Abstract
Background: Peripheral neuropathy (PN) represents a considerable and rapidly growing global health burden, with diabetic PN alone impacting nearly half of diabetic patients. Evidence from experimental studies highlighted that folate supplementation may protect nerve health by supporting myelin maintenance, minimizing oxidative stress, and enhancing neurotrophic factors. Nevertheless, its clinical efficacy and safety in managing PN have not yet been established. This study seeks to evaluate the role of folate in managing PN regarding the efficacy and safety endpoints. Methods: Up to July 2025, a comprehensive search of four electronic databases, encompassing PubMed, Scopus, Web of Science, and Cochrane Library, was executed, collecting studies evaluating the folate in managing PN. Outcomes included pain scores, symptom improvement endpoints, Neuropathy Total Symptom Score (NTSS) scores, epidermal nerve fiber density (ENFD), biomarkers, and side effects. Results: The narrative synthesis demonstrated consistent symptomatic benefits with pain reductions reaching 3 points, with symptom resolution rates of 87.5% and NTSS-6 score enhancements varying from 0.9 to 1.5 points. Notably, objective structural improvements in ENFD were observed, with increases reaching 97%. Furthermore, folate showed an excellent safety and tolerability profile with only one adverse event reported among 1367 individuals. Folate significantly decreased homocysteine and high-sensitivity C-reactive protein (hs-CRP) levels. Conclusions: Folate showed promising symptomatic benefits for peripheral neuropathy, with objective structural improvements (ENFD) and favourable biomarker changes (homocysteine, hs-CRP reduction), with an excellent safety profile.
1. Introduction
Peripheral neuropathy (PN) represents a significant and growing public health challenge, with an estimated prevalence of 2.4% in the general population, with estimates rising notably to about 8% among older adults. PN includes a wide range of clinical pathologies that mainly present with peripheral nervous system dysfunction [1]. Globally, diabetic peripheral neuropathy (DPN) is recognized as the most frequently encountered form of PN, affecting around 28% of adults in the United States [2,3]. Additionally, the longitudinal research has demonstrated that nearly 50% of patients with diabetes will develop DPN during their lifetime, with prevalence escalating with disease progression, rising from 26% five years post-diagnosis to 41% after ten years [4].
Patients with PN more commonly experience numbness and paresthesias, which are often accompanied by pain, muscle weakness, and reduced deep tendon reflexes. Typically, PN evolves over months to years, with some variants progressing more rapidly and severely. The pathophysiology of PN usually encompasses damage to the myelin-producing Schwann cells within peripheral nerves, especially in the segmental forms and to a lesser extent, in axonal types [5]. This disorder exhibits a wide range of severity and clinical features that reflect its involvement of motor, sensory, and autonomic fibers [6]. Notably, effective regeneration and continued maintenance of the myelin sheath after nerve injury are fundamental for the functional recovery of PN [5].
Peripheral neuropathy develops through a complex interplay of metabolic, vascular, and inflammatory mechanisms that result in axonal degeneration and demyelination. Chronic hyperglycemia, oxidative stress, and microvascular dysfunction contribute to Schwann cell injury, impaired axonal transport, and loss of protective myelin sheaths, leading to progressive sensory loss, pain, and motor deficits [5,6]. Despite this multifactorial pathogenesis, current standard treatments for PN remain largely symptom-focused. Pharmacologic options include serotonin–norepinephrine reuptake inhibitors (e.g., duloxetine), gabapentinoids (e.g., pregabalin), and tricyclic antidepressants, which provide variable pain relief but do not address underlying nerve damage or functional recovery [7,8]. Other agents, such as topical capsaicin and lidocaine patches, may reduce pain but are often limited by local irritation and short duration of effect. Importantly, there are no widely approved disease-modifying therapies that promote nerve regeneration or restore sensory function, underscoring the need to explore interventions with neuroprotective and regenerative potential, such as folate.
Folate deficiency has been identified as a potential risk factor for PN, with emerging evidence suggesting that even modestly low serum folate levels (6.8–13.5 nmol/L) may increase the risk of PN among adults [9,10]. Moreover, experimental studies have demonstrated a protective effect of folic acid against PN in diabetic rats by attenuating axonal degeneration and promoting remyelination [4]. Methyltetrahydrofolate reductase (MTHFR), an essential enzyme in folate metabolism, significantly influences plasma homocysteine and folate concentrations, and certain MTHFR gene polymorphisms have been linked to vascular complications in type 2 diabetes [11]. Beyond its metabolic role, folate exerts several neuroprotective effects that may be relevant for peripheral nerve health: it provides methyl groups necessary for DNA synthesis and repair, supports Schwann cell proliferation and myelin maintenance, and enhances neural stem cell differentiation and nerve growth factor (NGF) expression through activation of the mitogen-activated protein kinase (MAPK) pathway [12,13]. By lowering homocysteine, folate can reduce oxidative stress and endothelial dysfunction, improving microvascular perfusion and nitric oxide bioavailability, which are critical for nerve repair [14]. Deficiency of folic acid can impair nervous system development, limit neural stem cell function, and compromise nerve regeneration [15,16,17].
Among the various B-vitamins implicated in nerve health, folate has received particular attention because it is essential for one-carbon metabolism and methylation reactions critical to neuronal DNA repair and myelin synthesis, it lowers homocysteine (a neurotoxic and vasculopathic metabolite linked to nerve injury), and its biologically active form, L-methylfolate, readily crosses the blood–nerve barrier to support Schwann cell proliferation and axonal regeneration [4,9,10,11,12,13,14]. Observational and interventional studies also suggest that folate deficiency is independently associated with increased risk of peripheral neuropathy [9,10], making it a biologically and clinically compelling target.
While supplementation shows promise in enhancing neurodevelopment, reducing neuroinflammation, and promoting axonal repair, its clinical efficacy and safety in managing PN remain to be fully established. This study seeks to systemically synthesize the evidence from the available literature regarding the role of folate in managing PN.
2. Materials and Methods
This systematic investigation was executed with complete adherence to the guidelines declared in the Cochrane Handbook and PRISMA statement to guarantee an adequate execution and reporting of this systematic review and meta-analysis [18,19]. This study was prospectively registered under this identification number (https://doi.org/10.17605/OSF.IO/KFT2H, access date: 8 July 2025).
2.1. Criteria of Eligibility
Eligible studies included observational designs (prospective or retrospective) as well as randomized controlled trials, open-label, and single- or double-blind designs, written in English or Spanish. Acceptable comparators comprised placebo, no treatment, or non-drug interventions. Studies of all dosages, routes of administration, combinations, and durations were considered. Only human studies were included, involving male, female, or mixed populations. The study outcomes of interest were pain scores, symptomatic improvement outcomes, NTSS, its subdomains, epidermal nerve fiber density (ENFD), plasma biomarkers, and adverse events related to folate intervention. The exclusion criteria encompass conference abstracts, review articles, and foreign language studies.
2.2. Literature Search and Selection Process
The scope of our electronic search involved various databases, such as PubMed, Scopus, Web of Science, and Cochrane Library, from the origin of databases until July 2025. The detailed search strategy and results for each database are represented in Supplementary Table S1.
The gathered articles from the literature search were initially imported into EndNote v 21 (Clarivate Analytics, Philadelphia, PA, USA) to ensure proper identification and removal of duplicates. Consequently, two independent authors conducted a two-step screening process. First, the titles and abstracts of the eligible articles were evaluated using the Rayyan web [20]. Then, a comprehensive full-text assessment was employed for the relevant articles. Any emerging discrepancies among the authors were addressed through discussion.
2.3. Data Extraction
Two independent reviewers utilized a predesigned data extraction sheet to gather the relevant summary and baseline data, encompassing study design, country, recruitment period, folate active form and dose, sample size, age (years), male gender, body mass index (BMI), history of diabetic retinopathy, intervention period, outcomes, inclusion criteria, and conclusion. Discussion between the reviewers was employed to resolve any apparent conflicts.
2.4. Risk of Bias Assessment
The Cochrane Risk of Bias Two tool (ROB2, v2) was implemented to evaluate the risk of bias in RCTs [21]. The ROB2 tool comprehensively evaluates the quality of RCTs through critical methodological domains, including the randomization process, deviation from the planned interventions, incomplete outcome data, assessment of outcomes, selection of the reported results, and other sources of bias. Each RCT was categorized as having a low, some concerns, or high risk of bias, determined by a cumulative judgment score.
The Newcastle Ottawa scale (NOS) was utilized to evaluate the quality of double-arm observational studies [22]. It evaluated three essential domains, including population and exposure selection, comparability between cohorts, and outcomes assessment, with final evaluation for each study as having good, moderate, or poor quality. The National Institute of Health (NIH) tool was employed for assessing the quality of single-arm observational studies through the assessment of critical domains, such as a clear description of the study objective and population, exposure assessment more than once over time, and sufficiency of sample size [23]. Each study was finally classified as having good, fair, or poor quality.
2.5. Systematic Review Synthesis
Due to the marked variability among the included studies in terms of population characteristics, outcome measures, and follow-up durations, a meta-analytic approach was not appropriate. Instead, a narrative synthesis was conducted. For continuous variables, we summarized baseline, follow-up, and change data; for dichotomous variables, we reported improvement rates or changes in prevalence.
To address design-specific evidence evaluation, we stratified included studies by design type (randomized controlled trials vs. observational studies), presenting these stratifications in a Supplementary Table. This approach allows readers to assess the strength of evidence from RCTs separately from observational data.
To facilitate comparison across studies utilizing different measurement scales and to assess clinical relevance, we calculated standardized mean differences (SMD; Cohen’s d) for pain and neuropathic symptom outcomes where sufficient data were available. SMD effect sizes were interpreted as small (0.2), medium (0.5), or large (0.8) according to established conventions [24]. Additionally, we compared observed changes against minimal clinically important differences (MCID) derived from the literature: 2 points for pain scales (VAS/NRS 0-10) [25,26] and 1 point for NTSS-6 [27].
3. Results
3.1. Literature Search
A total of 8475 articles were retrieved from the search of four main databases; 2796 of them were eliminated as duplicates utilizing EndNote. As a result, 5679 articles were assessed through the title and abstract screening stage, excluding 5522 articles, and 157 articles proceeded to the full-text screening phase. Ultimately, 12 studies were included in this review, while 38 protocols, 47 conference abstracts, 33 studies with the wrong population, 14 studies with the wrong study designs, and 13 duplicates were excluded. Figure 1 illustrates the PRISMA flow diagram of the study selection process.
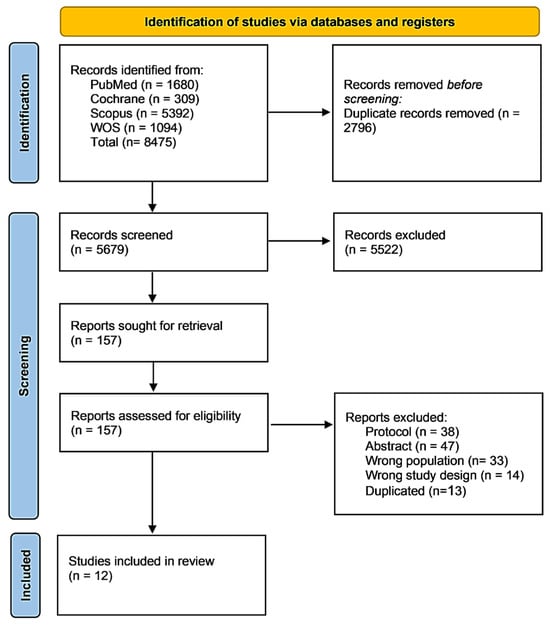
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram illustrating the systematic literature search and study selection process. The search was conducted across four electronic databases from database inception through July 2025, yielding a total of 8475 records. 12 studies met the inclusion criteria and were included in the final qualitative synthesis, comprising 3015 participants with peripheral neuropathy. Follow-up durations across included studies ranged from 2 to 12 months (median: 6 months).
3.2. Characteristics of the Included Studies
This systematic review involved 3015 patients with PN, either diabetic or not, collected from three RCTs, eight prospective observational studies, and one retrospective observational study [28,29,30,31,32,33,34,35,36,37,38,39]. The included studies covered various geographical areas, with three studies conducted in Asia, two studies performed in Portugal, and seven studies conducted in the USA. L-methyl folate (with or without calcium) was the predominant form assessed, with a folate dose ranging from 0.4 to 15 mg/day. Folate was primarily administered in combination with other active substances. In several studies, folate was delivered as part of multicomponent formulations; for example, LMF-MC-PLP (containing L-methylfolate, methylcobalamin, and pyridoxal-5′-phosphate) and UMP + folic acid + B12. The mean age of our study population was 58.3 years [SD = 4], ranging from 54 to 65.5 years. In-depth summary and baseline information of the included studies are presented in Table 1.

Table 1.
Showing summary and baseline characteristics of included studies and population:.
3.3. Risk of Bias Results
Three RCTs were assessed by the Cochrane ROB2 tool. Two studies were rated as having low risk of bias, and one showed some concerns due to issues related to the randomization process and selection of the reported outcomes, as outlined in Figure 2. Notably, NOS categorized two double-arm observational studies as having good quality, as shown in Supplementary Table S2. In the same context, NIH for single-arm observational studies rated four studies as having good quality and three as having fair quality, as presented in Supplementary Table S3.
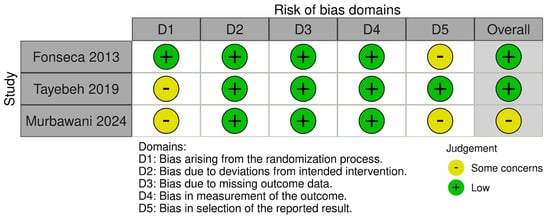
Figure 2.
Risk of bias evaluation across the three included randomized controlled trials (RCTs) using the Cochrane Risk of Bias 2 (ROB2) tool. Color coding: green = low risk, yellow = unclear risk, red = high risk. Assessment domains include random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other sources of bias. The three RCTs included in this assessment evaluated folate-based interventions over follow-up periods of 4 to 6 months in patients with diabetic peripheral neuropathy (DPN), with primary outcomes including Neuropathy Total Symptom Score-6 (NTSS-6, scale 0–21), pain scores (Visual Analog Scale 0–10), and biomarker changes (homocysteine, inflammatory markers).
3.4. Systematic Review
Design-stratified analysis (Supplementary Table S4) shows that RCT evidence (n = 6 treatment arms) demonstrates benefits with folate-based interventions, while a larger body of observational evidence (n = 11 studies) corroborates these findings. The heterogeneity in study populations, interventions, and outcome measures precluded meta-analysis but allowed for qualitative assessment of treatment effects across different neuropathy etiologies and folate formulations.
3.4.1. Pain
Folate administration showed meaningful reductions in pain among patients with PN across studies (Table 2). For instance, Trippe et al. [36] reported a 1.8-point decrease in pain severity (32%) over the follow-up period. In a study by Negrao et al. [35], the pain intensity was reduced from 5.9 ± 2.0 to 3.9 ± 2.1 following folate administration, while 7 points decreased the Pain Disability Questionnaire (PDQ) total score throughout follow-up. Notably, Jacobs et al. [31] reported a 3-point pain reduction with folate intervention compared to just 0.25 in the controls. Nevertheless, Fonseca et al. [29] found a minimal visual analogue scale (VAS) improvement among patients prescribed folate (−0.27) relative to those administered a placebo (−0.03).

Table 2.
Peripheral Neuropathy Treatment Outcomes Summary.
3.4.2. Symptom Improvement
Folate-based interventions revealed promising results regarding symptom improvement, NTSS, and NTSS subdomains (Table 2 and Table 3). In a study by Jacob et al. [31], the symptom resolution rates reached 87.5% with folate intervention compared to 25% in controls. In contrast, McNamara et al. [32] reported a 30.9% % improvement in monofilament sensation, while Yukawa et al. [38] reported an improvement of 66.7% in the neurological symptoms. Considerable improvements in NTSS-6 scores were noticed across studies. For example, Trippe et al. [36] reported a 35% reduction (−1.5 points) in NTSS-6 scores with folate intervention. Conversely, Fonseca et al. [29] reported slightly lower reductions estimated at 0.9 and 0.96 points over 16 and 24 weeks, respectively.

Table 3.
Epidermal Nerve Fiber Density, Biomarkers, and Safety Outcomes.
The subdomain results of the NTSS demonstrated substantial reductions in common neuropathic symptoms. Trippe et al. [36] reported a 41.9% reduction in the prevalence of burning pain, a 42.9% reduction in deep aching pain, and a 35.9% reduction in lancinating pain. In Negrao et al. [35], the severe numbness decreased from 43.8% to 4.4% (90% reduction), and severe tingling fell from 35.4% to 6.3% (82% reduction). Additionally, the severe burning pain completely resolved (100% reduction) while the electric shock pain reduced by 88.1% [35].
Standardized mean difference analysis revealed predominantly medium to large effect sizes for both pain (SMD range: 0.5–1.5) and NTSS-6 outcomes (SMD range: 0.5–0.8), indicating clinically meaningful improvements across heterogeneous measurement scales (Supplementary Table S5). Comparison against established minimal clinically important differences demonstrated that 75% of studies reporting pain outcomes achieved reductions exceeding the MCID threshold of 2 points, while 67% of studies reporting NTSS-6 outcomes exceeded the MCID threshold of 1 point. These findings confirm that the observed treatment effects are not only statistically significant but also clinically relevant for patients with diabetic peripheral neuropathy.
3.4.3. Epidermal Nerve Fiber Density
The current literature demonstrated modest enhancements in ENFD with folate-based intervention (Table 3. In McNamara et al. [32], the ENFD increases from 5.2 to 5.7 fibers/mm in the right foot (+ 11.5%) and from 4.7 to 5.7 in the left foot (+23.4%) following folate administration. Notably, the non-dominant limb showed a 20.8% increase in ENFD, while the dominant limb exhibited an 11.9% increase. Jacobs et al. [30] noticed a rise in the ENFD in the calf from 1.55 to 3.05 fibers/mm (97% increase), with 73% of patients showing increased ENFD.
3.4.4. Biomarkers
Folate supplementation was consistently associated with favorable biomarker changes across the included clinical trials (Table 3). Fonseca et al. [29] reported marked increases in serum total folate (+7.25 nmol/L) and 5-methyltetrahydrofolate (5-MTHF) (+229.70 nmol/L) together with significant reductions in homocysteine (−2.7 μmol/L; p = 0.001) and methylmalonic acid (MMA) (−63.29 nmol/L), indicating improved one-carbon metabolism and reduced functional vitamin B12 deficiency. Tayebeh et al. [28] observed a modest but significant homocysteine reduction (−0.1 μmol/L; p < 0.05) accompanied by increased serum folate concentrations (+2 ng/mL; p < 0.05) and improvement in nerve conduction parameters, including increased peroneal and sural amplitudes and velocities with shortened latencies. Murbawani et al. [33] demonstrated an additional anti-inflammatory effect, showing a mean high-sensitivity C-reactive protein (hs-CRP) decrease of −1.8 mg/L compared with a +1.2 mg/L rise in the placebo group (p = 0.01). Importantly, none of the studies reported clinically significant vitamin B12 depletion, mitigating the concern of folate masking B12 deficiency. Beyond these trial data, emerging evidence highlights that folate may also modulate neuro regeneration and microvascular repair by enhancing nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) expression, reducing oxidative stress markers such as malondialdehyde (MDA) and total antioxidant capacity (TAC), and improving endothelial nitric oxide bioavailability through tetrahydrobiopterin (BH4)-dependent nitric oxide synthase coupling [12,14,40]. Additionally, MTHFR C677T polymorphisms influence homocysteine response to folate supplementation and may predict treatment efficacy [41]. Collectively, these findings demonstrate that folate impacts both metabolic and neuro regenerative pathways, supporting its potential disease-modifying role in peripheral neuropathy.
3.4.5. Adverse Events
Generally, the folate-based intervention was extremely safe and well tolerated across the included studies (Table 3). Only one adverse event was reported in 1367 participants (0.07%), with no serious events or withdrawals due to side effects.
4. Discussion
The present systematic review comprehensively synthesizes the available evidence on the clinical efficacy and safety of folate for managing PN, collecting the evidence from 12 studies comprising 3015 patients. The narrative synthesis demonstrated symptomatic benefits, with pain reductions up to 3 points on standardized scales and substantial declines in burning pain, deep aching pain, and electric-shock-like pain. Notably, the symptom resolution rates reached 87.5% in some studies, with 66.7% improvement in neurological symptoms in another study. NTSS reductions ranged from 0.9 to 1.5 points, representing clinically meaningful symptomatic changes. In contrast, objective structural improvements were observed in ENFD, with increases up to 97%. Furthermore, biomarkers synthesize indicated rises in folate levels and reductions in homocysteine and MMA. Generally, folate showed an excellent safety profile, with only one adverse event reported among 1367 participants.
The observed clinical improvements can be supported by several complementary biological actions of folate on peripheral nerves. Folate reduces homocysteine, a metabolite that promotes oxidative stress and endothelial dysfunction, thereby improving microvascular perfusion and nitric oxide bioavailability essential for nerve repair [14]. It also donates methyl groups required for DNA synthesis and repair, supporting Schwann cell proliferation and myelin sheath restoration [5,12]. In preclinical models, folate activates the MAPK pathway, increasing the expression of neurotrophic factors such as NGF and BDNF, which promote neuronal survival and axonal regeneration [12,40]. Moreover, folate mitigates inflammation and oxidative damage while enhancing neurogenesis [9,38]. Genetic factors, particularly the MTHFR C677T polymorphism, influence homocysteine metabolism and may modulate individual response to supplementation [41]. These mechanistic pathways suggest that folate acts beyond pain relief to support nerve repair and vascular health, reinforcing its potential as a disease-modifying therapy for peripheral neuropathy. These clinical results are biologically consistent with preclinical evidence. Animal models of diabetic neuropathy demonstrate that folate supplementation preserves small nerve fibers, enhances myelin thickness, and reduces oxidative and inflammatory damage [4,42]. Cellular experiments confirm that folate can directly stimulate Schwann cell growth and NGF secretion, accelerate axonal sprouting, and restore mitochondrial and endothelial function under hyperglycemic and oxidative conditions [12,13,14]. Such data reinforce the concept that folate acts beyond symptomatic pain control to support structural nerve repair.
While the current approved treatments for PN, such as duloxetine and pregabalin, offer considerable pain relief, they primarily target symptom management without addressing nerve regeneration or sensory recovery [7,8]. Therefore, there is a pressing need to investigate disease-modifying intervention that could enhance nerve regeneration and functional recovery in patients with PN. Folate emerged as a promising therapeutic candidate for PN by facilitating nerve regeneration and slowing disease progression rather than focusing on symptomatic relief only [12]. A randomized trial by Fonseca et al. [29] revealed that the combination therapy with L-methylfolate, methylcobalamin, and pyridoxal-5′-phosphate (LMF-MC-PLP) significantly improved the neuropathy symptoms in DPN patients, with NTSS-6 scores reduced by up to 0.96 points at 24 weeks (p = 0.033). Although the primary sensory endpoint (vibration perception threshold) was not met, the transient reduction in neuropathy-related disability (NSD reduction of −0.78 at 16 weeks) implies a functional benefit for folate-based combination beyond symptomatic relief. Mechanistically, the LMF-MC-PLP combination significantly reduced homocysteine by 2.68 µmol/L compared to an increase of 0.48 µmol/L in the placebo group (p = 0.001), potentially reducing the oxidative stress and restoring vascular function that provides a better vascular health for nerve regeneration [14]. Notably, the safety profile was excellent, with no significant adverse events reported related to the study intervention and no study withdrawals, indicating a high tolerability. Nevertheless, the trial’s short duration and the absence of considerable sensory changes emphasize a significant limitation demanding long-term data.
An Iranian experience involving 80 patients with DPN (40 in the folate group and 40 in the placebo group) provides promising results regarding the disease-modifying effects of folic acid supplementation on PN [28]. Over 16 weeks, the daily administration of 1 mg of folic acid significantly increased the serum folate concentrations (8.1 vs. 10.1; p < 0.001) and reduced homocysteine levels (2.2 vs. 2.1; p < 0.001), supporting a plausible mechanism involving the suppression of oxidative stress pathways. Notably, these biochemical improvements translated to objective enhancements in nerve conduction parameters. In the folate group, sensory sural amplitude improved notably (3.3 vs. 2.4; p < 0.001) relative to placebo group, along with substantial gains in motor nerve function, encompassing increased amplitudes (e.g., peroneal 1.5 vs. 0.9; p = 0.001), conduction velocities (peroneal 30.2 vs. 29.9; p = 0.002), and decreased onset latencies (peroneal 5.4 vs. 5.2; p = 0.019). Although not restoring the nerve conduction velocity to normal values, these significant observations rigorously suggest that folic acid may modify the underlying disease process, not just provide symptomatic relief.
It is worth stating that beyond homocysteine-lowering, folate offers considerable neuroprotection effects through mitigating inflammation and oxidative damage and by promoting the expression of the nerve growth factor, which is crucial for neuronal regeneration and survival [4,40,42]. This trial also found no notable impact of folate on serum vitamin B12, emphasizing a reassuring safety profile that counters the prevalent concern of folate masking B12 deficiency [43].
Another observational experience by McNamara et al. [32] investigating the effects of the compound LMF-MC-PP within 123 patients with DPN strongly supported the available evidence regarding the potential disease-modifying effects of folate. Over 6 months of intervention, ENFD improved significantly, with a mean gain varying from 0.6 to 1.1 fibers/mm, contrasting notably with the expected annual decline of −0.68 fibers/mm/year reported in untreated DPN cohorts [32]. These structural enhancements were concomitant with meaningful clinical gains in sensation. For instance, the monofilament testing exhibited a substantial improvement, with 60 patients (48.8%) with intact sensation at baseline increasing to 95 (77.2%) after intervention, and of 63 patients with absent sensation at baseline, 38 (60.3%) restored intact sensation by 6 months. This robust parallel enhancement in ENFD and monofilament sensation highlights the likely disease-modifying effect of folate-based intervention rather than merely symptomatic improvement. Nevertheless, the study’s observational nature and the absence of a control group restrict the ability to draw definitive conclusions. Notably, using objective and quantifiable markers like ENFD strengthens confidence in the structural effects of folate.
Furthermore, the analgesic effects of folate therapy were also evident across several studies [29,31,35]. In a study by Negrao and his colleagues [35], The combination of a nutritional supplement containing uridine monophosphate, folic acid, and vitamin B12 significantly improved pain outcomes and had an excellent safety profile in patients with peripheral entrapment neuropathy. Patients experienced a significant reduction in global pain scores, from 17.3 ± 5.9 at baseline to 10.3 ± 6.1 at final assessment after two months, suggesting a considerable effect for this combination in reducing the burden of neuropathic pain. Notably, this supplement decreased the severity of neuropathic symptoms, as severe tingling or pricking declined from 35.4% of patients at baseline to just 6.3% at final assessment, while severe numbness fell from 43.8% to 4.4%. These significant improvements could be attributed to the synergistic action of nucleotides, which promotes the peripheral myelin sheath repair, and the roles of folic acid and vitamin B12 in accelerating nerve regeneration and the repair process [34,44,45].
4.1. Strengths and Drawbacks
This systematic review investigation provides the most extensive and updated evidence regarding the role of folate in managing PN, gathering the evidence from 12 studies incorporating 3015 patients. A key strength of this study is the inclusion of studies that were conducted across several geographical areas and distinct populations, supporting the generalizability and credibility of the findings. However, like any present research, this study is not free of limitations. First, the significant heterogeneity of population heterogeneity, outcomes assessment, and follow-up durations across the included studies hindered us from performing a meta-analysis. Second, including observational studies may have introduced selection bias and residual confounding, tempering our conclusions’ certainty.
A notable strength of our analysis is the assessment of clinical significance through standardized mean differences and comparison with established minimal clinically important difference thresholds. Despite the inability to perform quantitative meta-analysis due to methodological heterogeneity, the consistency of effect sizes and the high proportion of studies exceeding MCID thresholds provide robust evidence for clinically meaningful treatment effects. This approach addresses the challenge of interpreting results across studies employing different measurement scales and reinforces the clinical relevance of our findings beyond statistical significance alone.
Our design-stratified synthesis reveals important methodological considerations. While RCT evidence provides the highest certainty regarding treatment effects, the consistency observed across different study designs strengthens confidence in the therapeutic potential of folate-based interventions. However, the heterogeneity in interventions (different folate formulations, doses, and combination products) and outcome measures highlights the need for standardized approaches in future research. The diversity in study populations suggests potential applicability across various neuropathy etiologies, though dedicated trials in specific populations would strengthen the evidence base.
4.2. Advances in Clinical Knowledge and Future Suggestions
This systematic review expands clinical knowledge by demonstrating that folate-based interventions provide both symptomatic benefits and objective structural improvements in PN. Specifically, objective parameters such as nerve conduction velocity and epidermal nerve fiber density showed measurable improvements, suggesting potential disease-modifying effects beyond the symptomatic benefits observed in pain and neuropathic symptom scores. The excellent safety and tolerability profiles further support its applicability in clinical settings.
Nevertheless, the observed limitations across the included studies, such as significant heterogeneity, shorter follow-up durations, and reliance on observational studies, emphasize the demand for future large-scale, placebo-controlled randomized studies. These trials should focus on examining long-term functional and structural outcomes and investigating the optimal dosing strategies and combinations for better management of PN.
5. Conclusions
Folate-based regimens show a promising signal of symptomatic benefit in peripheral neuropathy, with several studies also suggesting objective improvements in nerve structure or function (ENFD, nerve conduction). The most consistent evidence comes from combination formulations (L-methylfolate with vitamins B12/B6 or UMP), while monotherapy data remains limited but biologically supportive. Randomized trials confirm homocysteine reduction and NTSS-6 improvement, though heterogeneity and small sample sizes temper certainty. Across 1367 participants reporting safety, only one mild adverse event was documented, suggesting an excellent safety profile.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17203299/s1, Supplementary Table S1: Detailed Search Results; Supplementary Table S2: Newcastle Ottawa Scale for observational studies; Supplementary Table S3: NIH for single-arm studies; Supplementary Table S4: Comprehensive Stratified Evidence Summary; Supplementary Table S5. Standardized Mean Differences, Minimal Clinically Important Differences, and Responder Rates for Pain and Neuropathic Symptom Outcomes.
Author Contributions
A.C.A.M.: Conceptualization, Investigation, Methodology, Formal Analysis, Writing—Review and Editing, Supervision, Validation, Visualization. M.G.M.A.: Conceptualization, Investigation, Methodology, Formal Analysis, Writing—Review and Editing, Supervision, Validation, Visualization. M.B.: Conceptualization, Investigation, Methodology, Formal Analysis, Data Curation, Writing—Original Draft, Validation, Visualization. G.M.: Conceptualization, Investigation, Methodology, Formal Analysis, Data Curation, Writing—Original Draft, Validation, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
The methodology of the systematic review and medical writing support for this article were funded by OPKO Health Spain S.L.U.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
A.C.A.M. and M.G.M.A. are employees of OPKO Health Spain S.L.U. and G.M. serves as a consultant for OPKO Health Spain S.L.U.
References
- Hughes, R.A.C. Peripheral neuropathy. BMJ 2002, 324, 466–469. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diab Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Çakici, N.; Fakkel, T.M.; van Neck, J.W.; Verhagen, A.P.; Coert, J.H. Systematic review of treatments for diabetic peripheral neuropathy. Diabet. Med. 2016, 33, 1466–1476. [Google Scholar] [CrossRef]
- Yilmaz, M.; Aktug, H.; Oltulu, F.; Erbas, O. Neuroprotective effects of folic acid on experimental diabetic peripheral neuropathy. Toxicol. Ind. Health 2016, 32, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Ide, C. Peripheral nerve regeneration. Neurosci. Res. 1996, 25, 101–121. [Google Scholar] [CrossRef]
- Hammi, C.; Yeung, B. Neuropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shahid, W.; Kumar, R.; Shaikh, A.; Kumar, S.; Jameel, R.; Fareed, S. Comparison of the Efficacy of Duloxetine and Pregabalin in Pain Relief Associated with Diabetic Neuropathy. Cureus 2019, 11, e5293. [Google Scholar] [CrossRef]
- Ziegler, D. Painful diabetic neuropathy: Advantage of novel drugs over old drugs? Diabetes Care 2009, 32 (Suppl. 2), S414–S419. [Google Scholar] [CrossRef]
- Reynolds, E.H. Chapter 61—The neurology of folic acid deficiency. In Handbook of Clinical Neurology; Biller, J., Ferro, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 120, pp. 927–943. [Google Scholar] [CrossRef]
- Hashim, I.A. Chapter 9—Neurological disorders. In Tutorials in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 241–270. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Han, L.; Ji, H.; Li, J. The effect of MTHFR C677T polymorphism on type 2 diabetes mellitus with vascular complications in Chinese Han population: A meta-analysis. Endocr. J. 2014, 61, 717–726. [Google Scholar] [CrossRef]
- Kang, W.-B.; Chen, Y.-J.; Lu, D.-Y.; Yan, J.-Z. Folic acid contributes to peripheral nerve injury repair by promoting Schwann cell proliferation, migration, and secretion of nerve growth factor. Neural Regen. Res. 2019, 14, 132–139. [Google Scholar] [CrossRef]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef]
- Topal, G.; Brunet, A.; Millanvoye, E.; Boucher, J.-L.; Rendu, F.; Devynck, M.-A.; Drutel, G.; Canet, E.; Wiernsperger, N.; Auguet, M.; et al. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic. Biol. Med. 2004, 36, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Xie, Y.; Guo, X.; Tang, X.; Wang, S.; Zheng, Y.; Huang, L.; Li, W.; Zhou, Q.; et al. Folic acid deficiency inhibits neural rosette formation and neuronal differentiation from rhesus monkey embryonic stem cells. J. Neurosci. Res. 2012, 90, 1382–1391. [Google Scholar] [CrossRef]
- Reynolds, E.H. Benefits and risks of folic acid to the nervous system. J. Neurol. Neurosurg. Psychiatry 2002, 72, 567–571. [Google Scholar] [CrossRef]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 6 April 2025).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- The BMJ. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. Available online: https://www.bmj.com/content/366/bmj.l4898 (accessed on 30 May 2025).
- Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 6 April 2025).
- Study Quality Assessment Tools|NHLBI, NIH n.d. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 6 April 2025).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef]
- Bastyr, E.J., 3rd; Price, K.L.; Bril, V.; MBBQ Study Group. Development and validity testing of the neuropathy total symptom score-6: Questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin. Ther. 2005, 27, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Tayebeh, M.; Khorvash, F.; Maracy, M.; Bellissimo, N.; Askari, G. Effect of folic acid supplementation on nerve conduction velocity in diabetic polyneuropathy patients. Neurol. Res. 2019, 41, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.A.; Lavery, L.A.; Thethi, T.K.; Daoud, Y.; DeSouza, C.; Ovalle, F.; Bottiglieri, T.; Messerli, F.H.; Boden, W.E.; Horton, E.S.; et al. Metanx in Type 2 Diabetes with Peripheral Neuropathy: A Randomized Trial. Am. J. Med. 2013, 126, 141–149. [Google Scholar] [CrossRef]
- Jacobs, A.M.; Cheng, D. Management of Diabetic Small-Fiber Neuropathy with Combination L-Methylfolate, Methylcobalamin, and Pyridoxal 5′-Phosphate. Rev. Neurol. Dis. 2011, 8, 39–47. [Google Scholar]
- Jacobs, A.M.; Cheng, D. Addition of Metanx in pregabalin partial responders for painful diabetic neuropathy. J. Diabetes Mellit. 2013, 3, 134–138. [Google Scholar] [CrossRef]
- McNamara, V.F.; Vinik, A.I.; Barrentine, L.; De Vol, E.B. Effectiveness of Metanx Prescription Medical Food on Small Nerve Fibers and Monofilament Sensation in Patients with Diabetic Peripheral Polyneuropathy. J. Diabetes Mellit. 2016, 6, 166–174. [Google Scholar] [CrossRef]
- Murbawani, E.A.; Probosari, E.; Muis, S.F.; Nugroho, H.S.H.; Sukmadianti, A.; Ardiaria, M. Folic Acid, Vitamin B6, B12 Co-supplementation Effect on Inflammatory Status of Diabetic Neuropathy Patients. Pak. J. Med. Health Sci. 2021, 15, 2044–2047. [Google Scholar] [CrossRef]
- Negrão, L.; Almeida, P.; Alcino, S.; Duro, H.; Libório, T.; Melo Silva, U.; Lemos, J.; Gomes, P.; Carvalho, M.; Costa, C.; et al. Effect of the Combination of Uridine Nucleotides, Folic Acid and Vitamin B12 on the Clinical Expression of Peripheral Neuropathies. Pain Manag. 2014, 4, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Negrão, L.; Nunes, P.; on behalf of the Portuguese Group for the Study of Peripheral Neuropathy. Uridine Monophosphate, Folic Acid and Vitamin B 12 in Patients with Symptomatic Peripheral Entrapment Neuropathies. Pain Manag. 2016, 6, 25–29. [Google Scholar] [CrossRef]
- Trippe, B.S.; Barrentine, L.W.; Curole, M.V.; Tipa, E. Nutritional management of patients with diabetic peripheral neuropathy with L-methylfolate-methylcobalamin-pyridoxal-5-phosphate: Results of a real-world patient experience trial. Curr. Med. Res. Opin. 2016, 32, 219–227. [Google Scholar] [CrossRef]
- Wade, R.L.; Cai, Q. Impact of L-Methylfolate Combination Therapy Among Diabetic Peripheral Neuropathy Patients. Am. J. Pharm. Benefits 2012, 4, 218–225. [Google Scholar]
- Yukawa, M.; Naka, H.; Murata, Y.; Katayama, S.; Kohriyama, T.; Mimori, Y.; Nakamura, S.; Kaji, R.; Nakashima, K.; Tsuji, S.; et al. Folic Acid-Responsive Neurological Diseases in Japan. J. Nutr. Sci. Vitaminol. 2001, 47, 181–187. [Google Scholar] [CrossRef]
- Walker, M.J.; Morris, L.M.; Cheng, D. Improvement of Cutaneous Sensitivity in Diabetic Peripheral Neuropathy with Combination L-Methylfolate, Methylcobalamin, and Pyridoxal 5′-Phosphate. Rev. Neurol. Dis. 2010, 7, 132–139. [Google Scholar]
- Ma, W.; Xiang, L.; Yu, H.-L.; Yuan, L.-H.; Guo, A.-M.; Xiao, Y.-X.; Chen, J.-G.; Zhang, X.-L.; Wang, S.-W.; Liu, R.-T.; et al. Neuroprotection of soyabean isoflavone co-administration with folic acid against beta-amyloid 1-40-induced neurotoxicity in rats. Br. J. Nutr. 2009, 102, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Mottaghi, T.; Khorvash, F.; Kheirollahi, M.; Maracy, M. The MTHFR C677T polymorphism influences the efficacy of folic acid supplementation on the nerve conduction studies in patients with diabetic polyneuropathy; A randomized, double blind, placebo-controlled study. J. Res. Med. Sci. 2019, 24, 36. [Google Scholar] [CrossRef]
- Mastroiacovo, P.; Leoncini, E. More folic acid, the five questions: Why, who, when, how much, and how. Biofactors 2011, 37, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Brantigan, C.O. Folate supplementation and the risk of masking vitamin B12 deficiency. JAMA 1997, 277, 884–885. [Google Scholar] [CrossRef]
- Wattig, B.; Schalow, G.; Heydenreich, F.; Warzok, R.; Cervós-Navarro, J. Enhancement of nerve fibre regeneration by nucleotides after peripheral nerve crush damage. Electrophysiologic and morphometric investigations. Arzneimittelforschung 1992, 42, 1075–1078. [Google Scholar]
- Wattig, B.; Schalow, G.; Madauss, M.; Heydenreich, F.; Warzok, R.; Cervós-Navarro, J. Acceleration of nerve and muscle regeneration by administration of nucleotides—Electroneurophysiological and morphometrical investigations. Acta Histochem. Suppl. 1992, 42, 333–339. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).