Antitumor, Antioxidant, and Hepatoprotective Effects of Grape Seed Oil Nanoemulsion as a Dietary Phytochemical Intervention in Ehrlich Solid Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Grape Seed Oil Nanoemulsion
2.2. Induction of EAC in Mice
2.3. Animal Handling and Experimental Protocol
- Group 1 (Control): received no treatment.
- Group 2 (GSO): received GSO orally at 4 mL/kg/day for 30 days.
- Group 3 (GSONE): received GSO nanoemulsion (GSONE) orally at the same dose.
- Group 4 (EST): inoculated intramuscularly with 0.2 mL of EAC cell suspension (2.5 × 106 cells) in the right thigh on day 1 and left untreated thereafter.
- Group 5 (EST + GSO): received daily oral GSO (4 mL/kg) for 30 days following EAC inoculation.
- Group 6 (EST + GSONE): received daily oral GSONE (4 mL/kg) for 30 days following EAC inoculation.
2.4. Assessment of Body Weight, Tumor Weight, and Tumor Volume
2.5. Sample Collection
2.6. Serum Biochemical Analysis
2.7. Assessment of Antioxidant Status and DNA Oxidative Damage
2.8. Gene Expression Analysis of Apoptotic Markers
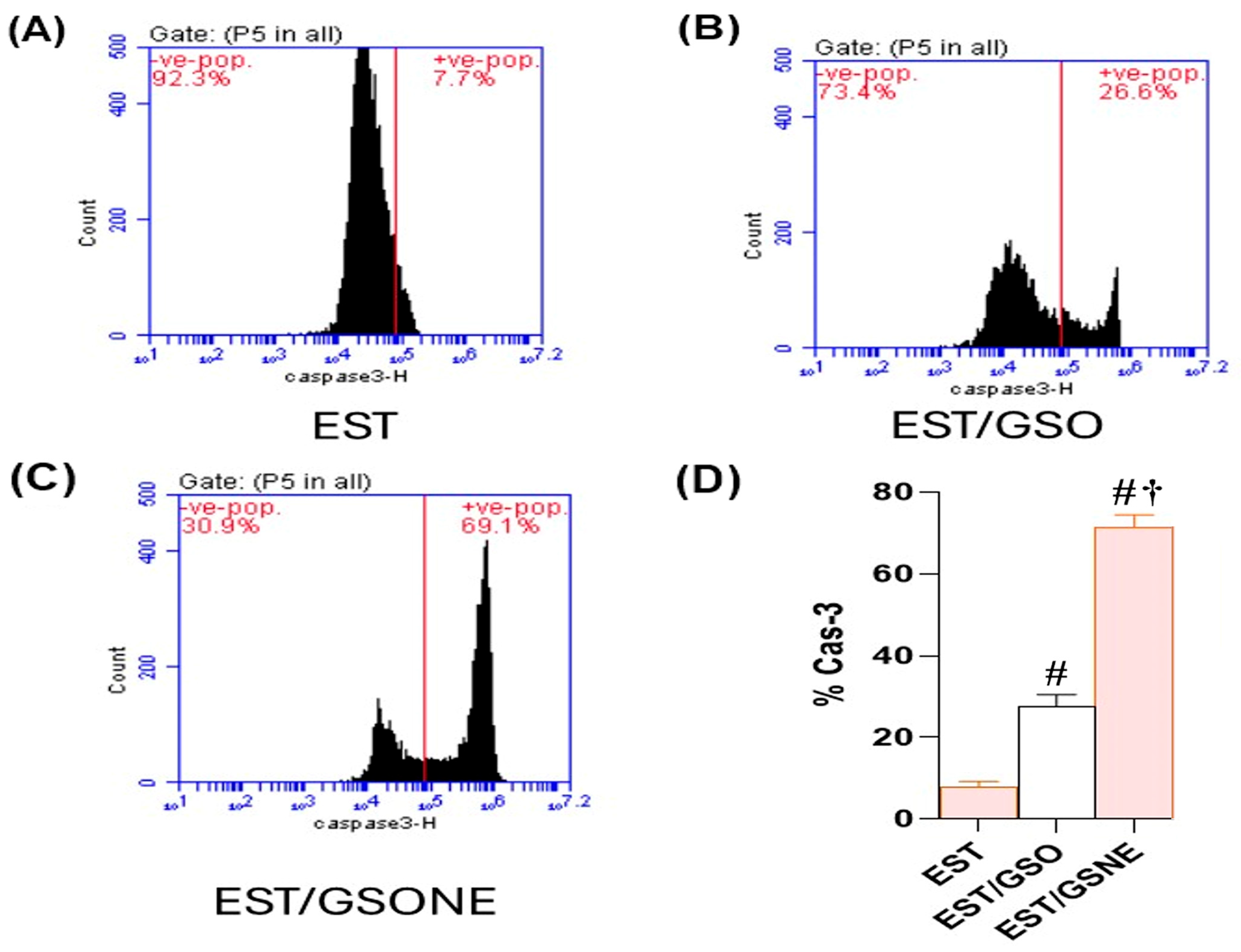
2.9. Flow Cytometric Analysis of p53 and Caspase-3 Expression
2.10. Histopathological Analysis
2.11. Transmission Electron Microscopy (TEM)
2.12. Statistical Analysis
3. Results
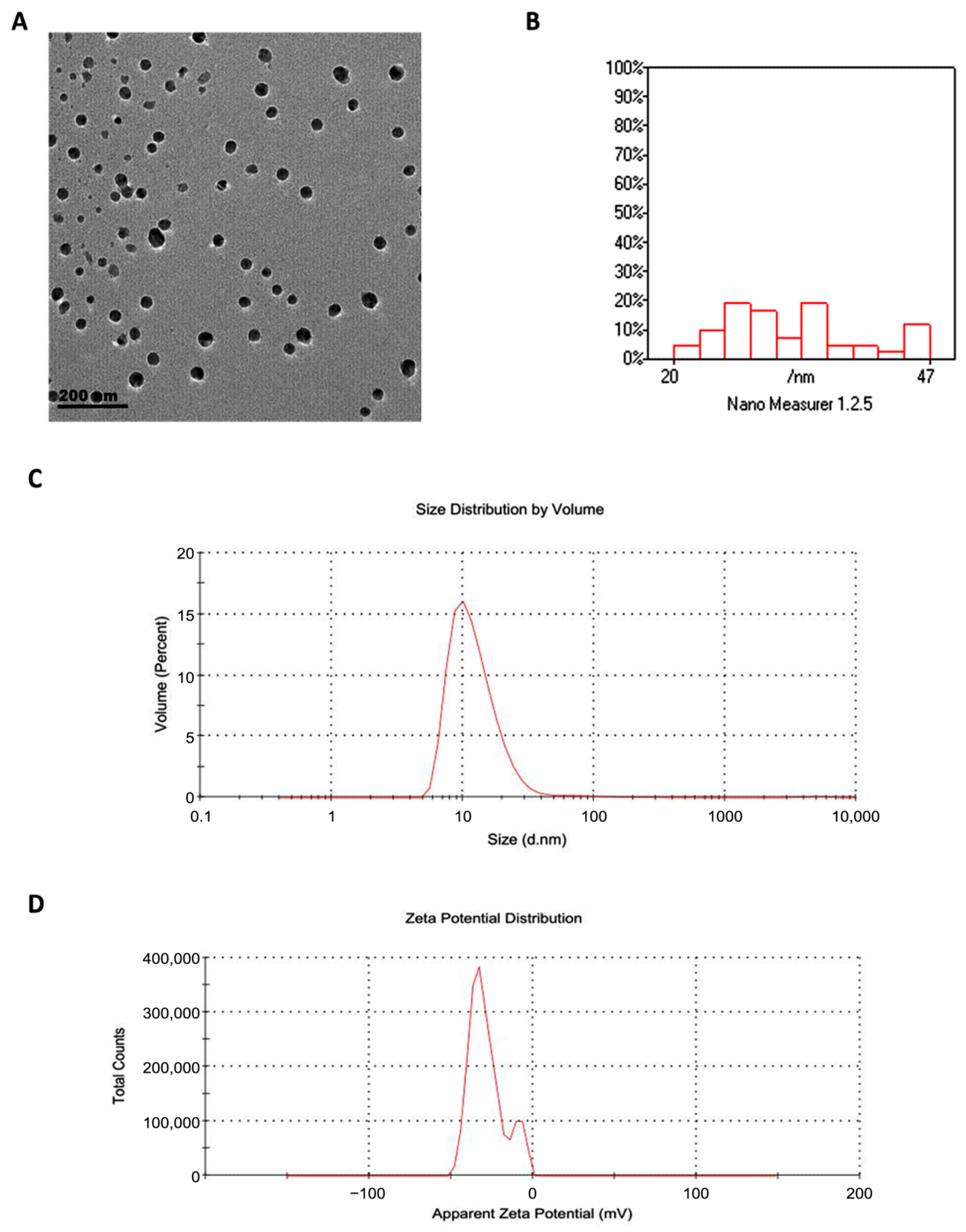
3.1. Characterization of Grape Seed Oil Nanoemulsion (GSONE)
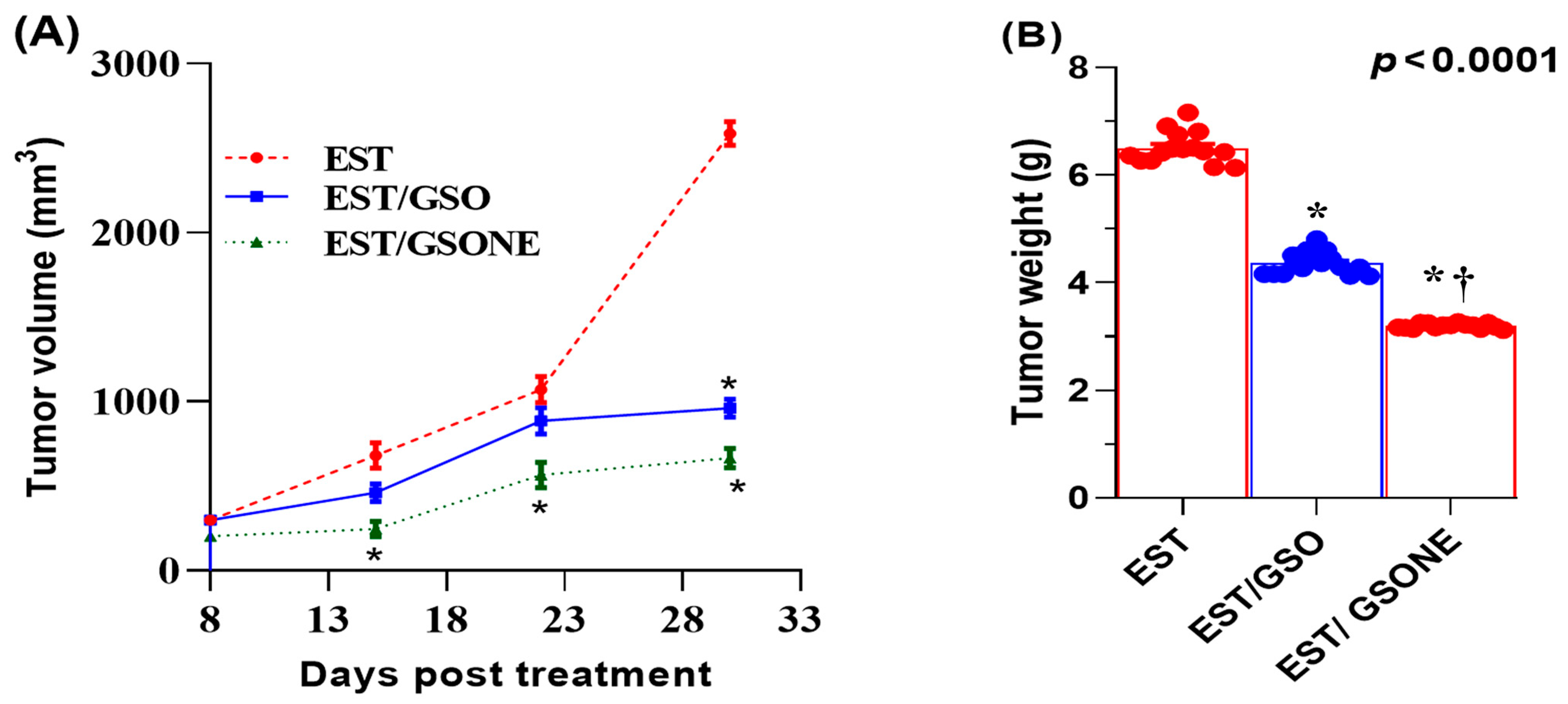
3.2. Growth Performance and Tumor Weight/Volume Changes
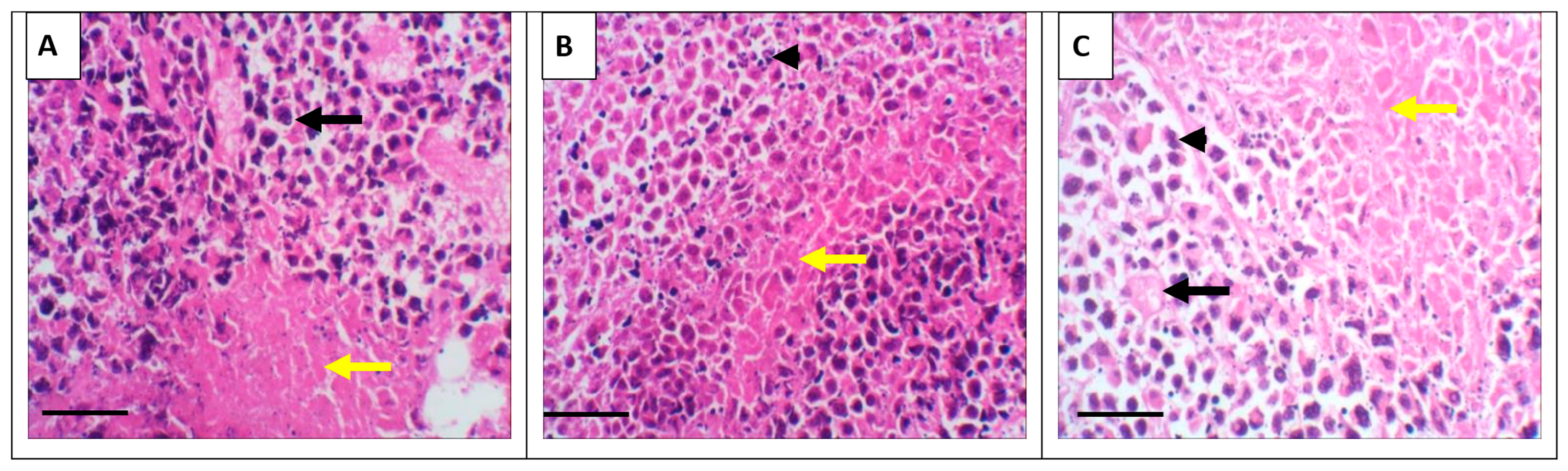
3.3. Tumor Histopathology
3.4. Blood Biochemical Parameters
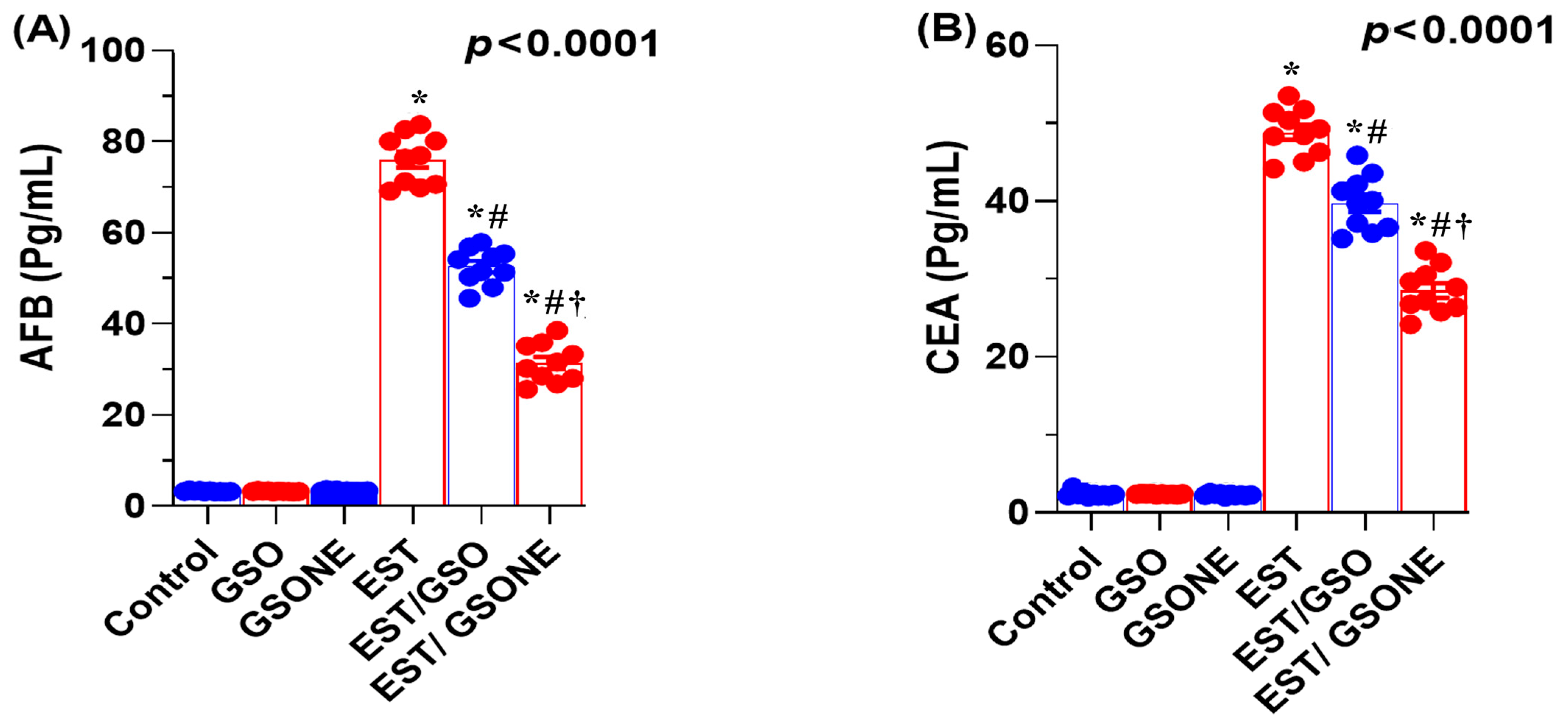
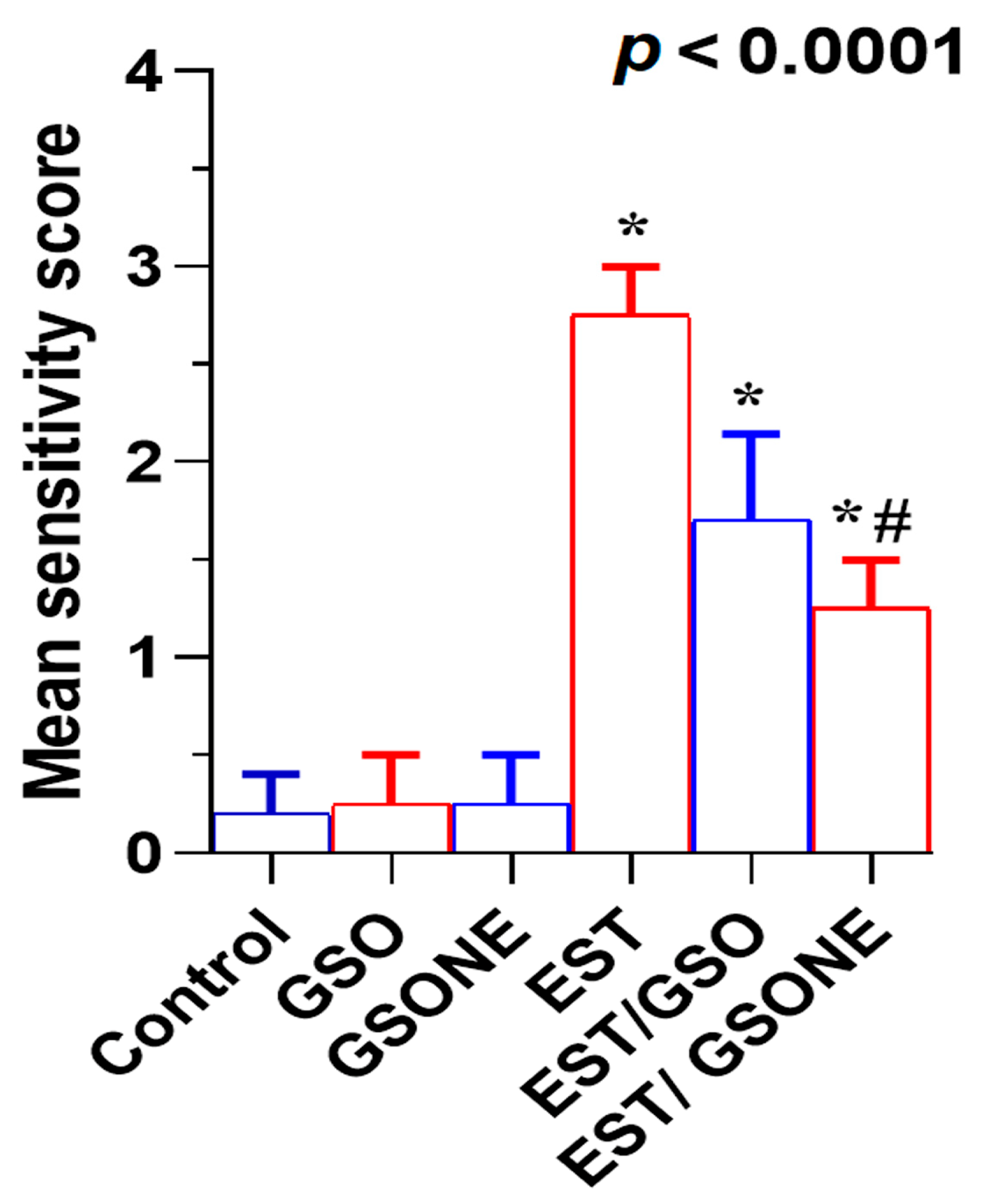
3.5. Tumor-Associated Biomarkers
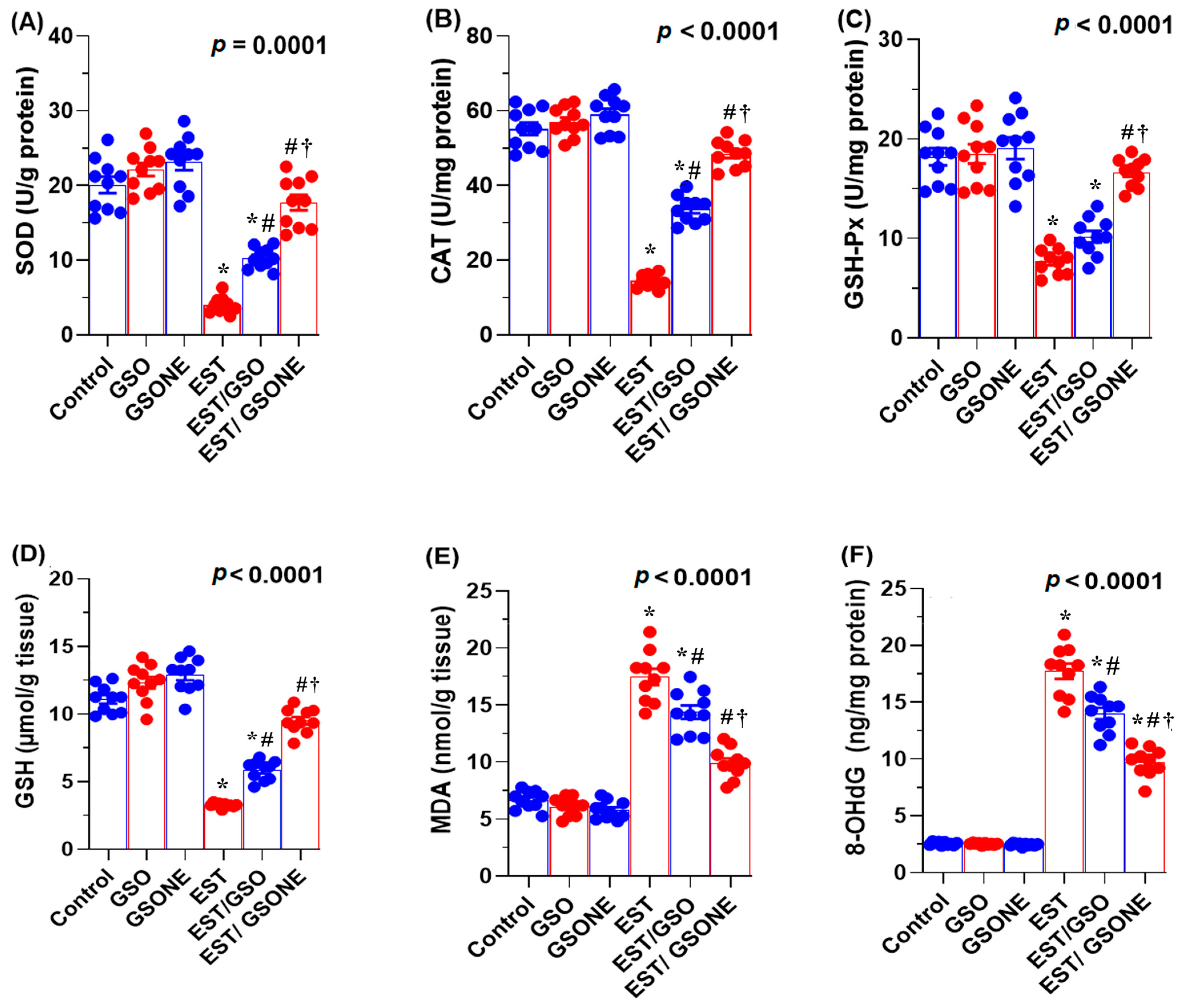
3.6. Redox Status and DNA Oxidative Damage
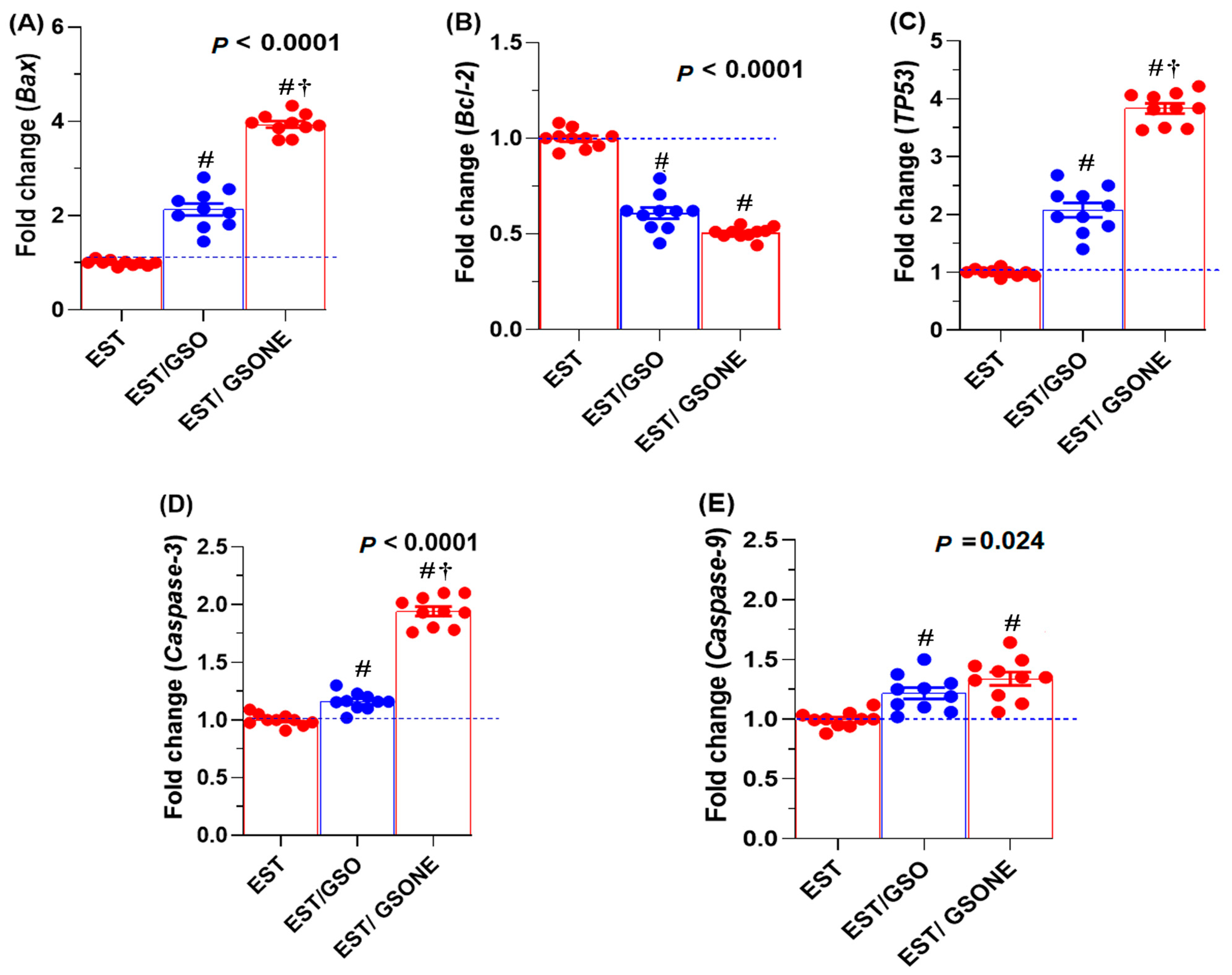
3.7. Apoptotic Gene Expression Profiles
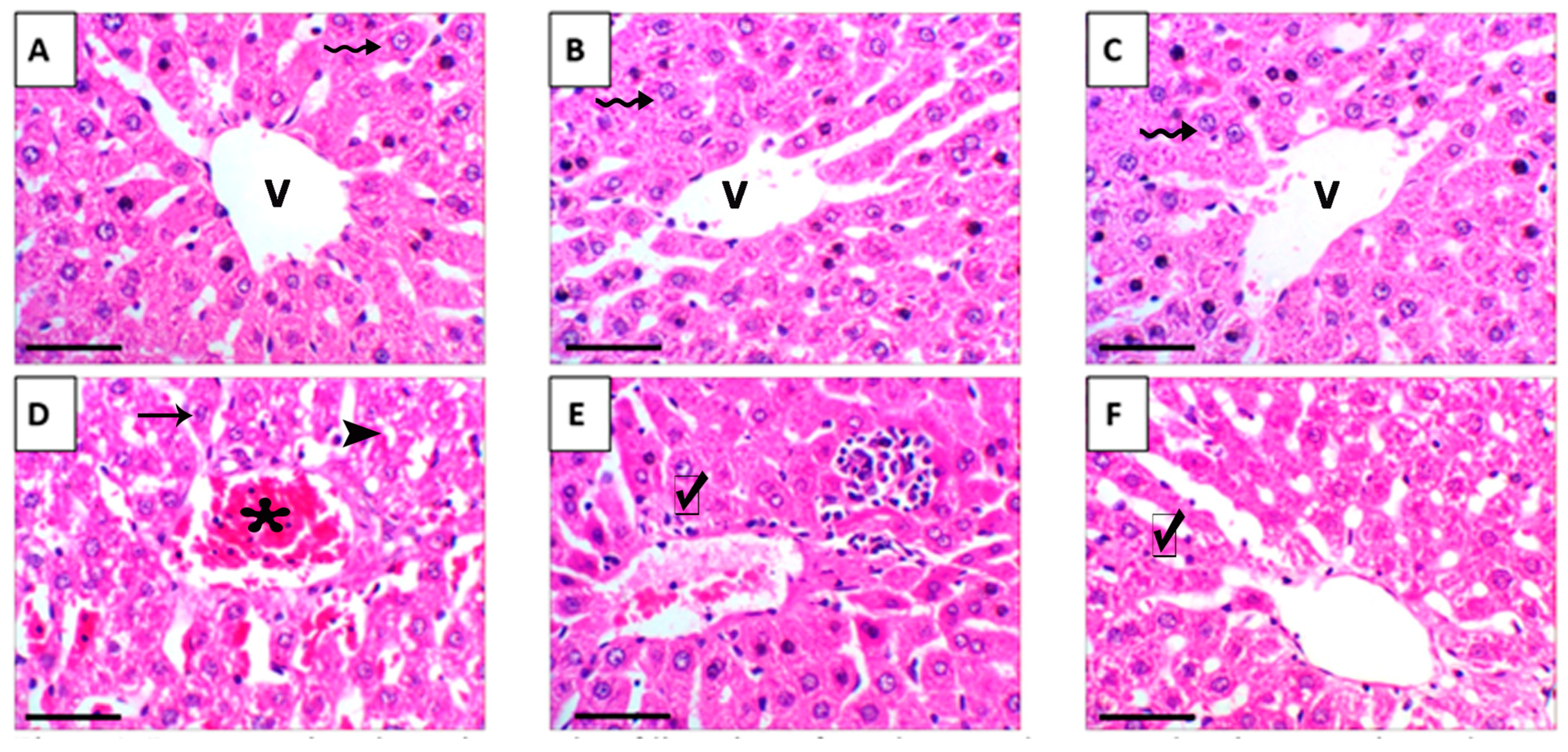
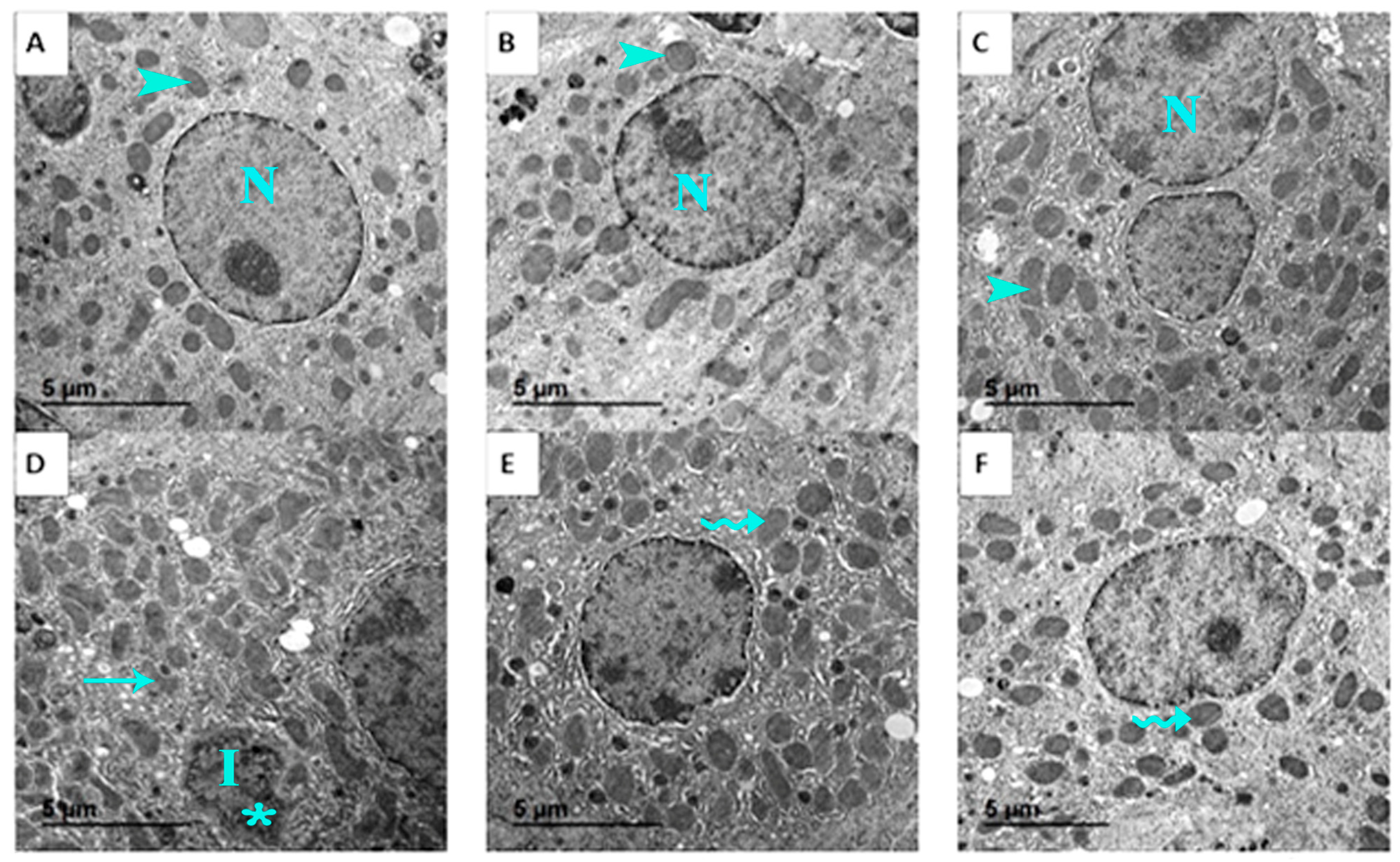
3.8. Liver Histopathology and Ultrastructural Changes
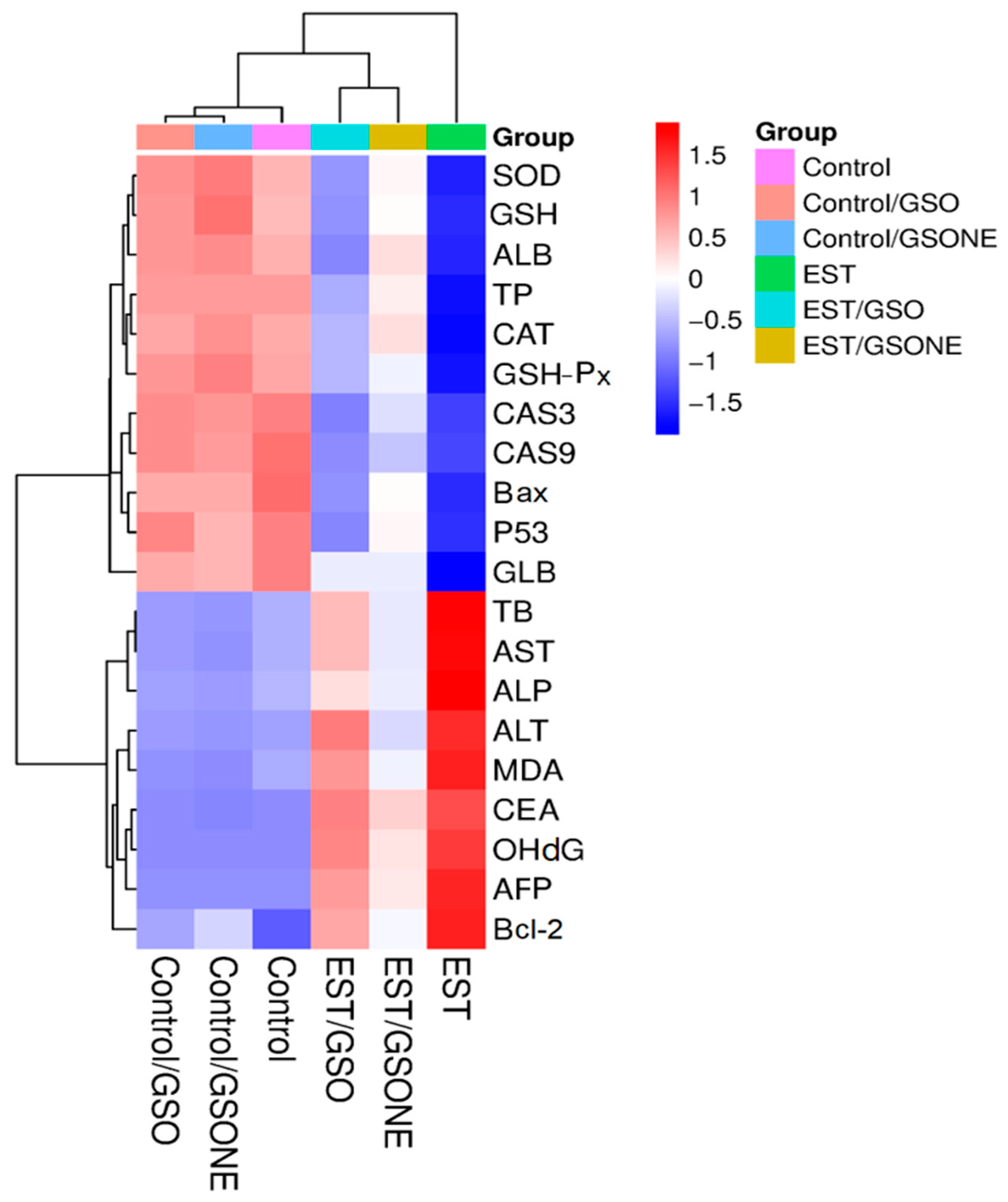
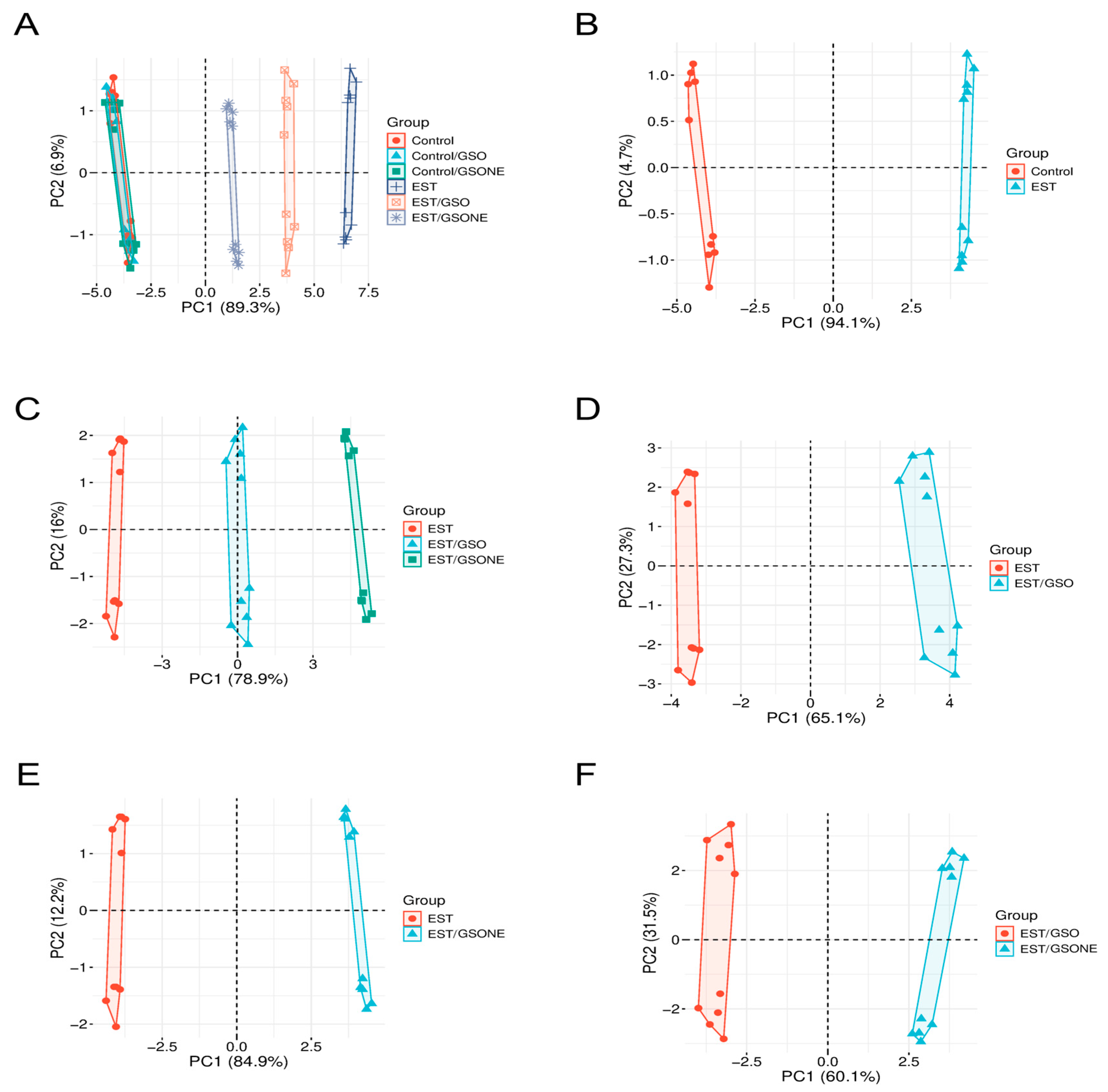
3.9. Multivariable Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| AFP | Alpha fetoprotein |
| AST | Aspartate aminotransferase |
| Bax | Bcl-2-associated x protein |
| Bcl-2 | B-cell lymphoma 2 |
| CEA | Carcinoembryonic antigen |
| CAT | Catalase |
| cDNA | Complementary DNA |
| DLS | Dynamic light scattering |
| EAC | Ehrlich Ascites Carcinoma |
| ELFA | Enzyme-linked fluorescent assay |
| EST | Ehrlich solid Tumor |
| GAPDH | Glyceraldehyde phosphate dehydrogenase |
| GSH | Glutathione (reduced) |
| GSO | Grape seed oil |
| GSONE | Grape seed oil nanoemulsion |
| GSH-Px | Glutathione peroxidase |
| H&E | Hematoxylin and eosin |
| MDA | Malondialdehyde |
| NCI | National Cancer Institute |
| p53 | Oncoprotein 53 |
| PBS | Phosphate-buffered saline |
| PCA | Principal component analysis |
| PDI | Polydispersity index |
| PE | Phycoerythrin |
| SE | Standard error |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
| TEM | Transmission electron microscopy |
| TP53 | Tumor protein 53 gene |
| TP | Total protein |
References
- Atuahene, D.; Mahama, K.; Sam, B.A.; Appiah, D.A.; Pandey, V.K.; Bela, K.; Harsányi, E.; Shaikh, A.M. Dietary targeting of cancer pathways: Role of bioactive compounds and nutraceuticals. Food Humanit. 2025, 5, 100748. [Google Scholar] [CrossRef]
- Li, L.; Jin, P.; Guan, Y.; Luo, M.; Wang, Y.; He, B.; Li, B.; He, K.; Cao, J.; Huang, C.; et al. Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals 2022, 15, 1540. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention-Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, A.; Rubio-Zarapuz, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F.; Beltrán-Velasco, A.I. Biomimetic Strategies for Nutraceutical Delivery: Advances in Bionanomedicine for Enhanced Nutritional Health. Biomimetics 2025, 10, 426. [Google Scholar] [CrossRef]
- Poureshaghi, F.; Eghlima, G.; Khanmohammadi, D.; Esmaeili, H.; Mirjalili, M.H. Variability in seed oil content, fatty acids profile, phytochemical properties, mineral and proximate composition of Iranian cultivars of Vitis vinifera L. Sci. Rep. 2025, 15, 23210. [Google Scholar] [CrossRef]
- Lin, Z.; Grasso, S. Exploring seed-based upcycled oils: Types, extraction processes, and emerging applications. Crit. Rev. Food Sci. Nutr. 2025, 1–20, ahead of print. [Google Scholar] [CrossRef]
- de Almeida Sousa Cruz, M.A.; de Barros Elias, M.; Calina, D.; Sharifi-Rad, J.; Teodoro, A.J. Insights into grape-derived health benefits: A comprehensive overview. Food Prod. Process. Nutr. 2024, 6, 91. [Google Scholar] [CrossRef]
- Dabetic, N.; Todorovic, V.; Djuricic, I.; Stankovic, J.; Basić, Z.; Vujovic, D.; Sobajic, S. Grape Seed Oil Characterization: A Novel Approach for Oil Quality Assessment. Eur. J. Lipid Sci. Technol. 2020, 122, 1900447. [Google Scholar] [CrossRef]
- Zhao, L.; Yagiz, Y.; Xu, C.; Fang, X.; Marshall, M.R. Identification and characterization of vitamin E isomers, phenolic compounds, fatty acid composition, and antioxidant activity in seed oils from different muscadine grape cultivars. J. Food Biochem. 2017, 41, e12384. [Google Scholar] [CrossRef]
- Bellili, S.; Jazi, S.; Nasr, S.; Dhifi, W.; Neves, M.A.; Miguel, M.G.C.; Mnif, W. Grape Seed Oil: Chemical Composition, Biological Properties and Health Benefits; Nova Science Publishers: Hauppauge, NY, USA, 2018; pp. 145–174. [Google Scholar]
- Mahanna, M.; Millan-Linares, M.C.; Grao-Cruces, E.; Claro, C.; Toscano, R.; Rodriguez-Martin, N.M.; Naranjo, M.C.; Montserrat-de la Paz, S. Resveratrol-enriched grape seed oil (Vitis vinifera L.) protects from white fat dysfunction in obese mice. J. Funct. Foods 2019, 62, 103546. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Liang, R.; Liu, W.; Chen, M.; Chen, J. Synergistic Anti-Inflammatory Effects of Lipophilic Grape Seed Proanthocyanidin and Camellia Oil Combination in LPS-Stimulated RAW264.7 Cells. Antioxidants 2022, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.A.; Mahsoub, F.; El Gamal, S.A.; Khamis, T.; Faroh, K.Y.; Abdelwarith, A.A.; Younis, E.M.; Saad, M.F.; Ali, H.S.; Davies, S.J.; et al. Chitosan-grape seed oil nanoemulsion enriched diet promotes performance, antioxidant-immune metrics and modifies immune- gene action and morphological architecture in Nile tilapia against Aeromonas veronii. Aquac. Rep. 2025, 41, 102697. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Castro, M.L.; Azevedo-Silva, J.; Valente, D.; Machado, A.; Ribeiro, T.; Ferreira, J.P.; Pintado, M.; Ramos, O.L.; Borges, S.; Baptista-Silva, S. Elevating Skincare Science: Grape Seed Extract Encapsulation for Dermatological Care. Molecules 2024, 29, 3717. [Google Scholar] [CrossRef]
- Rached, R.A.; Habre, M.; Salem, Y.; Khodeir, J.; Allaw, M.; Castangia, I.; Rajha, H.N.; Habre, L.; Feghali, J.; Touma, J.A.; et al. Clinical Trial to Evaluate the Effect of Grape Seed Extract-Loaded Hyalurosomes on Skin Wellness. Cosmetics 2025, 12, 38. [Google Scholar] [CrossRef]
- Nateghi, L.; Hosseini, E. Investigating the oxidative stability of grape seed oil using aqueous extract of pistachio green hull. J. Food Meas. Charact. 2023, 17, 4434–4447. [Google Scholar] [CrossRef]
- Bhutani, M.; Gaur, S.S.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Béla, K. Valorization of grape by-products: Insights into sustainable industrial and nutraceutical applications. Future Foods 2025, 12, 100710. [Google Scholar] [CrossRef]
- Böger, B.; Georgetti, S.; Kurozawa, L. Microencapsulation of grape seed oil by spray drying. Food Sci. Technol. 2018, 38, 263–270. [Google Scholar] [CrossRef]
- Mundo, J.L.M.; Zhou, H.; Tan, Y.; Liu, J.; McClements, D.J. Enhancing emulsion functionality using multilayer technology: Coating lipid droplets with saponin-polypeptide-polysaccharide layers by electrostatic deposition. Food Res. Int. 2021, 140, 109864. [Google Scholar] [CrossRef]
- Sepeidnameh, M.; Fazlara, A.; Hosseini, S.M.H.; Pourmahdi Borujeni, M. Encapsulation of grape seed oil in oil-in-water emulsion using multilayer technology: Investigation of physical stability, physicochemical and oxidative properties of emulsions under the influence of the number of layers. Curr. Res. Food Sci. 2024, 8, 100771. [Google Scholar] [CrossRef]
- Mutlu, N. Effects of grape seed oil nanoemulsion on physicochemical and antibacterial properties of gelatin-sodium alginate film blends. Int. J. Biol. Macromol. 2023, 237, 124207. [Google Scholar] [CrossRef]
- Radulski, D.R.; Stipp, M.C.; Galindo, C.M.; Acco, A. Features and applications of Ehrlich tumor model in cancer studies: A literature review. Transl. Breast Cancer Res. 2023, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, T.A.; El-Nagar, M.M.F.; El Mahdy, N.A.; Alherz, F.A.; Taher, R.; Osman, E.Y. Potential Antitumor Activity of Combined Lycopene and Sorafenib against Solid Ehrlich Carcinoma via Targeting Autophagy and Apoptosis and Suppressing Proliferation. Pharmaceuticals 2024, 17, 527. [Google Scholar] [CrossRef] [PubMed]
- Dahran, N.; Othman, M.S.; Ghoniem, M.E.; Samak, M.A.; Elabbasy, M.T.; Obeidat, S.T.; Aleid, G.M.; Abo Elnaga, S.; Khaled, A.M.; Altaleb, A.A.; et al. Evaluation of Vincamine Loaded with Silver Nanoparticles as a New Potential Therapeutic Agent Against Ehrlich’s Solid Carcinoma in Mice. Cells 2024, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Alfawaz, M.; Elmorsy, E.M.; Samy, A.; Shams, A.S.; Salem, M.A.; Shaalan, A.A.M.; Fawzy, M.S.; Hosny, N. Therapeutic Potential of Food-Derived Rutin Phytosome Nanoparticles: Antitumor, Antioxidant, and Anti-Inflammatory Activity in Ehrlich Ascites Carcinoma. Pharmaceuticals 2025, 18, 1410. [Google Scholar] [CrossRef]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- Aldubayan, M.A.; Elgharabawy, R.M.; Ahmed, A.S.; Tousson, E. Antineoplastic Activity and Curative Role of Avenanthramides against the Growth of Ehrlich Solid Tumors in Mice. Oxid. Med. Cell Longev. 2019, 2019, 5162687. [Google Scholar] [CrossRef]
- Sayed, H.M.; Said, M.M.; Morcos, N.Y.S.; El Gawish, M.A.; Ismail, A.F.M. Antitumor and Radiosensitizing Effects of Zinc Oxide-Caffeic Acid Nanoparticles against Solid Ehrlich Carcinoma in Female Mice. Integr. Cancer Ther. 2021, 20, 15347354211021920. [Google Scholar] [CrossRef]
- Uti, D.E.; Atangwho, I.J.; Alum, E.U.; Ntaobeten, E.; Obeten, U.N.; Bawa, I.; Agada, S.A.; Ukam, C.I.; Egbung, G.E. Antioxidants in cancer therapy mitigating lipid peroxidation without compromising treatment through nanotechnology. Discov. Nano 2025, 20, 70. [Google Scholar] [CrossRef]
- Yılmaz, S.; Doğanyiğit, Z.; Oflamaz, A.O.; Ateş, Ş.; Söylemez, E.S.A.; Nisari, M.; Farooqı, A.A. Determination of Rutin’s antitumoral effect on EAC solid tumor by AgNOR count and PI3K/AKT/mTOR signaling pathway. Med. Oncol. 2023, 40, 131. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; Tousson, E.; El-Masry, T.A.; Altwaijry, N.; Saleh, A. Ehrlich ascites carcinoma as model for studying the cardiac protective effects of curcumin nanoparticles against cardiac damage in female mice. Environ. Toxicol. 2021, 36, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. Methods Mol. Biol. 2011, 740, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.M.; Salem, A.A.M.; Eassawy, M.M.T. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of γ-irradiated rat. J. Photochem. Photobiol. B Biol. 2016, 160, 1–10. [Google Scholar] [CrossRef]
- Sannappa Gowda, N.G.; Shiragannavar, V.D.; Prabhuswamimath, S.C.; Tuladhar, S.; Chidambaram, S.B.; Santhekadur, P.K. Ehrlich Ascites carcinoma mice model for studying liver inflammation and fibrosis. Adv. Cancer Biol.-Metastasis 2022, 4, 100029. [Google Scholar] [CrossRef]
- Eltahir, Z.; Ibrahim, M.; Mohieldeen, M.Y.; Bayoumi, A.; Ahmed, S.M. Thymoquinone Nanoparticles (TQ-NPs) in Kidney Toxicity Induced by Ehrlich Ascites Carcinoma (EAC): An In Vivo Study. Can. J. Kidney Health Dis. 2024, 11, 20543581241258812. [Google Scholar] [CrossRef]
- Baris, M.M.; Serinan, E.; Calisir, M.; Simsek, K.; Aktas, S.; Yilmaz, O.; Ozdemir, S.K.; Secil, M. Xenograft Tumor Volume Measurement in Nude Mice: Estimation of 3D Ultrasound Volume Measurements Based on Manual Caliper Measurements. J. Basic Clin. Health Sci. 2020, 4, 90–95. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; Tulbah, F.S.A.; El-ghor, A.A.; Eissa, S.M. Suppression of tumor growth and apoptosis induction by pomegranate seed nanoemulsion in mice bearing solid Ehrlich carcinoma cells. Sci. Rep. 2023, 13, 5525. [Google Scholar] [CrossRef]
- Gencer, S.; Gür, C.; İleritürk, M.; Küçükler, S.; Akaras, N.; Şimşek, H.; Kandemir, F.M. The ameliorative effect of carvacrol on sodium arsenite-induced hepatotoxicity in rats: Possible role of Nrf2/HO-1, RAGE/NLRP3, Bax/Bcl-2/Caspase-3, and Beclin-1 pathways. J. Biochem. Mol. Toxicol. 2024, 38, e23863. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Liang, X.; Weng, J.; You, Z.; Wang, Y.; Wen, J.; Xia, Z.; Huang, S.; Luo, P.; Cheng, Q. Oxidative stress in cancer: From tumor and microenvironment remodeling to therapeutic frontiers. Mol. Cancer 2025, 24, 219. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Cebova, M.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Nedomova, S.; Baron, M.; Sochor, J. Health Effects of Grape Seed and Skin Extracts and Their Influence on Biochemical Markers. Molecules 2020, 25, 5311. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashmawy, G.M.; Labah, D.A.; Wahba, O.M.; Abdel Ghafar, M.T.; El-Feky, O.A. Cancer chemopreventive role of grape seed oil and cisplatin as a combination adjuvant therapy in the treatment of tongue squamous cell carcinoma: A biological in-vitro study. Arch. Oral Biol. 2023, 151, 105698. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, L.; Iacobazzi, R.M.; Quatrale, A.E.; Bergamini, C.; Denora, N.; Crupi, P.; Antonacci, D.; Mangia, A.; Simone, G.; Silvestris, N.; et al. Grape seed extracts modify the outcome of oxaliplatin in colon cancer cells by interfering with cellular mechanisms of drug cytotoxicity. Oncotarget 2017, 8, 50845–50863. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Heeba, G.H.; Elwy, H.M.; Murali, C.; El-Awady, R.; Amin, A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: In vivo and in vitro analyses. Sci. Rep. 2018, 8, 1270. [Google Scholar] [CrossRef]
- Chen, M.; Yu, S. Lipophilic Grape Seed Proanthocyanidin Exerts Anti-Proliferative and Pro-Apoptotic Effects on PC3 Human Prostate Cancer Cells and Suppresses PC3 Xenograft Tumor Growth in Vivo. J. Agric. Food Chem. 2019, 67, 229–235. [Google Scholar] [CrossRef]
- Suganya, M.; Gnanamangai, B.M.; Ravindran, B.; Chang, S.W.; Selvaraj, A.; Govindasamy, C.; Elsadek, M.F.; Ponmurugan, P. Antitumor effect of proanthocyanidin induced apoptosis in human colorectal cancer (HT-29) cells and its molecular docking studies. BMC Chem. 2019, 13, 21. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, J.; Huang, W. Grape Seed Proanthocyanidins Induce Apoptosis and Cell Cycle Arrest of HepG2 Cells Accompanied by Induction of the MAPK Pathway and NAG-1. Antioxidants 2020, 9, 1200. [Google Scholar] [CrossRef]
- Gašić, U.; Ćirić, I.; Pejčić, T.; Radenković, D.; Djordjević, V.; Radulović, S.; Tešić, Ž. Polyphenols as Possible Agents for Pancreatic Diseases. Antioxidants 2020, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.S.O.; Khan, E.; Elias, N.; Elshebiny, A.; Dou, Q. Updated Review on Natural Polyphenols: Molecular Mechanisms, Biological Effects, and Clinical Applications for Cancer Management. Biomolecules 2025, 15, 629. [Google Scholar] [CrossRef] [PubMed]
- Hirsa, M.; Fichna, J.; Tarasiuk-Zawadzka, A. Phytotherapy with Fruit Seed Extracts as a Promising Approach for the Treatment of Inflammation. Curr. Nutr. Rep. 2025, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, C.; Rapinel, V.; Nguyen-Thanh, B.; Bonet-García, R.; Bartier, M.; Claux, O.; Jacques, L.; Tabasso, S.; Barrajón-Catalán, E.; Fabiano-Tixier, A.-S. Sustainable grape seed oil processing: Green solvent extraction and by-product valorisation. Food Bioprod. Process. 2025, 149, 428–438. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Tanuku, S.; Velisila, D.; Thatraju, D.; Vadaga, A. Nanoemulsion Formulation Strategies for Enhanced Drug Delivery: Review Article. J. Pharma Insights Res. 2024, 2, 125–138. [Google Scholar] [CrossRef]
- Chavda, V.P.; Nalla, L.V.; Balar, P.; Bezbaruah, R.; Apostolopoulos, V.; Singla, R.K.; Khadela, A.; Vora, L.; Uversky, V.N. Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers 2023, 15, 1023. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Louisthelmy, R.; Maisel, K. Mechanisms of Nanoparticle Transport across Intestinal Tissue: An Oral Delivery Perspective. ACS Nano 2023, 17, 13044–13061. [Google Scholar] [CrossRef]
- Öztürk, K.; Kaplan, M.; Calis, S. Effects of nanoparticle size, shape, and zeta potential on drug delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef]
- Cahyani, D.M.; Mubarok, A.S.; Hariawan, B.S.; Amalina, I.; Drake, P.; Parumasivam, T.; Sahu, R.K.; Rijal, M.A.S.; Sari, R.; Miatmoko, A. Nanoparticle tools for maximizing oral drug delivery. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2025, 58, e14459. [Google Scholar] [CrossRef]
- Guo, S.; Liang, Y.; Liu, L.; Yin, M.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Research on the fate of polymeric nanoparticles in the process of the intestinal absorption based on model nanoparticles with various characteristics: Size, surface charge and pro-hydrophobics. J. Nanobiotechnol. 2021, 19, 32. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Roozitalab, G.; Yousefpoor, Y.; Abdollahi, A.; Safari, M.; Rasti, F.; Osanloo, M. Antioxidative, anticancer, and antibacterial activities of a nanoemulsion-based gel containing Myrtus communis L. essential oil. Chem. Pap. 2022, 76, 4261–4271. [Google Scholar] [CrossRef]
- Seyhan, V.; Barla Demirkoz, A.; Ner, M. Nanoemulsions: New Approaches in Cancer Therapy with Herbal Terpenes and Essential Oils; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–33. [Google Scholar]
- Rahaiee, S.; Assadpour, E.; Faridi Esfanjani, A.; Silva, A.S.; Jafari, S.M. Application of nano/microencapsulated phenolic compounds against cancer. Adv. Colloid Interface Sci. 2020, 279, 102153. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Kalam, N.; Shaikh, M.F.; Hasnain, M.; Hafiz, A.K.; Ansari, M.T. Nanoencapsulation of Polyphenols as Drugs and Supplements for Enhancing Therapeutic Profile—A Review. Curr. Mol. Pharmacol. 2021, 14, 77–107. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, J. Recent advance on the antitumor and antioxidant activity of grape seed extracts. Int. J. Wine Res. 2015, 2015, 63–67. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.-F.; Zheng, P.-S. Grape Seed Proanthocyanidins (GSPs) Inhibit the Growth of Cervical Cancer by Inducing Apoptosis Mediated by the Mitochondrial Pathway. PLoS ONE 2014, 9, e107045. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Zhang, X.; Zheng, Y.; Yu, F.; Liu, Y.; Wang, Y. Grape seed proanthocyanidins induce mitochondrial pathway-mediated apoptosis in human colorectal carcinoma cells. Oncol. Lett. 2017, 14, 5853–5860. [Google Scholar] [CrossRef]
- Ali, D.A.; Badr El-Din, N.K.; Abou-El-magd, R.F. Antioxidant and hepatoprotective activities of grape seeds and skin against Ehrlich solid tumor induced oxidative stress in mice. Egypt. J. Basic Appl. Sci. 2015, 2, 98–109. [Google Scholar] [CrossRef]
- Madbouly, N.; Ali, D.; Farid, A. Nanoparticles from grape seed extract inhibit inflammatory cytokines and ameliorate CCl4-induced hepatotoxicity. BMC Complement. Med. Ther. 2025, 25, 276. [Google Scholar] [CrossRef] [PubMed]
- Alhajlah, S. Effect of grape-derived products on the serum levels of enzymes mainly produced by the liver: A systematic review and meta-analysis of parallel randomized controlled trials. Phytother. Res. PTR 2024, 38, 3583–3593. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.; Lv, C.; Liu, B.; Yuan, C.; Huang, W.; Luo, Q.; Xiao, Y.; Sun, C.; Li, T.; et al. Modulation of Keap1-Nrf2-ARE signaling pathway by oxyresveratrol, a derivative of resveratrol from grape skin. Food Biosci. 2022, 50, 102162. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Wang, T.; Zhang, L.; Wang, H.; Lu, H.; Yang, R.; Ding, Y. Grape Seed Proanthocyanidins Protect Pancreatic β Cells Against Ferroptosis via the Nrf2 Pathway in Type 2 Diabetes. Biol. Trace Elem. Res. 2024, 202, 5531–5544. [Google Scholar] [CrossRef]
- Shrotriya, S.; Deep, G.; Lopert, P.; Patel, M.; Agarwal, R.; Agarwal, C. Grape seed extract targets mitochondrial electron transport chain complex III and induces oxidative and metabolic stress leading to cytoprotective autophagy and apoptotic death in human head and neck cancer cells. Mol. Carcinog. 2015, 54, 1734–1747. [Google Scholar] [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and cytotoxic effects of grape pomace and grape seed extracts on colorectal cancer cell lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Tousson, E.; Soliman, M.M.; El Sayed, I.E.T.; Abdel Aleem, A.A.H.; Elsharkawy, H.N. Grape seed extract ameliorated Ehrlich solid tumor-induced hepatic tissue and DNA damage with reduction of PCNA and P53 protein expression in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 44226–44238. [Google Scholar] [CrossRef]
- Yousefpoor, Y.; Esnaashari, S.S.; Baharifar, H.; Mehrabi, M.; Amani, A. Current challenges ahead in preparation, characterization, and pharmaceutical applications of nanoemulsions. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1920. [Google Scholar] [CrossRef]
- Goswami, A.; Rawat, R.; Pillai, P.; Saw, R.; Joshi, D.; Mandal, A. Formulation and characterization of nanoemulsions stabilized by nonionic surfactant and their application in enhanced oil recovery. Pet. Sci. Technol. 2023, 42, 2990–3008. [Google Scholar] [CrossRef]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.F.; Zhou, X. Lipid-based nanoparticles as drug delivery carriers for cancer therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- Bagheri Karimi, M. Preparation of Lipid Based Nanoparticles from Extracts of Grape Seeds and Evaluation of Their In Vitro Anticancer Effect. Ph.D. Thesis, Tabriz University of Medical Sciences, School of Pharmacy, Tabriz, Iran, 2024. [Google Scholar]
- Hashim, G.M.; Shahgolzari, M.; Hefferon, K.; Yavari, A.; Venkataraman, S. Plant-Derived Anticancer Therapeutics and Biopharmaceuticals. Bioengineering 2025, 12, 7. [Google Scholar]


| Gene | Sequences (5′-3′) | Length (bp) |
|---|---|---|
| Bax | F: GTCTCCGGCGAATTGGAGAT R: ACCCGGAAGAAGACCTCTCG | 100 |
| Bcl-2 | F: CATCGCCCTGTGGATGACTG R: GGCCATATAGTTCCACAAAGGC | 95 |
| TP53 | F: CCCCTGTCATCTTTTGTCCCT R: AGCTGGCAGAATAGCTTATTGAG | 137 |
| Caspase-3 | F: ACTGGAATGTCAGCTCGCAA R: GCAGTAGTCGCCTCTGAAGA | 270 |
| Caspase-9 | F: ACGTGAACTTCTGCCCTTCC R: GGTCGTTCTTCACCTCCACC | 117 |
| GAPDH | F: GTATCGGACGCCTGGTTAC R: CTTGCCGTGGGTAGAGTCAT | 128 |
| Score | Hepatic Lesions Were Graded Using an Integrated Semi-Quantitative Scoring System. |
|---|---|
| 0 (none) | Histological analysis revealed normal architecture. |
| 1 (mild) | Hepatocellular degeneration and necrosis were occasionally observed, accompanied by minimal or absent inflammatory infiltration and rare vascular congestion. |
| 2 (moderate) | Moderate vacuolar degeneration, scattered inflammatory cell infiltration, multifocal hepatocellular necrosis, and mild blood vessel congestion were shown in the hepatic tissue. |
| 3 (severe) | Diffuse and severe degenerative alterations were shown in hepatic tissue, along with widespread infiltration of leukocytes, extensive hepatocellular necrosis, and moderate to severe congestion of the hepatic vasculature. |
| Parameters | Control | GSO | GSONE | EST | EST/GSO | EST/GSONE | p-Values |
|---|---|---|---|---|---|---|---|
| IBW (g) | 21.86 ± 0.33 | 21.69 ± 0.29 | 21.44 ± 0.26 | 21.93 ± 0.38 | 21.56 ± 0.37 | 21.38 ± 0.48 | 0.746 |
| FBW (g) | 27.46 ± 0.24 | 27.86 ± 0.31 | 27.93 ± 0.22 | 27.38 ± 0.19 | 26.87 ± 0.25 | 27.34 ± 0.18 | 0.041 |
| NW(g) | 27.46 ± 0.24 | 27.86 ± 0.31 | 27.93 ± 0.22 | 20.62 ± 0.19 * | 22.43 ± 0.25 *# | 24.16 ± 0.12 *#† | 0.003 |
| BWG (g) | 5.60 ± 0.09 | 6.17 ± 0.02 | 6.49 ± 0.04 | −1.30 ± 0.19 * | 0.87 ± 0.12 *# | 2.79 ± 0.37 *#† | <0.0001 |
| Parameters | Control | GSO | GSONE | EST | EST/GSO | EST/GSONE | p-Values |
|---|---|---|---|---|---|---|---|
| TP (g/dL) | 6.88 ± 0.29 | 6.90 ± 0.31 | 6.92 ± 0.19 | 4.13 ± 0.13 * | 5.38 ± 0.32 *# | 6.19 ± 0.27 #† | 0.0146 |
| Alb (g/dL) | 3.79 ± 0.13 | 3.93 ± 0.17 | 3.98 ± 0.21 | 2.16 ± 0.07 * | 2.71 ± 0.12 *# | 3.53 ± 0.18 #† | 0.0273 |
| Glo (g/dL) | 3.09 ± 0.16 | 2.97 ± 0.14 | 2.94 ± 0.06 | 1.97 ± 0.06 * | 2.67 ± 0.10 # | 2.66 ± 0.09 # | 0.0047 |
| TB (mg/dL) | 0.42 ± 0.09 | 0.38 ± 0.07 | 0.36 ± 0.06 | 1.32 ± 0.25 * | 0.86 ± 0.03 *# | 0.58 ± 0.10 # | <0.0001 |
| ALP (U/L) | 88.32 ± 4.26 | 86.25 ± 5.18 | 85.41 ± 6.22 | 128.14 ± 7.52 * | 101.21 ± 6.91 # | 95.33 ± 4.93 # | 0.0026 |
| ALT (U/L) | 46.26 ± 3.24 | 45.21 ± 4.35 | 45.03 ± 5.11 | 89.25 ± 4.89 * | 78.29 ± 6.23 * | 54.26 ± 5.39 #† | 0.0001 |
| AST (U/L) | 76.32 ± 6.66 | 73.41 ± 4.20 | 72.27 ± 5.38 | 118.39 ± 6.15 * | 96.20 ± 4.44 *# | 84.06 ± 5.93 # | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, A.A.M.; Elmorsy, E.M.; Embaby, E.M.; Alfawaz, M.; Aly, N.M.; Shams, A.S.; Fawzy, M.S.; Hosny, N. Antitumor, Antioxidant, and Hepatoprotective Effects of Grape Seed Oil Nanoemulsion as a Dietary Phytochemical Intervention in Ehrlich Solid Tumors. Nutrients 2025, 17, 3450. https://doi.org/10.3390/nu17213450
Shaalan AAM, Elmorsy EM, Embaby EM, Alfawaz M, Aly NM, Shams AS, Fawzy MS, Hosny N. Antitumor, Antioxidant, and Hepatoprotective Effects of Grape Seed Oil Nanoemulsion as a Dietary Phytochemical Intervention in Ehrlich Solid Tumors. Nutrients. 2025; 17(21):3450. https://doi.org/10.3390/nu17213450
Chicago/Turabian StyleShaalan, Aly A. M., Ekramy M. Elmorsy, Eman M. Embaby, M. Alfawaz, Nagwa M. Aly, Ahmed S. Shams, Manal S. Fawzy, and Nora Hosny. 2025. "Antitumor, Antioxidant, and Hepatoprotective Effects of Grape Seed Oil Nanoemulsion as a Dietary Phytochemical Intervention in Ehrlich Solid Tumors" Nutrients 17, no. 21: 3450. https://doi.org/10.3390/nu17213450
APA StyleShaalan, A. A. M., Elmorsy, E. M., Embaby, E. M., Alfawaz, M., Aly, N. M., Shams, A. S., Fawzy, M. S., & Hosny, N. (2025). Antitumor, Antioxidant, and Hepatoprotective Effects of Grape Seed Oil Nanoemulsion as a Dietary Phytochemical Intervention in Ehrlich Solid Tumors. Nutrients, 17(21), 3450. https://doi.org/10.3390/nu17213450










