The Effects of 12-Week Prebiotic Supplementation on General Wellness and Exercise-Induced Gastrointestinal Symptoms in Recreationally Trained Endurance Athletes: A Triple-Blind Randomised Controlled Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
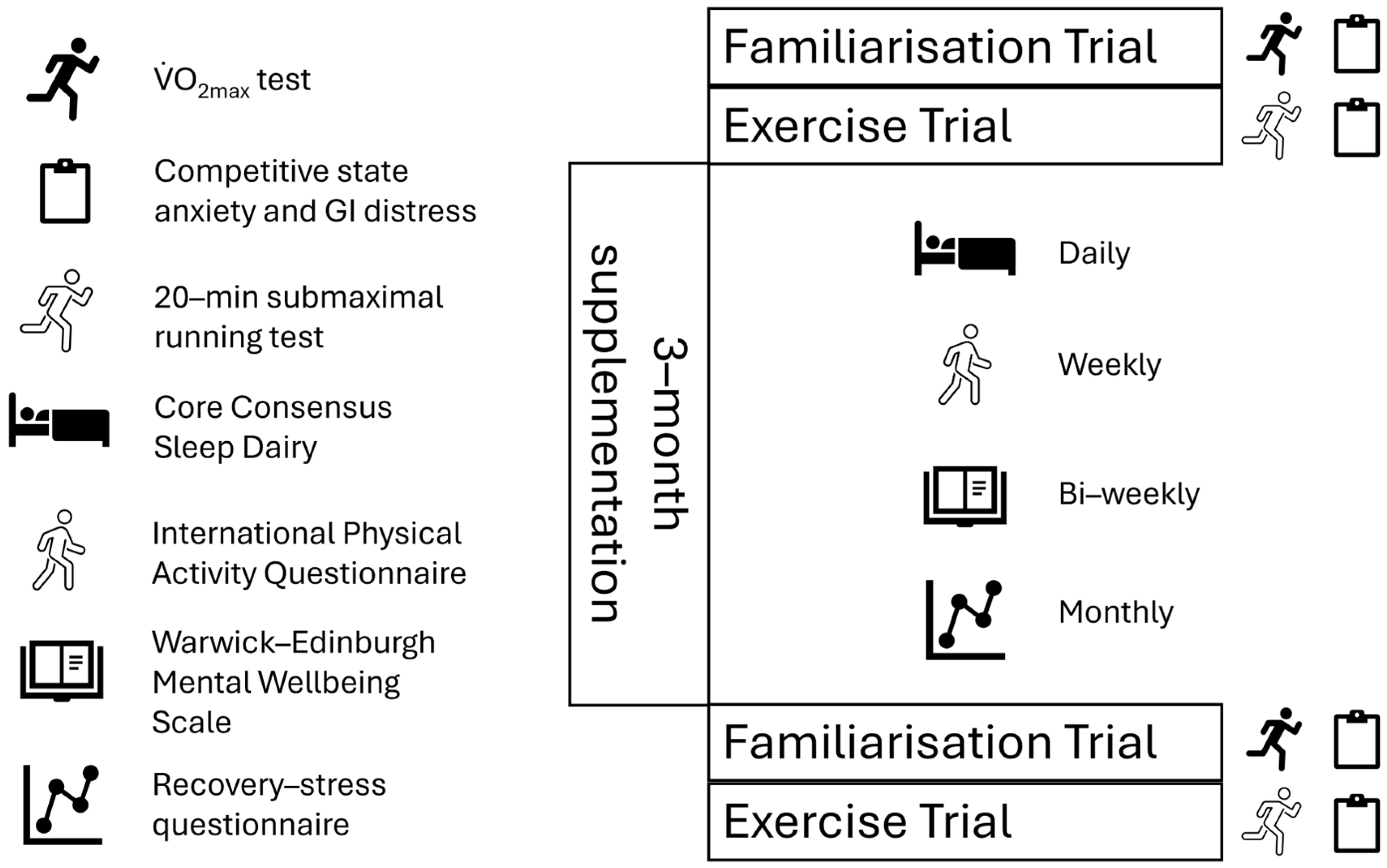
2.2. Experimental Overview
2.3. Maximum Rate of Oxygen Uptake Test and Familiarisation
2.4. Experimental Procedures
2.5. Statistical Analysis
3. Results
3.1. Summary and Adherence
3.2. Group, Time, and Group × Time Effects
3.3. Explanatory Power of Individual Differences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GOS | Galactooligosaccharide |
| GI | Gastrointestinal |
| VO2max | Maximal rate of oxygen uptake |
| CSAI-2 | Competitive State Anxiety Inventory-2 questionnaire |
| URTI | Upper respiratory tract infection |
| PAR-Q | Physical Activity Readiness Questionnaire |
| RPE | Rate of Perceived Exertion |
| mVAS | Modified Visual Analogue Scale |
| IPAQ-7 | International Physical Activity Questionnaire |
| WEMWBS | Warwick-Edinburgh Mental Wellbeing Scale |
| RESTQ | Recovery–stress questionnaire |
| LMM | Linear mixed modelling |
| AIC | Akaike Information Criterion |
| BIC | Bayesian Information Criterion |
| LM | Linear model |
| CI | 95% confidence interval |
References
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The athlete gut microbiome and its relevance to health and performance: A review. Sports Med. 2022, 52, 119–128. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef]
- Parker, C.; Hunter, K.A.; Johnson, M.A.; Sharpe, G.R.; Gibson, G.R.; Walton, G.E.; Williams, N.C. Effects of 24-week prebiotic intervention on self-reported upper respiratory symptoms, gastrointestinal symptoms, and markers of immunity in elite rugby union players. Eur. J. Sport. Sci. 2023, 23, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Sohn, W.; Lee, O.Y.; Lee, S.P.; Lee, K.N.; Jun, D.W.; Seo, J.G. Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014, 29, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Eyice, Ö.; Koumoutsos, I.; Lomer, M.C.; Irving, P.M.; Lindsay, J.O.; Whelan, K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients 2021, 13, 3598. [Google Scholar] [CrossRef]
- Wilson, B.; Rossi, M.; Kanno, T.; Parkes, G.C.; Anderson, S.; Mason, A.J.; Whelan, K. β-Galactooligosaccharide in conjunction with low FODMAP diet improves irritable bowel syndrome symptoms but reduces fecal bifidobacteria. Off. J. Am. Coll. Gastroenterol. 2020, 115, 906–915. [Google Scholar] [CrossRef]
- Nieman, D.C. Is infection risk linked to exercise workload? Med. Sci. Sports Exerc. 2000, 32, S406–S411. [Google Scholar] [CrossRef]
- Tobar, D.A. Overtraining and staleness: The importance of psychological monitoring. Int. J. Sport Exerc. Psychol. 2005, 3, 455–468. [Google Scholar] [CrossRef]
- Lakens, D. Sample Size Justification. Collabra Psychol. 2022, 8, 33267. [Google Scholar] [CrossRef]
- Reilly, T.; Brooks, G.A. Selective persistence of circadian rhythms in physiological responses to exercise. Chronobiol. Int. 1990, 7, 59–67. [Google Scholar] [CrossRef]
- Chantler, S.; Griffiths, A.; Matu, J.; Davison, G.; Jones, B.; Deighton, K. The effects of exercise on indirect markers of gut damage and permeability: A systematic review and meta-analysis. Sports Med. 2021, 51, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Doust, J.H. The Conconi test is not valid for estimation of the lactate turnpoint in runners. J. Sports Sci. 1997, 15, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.; Jones, A.M.; Barstow, T.J.; Burnley, M.; Williams, C.; Doust, J.H. Effect of endurance training on oxygen uptake kinetics during treadmill running. J. Appl. Physiol. 2000, 89, 1744–1752. [Google Scholar] [CrossRef]
- Bird, S.R.; Davison, R.C.R. (Eds.) Guidelines for the Physiological Testing of Athletes, 3rd ed.; British Association of Sport and Exercise Sciences: Leeds, UK, 1997. [Google Scholar]
- Martens, R.; Burton, D.; Vealey, R.S.; Bump, L.A.; Smith, D.E. Development and validation of the competitive state anxiety inventory 2. Compet. Anxiety Sport 1990, 3, 117–190. [Google Scholar]
- Gaskell, S.K.; Snipe, R.M.; Costa, R.J. Test–retest reliability of a modified visual analog scale assessment tool for determining incidence and severity of gastrointestinal symptoms in response to exercise stress. Int. J. Sport. Nutr. Exerc. Metab. 2019, 29, 411–419. [Google Scholar] [CrossRef]
- Costello, N.; Deighton, K.; Dyson, J.; McKenna, J.; Jones, B. Snap-N-Send: A valid and reliable method for assessing the energy intake of elite adolescent athletes. Eur. J. Sport. Sci. 2017, 17, 1044–1055. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh mental well-being scale (WEMWBS): Development and UK validation. Health Qual. Life Outcomes 2007, 5, 1–13. [Google Scholar] [CrossRef]
- Kellmann, M.; Kallus, K.W. Recovery-Stress Questionnaire for Athletes: User Manual; Human Kinetics: Champaign, IL, USA, 2001. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Tjoelker, R.S.; Chin, E.; Zwart, N.R.K.; Schoemaker, M.H.; Schaafsma, A.; Bovee, I. The effect of Galacto-Oligosaccharides (GOS) on self-perceived stress in apparently healthy but stressed Dutch women: A randomized controlled home-based trial. J. Neurol. Clin. Neurosci. 2023, 7, 24, abstract page. [Google Scholar]
- Freijy, T.M.; Cribb, L.; Oliver, G.; Metri, N.J.; Opie, R.S.; Jacka, F.N.; Sarris, J. Effects of a high-prebiotic diet versus probiotic supplements versus synbiotics on adult mental health: The “Gut Feelings” randomised controlled trial. Front. Neurosci. 2023, 16, 1097278. [Google Scholar] [CrossRef]
- Maheswaran, H.; Weich, S.; Powell, J.; Stewart-Brown, S. Evaluating the responsiveness of the Warwick Edinburgh Mental Well-Being Scale (WEMWBS): Group and individual level analysis. Health Qual. Life Outcomes 2012, 10, 156. [Google Scholar] [CrossRef]
- Vulevic, J.; Tzortzis, G.; Juric, A.; Gibson, G.R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol. Motil. 2018, 30, e13440. [Google Scholar] [CrossRef]
- Drakoularakou, A.; Tzortzis, G.; Rastall, R.A.; Gibson, G.R. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. Eur. J. Clin. Nutr. 2010, 64, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Wiącek, J.; Karolkiewicz, J. Different Approaches to Ergogenic, Pre-, and Probiotic Supplementation in Sports with Different Metabolism Characteristics: A Mini Review. Nutrients 2023, 15, 1541. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Tzortzis, G.; Vulevic, J.; I’Anson, K.; Gibson, G.R. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: A randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr. 2008, 87, 785–791. [Google Scholar] [CrossRef] [PubMed]
| Variable | Group | Time 1 (Mean ± SD) | Time 2 (Mean ± SD) | Change% (Cohen’s D) | Main Effect of Group (β, p-Value, CI) | Main Effect of Time (β, p-Value, CI) | Interaction (Group × Time) (β, p-Value, CI) | Marginal R2 | Conditional R2 | Explanatory Power of Random Effects (Change in r2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive A state | B-GOS | 32 ± 3 | 14 ± 5 | −55 (−4.56) | −0.11, p = 0.949, [−3.57, 3.34] | −17.56, p < 0.001, [−20.37, −14.75] * | 0.11, p = 0.954, [−3.66, 3.89] | 0.857 | 0.912 | 0.055 (6%) |
| PLA | 32 ± 3 | 14 ± 4 | −55 (−4.53) | |||||||
| Somatic A state | B-GOS | 12 ± 3 | 13 ± 5 | 3.6 (0.14) | −0.67, p = 0.647, [−3.56, 2.22] | 0.44, p = 0.639, [−1.47, 2.35] | −0.44, p = 0.739, [−3.06, 2.17] | 0.780 | 0.854 | 0.074 (7%) |
| PLA | 12 ± 3 | 12 ± 2 | 0 (0) | |||||||
| State self confidence | B-GOS | 29 ± 3 | 28 ± 5 | −0.8 (0.05) | −2.44, p = 0.230, [−6.46, 1.57] | −0.22, p = 0.881, [−3.11, 2.67] | −0.44, p = 0.832, [−4.25, 3.36] | 0.842 | 0.876 | 0.034 (3%) |
| PLA | 26 ± 4 | 26 ± 6 | −3 (0.15) | |||||||
| Overall gut discomfort | B-GOS | 1 ± 3 | 0.1 ± 0.3 | −90 (0.74) | −1.11, p = 0.073, [−2.30, 0.07] | −1.00, p = 0.127, [−2.32, 0.32] | 1.11, p = 0.227, [−0.67, 2.88] | - | - | - |
| PLA | 0.1 ± 0.4 | 0.1 ± 0.3 | 0 (0.08) | |||||||
| Upper GISs | B-GOS | 5 ± 10 | 3 ± 6 | −40 (0.31) | −3.44, p = 0.207, [−8.92, 2.04] | −1.89, p = 0.456, [−6.19, 2.41] | 1.78, p = 0.618, [−5.47, 9.03] | 0.062 | 0.199 | 0.137 (14%) |
| PLA | 1 ± 2 | 1 ± 2 | −8 (0.02) | |||||||
| Lower GISs | B-GOS | 6 ± 12 | 2 ± 5 | −66 (0.62) | −3.22, p = 0.285, [−9.24, 2.80] | −4.11, p = 0.129, [−9.33, 1.10] | 1.67, p = 0.654, [−5.90, 9.24] | 0.1 | 0.316 | 0.216 (22%) |
| PLA | 3 ± 4 | 2 ± 1 | −81 (0.37) | |||||||
| Other GISs | B-GOS | 2 ± 4 | 2 ± 3 | −18 (0.11) | −0.56, p = 0.691, [−3.46, 2.34] | −0.33, p = 0.774, [−2.50, 1.84] | 0.00, p = 1.000, [−3.23, 3.23] | 0.012 | 0.327 | 0.315 (32%) |
| PLA | 1 ± 2 | 1 ± 2 | −25 (0.11) | |||||||
| q3 SLEEP | B-GOS | 17 ± 10 | 12 ± 8 | −29 (0.58) | −3.57, p = 0.400, [−12.86, 5.72] | −5.02, p = 0.072, [−10.45, 0.42] | 0.04, p = 0.992, [−7.00, 7.08] | 0.131 | 0.683 | 0.552 (56%) |
| PLA | 14 ± 9 | 9 ± 8 | −36 (0.57) | |||||||
| q4 SLEEP | B-GOS | 2 ± 3 | 1 ± 2 | −37 (0.42) | −1.07, p = 0.257, [−2.97, 0.84] | −0.80, p = 0.093, [−1.72, 0.11] | 1.17, p = 0.072, [−0.05, 2.39] | 0.048 | 0.792 | 0.744 (74%) |
| PLA | 1 ± 1 | 1 ± 2 | 35 (0.19) | |||||||
| q8 SLEEP | B-GOS | 427 ± 67 | 371 ± 170 | −13 (0.59) | 43.65, p = 0.346, [−48.45, 135.76] | −55.65, p = 0.101, [−121.79, 10.48] | 44.90, p = 0.320, [−46.34, 136.13] | 0.164 | 0.614 | 0.450 (45%) |
| PLA | 471 ± 50 | 460 ± 45 | −2 (0.14) | |||||||
| q9 SLEEP | B-GOS | 3 ± 1 | 3 ± 1 | −14 (0.56) | 0.54, p = 0.192, [−0.28, 1.36] | −0.47, p = 0.192, [−1.17, 0.23] | 0.52, p = 0.285, [−0.49, 1.54] | 0.241 | 0.477 | 0.236 (24%) |
| PLA | 4 ± 1 | 4 ± 1 | 1.4 (0.06) | |||||||
| q10 SLEEP | B-GOS | 3 ± 1 | 3 ± 1 | −7 (0.22) | 0.59, p = 0.198, [−0.30, 1.48] | −0.20, p = 0.470, [−0.79, 0.39] | 0.11, p = 0.768, [−0.65, 0.88] | 0.134 | 0.694 | 0.560 (56%) |
| PLA | 3 ± 1 | 3 ± 1 | −3 (0.10) | |||||||
| q12a SLEEP | B-GOS | 0.6 ± 0.8 | 0.2 ± 0.4 | −63 (0.63) | −0.14, p = 0.635, [−0.72, 0.44] | −0.38, p = 0.068, [−0.77, 0.01] | 0.52, p = 0.065, [−0.02, 1.06] | 0.072 | 0.613 | 0.541 (54%) |
| PLA | 0.5 ± 0.4 | 0.6 ± 0.7 | 31 (0.24) | |||||||
| q13a SLEEP | B-GOS | 3 ± 2 | 3 ± 2 | −4 (0.05) | −0.39, p = 0.515, [−1.72, 0.95] | −0.79, p = 0.134, [−1.73, 0.15] | 0.79, p = 0.195, [−0.38, 1.97] | 0.063 | 0.774 | 0.711 (71%) |
| PLA | 3 ± 2 | 4 ± 3 | 40 (0.49) | |||||||
| q1 IPAQ | B-GOS | 3 ± 2 | 3 ± 3 | −5 (0.07) | −0.33, p = 0.789, [−2.85, 2.20] | −0.17, p = 0.715, [−1.10, 0.77] | 0.67, p = 0.312, [−0.64, 1.98] | 0.008 | 0.867 | 0.859 (86%) |
| PLA | 3 ± 2 | 3 ± 2 | 18 (0.22) | |||||||
| q2 IPAQ | B-GOS | 40 ± 24 | 42 ± 24 | 5 (0.05) | −7.17, p = 0.751, [−59.77, 45.42] | 2.17, p = 0.897, [−30.42, 34.76] | 34.50, p = 0.163, [−16.42, 85.43] | 0.12 | 0.522 | 0.402 (40%) |
| PLA | 33 ± 27 | 70 ± 72 | 112 (0.87) | |||||||
| q3 IPAQ | B-GOS | 2 ± 2 | 2 ± 2 | 22 (0.17) | 0.50, p = 0.638, [−1.61, 2.61] | 0.33, p = 0.738, [−1.77, 2.43] | −0.50, p = 0.723, [−3.48, 2.48] | 0.010 | 0.148 | 0.138 (14%) |
| PLA | 2 ± 2 | 2 ± 2 | −8 (0.08) | |||||||
| q4 IPAQ | B-GOS | 15 ± 23 | 39 ± 52 | 155 (0.72) | 18.00, p = 0.316, [−18.58, 54.58] | 23.83, p = 0.188, [−12.56, 60.23] | −22.17, p = 0.381, [−71.99, 27.65] | 0.086 | - | - |
| PLA | 33 ± 20 | 35 ± 29 | 5 (0.05) | |||||||
| q5 IPAQ | B-GOS | 5 ± 2 | 5 ± 2 | 0 (0) | 1.00, p = 0.344, [−1.09, 3.09] | −4.71 × 10−15, p = 1.000, [−2.05, 2.05] | −1.33, p = 0.372, [−4.36, 1.69] | 0.075 | - | - |
| PLA | 6 ± 2 | 5 ± 2 | −22 (0.68) | |||||||
| q6 IPAQ | B-GOS | 90 ± 84 | 45 ± 32 | −50 (0.20) | 20.00, p = 0.867, [−234.25, 274.25] | −45.00, p = 0.565, [−211.75, 121.75] | 168.33, p = 0.144, [−57.09, 393.75] | 0.109 | 0.629 | 0.520 (52%) |
| PLA | 110 ± 138 | 233 ± 416 | 112 (0.55) | |||||||
| q7 IPAQ | B-GOS | 280 ± 112 | 320 ± 124 | 14 (0.29) | 75.00, p = 0.322, [−107.56, 257.56] | 40.00, p = 0.392, [−56.78, 136.78] | −60.00, p = 0.365, [−195.64, 75.64] | 0.046 | 0.641 | 0.595 (60%) |
| PLA | 355 ± 164 | 335 ± 152 | −6 (0.13) | |||||||
| WEMWBS total score | B-GOS | 51 ± 10 | 53 ± 7 | 4 (0.33) | 0.25, p = 0.941, [−6.78, 7.28] | 2.17, p = 0.242, [−1.67, 6.00] | −0.42, p = 0.862, [−5.47, 4.63] | 0.025 | 0.756 | 0.731 (73%) |
| PLA | 51 ± 4 | 53 ± 6 | 3 (0.26) | |||||||
| General stress score | B-GOS | 5 ± 1 | 5 ± 1 | 3 (0.08) | 0.67, p = 0.447, [−1.14, 2.49] | 0.14, p = 0.796, [−0.99, 1.27] | −0.60, p = 0.406, [−2.14, 0.94] | 0.026 | 0.68 | 0.654 (65%) |
| PLA | 6 ± 2 | 5 ± 3 | −8 (0.27) | |||||||
| Recovery-related score | B-GOS | 13 ± 3 | 14 ± 4 | 9 (0.35) | 1.10, p = 0.530, [−2.48, 4.68] | 1.20, p = 0.278, [−1.13, 3.53] | −1.58, p = 0.282, [−4.64, 1.49] | 0.020 | 0.672 | 0.652 (65%) |
| PLA | 14 ± 3 | 14 ± 4 | −3 (0.11) | |||||||
| Sport-specific score | B-GOS | 2 ± 1 | 3 ± 1 | 26 (0.36) | 1.99, p = 0.026, [0.25, 3.72] * | 0.61, p = 0.505, [−1.25, 2.47] | −0.07, p = 0.954, [−2.53, 2.39] | 0.300 | - | - |
| PLA | 4 ± 1 | 5 ± 2 | 12 (0.32) | |||||||
| Sport-specific recovery score | B-GOS | 11 ± 2 | 13 ± 4 | 11 (0.36) | 0.58, p = 0.751, [−3.26, 4.42] | 1.29, p = 0.262, [−1.11, 3.70] | −1.54, p = 0.309, [−4.72, 1.64] | 0.018 | 0.682 | 0.664 (66%) |
| PLA | 12 ± 3 | 12 ± 5 | −2 (0.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gough, L.A.; Weldon, A.; Clark, C.C.T.; Young, A.; Roberts, C.J.; Clarke, N.D.; Brown, M.A.; Williams, R. The Effects of 12-Week Prebiotic Supplementation on General Wellness and Exercise-Induced Gastrointestinal Symptoms in Recreationally Trained Endurance Athletes: A Triple-Blind Randomised Controlled Pilot Trial. Nutrients 2025, 17, 3390. https://doi.org/10.3390/nu17213390
Gough LA, Weldon A, Clark CCT, Young A, Roberts CJ, Clarke ND, Brown MA, Williams R. The Effects of 12-Week Prebiotic Supplementation on General Wellness and Exercise-Induced Gastrointestinal Symptoms in Recreationally Trained Endurance Athletes: A Triple-Blind Randomised Controlled Pilot Trial. Nutrients. 2025; 17(21):3390. https://doi.org/10.3390/nu17213390
Chicago/Turabian StyleGough, Lewis A., Anthony Weldon, Cain C. T. Clark, Anthony Young, Charlie J. Roberts, Neil D. Clarke, Meghan A. Brown, and Rachel Williams. 2025. "The Effects of 12-Week Prebiotic Supplementation on General Wellness and Exercise-Induced Gastrointestinal Symptoms in Recreationally Trained Endurance Athletes: A Triple-Blind Randomised Controlled Pilot Trial" Nutrients 17, no. 21: 3390. https://doi.org/10.3390/nu17213390
APA StyleGough, L. A., Weldon, A., Clark, C. C. T., Young, A., Roberts, C. J., Clarke, N. D., Brown, M. A., & Williams, R. (2025). The Effects of 12-Week Prebiotic Supplementation on General Wellness and Exercise-Induced Gastrointestinal Symptoms in Recreationally Trained Endurance Athletes: A Triple-Blind Randomised Controlled Pilot Trial. Nutrients, 17(21), 3390. https://doi.org/10.3390/nu17213390







