Abstract
Background/Objectives: Acrylamide is a food contaminant formed during high-temperature cooking processes, leading to unintentional human exposure. Diet is the primary source for non-smokers, with potatoes, cereals, and coffee being the main contributors. While animal studies have demonstrated that acrylamide is neurotoxic, genotoxic, mutagenic, and cardiotoxic, its effects on human cardiovascular health remain poorly understood. This study aimed to evaluate the association between acrylamide exposure and cardiovascular risk. Methods: A comprehensive literature search was conducted across four databases without restrictions on publication year or language (last search: 1 July 2024). The risk of bias was assessed using the Joanna Briggs Institute critical appraisal tools. Results: In total, 28 studies were included, predominantly from the US NHANES sample and with cross-sectional designs. Higher acrylamide exposure was associated with an increased risk of cardiovascular mortality but was inversely associated with glucose and lipid levels, as well as key cardiovascular risk factors such as diabetes, obesity, and metabolic syndrome. Conversely, glycidamide—acrylamide’s most reactive metabolite—was positively associated with elevated glucose and lipid levels, higher systolic blood pressure, and increased obesity prevalence. Conclusions: These findings suggest that the adverse cardiovascular effects of acrylamide may be mediated by its conversion to glycidamide. Further research is necessary to fully elucidate the impact of acrylamide on cardiovascular health. Meanwhile, public health efforts should continue to focus on mitigation strategies within the food industry and raising public awareness about exposure.
1. Introduction
Acrylamide is a chemical substance used in gel electrophoresis, as well as in the production of crude oil, cosmetic additives, and other substances such as polyacrylamide, with applications in pulp and paper production, agriculture, food processing, mining, and as a flocculant in wastewater treatment. It is also present in tobacco smoke [1,2]. In addition, acrylamide has been classified as a food contaminant formed by the Maillard reaction between asparagine and sugars reducing during food processing at temperatures above 120 °C [3]. For this reason, diet is the main source of exposure in the non-smoking population, with potato products, cereal products, and coffee being the major contributors [3,4]. Therefore, humans are not only exposed to acrylamide through tobacco smoke, but also through other unintentional exposures via food, dermal contact, and inhalation [5].
After exposure, acrylamide reaches the systemic blood circulation and is metabolized in the liver via the mercapturic acid pathway and partially metabolized by cytochrome P450 2E1 (CYP2E1) to the more reactive epoxide glycidamide [6]. Glycidamide causes greater genotoxic and mutagenic damage than acrylamide [7]. The proportion of acrylamide converted to glycidamide varies according to activity, which is influenced by genetic factors, age, alcohol consumption, diet, and environmental exposure [6]. Both acrylamide and glycidamide are electrophilic and bind covalently to proteins such as hemoglobin (Hb) and DNA [8]. Hb adducts of acrylamide (HbAA) and of glycidamide (HbGA) provide a good estimate of long-term exposure. In contrast, the use of urinary mercapturic acid metabolites as biomarkers provides insight into short-term exposure to acrylamide (i.e., N-acetyl-S-(2-carbamoylethyl)-l-cysteine (AAMA) and N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA)) [9]. The conversion of acrylamide to glycidamide is represented by the HbGA/HbAA ratio [10].
In animal studies, acrylamide has been shown to be neurotoxic, genotoxic, mutagenic, and cardiotoxic [11], and the carcinogenic activity of acrylamide depends on its metabolism to glycidamide [8]. The association between acrylamide exposure and cancer has been extensively studied in humans, but the results are inconclusive for most types of cancer [12], with the possible exception of gynecological cancers [13]. Research on the association between acrylamide exposure and cardiovascular disease (CVD) in humans is scarce, and current evidence is mainly based on cross-sectional studies with mixed results. Therefore, the aim of this review was to examine the association between acrylamide exposure and CVD mortality, CVD risk, and cardiovascular risk factors.
2. Materials and Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [14]. The protocol is registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42023488410) [15].
2.1. Search Strategy
Four different databases were used for the literature search: Clarivate (Web of Science), Medline, PubMed, and Scopus, with no restrictions on the year of publication or language. The last search was updated on 1 July 2024. The search strategy included the following keywords: “acrylamide” or “glycidamide” and “humans” and (“cardiovascular” or “mortality” or “myocardial infarction” or “coronary heart disease” or “stroke” or “cerebrovascular” or “atherosclerosis” or “arterial injury” or “hypertension” or “diabetes” or “obesity” or “metabolic syndrome” or “insulin resistance” or “dyslipidemia” or “cholesterol” or “triglycerides”).
2.2. Study Selection
The study selection was performed by two of the co-authors (DMM and PG-C). After removing duplicates, the title and abstract were screened to identify eligible studies. After screening, full text reading was performed to select articles based on the inclusion criteria: (a) studies conducted in humans; (b) studies analyzing acrylamide exposure measured in urine (AAMA and GAMA) or blood (HbAA and HbGA) or from dietary intake; (c) studies providing association estimates between acrylamide exposure and cardiovascular risk factors, reporting OR, HR, RR, or linear regression coefficients (β); and (d) studies with any epidemiological design (longitudinal or cross-sectional). Studies were excluded if they were conducted in pregnant women and evaluated postnatal outcomes, did not report primary data, or evaluated other CVDs (e.g., peripheral arterial disease, rheumatic heart disease, etc.). Eligible studies were also identified by reviewing the reference lists of the selected studies. In the case of duplicate original data, the article with the most comprehensive information was included.
2.3. Outcomes
The main outcomes of this review were related to (a) CVD mortality and CVD risk; (b) diabetes mellitus (DM) and glucose metabolism; (c) dyslipidemia and lipid metabolism; (d) obesity and body composition; (e) hypertension (HTN) and blood pressure; and (f) metabolic syndrome (MetS).
2.4. Data Extraction
The following information was extracted from the studies: first author, year of publication, country and sample, sample size, age, percentage of men, acrylamide exposure (urine, blood, or dietary intake), outcome definition, and covariates in the fully adjusted model. The unit of comparison and the results of the association estimates (OR, HR, or RR) and linear regression coefficients (β) from the fully adjusted model were also extracted. Data extraction was performed by two of the co-authors (DMM and PG-C).
2.5. Risk of Bias
The risk of bias of the included studies was assessed using the Joanna Briggs Institute (JBI) critical appraisal tools [16]. The critical appraisal tool for cohort studies includes 11 questions with “Yes”, “No”, “Unclear” or “Not applicable” answers, and the tool for cross-sectional studies includes 8 questions. The overall risk of bias was established according to the percentage of “Yes” answers (the higher the score, the lower the risk of bias). The following cut-off points were used to determine the risk of bias of the studies: high risk of bias was considered if the score was less than 49%, moderate risk if the score was 50–69% and low risk if the score was more than 70%. Any discrepancies between the two co-authors (DMM and PG-C) were resolved by consensus.
2.6. Data Synthesis
The results were organized based on the primary outcome and for smokers and non-smokers, if this information was available. A meta-analysis could not be performed due to the heterogeneity of the studies. To better communicate the results, not only are the central estimators reported, but also the direction of the association using arrows (e.g., ↑ = higher prevalence, ↓ = lower prevalence).
3. Results
3.1. Search Results
The literature search identified 2530 articles, of which 984 were duplicates and 1546 were screened (Figure 1). After screening, 1485 articles were excluded based on the title and abstract, 61 were assessed for eligibility, and 11 were added from references. Of a total of 72 articles, 44 did not meet the inclusion criteria, and the reasons for exclusion are detailed in Table S1. Finally, 28 articles were included in this systematic review [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
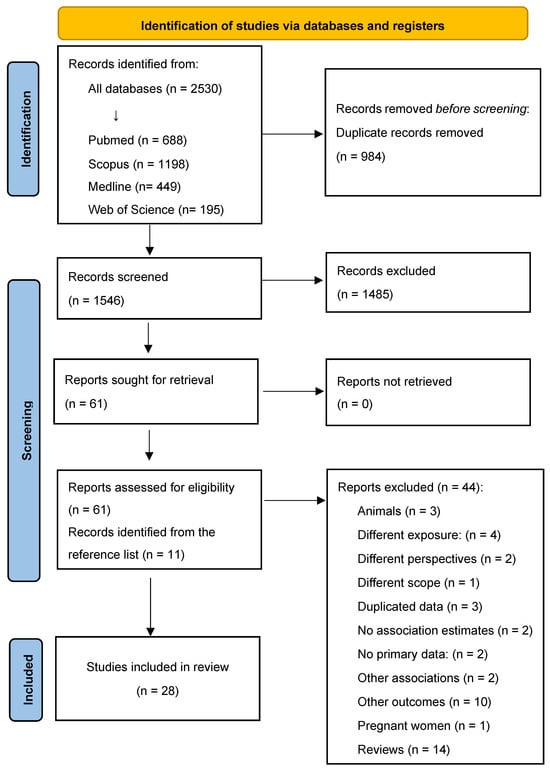
Figure 1.
Literature search and study selection.
3.2. Characteristics of the Included Studies
The articles were conducted in the USA (n = 22), China (n = 2), France (n = 1), Taiwan (n = 1), and Iran (n = 2), with 8 cohort studies and 20 cross-sectional studies. The sample sizes ranged from 696 to 72,585 participants. Most studies included participants aged ≥20 years. Two studies included adolescents [30,40], one study included participants with hyperglycemia [20], and two studies were conducted in men [17] and women [21] only. In most studies, acrylamide exposure was measured in blood (n = 12, corresponding to NHANES data) and urine (n = 12), while dietary exposure (n = 2) and cumulative occupational exposure (n = 2) were also considered (Table 1).

Table 1.
Characteristics of the included studies.
The mean HbAA ranged from 47.7 (US adults) to 65.0 pmol/g Hb (US adolescents) and HbGA ranged from 42.2 to 60.4 pmol/g Hb (US adults). Mean dietary acrylamide intake ranged from 32.6 (France) to 57 µg/day (Iran).
3.3. Risk of Bias
Most studies had a low (n = 23), moderate (n = 4), or high (n = 1) risk of bias (Table S2). The exposure measurement was performed in a valid and reliable way in most of the studies (urine or blood), except when dietary exposure was analyzed. Finally, for some of the cohort studies, follow-up was not long enough to examine the incidence of specific outcomes.
3.4. Association Between Acrylamide Exposure and CVD Mortality and CVD Risk
For CVD mortality, causes of death were defined according to ICD 8–10th. Six studies were included for this outcome [17,18,20,21,22,23] (Table 2). Among US male workers potentially exposed to acrylamide, a 12% lower risk of CVD mortality than expected was observed over the period 1925–2002 [17]. Comparing extreme categories of exposure, among participants with hyperglycemia from the NHANES sample, HbAA was associated with an 84% higher risk of CVD mortality [20]. In French women, dietary acrylamide intake was also associated with a 33% higher risk of CVD mortality [21]. And in Iran, AAMA was associated with a 68% higher risk of ischemic heart disease (IHD) mortality [23].

Table 2.
Association between acrylamide exposure and cardiovascular disease.
For CVD risk, four studies were included (partially overlapping) [25,26,27,28]. In the NHANES sample, it was observed that AAMA was associated with a 54% higher prevalence of self-reported CVD [28]. A study from China showed that those participants who were in the highest quartile of exposure to AAMA and GAMA had a significantly higher 10-year CVD risk (calculated using an algorithm) of 47% and 67%, respectively, compared with those in the lowest quartile [25] (Table 2 and Figure 2).
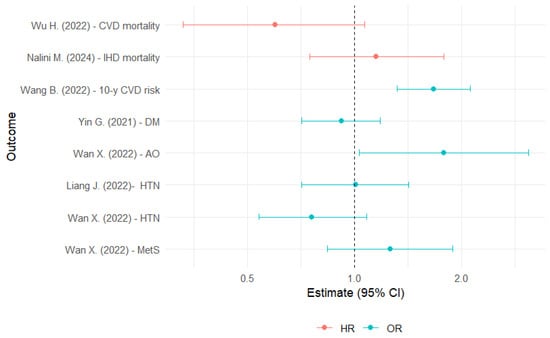
Figure 2.
Hazard ratios and odds ratios for the association between glycidamide exposure and cardiovascular risk factors [20,23,25,32,40,43]. AO: abdominal obesity; CI: confidence interval; CVD: cardiovascular disease; DM: diabetes mellitus; IHD: ischemic heart disease; HR: hazard ratio; HTN: hypertension; MetS: metabolic syndrome; OR: odds ratio.
3.5. Association Between Acrylamide Exposure and Diabetes and Glucose Metabolism
In most studies, glucose was analyzed as a continuous variable, and some also reported HbA1c, insulin, and HOMA-IR. DM was defined as fasting plasma glucose ≥ 126 mg/dL or 2 h plasma glucose ≥ 200 mg/dL, or use of medication for DM. Of the eight studies evaluating glucose metabolism [29,30,31,32,33,42,43,44], five analyzed data from different NHANES cycles (the samples partially overlapped) [29,32,42,43,44] (Table 3).

Table 3.
Association between acrylamide exposure and diabetes and glucose metabolism.
In China, those in the highest quartile of AAMA and ΣUAAM had higher glucose levels of about 0.20 and 0.17 mmol/L, respectively, compared with those in the lowest quartile [31]. In the NHANES sample, each unit increase in HbGA and HbGA/HbAA was associated with higher glucose levels of 2.51 and 3.12 mg/dL, respectively (Figure 3). HbAA and HbAA + HbGA were inversely associated with glucose. For HbA1c, HbAA was inversely associated, whereas HbGA and HbGA/HbAA were directly associated [43].
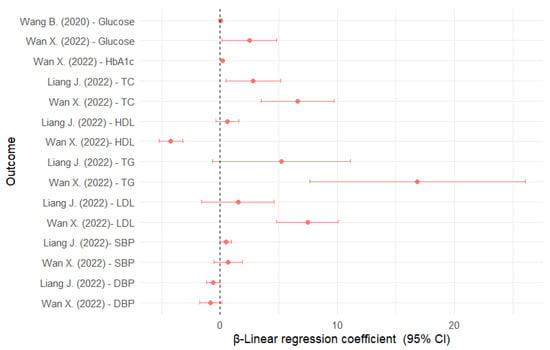
Figure 3.
Linear regression coefficients for the association between glycidamide and cardiovascular risk factors [31,40,43]. DBP: diastolic blood pressure; CI: confidence interval; HbA1c: glycated hemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; SBP: systolic blood pressure; TC: total cholesterol; TG: triglycerides.
In the NHANES sample, HbAA was associated with a 29% lower prevalence of DM, and HbGA/HbAA with a 95% higher prevalence when comparing the extreme quartiles [32].
3.6. Association Between Acrylamide Exposure and Dyslipidemia and Lipid Metabolism
Lipid metabolism was assessed with total cholesterol (TC), HDL, triglycerides (TG) and LDL as continuous variables. For these outcomes, eight studies were included [29,30,34,35,40,42,43,44], six of which analyzed data from the NHANES (partially overlapping) [29,34,35,40,42,43,44] (Table 4).

Table 4.
Association between acrylamide exposure and dyslipidemia and lipid metabolism.
In US adolescents, HbGA was associated with higher TC (2.83 mg/dL), but not associated with other serum lipids [40] (Figure 3). In US adults, HbGA was directly associated with TC (6.60 mg/dL), LDL (7.47 mg/dL), and TG (16.86 mg/dL), but inversely associated with HDL (−4.20 mg/dL). The results for HbAA were completely inverse to those for HbGA. The results for HbGA/HbAA were similar to those for HbGA [43]. Also, AAMA was associated with higher HDL levels (1.28 mg/dL) [35].
3.7. Association Between Acrylamide Exposure and Obesity and Body Composition
In most of the studies, obesity was considered as dichotomous. General obesity (GO) was defined as body mass index (BMI) ≥ 30 kg/m2 and abdominal obesity (AO) as waist circumference (WC) >102 cm in men and >88 cm in women. A total of nine studies were included [29,30,36,37,38,39,42,43,44], eight of which analyzed data from the NHANES (partially overlapping) [29,36,37,38,39,42,43,44] (Table 5).

Table 5.
Association between acrylamide exposure and obesity and body composition.
In US adults, HbAA was associated with a 64% lower prevalence of AO, whereas HbGA and HbGa/HbAA were associated with a 78% and 95% higher prevalence of AO [43] (Figure 2). AAMA was associated with a 21% lower prevalence of AO and GO [39].
3.8. Association Between Acrylamide Exposure and Hypertension and Blood Pressure
A total of five studies were included for this outcome [40,41,42,43,44], two of which partially overlapped [42,43] (Table 6). HTN was defined as blood pressure ≥ 130/80 mmHg or antihypertensive treatment. In the NHANES sample, HbAA and HbGA were not significantly associated with HTN in either adolescents or adults [40,43], but AAMA was associated with a 40% lower HTN prevalence [44]. When systolic blood pressure (SBP) was considered as a continuous variable, HbGA was associated with an increase of about 0.49 mmHg in US adolescents [40] and HbAA + HbGA with an increase of 1.29 mmHg in adults [43] (Figure 3).

Table 6.
Association between acrylamide exposure and hypertension and blood pressure.
3.9. Association Between Acrylamide Exposure and Metabolic Syndrome
MetS was defined by the NCEP-ATP III (three or more criteria) (Table 7). Three studies were included for this outcome [42,43]. In the NHANES sample, HbAA and AAMA were associated with a 40% and 22% lower prevalence of MetS, respectively [43,44], whereas HbGA/HbAA was associated with a 61% higher prevalence of MetS when comparing the extreme quartiles [43].

Table 7.
Association between acrylamide exposure and metabolic syndrome.
3.10. Association Between Acrylamide Exposure and Cardiovascular Risk Factors in Non-Smokers
A total of nine studies reported results in non-smokers [19,23,24,25,32,33,37,41,43] (Table S3). When comparing the extreme quartiles, HbGA and HbGA/HbAA were associated with a 78% and 88% lower risk of CVD mortality, respectively [19]. In contrast, AAMA was associated with a 2-fold higher risk of IHD mortality [23], and when assessing 10-year CVD risk, AAMA, GAMA, and ΣUAAM were associated with 50%, 73%, and 55% higher risk, respectively [25].
For metabolic diseases, comparing the extreme quartiles of exposure, HbAA was associated with a 54% lower prevalence of DM [32]. HbGA/HbAA was associated with a 55% higher prevalence of DM [32], a 3-fold higher prevalence of GO [37], and a 76% higher prevalence of MetS [43].
3.11. Association Between Acrylamide Exposure and Cardiovascular Risk Factors in Smokers
A total of seven studies reported results for smokers [23,24,32,33,37,41,43] (Table S4). Among those in the highest quartile of exposure, HbAA was associated with a 3.7-fold increased risk of self-reported CVD prevalence [24] and a 33% lower prevalence of DM [32] compared with those in the lowest quartile. HbGA/HbAA was associated with a 59% lower prevalence of CVD [24], a 2-fold higher prevalence of DM [32], and a 2.5-fold higher prevalence of GO [37].
4. Discussion
This systematic review included 28 articles, 19 of which were conducted on NHANES samples, some with partially overlapping populations. It was found that higher acrylamide exposure was associated with a higher risk of CVD mortality and CVD risk, but inversely associated with most cardiovascular risk factors (lower levels of glucose, HbA1c, TC, TG, and LDL, and lower prevalence of DM, obesity, HTN, and MetS), as well as positively associated with HDL levels. However, higher exposure to glycidamide (its most reactive metabolite) was positively associated with 10 y CVD risk and most cardiovascular risk factors (higher levels of glucose, HbA1c, TC, TG, LDL, SBP, and higher prevalence of AO), and inversely associated with HDL levels. Few studies performed stratified analyses based on smoking status.
Another interesting finding was that US adolescents had the highest HbAA levels (mean 65.0 pmol/g Hb) compared to US adults, although adolescents did not have the highest HbGA concentrations (low conversion from HbAA to HbGA). It is important to highlight that the percentage of conversion from acrylamide to glycidamide is influenced by the expression of liver CYP2E1, which is induced by alcohol consumption, drugs, physical development, hormonal status [6], obesity, and diabetes [45]. This could explain the lower conversion rate to glycidamide observed in US adolescents. It has also been shown that the consumption of fried potatoes, doughnuts, hot dogs, popcorn, and nachos is responsible for higher acrylamide exposure in adolescents [46,47,48], along with sociodemographic factors [47] associated with smoking and the consumption of ultra-processed foods. The increase in the consumption of ultra-processed foods in recent decades, especially among adolescents, remains a public health concern [49,50].
Several mechanisms may explain the association between acrylamide exposure and CVD risk. For instance, acrylamide may act as an obesogenic agent by inducing fat accumulation and increasing the expression of PPARγ in male mice [51,52]. The nuclear receptor PPARγ is expressed primarily in adipose tissue, hematopoietic cells, and colon cells [53]. PPARγ regulates adipogenesis, lipid metabolism, inflammation, and insulin sensitivity [54]. In female rats, acrylamide exposure significantly increased glucose levels, impaired glucose tolerance, and decreased hepatic glycogen content. Acrylamide has been shown to promote gluconeogenesis and glycogenolysis and to decrease glycolysis by up- or down-regulating specific genes involved in glucose metabolism [55].
Some outcomes show contradictory results depending on the biomarker used to assess acrylamide exposure. For instance, AAMA was associated with increased glucose levels, whereas HbAA was associated with decreased glucose levels. This discrepancy may arise from the fact that HbAA reflects long-term exposure to acrylamide, whereas AAMA reflects short-term exposure [9]. Furthermore, markers of acrylamide and its metabolite glycidamide often show opposite associations for most outcomes. In our findings, acrylamide was negatively associated with most cardiovascular risk factors, whereas glycidamide was positively associated with most of them. This highlights the critical role of the conversion of acrylamide to glycidamide, as glycidamide is more toxic and induces greater oxidative damage, which may explain its adverse cardiovascular associations.
The social relevance of studying acrylamide exposure is that it is a ubiquitous food processing contaminant to which the entire population is unintentionally exposed throughout life, including during the prenatal period, childhood, adolescent development, and adulthood. Furthermore, exposure to acrylamide comes not only from ultra-processed foods, but also from home-cooked and restaurant foods [56], even when using new cooking methods such as air frying [57].
Given the above mentioned, finding ways to mitigate acrylamide production and consumption is one of the most important areas of research in the food industry. The Commission Regulation (EU) 2017/2158 of 20 November 2017 published mandatory acrylamide mitigation measures for the catering and restaurants industry [58], and in the US, FDA guidelines have been issued for this purpose [59]. However, the population is largely unaware of acrylamide production in home-cooked foods and its presence in their daily diet. Moreover, browned foods are mistakenly perceived as more appealing and flavorful. For this reason, the Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN) developed recommendations for consumers to minimize the presence of acrylamide in home-cooked foods (e.g., reducing the final temperature and cooking time of foods, mainly for potatoes, biscuits, bread, and breaded foods) [60]. The food industry also developed the “Acrylamide Toolbox 2019” to control the presence of acrylamide in different food production processes [61].
This review has several strengths. First, it is the first study to synthesize the evidence on the association between acrylamide exposure and multiple cardiovascular outcomes. Second, it clearly describes the evidence for this association to date, without omitting negative findings. Third, the results encourage other researchers to provide evidence in this area to clarify the effects of acrylamide on cardiovascular health. Finally, these results are also of great interest for public health, highlighting the control of environmental and food contaminants as a strategy for CVD prevention. This is not only a matter for public health policy, but also for the food industry. As home-cooked food is also one of the most common sources of exposure, educating the population about healthier eating habits and cooking methods is an important part of these public health policies. The main conclusions of this review are summarized in Table 8.

Table 8.
Main conclusions of the review.
There are also some limitations. First, most of the studies included in this review are mainly based on the US NHANES, which may limit the generalizability and applicability of the findings to other populations. Second, although interest in acrylamide exposure has increased in recent years, the current evidence on its associations with CVD is scarce, fragmented, not standardized, and yields mixed results. Also, most of the results were not stratified according to tobacco consumption, which could be an important confounding factor. In addition, it seems that acrylamide and glycidamide are not associated in the same direction with certain cardiovascular risk factors. Thirdly, a meta-analysis could not be performed because of the differences in outcome measures and statistical approaches, the partly overlapping populations, and the lack of studies. Finally, except for mortality, most of the conclusions are based on a small number of studies and cross-sectional designs that do not allow us to establish causality.
5. Conclusions
Acrylamide exposure is associated with a higher risk of CVD mortality, 10-year CVD risk, and CVD prevalence. However, it is inversely associated with the prevalence of DM, GO, AO, HTN, and MetS. In addition, acrylamide is negatively associated with glucose, HbA1c, and lipid levels (TC, TG, and LDL) and positively associated with HDL. In contrast, glycidamide—its most reactive metabolite—is associated with adverse outcomes, including higher 10-year CVD risk, higher prevalence of AO, increased glucose and HbA1c levels, increased lipid levels (TC, TG, and LDL), higher SBP, and lower HDL levels. These findings suggest that the cardiovascular risks of acrylamide are primarily mediated by its conversion to glycidamide.
Further longitudinal studies are needed to clarify the long-term effects of acrylamide on the cardiovascular system. The findings of this review, which highlight the potential adverse effects of acrylamide exposure, are of high public health relevance, as they provide evidence of associations between key cardiovascular risk factors and disease outcomes. While regulatory measures, such as those implemented in the EU and the US, represent significant progress, additional efforts are needed to increase public awareness of how home-cooking practices contribute to acrylamide exposure. Educating consumers about strategies to minimize acrylamide formation, coupled with continued innovation in food industry practices, will be critical in addressing the broader health risks associated with this substance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16244279/s1, Table S1: Studies excluded; Table S2: Risk of bias of the studies according to the Joanna Briggs Institute critical appraisal tools; Table S3: Association between acrylamide exposure and cardiovascular risk factors in non-smokers; Table S4: Association between acrylamide exposure and cardiovascular risk factors in smokers.
Author Contributions
Conceptualization: P.G.-C.; Formal analysis: D.M.M.; Investigation: P.G.-C. and D.M.M.; Methodology: P.G.-C. and D.M.M.; Project administration, Supervision, and Validation: P.G.-C.; Visualization and Writing—original draft: D.M.M.; Writing—review and editing: J.R.-G., B.M.-F. and P.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
Data collection was funded by the following grants: PI23/240 (State Secretary of R + D and FEDER/FSE) and the CIBERESP, Instituto de Salud Carlos III. Madrid, Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Exon, J.H. A Review of the Toxicology of Acrylamide. J. Toxicol. Environ. Health Part B 2006, 9, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Substance Information—ECHA. Available online: https://echa.europa.eu/es/substance-information/-/substanceinfo/100.001.067 (accessed on 31 July 2024).
- Sarion, C.; Codină, G.G.; Dabija, A. Acrylamide in Bakery Products: A Review on Health Risks, Legal Regulations and Strategies to Reduce Its Formation. Int. J. Environ. Res. Public Health 2021, 18, 4332. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.J.; Tran, N. Exposure to Acrylamide. In Chemistry and Safety of Acrylamide in Food; Springer: Boston, MA, USA, 2005; pp. 63–76. [Google Scholar] [CrossRef]
- Peris-Camarasa, B.; Pardo, O.; Fernández, S.F.; Dualde, P.; Coscollà, C. Assessment of Acrylamide Exposure in Spain by Human Biomonitoring: Risk and Predictors of Exposure. Environ. Pollut. 2023, 331, 121896. [Google Scholar] [CrossRef] [PubMed]
- Schettgen, T.; Rossbach, B.; Kütting, B.; Letzel, S.; Drexler, H.; Angerer, J. Determination of Haemoglobin Adducts of Acrylamide and Glycidamide in Smoking and Non-Smoking Persons of the General Population. Int. J. Hyg. Environ. Health 2004, 207, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Pfeifer, G.P. Genotoxicity of Acrylamide and Glycidamide. J. Natl. Cancer Inst. 2004, 96, 1023–1029. [Google Scholar] [CrossRef]
- Pedersen, M.; Vryonidis, E.; Joensen, A.; Törnqvist, M. Hemoglobin Adducts of Acrylamide in Human Blood—What Has Been Done and What Is Next? Food Chem. Toxicol. 2022, 161, 112799. [Google Scholar] [CrossRef]
- Bjellaas, T.; Stølen, L.H.; Haugen, M.; Paulsen, J.E.; Alexander, J.; Lundanes, E.; Becher, G. Urinary Acrylamide Metabolites as Biomarkers for Short-Term Dietary Exposure to Acrylamide. Food Chem. Toxicol. 2007, 45, 1020–1026. [Google Scholar] [CrossRef]
- Obón-Santacana, M.; Lujan-Barroso, L.; Freisling, H.; Cadeau, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; Kaaks, R.; Fortner, R.T.; Boeing, H.; Ramón Quirós, J.; et al. Dietary and Lifestyle Determinants of Acrylamide and Glycidamide Hemoglobin Adducts in Non-Smoking Postmenopausal Women from the EPIC Cohort. Eur. J. Nutr. 2017, 56, 1157. [Google Scholar] [CrossRef]
- Shipp, A.; Lawrence, G.; Gentry, R.; McDonald, T.; Bartow, H.; Bounds, J.; Macdonald, N.; Clewell, H.; Allen, B.; Van Landingham, C. Acrylamide: Review of Toxicity Data and Dose-Response Analyses for Cancer and Noncancer Effects. Crit. Rev. Toxicol. 2006, 36, 481–608. [Google Scholar] [CrossRef]
- Filippini, T.; Halldorsson, T.I.; Capitão, C.; Martins, R.; Giannakou, K.; Hogervorst, J.; Vinceti, M.; Åkesson, A.; Leander, K.; Katsonouri, A.; et al. Dietary Acrylamide Exposure and Risk of Site-Specific Cancer: A Systematic Review and Dose-Response Meta-Analysis of Epidemiological Studies. Front. Nutr. 2022, 9, 875607. [Google Scholar] [CrossRef]
- Adani, G.; Filippini, T.; Wise, L.A.; Halldorsson, T.I.; Blaha, L.; Vinceti, M. Dietary Intake of Acrylamide and Risk of Breast, Endometrial, and Ovarian Cancers: A Systematic Review and Dose-Response Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 31 July 2024).
- JBI Critical Appraisal Tools|JBI. Available online: https://jbi.global/critical-appraisal-tools (accessed on 31 July 2024).
- Marsh, G.M.; Youk, A.O.; Buchanich, J.M.; Kant, I.J.; Swaen, G. Mortality Patterns among Workers Exposed to Acrylamide: Updated Follow Up. J. Occup. Environ. Med. 2007, 49, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Swaen, G.M.H.; Haidar, S.; Burns, C.J.; Bodner, K.; Parsons, T.; Collins, J.J.; Baase, C. Mortality Study Update of Acrylamide Workers. Occup. Environ. Med. 2007, 64, 396. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiao, J.; Wang, J.; Chen, X.; Zhang, Y. Associations of Hemoglobin Biomarker Levels of Acrylamide and All-Cause and Cardiovascular Disease Mortality among U.S. Adults: National Health and Nutrition Examination Survey 2003–2006. Environ. Pollut. 2018, 238, 852–858. [Google Scholar] [CrossRef]
- Wu, H.; Sun, X.; Jiang, H.; Hu, C.; Xu, J.; Sun, C.; Wei, W.; Han, T.; Jiang, W. The Association Between Exposure to Acrylamide and Mortalities of Cardiovascular Disease and All-Cause Among People with Hyperglycemia. Front. Cardiovasc. Med. 2022, 9, 930135. [Google Scholar] [CrossRef]
- Marques, C.; Frenoy, P.; Elbaz, A.; Laouali, N.; Shah, S.; Severi, G.; Mancini, F.R. Association between Dietary Intake of Acrylamide and Increased Risk of Mortality in Women: Evidence from the E3N Prospective Cohort. Sci. Total Environ. 2024, 906, 167514. [Google Scholar] [CrossRef]
- Feng, X.; Qiu, F.; Zheng, L.; Zhang, Y.; Wang, Y.; Wang, M.; Xia, H.; Tang, B.; Yan, C.; Liang, R. Exposure to Volatile Organic Compounds and Mortality in US Adults: A Population-Based Prospective Cohort Study. Sci. Total Environ. 2024, 928, 172512. [Google Scholar] [CrossRef]
- Nalini, M.; Poustchi, H.; Bhandari, D.; Chang, C.M.; Blount, B.C.; Wang, L.; Feng, J.; Gross, A.; Khoshnia, M.; Pourshams, A.; et al. Volatile Organic Compounds and Mortality from Ischemic Heart Disease: A Case-Cohort Study. Am. J. Prev. Cardiol. 2024, 19, 100700. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, M.; Zhuang, P.; Jiao, J.; Chen, X.; Wang, J.; Wu, Y. Exposure to Acrylamide and the Risk of Cardiovascular Diseases in the National Health and Nutrition Examination Survey 2003–2006. Environ. Int. 2018, 117, 154–163. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Yu, L.; Liu, W.; Song, J.; Fan, L.; Zhou, M.; Yang, M.; Ma, J.; Cheng, M.; et al. Acrylamide Exposure Increases Cardiovascular Risk of General Adult Population Probably by Inducing Oxidative Stress, Inflammation, and TGF-Β1: A Prospective Cohort Study. Environ. Int. 2022, 164, 107261. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Cheng, D.; Cao, Y.; Xie, X.; Zhou, J.; Wu, Y.; Li, X.; Yu, J.; Yang, B. Association between Urinary Metabolites of Volatile Organic Compounds and Cardiovascular Disease in the General Population from NHANES 2011–2018. Ecotoxicol. Environ. Saf. 2023, 264, 115412. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhu, X.; Li, F.; Guan, G.; Hui, R.; Zhu, L.; Pang, H.; Zhang, Y. Associations of Urinary Volatile Organic Compounds with Cardiovascular Disease among the General Adult Population. Int. J. Environ. Health Res. 2024, 34, 3876–3890. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xie, M.; Cheng, S.; Han, Y.; Li, P.; Guo, J. Associations between Specific Volatile Organic Chemical Exposures and Cardiovascular Disease Risks: Insights from NHANES. Front. Public Health 2024, 12, 1378444. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.C.; Kuo, H.K.; Hwang, J.J.; Lin, J.L.; Chen, P.C.; Lin, L.Y. Association among Acrylamide, Blood Insulin, and Insulin Resistance in Adults. Diabetes Care 2009, 32, 2206–2211. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, H.L.; Chen, Y.C.; Lien, G.W.; Lin, L.Y.; Wen, L.L.; Liao, C.C.; Chien, K.L.; Sung, F.C.; Chen, P.C.; et al. Positive Association between Urinary Levels of 8-Hydroxydeoxyguanosine and the Acrylamide Metabolite N-Acetyl-S-(Propionamide)-Cysteine in Adolescents and Young Adults. J. Hazard. Mater. 2013, 261, 372–377. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, W.; Yang, S.; Cao, L.; Zhu, C.; Ma, J.; Li, W.; Zhang, Z.; Xu, T.; Wang, X.; et al. Acrylamide Exposure and Oxidative DNA Damage, Lipid Peroxidation, and Fasting Plasma Glucose Alteration: Association and Mediation Analyses in Chinese Urban Adults. Diabetes Care 2020, 43, 1479–1486. [Google Scholar] [CrossRef]
- Yin, G.; Liao, S.; Gong, D.; Qiu, H. Association of Acrylamide and Glycidamide Haemoglobin Adduct Levels with Diabetes Mellitus in the General Population. Environ. Pollut. 2021, 277, 116816. [Google Scholar] [CrossRef]
- Hosseini-Esfahani, F.; Beheshti, N.; Nematollahi, A.; Koochakpoor, G.; Verij-Kazemi, S.; Mirmiran, P.; Azizi, F. The Association between Dietary Acrylamide Intake and the Risk of Type 2 Diabetes Incidence in the Tehran Lipid and Glucose Study. Sci. Rep. 2023, 13, 8235. [Google Scholar] [CrossRef]
- Cheang, I.; Liao, S.; Zhu, X.; Lu, X.; Zhu, Q.; Yao, W.; Zhou, Y.; Zhang, H.; Li, X. Association of Acrylamide Hemoglobin Biomarkers with Serum Lipid Levels in General US Population: NHANES 2013–2016. Ecotoxicol. Environ. Saf. 2021, 214, 112111. [Google Scholar] [CrossRef]
- Chen, W.Y.; Fu, Y.P.; Tu, H.; Zhong, W.; Zhou, L. The Association between Exposure to Volatile Organic Compounds and Serum Lipids in the US Adult Population. Lipids Health Dis. 2023, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.L.; Lin, L.Y.; Chen, P.C.; Su, T.C.; Lin, C.Y. Negative Association between Acrylamide Exposure and Body Composition in Adults: NHANES, 2003–2004. Nutr. Diabetes 2017, 7, e246. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhuang, P.; Jiao, J.; Wang, J.; Zhang, Y. Association of Acrylamide Hemoglobin Biomarkers with Obesity, Abdominal Obesity and Overweight in General US Population: NHANES 2003–2006. Sci. Total Environ. 2018, 631–632, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Xu, F.; Shi, S.; Liao, S.; Tang, X.; Zhang, H.; Zhou, Y.; Li, X. Vitamin D Mediates the Association between Acrylamide Hemoglobin Biomarkers and Obesity. Environ. Sci. Pollut. Res. Int. 2022, 29, 17162–17172. [Google Scholar] [CrossRef]
- Lei, T.; Qian, H.; Yang, J.; Hu, Y. The Association Analysis between Exposure to Volatile Organic Chemicals and Obesity in the General USA Population: A Cross-Sectional Study from NHANES Program. Chemosphere 2023, 315, 137738. [Google Scholar] [CrossRef]
- Liang, J.; Xu, C.; Liu, Q.; Weng, Z.; Zhang, X.; Xu, J.; Gu, A. Total Cholesterol: A Potential Mediator of the Association between Exposure to Acrylamide and Hypertension Risk in Adolescent Females. Environ. Sci. Pollut. Res. 2022, 29, 38425–38434. [Google Scholar] [CrossRef]
- McGraw, K.E.; Konkle, S.L.; Riggs, D.W.; Rai, S.N.; DeJarnett, N.; Xie, Z.; Keith, R.J.; Oshunbade, A.; Hall, M.E.; Shimbo, D.; et al. Exposure to Volatile Organic Compounds Is Associated with Hypertension in Black Adults: The Jackson Heart Study. Environ. Res. 2023, 223, 115384. [Google Scholar] [CrossRef]
- Hung, C.C.; Cheng, Y.W.; Chen, W.L.; Fang, W.H. Negative Association between Acrylamide Exposure and Metabolic Syndrome Markers in Adult Population. Int. J. Environ. Res. Public Health 2021, 18, 11949. [Google Scholar] [CrossRef]
- Wan, X.; Zhu, F.; Zhuang, P.; Liu, X.; Zhang, L.; Jia, W.; Jiao, J.; Xu, C.; Zhang, Y. Associations of Hemoglobin Adducts of Acrylamide and Glycidamide with Prevalent Metabolic Syndrome in a Nationwide Population-Based Study. J. Agric. Food Chem. 2022, 70, 8755–8766. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Y.; Liu, J.; Liu, Z.; Shi, R. Associations of Individual and Mixture Exposure to Volatile Organic Compounds with Metabolic Syndrome and Its Components among US Adults. Chemosphere 2024, 347, 140683. [Google Scholar] [CrossRef]
- Tanaka, E.; Terada, M.; Misawa, S. Cytochrome P450 2E1: Its Clinical and Toxicological Role. J. Clin. Pharm. Ther. 2000, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Vryonidis, E.; Törnqvist, M.; Lignell, S.; Rosén, J.; Aasa, J. Estimation of Intake and Quantification of Hemoglobin Adducts of Acrylamide in Adolescents in Sweden. Front. Nutr. 2024, 11, 1371612. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.F.; Poteser, M.; Govarts, E.; Pardo, O.; Coscollà, C.; Schettgen, T.; Vogel, N.; Weber, T.; Murawski, A.; Kolossa-Gehring, M.; et al. Determinants of Exposure to Acrylamide in European Children and Adults Based on Urinary Biomarkers: Results from the “European Human Biomonitoring Initiative” HBM4EU Participating Studies. Sci. Rep. 2023, 13, 21291. [Google Scholar] [CrossRef] [PubMed]
- Duke, T.J.; Ruestow, P.S.; Marsh, G.M. The Influence of Demographic, Physical, Behavioral, and Dietary Factors on Hemoglobin Adduct Levels of Acrylamide and Glycidamide in the General U.S. Population. Crit. Rev. Food Sci. Nutr. 2018, 58, 700–710. [Google Scholar] [CrossRef]
- Wang, L.; Martínez Steele, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in Consumption of Ultraprocessed Foods Among US Youths Aged 2–19 Years, 1999–2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef]
- Lauria, F.; Dello Russo, M.; Formisano, A.; De Henauw, S.; Hebestreit, A.; Hunsberger, M.; Krogh, V.; Intemann, T.; Lissner, L.; Molnar, D.; et al. Ultra-Processed Foods Consumption and Diet Quality of European Children, Adolescents and Adults: Results from the I.Family Study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3031–3043. [Google Scholar] [CrossRef]
- Lee, H.W.; Pyo, S. Acrylamide Induces Adipocyte Differentiation and Obesity in Mice. Chem. Biol. Interact. 2019, 298, 24–34. [Google Scholar] [CrossRef]
- Egusquiza, R.J.; Blumberg, B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology 2020, 161, bqaa024. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty Acids, Eicosanoids and PPAR Gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Wang, Q.; Imam, M.U.; Yida, Z.; Wang, F. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) as a Target for Concurrent Management of Diabetes and Obesity-Related Cancer. Curr. Pharm. Des. 2017, 23, 3677–3688. [Google Scholar] [CrossRef]
- Yue, Z.; Chen, Y.; Song, Y.; Zhang, J.; Yang, X.; Wang, J.; Li, L.; Sun, Z. Effect of Acrylamide on Glucose Homeostasis in Female Rats and Its Mechanisms. Food Chem. Toxicol. 2020, 135, 110894. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.; Gujska, E.; Czarnowska-Kujawska, M.; Nowak, F. Effect of Different Home-Cooking Methods on Acrylamide Formation in Pre-Prepared Croquettes. J. Food Compos. Anal. 2017, 56, 134–139. [Google Scholar] [CrossRef]
- Navruz-Varlı, S.; Mortaş, H. Acrylamide Formation in Air-Fried versus Deep and Oven-Fried Potatoes. Front. Nutr. 2023, 10, 1297069. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2017/2158 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food.|FAOLEX. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC171340/ (accessed on 31 July 2024).
- Guidance for Industry: Acrylamide in Foods|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-acrylamide-foods (accessed on 31 July 2024).
- Aesan—Agencia Española de Seguridad Alimentaria y Nutrición. Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/campanyas/acrilamida.htm (accessed on 31 July 2024).
- Acrylamide Toolbox—FoodDrinkEurope: FoodDrinkEurope. Available online: https://www.fooddrinkeurope.eu/resource/acrylamide-toolbox/ (accessed on 31 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).