A Systematic Review of Palmitate-Mediated Insulin Resistance in C2C12 Myotubes
Abstract
1. Introduction
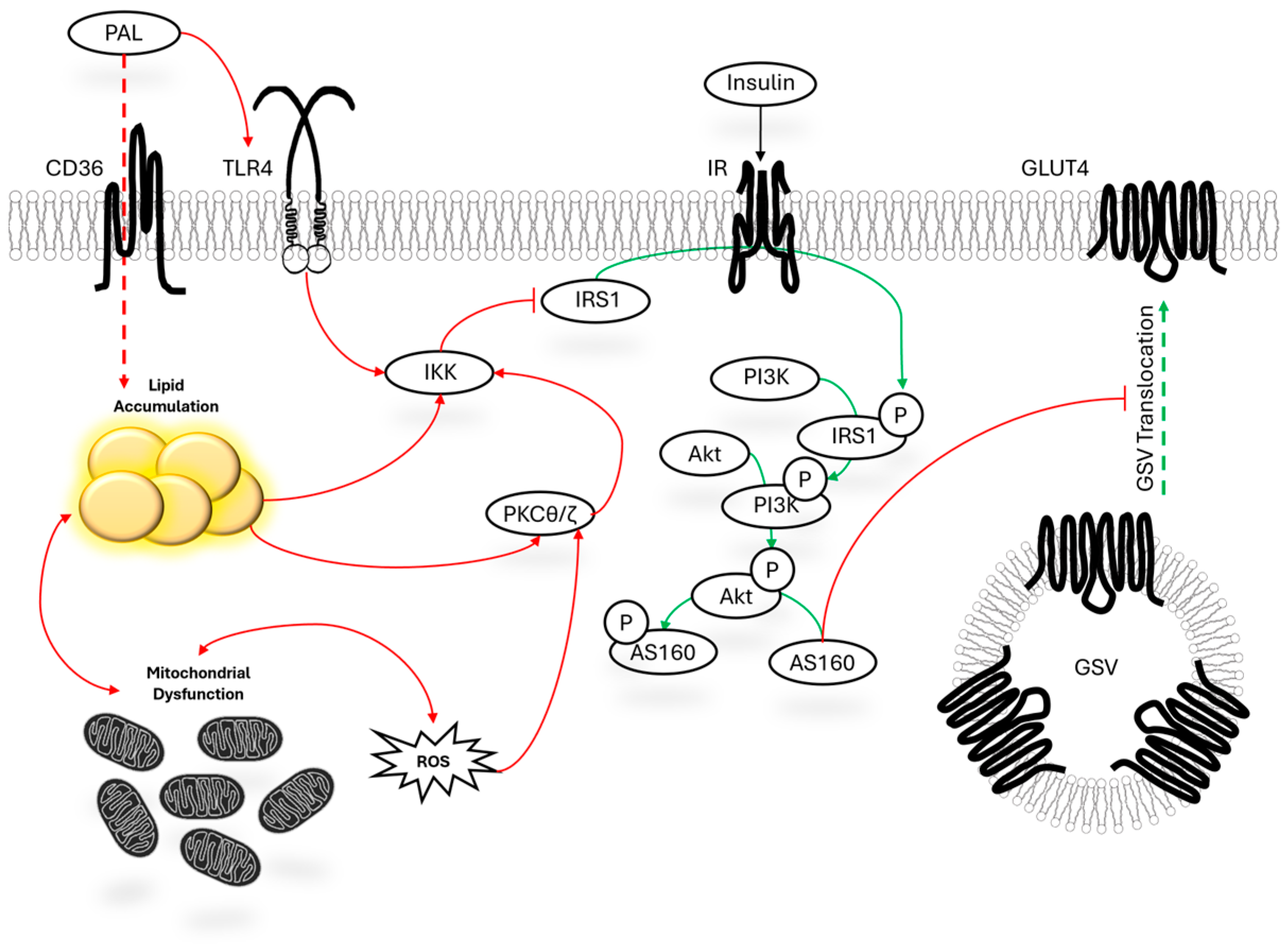
1.1. Mechanistic Overview of Palmitate-Mediated Insulin Resistance
1.2. Aims and Scope of Review
2. Materials and Methods
2.1. General Article Search Procedure and Eligibility Criteria
2.2. Secondary Article Screening and Inclusion Procedure
3. Results
3.1. Search Results
3.2. Organization of Data
3.3. Effect of Pharmacokinetically Attainable Levels of Palmitate on Insulin Signaling
3.4. Effect of Moderately Higher than Pharmacokinetically Attainable Levels of Palmitate on Insulin Signaling
3.5. Effect of “Proof-of-Concept” Levels of Palmitate on Insulin Signaling
4. Discussion
4.1. IRS
4.2. Akt
4.3. GLUT4
4.4. Glucose Uptake
4.5. Limitations and Considerations
4.6. Strengths and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | 3 dimensional |
| Glut4 | glucose transporter member 4 |
| GSV | glut4 storage vesicles |
| HG | high glucose |
| Iκκ | I kappa B kinase |
| IRS1 | insulin receptor substrate 1 (protein) |
| IR | insulin receptor (protein) |
| LG | low glucose |
| PI3K | phosphatidylinositol 3-Kinase (protein) |
| PAkt | phosphorylated Akt |
| PKC | protein kinase c |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SEM | standard error of the mean |
| TLR4 | toll-like receptor 4 |
| VC | visual confirmation |
References
- Yudhani, R.D.; Sari, Y.; Nugrahaningsih, D.A.A.; Sholikhah, E.N.; Rochmanti, M.; Purba, A.K.R.; Khotimah, H.; Nugrahenny, D.; Mustofa, M. Insulin Resistance Model: A Recent Update. J. Obes. 2023, 2023, 1964732. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Schmitz-Peiffer, C.; Craig, D.L.; Biden, T.J. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 1999, 274, 24202–24210. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, X.; He, Q.; Tadese, D.A.; Cao, K.; Gao, J.; Xu, Q.; Cheng, R.; Lu, Q.; Chen, Y.; et al. High-fat diet increases circulating palmitic acid produced by gut Bacteroides thetaiotaomicron to promote thrombosis. Cell Rep. Med. 2025, 6, 102260. [Google Scholar] [CrossRef] [PubMed]
- Normand-Lauzière, F.; Frisch, F.; Labbé, S.M.; Bherer, P.; Gagnon, R.; Cunnane, S.C.; Carpentier, A.C. Increased postprandial nonesterified fatty acid appearance and oxidation in type 2 diabetes is not fully established in offspring of diabetic subjects. PLoS ONE 2010, 5, e10956. [Google Scholar] [CrossRef]
- de Wilde, J.; Smit, E.; Snepvangers, F.J.; de Wit, N.W.; Mohren, R.; Hulshof, M.F.; Mariman, E.C. Adipophilin protein expression in muscle—A possible protective role against insulin resistance. FEBS J. 2010, 277, 761–773. [Google Scholar] [CrossRef]
- Cheon, H.G.; Cho, Y.S. Protection of palmitic acid-mediated lipotoxicity by arachidonic acid via channeling of palmitic acid into triglycerides in C2C12. J. Biomed. Sci. 2014, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Han, I.S.; Hsieh, P.S.; Tsai, M.C.; Chien, H.C. Dichloroacetate, a pyruvate dehydrogenase activator, alleviates high-fat-induced impairment of myogenic differentiation in skeletal muscles. Basic Clin. Pharmacol. Toxicol. 2025, 136, e14102. [Google Scholar] [CrossRef]
- Chen, X.; Xu, S.; Wei, S.; Deng, Y.; Li, Y.; Yang, F.; Liu, P. Comparative Proteomic Study of Fatty Acid-treated Myoblasts Reveals Role of Cox-2 in Palmitate-induced Insulin Resistance. Sci. Rep. 2016, 6, 21454. [Google Scholar] [CrossRef]
- Chavez, J.A.; Holland, W.L.; Bär, J.; Sandhoff, K.; Summers, S.A. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 2005, 280, 20148–20153. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.J.; Choi, K.J.; Jung, W.H.; Park, S.B.; Koh, B.; Hoe, K.L.; Kim, K.Y. Development of 3D Muscle Cell Culture-Based Screening System for Metabolic Syndrome Drug Research. Tissue Eng. Part C Methods 2025, 31, 53–64. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, M.H.; Ahn, J.H.; Lee, S.J.; Lee, J.H.; Eum, W.S.; Choi, S.Y.; Kwon, H.Y. The Stimulatory Effect of Essential Fatty Acids on Glucose Uptake Involves Both Akt and AMPK Activation in C2C12 Skeletal Muscle Cells. Korean J. Physiol. Pharmacol. 2014, 18, 255–261. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, K.; Wu, Y.; Li, J.; Ma, J.; Wang, L.; Zhang, C.; Wei, Y.; Yang, Y. Lycium barbarum polysaccharide mitigates high-fat-diet-induced skeletal muscle atrophy by promoting AMPK/PINK1/Parkin-mediated mitophagy. Int. J. Biol. Macromol. 2025, 301, 140488. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, S.; Adulcikas, J.; Henstridge, D.C.; Sonda, S.; Sohal, S.S.; Myers, S. The Zinc Transporter Zip7 Is Downregulated in Skeletal Muscle of Insulin-Resistant Cells and in Mice Fed a High-Fat Diet. Cells 2019, 8, 663. [Google Scholar] [CrossRef]
- Pan, X.; Liu, C.; Wang, X.; Zhao, M.; Zhang, Z.; Zhang, X.; Wang, C.; Song, G. Resveratrol improves palmitic acid-induced insulin resistance via the DDIT4/mTOR pathway in C2C12 cells. Mol. Med. Rep. 2023, 28, 181. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Y.; Yuan, Q.; Gao, Y.; Wang, Y.; Wang, X.; Cui, X.; Xu, P.; Ji, C.; Guo, X.; et al. Dynamic transcriptome profile in db/db skeletal muscle reveal critical roles for long noncoding RNA regulator. Int. J. Biochem. Cell Biol. 2018, 104, 14–24. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Curi, R.; Maechler, P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J. Cell. Physiol. 2010, 222, 187–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.P.; Gao, Y.F.; Fan, Z.M.; Liu, M.Y.; Cai, X.Y.; Xia, Z.K.; Gao, C.L. Silencing miR-106b improves palmitic acid-induced mitochondrial dysfunction and insulin resistance in skeletal myocytes. Mol. Med. Rep. 2015, 11, 3834–3841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, X.T.; Wang, T.Z.; Leng, J.; Chen, Y.; Liu, J.B.; Liu, Y.; Wang, W.J. Palmitate contributes to insulin resistance through downregulation of the Src-mediated phosphorylation of Akt in C2C12 myotubes. Biosci. Biotechnol. Biochem. 2012, 76, 1356–1361. [Google Scholar] [CrossRef]
- Kopp, E.L.; Deussen, D.N.; Cuomo, R.; Lorenz, R.; Roth, D.M.; Mahata, S.K.; Patel, H.H. Modeling and Phenotyping Acute and Chronic Type 2 Diabetes Mellitus In Vitro in Rodent Heart and Skeletal Muscle Cells. Cells 2023, 12, 2786. [Google Scholar] [CrossRef]
- Mok, J.; Park, T.S.; Kim, S.; Kim, D.; Choi, C.S.; Park, J. Prokineticin receptor 1 ameliorates insulin resistance in skeletal muscle. FASEB J. 2021, 35, e21179. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-W.; Huang, W.-C.; Yu, W.-J.; Chang, S.-J. Toona sinensis ameliorates insulin resistance via AMPK and PPAR gamma pathways. Food Funct. 2015, 6, 1855–1864. [Google Scholar] [CrossRef]
- Kirk-Ballard, H.; Wang, Z.Q.; Acharya, P.; Zhang, X.H.; Yu, Y.; Kilroy, G.; Ribnicky, D.; Cefalu, W.T.; Floyd, Z.E. An extract of Artemisia dracunculus L. inhibits ubiquitin-proteasome activity and preserves skeletal muscle mass in a murine model of diabetes. PLoS ONE 2013, 8, e57112. [Google Scholar] [CrossRef]
- Gone, G.B.; Go, G.; Nam, G.; Jeong, W.; Kim, H.; Lee, S.; Chung, S.J. Exploring the Anti-Diabetic Potential of Quercetagitrin through Dual Inhibition of PTPN6 and PTPN9. Nutrients 2024, 16, 647. [Google Scholar] [CrossRef] [PubMed]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef]
- Parvaneh, L.; Meshkani, R.; Bakhtiyari, S.; Mohammadtaghvaie, N.; Gorganifiruzjaee, S.; Taheripak, G.; Golestani, A.; Foruzandeh, M.; Larijani, B.; Taghikhani, M. Palmitate and inflammatory state additively induce the expression of PTP1B in muscle cells. Biochem. Biophys. Res. Commun. 2010, 396, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, M.L.; Ono-Moore, K.D.; Sobhi, H.F.; Adams, S.H. Carnitine palmitoyltransferase 2 knockout potentiates palmitate-induced insulin resistance in C2C12. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E265–E275. [Google Scholar] [CrossRef]
- Tatebe, J.; Morita, T. Enhancement of TNF-α expression and inhibition of glucose uptake by nicotine in the presence of a free fatty acid in C2C12 skeletal myocytes. Horm. Metab. Res. 2011, 43, 11–16. [Google Scholar] [CrossRef]
- Gwon, H.J.; Cho, W.; Choi, S.W.; Lim, D.S.; Tanriverdi, E.; El-Aty, A.M.A.; Jeong, J.H.; Jung, T.W. Donepezil improves skeletal muscle insulin resistance in obese mice via the AMPK/FGF21-mediated suppression of inflammation and ferroptosis. Arch. Pharmacal Res. 2024, 47, 940–953. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, C.; Li, F.; Yan, Y.; Wu, Y.; Li, B.; Tong, H.; Lang, J. Fucoxanthin Mitigates High-Fat-Induced Lipid Deposition and Insulin Resistance in Skeletal Muscle through Inhibiting PKM1 Activity. J. Agric. Food Chem. 2024, 72, 18013–18026. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Kim, Y.J.; Cho, W.; Lim, D.S.; Gwon, H.J.; El-Aty, A.M.A.; Nas, M.A.; Jeong, J.H.; Jung, T.W. Interleukin 38 improves insulin resistance in hyperlipidemic skeletal muscle cells via PPARδ/SIRT1-mediated suppression of STAT3 signaling and oxidative stress. Biochem. Biophys. Res. Commun. 2024, 722, 150158. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yu, F.; Pan, Y.; Zhang, Z.; He, Y.; Yang, H.; Zhou, P. Proteoglycan Extracted from Ganoderma lucidum Ameliorated Diabetes-Induced Muscle Atrophy via the AMPK/SIRT1 Pathway In Vivo and In Vitro. ACS Omega 2023, 8, 30359–30373. [Google Scholar] [CrossRef]
- Oh, H.; Cho, W.; El-Aty, A.M.A.; Bayram, C.; Jeong, J.H.; Jung, T.W. Resolvin D3 improves the impairment of insulin signaling in skeletal muscle and nonalcoholic fatty liver disease through AMPK/autophagy-associated attenuation of ER stress. Biochem. Pharmacol. 2022, 203, 115203. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.; Park, S.Y.; Cho, W.; Oh, H.; Lee, H.J.; El-Aty, A.M.A.; Hacimuftuoglu, A.; Jeong, J.H. Stachydrine alleviates lipid-induced skeletal muscle insulin resistance via AMPK/HO-1-mediated suppression of inflammation and endoplasmic reticulum stress. J. Endocrinol. Investig. 2022, 45, 2181–2191. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.J.; Song, J.H.; Shin, Y.K.; El-Aty, A.M.A.; Ramadan, A.; Hacimuftuoglu, A.; Jeong, J.H.; Jung, T.W. Dimethyl itaconate attenuates palmitate-induced insulin resistance in skeletal muscle cells through the AMPK/FGF21/PPARδ-mediated suppression of inflammation. Life Sci. 2021, 287, 120129. [Google Scholar] [CrossRef]
- Pesta, D.; Jelenik, T.; Zaharia, O.P.; Bobrov, P.; Görgens, S.; Bódis, K.; Karusheva, Y.; Jakovljevic, N.K.; Lalic, N.M.; Markgraf, D.F.; et al. NDUFB6 Polymorphism Is Associated with Physical Activity-Mediated Metabolic Changes in Type 2 Diabetes. Front. Endocrinol. 2021, 12, 693683. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, H.J.; Pyun, D.H.; Kim, T.J.; Bang, J.S.; Song, J.H.; Shin, Y.K.; El-Aty, A.M.A.; Jeong, J.H. Capmatinib improves insulin sensitivity and inflammation in palmitate-treated C2C12 myocytes through the PPARδ/p38-dependent pathway. Mol. Cell Endocrinol. 2021, 534, 111364. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Lee, H.J.; Pyun, D.H.; El-Aty, A.M.A.; Jeong, J.H.; Jung, T.W. Valdecoxib improves lipid-induced skeletal muscle insulin resistance via simultaneous suppression of inflammation and endoplasmic reticulum stress. Biochem. Pharmacol. 2021, 188, 114557. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, L.; Li, X.; Wu, Y.; Yin, F.; Liu, J. Trilobatin ameliorates insulin resistance through IRS-AKT-GLUT4 signaling pathway in C2C12 myotubes and ob/ob mice. Chin. Med. 2020, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.J.; Lo, Y.H.; Yang, J.S.; Kuo, S.C.; Tsai, S.C. Curcumin Derivative MTH-3 Regulates Palmitate-induced Insulin Resistance in Mouse Myoblast C2C12 Cells. Vivo 2021, 35, 3181–3191. [Google Scholar] [CrossRef]
- Chuang, W.T.; Yen, C.C.; Huang, C.S.; Chen, H.W.; Lii, C.K. Benzyl Isothiocyanate Ameliorates High-Fat Diet-Induced Hyperglycemia by Enhancing Nrf2-Dependent Antioxidant Defense-Mediated IRS-1/AKT/TBC1D1 Signaling and GLUT4 Expression in Skeletal Muscle. J. Agric. Food Chem. 2020, 68, 15228–15238. [Google Scholar] [CrossRef]
- Park, J.; Jung, T.W.; Chung, Y.H.; Park, E.S.; Jeong, J.H. 1,2-Dilinoleoyl-sn-glycero-3-phosphocholine increases insulin sensitivity in palmitate-treated myotubes and induces lipolysis in adipocytes. Biochem. Biophys. Res. Commun. 2020, 533, 162–167. [Google Scholar] [CrossRef]
- Sun, J.L.; Park, J.; Lee, T.; Jeong, J.H.; Jung, T.W. DEL-1 ameliorates high-fat diet-induced insulin resistance in mouse skeletal muscle through SIRT1/SERCA2-mediated ER stress suppression. Biochem. Pharmacol. 2020, 171, 113730. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Lee, S.H.; Kim, H.C.; Bang, J.S.; El-Aty, A.M.A.; Hacımüftüoğlu, A.; Shin, Y.K.; Jeong, J.H. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kulabas, S.S.; Ipek, H.; Tufekci, A.R.; Arslan, S.; Demirtas, I.; Ekren, R.; Sezerman, U.; Tumer, T.B. Ameliorative potential of Lavandula stoechas in metabolic syndrome via multitarget interactions. J. Ethnopharmacol. 2018, 223, 88–98. [Google Scholar] [CrossRef]
- Yang, M.; Wei, D.; Mo, C.; Zhang, J.; Wang, X.; Han, X.; Wang, Z.; Xiao, H. Saturated fatty acid palmitate-induced insulin resistance is accompanied with myotube loss and the impaired expression of health benefit myokine genes in C2C12 myotubes. Lipids Health Dis. 2013, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Hwang, H.J.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Choi, K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARδ-dependent pathway in mice. Diabetologia 2015, 58, 2096–2105. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.C.; El-Aty, A.M.A.; Jeong, J.H. Protectin DX ameliorates palmitate- or high-fat diet-induced insulin resistance and inflammation through an AMPK-PPARα-dependent pathway in mice. Sci. Rep. 2017, 7, 1397. [Google Scholar] [CrossRef]
- Yang, C.; Aye, C.C.; Li, X.; Ramos, A.D.; Zorzano, A.; Mora, S. Mitochondrial dysfunction in insulin resistance: Differential contributions of chronic insulin and saturated fatty acid exposure in muscle cells. Biosci. Rep. 2012, 32, 465–478. [Google Scholar] [CrossRef]
- Pyun, D.H.; Kim, T.J.; Park, S.Y.; Lee, H.J.; El-Aty, A.M.A.; Jeong, J.H.; Jung, T.W. Patchouli alcohol ameliorates skeletal muscle insulin resistance and NAFLD via AMPK/SIRT1-mediated suppression of inflammation. Mol. Cell Endocrinol. 2021, 538, 111464. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Park, J.; Sun, J.L.; Ahn, S.H.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Kim, H.C.; Shim, J.H.; Shin, S.; Jeong, J.H. Administration of kynurenic acid reduces hyperlipidemia-induced inflammation and insulin resistance in skeletal muscle and adipocytes. Mol. Cell Endocrinol. 2020, 518, 110928. [Google Scholar] [CrossRef]
- de Figueiredo, A.S.P.; Salmon, A.B.; Bruno, F.; Jimenez, F.; Martinez, H.G.; Halade, G.V.; Ahuja, S.S.; Clark, R.A.; DeFronzo, R.A.; Abboud, H.E.; et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J. Biol. Chem. 2015, 290, 13427–13439. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Z.F.; Ding, Y.; Wang, J.B.; Li, Y. Astragalus polysaccharide improves palmitate-induced insulin resistance by inhibiting PTP1B and NF-κB in C2C12 myotubes. Molecules 2012, 17, 7083–7092. [Google Scholar] [CrossRef]
- Kim, J.; Son, J.; Ahn, D.; Nam, G.; Zhao, X.; Park, H.; Jeong, W.; Chung, S.J. Structure-Activity Relationship of Synthetic Ginkgolic Acid Analogs for Treating Type 2 Diabetes by PTPN9 Inhibition. Int. J. Mol. Sci. 2022, 23, 3927. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.M.; Beall, C. Extracellular ATP Increases Glucose Metabolism in Skeletal Muscle Cells in a P2 Receptor Dependent Manner but Does Not Contribute to Palmitate-Induced Insulin Resistance. Front. Physiol. 2020, 11, 567378. [Google Scholar] [CrossRef] [PubMed]
- Bosquet, A.; Girona, J.; Guaita-Esteruelas, S.; Heras, M.; Saavedra-García, P.; Martínez-Micaelo, N.; Masana, L.; Rodríguez-Calvo, R. FABP4 inhibitor BMS309403 decreases saturated-fatty-acid-induced endoplasmic reticulum stress-associated inflammation in skeletal muscle by reducing p38 MAPK activation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2018, 1863, 604–613. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.M.; Lee, S.; Lee, H.J.; Yang, H.S.; Yeo, J.; Lee, K.R.; Choi, B.H.; Hong, E.K. Hispidin rescues palmitate-induced insulin resistance in C2C12 myotubes. Mol. Med. Rep. 2017, 16, 4229–4234. [Google Scholar] [CrossRef][Green Version]
- Zhou, Q.; Du, J.; Hu, Z.; Walsh, K.; Wang, X.H. Evidence for adipose-muscle cross talk: Opposing regulation of muscle proteolysis by adiponectin and Fatty acids. Endocrinology 2007, 148, 5696–5705. [Google Scholar] [CrossRef]
- Hassan, R.H.; Hainault, I.; Vilquin, J.T.; Samama, C.; Lasnier, F.; Ferre, P.; Foufelle, F.; Hajduch, E. Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia 2012, 55, 204–214. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, W.; Li, D.; Guo, Y.; Ding, H. Overactivation of NF-κB impairs insulin sensitivity and mediates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Endocrine 2010, 37, 157–166. [Google Scholar] [CrossRef]
- Qin, H.; Liu, Y.; Lu, N.; Li, Y.; Sun, C.H. cis-9,trans-11-Conjugated linoleic acid activates AMP-activated protein kinase in attenuation of insulin resistance in C2C12 myotubes. J. Agric. Food Chem. 2009, 57, 4452–4458. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Liu, H.W.; Chen, Y.T.; Chen, Y.A.; Chen, Y.J.; Chang, S.J. Resveratrol protects muscle cells against palmitate-induced cellular senescence and insulin resistance through ameliorating autophagic flux. J. Food Drug Anal. 2018, 26, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, X.; Zhou, Y.; Gu, Y.; Ding, Y.; Luo, J.; Pang, N.; Sun, Y.; Pei, L.; Pan, J.; et al. Improving Mitochondrial Function in Skeletal Muscle Contributes to the Amelioration of Insulin Resistance by Nicotinamide Riboside. Int. J. Mol. Sci. 2023, 24, 10015. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, S.; Gong, C.; Chen, L. Neuregulin-1β increases glucose uptake and promotes GLUT4 translocation in palmitate-treated C2C12 myotubes by activating PI3K/AKT signaling pathway. Front. Pharmacol. 2023, 13, 1066279. [Google Scholar] [CrossRef]
- Jiao, Y.; Williams, A.; Wei, N. Quercetin ameliorated insulin resistance via regulating METTL3-mediated N6-methyladenosine modification of PRKD2 mRNA in skeletal muscle and C2C12 myocyte cell line. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2655–2668. [Google Scholar] [CrossRef]
- He, Q.; Chen, B.; Wang, G.; Zhou, D.; Zeng, H.; Li, X.; Song, Y.; Yu, X.; Liang, W.; Chen, H.; et al. Co-Crystal of Rosiglitazone With Berberine Ameliorates Hyperglycemia and Insulin Resistance Through the PI3K/AKT/TXNIP Pathway. Front. Pharmacol. 2022, 13, 842879. [Google Scholar] [CrossRef]
- Hu, M.M.; Zheng, W.Y.; Cheng, M.H.; Song, Z.Y.; Shaukat, H.; Atta, M.; Qin, H. Sesamol Reverses Myofiber-Type Conversion in Obese States via Activating the SIRT1/AMPK Signal Pathway. J. Agric. Food Chem. 2022, 70, 2253–2264. [Google Scholar] [CrossRef]
- Nan, J.; Lee, J.S.; Lee, S.A.; Lee, D.S.; Park, K.S.; Chung, S.S. An Essential Role of the N-Terminal Region of ACSL1 in Linking Free Fatty Acids to Mitochondrial β-Oxidation in C2C12 Myotubes. Mol. Cells 2021, 44, 637–646. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Zhang, T.; Pan, R.; Lin, L. Myricanol modulates skeletal muscle-adipose tissue crosstalk to alleviate high-fat diet-induced obesity and insulin resistance. Br. J. Pharmacol. 2019, 176, 3983–4001. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, X.; Yuan, H.; Guan, H.; Li, Y.; Niu, Y. Mangiferin Improved Palmitate-Induced-Insulin Resistance by Promoting Free Fatty Acid Metabolism in HepG2 and C2C12 Cells via PPARα: Mangiferin Improved Insulin Resistance. J. Diabetes Res. 2019, 2019, 2052675. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, S.E.; Lee, H.B.; Song, M.W.; Kim, Y.H.; Jeong, J.Y.; Kang, Y.; Kim, H.J.; Kim, T.H.; Jeon, J.Y.; et al. A Class I Histone Deacetylase Inhibitor Attenuates Insulin Resistance and Inflammation in Palmitate-Treated C2C12 Myotubes and Muscle of HF/HFr Diet Mice. Front. Pharmacol. 2020, 11, 601448. [Google Scholar] [CrossRef]
- Chien, H.C.; Greenhaff, P.L.; Constantin-Teodosiu, D. PPARδ and FOXO1 Mediate Palmitate-Induced Inhibition of Muscle Pyruvate Dehydrogenase Complex and CHO Oxidation, Events Reversed by Electrical Pulse Stimulation. Int. J. Mol. Sci. 2020, 21, 5942. [Google Scholar] [CrossRef]
- Smimmo, M.; Casale, V.; D’Andrea, D.; Bello, I.; Iaccarino, N.; Romano, F.; Brancaleone, V.; Panza, E.; Bianca, R.D.d.V.; Katsouda, A.; et al. Defective protein persulfidation is involved in obesity associated skeletal muscle dysfunction: Role of SIRT-1. Redox Biol. 2025, 83, 103645. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Han, I.S.; Chen, T.H.; Hsieh, P.S.; Tsai, M.C.; Chien, H.C. Pharmacological activation of pyruvate dehydrogenase by dichloroacetate protects against obesity-induced muscle atrophy in vitro and in vivo. Eur. J. Pharmacol. 2024, 979, 176854. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Xie, Q.; Cheng, Q.; Tang, W.; Mao, L. EPA but not DHA improve systemic IR through activating muscle IL-6/AMPK pathway in high-fat diet-fed mice. J. Funct. Foods 2025, 127, 106749. [Google Scholar] [CrossRef]
- Kwon, B.; Querfurth, H.W. Palmitate activates mTOR/p70S6K through AMPK inhibition and hypophosphorylation of raptor in skeletal muscle cells: Reversal by oleate is similar to metformin. Biochimie 2015, 118, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, X.; Lan, F.; Zhou, T.; Cai, H.; Sun, H.; Kong, W. Hydrogen Sulphide Treatment Increases Insulin Sensitivity and Improves Oxidant Metabolism through the CaMKKbeta-AMPK Pathway in PA-Induced IR C2C12 Cells. Sci. Rep. 2017, 7, 13248. [Google Scholar] [CrossRef]
- Tardif, N.; Salles, J.; Guillet, C.; Tordjman, J.; Reggio, S.; Landrier, J.F.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; Migne, C.; et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2α activation. Aging Cell 2014, 13, 1001–1011. [Google Scholar] [CrossRef]
- Dai, F.; Jiang, T.; Bao, Y.Y.; Chen, G.J.; Chen, L.; Zhang, Q.; Lu, Y.X. Fenofibrate improves high-fat diet-induced and palmitate-induced endoplasmic reticulum stress and inflammation in skeletal muscle. Life Sci. 2016, 157, 158–167. [Google Scholar] [CrossRef]
- D’Souza, K.; Mercer, A.; Mawhinney, H.; Pulinilkunnil, T.; Udenigwe, C.C.; Kienesberger, P.C. Whey Peptides Stimulate Differentiation and Lipid Metabolism in Adipocytes and Ameliorate Lipotoxicity-Induced Insulin Resistance in Muscle Cells. Nutrients 2020, 12, 425. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Li, L.; Wang, R.; Luo, S.; Li, G. Stevioside Ameliorates Palmitic Acid-Induced Abnormal Glucose Uptake via the PDK4/AMPK/TBC1D1 Pathway in C2C12 Myotubes. Endocrinol. Diabetes Metab. 2024, 7, e00482. [Google Scholar] [CrossRef]
- Jakovljevic, N.K.; Pavlovic, K.; Zujovic, T.; Kravic-Stevovic, T.; Jotic, A.; Markovic, I.; Lalic, N.M. In vitro models of insulin resistance: Mitochondrial coupling is differently affected in liver and muscle cells. Mitochondrion 2021, 61, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, Q.; Jiang, C.; Chen, C.; Liu, Y.; Chen, Y.; Zeng, Y. MicroRNA-29a is involved lipid metabolism dysfunction and insulin resistance in C2C12 myotubes by targeting PPARδ. Mol. Med. Rep. 2018, 17, 8493–8501. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, J.; Chen, Q.N.; Lyu, A.K.; Chen, J.L.; Sun, Y.; Lyu, Q.; Zhao, Y.X.; Guo, A.; Liao, Z.Y.; et al. Type 2 diabetes-induced overactivation of P300 contributes to skeletal muscle atrophy by inhibiting autophagic flux. Life Sci. 2020, 258, 118243. [Google Scholar] [CrossRef] [PubMed]
- Nieuwoudt, S.; Mulya, A.; Fealy, C.E.; Martelli, E.; Dasarathy, S.; Prasad, S.V.N.; Kirwan, J.P. In vitro contraction protects against palmitate-induced insulin resistance in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 2017, 313, C575–C583. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, H.; Jung, C.H.; Lee, S.J.; Ha, T.Y.; Ahn, J. Chicoric acid mitigates impaired insulin sensitivity by improving mitochondrial function. Biosci. Biotechnol. Biochem. 2018, 82, 1197–1206. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Na, H.-M.; Peng, G.; Pu, J.; Liu, P. Alteration of microRNA expression correlates to fatty acid-mediated insulin resistance in mouse myotblasts. Mol. Biosyst. 2011, 7, 871–877. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Wang, F.; Yang, H.; Yang, R.; Wang, X.; Wang, Y. Selenium-enriched exopolysaccharides improve skeletal muscle glucose uptake of diabetic KKAy mice via AMPK pathway. J. Physiol. Biochem. 2014, 70, 547–554. [Google Scholar] [CrossRef]
- Paez, H.G.; Ferrandi, P.J.; Pitzer, C.R.; Mohamed, J.S.; Alway, S.E. Loss of NOR-1 represses muscle metabolism through mTORC1-mediated signaling and mitochondrial gene expression in C2C12 myotubes. FASEB J. 2023, 37, e23050. [Google Scholar] [CrossRef]
- Jia, X.K.; Huang, J.F.; Huang, X.Q.; Li, X.Y.; Huang, M.Q.; Zhu, H.C.; Li, G.P.; Lan, M.L.; Yu, Z.W.; Xu, W.; et al. Alismatis Rhizoma Triterpenes Alleviate High-Fat Diet-Induced Insulin Resistance in Skeletal Muscle of Mice. Evid. Based Complement. Alternat Med. 2021, 2021, 8857687. [Google Scholar] [CrossRef]
- Rustamov, J.; Roh, Y.S.; Hong, J.T.; Yoo, H.S. GT-11 impairs insulin signaling through modulation of sphingolipid metabolism in C2C12 myotubes. Life Sci. 2024, 342, 122534. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudière, J.P.; Jouve, C.; Capel, F. Modulation of Insulin Resistance and the Adipocyte-Skeletal Muscle Cell Cross-Talk by LCn-3PUFA. Int. J. Mol. Sci. 2018, 19, 2778. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Hanayama, M.; Mori, K.; Sato, R. Grifola frondosa (Maitake) extract activates PPARδ and improves glucose intolerance in high-fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2018, 82, 1550–1559. [Google Scholar] [CrossRef]
- Botteri, G.; Salvadó, L.; Gumà, A.; Hamilton, D.L.; Meakin, P.J.; Montagut, G.; Ashford, M.L.J.; Ceperuelo-Mallafré, V.; Fernández-Veledo, S.; Vendrell, J.; et al. The BACE1 product sAPPβ induces ER stress and inflammation and impairs insulin signaling. Metabolism 2018, 85, 59–75. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Li, C.; Tang, Y.; Jiang, Z.; Yang, M.; Ni, C.L.; Li, D.; Chen, L.; Niu, W. Liraglutide ameliorates palmitate-induced insulin resistance through inhibiting the IRS-1 serine phosphorylation in mouse skeletal muscle cells. J. Endocrinol. Investig. 2018, 41, 1097–1102. [Google Scholar] [CrossRef]
- Qin, X.; Li, X.; Liu, C.; Chen, Z. A novel mechanism of pre-transplant insulin resistance contributing to post-transplant complications: Cyclosporin A-induced O-GlcNAcylation. Biochem. Biophys. Res. Commun. 2017, 492, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Capel, F.; Cheraiti, N.; Acquaviva, C.; Hénique, C.; Bertrand-Michel, J.; Vianey-Saban, C.; Prip-Buus, C.; Morio, B. Oleate dose-dependently regulates palmitate metabolism and insulin signaling in C2C12 myotubes. Biochim. Biophys. Acta 2016, 1861, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kaddai, V.; Ching, J.; Fridianto, K.T.; Sieli, R.J.; Sugii, S.; Summers, S.A. A Role for Ceramides, but Not Sphingomyelins, as Antagonists of Insulin Signaling and Mitochondrial Metabolism in C2C12 Myotubes. J. Biol. Chem. 2016, 291, 23978–23988. [Google Scholar] [CrossRef] [PubMed]
- Pinel, A.; Rigaudière, J.P.; Laillet, B.; Pouyet, C.; Malpuech-Brugère, C.; Prip-Buus, C.; Morio, B.; Capel, F. N-3PUFA differentially modulate palmitate-induced lipotoxicity through alterations of its metabolism in C2C12 muscle cells. Biochim. Biophys. Acta 2016, 1861, 12–20. [Google Scholar] [CrossRef]
- Capel, F.; Acquaviva, C.; Pitois, E.; Laillet, B.; Rigaudière, J.P.; Jouve, C.; Pouyet, C.; Gladine, C.; Comte, B.; Saban, C.V.; et al. DHA at nutritional doses restores insulin sensitivity in skeletal muscle by preventing lipotoxicity and inflammation. J. Nutr. Biochem. 2015, 26, 949–959. [Google Scholar] [CrossRef]
- Salvadó, L.; Coll, T.; Gómez-Foix, A.M.; Salmerón, E.; Barroso, E.; Palomer, X.; Vázquez-Carrera, M. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 2013, 56, 1372–1382. [Google Scholar] [CrossRef]
- Jing, Y.Y.; Li, D.; Wu, F.; Gong, L.L.; Li, R. GDF11 does not improve the palmitate induced insulin resistance in C2C12. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1795–1802. [Google Scholar]
- Karimfar, M.H.; Haghani, K.; Babakhani, A.; Bakhtiyari, S. Rosiglitazone, but not epigallocatechin-3-gallate, attenuates the decrease in PGC-1α protein levels in palmitate-induced insulin-resistant C2C12 cells. Lipids 2015, 50, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyari, S.; Meshkani, R.; Taghikhani, M.; Larijani, B.; Adeli, K. Protein tyrosine phosphatase-1B (PTP-1B) knockdown improves palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Lipids 2010, 45, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Planavila, A.; Laguna, J.C.; Vázquez-Carrera, M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology 2005, 146, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Henique, C.; Mansouri, A.; Fumey, G.; Lenoir, V.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; Cohen, I. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 2010, 285, 36818–36827. [Google Scholar] [CrossRef]
- Coll, T.; Alvarez-Guardia, D.; Barroso, E.; Gómez-Foix, A.M.; Palomer, X.; Laguna, J.C.; Vázquez-Carrera, M. Activation of peroxisome proliferator-activated receptor-δ by GW501516 prevents fatty acid-induced nuclear factor-κB activation and insulin resistance in skeletal muscle cells. Endocrinology 2010, 151, 1560–1569. [Google Scholar] [CrossRef]
- Feng, X.T.; Wang, T.Z.; Chen, Y.; Liu, J.B.; Liu, Y.; Wang, W.J. Pollen Typhae total flavone improves insulin-induced glucose uptake through the β-arrestin-2-mediated signaling in C2C12 myotubes. Int. J. Mol. Med. 2012, 30, 914–922. [Google Scholar] [CrossRef]
- Gorgani-Firuzjaee, S.; Bakhtiyari, S.; Golestani, A.; Meshkani, R. Leukocyte antigen-related inhibition attenuates palmitate-induced insulin resistance in muscle cells. J. Endocrinol. 2012, 215, 71–77. [Google Scholar] [CrossRef]
- Li, T.; Lv, Q.; Liu, C.; Li, C.; Xie, X.; Zhang, W. The Lipophilic Extract from Ginkgo biloba L. Leaves Promotes Glucose Uptake and Alleviates Palmitate-Induced Insulin Resistance in C2C12 Myotubes. Molecules 2024, 29, 1605. [Google Scholar] [CrossRef]
- Aswad, H.; Forterre, A.; Wiklander, O.P.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamazière, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef]
- Rivera, M.E.; Vaughan, R.A. Comparing the effects of palmitate, insulin, and palmitate-insulin co-treatment on myotube metabolism and insulin resistance. Lipids 2021, 56, 563–578. [Google Scholar] [CrossRef]
- Wu, S.J.; Tung, Y.J.; Ng, L.T. Anti-diabetic effects of Grifola frondosa bioactive compound and its related molecular signaling pathways in palmitate-induced C2C12 cells. J. Ethnopharmacol. 2020, 260, 112962. [Google Scholar] [CrossRef]
- Song, L.; Huang, K.; Tian, D.; Liu, X.; Huang, R.; Luo, J.; Zhang, M.; Lu, J.; Gui, M.; Ma, X. Epicatechin ameliorates palmitate-induced insulin resistance in C2C12 myogenic cells by alleviating oxidative stress and activating the AMPK/ACC pathway. CyTA J. Food 2024, 22, 2401591. [Google Scholar] [CrossRef]
- Shree, N.; Bhonde, R.R. Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Reverse Insulin Resistance in Cellular Models. J. Cell. Biochem. 2017, 118, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Shen, P.; Liu, S.; Wang, J.; Zeng, J.; Du, C. Vitamin D alleviates skeletal muscle loss and insulin resistance by inducing vitamin D receptor expression and regulating the AMPK/SIRT1 signaling pathway in mice. Food Sci. Technol. 2022, 42, e47921. [Google Scholar] [CrossRef]
- Silva, G.; Silva, S.S.D.; Guimarães, D.S.P.S.; Cruz, M.V.D.; Silveira, L.R.; Rocha-Vieira, E.; Amorim, F.T.; Magalhaes, F.d.C. The dose-effect response of combined red and infrared photobiomodulation on insulin resistance in skeletal muscle cells. Biochem. Biophys. Rep. 2024, 40, 101831. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, C.F.; Liu, C.H.; Chen, S.C.; Liu, Y.F.; Lv, Q.H.; Zhang, W. Extract of Phyllanthus emblica L. fruit stimulates basal glucose uptake and ameliorates palmitate-induced insulin resistance through AMPK activation in C2C12 myotubes. BMC Complement. Med. Ther. 2024, 24, 296. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, B.; Du, H.; Zhu, R.; Sun, X.; Fan, X.; Wei, X.; Yang, D.; Oh, Y.; Fan, L.; et al. High-fat diet and palmitate inhibits FNDC5 expression via AMPK-Zfp57 pathway in mouse muscle cells. Chem. Biol. Interact. 2023, 369, 110265. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Valentine, R.J. Saturated Fatty Acid-Induced Endoplasmic Reticulum Stress and Insulin Resistance Are Prevented by Imoxin in C2C12 Myotubes. Front. Physiol. 2022, 13, 842819. [Google Scholar] [CrossRef]
- Rios-Morales, M.; Vieira-Lara, M.A.; Homan, E.; Langelaar-Makkinje, M.; Gerding, A.; Li, Z.; Huijkman, N.; Rensen, P.C.N.; Wolters, J.C.; Reijngoud, D.J.; et al. Butyrate oxidation attenuates the butyrate-induced improvement of insulin sensitivity in myotubes. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166476. [Google Scholar] [CrossRef]
- Li, B.; Ye, J.; Liu, R.; Weng, L.; Cao, Y.; Jia, S.; Xu, C.; Liu, Y.; Yan, S.; Zheng, M. Programmed cell death 5 improves skeletal muscle insulin resistance by inhibiting IRS-1 ubiquitination through stabilization of MDM2. Life Sci. 2021, 285, 119918. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.; Spagnol, A.R.; Frias, F.T.; de Mendonça, M.; Araújo, H.N.; Guimarães, D.; Silva, W.J.; Bolin, A.P.; Murata, G.M.; Silveira, L. Intramuscular Injection of miR-1 Reduces Insulin Resistance in Obese Mice. Front. Physiol. 2021, 12, 676265. [Google Scholar] [CrossRef]
- Muñoz, V.R.; Gaspar, R.C.; Severino, M.B.; Macêdo, A.P.A.; Simabuco, F.M.; Ropelle, E.R.; Cintra, D.E.; da Silva, A.S.R.; Kim, Y.B.; Pauli, J.R. Exercise Counterbalances Rho/ROCK2 Signaling Impairment in the Skeletal Muscle and Ameliorates Insulin Sensitivity in Obese Mice. Front. Immunol. 2021, 12, 702025. [Google Scholar] [CrossRef]
- Guo, A.; Li, K.; Tian, H.C.; Fan, Z.; Chen, Q.N.; Yang, Y.F.; Yu, J.; Wu, Y.X.; Xiao, Q. FGF19 protects skeletal muscle against obesity-induced muscle atrophy, metabolic derangement and abnormal irisin levels via the AMPK/SIRT-1/PGC-α pathway. J. Cell Mol. Med. 2021, 25, 3585–3600. [Google Scholar] [CrossRef]
- Luo, J.; Hou, Y.; Xie, M.; Ma, W.; Shi, D.; Jiang, B. CYC31, A Natural Bromophenol PTP1B Inhibitor, Activates Insulin Signaling and Improves Long Chain-Fatty Acid Oxidation in C2C12 Myotubes. Mar. Drugs 2020, 18, 267. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lee, T.Y.; Leu, Y.L.; Wang, S.H. Pigment epithelium-derived factor inhibits adipogenesis in 3T3-L1 adipocytes and protects against high-fat diet-induced obesity and metabolic disorders in mice. Transl. Res. 2019, 210, 26–42. [Google Scholar] [CrossRef]
- Guo, Q.; Wei, X.; Hu, H.; Yang, D.; Zhang, B.; Fan, X.; Liu, J.; He, H.; Oh, Y.; Wu, Q.; et al. The saturated fatty acid palmitate induces insulin resistance through Smad3-mediated down-regulation of FNDC5 in myotubes. Biochem. Biophys. Res. Commun. 2019, 520, 619–626. [Google Scholar] [CrossRef]
- Liu, Q.; Li, R.; Chen, G.; Wang, J.; Hu, B.; Li, C.; Zhu, X.; Lu, Y. Inhibitory effect of 17β-estradiol on triglyceride synthesis in skeletal muscle cells is dependent on ESR1 and not ESR2. Mol. Med. Rep. 2019, 19, 5087–5096. [Google Scholar] [CrossRef]
- Bakhtiyari, S.; Zaherara, M.; Haghani, K.; Khatami, M.; Rashidinejad, A. The Phosphorylation of IRS1S307 and AktS473 Molecules in Insulin-Resistant C2C12 Cells Induced with Palmitate Is Influenced by Epigallocatechin Gallate from Green Tea. Lipids 2019, 54, 141–148. [Google Scholar] [CrossRef]
- Huang, S.; Wang, X.; Zhang, Q.; Liu, J.; Leng, Y. Downregulation of lipin-1 induces insulin resistance by increasing intracellular ceramide accumulation in C2C12 myotubes. Int. J. Biol. Sci. 2017, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dziewulska, A.; Dobrzyn, P.; Jazurek, M.; Pyrkowska, A.; Ntambi, J.M.; Dobrzyn, A. Monounsaturated fatty acids are required for membrane translocation of protein kinase C-theta induced by lipid overload in skeletal muscle. Mol. Membr. Biol. 2012, 29, 309–320. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Yu, J.S.; Hwang, J.Y.; So, H.M.; Seo, S.O.; Kim, J.K.; Jang, T.S.; Chung, S.J.; Kim, K.H. Phloridzin Acts as an Inhibitor of Protein-Tyrosine Phosphatase MEG2 Relevant to Insulin Resistance. Molecules 2021, 26, 1612. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chiang, C.F.; Lin, F.H.; Kuo, F.C.; Su, S.C.; Huang, C.L.; Li, P.F.; Liu, J.S.; Lu, C.H.; Hsieh, C.H.; et al. PDIA4, a new endoplasmic reticulum stress protein, modulates insulin resistance and inflammation in skeletal muscle. Front. Endocrinol. 2022, 13, 1053882. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hirasaka, K.; Kohno, S.; Tomida, C.; Haruna, M.; Uchida, T.; Ohno, A.; Oarada, M.; Teshima-Kondo, S.; Okumura, Y.; et al. Capric Acid Up-Regulates UCP3 Expression without PDK4 Induction in Mouse C2C12 Myotubes. J. Nutr. Sci. Vitaminol. 2016, 62, 32–39. [Google Scholar] [CrossRef]
- Tang, H.; Deng, S.; Cai, J.-G.; Ma, X.-N.; Liu, M.; Zhou, L. Muscle-derived IL-6 improved insulin resistance of C2C12 cells through activating AMPK and inhibiting p38MAPK signal pathway in vitro. Int. J. Diabetes Dev. Ctries. 2019, 39, 486–498. [Google Scholar] [CrossRef]
- Rieusset, J.; Chauvin, M.A.; Durand, A.; Bravard, A.; Laugerette, F.; Michalski, M.C.; Vidal, H. Reduction of endoplasmic reticulum stress using chemical chaperones or Grp78 overexpression does not protect muscle cells from palmitate-induced insulin resistance. Biochem. Biophys. Res. Commun. 2012, 417, 439–445. [Google Scholar] [CrossRef]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef]
- de Hart, N.M.; Petrocelli, J.J.; Nicholson, R.J.; Yee, E.M.; Ferrara, P.J.; Bastian, E.D.; Ward, L.S.; Petersen, B.L.; Summers, S.A.; Drummond, M.J. Palmitate-Induced Inflammation and Myotube Atrophy in C2C12 Cells Are Prevented by the Whey Bioactive Peptide, Glycomacropeptide. J. Nutr. 2023, 153, 2915–2928. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Riojas, R.A.; Xin, X.; Gao, Z.; Zeng, R.; Wu, J.; Dong, L.Q.; Liu, F. Protein kinase C θ (PKCθ)-dependent phosphorylation of PDK1 at Ser504 and Ser532 contributes to palmitate-induced insulin resistance. J. Biol. Chem. 2009, 284, 2038–2044. [Google Scholar] [CrossRef]
- Sarma, P.; Kashyap, B.; Gurumayum, S.; Sarma, S.; Baruah, P.; Swargiary, D.; Saikia, A.; Deka, R.C.; Borah, J.C. Antihyperglycemic Potential of Quercetin-3-glucoside Isolated from Leucaena leucocephala Seedpods via the SIRT1/AMPK/GLUT4 Signaling Cascade. ACS Omega 2024, 9, 32429–32443. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Bang, M.-H.; Kim, H.; Imm, J.-Y. Improvement of palmitate-induced insulin resistance in C2C12 skeletal muscle cells using Platycodon grandiflorum seed extracts. Food Biosci. 2018, 25, 61–67. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chuang, J.H.; Yang, W.C.; Yin, Y.; Lin, Y. Ceramide inhibits insulin-stimulated Akt phosphorylation through activation of Rheb/mTORC1/S6K signaling in skeletal muscle. Cell. Signal 2014, 26, 1400–1408. [Google Scholar] [CrossRef]
- Bandet, C.L.; Tan-Chen, S.; Ali-Berrada, S.; Campana, M.; Poirier, M.; Blachnio-Zabielska, A.; Pais-de-Barros, J.P.; Rouch, C.; Ferré, P.; Foufelle, F.; et al. Ceramide analog C2-cer induces a loss in insulin sensitivity in muscle cells through the salvage/recycling pathway. J. Biol. Chem. 2023, 299, 104815. [Google Scholar] [CrossRef]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Eburicoic Acid, a Triterpenoid Compound from Antrodia camphorata, Displays Antidiabetic and Antihyperlipidemic Effects in Palmitate-Treated C2C12 Myotubes and in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2017, 18, 2314. [Google Scholar] [CrossRef] [PubMed]
- Feraco, A.; Gorini, S.; Mammi, C.; Lombardo, M.; Armani, A.; Caprio, M. Neutral Effect of Skeletal Muscle Mineralocorticoid Receptor on Glucose Metabolism in Mice. Int. J. Mol. Sci. 2023, 24, 7412. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Mthembu, S.X.; Muller, C.J.; Ziqubu, K.; Muvhulawa, N.; Modibedi, R.V.; Tiano, L.; Dludla, P.V. Aspalathin alleviates skeletal muscle insulin resistance and mitochondrial dysfunction. Physiol. Res. 2022, 71, 643–656. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Li, P.; Liu, C.; Li, L. N1-methylnicotinamide ameliorates insulin resistance in skeletal muscle of type 2 diabetic mice by activating the SIRT1/PGC-1α signaling pathway. Mol. Med. Rep. 2021, 23, 270. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Huang, Q.; Duan, H.; Zhao, G.; Liu, L.; Li, Y. Flavonoids from Sophora alopecuroides L. improve palmitate-induced insulin resistance by inhibiting PTP1B activity in vitro. Bioorganic Med. Chem. Lett. 2021, 35, 127775. [Google Scholar] [CrossRef]
- Bitsi, S. The chemokine CXCL16 can rescue the defects in insulin signaling and sensitivity caused by palmitate in C2C12 myotubes. Cytokine 2020, 133, 155154. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, M.; de Sousa, É.; da Paixão, A.O.; Santos, B.A.D.; Spagnol, A.R.; Murata, G.M.; Araújo, H.N.; de Lima, T.I.; Guimarães, D.S.P.S.F.; Silveira, L.R.; et al. MicroRNA miR-222 mediates pioglitazone beneficial effects on skeletal muscle of diet-induced obese mice. Mol. Cell. Endocrinol. 2020, 501, 110661. [Google Scholar] [CrossRef]
- Huang, S.; Ma, S.; Ning, M.; Yang, W.; Ye, Y.; Zhang, L.; Shen, J.; Leng, Y. TGR5 agonist ameliorates insulin resistance in the skeletal muscles and improves glucose homeostasis in diabetic mice. Metabolism 2019, 99, 45–56. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. Aqueous-Methanol Extracts of Orange-Fleshed Sweet Potato (Ipomoea batatas) Ameliorate Oxidative Stress and Modulate Type 2 Diabetes Associated Genes in Insulin Resistant C2C12 Cells. Molecules 2018, 23, 2058. [Google Scholar] [CrossRef]
- Xu, Q.; Luo, J.; Wu, N.; Zhang, R.; Shi, D. BPN, a marine-derived PTP1B inhibitor, activates insulin signaling and improves insulin resistance in C2C12 myotubes. Int. J. Biol. Macromol. 2018, 106, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gu, Y.; Lang, H.; Wang, X.; Chen, K.; Gong, X.; Zhou, M.; Ran, L.; Zhu, J.; Mi, M. Dihydromyricetin prevents obesity-induced slow-twitch-fiber reduction partially via FLCN/FNIP1/AMPK pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zheng, J.; Chen, L.; Gu, B.; Huang, S. Astragaloside IV facilitates glucose transport in C2C12 myotubes through the IRS1/AKT pathway and suppresses the palmitate-induced activation of the IKK/IκBα pathway. Int. J. Mol. Med. 2016, 37, 1697–1705. [Google Scholar] [CrossRef]
- Haghani, K.; Pashaei, S.; Vakili, S.; Taheripak, G.; Bakhtiyari, S. TNF-α knockdown alleviates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2015, 460, 977–982. [Google Scholar] [CrossRef]
- Li, H.B.; Yang, Y.R.; Mo, Z.J.; Ding, Y.; Jiang, W.J. Silibinin improves palmitate-induced insulin resistance in C2C12 myotubes by attenuating IRS-1/PI3K/Akt pathway inhibition. Braz. J. Med. Biol. Res. 2015, 48, 440–446. [Google Scholar] [CrossRef]
- Chen, P.Y.; Wang, J.; Lin, Y.C.; Li, C.C.; Tsai, C.W.; Liu, T.C.; Chen, H.W.; Huang, C.S.; Lii, C.K.; Liu, K.L. 18-carbon polyunsaturated fatty acids ameliorate palmitate-induced inflammation and insulin resistance in mouse C2C12 myotubes. J. Nutr. Biochem. 2015, 26, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.; Khoury, R.; Blachnio-Zabielska, A.; Turban, S.; Loiseau, N.; Lipina, C.; Stretton, C.; Bourron, O.; Ferré, P.; Foufelle, F.; et al. Characterising the inhibitory actions of ceramide upon insulin signaling in different skeletal muscle cell models: A mechanistic insight. PLoS ONE 2014, 9, e101865. [Google Scholar] [CrossRef]
- Fabre, O.; Breuker, C.; Amouzou, C.; Salehzada, T.; Kitzmann, M.; Mercier, J.; Bisbal, C. Defects in TLR3 expression and RNase L activation lead to decreased MnSOD expression and insulin resistance in muscle cells of obese people. Cell Death Dis. 2014, 5, e1136. [Google Scholar] [CrossRef]
- Mazibuko, S.E.; Muller, C.J.; Joubert, E.; de Beer, D.; Johnson, R.; Opoku, A.R.; Louw, J. Amelioration of palmitate-induced insulin resistance in C2C12 muscle cells by rooibos (Aspalathus linearis). Phytomedicine 2013, 20, 813–819. [Google Scholar] [CrossRef]
- Peterson, J.M.; Wang, Y.; Bryner, R.W.; Williamson, D.L.; Alway, S.E. Bax signaling regulates palmitate-mediated apoptosis in C2C12 myotubes. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1307–E1314. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 2003, 419, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Planavila, A.; Sánchez, R.M.; Merlos, M.; Laguna, J.C.; Vázquez-Carrera, M. Palmitate induces tumor necrosis factor-α expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-κB activation. Endocrinology 2006, 147, 552–561. [Google Scholar] [CrossRef]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Yu, H.; Feng, X.; Wang, P.; Chen, J.; Pu, Y.; Zhao, Y.; He, H.; Zhong, J.; et al. Telmisartan improves insulin resistance of skeletal muscle through peroxisome proliferator-activated receptor-δ activation. Diabetes 2013, 62, 762–774. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, P.Y.; Wu, Y.L.; Chen, C.W.; Chen, H.W.; Lii, C.K.; Sun, H.L.; Liu, K.L. Long-chain polyunsaturated fatty acids amend palmitate-induced inflammation and insulin resistance in mouse C2C12 myotubes. Food Funct. 2016, 7, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, J.; Meng, X. Aronia melanocarpa anthocyanin extracts are an effective regulator of suppressor of cytokine signaling 3-dependent insulin resistance in HepG2 and C2C12 cells. J. Funct. Foods 2020, 75, 104258. [Google Scholar] [CrossRef]
- Swargiary, D.; Kashyap, B.; Sarma, P.; Ahmed, S.A.; Gurumayum, S.; Barge, S.R.; Basumatary, D.; Borah, J.C. Free radical scavenging polyphenols isolated from Phyllanthus niruri L. ameliorates hyperglycemia via SIRT1 induction and GLUT4 translocation in in vitro and in vivo models. Fitoterapia 2024, 173, 105803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dun, Y.; You, B.; Qiu, L.; Ripley-Gonzalez, J.W.; Cheng, J.; Fu, S.; Li, C.; Liu, S. Trimetazidine and exercise offer analogous improvements to the skeletal muscle insulin resistance of mice through Nrf2 signaling. BMJ Open Diabetes Res. Care 2022, 10, e002699. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, C.G.; Zhou, X.; Ding, S. Transcription factor EB enhances autophagy and ameliorates palmitate-induced insulin resistance at least partly via upregulating AMPK activity in skeletal muscle cells. Clin. Exp. Pharmacol. Physiol. 2022, 49, 302–310. [Google Scholar] [CrossRef]
- Han, M.; You, L.; Wu, Y.; Gu, N.; Wang, Y.; Feng, X.; Xiang, L.; Chen, Y.; Zeng, Y.; Zhong, T. RNA-sequencing analysis reveals the potential contribution of lncRNAs in palmitic acid-induced insulin resistance of skeletal muscle cells. Biosci. Rep. 2020, 40, BSR20192523. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Tan, J.S. Improvement of mitochondrial function by celastrol in palmitate-treated C2C12 myotubes via activation of PI3K-Akt signaling pathway. Biomed. Pharmacother. 2017, 93, 903–912. [Google Scholar] [CrossRef]
- Kwak, H.J.; Choi, H.E.; Cheon, H.G. 5-LO inhibition ameliorates palmitic acid-induced ER stress, oxidative stress and insulin resistance via AMPK activation in murine myotubes. Sci. Rep. 2017, 7, 5025. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Yuan, H.; Niu, Y.; Fu, L. Sestrin 2 induces autophagy and attenuates insulin resistance by regulating AMPK signaling in C2C12 myotubes. Exp. Cell Res. 2017, 354, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.J.; Choi, H.E.; Jang, J.; Park, S.K.; Bae, Y.A.; Cheon, H.G. Bortezomib attenuates palmitic acid-induced ER stress, inflammation and insulin resistance in myotubes via AMPK dependent mechanism. Cell. Signal 2016, 28, 788–797. [Google Scholar] [CrossRef]
- Meshkani, R.; Sadeghi, A.; Taheripak, G.; Zarghooni, M.; Gerayesh-Nejad, S.; Bakhtiyari, S. Rosiglitazone, a PPARγ agonist, ameliorates palmitate-induced insulin resistance and apoptosis in skeletal muscle cells. Cell Biochem. Funct. 2014, 32, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Dymkowska, D.; Drabarek, B.; Jakubczyk, J.; Wojciechowska, S.; Zabłocki, K. Potassium channel openers prevent palmitate-induced insulin resistance in C2C12 myotubes. Arch. Biochem. Biophys. 2014, 541, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Senn, J.J. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J. Biol. Chem. 2006, 281, 26865–26875. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, J.; Zheng, L.; Su, H.; Cao, S.; Jiang, X.; Liu, X.; Liu, W.; Wang, Z.; Meng, F.; et al. Dysfunction of Akt/FoxO3a/Atg7 regulatory loop magnifies obesity-regulated muscular mass decline. Mol. Metab. 2024, 81, 101892. [Google Scholar] [CrossRef]
- D’Souza, K.; Nzirorera, C.; Cowie, A.M.; Varghese, G.P.; Trivedi, P.; Eichmann, T.O.; Biswas, D.; Touaibia, M.; Morris, A.J.; Aidinis, V.; et al. Autotaxin-LPA signaling contributes to obesity-induced insulin resistance in muscle and impairs mitochondrial metabolism. J. Lipid Res. 2018, 59, 1805–1817. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, H.; Niu, Y.; Niu, W.; Fu, L. The role of AMPK/mTOR/S6K1 signaling axis in mediating the physiological process of exercise-induced insulin sensitization in skeletal muscle of C57BL/6 mice. Biochim. Biophys. Acta 2012, 1822, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Kadotani, A.; Tsuchiya, Y.; Hatakeyama, H.; Katagiri, H.; Kanzaki, M. Different impacts of saturated and unsaturated free fatty acids on COX-2 expression in C2C12 myotubes. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1291–E1303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ragheb, R.; Shanab, G.M.; Medhat, A.M.; Seoudi, D.M.; Adeli, K.; Fantus, I.G. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: Evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem. Biophys. Res. Commun. 2009, 389, 211–216. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Hatakeyama, H.; Emoto, N.; Wagatsuma, F.; Matsushita, S.; Kanzaki, M. Palmitate-induced down-regulation of sortilin and impaired GLUT4 trafficking in C2C12 myotubes. J. Biol. Chem. 2010, 285, 34371–34381. [Google Scholar] [CrossRef]
- Kusudo, T.; Kontani, Y.; Kataoka, N.; Ando, F.; Shimokata, H.; Yamashita, H. Fatty acid-binding protein 3 stimulates glucose uptake by facilitating AS160 phosphorylation in mouse muscle cells. Genes Cells 2011, 16, 681–691. [Google Scholar] [CrossRef]
- Pierre, N.; Fernández-Verdejo, R.; Regnier, P.; Vanmechelen, S.; Demeulder, B.; Francaux, M. IRE1α and TRB3 do not contribute to the disruption of proximal insulin signaling caused by palmitate in C2C12 myotubes. Cell Biol. Int. 2016, 40, 91–99. [Google Scholar] [CrossRef]
- Vong, S.-K.; Chen, C.-M.; Das, A.; Shyur, L.-F.; Ju, Y.-M.; Hsieh, H.-M.; Yang, S.-H.; Varga, V.; Li, S.-C. Stimulation of glucose uptake by edible Pholiota nameko (T.Itô) S.Ito & S.Imai extract counteracts palmitate-induced insulin resistance in C2C12 myotubes. Front. Sustain. Food Syst. 2025, 9, 1520875. [Google Scholar]
- Kim, K.-S.; Choi, Y.K.; Kim, M.J.; Yun, C.-K.; Hwang, J.W.; Min, K.; Jung, S.Y.; Soo-kyung, K.; Cho, Y.-W.; Choi, Y.-S. Effect of Mitochondrial Transfer via Centrifugation on Insulin Resistance in C2C12 Cells. J. Endocrinol. Metab. 2024, 14, 207–212. [Google Scholar] [CrossRef]
- Xiang, J.; Qin, L.; Zhong, J.; Xia, N.; Liang, Y. GLP-1RA Liraglutide and Semaglutide Improves Obesity-Induced Muscle Atrophy via SIRT1 Pathway. Diabetes Metab. Syndr. Obes. 2023, 16, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Choi, Y.K.; Kim, M.J.; Hwang, J.W.; Min, K.; Jung, S.Y.; Kim, S.K.; Choi, Y.S.; Cho, Y.W. Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell. Diabetes Metab. J. 2021, 45, 260–269. [Google Scholar] [CrossRef]
- Sun, Y.N.; Yang, Z.X.; Ren, F.Z.; Fang, B. FGF19 alleviates palmitate-induced atrophy in C2C12 cells by inhibiting mitochondrial overload and insulin resistance. Int. J. Biol. Macromol. 2020, 158, 401–407. [Google Scholar] [CrossRef]
- Zhou, Q.G.; Hou, F.F.; Guo, Z.J.; Liang, M.; Wang, G.B.; Zhang, X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes Metab. Res. Rev. 2008, 24, 459–464. [Google Scholar] [CrossRef]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell. Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef] [PubMed]

| Palmitate Concentration | Duration | Vehicle | Insulin Stimulation | Insulin Receptor Substrate (pIRS) | Protein Kinase B (pAkt) | Glucose Transporter 4 (GLUT4) | Glucose Uptake | Reference |
|---|---|---|---|---|---|---|---|---|
| 0.05 mM | 16 h | BSA | 17.2 nM × 15 min | ↓ (≈24.6 ± 10%) | De Wilde et al., 2010 [7] | |||
| 0.0625 mM | 24 h | BSA | No | ↓VC | Cheon et al., 2014 [8] | |||
| 0.0625 mM | 24 h | BSA | 100 nM × 30 min | ↓VC | Cheon et al., 2014 [8] | |||
| 0.075 mM | 24 h | BSA | No | ↓ (≈39.1 ± 10%) | ↓ (≈52.5 ± 10%) | Huang et al., 2024 [9] | ||
| 0.1 mM | 12 h | BSA | Unknown | ↔ VC | Chen et al., 2016 [10] | |||
| 0.1 mM | 16 h | BSA | 17.2 nM × 15 min | ↓ (≈32.0 ± 10%) | De Wilde et al., 2010 [7] | |||
| 0.1 mM | 16 h | BSA | 100 nM × 10 min | ↓ VC | Chavez et al., 2005 [11] | |||
| 0.1 mM | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.1 mM | 16 h | BSA | 200 nM × 20 min | ↓ (≈61.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.1 mM (3D) | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.1 mM (3D) | 16 h | BSA | 200 nM × 20 min | ↓ (≈50.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.1 mM | 18 h | BSA | No | ↓ (≈83.6 ± 5%) | Park et al., 2014 [13] | |||
| 0.1 mM | 18 h | BSA | 100 nM × 10 min | ↓ (≈61.4 ± 5%) | Park et al., 2014 [13] | |||
| 0.1 mM | 24 h | BSA | No | ↓ (≈65.7 ± 5%) | ↓ (≈60.5 ± 5%) | ↓ (≈60.0 ± 5%) | Ren et al., 2025 [14] | |
| 0.1 mM | 24 h | BSA | 10 nM × 30 min | ↓ (≈85.7 ± 5%) | ↓ (≈84.0 ± 5%) | Norouzi et al., 2019 [15] | ||
| 0.1 mM | 24 h | BSA | 100 nM × 30 min | ↔ (≈108.3 ± 5%) | ↔ (≈85.3 ± 5%) | ↔ (≈92.5 ± 5%) | ↔ (≈109.0 ± 5%) | Pan et al., 2023 [16] |
| 0.1 mM | 24 h | BSA | No | ↔ (≈72.7 ± 5%) | ↔ (≈100.0 ± 5%) | Zhang et al., 2018 [17] | ||
| 0.1 mM | 24 h | BSA | No | ↔ (≈95.0 ± 5%) | Hirabara et al., 2010 [18] | |||
| 0.1 mM | 24 h | BSA | 7 nM × 30 min | ↓ (≈51.0 ± 5%) | Hirabara et al., 2010 [18] | |||
| 0.1 mM | 24 h | BSA | 100 nM × 30 min | ↔ (≈93.0 ± 10%) | ↔ (≈96.0 ± 5%) | Zhang et al., 2015 [19] | ||
| 0.125 mM | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Feng et al., 2012 [20] | |||
| 0.125 mM | 16 h | BSA | 100 nM × 30 min | ↔ (≈91.6 ± 5%) SEM | Feng et al., 2012 [20] | |||
| 0.125 mM | 24 h | BSA | No | ↓ VC | ↓ (≈71.8 ± 5%) SEM | Cheon et al., 2014 [8] | ||
| 0.125 mM | 24 h | BSA | 100 nM × 30 min | ↓ VC | ↓ (≈67.0 ± 5%) SEM | Cheon et al., 2014 [8] | ||
| 0.15 mM | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.15 mM | 16 h | BSA | 200 nM × 20 min | ↓ (≈61.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.15 mM (3D) | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.15 mM (3D) | 16 h | BSA | 200 nM × 20 min | ↓ (≈35.7 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.15 mM (LG) | 24 h | BSA | No | ↔ (≈86.2 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.15 mM (LG) | 24 h | BSA | 100 nM × 20 min | ↔ (≈84.6 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.15 mM (HG) | 24 h | BSA | No | ↓ (≈68.8 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.15 mM (HG) | 24 h | BSA | 100 nM × 20 min | ↓ (≈82.8 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.15 mM (LG) | 96 h | BSA | No | ↔ (≈113.6 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.15 mM (LG) | 96 h | BSA | 100 nM × 20 min | ↔ (≈87.9 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.15 mM (HG) | 96 h | BSA | No | ↓ (≈67.2 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.15 mM (HG) | 96 h | BSA | 100 nM × 20 min | ↓ (≈77.4 ± 5%) | Kopp et al., 2023 [21] | |||
| 0.2 mM | 8 h | BSA | No | ↔ (≈94.4 ± 5%) | ↔ (≈30.7 ± 5%) | Mok et al., 2020 [22] | ||
| 0.2 mM | 8 h | BSA | 100 nM × 60 min | ↓ (≈49.3 ± 5%) | ↓ (≈44.8 ± 5%) | Mok et al., 2020 [22] | ||
| 0.2 mM | 12 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.2 mM | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.2 mM | 16 h | BSA | 200 nM × 20 min | ↓ (≈55.9 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.2 mM (3D) | 16 h | BSA | No | ↔ (≈118.2 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.2 mM (3D) | 16 h | BSA | 200 nM × 20 min | ↓ (≈35.7 ± 5%) SEM | Na et al., 2025 [12] | |||
| 0.2 mM | 16 h | BSA | No | ↔ (≈55.6 ± 5%) SEM | ↓ (≈78.9 ± 5%) SEM | Liu et al., 2015 [23] | ||
| 0.2 mM | 16 h | BSA | 200 nM × 30 min | ↓ (≈21.8 ± 5%) SEM | ↓ (≈70.4 ± 5%) SEM | Liu et al., 2015 [23] | ||
| 0.2 mM | 16 h | BSA | 100 nM × 120 min | ↓VC | Kirk-Ballard et al., 2014 [24] | |||
| 0.2 mM | 16 h | BSA | 50 nM × 15 min | ↓ (≈7.8 ± 5%) | Gone et al., 2024 [25] | |||
| 0.2 mM | 16 h | BSA | 17.2 nM × 15 min | ↓ (≈17.5 ± 5%) | De Wilde et al., 2010 [7] | |||
| 0.2 mM | 16 h | NR | 10 nM × 30 min | ↓ (≈44.2 ± 5%) SEM | Jheng et al., 2012 [26] | |||
| 0.2 mM | 16 h | EtOH | No | ↔ (≈97.1 ± 5%) | Parvaneh et al., 2010 [27] | |||
| 0.2 mM | 16 h | EtOH | 100 nM × 30 min | ↔ (≈100.0 ± 5%) | Parvaneh et al., 2010 [27] | |||
| 0.2 mM | 19 h | BSA | 10 nM × 10 min | ↓ VC | Blackburn et al., 2020 [28] | |||
| 0.2 mM | 19 h | BSA | 100 nM × 10 min | ↓ VC | Blackburn et al., 2020 [28] | |||
| 0.2 mM | 24 h | BSA | No | ↔ (≈103.4 ± 5%) | Tatebe et al., 2011 [29] | |||
| 0.2 mM | 24 h | BSA | 100 nM × 10 min | ↔ (≈88.4 ± 5%) | ↔ (≈105.9 ± 5%) | ↔ (≈100.0 ± 5%) | Tatebe et al., 2011 [29] | |
| 0.2 mM | 24 h | BSA | 10 nM × 30 min | ↓ (≈22.8 ± 5%) | ↓ (≈56.8 ± 5%) | Norouzi et al., 2019 [15] | ||
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈21.0 ± 5%) | ↓ (≈10.5 ± 5%) | ↓ (≈47.7 ± 5%) | Gwon et al., 2024 [30] | |
| 0.2 mM | 24 h | BSA | No | ↔ (≈96.9 ± 5%) | Wu et al., 2024 [31] | |||
| 0.2 mM | 24 h | BSA | Yes, unknown conditions | ↓ (≈59.6 ± 5%) | ↓ (≈73.6 ± 5%) | Wu et al., 2024 [31] | ||
| 0.2 mM | 24 h | BSA | 50 nM × 10 min | ↓ (≈45.7 ± 5%) | ↓ (≈57.1 ± 5%) | ↓ (≈43.5 ± 5%) | Sun et al., 2024 [32] | |
| 0.2 mM | 24 h | BSA | 100 nM × 10 min | ↓ (≈47.7 ± 5%) SEM | ↓ (≈66.2 ± 5%) SEM | Li et al., 2023 [33] | ||
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈20.9 ± 5%) | ↓ (≈27.1 ± 5%) | ↓ (≈43.6 ± 5%) | Oh et al., 2022 [34] | |
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈38.0 ± 5%) SEM | ↓ (≈17.5 ± 5%) SEM | ↓ (≈42.1 ± 5%) SEM | Jung et al., 2022 [35] | |
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈30.6 ± 10%) SEM | ↓ (≈26.5 ± 10%) SEM | ↓ (≈52.5 ± 5%) SEM | Park et al., 2021 [36] | |
| 0.2 mM | 24 h | BSA | No | ↔ (≈72.7 ± 5%) | Pesta et al., 2021 [37] | |||
| 0.2 mM | 24 h | BSA | 100 nM × 10 min | ↓ (≈57.6 ± 5%) | Pesta et al., 2021 [37] | |||
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈24.0 ± 5%) SEM | ↓ (≈38.8 ± 5%) SEM | ↓ (≈40.3 ± 5%) SEM | Jung et al., 2021 [38] | |
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈50.6 ± 5%) SEM | ↓ (≈23.3 ± 5%) SEM | ↓ (≈44.2 ± 5%) SEM | Kim et al., 2021 [39] | |
| 0.2 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈46.9 ± 5%) | ↓ (≈78.8 ± 5%) | ↓ (≈69.3 ± 5%) | Liu et al., 2020 [40] | |
| 0.2 mM | 24 h | BSA | 100 nM × 10 min | ↓ (≈81.9 ± 5%) | Chiu et al., 2021 [41] | |||
| 0.2 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈45.0 ± 5%) | ↓ (≈36.4 ± 5%) | ↓ (≈59.4 ± 5%) | Chuang et al., 2020 [42] | |
| 0.2 mM | 24 h | BSA | Unknown × 15 min | ↓ (≈33.3 ± 5%) SEM | ↓ (≈52.4 ± 5%) | Park et al., 2020 [43] | ||
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈43.8 ± 5%) SEM | ↓ (≈17.6 ± 5%) SEM | ↓ (≈30.3 ± 5%) SEM | Sun et al., 2020 [44] | |
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈6.0 ± 5%) SEM | ↓ (≈20.8 ± 5%) SEM | ↓ (≈34.1 ± 5%) SEM | Jung et al., 2017 [45] | |
| 0.2 mM | 24 h | BSA | Unknown | ↓ (≈76.8 ± 5%) SEM | ↓ (≈57.7 ± 5%) SEM | ↓ (≈51.9 ± 5%) SEM | Kulabas et al., 2018 [46] | |
| 0.2 mM | 24 h | BSA | 100 nM × 15 min | ↓ VC | ↔ (≈57.5 ± 5%) SEM | ↓ (≈85.1 ± 5%) SEM | Yang et al., 2013 [47] | |
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈48.8 ± 5%) SEM | ↓ (≈33.3 ± 5%) SEM | Jung et al., 2015 [48] | ||
| 0.2 mM | 24 h | BSA | 10 nM × 3 min | ↓ (≈75.8 ± 5%) SEM | ↓ (≈68.0 ± 5%) SEM | ↓ (≈42.3 ± 5%) SEM | Jung et al., 2017 [49] | |
| 0.2 mM | 24 h | BSA | No | ↔ (≈141.3 ± 5%) SEM | Zhang et al., 2015 [19] | |||
| 0.2 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈60.4 ± 5%) | ↓ (≈82.3 ± 5%) | Zhang et al., 2015 [19] | ||
| 0.2 mM | 24 h | BSA | No | ↓ (≈46.1 ± 5%) SEM | Yang et al., 2012 [50] | |||
| 0.2 mM | 48 h | BSA | 10 nM × 3 min | ↓ (≈24.3 ± 5%) SEM | ↓ (≈19.5 ± 5%) SEM | ↓ (≈40.9 ± 5%) SEM | Pyun et al., 2021 [51] | |
| 0.2 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈58.8 ± 5%) SEM | Yang et al., 2012 [50] | |||
| 0.2 mM | 48 h | BSA | 10 nM × 3 min | ↓ (≈24.3 ± 5%) | ↓ (≈18.3 ± 5%) | ↓ (≈41.0 ± 5%) | Jung et al., 2020 [52] | |
| 0.2 mM | 48 h | BSA | No | ↔ VC | ↔ (≈104.1 ± 5%) | de Figueiredo et al., 2015 [53] | ||
| 0.2 mM | 48 h | BSA | 100 nM × 30 min | ↓ (≈50.0 ± 5%) | ↓ (≈67.3 ± 5%) | de Figueiredo et al., 2015 [53] | ||
| 0.25 mM | 12 h | BSA | 100 nM × 30 min | ↔ (≈95.3 ± 5%) | Zhao et al., 2012 [54] | |||
| 0.25 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈46.6 ± 5%) | Kim et al., 2022 [55] | |||
| 0.25 mM | 16 h | BSA | 20 nM × 60 min (with amino acids) | ↓ (≈48.4 ± 5%) SEM | Cruz et al., 2020 [56] | |||
| 0.25 mM | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Bosquet et al., 2018 [57] | |||
| 0.25 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈50.4 ± 5%) SEM | Bosquet et al., 2018 [57] | |||
| 0.25 mM | 16 h | Unconjugated | 100 nM × 30 min | ↔ (≈100.0 ± 5%) SEM | Lee et al., 2017 [58] | |||
| 0.25 mM | 16 h | BSA | 100 nM × 30 min | ↔ (≈78.4 ± 5%) SEM | Lee et al., 2017 [58] | |||
| 0.25 mM | 16 h | BSA | No | ↓ (≈74.0 ± 5%) SEM | Zhou et al., 2007 [59] | |||
| 0.25 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈50.0 ± 5%) SEM | Hage Hassan et al., 2012 [60] | |||
| 0.25 mM | 16 h | BSA | No | ↔ (≈96.8 ± 5%) | Zhang et al., 2010 [61] | |||
| 0.25 mM | 16 h | BSA | 100 nM × 20 min | ↔ (≈96.0 ± 5%) | Zhang et al., 2010 [61] | |||
| 0.25 mM | 16 h | BSA | 100 nM × 10 min | ↓ VC | Chavez et al., 2005 [11] | |||
| 0.25 mM | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Feng et al., 2012 [20] | |||
| 0.25 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈87.4 ± 5%) SEM | Feng et al., 2012 [20] | |||
| 0.25 mM | 18 h | BSA | No | ↓ (≈61.8 ± 5%) | Park et al., 2014 [13] | |||
| 0.25 mM | 18 h | BSA | 100 nM × 10 min | ↓ (≈57.8 ± 5%) | ↓ (≈42.7 ± 5%) | Park et al., 2014 [13] | ||
| 0.25 mM | 18 h | BSA | 100 nM × 30 min | ↔ VC | ↓ (≈65.1 ± 5%) SEM | Qin et al., 2009 [62] | ||
| 0.25 mM | 24 h | BSA | No | ↓ VC | ↓ (≈65.6 ± 5%) SEM | Cheon et al., 2014 [8] | ||
| 0.25 mM | 24 h | BSA | 100 nM × 30 min | ↓ VC | ↓ (≈54.8 ± 5%) SEM | Cheon et al., 2014 [8] | ||
| 0.25 mM | 24 h | BSA | ↓ (≈61.2 ± 5%) SEM | Chang et al., 2018 [63] | ||||
| 0.25 mM | 24 h | BSA | 100 nM × 30 min | ↔ (≈112.5 ± 5%) | ↔ (≈78.0 ± 5%) | ↔ (≈85.0 ± 5%) | ↔ (≈113.6 ± 5%) | Pan et al., 2023 [16] |
| 0.25 mM | 24 h | BSA | 100 nM × 30 min | ↔ (≈94.7 ± 5%) SEM | ↓ (≈90.5 ± 5%) SEM | ↓ VC | Li et al., 2023 [64] | |
| 0.25 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈55.9 ± 5%) SEM | ↓ (≈68.8 ± 5%) SEM | ↓ (≈72.7 ± 5%) SEM | Yu et al., 2022 [65] | |
| 0.25 mM | 24 h | BSA | No | ↓ (≈50.0 ± 5%) SEM | Jiao et al., 2022 [66] | |||
| 0.25 mM | 24 h | BSA | 100 nM × unknown | ↓ (≈38.6 ± 5%) SEM | Jiao et al., 2022 [66] | |||
| 0.25 mM | 24 h | BSA | No | ↓ (≈54.5 ± 5%) | He et al., 2022 [67] | |||
| 0.25 mM | 24 h | BSA | 100 nM × 60 min | ↓ (≈47.8 ± 5%) SEM | Hu et al., 2022 [68] | |||
| 0.25 mM | 24 h | BSA | No | ↔ (≈100.0 ± 5%) | Nan et al., 2021 [69] | |||
| 0.25 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈45.7 ± 5%) | Nan et al., 2021 [69] | |||
| 0.25 mM | 24 h | BSA | No | ↓ (≈32.2 ± 5%) | ↔ (≈70.9 ± 5%) | Shen et al., 2019 [70] | ||
| 0.25 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈27.2 ± 5%) | ↓ (≈30.3 ± 5%) | ↓ (≈51.4 ± 5%) | Shen et al., 2019 [70] | |
| 0.25 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈60.4 ± 5%) | ↓ (≈55.3 ± 5%) | ↓ VC | Zhang et al., 2019 [71] | |
| 0.25 mM | 24 h | BSA | No | ↔ (≈109.0 ± 5%) | Fujiwara et al., 2017 [72] | |||
| 0.25 mM | 24 h | BSA | 100 nM × 20 min | ↓ (≈47.2 ± 5%) | Fujiwara et al., 2017 [72] |
| Palmitate Concentration | Duration | Vehicle | Insulin Stimulation | Insulin Receptor Substrate (pIRS) | Protein Kinase B (pAkt) | Glucose Transporter 4 (GLUT4) | Glucose Uptake | Reference |
|---|---|---|---|---|---|---|---|---|
| 0.3 mM | 12 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.3 mM | 16 h | BSA | No | ↔ (≈75.0 ± 5%) SEM | Lee et al., 2020 [73] | |||
| 0.3 mM | 16 h | BSA | 100 nM × 120 min | ↓ (≈84.6 ± 5%) SEM | Lee et al., 2020 [73] | |||
| 0.3 mM | 16 h | BSA | No | ↓ (≈38.4 ± 5%) SEM | Chien et al., 2020 [74] | |||
| 0.3 mM | 18 h | BSA | 100 nM × 15 min | ↓ (≈61.7 ± 5%) | Smimmo et al., 2025 [75] | |||
| 0.3 mM | 24 h | BSA | No | ↓ (≈36.3 ± 5%) | Yang et al., 2024 [76] | |||
| 0.3 mM | 24 h | BSA | No | ↓ (≈46.9 ± 5%) | ↓ (≈65.7 ± 5%) | Mao et al., 2025 [77] | ||
| 0.3 mM | 24 h | BSA | 10 nM × 30 min | ↓ (≈25.7 ± 5%) | ↓ (≈36.3 ± 5%) | Norouzi et al., 2019 [15] | ||
| 0.3 mM | 24 h * | BSA | 20 nM × 15 min | ↓ (≈56.8 ± 5%) | Kwon et al., 2015 [78] | |||
| 0.35 mM | 18 h | BSA | No | ↔ (≈92.8 ± 5%) SEM | Chen et al., 2017 [79] | |||
| 0.35 mM | 18 h | BSA | 10 nM × 10 min | ↓ (≈50.0 ± 5%) SEM | ↓ (≈78.1 ± 5%) SEM | Chen et al., 2017 [79] | ||
| 0.375 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈59.1 ± 5%) SEM | Tardif et al., 2014 [80] | |||
| 0.4 mM | 6 h | BSA | No | ↔ (≈89.2 ± 5%) | Dai et al., 2016 [81] | |||
| 0.4 mM | 6 h | BSA | 1 µM × 18 min | ↓ (≈71.4 ± 5%) | Dai et al., 2016 [81] | |||
| 0.4 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈67.6 ± 5%) | Dai et al., 2016 [81] | |||
| 0.4 mM | 12 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.4 mM | 16 h | BSA | No | ↔ (≈50.0 ± 5%) | ↔ (≈79.2 ± 5%) SEM | Lee et al., 2020 [73] | ||
| 0.4 mM | 16 h | BSA | 100 nM × 120 min | ↓ (≈66.1 ± 5%) SEM | ↓ (≈66.6 ± 5%) SEM | Lee et al., 2020 [73] | ||
| 0.4 mM | 16 h | BSA | 17.2 nM × 15 min | ↓ (≈15.2 ± 5%) | De Wilde et al., 2010 [7] | |||
| 0.4 mM | 18 h | BSA | 100 nM × 15 min | ↓ (≈53.0 ± 5%) | ↓ (≈63.6 ± 5%) | D’Souza et al., 2020 [82] | ||
| 0.4 mM | 24 h | BSA | No | ↓ (≈59.5 ± 5%) | ↓ (≈79.6 ± 5%) | Zhang et al., 2024 [83] | ||
| 0.4 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈85.5 ± 5%) | Zhang et al., 2024 [83] | |||
| 0.4 mM | 24 h | BSA | No | ↔ (≈66.6 ± 5%) SEM | Jakovljevic et al., 2021 [84] | |||
| 0.4 mM | 24 h | BSA | 120 nM × 15 min | ↓ (≈47.6 ± 5%) SEM | Jakovljevic et al., 2021 [84] | |||
| 0.4 mM | 24 h | BSA | 100 nM × 10 min | ↓ (≈58.3 ± 5%) | ↓ (≈51.6 ± 5%) | Chiu et al., 2021 [41] | ||
| 0.4 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈38.5 ± 5%) | ↓ (≈68.8 ± 5%) | ↓ (≈60.0 ± 5%) | Liu et al., 2020 [40] | |
| 0.4 mM | 24 h | BSA | 100 nM × 30 min | ↓ VC | ↓ VC | Wu et al., 2018 [85] | ||
| 0.4 mM | 24 h | BSA | 100 nM × 15 min | ↓ VC | ↓ (≈30.3 ± 5%) SEM | ↔ (≈75.5 ± 5%) SEM | Yang et al., 2013 [47] | |
| 0.4 mM | 24 h | BSA | No | ↓ (≈30.7 ± 5%) SEM | Yang et al., 2012 [50] | |||
| 0.4 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈38.8 ± 5%) SEM | Yang et al., 2012 [50] | |||
| 0.4 mM | 36 h | BSA | No | (≈167.1 ± 5%) SEM | Fan et al., 2021 [86] | |||
| 0.5 mM | 0.5 h | BSA | Unknown | ↔ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 1 h | BSA | Unknown | ↔ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 2 h | BSA | Unknown | ↔ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 4 h | BSA | No | ↑ (≈50.0 ± 5%) | Nieuwoudt et al., 2017 [87] | |||
| 0.5 mM | 4 h | BSA | 1 µM × 30 min | ↓ (≈71.8 ± 5%) | Nieuwoudt et al., 2017 [87] | |||
| 0.5 mM | 4 h | BSA | 1 µM × 10 min | ↔ (≈114.8 ± 5%) | Nieuwoudt et al., 2017 [87] | |||
| 0.5 mM | 4 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 6 h | BSA | No | ↑ (≈1060.0 ± 5%) | ↔ (≈94.8 ± 5%) | Kim et al., 2018 [88] | ||
| 0.5 mM | 6 h | BSA | 100 nM × 15 min | ↓ (≈58.0 ± 5%) | ↓ (≈73.4 ± 5%) | Kim et al., 2018 [88] | ||
| 0.5 mM | 6 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 8 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 10 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 12 h | BSA | No | ↔ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 12 h | BSA | Unknown | ↓ VC | Chen et al., 2016 [10] | |||
| 0.5 mM | 12 h | BSA | No | ↓ VC | Li et al., 2011 [89] | |||
| 0.5 mM | 12 h | BSA | 100 nM × 5 min | ↓ VC | Li et al., 2011 [89] | |||
| 0.5 mM | 12 h | BSA | No | ↔ (100.0 ± 5%) | ↔ (100.0 ± 5%) | Zhao et al., 2012 [54] | ||
| 0.5 mM | 12 h | BSA | 100 nM × 30 min | ↑ (≈356.5 ± 5%) | ↓ (≈52.9 ± 10%) | ↓ (≈68.4 ± 5%) | Zhao et al., 2012 [54] | |
| 0.5 mM | 16 h | BSA | 100 nM × 60 min | ↓ VC | ↓ (≈26.2 ± 5%) SEM | Zhou et al., 2014 [90] | ||
| 0.5 mM | 16 h | BSA | No | ↔ (≈100.0 ± 5%) SEM | Paez et al., 2023 [91] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 120 min | ↓ (≈55.1 ± 5%) SEM | Paez et al., 2023 [91] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↑ (110.0 ± 5%) SEM | ↓ (77.1 ± 5%) SEM | ↓ (84.3 ± 5%) SEM | Jia et al., 2021 [92] | |
| 0.5 mM | 16 h | BSA | 100 ng/mL × 15 min | ↓ (≈36.2 ± 10%) | Rustamov et al., 2024 [93] | |||
| 0.5 mM | 16 h | BSA | 20 nM × 60 min (with amino acids) | ↓ (≈25.7 ± 5%) SEM | Cruz et al., 2020 [56] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈58.6 ± 5%) | Pinel et al., 2018 [94] | |||
| 0.5 mM | 16 h | BSA | 1 µg/mL × 4 h | ↓ (≈76.8 ± 5%) | Pinel et al., 2018 [94] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↑ (≈356.5 ± 5%) | ↓ (≈27.7 ± 5%) | Aoki et al. [95] | ||
| 0.5 mM | 16 h | Unconjugated | 100 nM × 30 min | ↔ (≈100.0 ± 5%) SEM | Botteri et al., 2018 [96] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈56.8 ± 5%) SEM | Lee et al., 2017 [58] | |||
| 0.5 mM | 16 h | BSA | No | ↔ (≈125.0 ± 5%) | ↔ (≈66.6 ± 5%) | ↔ (≈90.0 ± 5%) | Lee et al., 2017 [58] | |
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↑ (≈200.0 ± 5%) | ↓ (≈42.8 ± 5%) | ↓ (≈63.7 ± 5%) | Li et al., 2018 [97] | |
| 0.5 mM | 16 h | BSA | No | ↔ (≈83.3 ± 5%) | Li et al., 2018 [97] | |||
| 0.5 mM | 16 h | BSA | Yes, unknown conditions | ↓ (≈63.6 ± 5%) | Qin et al., 2017 [98] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↑ VC | ↓ VC | ↓ (≈61.1 ± 5%) SEM | Qin et al., 2017 [98] | |
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈62.9 ± 5%) | Lee et al., 2017 [58] | |||
| 0.5 mM | 16 h | BSA | 1 nM × 15 min | ↓ VC | Capel et al., 2016 [99] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 15 min | ↓ Thr308 (≈54.0 ± 5%) | Capel et al., 2016 [99] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 15 min | ↓ Ser473 (≈60.0 ± 5%) | ↓ (≈80.7 ± 5%) | Park et al., 2016 [100] | ||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ Thr308 (≈34.3 ± 5%) | Pinel et al., 2015 [101] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ Ser473 (≈61.667 ± 5%) | ↓ (≈56.0 ± 5%) | Capel et al., 2015 [102] | ||
| 0.5 mM | 16 h | BSA | 100 nM × >10 min | ↓ (≈75.0 ± 5%) | ↓ (≈56.6 ± 5%) | Salvado et al., 2013 [103] | ||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈32.2 ± 5%) | ↓ (≈51.5 ± 5%) | Jing et al., 2017 [104] | ||
| 0.5 mM | 16 h | BSA | No | ↔ (≈86.2 ± 5%) | Karimfar et al., 2015 [105] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈51.0 ± 5%) | Karimfar et al., 2015 [105] | |||
| 0.5 mM | 16 h | BSA | No | ↑ (≈193.7 ± 5%) SEM | ↓ (≈80.0 ± 5%) SEM | Zhou et al., 2007 [59] | ||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈81.9 ± 5%) | ↓ (≈72.5 ± 5%) | ↓ (≈75.6 ± 5%) | Bakhtiyari et al. [106] | |
| 0.5 mM | 16 h | BSA | No | ↔ (≈87.5 ± 5%) | Zhang et al., 2010 [61] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 20 min | ↓ (≈85.3 ± 5%) | Zhang et al., 2010 [61] | |||
| 0.5 mM | 16 h | BSA | No | ↓ (≈236.3 ± 5%) | ↔ (≈154.3 ± 5%) | Jove et al., 2005 [107] | ||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈65.3 ± 5%) | Jove et al., 2005 [107] | |||
| 0.5 mM | 16 h | BSA | No | ↔ (83.3 ± 5%) SEM | Henique et al., 2010 [108] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ (49.1 ± 5%) SEM | Henique et al., 2010 [108] | |||
| 0.5 mM | 16 h | BSA | No | ↑ VC | Coll et al., 2010 [109] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈78.9 ± 5%) | ↓ (≈61.3 ± 5%) | Coll et al., 2010 [109] | ||
| 0.5 mM * | 16 h | BSA | No | ↓ (≈43.1 ± 5%) | Zhou et al., 2007 [59] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈79.5 ± 5%) SEM | Hage Hassan et al., 2012 [60] | |||
| 0.5 mM | 16 h | BSA | No | ↔ (≈103.2 ± 5%) SEM | ↔ (≈100.0 ± 5%) SEM | Feng et al., 2012 [20] | ||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈60.4 ± 5%) SEM | ↓ (≈80.0 ± 5%) SEM | Feng et al., 2012 [20] | ||
| 0.5 mM | 16 h | BSA | No | ↔ (≈101.5 ± 5%) SEM | Feng et al., 2012 [110] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 20 min | ↓ (≈82.8 ± 5%) SEM | Feng et al., 2012 [110] | |||
| 0.5 mM | 16 h | BSA | No | ↔ (≈97.3 ± 5%) SEM | Gorgani-Firuzjaee et al., 2012 [111] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 30 min | ↓ (76.0 ± 5%) SEM | Gorgani-Firuzjaee et al., 2012 [111] | |||
| 0.5 mM | 16 h | BSA | 100 nM × 15 min | ↓ (≈70.4 ± 5%) SEM | ↓ (≈68.7 ± 5%) SEM | Gorgani-Firuzjaee et al., 2012 [111] | ||
| 0.5 mM | 18 h | BSA | No | ↓ (≈41.8 ± 5%) | Park et al., 2014 [13] | |||
| 0.5 mM | 18 h | BSA | 100 nM × 10 min | ↓ (≈50.5 ± 5%) | ↓ (≈23.9 ± 5%) | Park et al., 2014 [13] | ||
| 0.5 mM | 18 h | BSA | 100 nM × 30 min | ↓ (≈58.1 ± 5%) | ↓ (≈62.5 ± 5%) | ↓ (≈61.5 ± 5%) | Li et al., 2024 [112] | |
| 0.5 mM | 18 h | No | 10 nM × 20 min | ↓ (≈66.6 ± 5%) SEM | Aswad et al., 2014 [113] | |||
| 0.5 mM | 18 h | BSA | 10 nM × 20 min | ↓ (≈65.4 ± 5%) SEM | Aswad et al., 2014 [113] | |||
| 0.5 mM | 19 h | BSA | 10 nM × 10 min | ↓ VC | Blackburn et al., 2020 [28] | |||
| 0.5 mM | 19 h | BSA | 100 nM × 10 min | ↓ VC | Blackburn et al., 2020 [28] | |||
| 0.5 mM | 24 h | EtOH | 100 nM × 30 min | ↔ (≈91.0 ± 5%) | ↔ (≈86.6 ± 5%) | Rivera et al., 2021 [114] | ||
| 0.5 mM | 24 h | DMSO | No | ↓ (≈83.8 ± 5%) | ↑ (≈134.4 ± 5%) | ↓ (≈81.4 ± 5%) | Wu et al., 2020 [115] | |
| 0.5 mM | 24 h | BSA | 100 nM × 24 h | ↓ (≈43.2 ± 5%) | ↓ (≈66.6 ± 5%) | Song et al., 2024 [116] | ||
| 0.5 mM | 24 h | BSA | 100 nM × 15 min | ↓VC | ↓VC | ↑ (≈231.5 ± 5%) | Shree et al., 2016 [117] | |
| 0.5 mM | 24 h | BSA | No | ↓ (≈72.6 ± 5%) | Li et al., 2022 [118] | |||
| 0.5 mM | 24 h | BSA | 1 µg/mL × 30 min | ↓ (≈62.6 ± 5%) | Li et al., 2022 [118] | |||
| 0.5 mM | 24 h | BSA | No | ↓VC | ↓ (≈67.1 ± 5%) SEM | Cheon et al., 2014 [8] | ||
| 0.5 mM | 24 h | BSA | 100 nM × 30 min | ↓VC | ↓ (≈52.4 ± 5%) SEM | Cheon et al., 2014 [8] | ||
| 0.5 mM | 24 h | BSA | No | ↔ (≈72.7 ± 5%) | Silva et al., 2024 [119] | |||
| 0.5 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈39.2 ± 5%) | Silva et al., 2024 [119] | |||
| 0.5 mM | 24 h | BSA | No | ↓ VC | Li et al., 2024 [120] | |||
| 0.5 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈59.5 ± 5%) | ↓ (≈34.9 ± 5%) | Li et al., 2024 [120] | ||
| 0.5 mM | 24 h | BSA | 100 nM × 30 min | ↑ (≈204.1 ± 5%) | ↓ (≈46.3 ± 5%) | ↓ (≈30.0 ± 5%) | ↑ (≈140.9 ± 5%) | Pan et al., 2023 [16] |
| 0.5 mM | 24 h | BSA | No | ↓ (≈91.5 ± 5%) SEM | Guo et al., 2023 [121] | |||
| 0.5 mM | 24 h | BSA | 1 µM × 60 min | ↓ (≈77.7 ± 5%) SEM | Guo et al., 2023 [121] | |||
| 0.5 mM | 24 h | BSA | No | ↔ (≈81.2 ± 5%) SEM | ↔ (≈80.0 ± 5%) SEM | ↔ (≈78.2 ± 5%) SEM | Eo et al., 2022 [122] | |
| 0.5 mM | 24 h | BSA | 50 nM × 15 min | ↔ (≈68.4 ± 5%) SEM | ↓ (≈45.2 ± 5%) SEM | ↓ (≈64.7 ± 5%) SEM | Eo et al., 2022 [122] | |
| 0.5 mM | 24 h | BSA | No | ↔ Thr308 (≈138.4 ± 5%) SEM | Rios-Morales et al., 2022 [123] | |||
| 0.5 mM | 24 h | BSA | No | ↔ Ser473(≈70.0 ± 5%) SEM | Rios-Morales et al., 2022 [123] | |||
| 0.5 mM | 24 h | BSA | 100 nM × 20 min | ↓ (≈71.4 ± 5%) SEM | Rios-Morales et al., 2022 [123] | |||
| 0.5 mM | 24 h | BSA | No | ↔ (≈85.7 ± 5%) SEM | Li et al., 2021 [124] | |||
| 0.5 mM | 24 h | BSA | 100 nM × 20 min | ↓ (≈38.8 ± 5%) SEM | Li et al., 2021 [124] | |||
| 0.5 mM | 24 h | BSA | 7 nM × 15 min | ↓ (≈21.9 ± 5%) | Rodrigues et al., 2021 [125] | |||
| 0.5 mM | 24 h | BSA | 200 nM × 20 min | ↓ (≈51.0 ± 5%) | ↓ (≈67.9 ± 5%) | Munoz et al., 2021 [126] | ||
| 0.5 mM | 24 h | BSA | No | ↓ (≈42.3 ± 5%) | ↓ (≈63.3 ± 5%) | Guo 2021 et al. [127] | ||
| 0.5 mM | 24 h | BSA | 10 nM × 15 min | ↓ (≈80.0 ± 5%) | ↓ (≈69.3 ± 5%) | Luo et al., 2020 [128] | ||
| 0.5 mM | 24 h | BSA | 100 nM × unknown | ↓ (≈65.3 ± 5%) | ↓ (≈54.0 ± 5%) | Chen et al., 2019 [129] | ||
| 0.5 mM | 24 h | BSA | No | ↓ (≈63.1 ± 5%) | Guo et al., 2020 [130] | |||
| 0.5 mM | 24 h | BSA | 1 µM × 60 min | ↓ (≈75.4 ± 5%) | Guo et al., 2020 [130] | |||
| 0.5 mM | 24 h | BSA | unknown | ↓ (≈47.3 ± 5%) | Liu et al., 2019 [131] | |||
| 0.5 mM | 24 h | BSA | 100 nM × unknown | ↓ (≈52.8 ± 5%) | ↓ (≈30.1 ± 5%) | Bakhtiyari et al., 2019 [132] | ||
| 0.5 mM | 24 h | BSA | No | ↓ (≈61.3 ± 5%) | ↓ (≈55.0 ± 5%) | Zhang et al., 2018 [17] | ||
| 0.5 mM | 24 h | BSA | 10 nM × 30 min | ↓ (≈51.2 ± 5%) | Huang et al., 2017 [133] | |||
| 0.5 mM | 24 h | BSA | 100 nM × 10 min | ↔ VC | Dziewulska et al., 2012 [134] | |||
| 0.5 mM | 24 h | BSA | 100 nM × 10 min | ↓ (≈17.9 ± 5%) | Dziewulska et al., 2012 [134] | |||
| 0.5 mM | 18 h + 9 h | No | 10 nM × 20 min | ↓ (≈0.00 ± 5%) | Aswad et al., 2014 [113] | |||
| 0.5 mM | 18 h + 9 h | BSA | 10 nM × 20 min | ↓ (≈25.0 ± 5%) | Aswad et al., 2014 [113] | |||
| 0.5 mM | 48 h | BSA | 100 nM × 30 min | ↓ (≈45.4 ± 5%) SEM | ↓ (≈55.5 ± 5%) | Yoon et al., 2021 [135] |
| Palmitate Concentration | Duration | Vehicle | Insulin Stimulation | Insulin Receptor Substrate (pIRS) | Protein Kinase B (pAkt) | Glucose Transporter 4 (GLUT4) | Glucose Uptake | Reference |
|---|---|---|---|---|---|---|---|---|
| 0.6 mM | 24 h | BSA | No | ↔ (≈75.4 ± 5%) SEM | Lee et al., 2022 [136] | |||
| 0.6 mM | 24 h | BSA | 100 nM × 15 min | ↓ (≈0.00 ± 5%) | ↓ (≈62.5 ± 5%) | Lee et al., 2022 [136] | ||
| 0.6 mM | 24 h | BSA | 100 nM × 15 min | ↓ VC | ↓ (≈21.2 ± 5%) SEM | ↓ (≈73.4 ± 5%) SEM | Yang et al., 2013 [47] | |
| 0.6 mM | 24 h | BSA | No | ↓ (≈21.7 ± 5%) | Abe et al., 2016 [137] | |||
| 0.6 mM | 24 h | BSA | 100 nM × 60 min | ↓ (≈37.6 ± 5%) | Abe et al., 2016 [137] | |||
| 0.6 mM | 24 h | BSA | No | ↓ (≈30.7 ± 5%) SEM | Yang et al., 2012 [50] | |||
| 0.6 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈29.4 ± 5%) SEM | Yang et al., 2012 [50] | |||
| 0.6 mM | 48 h | BSA | unknown | ↓ (≈24.4 ± 5%) | Tang et al., 2018 [138] | |||
| 0.7 mM | 18 h | BSA | No | ↔ (≈100.0 ± 5%) | Rieusset et al., 2012 [139] | |||
| 0.7 mM | 18 h | BSA | 10 nM × 20 min | ↓ (≈29.2 ± 5%) | Rieusset et al., 2012 [139] | |||
| 0.75 mM | 1 h | BSA | 100 nM × 10 min | ↓ VC | Hage Hassan et al., 2012 [60] | |||
| 0.75 mM | 2 h | BSA | No | ↑ (≈266.6 ± 5%) | ↔ (≈100.0 ± 5%) | ↓ (≈71.7 ± 5%) | Schmitz-Peiffer et al., 1999 [4] | |
| 0.75 mM | 2 h | BSA | 100 nM × 10 min | ↔ (≈93.7 ± 5%) | ↓ (≈58.9 ± 5%) | ↓ (≈77.1 ± 5%) | Schmitz-Peiffer et al., 1999 [4] | |
| 0.75 mM | 2 h | BSA | 100 nM × 10 min | ↓ VC | Hage Hassan et al., 2012 [60] | |||
| 0.75 mM | 4 h | BSA | 100 nM × 10 min | ↓ VC | Hage Hassan et al., 2012 [60] | |||
| 0.75 mM | 5 h | BSA | No | ↑ (≈268.5 ± 5%) | ↔ VC | Deng et al., 2012 [140] | ||
| 0.75 mM | 5 h | BSA | 100 nM × 10 min | ↓ (≈58.1 ± 5%) | ↔ VC | Deng et al., 2012 [140] | ||
| 0.75 mM | 6 h | BSA | 10 nM × 10 min | ↔ (≈85.7 ± 5%) | De Hart et al., 2023 [141] | |||
| 0.75 mM | 6 h | BSA | 10 nM × 10 min | ↓ VC | Wang et al., 2009 [142] | |||
| 0.75 mM | 8 h | BSA | 100 nM × 60 min | ↓(≈54.5 ± 5%) | ↓(≈45.1 ± 5%) | Samra et al., 2024 [143] | ||
| 0.75 mM | 12 h | BSA | 10 nM × 10 min | ↔ (≈71.4 ± 5%) | De Hart et al., 2023 [141] | |||
| 0.75 mM | 12 h | BSA | 10 nM × 10 min | ↓ VC | Wang et al., 2009 [142] | |||
| 0.75 mM | 16 h | EtOH | No | ↔ (≈92.5 ± 5%) | Parvaneh et al., 2010 [27] | |||
| 0.75 mM | 16 h | EtOH | 100 nM × 30 min | ↓ (≈71.6 ± 5%) | Parvaneh et al., 2010 [27] | |||
| 0.75 mM | 16 h | BSA | No | ↓ (≈43.8 ± 5%) | ↓ (≈32.5 ± 5%) | ↓ (≈61.7 ± 5%) | Yoon et al., 2018 [144] | |
| 0.75 mM | 16 h | BSA | 1 µM × unknown | ↓ (≈44.6 ± 5%) | ↓ (≈74.5 ± 5%) | Yoon et al., 2018 [144] | ||
| 0.75 mM | 16 h | BSA | 100 nM × 15 min | ↓ (≈80.8 ± 5%) SEM | ↓ (≈67.6 ± 5%) SEM | Hsieh et al., 2014 [145] | ||
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ VC | Bandet et al., 2023 [146] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈56.8 ± 5%) | ↓ (≈62.0 ± 5%) | Lin et al., 2017 [147] | ||
| 0.75 mM | 16 h | BSA | 100 nM × 15 min | ↓ (≈53.2 ± 5%) | ↓ (≈8.6 ± 5%) | Feraco et al., 2023 [148] | ||
| 0.75 mM | 16 h | BSA | 1 µM × 10 min | ↓ (≈6.3 ± 5%) | ↓ (≈47.9 ± 5%) | Mazibuko-Mbeje et al., 2022 [149] | ||
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈60.6 ± 5%) | ↓ (≈44.4 ± 5%) | ↓ (≈80.0 ± 5%) | Chen et al., 2021 [150] | |
| 0.75 mM | 16 h | BSA | 100 nM × unknown | ↓ (≈70.8 ± 5%) | Zhang et al., 2021 [151] | |||
| 0.75 mM | 16 h | BSA | No | ↔ (≈70.0 ± 5%) SEM | Bitsi, 2020 [152] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈55.1 ± 5%) SEM | Bitsi, 2020 [152] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 15 min | ↓ (≈47.8 ± 5%) SEM | de Mendonça et al., 2020 [153] | |||
| 0.75 mM | 16 h | BSA | No | ↔ (≈112.1 ± 5%) SEM | Huang et al., 2019 [154] | |||
| 0.75 mM | 16 h | BSA | 10 nM × 30 min | ↓ Thr308 (≈57.5 ± 5%) SEM | Huang et al., 2019 [154] | |||
| 0.75 mM | 16 h | BSA | 10 nM × 30 min | ↓ Ser473 (≈44.1 ± 5%) SEM | ↓ (≈95.3 ± 5%) SEM | Huang et al., 2019 [154] | ||
| 0.75 mM | 16 h | BSA | No | ↓ (≈6.3 ± 5%) | Ayeleso et al., 2018 [155] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈59.0 ± 5%) | ↔ (≈70.8 ± 5%) | Xu et al., 2017 [156] | ||
| 0.75 mM | 16 h | Unconjugated | 100 nM × 30 min | ↓ (≈92.0 ± 5%) SEM | Lee et al., 2017 [58] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈31.8 ± 5%) SEM | Lee et al., 2017 [58] | |||
| 0.75 mM | 16 h | BSA | Yes, unknown conditions | ↓ (≈32.0 ± 5%) | ↓ (≈36.0 ± 5%) | ↓ (≈56.5 ± 5%) | Zhou et al., 2017 [157] | |
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈26.3 ± 5%) | ↓ Thr308 (≈54.0 ± 5%) | ↓ (≈13.7 ± 5%) | ↓ (≈71.4 ± 5%) | Zhu et al. [158] |
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ Ser473 (≈64.8 ± 5%) | Zhu et al. [158] | |||
| 0.75 mM | 16 h | BSA | No | ↔ (≈93.3 ± 5%) | Haghani et al., 2015 [159] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈58.9 ± 5%) | ↓ (≈38.6 ± 5%) | ↓ (≈62.7 ± 5%) | Haghani et al., 2015 [159] | |
| 0.75 mM | 16 h | BSA | No | ↔ (≈89.6 ± 5%) | Li et al. [160] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈40.4 ± 5%) | ↓ (≈36.9 ± 5%) | ↓ VC | ↓ (≈70.2 ± 5%) | Li et al. [160] |
| 0.75 mM | 16 h | BSA | No | ↓ (≈40.0 ± 5%) | ↓ (≈42.0 ± 5%) | ↓ (≈59.5 ± 5%) | Chen et al., 2015 [161] | |
| 0.75 mM | 16 h | BSA | 10 nM × 15 min | ↓ (≈55.3 ± 5%) | ↓ (≈43.4 ± 5%) | ↓ (≈30.5 ± 5%) | Chen et al., 2015 [161] | |
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈40.0 ± 5%) | Mahfouz et al., 2014 [162] | |||
| 0.75 mM | 16 h | BSA | No | ↓ VC | Fabre et al., 2014 [163] | |||
| 0.75 mM | 16 h | BSA | 1 nM × 10 min | ↓ VC | Fabre et al., 2014 [163] | |||
| 0.75 mM | 16 h | BSA | No | ↓ (≈33.3 ± 5%) | ↓ (≈33.3 ± 5%) | ↓ (≈33.3 ± 5%) | Mazibuko et al., 2013 [164] | |
| 0.75 mM | 16 h | BSA | 1 µM × 15 min | ↓ (≈60.7 ± 5%) | ↓ (≈15.9 ± 5%) | ↓ (≈23.0 ± 5%) | Mazibuko et al., 2013 [164] | |
| 0.75 mM | 16 h | BSA | No | ↔ (≈93.7 ± 5%) | Zhang et al., 2010 [61] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 20 min | ↓ (≈72.0 ± 5%) | Zhang et al., 2010 [61] | |||
| 0.75 mM * | 16 h | BSA | 100 nM × 20 min | ↑ (≈266.6 ± 5%) | ↓ (≈43.5 ± 5%) | ↑ (≈127.7 ± 5%) | ↓ (≈73.7 ± 5%) | Zhang et al., 2010 [61] |
| 0.75 mM | 16 h | EtOH | No | ↓ (≈50.0 ± 5%) | Peterson et al., 2008 [165] | |||
| 0.75 mM | 16 h | BSA | No | ↓ VC | Chavez et al., 2003 [166] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ VC | Chavez et al., 2003 [166] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ VC | Chavez et al., 2005 [11] | |||
| 0.75 mM * | 16 h | BSA | 100 nM × 10 min | ↓ VC | Chavez et al., 2005 [11] | |||
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈20.0 ± 5%) | ↓ VC | Jove et al., 2006 [167] | ||
| 0.75 mM | 16 h | BSA | No | ↓ VC | ↓ VC | Deng et al., 2012 [140] | ||
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ VC | ↓ VC | ↓ (≈85.4 ± 5%) | Deng et al., 2012 [140] | |
| 0.75 mM | 16 h | BSA | 100 nM × 10 min | ↓ VC | Hage Hassan et al., 2012 [60] | |||
| 0.75 mM * | 16 h | BSA | 100 nM × 10 min | ↓ (≈48. ± 5%) | Hage Hassan et al., 2012 [60] | |||
| 0.75 mM | 18 h | BSA | Unknown | ↓ (≈77.1 ± 5%) | Aguer et al., 2014 [168] | |||
| 0.75 mM * | 18 h | BSA | 10 nM × 10 min | ↓ (≈11.1 ± 5%) | Wang et al., 2009 [142] | |||
| 0.75 mM | 18 h | BSA | 10 nM × 10 min | ↓ (≈32.1 ± 5%) | Wang et al., 2009 [142] | |||
| 0.75 mM | 18 h | BSA | No | ↔ (≈100.0 ± 5%) | ↔ (≈100.0 ± 5%) | Li et al., 2013 [169] | ||
| 0.75 mM | 18 h | BSA | 100 nM × 30 min | ↓ (≈16.6 ± 5%) | ↓ (≈21.6 ± 5%) | Li et al., 2013 [169] | ||
| 0.75 mM | 19 h | BSA | 10 nM × 10 min | ↓ VC | Blackburn et al., 2020 [28] | |||
| 0.75 mM | 19 h | BSA | 100 nM × 10 min | ↓ VC | Blackburn et al., 2020 [28] | |||
| 0.75 mM | 24 h | BSA | 100 nM × 15 min | ↓ VC | ↓ VC | ↓ (≈60.6 ± 5%) | Chen et al., 2016 [170] | |
| 0.75 mM | 24 h | BSA | 100 nM × 30 min | ↑ (≈164.1 ± 5%) | ↓ (≈50.7 ± 5%) | Chen et al., 2020 [171] | ||
| 0.75 mM | 24 h | BSA | No | ↓ (≈63.1 ± 5%) | Zhang et al., 2024 [83] | |||
| 0.75 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈47.3 ± 5%) | Zhang et al., 2024 [83] | |||
| 0.75 mM | 24 h | BSA | 100 nM × 10 min | ↓ (≈70.0 ± 5%) | Swargiary et al., 2024 [172] | |||
| 0.75 mM | 24 h | BSA | 10 nM × 10 min | ↓ (≈56.5 ± 5%) | De Hart et al., 2023 [141] | |||
| 0.75 mM | 24 h | BSA | No | ↔ (≈89.4 ± 5%) | Zhang et al., 2021 [173] | |||
| 0.75 mM | 24 h | BSA | 30 mU/mL × 60 min | ↓ (≈18.1 ± 5%) | ↓ (≈17.6 ± 5%) | ↓ (≈66.6 ± 5%) | Zhang et al., 2021 [173] | |
| 0.75 mM | 24 h | BSA | No | ↓ (≈53.1 ± 5%) | ↓ (≈67.2 ± 5%) | Wang et al., 2021 [174] | ||
| 0.75 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈52.0 ± 5%) | ↓ (≈61.1 ± 5%) | Wang et al., 2021 [174] | ||
| 0.75 mM | 24 h | BSA | No | ↓ VC | ↓ VC | Han et al., 2020 [175] | ||
| 0.75 mM | 24 h | BSA | Yes, unknown | ↓ VC | ↓ VC | Han et al., 2020 [175] | ||
| 0.75 mM | 24 h | BSA | 100 nM × unknown | ↓ (≈14.0 ± 5%) | Chen et al., 2019 [129] | |||
| 0.75 mM | 24 h | BSA | No | ↓ (≈40.9 ± 5%) | ↓ (≈51.7 ± 5%) | Zhang et al., 2018 [17] | ||
| 0.75 mM | 24 h | BSA | No | ↓ (≈28.5 ± 5%) | Bakar et al., 2017 [176] | |||
| 0.75 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈38.2 ± 5%) | ↓ (≈20.0 ± 5%) | Bakar et al., 2017 [176] | ||
| 0.75 mM | 24 h | BSA | No | ↑ VC | ↓ VC | ↓ (≈64.8 ± 5%) | Kwak et al., 2017 [177] | |
| 0.75 mM | 24 h | BSA | 1 μg/mL × 30 min | ↑ VC | ↓ VC | ↓ (≈57.1 ± 5%) | Kwak et al., 2017 [177] | |
| 0.75 mM | 24 h | BSA | 10 nM × 30 min | ↓ (≈25.6 ± 5%) | Huang et al., 2017 [133] | |||
| 0.75 mM | 24 h | BSA | No | ↔ (≈236.0 ± 5%) | ↔ (≈50.0 ± 5%) | ↔ (≈47.8 ± 5%) | Li et al., 2017 [178] | |
| 0.75 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈256.0 ± 5%) | ↓ (≈45.7 ± 5%) | ↓ (≈40.4 ± 5%) | Li et al., 2017 [178] | |
| 0.75 mM | 24 h | BSA | No | ↓ (≈21.4 ± 5%) | ↓ (≈11.1 ± 5%) | ↔ (≈92.3 ± 5%) | ↔ (≈78.5 ± 5%) | Kwak et al., 2016 [179] |
| 0.75 mM | 24 h | BSA | 1 μg/mL × 30 min | ↓ (≈21.5 ± 5%) | ↓ (≈39.2 ± 5%) | ↓ (≈50.0 ± 5%) | ↓ (≈61.3 ± 5%) | Kwak et al., 2016 [179] |
| 0.75 mM | 24 h | BSA | No | ↔ (≈90.0 ± 5%) | Meshkani et al., 2014 [180] | |||
| 0.75 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈62.9 ± 5%) | ↓ (≈70.5 ± 5%) | Meshkani et al., 2014 [180] | ||
| 0.75 mM | 24 h | BSA | No | ↓ VC | Dymkowska et al., 2014 [181] | |||
| 0.75 mM | 24 h | BSA | 10 nM × 20 min | ↓ (≈51.7 ± 5%) | ↓ (≈83.0 ± 5%) | Dymkowska et al., 2014 [181] | ||
| 0.75 mM | 24 h | BSA | 10 nM × 10 min | ↓ (≈9.7 ± 5%) | Wang et al., 2009 [142] | |||
| 0.75 mM | 24 h | BSA | 10 nM × 10 min | ↓ (≈16.3 ± 5%) | Senn et al., 2006 [182] | |||
| 0.75 mM | 48 h | BSA | No | ↓ (≈39.1 ± 5%) | Yu et al., 2024 [183] | |||
| 0.8 mM | 18 h | BSA | No | ↔ (≈90.9 ± 5%) | D’Souza et al., 2018 [184] | |||
| 0.8 mM | 18 h | BSA | 20 nM × 15 min | ↓ (≈70.2 ± 5%) | D’Souza et al., 2018 [184] | |||
| 0.8 mM | 18 h | BSA | 200 nM × 30 min | ↑ (≈271.4 ± 5%) | ↑ (≈131.8 ± 5%) | Lui et al., 2012 [185] | ||
| 1 mM | 16 h | BSA | No | ↓ VC | ↑ (≈207.4 ± 5%) SEM | Kadotani et al., 2008 [186] | ||
| 1 mM | 16 h | BSA | 2 nM × 30 min | ↓ VC | Kadotani et al., 2008 [186] | |||
| 1 mM | 16 h | BSA | 10 nM × 30 min | ↓ VC | Kadotani et al., 2008 [186] | |||
| 1 mM | 16 h | BSA | 100 nM × 30 min | ↓ VC | ↓ (≈78.2 ± 5%) SEM | Kadotani et al., 2008 [186] | ||
| 1 mM | 16 h | Unconjugated | 100 nM × 30 min | ↓ (≈84.0 ± 5%) SEM | Lee et al., 2017 [58] | |||
| 1 mM | 16 h | BSA | 100 nM × 30 min | ↓ (≈30.6 ± 5%) SEM | Lee et al., 2017 [58] | |||
| 1 mM | 16 h | BSA | No | ↓ (≈76.1 ± 5%) | Zhou et al., 2007 [59] | |||
| 1 mM | 16 h | BSA | No | ↑ (≈191.8 ± 5%) | ↔ (≈150.0 ± 5%) | ↓ (≈75.8 ± 5%) | Ragheb et al., 2009 [187] | |
| 1 mM | 16 h | BSA | 100 nM × 10 min | ↑ (≈159.0 ± 5%) | ↓ (≈72.2 ± 5%) | ↓ (≈60.0 ± 5%) | Ragheb et al., 2009 [187] | |
| 1 mM | 16 h | BSA | 100 nM × 30 min | ↓ VC | Tsuchiya et al., 2010 [188] | |||
| 1 mM | 16 h | BSA | 100 nM × 10 min | ↓ (≈42.8 ± 5%) | Kusudo et al., 2011 [189] | |||
| 1 mM | 16 h | BSA | No | ↔ (≈90.6 ± 5%) | Zhang et al., 2010 [61] | |||
| 1 mM | 16 h | BSA | 100 nM × 20 min | ↓ (≈72.0 ± 5%) | Zhang et al., 2010 [61] | |||
| 1 mM | 17 h | BSA | 100 nM × 10 min | ↓ Thr308 (≈16.6 ± 5%) | Pierre et al., 2016 [190] | |||
| 1 mM | 17 h | BSA | 100 nM × 10 min | ↓ Ser473 (≈29.6 ± 5%) | Pierre et al., 2016 [190] | |||
| 1 mM | 24 h | BSA | No | ↔ (≈103.2 ± 5%) | ↔ (≈119.2 ± 5%) | Vong et al., 2025 [191] | ||
| 1 mM | 24 h | BSA | 500 nM × unknown | ↓ (≈80.8 ± 5%) | ↓ (≈81.6 ± 5%) | Vong et al., 2025 [191] | ||
| 1 mM | 24 h | BSA | 100 nM × 30 min | ↓ (≈37.9 ± 5%) | Kim et al., 2024 [192] | |||
| 1 mM | 24 h | BSA | No | ↓ (≈24.5 ± 5%) | ↓ (≈40.0 ± 5%) | Xiang et al., 2023 [193] | ||
| 1 mM | 24 h | BSA | 100 nM × 30 min | ↓ Cytosolic (≈78.1 ± 5%) | Kim et al., 2020 [194] | |||
| 1 mM | 24 h | BSA | 100 nM × 30 min | ↑ (≈147.2 ± 5%) | ↓ (≈36.6 ± 5%) | ↓ Membranous (≈42.4 ± 5%) | ↓ (≈53.2 ± 5%) | Sun et al., 2020 [195] |
| 1 mM | 48 h | BSA | 17 nM × 15 min | ↓ (≈24.5 ± 5%) | ↓ (≈20.8 ± 5%) | Zhou et al., 2008 [196] | ||
| 1 mM | 48 h | BSA | 100 nM × 15 min | ↓ (≈40.7 ± 5%) | ↓ (≈68.6 ± 5%) | ↓ (≈42.6 ± 5%) | Sun et al., 2020 [195] | |
| 1.2 mM | 16 h | BSA | 100 nM × 15 min | ↓ (≈72.3 ± 5%) SEM | ↓ (≈55.8 ± 5%) SEM | Hsieh et al., 2014 [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmerman, J.M.; Klein, A.J.; Travis, K.B.; Vaughan, R.A. A Systematic Review of Palmitate-Mediated Insulin Resistance in C2C12 Myotubes. Nutrients 2025, 17, 3619. https://doi.org/10.3390/nu17223619
Zimmerman JM, Klein AJ, Travis KB, Vaughan RA. A Systematic Review of Palmitate-Mediated Insulin Resistance in C2C12 Myotubes. Nutrients. 2025; 17(22):3619. https://doi.org/10.3390/nu17223619
Chicago/Turabian StyleZimmerman, John M., Alexa J. Klein, Kipton B. Travis, and Roger A. Vaughan. 2025. "A Systematic Review of Palmitate-Mediated Insulin Resistance in C2C12 Myotubes" Nutrients 17, no. 22: 3619. https://doi.org/10.3390/nu17223619
APA StyleZimmerman, J. M., Klein, A. J., Travis, K. B., & Vaughan, R. A. (2025). A Systematic Review of Palmitate-Mediated Insulin Resistance in C2C12 Myotubes. Nutrients, 17(22), 3619. https://doi.org/10.3390/nu17223619







