. The original hypothesis has progressively evolved into a more mechanistic framework, recognizing the cumulative influence of industrialization on the human microbiome while also identifying microbial signatures shared across distinct allergic manifestations []. Emerging evidence highlights the role of microbial diversity, environmental pollutants, and timing and route of allergen exposure in modulating allergic risk [,,,]. In general terms, it is assumed that increased food diversity in the first year is associated with reduced allergic sensitization and allergy outcomes in children [,]. This protective effect is thought to be mediated, at least in part, by the positive influence that enhanced exposure to dietary components exerts on gut microbial multiplicity and function [,]. The overall composition of the infant diet is an important factor in the development of allergic disease, with higher intake of fruits, vegetables, and home-prepared foods and, in general, higher healthy dietary scores in non-allergic children than in children who have a food allergy []. In contrast, Western dietary patterns are linked to reduced microbial diversity, potentially impairing mucosal immune tolerance and promoting proinflammatory responses to food antigens [].
Diet is increasingly acknowledged as a modifiable factor in the prevention and management of allergic diseases. Many effects of dietary components are mediated by their impact on the intestinal microbiome []. There is evidence that diet shapes the intestinal microbiota of mice across diverse genotypes in a dose-dependent manner []. Similarly, in humans, diets exclusively composed of either animal- or plant-based products consistently modify the microbiome despite inter-individual variability []. Diet modulates microbial composition and can either promote eubiosis or contribute to dysbiosis, depending on both the pre-existing state of the microbiota and the nature, duration, and quality of dietary exposure []. Therefore, dietary responses remain highly personalized, highlighting the necessity for tailored nutritional interventions to modulate microbiome composition []. Multiple interactions exist between diet, microbiota, and immune responses that range from direct or indirect (metabolite-mediated) diet-induced changes in microbiota composition and activity to the generation of diet-derived microbial metabolites with immunomodulatory properties []. Furthermore, beyond its influence on microbial ecology, diet can directly modulate immune function, although the precise mechanisms through which specific nutrients regulate immune cell development and systemic immune responses remain incompletely understood [].
The small intestine, the primary site of nutrient absorption, serves as a key location for oral sensitization to dietary proteins. In murine models of food allergy, the small intestine has been identified as a critical locus for the initiation and amplification of localized inflammatory responses following allergen exposure []. Compared to the colon, the microbiota of the small intestine is less well characterized. The continuous influx of digestive enzymes and bile, along with intermittent food delivery, shapes a microbial community with lower biomass and reduced diversity but highly adaptable to fluctuating environmental conditions and very susceptible to dietary influences [].
This review explores the interplay between diet and immune responses, with a specific focus on the cellular and molecular mechanisms through which specific nutrients modulate immune responses and contribute to the establishment of oral tolerance. The analysis integrates findings from in vitro experiments, animal models, and, where available, epidemiological studies, acknowledging the limited availability of controlled human intervention trials. Particular attention is given to the impact of distinct dietary components on epithelial cell function and mucosal immunity in the small intestine due to their relevance in the pathophysiology and prevention of allergic disorders.
2. Vitamins
2.1. Vitamins D and E
Vitamin D insufficiency, prevalent worldwide, has been proposed to partly explain the increases in asthma and allergic diseases that we have witnessed during the past years []. Reduced maternal vitamin D levels during pregnancy, as well as vitamin D insufficiency in infancy, have been associated with an increased risk of developing food allergies, particularly during the second year of life []. Some studies have suggested that vitamin D, by signalling through the vitamin D receptor (VDR), a member of the nuclear receptor family of ligand-regulated transcription factors, can promote oral tolerance by favouring intestinal barrier function, inducting antimicrobial peptides, dampening IgE-mediated mast cell activation, driving a tolerogenic phenotype on dendritic cells (DCs), and acting directly on T cells to promote regulatory T (Treg) cells (supporting forkhead box P3 (Foxp3) and Cytotoxic T-lymphocyte associated protein 4 (CTLA-4) expression, as well as IL-10 and TGF-β production) [,,,]. Peripherally induced Treg cells play a critical role in establishing tolerance to exogenous antigens by controlling Th2 inflammation towards non-self-substances at mucosal surfaces []. These cells are typically characterized as CD4+ T cells expressing the IL-2 receptor α-chain (CD25) and the transcription Foxp3, which is widely regarded as a canonical marker of Treg cell identity, although, beyond Foxp3+ Treg cells, several distinct subsets of regulatory T cells have been identified, defined by the expression of additional lineage-determining transcription factors and by their secretion of immunosuppressive cytokines such as IL-10 and TGF-β []. However, the effect of vitamin D on Treg cells is complex and depends on the dose, environmental cellular milieu and on factors such as the age of the individual, pregnancy, and health or disease status, thus many questions remain regarding its role [,].
Maternal supplementation with α-tocopherol (α-T), a form of vitamin E, reduces development of allergic responses in offspring from allergic mice []. Similarly, early-life levels of α-T have been negatively associated with asthma development in children, particularly when levels of γ-tocopherol (γ-T) are low. Elevated γ-T concentrations seem to counteract the protective effects of α-T, suggesting that these two isoforms have opposing effects on the allergic response []. Results regarding food allergy are currently limited to animal models, showing that maternal supplementation with α-T reduces the incidence of food allergy and anaphylaxis in neonatal mice with skin barrier defects [].
2.2. Retinoic Acid
Retinoic acid (RA), a vitamin A metabolite, plays a critical role in facilitating Foxp3+ Treg cell generation by acting to enhance signals derived from TGF-β, which is the limiting factor in this process [,]. Beyond augmenting TGF-β signalling, RA contributes to the stabilization of Treg cells by releasing IL-6 inhibition via the blockade of the expression of the IL-6 receptor [,]. Moreover, RA suppresses cytokine production by memory CD4+ T cells, particularly IL-4, and thus, it limits their capacity to antagonize TGF-β-mediated Treg conversion [,,]. Notably, RA signalling also promotes the expression of Rorc, the gene encoding the transcription factor RAR-related orphan receptor gamma t (RORγt), thereby facilitating the differentiation of Foxp3+RORγt+ T reg cells. This subset constitutes the predominant population of colonic Treg cells in both mice and humans and plays a pivotal role in maintaining oral tolerance to commensal microbiota, as well as in regulating immune responses in the context of food allergy []. In the absence of TGF-β, in vitro, RA induces the differentiation of Foxp3−IL-10+ type 1 regulatory T (Tr1) cells, with immunosuppressive functions, even under inflammatory conditions []. Similarly, without TGF-β, RA induces the in vitro conversion of human group 2 innate lymphoid cells (ILC2s) into regulatory ILCs (ILCregs), which suppress CD4+ T cell and ILC2 proliferation via IL-10 production and express CTLA-4 and CD25, but not Foxp3 [,].
RA is produced through a two-step oxidation of vitamin A, catalyzed successively by widely expressed alcohol dehydrogenases and tightly regulated aldehyde dehydrogenases. Three different isoforms of the enzyme retinaldehyde dehydrogenase (RALDH), that oxidizes retinal into RA, have been described in mammal cells []. RA, due to its short half-life, must be synthesized locally, in close proximity to its target cells, and its concentration exhibits a decreasing gradient from proximal to distal regions of the intestine, which correlates with the RALDH activity of resident cells [,]. In mice, DCs from the lamina propria and intestinal lymphoid organs have for long been considered to be functionally specialized in the differentiation of Foxp3+ Treg cells largely due to their capacity to produce high levels of active TGF-β and express Aldh1a2 (the gene encoding RALDH2) [,]. In addition to DCs, macrophages also express RALDH enzymes and contribute to a microenvironment conducive to mucosal tolerance [,].
RA is both necessary and sufficient to induce Aldh1a2 expression in DC precursors, thereby enhancing their autocrine production of RA and active TGF-β [,,,] (Figure 1). This RA-dependent positive feedback loop suggests that, in vivo, cells capable of constitutive RA production, independent of RA signalling, such as intestinal epithelial cells (IECs) and stromal cells from the lamina propria and mesenteric lymph nodes serve as an initial source to educate DCs [,]. IECs are a key source of RA, expressing high levels of Aldh1a1 and converting dietary retinol absorbed from the intestinal lumen into RA. Retinol may also be metabolized into retinyl esters, stored in the liver, and later mobilized via the circulation or secreted in the bile into the small intestine [] (Figure 1). RA and TGF-β produced by IECs imprint DCs with tolerogenic properties, promoting Treg cell induction both in murine models and in vitro or ex vivo human systems [,]. Furthermore, IECs secrete serum amyloid A (SAA) proteins, which are retinol binding proteins that transport retinol to intestinal DCs. DCs acquire retinol for conversion to RA through the LDL receptor-related protein 1 (LRP1) that mediates the uptake of SAA-retinol complexes [].
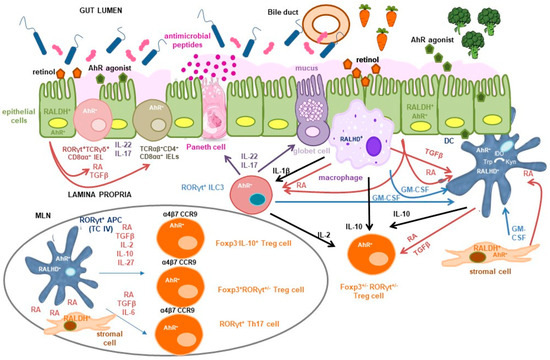
Figure 1.
Tolerogenic signals delivered by vitamin A and indole derivatives in the small intestine. Dietary retinol absorbed from the intestinal lumen or secreted in the bile into the small intestine is metabolized into retinoic acid (RA) by constitutively retinaldehyde dehydrogenase (RALDH)-expressing cells, including intestinal epithelial cells (IECs) and lamina propria stromal cells. This serves as a primary source of RA that promotes its own synthesis by macrophages and dendritic cells (DCs). IL-1β, released by macrophages in response to microbial stimuli activates group 3 innate lymphoid cells (ILC3s) to produce granulocyte-macrophage colony-stimulating factor (GM-CSF), which together with GM-CSF produced by macrophages themselves and stromal cells, further enhances RALDH activity and induces RA and TGF-β secretion by DCs. RA directly activates RORγt+ cells, including ILC3s and TCRγδ+CD8αα+ intraepithelial lymphocytes (IELs), promoting secretion of IL-22 and IL-17. This cytokine cascade supports antimicrobial peptide and mucus production, thereby reinforcing epithelial barrier integrity. RA and TGF-β also contribute to the differentiation of tolerogenic TCRαβ+CD4+CD8αα+ double-positive IELs. Within intestinal lymphoid tissues such as mesenteric lymph nodes (MLN), RA facilitates regulatory T (Treg) cell induction by RORγt+ antigen-presenting cells (APCs) specific to microbial and dietary antigens. The relative concentrations of TGF-β, RA, IL-10, and IL-27 influence the differentiation of Foxp3−IL-10+, Foxp3+RORγt−, and Foxp3+RORγt+ Treg cell subsets. Additionally, RA, in concert with TGF-β and IL-6, promotes Th17 cell differentiation under homeostatic conditions, enabling IL-22 and IL-17 production to maintain epithelial integrity. RA modulates the pathogenic potential of Th17 cells by downregulating IL-6 and IL-23 receptor expression. It also induces intestinal homing receptors (α4β7 and CCR9) on activated T cells. Conventional DCs may deliver proliferative cues required for Treg cell maintenance, proliferation and function in the intestinal lamina propria. Indole compounds, derived from cruciferous vegetables or protein metabolism, activate the aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor expressed across IECs, stromal cells, and immune populations. In DCs, AhR activation suppresses proinflammatory cytokine production and enhances RA synthesis. It also upregulates indoleamine 2,3-dioxygenase (IDO), facilitating tryptophan (Trp) conversion to kynurenine (Kyn), an AhR agonist, and promotes TGF-β and IL-27 production, thereby influencing Treg cell differentiation. AhR signalling is critical for the development and function of RORγt+ cells, including ILC3s and Th17 cells, and supports the maintenance of natural IELs and the adaptation of induced IELs. As a transcriptional regulator of RORγt+ APCs, AhR may contribute to the induction of Foxp3−IL-10+, Foxp3+RORγt−, and Foxp3+RORγt+ Treg cells. It also modulates the balance between Treg and Th17 cells and regulates Treg cell intestinal homing and function. The co-expression of AhR and RA signalling pathways in multiple cell types suggests potential cross-regulation, with AhR influencing RA biosynthesis and RA modulating AhR activity, possibly through shared gene regulatory networks.
Additional factors act synergistically to induce Aldh1a2 expression and RALDH activity in intestinal DCs. Among these, granulocyte-macrophage colony-stimulating factor (GM-CSF; also known as colony-stimulating factor 2, Csf2) plays a key role. GM-CSF is produced by lamina propria macrophages, stromal cells and, notably, group 3 innate lymphoid cells (ILC3s) [,,]. GM-CSF deficiency in mice also leads to impaired TGF-β production by DCs and reduced IL-10 secretion by macrophages, collectively compromising the differentiation of Foxp3+ Treg cells in both the small and large intestine []. GM-CSF production is regulated by signals from the commensal microbiota, which activate Toll-like receptors (TLRs) on intestinal macrophages, promoting IL-1β secretion. IL-1β, in turn, stimulates ILC3s via the IL-1β receptor pathway, driving GM-CSF production [] (Figure 1). Additionally, microbiota-induced IL-1β signalling promotes IL-2 production by ILC3s, which contributes to the maintenance of Treg cells and the induction of oral tolerance to dietary antigens in the small intestine []. Beyond its indirect effects through GM-CSF, TLR signalling itself contributes directly to DC education by inducing Aldh1a2 expression, as shown in murine DCs and in human DCs in vitro [,,,] (Figure 1). Interestingly, RA induces suppressor of cytokine signalling 3 (SOCS3) expression in DCs, which inhibits the TLR-driven production of proinflammatory cytokines that negatively affect Treg cell induction []. Furthermore, IL-4, which typically antagonizes Foxp3 expression, has been shown to synergize with RA to enhance the capacity of DCs to promote Foxp3+ Treg differentiation in the presence of TGF-β []. IL-4 and GM-CSF also act synergistically to strongly upregulate Aldh1a2 expression and RALDH activity in murine bone marrow-derived DCs, effects that are further amplified by TLR stimulation and RA itself [].
RA plays other roles in the establishment and maintenance of mucosal tolerance []. One of its key functions is the induction of gut-homing receptors, such as CCR9 and α4β7 integrin, on activated B and T cells, including Treg cells, thereby promoting their trafficking to the intestinal mucosa [] (Figure 1). In B cells, RA synergizes with IL-6 to facilitate class-switch recombination to IgA, the principal antibody isotype secreted across mucosal surfaces. IgA modulates microbial colonization and has been proposed to inhibit allergic responses by preventing IgE binding to allergens [], although other studies have questioned this protective role []. RA also impacts innate immunity by contributing to the development and transcriptional programming of conventional DCs in the bone marrow, and by enhancing their capacity to migrate to intestinal tissues [,,]. Additionally, it facilitates the transmigration of mature proinflammatory DCs from the lamina propria to the intestinal epithelium where they acquire tolerogenic properties imprinted by both RA and mucus [].
As mentioned above, RA signalling directly influences the differentiation and function of RORγt-expressing cells []. In addition to Foxp3+RORγt+ Treg cells, these include several other T cell subsets, such as natural T cell receptor (TCR)γδ+CD8αα+ intraepithelial lymphocytes (IELs), invariant natural killer T (iNKT) cells, and Th17 cells, as well as ILC3s, a population that comprises lymphoid tissue inducer (LTi) cells. These cell types cooperate to maintain intestinal barrier integrity primarily through the secretion of IL-22 and IL-17 []. RA influences intestinal immune responses by promoting IL-22 synthesis by ILC3s and TCRγδ+ CD8αα+IELs [] (Figure 1). IL-22, constitutively produced in the small intestine and inducible in the colon under inflammatory conditions, acts specifically on IECs []. It promotes the production of antimicrobial peptides and mucus, facilitates epithelial repair, enhances symbiosis with commensal microbiota through IEC fucosylation, and aids in pathogen clearance []. Similarly, IL-17 enhances antimicrobial peptide production and contributes to barrier integrity and protection against pathogens []. Despite their protective roles, both IL-22 and IL-17 are implicated in the pathogenesis of various chronic inflammatory conditions, reflecting their context-dependent functions []. Interestingly, RA also induces the expression of IL-22 binding protein (IL-22BP), a soluble inhibitory receptor that neutralizes IL-22 activity, thereby serving as a regulatory mechanism to modulate IL-22-driven responses [].
Recent murine studies have identified RORγt+ antigen-presenting cells (APCs), such as ILC3s [], extrathymic autoimmune regulator (AIRE)-expressing cells or Janus cells [], and Thetis cells (four different subsets with a hybrid phenotype between medullary thymic epithelial-like cells and DCs, with or without AIRE expression) [] as key mediators in the induction of Foxp3+RORγt+ Treg cells that confer tolerance towards the microbiota. More recently, RORγt+ APCs have also been implicated in the induction of peripheral Treg cells specific to dietary antigens in mice, thereby contributing to oral tolerance to food. Notably, a distinct subset of Thetis cells, designated TC IV, has been shown to induce both Foxp3+RORγt+ and Foxp3+RORγt− food antigen-specific Treg cells. This induction occurs prominently during the weaning period, a critical developmental window for Treg cell expansion and establishment of long-term immune tolerance, but also persists into adulthood, independent of microbiota composition [,,]. Importantly, TC IV cells have also been identified in human tissues such as mesenteric lymph nodes, intestinal lamina propria and tonsils [,]. Despite some divergences in nomenclature and phenotype, other recent papers have also identified and described in mice the same or a closely similar tolerogenic APC type [,,].
TC IV cells, predominantly enriched in lymph nodes draining the small and large intestine, are characterized by high levels of IL-2, MHCII, αvβ8 integrin, a potent activator of TGF-β, and RALDH2, which makes them responsive to RA, that enhances their tolerogenic function [,] (Figure 1). In contrast to their previously assumed roles, ILC3s and conventional DCs appear to be dispensable for establishing intestinal tolerance to dietary antigens across both early life and adult stages, prompting further investigation into their precise contributions to this process [,,,]. Nonetheless, DCs remain critical for maintaining other antigen-specific CD4+ T cell responses, indicating a functional specialization among APC subsets. Notably, migratory DCs that transport antigens from the lamina propria to gut-draining lymph nodes may provide proliferative signals essential for the maintenance of Treg cells, following their initial differentiation driven by RORγt+ APCs [,,]. Furthermore, these DCs may contribute to Treg cell survival and functionality within the small intestinal lamina propria by delivering costimulatory cues within a tolerogenic microenvironment [] (Figure 1).
3. Carbohydrates
3.1. Simple Sugars
Childhood consumption of high-fructose foods, sugar-sweetened beverages and high-carbohydrate ultra-processed products has been associated with an increased risk of allergic conditions, including food allergies []. A key factor implicated in this trend is the presence of advanced glycation end-products (AGEs), prevalent in Western diets and formed under high-sugar conditions. The consumption of AGEs has been positively associated with self-reported food allergy []. AGEs are hypothesized to trigger innate immune pathways that contribute to the initiation and amplification of allergic responses []. In vitro studies using human cell models have demonstrated that AGEs disrupt epithelial barrier integrity and elicit proinflammatory and Th2-skewed immune responses, pointing to a potential role in the observed rise in food allergy incidence []. In contrast to dietary sugar, that increases the inflammatory tone of the intestine [], longer chain, non-digestible carbohydrates are highly beneficial to intestinal and immune health [].
3.2. Fibre
Western diet is characterized by a low fibre intake, in most cases below the recommended daily range of 28–35 g for adults []. Fibre includes a broad array of carbohydrates that are not digestible by human enzymes or absorbable in the small intestine and provide an important substrate to the microbiota that inhabits the colon (in fact, it is its main energy source) to produce absorbable short-chain fatty acids (SCFAs). In mice only harbouring human-derived microbial communities, the transition from a low-fat, plant polysaccharide-rich diet to a high-fat, high-sugar Western diet causes significant alterations in microbiota structure and microbial metabolic pathways within a single day []. Diets low in fibre reduce the richness of the microbial community in the large intestine, causing dysbiosis and, in consequence, several diseases characterized by inflammation []. Notably, distinct dietary fibre structures elicit divergent and highly specific effects on microbiome composition, leading to shifts in the production of either propionate or butyrate or other bacteria-derived metabolites []. Thus, while dietary fibres are generally beneficial, certain components may trigger adverse immunological outcomes under specific conditions. For instance, it has been reported that inulin at a high concentration induces alterations in microbiota composition and metabolic activity that elevate bile acid levels (particularly cholic acid) and stimulates IL-33 production by stromal cells. This, in turn, activates IL-5-producing ILC2s, leading to excessive type 2 inflammation and allergy-prone responses in mice []. On the other hand, the immunological effects of dietary fibres are further modulated by the microbial fermentative capacity. In healthy individuals, a 17-week high-fibre dietary intervention revealed distinct immune response patterns linked to baseline microbial diversity []. Accordingly, individuals with low microbial gene richness show less improvement in inflammatory markers following fibre-rich dietary interventions []. Furthermore, in subjects lacking key fermentative microbes, certain fibres may have detrimental effects, due to unprocessed substrate accumulation or altered metabolite profiles that can exacerbate inflammation []. These findings underscore the complex, personalized nature of diet-microbiota-immune interactions and suggest that baseline microbiome composition may predict individual immunological responses to high-fibre diets.
A literature survey on epidemiological and intervention studies dealing with the consumption of dietary fibre for preventing or treating allergic diseases in humans did not identify any focused in food allergy. Nonetheless, studies in animal models have shown that fibre feeding confers protection from food allergy and increases the safety and effectiveness of oral immunotherapy protocols, mainly through the release of SCFAs by the colonic microbiota [,]. SCFAs, particularly butyrate, act through a combination of different immune and non-immune tolerogenic mechanisms to support intestinal homeostasis and prevent allergic sensitization []. Indeed, the microbiome of infants who later developed allergic sensitization is characterized by a reduced abundance of genes encoding enzymes involved in complex carbohydrate degradation and SCFA biosynthesis, particularly those responsible for butyrate production, impaired mucus integrity, heightened oxidative potential, and elevated levels of biogenic amines [,,]. Interestingly, although absorbed SCFAs are largely metabolized in the liver and appear in only trace amounts in the peripheral circulation, they are found at exceptionally high concentrations in the portal venous system, particularly in small blood vessels draining the colonic mucosa. This localized enrichment suggests that SCFAs may influence the function of circulating immune cells as they transit through the portal circulation []. IECs and immune cells detect SCFA signals via G protein-coupled receptors (GPCRs) located on their surfaces or internalize SCFAs through monocarboxylate transporters. Inside the cells, SCFAs function as histone deacetylase (HDAC) inhibitors, inducing epigenetic changes that result in chromatin remodelling and the activation of diverse gene expression programmes, and promote metabolic changes that result in energy supply and cell activation [].
Butyrate induces RALDH activity and increases RA conversion and active TGF-β production in human and mouse IEC lines and DCs [,,]. As mentioned, this facilitates the differentiation of naive CD4+ T cells into colonic Foxp3+ Treg cells [,,]. Furthermore, SCFAs directly modulate colonic Treg cells in mice, increasing their expression of Foxp3 and Il10, proliferation and suppressive function [,]. In addition, through its conversion into acetyl-CoA, acetate induces the differentiation of immunosuppressive IL-10 producing B cells, in vivo and in vitro in both mice and humans, which effectively promote the differentiation of Treg cells []. Regarding the eliciting phase of food allergy, SCFAs suppress IgE-mediated activation of human and mouse mast cells in vitro and in vivo in the mouse, through a mechanism that involves GPR109A, prostaglandin 2 (PGE2), and epigenetic regulation (HDAC inhibition) [,].
A diet rich in saturated fats and simple carbohydrates, but depleted of dietary fibre, reduces growth rate and increases penetrability in the inner colonic mucus layer of mice, linked to loss of defined fibre-dependent bacteria that are beneficial for maintaining mucus function []. In particular, Blautia coccoides stimulates mucus growth through the production of propionate and acetate via activation of GPR43 []. Furthermore, dietary fibre deficiency compels the microbiota to utilize mucus glycoproteins as an alternative nutrient source, leading to the degradation of the colonic mucus barrier []. This fibre deficiency-driven expansion of mucin-degrading bacteria contributes to colonic barrier dysfunction and promotes a type 2 immune profile, potentially exacerbating food allergy susceptibility []. Indeed, in mice, mucin-2 induces tolerogenic factors in IECs and enhances IL-10, TGF-β, and RALDH2 expression in DCs, thereby supporting a regulatory immune environment []. SCFAs also modulate the proliferation and function of colonic ILC3s through activation of GPR43. This interaction promotes the production of IL-22, which in turn enhances the expression of mucus-associated proteins and antimicrobial peptides, thereby supporting the maintenance of epithelial barrier integrity and overall intestinal homeostasis []. Another pathway that contributes to intestinal homeostasis involves the ligand-activated transcription factor aryl hydrocarbon receptor (AhR), which is activated by indoles []. AhR plays a critical role in the development of ILC3s and Th17 cells, the production of IL-22, and the promotion of Treg cell homing and function []. Interestingly, the SCFA butyrate has also been shown to activate AhR in IECs, likely in synergy with its HDAC inhibitory activity, thereby further supporting intestinal health [,].
A low fibre diet also changes the composition of the microbiota at the small intestinal level, causing, in mice, a decrease in fibre fermenters. In adult mice, reintroduction of fibre in the diet restores the balance, although constant low-fibre diets cause defective vertical transmission of key microbes to offspring that can only be overcome by combining fibre supplementation with a fecal transplant []. Oral immunotherapy combining allergen with an inulin-based gel effectively restores the dysregulated ileal microbiome in sensitized mice, increasing the abundance of key metabolites such as guanosine and inducing long-lasting regulatory immune responses []. In particular, fibre contributes to the development of Segmented Filamentous Bacteria (SFB), a group of Clostridium-related organisms that attach to the ileal epithelium of their hosts []. SFB anchor to the surface of the small intestinal epithelium, without inducing an inflammatory response, and impact the generation of innate and acquired immunity. SFB colonization stimulates the production of antimicrobial factors and chemokines, induces the development of gut lymphoid tissue and increases fecal IgA concentrations in the lamina propria []. Notably, SFB enhance the number and effector activity of RORγt+ Th17 cells in the lamina propria. RORγt+Foxp3+ Treg cells are also induced by SFB in the small intestine, albeit at significantly lower frequencies compared to Th17 cells []. In addition to impair the differentiation of Th17 cells, low numbers of SFB also affect the development of TCRαβ+CD4+CD8αα+ IELs [,].
Colonization of the mouse small intestine with SFB as a single commensal microbe is sufficient to induce the differentiation of Th17 cells []. The TCR repertoire of intestinal Th17 cells has a minimal overlap with that of other intestinal CD4+ T cells, with most Th17 cells recognizing antigens encoded by SFB []. Th17 cells, characterized by the expression of the transcription factor RORγt and the production of IL-17 and IL-22, are specialized in the defence against extracellular pathogens, particularly at mucosal and epithelial barriers, although excessive activation leads to severe inflammatory and autoimmune disorders []. Although Th17 cells were originally characterized by their proinflammatory functions, accumulating evidence highlights the existence of non-pathogenic Th17 subsets that contribute to epithelial barrier maintenance and mucosal equilibrium. In fact, both IL-22 and IL-17 are involved in restraining the overgrowth of SFB and other bacterial species, revealing a balancing effect that helps to preserve intestinal homeostasis []. The functional dichotomy of Th17 cells, protective versus pathogenic, is largely context-dependent and influenced by the local microenvironment and the specific cues present during their differentiation [,]. RA plays a central role in this process by promoting the differentiation and stabilization of RORγt+Foxp3+ Treg cells while concurrently downregulating the expression of the IL-23 receptor, a key driver of pathogenic Th17 cell differentiation [,] (Figure 1).
A complementary function of SFB has to do with the development of tolerogenic TCRαβ+CD4+CD8αα+ T cells, referred to as double positive intraepithelial lymphocytes (DP IELs) or CD4+ IELs. These are induced IELs that derive from antigen-experienced CD4+ T cells, including Treg cells, that accumulate in the intestinal epithelium and reactivate the CD8 programme by acquisition of the CD8-lineage transcription factor Runx3 and loss of the CD4-lineage transcription factor T helper inducing POZ-Kruppel Factor (ThPOK) [,,,,]. DP IELs display a regulatory function and collaborate with lamina propria Treg cells to favour tolerance to dietary antigens and protect against intestinal inflammation [,], albeit they may also exert cytotoxic activities depending on the context [,]. DP ILEs differentiate under the stimulus of the microbiota, with a diverse set of bacterial, dietary and environmental antigens and metabolites contributing to their optimal development [,,,]. TCR expression on CD4+ T cells and MHCII expression on IECs are required for the differentiation, but not maintenance, of conventional and Treg CD4+ T cells into DP IELs, suggesting that cognate antigen recognition is dispensable for their function []. Induction of an IEL phenotype also relies on intestinal signals like TGF-β and RA, which regulate CD103 expression for epithelial adhesion, and transcriptional cues including T-bet and IFN-γ or IL-27 signalling [,] (Figure 1).
The mechanism by which SFB induce DP IELs in the small intestine involves the production of IFN-γ, which drives the expression of MHCII on IECs and their antigen presentation ability, ultimately promoting the final maturation of CD4+ IELs intro double positive cells, although the source of IFN-γ may depend on the context []. According to Brabec et al. [], IFN-γ can be produced by lamina propria Th1 cells, inducing the massive expression of MHCII on IECs along with a SFB-specific IEL response. This cognate DP IELs contribute to the renewal of the SFB-stressed gut epithelium by increasing its turnover rate, thereby complementing the Th17-mediated response aimed to control SFB colonization. On the other hand, Rodriguez-Marino et al. [] suggested that group 1 innate lymphoid cells (ILC1s) and NKT cells produce IFN-γ in the absence of T cells under homeostatic conditions. In this way, SFB-induced MHCII expression enables IECs to present diverse microbial and dietary antigens, fostering a regulatory role for DP IELs.
SFB are widely present among vertebrates where they contribute to immune maturation, but it remains to be determined whether a human homologue of SFB exists with a function similar to that observed in mice. Although a human-specific SFB genome has been identified, its functional significance remains to be elucidated []. In humans, SFB constitute a minor fraction of the microbiota, with their peak abundance occurring between 21 and 36 months of age. This period coincides with increased expression of sIgA and Th17-associated markers, suggesting a potential role in postnatal immune development []. Regarding Th17 cell induction, human-derived Bifidobacterium adolescentis, along with other bifidobacterial species, has been shown to promote small intestinal Th17 cell differentiation. However, the mechanism of Th17 cell accumulation likely differs from that mediated by SFB []. B. adolescentis possesses polysaccharide transporters and degradation enzymes, but the relationship between dietary fibre and its immunomodulatory potential is yet to be fully established.
Dietary fibre may also exert microbiota-independent effects by modulating cells of the innate immune system or by protecting intestinal barrier function through actions on IEC maturation and tight junction proteins. These processes also depend strongly on the physicochemical properties of the fibre [,]. Microbiota-independent effects of fibre on innate cells rely on interactions with pattern recognition receptors (PRR) that bind carbohydrate structures and induce a variety of cellular responses, including production of cytokines and chemokines, which shape downstream adaptive immune responses. Key PRRs involved include C-type lectin receptors, such as dectin-1, dectin-2, and DC-SIGN [], as well as TLRs [,]. Recognition of glycan structures from common allergens (for instance, peanut) by specific C-type lectin receptors can promote allergic responses. However, depending on the receptor involved, the physical nature of the carbohydrate ligand, and the strength of the engagement, activation of these receptors can also induce immune regulation and tolerance []. Similarly, TLR signalling plays dual roles, contributing to both allergy-associated and protective immune responses, influenced by genetic predisposition, environmental factors, and ligand characteristics [].
In addition to their effects on immune cells, non-digestible polysaccharides have been shown to interact directly with surface receptors on IECs in in vitro cultures, such as TLRs, calcium-sensing receptors, and mannose receptors. These interactions activate a range of intracellular signalling pathways (including AMP-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), and protein kinase (PK) pathways), which play key roles in the regulation of tight junction integrity and epithelial barrier function [,,]. Dietary fibre also contributes to the strengthening of the epithelial barrier by modulating membrane composition, independently of changes in tight junction protein abundance, and by mitigating inflammation, further supporting barrier integrity and intestinal homeostasis [,]. Although in vivo studies remain limited, evidence supports the existence of rapid, microbiota-independent, beneficial effects of certain dietary fibres, such as psyllium, on intestinal barrier function. Psyllium exerts these effects in germ-free mice, albeit to a lesser extent than in specific pathogen-free counterparts, indicating that both microbiota-dependent and -independent mechanisms contribute to fibre-mediated barrier enhancement []. Similarly, fibre deprivation in germ-free mice has been shown to increase the expression of genes encoding the colonic type 2-inducing alarmins Il25 and Il33 []. A high rate of epithelial turnover and the subsequent increase in goblet cell numbers in the small intestine has been implicated in the effects of soluble fibres on baseline secretion of luminal sialylated mucins in rats []. However, other studies suggest that the proliferative effects of soluble fibre (specifically inulin at high concentrations) on colonic epithelial cells are strictly microbiota-dependent [].
Oligosaccharides, key dynamic components of human milk, are well recognized for their beneficial effects on gastrointestinal mucosal barrier integrity, mucin production, and neonatal immune development, in addition to their established prebiotic properties []. While the majority of milk oligosaccharides transit undigested to the large intestine, approximately 1–3% are detectable in the blood and urine of breastfed infants, indicating partial absorption within the gastrointestinal tract at concentrations potentially sufficient to exert systemic immunomodulatory effects, although the underlying mechanisms remain incompletely understood []. The intestinal mucosal epithelium expresses high levels of galectins, a family of glycan-binding proteins with diverse extracellular and intracellular roles in regulating epithelial function as well as innate and adaptive immune responses []. Milk-derived lactose and oligosaccharides can inhibit the extracellular binding of galectins to their glycan ligands, thereby functioning as selective galectin inhibitors with therapeutic potential in a range of pathological conditions, or modulate galectin expression within the gastrointestinal epithelium [,]. Dietary intervention with oligosaccharides has been shown to suppress allergic effector responses in casein-sensitized mice []. The biological basis involves the secretion of galectin-9 by IECs, a soluble lectin that directly suppresses mast cell degranulation and promotes Th1 and Treg cell responses that counteract excessive type 2 immunity, preventing acute allergic symptoms in mice []. The mechanism by which galectin-9 fosters regulatory responses in vitro involves the induction of RALDH activity in DCs []. In humans, galectin-9 expression in small intestinal tissues positively correlates with serum levels of galectin-9 and IL-10, as well as with increased frequencies of peripheral Treg cells in non-allergic compared to food allergic individuals []. Furthermore, galectin-9 can restore the defective induction of IL10 expression in naive peripheral B cells in food allergic patients, re-establishing the immune-suppressive function of Breg cells [].
4. Phytochemicals
4.1. Indoles
Different plant-based foods contain ligands for the AhR, predominantly in the form of indole derivatives. Cruciferous vegetables, such as broccoli and Brussels sprouts, are particularly rich in indole-3-carbinol (I3C), which is converted in the acidic environment of the stomach into high-affinity AhR ligands []. AhR is broadly expressed in immune cells, IECs and stromal cells, and it plays a pivotal role in regulating mucosal immune responses.
As mentioned, AhR signalling is essential for the development and function of RORγt+ ILC3s and Th17 cells, as well as for the production of IL-22 [] (Figure 1). Mice lacking AhR specifically in RORγt+ cells exhibit increased intestinal permeability to peanut allergens and heightened allergic responses, attributed to both IL-22-dependent and -independent contributions to epithelial barrier function []. However, barrier enhancement alone is insufficient to fully protect against food allergy, suggesting the involvement of additional immune-regulatory mechanisms []. Furthermore, analysis of a chromatin-accessible region within the Rorc locus, highly conserved between murine and human genomes, in the recently identified Treg cell inducers RORγt+ APCs, revealed a significant enrichment of AhR binding motifs. This finding implicates AhR as a key transcriptional regulator of RORγt+ APCs, also suggesting the potential involvement of TC IV cells in the pathogenesis associated with AhR-deficient mice []. Moreover, the data support a model in which both AhR and RORγt cooperatively regulate gene expression programmes that confer tolerogenic properties to these APCs []. In conventional DCs, where AhR expression is notably high, ligand-mediated activation promotes a tolerogenic phenotype characterized by enhanced RA synthesis and suppression of proinflammatory cytokines, thereby facilitating the differentiation of Foxp3+ Treg cells [] (Figure 1).
AhR, along with signals such as TGF-β, TLR ligands, or interactions with Treg cells via CTLA-4, can induce DCs to express indoleamine 2,3-dioxygenase (IDO), which catalyzes the conversion of Trp into kynurenine (Kyn), which is itself an AhR agonist [,] (Figure 1). AhR signalling in naïve CD4+ T cells contributes to the differentiation of Treg cells capable of suppressing inflammation and autoimmunity []. AhR signalling also promotes the differentiation of IL-27-driven IL-10 producing Tr1 cells in vivo []. Moreover, AhR is expressed in peripherally induced Treg cells, where it influences gut homing and supports in vivo suppressive capacity [,] (Figure 1). Recent evidence also indicates that indole-containing phytochemicals stimulate IECs to express MHCII molecules via AhR signalling. These MHCII+ IECs interact with thymus-derived natural Treg cells, promoting their accumulation at the crypt tops of the colon []. Nutritional AhR ligands are also critical for IELs. Experiments in mice have shown that natural IELs rely on AhR signalling for tissue maintenance [], while induced IELs use AhR pathways for local adaptation [] (Figure 1). Consequently, diets lacking AhR ligands impair IEL maintenance, disturb microbial homeostasis, and heighten susceptibility to epithelial injury and immune activation [].
Dietary supplementation with I3C alleviates allergic symptoms in peanut-sensitized mice, partially through the upregulation of Aldh1 gene expression in the small intestine []. AhR activation has been shown to suppress food allergic responses in mice, primarily by modulating the frequency rather than the absolute number of Foxp3+ Treg cells []. Targeted deletion of Ahr in Treg cells eliminates the capacity of indole derivatives to induce RORγt+ Treg cell differentiation and confer protection against food allergy []. However, the pleiotropic effects of AhR ligands across multiple immune and epithelial cell types, combined with ligand-specific activity and variations in the route of administration, may account for inconsistencies between in vitro and in vivo findings and the variability observed across different experimental animal models [].
4.2. Antioxidants: Isoflavones and Polyphenols
In human studies, increased oxidative stress prior to allergen exposure and inadequate antioxidant responses have been associated with a heightened susceptibility to occupational allergies []. At least in the case of inhalant allergens, an antioxidant-rich diet may contribute to allergy prevention []. In animal models, dietary isoflavones have been shown to suppress allergic sensitization and confer protection against peanut allergy [,]. Similarly, the anti-allergic potential of dietary polyphenols has been extensively investigated, with numerous studies documenting their anti-inflammatory, antioxidant, immunomodulatory, and microbiota-modifying effects []. Polyphenols can interact with food allergens through covalent and non-covalent binding, potentially altering protein conformation, promoting aggregation or crosslinking, and enhancing digestibility while reducing IgE-binding capacity. Such interactions have been proposed as a basis for developing hypoallergenic food products or novel immunotherapeutic approaches []. Experimental studies in murine models have shown that polyphenol administration during or after sensitization can reduce allergen-specific IgE levels, allergic symptoms, mast cell protease and histamine release, and Th2 cytokine production upon challenge, but the mechanism of action either at the cellular or molecular level is unclear [,,,,,]. Among the best supported properties of polyphenols is the inhibition of mast cell degranulation by acting on the high-affinity IgE receptor (FcεRI)-mediated pathway []. Depending on their structure, polyphenols modulate mast cell signal transduction via multiple mechanisms, including the downregulation of FcεRI surface expression, direct binding to FcεRI, and inhibition of key signalling molecules, such as Lyn or Syk kinases [,,,]. They also interfere with downstream signalling cascades involving MAPKs, protein kinase B (Akt), and Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), whose activation and nuclear translocation promotes the synthesis of inflammatory substances and reduce calcium influx by modulating calcium channel protein expression, a process critical for degranulation [,]. However, a more comprehensive understanding of the structural characteristics of polyphenols as they relate to absorption, metabolism, and bioavailability in humans is essential to fully assess their potential as antiallergic agents []. Researchers are beginning to decipher the specialized multi-enzyme system of specific members of the human gut microbiome, able to liberate aglycones from dietary phenolic glycosides with a broad diversity of effector functions, including the regulation of intestinal inflammation or, conversely, contributing to the increasing incidence of allergic diseases [].
5. Proteins and Nucleic Acids
The essential amino acid L-tryptophan (Trp), an indole derivative, serves as a structural component of proteins and a precursor to a wide array of biologically active metabolites. Trp is metabolized primarily via four major pathways: the Kyn pathway, which accounts for approximately 95% of total Trp degradation, the serotonin pathway (also leading to melatonin synthesis), and the tryptamine and indole-pyruvate pathways []. The Kyn pathway is regulated at its rate-limiting step by IDO and tryptophan dioxygenase (TDO), and generates Kyn, a well-characterized agonist of AhR. As mentioned, activation of AhR modulates transcriptional programmes involved in multiple biological processes including immune responses. In parallel, bacterial metabolism of dietary Trp yields indole-based metabolites, another important class of AhR ligands. For instance, a Trp-rich diet promotes DP IEL development by upregulating Thpok via AhR activation in T cells, driven by indole derivatives produced by Lactobacillus reuteri []. Importantly, Trp metabolism is both shaped by the gut microbiota and, reciprocally, exerts significant effects on microbial composition and function through AhR-dependent and -independent pathways, in contrast with plant-derived I3C, which exhibits more limited microbiota-modulating capabilities [,,]. Of note, high protein-diets reduce microbial diversity, increase microbial density and decrease intestinal barrier function, likely enhancing the antigenic load under conditions potentially leading to allergic reactions [].
Experiments in mice have shown that, under physiological conditions, proteins are the principal drivers of peripherally induced Treg cell development in the small intestine, the primary site of nutrient absorption. These Treg cells are essential for establishing and maintaining the default state of oral tolerance, although their lifespan is relatively short compared to that of colonic Treg cells, which are predominantly induced by commensal microbial antigens, as they undergo rapid turnover upon withdrawal of dietary antigens []. Exposure of naïve, food antigen-specific CD4+ T cells to dietary peptides in homeostatic settings is thought to drive their differentiation into a heterogeneous population of hyporesponsive cells that lack canonical T helper lineage-defining markers and inflammatory effector functions, yet retain the potential to convert into Treg cells []. Dietary peptides in the duodenum trigger the cleavage of gasdermin D (GSDMD) in IECs, generating a non-pyroptotic fragment that enters the nucleus and promotes the transcriptional activation of class II transactivator (CIITA) and MHCII genes and the development of Tr1 cells, helping to maintain immune tolerance to food []. Furthermore, food-derived peptides contribute to epithelial barrier protection and, via TLR activation among other possible mechanisms, deliver tolerogenic signals to DCs. This stimulation induces RALDH enzyme activity, thereby promoting the generation of TGF-β-producing Foxp3+RORγt+ Treg cells [,,]. In mice maintained on an antigen-free diet supplemented with amino acids, impairment in Treg cell generation and loss of oral tolerance is associated with profound transcriptional reprogramming of DCs in the small intestine []. These changes hinder key functional attributes of DCs, including their capacity for maturation, migration, activation, and induction of Treg cells in vitro. While protein deprivation is the primary driver of these alterations, shifts in the composition of the gut microbiota secondary to dietary changes also contribute to the modulation of DC function []. Lack of dietary antigens may also contribute to exacerbated type 2 responses in the small intestine through enhanced IL-25 production by tuft cells and subsequent ILC2 expansion [].
In addition, compared with a diet devoid of whole protein, a protein-containing diet promotes clonal selection, maturation, epithelial adaptation, and cytotoxic programming of antigen-experienced conventional CD4+ T cells and Treg cells in the small intestine []. Increased diversity of dietary protein may have an additive influence. Notably, these effects occur independently of the microbiota, which, while not essential for DP IEL differentiation, serves to amplify the response through microbial stimulation []. The accumulation of DP IELs in the small intestine in response to dietary antigens strongly suggests their involvement in mediating tolerogenic responses to food []. Consistent with this tolerogenic function, the co-receptor CD8αα, expressed by DP IELs, has been shown to attenuate TCR antigen sensitivity [], potentially restraining the cytotoxic effector mechanisms acquired during dietary protein-induced DP IEL differentiation. Furthermore, Lockhart et al. [] demonstrated that during steady-state tolerance, DP IELs predominate; however, when this tolerance state is challenged with a Th2-driven inflammatory stimulus, there is a clonal expansion and accumulation of lamina propria Treg cells specific for dietary antigens, indicating a functional division of labour between these two T cell subsets.
Other diet-derived factors also contribute to the development and maintenance of natural IELs specifically in the small intestine. Notably, dietary nucleic acids activate innate receptors for RNA and DNA to induce IL-15 production by IECs. This cytokine response enhances the proliferation and survival of natural IELs independently of microbial signals []. Interestingly, nucleic acid supplementation promotes, through natural IELs (with the participation of both TCRγδ+CD8αα+ and TCRαβ+CD8αα+ subsets), oral tolerance in mouse models of food allergy by producing TGF-β1 that supports Treg cell induction by small intestinal DCs [].
6. Lipids
Dietary fat increases intestinal permeability, promoting bacterial translocation, metabolic endotoxemia, and low-grade inflammation []. This compromised barrier facilitates allergen passage and disrupts antigen presentation, potentially impairing oral tolerance and enhancing food sensitization []. A high-fat, Western-style diet can trigger trained-immunity resulting in long-lasting enhanced innate immune responses to secondary inflammatory stimuli []. It also rapidly alters gut microbiota composition, gene expression and metabolic activity [,,]. The type of fatty acids in a high-fat diet distinctly shapes the gut microbiota of mice: saturated fats (e.g., palm oil) have been reported to reduce beneficial Bacteroidetes, monounsaturated fats (e.g., olive oil) increase Bacteroidaceae, and omega-3 polyunsaturated fats (e.g., from flaxseed or fish oil) promote bifidogenic effects []. Nevertheless, though fat intake clearly impacts the microbiota, predictive bacterial markers for metabolic dysfunction remain undefined []. Among other consequences, reduced microbiome diversity induced by a high-fat diet suppresses MHCII expression in IECs via TLR2/ Myeloid differentiation primary response 88 (MyD88) and IFN-γ signalling pathways [] that, as previously noted, may have implications for the development of DP IELs and Tr1 cells. Microbial alterations induced by a high fat diet in mice enhance susceptibility to food allergy []. Moreover, high-fat paternal diets alter microbiota and immune programming in offspring, increasing the risk of allergic sensitization via inherited dysbiosis, colonic inflammation, and reduced Treg cell frequencies [,]. In humans, maternal fat intake is linked to neonatal microbiome changes persisting until 4 to 6 weeks of age [].
n-3 Polyunsaturated Fatty Acids
Among dietary factors implicated in the rising prevalence of food allergy is the increased intake of n-6 polyunsaturated fatty acids (PUFAs) relative to n-3 PUFAs in Western diets during the latter half of the 20th century, primarily due to the replacement of animal fats with vegetable oils and margarines []. Linoleic acid (LA, 18:2n-6), abundant in corn, sunflower, and soybean oils, is the main n-6 PUFA, while α-linolenic acid (ALA, 18:3n-3), found in green plants, flaxseed, and walnuts, is the principal n-3 PUFA [] (Russo, 2009). n-6 PUFA-derived mediators such as leukotrienes and prostanoids are proinflammatory and contribute to allergic disease [,]. However, n-3 PUFAs (mainly eicosapentaenoic acid, EPA, 20:5n-3, and docosahexanoic acid, DPA, 22:5n-3) compete as substrates with n-6 PUFAs, leading to less potent or anti-inflammatory mediators, like resolvins and protectins [], and modulate the expression of proinflammatory genes [].
Populations with diets high in marine n-3 PUFAs show lower incidence of inflammatory diseases []. Maternal fish intake and higher EPA levels in breast milk have been associated with reduced childhood allergy risk in observational studies, though the effects of fish intake during infancy and childhood are inconsistent []. On their part, meta-analyses of n3-PUFA supplementation during pregnancy and lactation or early life show mixed evidence regarding allergy prevention [,,,,,,,,]. A recent study again highlights maternal n-3 supplementation during pregnancy and lactation as a potential strategy to reduce the risk of food allergies in offspring, particularly for egg and peanut sensitization, whereas childhood intake shows no comparable benefit [].
n-3 PUFAs, EPA and DHA, inhibit LPS-induced maturation of DCs, reducing MHCII and co-stimulatory molecules (CD40, CD80, CD86), and thereby dampening T cell activation and proinflammatory cytokine production that supports Th1 responses [,]. These effects are partly mediated by suppression of the NF-κB pathway, a key regulator of inflammatory gene expression [], with DHA being more effective than EPA in reducing NF-κB activation []. n-3 PUFAs also modulate adaptive immunity by altering T cell activation thresholds. In n-3 PUFA-enriched T cells, signalling through the TCR and CD28 is reduced, leading to impaired proliferation and cytokine secretion. However, these T cells can still respond under stronger stimulation []. This modulation is attributed to the incorporation of n-3 PUFAs into lipid rafts, ordered microdomains in the plasma membrane rich in cholesterol and sphingolipids, which disrupts raft organization and the recruitment and activation of signal-transducing proteins [].
In vivo models of food allergy show that n-3 PUFA supplementation suppresses both Th1 and Th2 responses [,]. Fish oil supplementation increases tolerogenic CD11b+CD103+ DCs and intestinal Foxp3+ Treg cells, with DHA being more effective than EPA in preventing allergic responses and IgE/IgG1 production [,,]. Offspring of mice fed diets rich in n-3 PUFAs exhibit altered gut microbiota, increased splenic IL-10 expression, and reduced allergen-specific IgE levels following sensitization, accompanied by attenuated allergic responses upon allergen challenge [,]. While fish oil has been reported to confer protective effects during both sensitization and effector phases of food allergy [], other murine studies suggest that n-3 PUFAs suppress allergic symptoms independently of specific antibody levels by inhibiting mast cell activation [,]. DHA inhibits IgE class switching and production in human B cells through downregulation of CD40- and IL-4-mediated NF-κB and STAT6 signalling, without affecting IgA or IgG production []. Given that FcεRI signalling in mast cells depends on lipid raft integrity, n-3 PUFAs disrupt FcεRI localization within rafts, thereby interfering with degranulation, cytokine release, and the secretion of IL-4 and IL-13, ultimately reducing allergic symptom severity [,,].
7. Conclusions and Future Directions
The rising incidence of food allergies represents a multifactorial challenge, driven by complex interactions among dietary habits, immunological development, and environmental exposures. A comprehensive understanding of how nutritional inputs together with environmental conditions, with or without microbial concurrence, shape immune tolerance is essential for devising effective prevention and intervention strategies. Specific dietary components, particularly vitamin A, fibre, indole compounds, and proteins, interact with the immune system through both microbiota-dependent and independent pathways to promote intestinal homeostasis. Two pivotal signalling pathways, mediated by RA and AhR ligands, converge to regulate the differentiation and function of RORγt-expressing immune cells, including ILC3s, TCRγδ+CD8αα+ IELs, and Th17 cells. These cell populations play indispensable roles in the development of secondary lymphoid structures and in fortifying the intestinal epithelial barrier through mechanisms such as tight junction maintenance, antimicrobial peptide secretion, and mucus production. RA and AhR ligands also critically influence the homing and functional specialization of different Treg cell subsets, including Foxp3+RORγt+, Foxp3+RORγt−, and Foxp3−IL-10− Treg cells. These cells orchestrate active immune regulation essential for peripheral tolerance to dietary antigens. Additionally, RA and AhR signalling contribute to the development and tissue adaptation of CD4+ IELs, which collaborate with Treg cells to sustain intestinal immune equilibrium. Emerging evidence also implicates RORγt+ APCs in the induction of peripheral Treg cells during critical developmental windows, such as weaning. Diet-derived microbial metabolites and signals from the commensal microbiota further complemented these effects, modulating the immune function conductive to oral tolerance, reflecting the complexity of host–microbe–nutrient interactions.
Although many mechanistic pathways remain incompletely understood, current evidence suggests that overall dietary diversity may exert a more profound immunological impact than any single nutrient. This is likely due to the intricate interplay among multiple dietary constituents, host physiology, disease states, and microbial ecology in maintaining a tolerogenic mucosal environment. The concept of “precision nutrition”, tailoring dietary recommendations based on individual or population-level factors such as genetics, microbiome composition, dietary habits, and socioeconomic context, offers promising avenues for personalized allergy prevention and management. Future research should prioritize longitudinal cohort studies and targeted interventions that address microbiome restoration, optimal timing of allergen introduction, dietary modulation, and environmental mitigation. Such efforts will be critical in advancing our understanding of food allergy pathogenesis and in developing holistic, evidence-based strategies for reducing allergy burden across diverse populations.
Funding
This work was supported by Ministerio de Ciencia, Innovación y Universidades under grant numbers PID2022-142298OA-I00 and PID2024-157139OB-I00.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author reports there are no competing interests to declare. The author has read and agreed to the published version of the manuscript.
References
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Spolidoro, G.C.I.; Amera, Y.T.; Ali, M.M.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Frequency of food allergy in Europe: An updated systematic review and meta-analysis. Allergy 2023, 78, 351–368. [Google Scholar] [CrossRef]
- Cabieses, B.; Uphoff, E.; Pinart, M.; Antó, J.M.; Wright, J. A systematic review on the development of asthma and allergic diseases in relation to international immigration: The leading role of the environment confirmed. PLoS ONE 2014, 9, e105347. [Google Scholar] [CrossRef]
- Lawson, L.P.; Parameswaran, S.; Panganiban, R.A.; Constantine, G.M.; Weirauch, M.T.; Kottyan, L.C. Update on the genetics of allergic diseases. J. Allergy Clin. Immunol. 2025, 155, 1738–1752. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Hoskinson, C.; Petersen, C.; Turvey, S.E. How the early life microbiome shapes immune programming in childhood asthma and allergies. Mucosal Immunol. 2025, 18, 26–35. [Google Scholar] [CrossRef]
- Pacheco, S.E.; Guidos-Fogelbach, G.; Annesi-Maesano, I.; Pawankar, R.; D’ Amato, G.; Latour-Staffeld, P.; Urrutia-Pereira, M.; Kesic, M.J.; Hernandez, M.L.; American Academy of Allergy, Asthma & Immunology Environmental Exposures and Respiratory Health Committee. Climate change and global issues in allergy and immunology. J. Allergy Clin. Immunol. 2021, 148, 1366–1377. [Google Scholar] [CrossRef]
- Collins, N.; Belkaid, Y. Control of immunity via nutritional interventions. Immunity 2022, 55, 210–223. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Emmerson, L.N. Epithelial barrier regulation, antigen sampling and food allergy. J. Allergy Clin. Immunol. 2022, 150, 493–502. [Google Scholar] [CrossRef]
- Sozener, Z.C.; Ozturk, B.O.; Cerci, P.; Turk, M.; Akin, B.G.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Takkinen, H.M.; Kaila, M.; Erkkola, M.; Ahonen, S.; Pekkanen, J.; Simell, O.; Veijola, R.; Ilonen, J.; Hyöty, H.; et al. Food diversity in infancy and the risk of childhood asthma and allergies. J. Allergy Clin. Immunol. 2014, 133, 1084–1091. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Déjardin, F.; Sparwasser, T.; Bérard, M.; Cerf-Bensussan, N.; Eberl, G. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 2019, 50, 1276–1288.e5. [Google Scholar] [CrossRef]
- Stephen-Victor, E.; Crestani, E.; Chatila, T.A. Dietary and microbial determinants in food allergy. Immunity 2020, 53, 277–289. [Google Scholar] [CrossRef]
- Grimshaw, K.E.; Maskell, J.; Oliver, E.M.; Morris, R.C.; Foote, K.D.; Mills, E.N.; Margetts, B.M.; Roberts, G. Diet and food allergy development during infancy: Birth cohort study findings using prospective food diary data. J. Allergy Clin. Immunol. 2014, 133, 511–519. [Google Scholar] [CrossRef]
- Skypala, I.; Vlieg-Boerstra, B. Food intolerance and allergy: Increased incidence or contemporary inadequate diets? Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 442–447. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Collins, N.; Guo, C.J.; Artis, D. Nutritional regulation of microbiota-derived metabolites: Implications for immunity and inflammation. Immunity 2024, 57, 14–27. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Personalized Microbiome Class Students; et al. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef]
- Alexander, M.; Turnbaugh, P.J. Deconstructing mechanisms of diet-microbiome-immune interactions. Immunity 2020, 53, 264–276. [Google Scholar] [CrossRef]
- Leung, A.S.; Xing, Y.; Fernández-Rivas, M.; Wong, G.W. The relationship between dietary patterns and the epidemiology of food allergy. Allergy 2025, 80, 690–702. [Google Scholar] [CrossRef]
- Lockhart, A.; Reed, A.; Rezende de Castro, T.; Herman, C.; Campos Canesso, M.C.; Mucida, D. Dietary protein shapes the profile and repertoire of intestinal CD4+ T cells. J. Exp. Med. 2023, 220, e20221816. [Google Scholar] [CrossRef]
- Kastl, A.J., Jr.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The structure and function of the human small intestinal microbiota: Current understanding and future directions. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 33–45. [Google Scholar] [CrossRef]
- Mirzakhani, H.; Al-Garawi, A.; Weiss, S.T.; Litonjua, A.A. Vitamin D and the development of allergic disease: How important is it? Clin. Exp. Allergy 2015, 45, 114–125. [Google Scholar] [CrossRef]
- Psaroulaki, E.; Katsaras, G.N.; Samartzi, P.; Chatziravdeli, V.; Psaroulaki, D.; Oikonomou, E.; Tsitsani, P. Association of food allergy in children with vitamin D insufficiency: A systematic review and meta-analysis. Eur. J. Pediatr. 2023, 182, 1533–1554. [Google Scholar] [CrossRef]
- Chambers, E.S.; Hawrylowicz, C.M. The impact of vitamin D on regulatory T cells. Curr. Allergy Asthma Rep. 2011, 11, 29–36. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.W.; Hwang, K.A.; Shin, M.S.; Lee, S.H.; Kim, W.U.; Kang, I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef]
- Yip, K.H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.A.; Anderson, P.H.; Samuel, M.S.; et al. Mechanisms of vitamin D3 metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364.e14. [Google Scholar] [CrossRef]
- Mailhot, G.; White, J.H. Vitamin D and immunity in infants and children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Niec, R.E.; Kim, H.Y.; Treuting, P.; Chinen, T.; Zheng, Y.; Umetsu, D.T.; Rudensky, A.Y. Extrathymically generated regulatory T cells control mucosal Th2 inflammation. Nature 2012, 482, 395–399. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Hernandez, J.D.; Boyd, S.D.; Galli, S.J.; Nadeau, K.C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016, 137, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Suaini, N.H.; Zhang, Y.; Vuillermin, P.J.; Allen, K.J.; Harrison, L.C. Immune modulation by vitamin D and its relevance to food allergy. Nutrients 2015, 7, 6088–6108. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G.B. Cellular and molecular mechanisms of vitamin D in food allergy. J. Cell. Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Berdnikovs, S.; Soveg, F.W.; Cook-Mills, J.M. α-Tocopherol supplementation of allergic female mice inhibits development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L482–L496. [Google Scholar] [CrossRef]
- Kumar, R.; Ferrie, R.P.; Balmert, L.C.; Kienzl, M.; Rifas-Shiman, S.L.; Gold, D.R.; Sordillo, J.E.; Kleinman, K.; Camargo, C.A., Jr.; Litonjua, A.A.; et al. Associations of α- and γ-tocopherol during early life with lung function in childhood. J. Allergy Clin. Immunol. 2020, 146, 1349–1357.e3. [Google Scholar] [CrossRef] [PubMed]
- Kosins, A.E.; Gao, H.; Blankenship, R.L.; Emmerson, L.N.; Ochoa, J.A.; Cook-Mills, J.M. Maternal supplementation with α-tocopherol inhibits the development of offspring food allergy, H1R signaling and ultimately anaphylaxis early in life. J. Immunol. 2025, 214, 199–210. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Sun, C.M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Hill, J.A.; Hall, J.A.; Sun, C.M.; Cai, Q.; Ghyselinck, N.; Chambon, P.; Belkaid, Y.; Mathis, D.; Benoist, C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity 2008, 29, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Nolting, J.; Daniel, C.; Reuter, S.; Stuelten, C.; Li, P.; Sucov, H.; Kim, B.G.; Letterio, J.J.; Kretschmer, K.; Kim, H.J.; et al. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J. Exp. Med. 2009, 206, 2131–2139. [Google Scholar] [CrossRef]
- Lee, S.; Park, K.; Kim, J.; Min, H.; Seong, R.H. Foxp3 expression in induced regulatory T cells is stabilized by C/EBP in inflammatory environments. EMBO Rep. 2018, 19, e45995. [Google Scholar] [CrossRef] [PubMed]
- López-Fandiño, R.; Molina, E.; Lozano-Ojalvo, D. Intestinal factors promoting the development of RORγt+ cells and oral tolerance. Front. Immunol. 2023, 14, 1294292. [Google Scholar] [CrossRef]
- Bakdash, G.; Vogelpoel, L.T.; van Capel, T.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef]
- Morita, H.; Kubo, T.; Rückert, B.; Ravindran, A.; Soyka, M.B.; Rinaldi, A.O.; Sugita, K.; Wawrzyniak, M.; Wawrzyniak, P.; Motomura, K.; et al. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J. Allergy Clin. Immunol. 2019, 143, 2190–2201.e9. [Google Scholar] [CrossRef]
- Golebski, K.; Layhadi, J.A.; Sahiner, U.; Steveling-Klein, E.H.; Lenormand, M.M.; Li, R.C.Y.; Bal, S.M.; Heesters, B.A.; Vilà-Nadal, G.; Hunewald, O.; et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307.e7. [Google Scholar] [CrossRef]
- Cassani, B.; Villablanca, E.J.; De Calisto, J.; Wang, S.; Mora, J.R. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Aspects Med. 2012, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Villablanca, E.J.; Wang, S.; de Calisto, J.; Gomes, D.C.; Kane, M.A.; Napoli, J.L.; Blaner, W.S.; Kagechika, H.; Blomhoff, R.; Rosemblatt, M.; et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology 2011, 141, 176–185. [Google Scholar] [CrossRef]
- Jaensson-Gyllenbäck, E.; Kotarsky, K.; Zapata, F.; Persson, E.K.; Gundersen, T.E.; Blomhoff, R.; Agace, W.W. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 2011, 4, 438–447. [Google Scholar] [CrossRef]
- Denning, T.L.; Wang, Y.C.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef]
- Denning, T.L.; Norris, B.A.; Medina-Contreras, O.; Manicassamy, S.; Geem, D.; Madan, R.; Karp, C.L.; Pulendran, B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 2011, 187, 733–747. [Google Scholar] [CrossRef]
- Yokota, A.; Takeuchi, H.; Maeda, N.; Ohoka, Y.; Kato, C.; Song, S.Y.; Iwata, M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int. Immunol. 2009, 21, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Cong, Y.; Qin, H.; Benveniste, E.N.; Elson, C.O. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J. Immunol. 2010, 185, 5915–5925. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, S.I.; Ahrendt, M.; Bode, U.; Wahl, B.; Kremmer, E.; Förster, R.; Pabst, O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 2008, 205, 2483–2490. [Google Scholar] [CrossRef]
- Vicente-Suarez, I.; Larange, A.; Reardon, C.; Matho, M.; Feau, S.; Chodaczek, G.; Park, Y.; Obata, Y.; Gold, R.; Wang-Zhu, Y.; et al. Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol. 2015, 8, 141–451. [Google Scholar] [CrossRef] [PubMed]
- Agace, W.W.; Persson, E.K. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 2012, 33, 42–48. [Google Scholar] [CrossRef]
- Iliev, I.D.; Mileti, E.; Matteoli, G.; Chieppa, M.; Rescigno, M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009, 2, 340–350. [Google Scholar] [CrossRef]
- Iliev, I.D.; Spadoni, I.; Mileti, E.; Matteoli, G.; Sonzogni, A.; Sampietro, G.M.; Foschi, D.; Caprioli, F.; Viale, G.; Rescigno, M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 2009, 58, 1481–1489. [Google Scholar] [CrossRef]
- Bang, Y.J.; Hu, Z.; Li, Y.; Gattu, S.; Ruhn, K.A.; Raj, P.; Herz, J.; Hooper, L.V. Serum amyloid A delivers retinol to intestinal myeloid cells to promote adaptive immunity. Science 2021, 373, eabf9232. [Google Scholar] [CrossRef]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343, 1249288. [Google Scholar] [CrossRef]
- Zhou, L.; Chu, C.; Teng, F.; Bessman, N.J.; Goc, J.; Santosa, E.K.; Putzel, G.G.; Kabata, H.; Kelsen, J.R.; Baldassano, R.N.; et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 2019, 568, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Villablanca, E.J.; De Calisto, J.; Gomes, D.C.; Nguyen, D.D.; Mizoguchi, E.; Kagan, J.C.; Reinecker, H.C.; Hacohen, N.; Nagler, C.; et al. MyD88-dependent TLR1/2 signals educate dendritic cells with gut-specific imprinting properties. J. Immunol. 2011, 187, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Fujimoto, K.; Jang, M.H.; Yang, B.G.; Jung, Y.J.; Nishiyama, M.; Sato, S.; Tsujimura, T.; Yamamoto, M.; Yokota, Y.; et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008, 9, 769–776. [Google Scholar] [CrossRef]
- Manicassamy, S.; Ravindran, R.; Deng, J.; Oluoch, H.; Denning, T.L.; Kasturi, S.P.; Rosenthal, K.M.; Evavold, B.D.; Pulendran, B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 2009, 15, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Buttrick, T.; Bassil, R.; Zhu, C.; Olah, M.; Wu, C.; Xiao, S.; Orent, W.; Elyaman, W.; Khoury, S.J. IL-4 and retinoic acid synergistically induce regulatory dendritic cells expressing Aldh1a2. J. Immunol. 2013, 191, 3139–3151. [Google Scholar] [CrossRef]
- Erkelens, M.N.; Mebius, R.E. Retinoic acid and immune homeostasis: A balancing act. Trends Immunol. 2017, 38, 168–180. [Google Scholar] [CrossRef]
- Benson, M.J.; Pino-Lagos, K.; Rosemblatt, M.; Noelle, R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007, 204, 1765–1774. [Google Scholar] [CrossRef]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J.J. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef]
- Liu, E.G.; Zhang, B.; Martin, V.; Anthonypillai, J.; Kraft, M.; Grishin, A.; Grishina, G.; Catanzaro, J.R.; Chinthrajah, S.; Sindher, T.; et al. Food-specific immunoglobulin A does not correlate with natural tolerance to peanut or egg allergens. Sci. Transl. Med. 2022, 14, eabq0599. [Google Scholar] [CrossRef]
- Zeng, R.; Bscheider, M.; Lahl, K.; Lee, M.; Butcher, E.C. Generation and transcriptional programming of intestinal dendritic cells: Essential role of retinoic acid. Mucosal Immunol. 2016, 9, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Oderup, C.; Yuan, R.; Lee, M.; Habtezion, A.; Hadeiba, H.; Butcher, E.C. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal Immunol. 2013, 6, 847–856. [Google Scholar] [CrossRef]
- Rivera, C.A.; Randrian, V.; Richer, W.; Gerber-Ferder, Y.; Delgado, M.G.; Chikina, A.S.; Frede, A.; Sorini, C.; Maurin, M.; Kammoun-Chaari, H.; et al. Epithelial colonization by gut dendritic cells promotes their functional diversification. Immunity 2022, 55, 129–144.e8. [Google Scholar] [CrossRef] [PubMed]
- van de Pavert, S.A.; Ferreira, M.; Domingues, R.G.; Ribeiro, H.; Molenaar, R.; Moreira-Santos, L.; Almeida, F.F.; Ibiza, S.; Barbosa, I.; Goverse, G.; et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014, 508, 123–127. [Google Scholar] [CrossRef]
- Eberl, G. RORγt, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol. 2017, 10, 27–34. [Google Scholar] [CrossRef]
- Mielke, L.A.; Jones, S.A.; Raverdeau, M.; Higgs, R.; Stefanska, A.; Groom, J.R.; Misiak, A.; Dungan, L.S.; Sutton, C.E.; Streubel, G.; et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 2013, 210, 1117–1124. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef]
- Keir, M.; Yi, T.; Lu, T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Martin, J.C.; Bériou, G.; Heslan, M.; Chauvin, C.; Utriainen, L.; Aumeunier, A.; Scott, C.L.; Mowat, A.; Cerovic, V.; Houston, S.A.; et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 2014, 7, 101–113. [Google Scholar] [CrossRef]
- Lyu, M.; Suzuki, H.; Kang, L.; Gaspal, F.; Zhou, W.; Goc, J.; Zhou, L.; Zhou, J.; Zhang, W.; JRI Live Cell Bank; et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 2022, 610, 744–751. [Google Scholar] [CrossRef]
- Kedmi, R.; Najar, T.A.; Mesa, K.R.; Grayson, A.; Kroehling, L.; Hao, Y.; Hao, S.; Pokrovskii, M.; Xu, M.; Talbot, J.; et al. A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nature 2022, 610, 737–743. [Google Scholar] [CrossRef]
- Akagbosu, B.; Tayyebi, Z.; Shibu, G.; Paucar Iza, Y.A.; Deep, D.; Parisotto, Y.F.; Fisher, L.; Pasolli, H.A.; Thevin, V.; Elmentaite, R.; et al. Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota. Nature 2022, 610, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Cabric, V.; Franco Parisotto, Y.; Park, T.; Akagbosu, B.; Zhao, Z.; Lo, Y.; Shibu, G.; Fisher, L.; Paucar Iza, Y.A.; Leslie, C.; et al. A wave of Thetis cells imparts tolerance to food antigens early in life. Science 2025, 389, 268–274. [Google Scholar] [CrossRef]
- Fu, L.; Upadhyay, R.; Pokrovskii, M.; Chen, F.M.; Romero-Meza, G.; Griesemer, A.; Littman, D.R. PRDM16-dependent antigen-presenting cells induce tolerance to gut antigens. Nature 2025, 642, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.F.; Wu, S.; Trsan, T.; Panda, S.K.; Fachi, J.L.; Liu, Y.; Du, S.; de Oliveira, S.; Antonova, A.U.; Khantakova, D.; et al. Rorγt-positive dendritic cells are required for the induction of peripheral regulatory T cells in response to oral antigens. Cell 2025, 188, 2720–2737.e22. [Google Scholar] [CrossRef]
- Narasimhan, H.; Richter, M.L.; Shakiba, R.; Papaioannou, N.E.; Stehle, C.; Ravi Rengarajan, K.; Ulmert, I.; Kendirli, A.; de la Rosa, C.; Kuo, P.Y.; et al. RORγt-expressing dendritic cells are functionally versatile and evolutionarily conserved antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2025, 122, e2417308122. [Google Scholar] [CrossRef] [PubMed]
- Rudnitsky, A.; Oh, H.; Margolin, M.; Dassa, B.; Shteinberg, I.; Stoler-Barak, L.; Shulman, Z.; Kedmi, R. A coordinated cellular network regulates tolerance to food. Nature 2025, 644, 231–240. [Google Scholar] [CrossRef]
- Sun, I.H.; Qualls, A.E.; Yin, H.S.; Wang, J.; Arvedson, M.P.; Germino, J.; Horner, N.K.; Zhong, S.; Du, J.; Valdearcos, M.; et al. RORγt eTACs mediate oral tolerance and Treg induction. J. Exp. Med. 2025, 222, e20250573. [Google Scholar] [CrossRef]
- Cabric, V.; Brown, C.C. Thetis cells: Regulators of intestinal immune tolerance. Curr. Opin. Immunol. 2025, 95, 102570. [Google Scholar] [CrossRef] [PubMed]
- Campos Canesso, M.C.; de Castro, T.B.R.; Nakandakari-Higa, S.; Lockhart, A.; Luehr, J.; Bortolatto, J.; Parsa, R.; Esterházy, D.; Lyu, M.; Liu, T.T.; et al. Identification of antigen-presenting cell-T cell interactions driving immune responses to food. Science 2025, 387, eado5088. [Google Scholar] [CrossRef]
- Cruz-Morales, E.; Hart, A.P.; Fossett, G.M.; Laufer, T.M. Helios+ and RORγt+ Treg populations are differentially regulated by MHCII, CD28, and ICOS to shape the intestinal Treg pool. Mucosal Immunol. 2023, 16, 264–274. [Google Scholar] [CrossRef]
- Berni Canani, R.; Carucci, L.; Coppola, S.; D’Auria, E.; O’Mahony, L.; Roth-Walter, F.; Vassilopolou, E.; Agostoni, C.; Agache, I.; Akdis, C.; et al. Ultra-processed foods, allergy outcomes and underlying mechanisms in children: An EAACI task force report. Pediatr. Allergy Immunol. 2024, 35, e14231. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, G.; Jiang, Y.; Shi, H.; Yang, X.; Gao, Z.; Wang, Q.; Sun, J.; Wang, C.; Li, Q.; et al. Dietary advanced glycation end-products promote food allergy by disrupting intestinal barrier and enhancing Th2 immunity. Nat. Commun. 2025, 16, 4960. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Masilamani, M.; Li, X.M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Paparo, L.; Coppola, S.; Nocerino, R.; Pisapia, L.; Picariello, G.; Cortese, M.; Voto, L.; Maglio, M.; Miele, E.; Carucci, L.; et al. How dietary advanced glycation end products could facilitate the occurrence of food allergy. J. Allergy Clin. Immunol. 2024, 153, 742–758. [Google Scholar] [CrossRef]
- Racine, A.; Carbonnel, F.; Chan, S.S.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary patterns and risk of inflammatory bowel disease in Europe: Results from the EPIC study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of dietary fiber in promoting immune health-An EAACI position paper. Allergy 2022, 77, 3185–3198. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Scientific opinion on dietary reference values for carbohydrates and dietary fiber. EFSA panel on dietetic products, nutrition, and allergies. EFSA J. 2010, 8, 1462. [Google Scholar]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.K.; Bording-Jorgensen, M.; Santer, D.M.; Zhang, Z.; Valcheva, R.; Rieger, A.M.; Sung-Ho Kim, J.; Dijk, S.I.; Mahmood, R.; Ogungbola, O.; et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 2023, 164, 228–240. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, L.; Bol-Schoenmakers, M.; Giustarini, G.; Vonk, M.M.; van Esch, B.C.A.M.; Knippels, L.M.J.; Garssen, J.; Smit, J.J.; Pieters, R.H.H. Dietary supplementation with nondigestible oligosaccharides reduces allergic symptoms and supports low dose oral immunotherapy in a peanut allergy mouse model. Mol. Nutr. Food Res. 2018, 62, e1800369. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy 2021, 76, 1398–1415. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D.; Cait, J.; Sbihi, H.; Stiemsma, L.; Subbarao, P.; Mandhane, P.J.; et al. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647.e3. [Google Scholar] [CrossRef] [PubMed]
- Hoskinson, C.; Dai, D.L.Y.; Del Bel, K.L.; Becker, A.B.; Moraes, T.J.; Mandhane, P.J.; Finlay, B.B.; Simons, E.; Kozyrskyj, A.L.; Azad, M.B.; et al. Delayed gut microbiota maturation in the first year of life is a hallmark of pediatric allergic disease. Nat. Commun. 2023, 14, 4785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mackay, C.R. High metabolite concentrations in portal venous blood as a possible mechanism for microbiota effects on the immune system and Western diseases. J. Allergy Clin. Immunol. 2024, 153, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Schilderink, R.; Verseijden, C.; Seppen, J.; Muncan, V.; van den Brink, G.R.; Lambers, T.T.; van Tol, E.A.; de Jonge, W.J. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G1138–G1146. [Google Scholar] [CrossRef]
- Goverse, G.; Molenaar, R.; Macia, L.; Tan, J.; Erkelens, M.N.; Konijn, T.; Knippenberg, M.; Cook, E.C.L.; Hanekamp, D.; Veldhoen, M.; et al. Diet-derived short chain fatty acids stimulate intestinal epithelial cells to induce mucosal tolerogenic dendritic cells. J. Immunol. 2017, 198, 2172–2181. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci. Rep. 2018, 8, 9742. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. The microbiota regulates type 2 immunity through RORγt⁺ T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Daïen, C.I.; Tan, J.; Audo, R.; Mielle, J.; Quek, L.E.; Krycer, J.R.; Angelatos, A.; Duraes, M.; Pinget, G.; Ni, D.; et al. Gut-derived acetate promotes B10 cells with antiinflammatory effects. JCI Insight 2021, 6, e144156. [Google Scholar] [CrossRef]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.J.; Junt, T.; Tam, S.Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef]
- Nagata, K.; Ando, D.; Ashikari, T.; Ito, K.; Miura, R.; Fujigaki, I.; Goto, Y.; Ando, M.; Ito, N.; Kawazoe, H.; et al. Butyrate, valerate, and niacin ameliorate anaphylaxis by suppressing IgE-dependent mast cell activation: Roles of GPR109A, PGE2, and epigenetic regulation. J. Immunol. 2024, 212, 771–784. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef]
- Holmberg, S.M.; Feeney, R.H.; Prasoodanan, P.K.V.; Puértolas-Balint, F.; Singh, D.K.; Wongkuna, S.; Zandbergen, L.; Hauner, H.; Brandl, B.; Nieminen, A.I.; et al. The gut commensal Blautia maintains colonic mucus function under low-fiber consumption through secretion of short-chain fatty acids. Nat. Commun. 2024, 15, 3502. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Parrish, A.; Boudaud, M.; Grant, E.T.; Willieme, S.; Neumann, M.; Wolter, M.; Craig, S.Z.; De Sciscio, A.; Cosma, A.; Hunewald, O.; et al. Akkermansia muciniphila exacerbates food allergy in fibre-deprived mice. Nat. Microbiol. 2023, 8, 1863–1879. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef]
- Chun, E.; Lavoie, S.; Fonseca-Pereira, D.; Bae, S.; Michaud, M.; Hoveyda, H.R.; Fraser, G.L.; Comeau, C.A.G.; Glickman, J.N.; Fuller, M.H.; et al. Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 2019, 51, 871–884.e6. [Google Scholar] [CrossRef]
- Zhou, L. AHR Function in lymphocytes: Emerging concepts. Trends Immunol. 2016, 37, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Shah, K.; Wincent, E. AHR in the intestinal microenvironment: Safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Cheng, Y.; Park, H.; Davidson, L.A.; Callaway, E.S.; Chapkin, R.S.; Jayaraman, A.; Asante, A.; Allred, C.; Weaver, E.A.; et al. Short chain fatty acids enhance aryl hydrocarbon (Ah) responsiveness in mouse colonocytes and Caco-2 human colon cancer cells. Sci. Rep. 2017, 7, 10163. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef]
- Royer, C.J.; Rodriguez-Marino, N.; Yaceczko, M.D.; Rivera-Rodriguez, D.E.; Ziegler, T.R.; Cervantes-Barragan, L. Low dietary fiber intake impairs small intestinal Th17 and intraepithelial T cell development over generations. Cell Rep. 2023, 42, 113140. [Google Scholar] [CrossRef]
- Han, K.; Xie, F.; Animasahun, O.; Nenwani, M.; Kitamoto, S.; Kim, Y.; Phoo, M.T.; Xu, J.; Wuchu, F.; Omoloja, K.; et al. Inulin-gel-based oral immunotherapy remodels the small intestinal microbiome and suppresses food allergy. Nat. Mater. 2024, 23, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Schnupf, P.; Gaboriau-Routhiau, V.; Cerf-Bensussan, N. Host interactions with Segmented Filamentous Bacteria: An unusual trade-off that drives the post-natal maturation of the gut immune system. Semin. Immunol. 2013, 25, 342–351. [Google Scholar] [CrossRef]
- Rodriguez-Marino, N.; Royer, C.J.; Rivera-Rodriguez, D.E.; Seto, E.; Gracien, I.; Jones, R.M.; Scharer, C.D.; Gracz, A.D.; Cervantes-Barragan, L. Dietary fiber promotes antigen presentation on intestinal epithelial cells and development of small intestinal CD4+CD8αα+ intraepithelial T cells. Mucosal Immunol. 2024, 17, 1301–1313. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Yang, Y.; Torchinsky, M.B.; Gobert, M.; Xiong, H.; Xu, M.; Linehan, J.L.; Alonzo, F.; Ng, C.; Chen, A.; Lin, X.; et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 2014, 510, 152–156. [Google Scholar] [CrossRef]
- Schnupf, P.; Gaboriau-Routhiau, V.; Sansonetti, P.J.; Cerf-Bensussan, N. Segmented filamentous bacteria, Th17 inducers and helpers in a hostile world. Curr. Opin. Microbiol. 2017, 35, 100–109. [Google Scholar] [CrossRef]
- Lochner, M.; Wang, A.; Sparwasser, T. The special relationship in the development and function of T helper 17 and regulatory T cells. Prog. Mol. Biol. Transl. Sci. 2015, 136, 99–129. [Google Scholar]
- Ohnmacht, C. Tolerance to the intestinal microbiota mediated by ROR(γt)+ cells. Trends Immunol. 2016, 37, 477–486. [Google Scholar] [CrossRef]
- Xiao, S.; Jin, H.; Korn, T.; Liu, S.M.; Oukka, M.; Lim, B.; Kuchroo, V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008, 181, 2277–2284. [Google Scholar] [CrossRef]
- Mucida, D.; Husain, M.M.; Muroi, S.; van Wijk, F.; Shinnakasu, R.; Naoe, Y.; Reis, B.S.; Huang, Y.; Lambolez, F.; Docherty, M.; et al. Transcriptional reprogramming of mature CD4⁺ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 2013, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Rogoz, A.; Costa-Pinto, F.A.; Taniuchi, I.; Mucida, D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4⁺ T cell immunity. Nat. Immunol. 2013, 14, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Hoytema van Konijnenburg, D.P.; Grivennikov, S.I.; Mucida, D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity 2014, 41, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Sujino, T.; London, M.; Hoytema van Konijnenburg, D.P.; Rendon, T.; Buch, T.; Silva, H.M.; Lafaille, J.J.; Reis, B.S.; Mucida, D. Tissue adaptation of regulatory and intraepithelial CD4⁺ T cells controls gut inflammation. Science 2016, 352, 1581–1586. [Google Scholar] [CrossRef]
- Bilate, A.M.; Bousbaine, D.; Mesin, L.; Agudelo, M.; Leube, J.; Kratzert, A.; Dougan, S.K.; Victora, G.D.; Ploegh, H.L. Tissue-specific emergence of regulatory and intraepithelial T cells from a clonal T cell precursor. Sci. Immunol. 2016, 1, eaaf7471. [Google Scholar] [CrossRef] [PubMed]
- Bousbaine, D.; Fisch, L.I.; London, M.; Bhagchandani, P.; Rezende de Castro, T.B.; Mimee, M.; Olesen, S.; Reis, B.S.; VanInsberghe, D.; Bortolatto, J.; et al. A conserved Bacteroidetes antigen induces anti-inflammatory intestinal T lymphocytes. Science 2022, 377, 660–666. [Google Scholar] [CrossRef]
- Bilate, A.M.; London, M.; Castro, T.B.R.; Mesin, L.; Bortolatto, J.; Kongthong, S.; Harnagel, A.; Victora, G.D.; Mucida, D. T Cell receptor is required for differentiation, but not maintenance, of intestinal CD4+ intraepithelial lymphocytes. Immunity 2020, 53, 1001–1014.e20. [Google Scholar] [CrossRef]
- Dobeš, J.; Brabec, T. Dietary influence and immune balance: Regulating CD4+ IEL responses and MHCII in the gut. Mucosal Immunol. 2025, 18, 36–38. [Google Scholar] [CrossRef]
- Brabec, T.; Schwarzer, M.; Kováčová, K.; Dobešová, M.; Schierová, D.; Březina, J.; Pacáková, I.; Šrůtková, D.; Ben-Nun, O.; Goldfarb, Y.; et al. Segmented filamentous bacteria-induced epithelial MHCII regulates cognate CD4+ IELs and epithelial turnover. J. Exp. Med. 2024, 221, e20230194. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, H.; Hugerth, L.W.; Sundh, J.; Lundin, E.; Andersson, A.F. Genome sequence of segmented filamentous bacteria present in the human intestine. Commun. Biol. 2020, 3, 485. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, H.; Shu, X.; Yin, Y.; Li, J.; Qin, J.; Chen, L.; Peng, K.; Xu, F.; Gu, W.; et al. Presence of Segmented Filamentous Bacteria in human children and its potential role in the modulation of human gut immunity. Front. Microbiol. 2018, 9, 1403. [Google Scholar] [CrossRef]
- Tan, T.G.; Sefik, E.; Geva-Zatorsky, N.; Kua, L.; Naskar, D.; Teng, F.; Pasman, L.; Ortiz-Lopez, A.; Jupp, R.; Wu, H.J.; et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E8141–E8150. [Google Scholar] [CrossRef]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, X.; Guo, L.; Ren, M.; Kang, W.; Ma, C.; Waterhouse, G.I.N.; Sun-Waterhouse, D. The potential of dietary fiber in building immunity against gastrointestinal and respiratory disorders. Crit. Rev. Food Sci. Nutr. 2024, 64, 13318–13336. [Google Scholar] [CrossRef]
- Rainer, H.; Goretzki, A.; Lin, Y.J.; Schiller, H.R.; Krause, M.; Döring, S.; Strecker, D.; Junker, A.C.; Wolfheimer, S.; Toda, M.; et al. Characterization of the immune-modulating properties of different β-glucans on myeloid dendritic cells. Int. J. Mol. Sci. 2024, 25, 9914. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Sahasrabudhe, N.M.; Rösch, C.; Schols, H.A.; Faas, M.M.; de Vos, P. The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells. Mol. Nutr. Food Res. 2015, 59, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Kanjan, P.; Sahasrabudhe, N.M.; de Haan, B.J.; de Vos, P. Immune effects of β-glucan are determined by combined effects on Dectin-1, TLR2, 4 and 5. J. Funct. Foods 2017, 37, 433–440. [Google Scholar] [CrossRef]
- Angelina, A.; Martín-Cruz, L.; de la Rocha-Muñoz, A.; Lavín-Plaza, B.; Palomares, O. C-Type lectin receptor mediated modulation of T2 immune responses to allergens. Curr. Allergy Asthma Rep. 2023, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Wenger, M.; Grosse-Kathoefer, S.; Kraiem, A.; Pelamatti, E.; Nunes, N.; Pointner, L.; Aglas, L. When the allergy alarm bells toll: The role of Toll-like receptors in allergic diseases and treatment. Front. Mol. Biosci. 2023, 10, 1204025. [Google Scholar] [CrossRef]
- Vogt, L.M.; Sahasrabudhe, N.M.; Ramasamy, U.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Schols, H.A.; de Vos, P. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J. Funct. Foods 2016, 22, 398–407. [Google Scholar] [CrossRef]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef]
- Wongkrasant, P.; Pongkorpsakol, P.; Ariyadamrongkwan, J.; Meesomboon, R.; Satitsri, S.; Pichyangkura, R.; Barrett, K.E.; Muanprasat, C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed. Pharmacother. 2020, 129, 110415. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary fiber pectin directly blocks Toll-like receptor 2-1 and prevents doxorubicin-induced ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef]
- Mavrogeni, M.E.; Asadpoor, M.; Henricks, P.A.J.; Keshavarzian, A.; Folkerts, G.; Braber, S. Direct action of non-digestible oligosaccharides against a leaky gut. Nutrients 2022, 14, 4699. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 2018, 154, 1037–1046.e2. [Google Scholar] [CrossRef]
- Hino, S.; Takemura, N.; Sonoyama, K.; Morita, A.; Kawagishi, H.; Aoe, S.; Morita, T. Small intestinal goblet cell proliferation induced by ingestion of soluble and insoluble dietary fiber is characterized by an increase in sialylated mucins in rats. J. Nutr. 2012, 142, 1429–1436. [Google Scholar] [CrossRef]
- Corrêa, R.O.; Castro, P.R.; Fachi, J.L.; Nirello, V.D.; El-Sahhar, S.; Imada, S.; Pereira, G.V.; Pral, L.P.; Araújo, N.V.P.; Fernandes, M.F.; et al. Inulin diet uncovers complex diet-microbiota-immune cell interactions remodeling the gut epithelium. Microbiome 2023, 11, 90. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Next-generation functions and questions. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef]
- Urashima, T.; Sato, S.; Nio-Kobayashi, J.; Hirabayashi, J. Why does breast milk contain a large amount of “galectin stripper”, milk oligosaccharides? What is their mysterious function? Glycoforum 2021, 24, A13. [Google Scholar]
- Cagnoni, A.J.; Massaro, M.; Cutine, A.M.; Gimeno, A.; Pérez-Sáez, J.M.; Manselle Cocco, M.N.; Maller, S.M.; Di Lella, S.; Jiménez-Barbero, J.; Ardá, A.; et al. Exploring galectin interactions with human milk oligosaccharides and blood group antigens identifies BGA6 as a functional galectin-4 ligand. J. Biol. Chem. 2024, 300, 107573. [Google Scholar] [CrossRef]
- Schouten, B.; van Esch, B.C.; Hofman, G.A.; de Kivit, S.; Boon, L.; Knippels, L.M.; Garssen, J.; Willemsen, L.E. A potential role for CD25+ regulatory T-cells in the protection against casein allergy by dietary non-digestible carbohydrates. Br. J. Nutr. 2012, 107, 96–105. [Google Scholar] [CrossRef]
- de Kivit, S.; Saeland, E.; Kraneveld, A.D.; van de Kant, H.J.; Schouten, B.; van Esch, B.C.; Knol, J.; Sprikkelman, A.B.; van der Aa, L.B.; Knippels, L.M.; et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy 2012, 67, 343–352. [Google Scholar] [CrossRef]
- de Kivit, S.; Kostadinova, A.I.; Kerperien, J.; Ayechu Muruzabal, V.; Morgan, M.E.; Knippels, L.M.J.; Kraneveld, A.D.; Garssen, J.; Willemsen, L.E.M. Galectin-9 produced by intestinal epithelial cells enhances aldehyde dehydrogenase activity in dendritic cells in a PI3K- and p38-dependent manner. J. Innate Immun. 2017, 9, 609–620. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Wang, X.; Zhang, S.; Yao, W.; Liu, J.; Liu, Z.; Yang, P. Galectin-9 in synergy with NF-κB inhibition restores immune regulatory capability in dendritic cells of subjects with food allergy. Clin. Exp. Immunol. 2023, 213, 155–163. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Song, S.; Feng, S.; Feng, X.; Liu, Y.; Yang, P.; Zheng, P. Galectin 9 rescues the inducibility of IL-10 expression in regulatory B cells of patients with food allergy. Sci. Rep. 2025, 15, 196. [Google Scholar] [CrossRef]
- De Juan, A.; Segura, E. Modulation of immune responses by nutritional ligands of aryl hydrocarbon receptor. Front. Immunol. 2021, 12, 645168. [Google Scholar] [CrossRef]
- Kemter, A.M.; Patry, R.T.; Arnold, J.; Hesser, L.A.; Campbell, E.; Ionescu, E.; Mimee, M.; Wang, S.; Nagler, C.R. Commensal bacteria signal through TLR5 and AhR to improve barrier integrity and prevent allergic responses to food. Cell Rep. 2023, 42, 113153. [Google Scholar] [CrossRef] [PubMed]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef]
- Quintana, F.J.; Murugaiyan, G.; Farez, M.F.; Mitsdoerffer, M.; Tukpah, A.M.; Burns, E.J.; Weiner, H.L. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2010, 107, 20768–20773. [Google Scholar] [CrossRef]
- Onodera, T.; Jang, M.H.; Guo, Z.; Yamasaki, M.; Hirata, T.; Bai, Z.; Tsuji, N.M.; Nagakubo, D.; Yoshie, O.; Sakaguchi, S.; et al. Constitutive expression of IDO by dendritic cells of mesenteric lymph nodes: Functional involvement of the CTLA-4/B7 and CCL22/CCR4 interactions. J. Immunol. 2009, 183, 5608–5614. [Google Scholar] [CrossRef]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef]
- Goettel, J.A.; Gandhi, R.; Kenison, J.E.; Yeste, A.; Murugaiyan, G.; Sambanthamoorthy, S.; Griffith, A.E.; Patel, B.; Shouval, D.S.; Weiner, H.L.; et al. AHR activation is protective against colitis driven by t cells in humanized mice. Cell Rep. 2016, 17, 1318–1329. [Google Scholar] [CrossRef]
- Apetoh, L.; Quintana, F.J.; Pot, C.; Joller, N.; Xiao, S.; Kumar, D.; Burns, E.J.; Sherr, D.H.; Weiner, H.L.; Kuchroo, V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010, 11, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Qiu, J.; Bostick, J.W.; Ueda, A.; Schjerven, H.; Li, S.; Jobin, C.; Chen, Z.E.; Zhou, L. The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep. 2017, 21, 2277–2290. [Google Scholar] [CrossRef]
- Rankin, L.C.; Kaiser, K.A.; de Los Santos-Alexis, K.; Park, H.; Uhlemann, A.C.; Gray, D.H.D.; Arpaia, N. Dietary tryptophan deficiency promotes gut RORγt+ Treg cells at the expense of Gata3+ Treg cells and alters commensal microbiota metabolism. Cell Rep. 2023, 42, 112135. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Sujino, T.; Miyamoto, K.; Harada, Y.; Tanemoto, S.; Ono, K.; Umeda, S.; Yoshida, K.; Teratani, T.; Suzuki, T.; et al. Aryl hydrocarbon receptor signals in epithelial cells govern the recruitment and location of Helios+ Tregs in the gut. Cell Rep. 2022, 39, 110773. [Google Scholar] [CrossRef]
- Li, Y.; Innocentin, S.; Withers, D.R.; Roberts, N.A.; Gallagher, A.R.; Grigorieva, E.F.; Wilhelm, C.; Veldhoen, M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 2011, 147, 629–640. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef]
- Hammerschmidt-Kamper, C.; Biljes, D.; Merches, K.; Steiner, I.; Daldrup, T.; Bol-Schoenmakers, M.; Pieters, R.H.H.; Esser, C. Indole-3-carbinol, a plant nutrient and AhR-Ligand precursor, supports oral tolerance against OVA and improves peanut allergy symptoms in mice. PLoS ONE 2017, 12, e0180321. [Google Scholar] [CrossRef]
- Schulz, V.J.; Smit, J.J.; Willemsen, K.J.; Fiechter, D.; Hassing, I.; Bleumink, R.; Boon, L.; van den Berg, M.; van Duursen, M.B.; Pieters, R.H. Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model. Toxicol. Sci. 2011, 123, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Kuziel, G.A.; Martinez-Blanco, M.; Jugder, B.E.; Benamar, M.; Wang, Z.; Chen, Q.; Lozano, G.L.; Abdel-Gadir, A.; Cui, Y.; et al. RELMβ sets the threshold for microbiome-dependent oral tolerance. Nature 2025, 638, 760–768. [Google Scholar] [CrossRef]
- Duarte, J.H.; Di Meglio, P.; Hirota, K.; Ahlfors, H.; Stockinger, B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS ONE 2013, 8, e79819. [Google Scholar] [CrossRef] [PubMed]
- Utsch, L.; Folisi, C.; Akkerdaas, J.H.; Logiantara, A.; van de Pol, M.A.; van der Zee, J.S.; Krop, E.J.; Lutter, R.; van Ree, R.; van Rijt, L.S. Allergic sensitization is associated with inadequate antioxidant responses in mice and men. Allergy 2015, 70, 1246–1258. [Google Scholar] [CrossRef]
- Gref, A.; Rautiainen, S.; Gruzieva, O.; Håkansson, N.; Kull, I.; Pershagen, G.; Wickman, M.; Wolk, A.; Melén, E.; Bergström, A. Dietary total antioxidant capacity in early school age and subsequent allergic disease. Clin. Exp. Allergy 2017, 47, 751–759. [Google Scholar] [CrossRef]
- Masilamani, M.; Wei, J.; Bhatt, S.; Paul, M.; Yakir, S.; Sampson, H.A. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J. Allergy Clin. Immunol. 2011, 128, 1242–1250.e1. [Google Scholar] [CrossRef]
- Chang, L.M.; Song, Y.; Li, X.M.; Sampson, H.A.; Masilamani, M. Dietary elimination of soybean components enhances allergic immune response to peanuts in BALB/c mice. Int. Arch. Allergy Immunol. 2015, 166, 304–310. [Google Scholar] [CrossRef]
- Dębińska, A.; Sozańska, B. Dietary polyphenols-natural bioactive compounds with potential for preventing and treating some allergic conditions. Nutrients 2023, 15, 4823. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Y.; He, S.; Wu, Z.; Jin, R. Food allergenic protein conjugation with plant polyphenols for allergenicity reduction. Curr. Opin. Food Sci. 2022, 43, 36–42. [Google Scholar] [CrossRef]
- Abril-Gil, M.; Massot-Cladera, M.; Pérez-Cano, F.J.; Castellote, C.; Franch, A.; Castell, M. A diet enriched with cocoa prevents IgE synthesis in a rat allergy model. Pharmacol. Res. 2012, 65, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Demont, A.; Actis-Goretta, L.; Holvoet, S.; Lévêques, A.; Lepage, M.; Nutten, S.; Mercenier, A. Identification of epicatechin as one of the key bioactive constituents of polyphenol-enriched extracts that demonstrate an anti-allergic effect in a murine model of food allergy. Br. J. Nutr. 2014, 112, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Liu, Q.M.; Gao, Y.Y.; Liu, B.; Liu, H.; Cao, M.J.; Yang, X.W.; Liu, G.M. Attenuation of allergic responses following treatment with resveratrol in anaphylactic models and IgE-mediated mast cells. Food Funct. 2019, 10, 2030–2039. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Liu, Q.M.; Liu, B.; Shu, Z.D.; Han, J.; Liu, H.; Cao, M.J.; Yang, X.W.; Gu, W.; Liu, G.M. Dihydromyricetin inhibited ovalbumin-induced mice allergic responses by suppressing the activation of mast cells. Food Funct. 2019, 10, 7131–7141. [Google Scholar] [CrossRef]
- Mine, Y.; Majumder, K.; Jin, Y.; Zeng, Y. Chinese sweet tea (Rubus suavissimus) polyphenols attenuate the allergic responses in a Balb/c mouse model of egg allergy. J. Funct. Foods 2020, 67, 103827. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, X.; Tian, L.; Li, L. Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice. Food Sci. Hum. Wellness 2023, 12, 882–888. [Google Scholar] [CrossRef]
- Li, Y.; Prabhakaran, P.; Wang, Y.; Li, D.; Sun-Waterhouse, D.; Li, F. Insights into food allergy mechanisms and the anti-allergic potential of natural polyphenols in regulating FcεRI-mediated mast cell degranulation. Food Chem. 2025, 490, 145099. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Je, I.G.; Song, J.; Fei, X.; Lee, S.; Yang, H.; Kang, W.; Jang, Y.H.; Seo, S.Y.; Kim, S.H. SG-SP1 suppresses mast cell-mediated allergic inflammation via inhibition of FcεRI signaling. Front. Immunol. 2020, 11, 50. [Google Scholar] [CrossRef]
- Crozier, R.W.E.; Yousef, M.; Coish, J.M.; Fajardo, V.A.; Tsiani, E.; MacNeil, A.J. Carnosic acid inhibits secretion of allergic inflammatory mediators in IgE-activated mast cells via direct regulation of Syk activation. J. Biol. Chem. 2023, 299, 102867. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Araumi, S.; Ando, D.; Ito, N.; Ando, M.; Ikeda, Y.; Takahashi, M.; Noguchi, S.; Yasuda, Y.; Nakano, N.; et al. Kaempferol suppresses the activation of mast cells by modulating the expression of FcεRI and SHIP1. Int. J. Mol. Sci. 2023, 24, 5997. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Jin, M.; Choi, Y.A.; Choi, J.K.; Kwon, T.K.; Khang, D.; Kim, S.H. Aspalathin, a primary rooibos flavonoid, alleviates mast cell-mediated allergic inflammation by the inhibition of FcεRI signaling pathway. Inflammation 2025, 48, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Kang, M.J.; Kang, C.W.; Kim, G.D. Kaempferol-3-O-β-rutinoside suppresses the inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. Int. J. Mol. Med. 2019, 44, 2321–2328. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Hu, S.; Ding, Y.; Jia, Q.; Zhu, J.; An, H. Kaempferol ameliorates secretagogue-induced pseudo-allergic reactions via inhibiting intracellular calcium fluctuation. J. Pharm. Pharmacol. 2020, 72, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological properties of polyphenols: Bioavailability, mechanisms of action, and biological effects in in vitro studies, animal models, and humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef]
- Kuziel, G.A.; Lozano, G.L.; Simian, C.; Li, L.; Manion, J.; Stephen-Victor, E.; Chatila, T.; Dong, M.; Weng, J.K.; Rakoff-Nahoum, S. Functional diversification of dietary plant small molecules by the gut microbiome. Cell 2025, 188, 1967–1983.e22. [Google Scholar] [CrossRef] [PubMed]
- Sonner, J.K.; Keil, M.; Falk-Paulsen, M.; Mishra, N.; Rehman, A.; Kramer, M.; Deumelandt, K.; Röwe, J.; Sanghvi, K.; Wolf, L.; et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat. Commun. 2019, 10, 4877. [Google Scholar] [CrossRef]
- Schanz, O.; Chijiiwa, R.; Cengiz, S.C.; Majlesain, Y.; Weighardt, H.; Takeyama, H.; Förster, I. Dietary AhR ligands regulate AhRR expression in intestinal immune cells and intestinal microbiota composition. Int. J. Mol. Sci. 2020, 21, 3189. [Google Scholar] [CrossRef]
- Pei, T.; Li, W.; Zhou, Z.; Zhang, Q.; Yu, G.; Yin, S.; Chen, H.; Tang, J. The relationship between tryptophan metabolism and gut microbiota: Interaction mechanism and potential effects in infection treatment. Microbiol. Res. 2025, 298, 128211. [Google Scholar] [CrossRef]
- Kim, K.S.; Hong, S.W.; Han, D.; Yi, J.; Jung, J.; Yang, B.G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef]
- Hong, S.W.; Krueger, P.D.; Osum, K.C.; Dileepan, T.; Herman, A.; Mueller, D.L.; Jenkins, M.K. Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction. Nature 2022, 607, 762–768. [Google Scholar] [CrossRef]
- He, K.; Wan, T.; Wang, D.; Hu, J.; Zhou, T.; Tao, W.; Wei, Z.; Lu, Q.; Zhou, R.; Tian, Z.; et al. Gasdermin D licenses MHCII induction to maintain food tolerance in small intestine. Cell 2023, 186, 3033–3048.e20. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Martínez-Blanco, M.; Pérez-Rodríguez, L.; Molina, E.; López-Fandiño, R. Oral immunotherapy with egg peptides induces innate and adaptive tolerogenic responses. Mol. Nutr. Food Res. 2019, 63, e1900144. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Mártinez-Blanco, M.; Pérez-Rodríguez, L.; Molina, E.; Peláez, C.; Requena, T.; López-Fandiño, R. Egg white peptide-based immunotherapy enhances vitamin A metabolism and induces RORγt+ regulatory T cells. J. Funct. Foods 2019, 52, 204–211. [Google Scholar] [CrossRef]
- Martínez-Blanco, M.; Pérez-Rodríguez, L.; Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Ovalbumin-derived peptides activate retinoic acid signalling pathways and induce regulatory responses through Toll-like receptor interactions. Nutrients 2020, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Moreira, T.G.; Cox, L.M.; Da Silva, P.; Mangani, D.; De Oliveira, M.G.; Escobar, G.; Lanser, T.B.; Murphy, L.; Lobo, E.L.C.; Milstein, O.; et al. Dietary protein modulates intestinal dendritic cells to establish mucosal homeostasis. Mucosal Immunol. 2024, 17, 911–922. [Google Scholar] [CrossRef]
- Lee, M.; Ko, H.J.; Hong, S.W.; Park, J.; Ham, S.; Kim, M.; Kwon, D.I.; Lee, M.S.; Roh, T.Y.; Soon Kim, K.; et al. Dietary antigens suppress the proliferation of type 2 innate lymphoid cells by restraining homeostatic IL-25 production. Sci. Rep. 2022, 12, 7443. [Google Scholar] [CrossRef]
- Lockhart, A.; Mucida, D.; Bilate, A.M. Intraepithelial lymphocytes of the intestine. Annu. Rev. Immunol. 2024, 42, 289–316. [Google Scholar] [CrossRef]
- Cheroutre, H.; Lambolez, F. Doubting the TCR coreceptor function of CD8αα. Immunity 2008, 28, 149–159. [Google Scholar] [CrossRef]
- Yang, T.; Li, T.; Xing, Y.; Cao, M.; Zhang, M.; Leng, Q.; Qiu, J.; Song, X.; Chen, J.; Hu, G.; et al. Dietary nucleic acids promote oral tolerance through innate sensing pathways in mice. Nat. Commun. 2024, 15, 9461. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability-a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- López-Fandiño, R. Role of dietary lipids in food allergy. Crit. Rev. Food Sci. Nutr. 2020, 60, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-dependent innate immune reprogramming. Cell 2018, 172, 162–175.e14. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest Liver Physiol. 2015, 308, G840–G851. [Google Scholar] [CrossRef]
- Patterson, E.; O’ Doherty, R.M.; Murphy, E.F.; Wall, R.; O’ Sullivan, O.; Nilaweera, K.; Fitzgerald, G.F.; Cotter, P.D.; Ross, R.P.; Stanton, C. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br. J. Nutr. 2014, 111, 1905–1917. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef]
- Caruso, R.; Chen, G.Y. High fat stems tumor immune surveillance. Cell Rep. Med. 2021, 2, 100483. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Bonilla-Rosso, G.; Chung, C.K.K.; Bäriswyl, L.; Rodriguez, M.P.; Kim, B.S.; Engel, P.; Noti, M. High dietary fat intake induces a microbiota signature that promotes food allergy. J. Allergy Clin. Immunol. 2019, 144, 157–170.e8. [Google Scholar] [CrossRef]
- Odaka, Y.; Nakano, M.; Tanaka, T.; Kaburagi, T.; Yoshino, H.; Sato-Mito, N.; Sato, K. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity 2010, 18, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 2013, 191, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef]
- Samuchiwal, S.K.; Boyce, J.A. Role of lipid mediators and control of lymphocyte responses in type 2 immunopathology. J. Allergy Clin. Immunol. 2018, 141, 1182–1190. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Frankhouser, D.E.; Steck, S.; Sovic, M.G.; Belury, M.A.; Wang, Q.; Clinton, S.K.; Bundschuh, R.; Yan, P.S.; Yee, L.D. Dietary omega-3 fatty acid intake impacts peripheral blood DNA methylation -anti-inflammatory effects and individual variability in a pilot study. J. Nutr. Biochem. 2022, 99, 108839. [Google Scholar] [CrossRef]
- Tedeschi, S.K.; Bathon, J.M.; Giles, J.T.; Lin, T.C.; Yoshida, K.; Solomon, D.H. Relationship between fish consumption and disease activity in rheumatoid arthritis. Arthritis Care Res. 2018, 70, 327–332. [Google Scholar] [CrossRef]
- Kremmyda, L.S.; Vlachava, M.; Noakes, P.S.; Diaper, N.D.; Miles, E.A.; Calder, P.C. Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: A systematic review. Clin. Rev. Allergy Immunol. 2011, 41, 36–66. [Google Scholar] [CrossRef]
- Anandan, C.; Nurmatov, U.; Sheikh, A. Omega 3 and 6 oils for primary prevention of allergic disease: Systematic review and meta-analysis. Allergy 2009, 64, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Klemens, C.M.; Berman, D.R.; Mozurkewich, E.L. The effect of perinatal omega-3 fatty acid supplementation on inflammatory markers and allergic diseases: A systematic review. BJOG 2011, 118, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.W.; Makrides, M.; Collins, C.T. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst. Rev. 2015, 2015, CD010085. [Google Scholar] [CrossRef] [PubMed]
- Best, K.P.; Gold, M.; Kennedy, D.; Martin, J.; Makrides, M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Sinn, J.K.; Osborn, D.A. Polyunsaturated fatty acid supplementation in infancy for the prevention of allergy. Cochrane Database Syst. Rev. 2016, 2016, CD010112. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, B.; Li, J.; Luo, C.Q.; Zhang, Q.; Chen, J.L.; Sinha, A.; Li, Z.Y. Fish intake during pregnancy or infancy and allergic outcomes in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2017, 28, 152–161. [Google Scholar] [CrossRef]
- Vahdaninia, M.; Mackenzie, H.; Dean, T.; Helps, S. ω-3 LCPUFA supplementation during pregnancy and risk of allergic outcomes or sensitization in offspring: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2019, 122, 302–313.e2. [Google Scholar] [CrossRef]
- Wu, W.; Lin, L.; Shi, B.; Jing, J.; Cai, L. The effects of early life polyunsaturated fatty acids and ruminant trans fatty acids on allergic diseases: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 1802–1815. [Google Scholar] [CrossRef]
- Bärebring, L.; Nwaru, B.I.; Lamberg-Allardt, C.; Thorisdottir, B.; Ramel, A.; Söderlund, F.; Arnesen, E.K.; Dierkes, J.; Åkesson, A. Supplementation with long chain n-3 fatty acids during pregnancy, lactation, or infancy in relation to risk of asthma and atopic disease during childhood: A systematic review and meta-analysis of randomized controlled clinical trials. Food Nutr. Res. 2022, 66, 10–29219. [Google Scholar] [CrossRef]
- Huynh, L.B.P.; Nguyen, N.N.; Fan, H.Y.; Huang, S.Y.; Huang, C.H.; Chen, Y.C. Maternal omega-3 supplementation during pregnancy, but not childhood supplementation, reduces the risk of food allergy diseases in offspring. J. Allergy Clin. Immunol. Pract. 2023, 11, 2862–2871.e8. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Vassiliou, E.; Adhikary, S.; Toscano, M.G.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010, 9, 12. [Google Scholar] [CrossRef]
- Draper, E.; Reynolds, C.M.; Canavan, M.; Mills, K.H.; Loscher, C.E.; Roche, H.M. Omega-3 fatty acids attenuate dendritic cell function via NF-κB independent of PPARγ. J. Nutr. Biochem. 2011, 22, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef]
- Brix, S.; Lund, P.; Kjaer, T.M.; Straarup, E.M.; Hellgren, L.I.; Frøkiaer, H. CD4+ T-cell activation is differentially modulated by bacteria-primed dendritic cells, but is generally down-regulated by n-3 polyunsaturated fatty acids. Immunology 2010, 129, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; McMurray, D.N.; Chapkin, R.S. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur. J. Pharmacol. 2016, 785, 2–9. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.; Madsen, N.; Vliagoftis, H.; Field, C.; Cameron, L. n-3 Fatty acids inhibit transcription of human IL-13: Implications for development of T helper type 2 immune responses. Br. J. Nutr. 2013, 109, 990–1000. [Google Scholar] [CrossRef]
- van den Elsen, L.; van Esch, B.; Hofman, G.; van de Heijning, B.; Garssen, J.; Willemsen, L. Dietary long chain n-3 polyunsaturated fatty acid induced regulatory T-cells contribute to the prevention of oral sensitization to cow’s milk protein in mice. Clin. Transl. Allergy. 2013, 3 (Suppl. S3), O18. [Google Scholar] [CrossRef]
- van den Elsen, L.W.; Meulenbroek, L.A.; van Esch, B.C.; Hofman, G.A.; Boon, L.; Garssen, J.; Willemsen, L.E. CD25+ regulatory T cells transfer n-3 long chain polyunsaturated fatty acids-induced tolerance in mice allergic to cow’s milk protein. Allergy 2013, 68, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, L.W.; Bol-Schoenmakers, M.; van Esch, B.C.; Hofman, G.A.; van de Heijning, B.J.; Pieters, R.H.; Smit, J.J.; Garssen, J.; Willemsen, L.E. DHA-rich tuna oil effectively suppresses allergic symptoms in mice allergic to whey or peanut. J. Nutr. 2014, 144, 1970–1976. [Google Scholar] [CrossRef]
- Richard, C.; Lewis, E.D.; Goruk, S.; Field, C.J. A dietary supply of docosahexaenoic acid early in life is essential for immune development and the establishment of oral tolerance in female rat offspring. J. Nutr. 2016, 146, 2398–2406. [Google Scholar] [CrossRef]
- de Matos, O.G.; Amaral, S.S.; Pereira da Silva, P.E.; Perez, D.A.; Alvarenga, D.M.; Ferreira, A.V.; Alvarez-Leite, J.; Menezes, G.B.; Cara, D.C. Dietary supplementation with omega-3-PUFA-rich fish oil reduces signs of food allergy in ovalbumin-sensitized mice. Clin. Dev. Immunol. 2012, 2012, 236564. [Google Scholar] [CrossRef]
- Thang, C.L.; Boye, J.I.; Shi, H.N.; Zhao, X. Effects of supplementing different ratios of omega-3 and omega-6 fatty acids in western-style diets on cow’s milk protein allergy in a mouse model. Mol. Nutr. Food Res. 2013, 57, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Arita, M.; Hayasaka, T.; Harada, T.; Iwamoto, R.; Nagasawa, R.; Shikata, S.; Nagatake, T.; Suzuki, H.; Hashimoto, E.; et al. Dietary ω3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci. Rep. 2015, 5, 9750. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.; Hilt, K.; Milovanovic, M.; Ernst, D.; Rühl, R.; Worm, M. Inhibition of IgE production by docosahexaenoic acid is mediated by direct interference with STAT6 and NFκB pathway in human B cells. J. Nutr. Biochem. 2011, 22, 269–275. [Google Scholar] [CrossRef]
- Wang, X.; Ma, D.W.; Kang, J.X.; Kulka, M. n-3 Polyunsaturated fatty acids inhibit Fc ε receptor I-mediated mast cell activation. J. Nutr. Biochem. 2015, 26, 1580–1588. [Google Scholar] [CrossRef]
- Park, B.K.; Park, S.; Park, J.B.; Park, M.C.; Min, T.S.; Jin, M. Omega-3 fatty acids suppress Th2-associated cytokine gene expressions and GATA transcription factors in mast cells. J. Nutr. Biochem. 2013, 24, 868–876. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, L.W.; Nusse, Y.; Balvers, M.; Redegeld, F.A.; Knol, E.F.; Garssen, J.; Willemsen, L.E. n-3 Long-chain PUFA reduce allergy-related mediator release by human mast cells in vitro via inhibition of reactive oxygen species. Br. J. Nutr. 2013, 109, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).