Highlights
- Clinical trials show that high-amylose rice lowers postprandial blood glucose concentrations and glycemic index.
- Further, the effectiveness of postprandial blood glucose control is shown as amylose content increases.
- Overall, high-amylose rice holds promise for blood sugar management.
Abstract
Background/Objectives: Rice is a major staple in the diets of East Asian populations. Numerous meta-analyses have shown an association between high white rice consumption and a higher risk of diabetes. High-amylose rice (varieties with over 25% amylose content) is absorbed more slowly in the gut compared to low-amylose rice, and it results in lower levels of postprandial blood glucose. Various intervention studies have investigated the effects of high-amylose rice consumption on postprandial blood glucose and the glycemic index. The quantity of the research suggests that a comprehensive review of these diverse findings is necessary. Methods and Results: We reviewed 17 clinical trials, most of which showed that high-amylose rice ingestion results in lower postprandial blood glucose levels and glycemic index compared to low-amylose rice diets. Although they differed in their sample size, study design, rice type and quantity, and amylose content, most of these studies suggested that there is a reasonable effect of high-amylose rice consumption on postprandial blood glucose. In particular, the effect on blood glucose suppression tended to be related to the amylose content. However, long-term intake studies are still limited and require further investigation. Conclusions: In conclusion, high-amylose rice shows promise for blood glucose management.
1. Introduction
The International Diabetes Federation (IDF) projected, in its Diabetes Atlas, that over 90% of global diabetes cases are attributed to Type 2 diabetes, with recent estimates indicating that the diabetic population is anticipated to reach 783 million by the year 2045 [1]. In Japan, one of the world’s fastest-aging nations, the estimated number of individuals with glucose intolerance-related health issues, including diabetes, has been continuously increasing over the past three decades [2]. The fundamental approach for managing diabetes encompasses dietary modifications and regular exercise, complemented by pharmacological intervention methods. Despite the diverse array of antihyperglycemic agents at our disposal, over 50% of individuals diagnosed with Type 2 diabetes mellitus fail to attain internationally acknowledged blood glucose targets [3]. The management of hyperglycemia in Type 2 diabetes (2022) consensus report advocates that the incorporation of lifestyle adjustments, including dietary modifications, is a crucial component of the therapeutic regimen [4].
Rice is a staple food in East Asian diets, and numerous associations between diabetes and dietary habits have been documented, with particular emphasis being placed on the link between the consumption of white rice and the risk of developing diabetes [5,6,7]. Both domestic and international studies, supported by multiple meta-analyses, have demonstrated a notable association between elevated white rice consumption and an augmented risk of diabetes [8,9,10]. However, there are also low-glycemic index (GI) foods, such as brown rice and high-amylose rice (varieties with an amylose content exceeding 25%), which are digested slowly in the intestines and do not raise postprandial blood glucose levels [11,12]. As shown in Figure 1, unlike the highly branched structure of amylopectin, the linear structure of amylose makes it resistant to hydrolysis via α-amylase, resulting in a slower breakdown rate; this characteristic classifies it as type 2 resistant starch, which contributes to its ability to moderate blood glucose response [13,14].
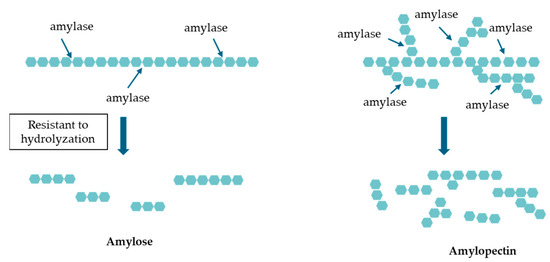
Figure 1.
Hydrolysis of amylose and amylopectin. Comparison of the amylose and amylopectin digestion mechanisms. Amylose’s linear structure resists hydrolysis via amylase, slowing glucose release while amylopectin’s branched structure allows for faster enzymatic digestion, leading to rapid blood glucose elevation.
Numerous studies have investigated the mitigation of elevated postprandial blood glucose levels and GI regarding high-amylose rice consumption [15,16,17,18]. However, these studies have varied in their sample size, study design, rice type and volume, and amylose content. To draw meaningful conclusions regarding the impact of high-amylose rice on postprandial blood glucose levels, there is a need to systematically organize these studies’ findings. To date, a comprehensive review of the results of previous studies has been lacking. Consequently, the present study employed both national and international literature databases to conduct a review of the data associated with the consumption of high-amylose rice and its impact on postprandial blood glucose levels.
2. Materials and Methods
2.1. Search Methods for Articles
This review followed the PRISMA statement guidelines [19]. Literature related to high-amylose rice and its impact on postprandial blood glucose levels was gathered. The literature search included articles published between January 1984 and April 2024, and it was conducted using PubMed, Google Scholar, and Ichushi Web (Japanese), which is developed and maintained by the NPO Central Journal of Medical Science. The keywords used for the search were “high amylose rice”, “glucose”, and “glycemic index”.
2.2. Screening and Selection of Articles
Articles were screened using the following inclusion criteria: (1) the study design was a clinical trial; (2) the exposure was the consumption of high-amylose rice; and (3) the outcomes included changes in postprandial blood glucose levels and the GI. The following were excluded: (1) studies involving animal subjects; (2) processed products containing high-amylose rice (e.g., cakes, porridge); and (3) duplicate publications, books, conference abstracts, and similar materials.
2.3. Article Information Summary
From each article, a multitude of information was extracted and summarized, including, but not limited to, the study design, country/region where the study was conducted, participant characteristics (age, gender, disease status, and sample size), study methods (intervention, exposure, and control), outcome assessment/results (mean postprandial area under the curve (AUC)/incremental area under the curve (IAUC) values, GI, and glycemic load), and rice amylose content (Table 1 and Table 2).

Table 1.
Description of studies showing significant effects of high-amylose rice on the AUC/IAUC of the postprandial glucose levels.

Table 2.
Description of studies showing no significant effects or unmeasured outcomes of high-amylose rice on the AUC/IAUC of the postprandial glucose levels.
3. Results
3.1. Literature Search
The literature search yielded a total of 1254 articles, comprising 98 from PubMed, 1150 from Google Scholar, and 6 from Ichushi Web. Following a thorough screening process involving titles, abstracts, and related criteria, 21 articles (i.e., 21 studies) were selected for a comprehensive full-text review. Subsequently, a total of 17 articles were used after 4 articles were excluded that did not align with our predefined inclusion criteria (pertaining to study design, intervention, subjects, or outcomes) (Figure 2).
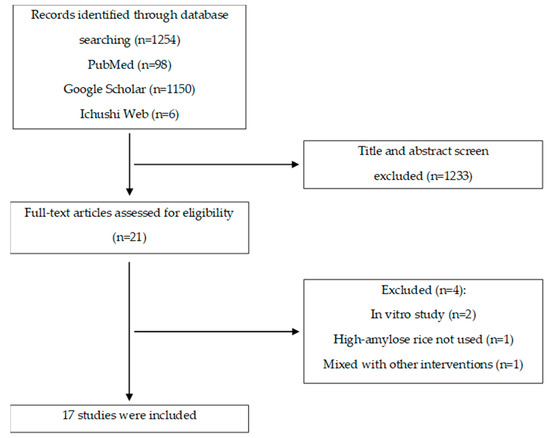
Figure 2.
Flow diagram of the study selection processes.
3.2. Study Characteristics
The 17 selected studies [15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32] originated from various countries (as shown in Table 1 and Table 2): 7 were from Japan, 3 from the United States, 2 from Bangladesh, and 1 each from Malaysia, Denmark, Australia, the Philippines, and Sri Lanka. Regarding the individuals included in the respective studies, 15 involved healthy adults, while 2 involved individuals with Type 2 diabetes. A total of 16 of the studies compared postprandial glucose AUC or IAUC, and 10 documented GI values.
3.3. Effect of High-Amylose Rice Consumption on Glucose AUC or IAUC
In the studies comparing the AUC or IAUC, 9 out of 14 reported significantly lower postprandial glucose AUC or IAUC in individuals after consuming high-amylose rice compared to those who consumed control rice (low-to-middle-amylose rice) (Table 1) [15,16,20,21,24,25,26,27,28]. Two of these studies involved diabetic individuals and demonstrated the effectiveness of high-amylose rice in inhibiting blood glucose elevation [20,21]. Moreover, among the seven studies featuring an amylose content exceeding 27%, six studies showed a significantly lower glucose AUC or IAUC in individuals after consuming high-amylose rice [15,20,21,24,26,27]. Additionally, among the seven studies featuring relatively low amylose content (<27%), four highlighted the insignificant effect that high-amylose rice consumption had on postprandial glucose levels [17,18,31,32].
Regarding studies using glucose as the reference food, a total of five studies were selected. Among these studies, two reported numerical values. The median IAUC level observed in individuals after consuming glucose was 4325 (range: 2955–5695) mg×min/dL while the median IAUC for those who consumed high-amylose rice was 2658 (range: 1969–2800) mg×min/dL [22,31]. Among the five studies reporting significant differences due to diet, compared to those who consumed normal rice where the median IAUC observed was 3518 (range: 2502–4248) mg×min/dL while those whose diet focused on high-amylose rice displayed a median of 1767 (range: 1389–3384) mg×min/dL [16,24,26,27]. The median AUC for individuals who consumed normal rice was 8240 (range: 3519–12,960) mg×min/dL, and it was 2419 (range: 2170–11,640) mg×min/dL for those who consumed high-amylose rice [15,16].
3.4. Effect of High-Amylose Rice Consumption on the GI
A total of ten of the selected papers focused on documenting the GI values, as shown in Table 1 and Table 2. Among the five studies calculating GI based on glucose consumption, two reported that high-amylose rice, when consumed, aids in lowering the GI (i.e., “low GI food”) [22,24], whereas two classified it as a “medium GI food” [23,30]. In these ten studies, the median GI value calculated with glucose as the reference food was 61 (range: 43–86), while that calculated with normal rice or white bread as the reference food was 50 (range: 45–86).
4. Discussion
In this study, 17 clinical trials were reviewed, with sample sizes ranging from 4 to 33 participants. Most of the results showed that, compared to those consuming normal rice, individuals consuming high-amylose rice exhibited lower postprandial blood glucose levels and GI. Regarding the GI, most of the studies indicated that high-amylose rice belongs in the category of medium-to-low GI foods. Compared to normal white rice or white bread, high-amylose rice consumption led to a lower GI in participants. Although the results of AUC and IAUC for postprandial blood glucose levels after consuming high-amylose rice were not significant in individual studies, a significant reduction in the initial blood glucose levels and peak blood glucose levels was observed, and, in some studies, the overall insulin secretion responses were significantly lower [17,32]. Overall, these data indicate the potential for high-amylose rice to delay glucose digestion and absorption.
Potential reasons for the inconsistency in these findings may be the amylose content levels in experimental diets and the ethnicity of participating individuals. In our review, the effects on the inhibition of postprandial blood glucose elevation were more pronounced in the studies where the content of amylose in rice exceeded 27% [15,20,21,22,23,24,26,27]. In particular, as observed in the studies by Fatema K. et al. and Pathiraje P. et al., among various high-amylose rice types, the rice with the lowest amylose content (24.5%) had the highest GI (GI = 73). However, when the amylose content exceeded 27%, the GI was consistently below 70, which suggests a correlation between the effectiveness of inhibiting the blood glucose elevation and amylose content [22,23]. Previous studies found that the hydrolysis rate of starch in high-amylose rice is significantly lower than that in normal rice [33]. Moreover, as the amylose content increases, the hydrolysis rate slows down, resulting in reduced digestion and absorption rates [34]. This may explain why higher amylose content is associated with providing better glycemic control. The majority of participants were Asian in the studies and no significant differences were shown. Therefore, the results may be due to ethnic differences as Asians tend to have lower insulin sensitivity and higher insulin resistance than Caucasians, according to relevant research [35]. Additionally, both studies focusing on Type 2 diabetes demonstrated a lower increase in postprandial blood glucose levels compared to individuals with normal rice diets [20,21]. This suggests that high-amylose rice may have a more marked (and beneficial) effect in diabetic patients. Consistent with previous reviews, the higher proportion of amylose could be associated with lower postprandial blood glucose [36]. Moreover, the present review further explores this relationship and suggests that an amylose content of approximately 27% may play a significant role in influencing postprandial blood glucose. Unlike earlier reviews that primarily provided a general evaluation of various rice types or starchy foods, this review discusses whether high-amylose rice specifically contributes to the suppression of postprandial hyperglycemia [36,37].
It must be noted that this study had some limitations. Firstly, a limited number of published papers fit the search criteria, and the rice cooking methods varied across different studies. For instance, in the Goddard M.S. et al. and Juliano B.O. et al. studies, microwave-cooked rice was utilized [17,29], while the participants in the studies of Zenel A.M. et al. and Saito Y. et al. consumed refrigerated and reheated rice [15,26]. Previous research indicates that different cooking and processing methods can influence the digestibility and postprandial glycemic responses of various rice varieties [38,39,40]. Additionally, studies by Yamaguchi T. et al. and Mori H. et al. used the same high-amylose rice as the test food, but differences such as water-to-rice ratios in the processing methods used led to varying outcomes [25,32]. Furthermore, the literature selection process in the present review was constrained by the keywords and the types of test foods. Studies from previous related reviews, such as those focusing on resistant starch rice, were excluded not only due to differences in keywords but also because they did not clearly identify high-amylose rice as the main subject [41]. Similarly, studies involving meals that combined high-amylose rice with other ingredients, such as amylomaize starch, were excluded as they did not solely focus on high-amylose rice [42]. Secondly, factors such as the participants’ characteristics, particularly whether they are undergoing diabetic treatment or not, warrant further investigation.
Multiple studies have indicated the beneficial effects of high-amylose rice in inhibiting postprandial blood glucose elevation [15,16,20,21,22,23,24,25,26,27,28], with two studies showing particular effectiveness in diabetic patients [20,21], thus suggesting its potential value in diabetic dietary management. However, the data on this topic remain limited, and further research is required. Additionally, the unique texture of high-amylose rice and the culinary preferences may limit consistent rice consumption by certain individuals.
5. Conclusions
Overall, high-amylose rice holds promise for blood sugar management. In conclusion, a tendency toward lower postprandial blood glucose levels was observed as a result of high-amylose-focused diets, suggesting that high-amylose rice may have a beneficial effect on postprandial hyperglycemia.
Author Contributions
J.L.: conceptualization ideas, methodology, and writing—original draft; K.Y.: investigation; M.S.: investigation; K.M.: conceptualization of ideas, methodology, writing—review and editing, and formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are derived from publicly available resources. These data were obtained from the following public domain sources: PubMed, available at https://pubmed.ncbi.nlm.nih.gov. Google Scholar, available at https://scholar.google.com. Ichushi Web, available at https://search.jamas.or.jp/. References to the articles retrieved from these platforms are provided in the manuscript.
Acknowledgments
Throughout the writing of this dissertation, the authors received a great deal of support and assistance. We would particularly like to acknowledge the members of the Laboratory of Community Health and Nutrition, Ehime University, for their wonderful collaboration and patient support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The International Diabetes Federation. IDF Diabetes Atlas 10th Edition. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 3 February 2024).
- Summary of National Health and Nutrition Survey Results. Available online: https://www.mhlw.go.jp/content/10900000/000687163.pdf (accessed on 3 February 2024). (In Japanese).
- Hoerger, T.J.; Segel, J.E.; Gregg, E.W.; Saaddine, J.B. Is glycemic control improving in U.S. adults? Diabetes Care 2008, 31, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.Y.H.; Koh, W.P.; Yuan, J.M.; van Dam, R.M. Rice intake and risk of type 2 diabetes: The Singapore Chinese Health Study. Eur. J. Nutr. 2019, 58, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.; Lin, X.; Jia, W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014, 2, 980–991. [Google Scholar] [CrossRef]
- Venn, B.S.; Williams, S.M.; Mann, J.I. Comparison of postprandial glycaemia in Asians and Caucasians. Diabet. Med. 2010, 27, 1205–1208. [Google Scholar] [CrossRef]
- Ren, G.; Qi, J.; Zou, Y. Association between intake of white rice and incident type 2 diabetes—An updated meta-analysis. Diabetes Res. Clin. Pract. 2021, 172, 108651. [Google Scholar] [CrossRef]
- Yu, J.Y.; Balaji, B.; Tinajero, M.; Jarvis, S.; Khan, T.; Vasudevan, S.; Ranawana, V.; Poobalan, A.; Bhupathiraju, S.; Sun, Q.; et al. White rice, brown rice and the risk of type 2 diabetes: A systematic review and meta-analysis. BMJ Open 2022, 12, e065426. [Google Scholar] [CrossRef]
- Bhavadharini, B.; Mohan, V.; Dehghan, M.; Rangarajan, S.; Swaminathan, S.; Rosengren, A.; Wielgosz, A.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. White Rice Intake and Incident Diabetes: A Study of 132,373 Participants in 21 Countries. Diabetes Care 2020, 43, 2643–2650. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Frei, M.; Siddhuraju, P.; Becker, K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003, 83, 395–402. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Khaneghah, A.M.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods. 2022, 93, 105094. [Google Scholar] [CrossRef]
- Ebihara, K. Nutritional and physiological functions of resistant starch. J. Cookery Sci. Jpn. 2014, 47, 49–52. (In Japanese) [Google Scholar] [CrossRef]
- Zenel, A.M.; Stewart, M.L. High Amylose White Rice Reduces Post-Prandial Glycemic Response but Not Appetite in Humans. Nutrients 2015, 7, 5362–5374. [Google Scholar] [CrossRef] [PubMed]
- Unno, R.; Hayashi, Y.; Takagi, R.; Takano, K.; Kawamori, R. Effects of high amylose rice “Yuki no Ho” on postprandial blood glucose and insulin secretion in healthy adults. Nihon Byotai Eiyo Gakkaishi (J. Metab. Clin. Nutr. Jpn.) 2012, 15, 167–173. (In Japanese) [Google Scholar]
- Goddard, M.S.; Young, G.; Marcus, R. The effect of amylose content on insulin and glucose responses to ingested rice. Am. J. Clin. Nutr. 1984, 39, 388–392. [Google Scholar] [CrossRef]
- Maruyama, K.; Minakuchi, S.; Tomooka, K.; Tanigawa, T. Examination of the glycemic index for the high-amylose rice variety “Hoshinishiki” grown in Ehime prefecture. J. Nutr. Sci. 2019, 72, 85–89. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Larsen, H.N.; Christensen, C.; Rasmussen, O.W.; Tetens, I.H.; Choudhury, N.H.; Thilsted, S.H.; Hermansen, K. Influence of parboiling and physico-chemical characteristics of rice on the glycaemic index in non-insulin-dependent diabetic subjects. Eur. J. Clin. Nutr. 1996, 50, 22–27. [Google Scholar]
- Parvin, S.; Hasan, Q.; Knudsen, K.E.B.; Ali, L. Effects of Parboiling and Physico-Chemical Characteristics of Rice on the Glycemic and Insulinemic Indices in Type 2 Diabetic Subjects. Ibrahim Med. Coll. J. 2009, 2, 12–16. [Google Scholar] [CrossRef]
- Fatema, K.; Rahman, F.; Sumi, N.; Kobura, K.; Ali, L. Glycemic index of three common varieties of Bangladeshi rice in healthy subjects. Afr. J. Food Sci. 2010, 4, 531–535. [Google Scholar]
- Pathiraje, P.; Madhujith, W.M.; Chandrasekara, A.; Nissanka, S.P. The effect of rice variety and parboiling on in vivo glycemic response. Trop. Agric. Res. 2010, 22, 26–33. [Google Scholar] [CrossRef][Green Version]
- Trinidad, T.P.; Mallillin, A.C.; Encabo, R.R.; Sagum, R.S.; Felix, A.D.; Juliano, B.O. The effect of apparent amylose content and dietary fibre on the glycemic response of different varieties of cooked milled and brown rice. Int. J. Food Sci. Nutr. 2013, 64, 89–93. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Enoki, Y.; Sasagawa, K.; Fujimura, S. Evaluation of Postprandial Glycemic Response and Physical Properties of High-Amylose Rice “Koshinokaori”. J. Nutr. Sci. Vitaminol. 2019, 65, S117–S121. [Google Scholar] [CrossRef]
- Saito, Y.; Watanabe, T.; Sasaki, T.; Watanabe, K.; Hirayama, M.; Fujita, N. Effects of single ingestion of rice cracker and cooked rice with high resistant starch on postprandial glucose and insulin responses in healthy adults: Two randomized, single-blind, cross-over trials. Biosci. Biotechnol. Biochem. 2020, 84, 365–371. [Google Scholar] [CrossRef]
- Tamura, A.; Kontai, Y.; Enoki, Y.; Maejima, D. Inhibitory effects of gelatinized high-amylose rice ‘Koshi-no-kaori’ on post-prandial blood glucose elevation and improvement of taste. Nihon Byotai Eiyo Gakkaishi (J. Metab. Clin. Nutr. Jpn.) 2021, 24, 29–39. (In Japanese) [Google Scholar]
- Yamaguchi, T.; Kobayashi, M.; Mizutani, M.; Fujimura, S.; Enoki, Y. Effect of cooking conditions on postprandial glycemic response and eating qualities of high-amylose rice “Koshinokaori”. Food Sci. Technol. Res. 2021, 27, 161–167. [Google Scholar] [CrossRef]
- Juliano, B.O.; Goddard, M.S. Cause of varietal difference in insulin and glucose responses to ingested rice. Plant Food Hum. Nutr. 1986, 36, 35–41. [Google Scholar] [CrossRef]
- Miller, J.B.; Pang, E.; Bramall, L. Rice: A high or low glycemic index food? Am. J. Clin. Nutr. 1992, 56, 1034–1036. [Google Scholar] [CrossRef]
- Karupaiah, T.; Aik, C.K.; Heen, T.C.; Subramaniam, S.; Bhuiyan, A.R.; Fasahat, P.; Zain, A.M.; Ratnam, W. A transgressive brown rice mediates favourable glycaemic and insulin responses. J. Sci. Food Agric. 2011, 91, 1951–1956. [Google Scholar] [CrossRef]
- Mori, H.; Mori, M.; Ikuta, Y.; Enoki, Y.; Sasagawa, K.; Tanaka, S.; Kida, N.; Nomura, T.; Yamori, Y. Investigation of blood glucose response during consumption of “Koshi-no-kaori”, a Japanese high amylose rice variety with different cooking methods. Nihon Byotai Eiyo Gakkaishi (J. Metab. Clin. Nutr. Jpn.) 2018, 21, 237–246. (In Japanese) [Google Scholar]
- Stevnebø, A.; Sahlström, S.; Svihus, B. Starch structure and degree of starch hydrolysis of small and large starch granules from barley varieties with varying amylose content. Anim. Feed. Sci. Technol. 2006, 130, 23–38. [Google Scholar] [CrossRef]
- Shu, X.; Jia, L.; Ye, H.; Li, C.; Wu, D. Slow digestion properties of rice different in resistant starch. J. Agric. Food Chem. 2009, 57, 7552–7559. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, S.; Tabák, A.G.; Akbaraly, T.N.; Hulmán, A.; Kivimäki, M.; Forouhi, N.G.; Iso, H.; Brunner, E.J. Age trajectories of glycaemic traits in non-diabetic South Asian and white individuals: The Whitehall II cohort study. Diabetologia 2015, 58, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Boers, H.M.; Seijen Ten Hoorn, J.; Mela, D.J. A systematic review of the influence of rice characteristics and processing methods on postprandial glycaemic and insulinaemic responses. Br. J. Nutr. 2015, 114, 1035–1045. [Google Scholar] [CrossRef]
- Cai, M.; Dou, B.; Pugh, J.E.; Lett, A.M.; Frost, G.S. The impact of starchy food structure on postprandial glycemic response and appetite: A systematic review with meta-analysis of randomized crossover trials. Am. J. Clin. Nutr. 2021, 114, 472–487. [Google Scholar] [CrossRef]
- Gunathilaka, M.D.; Ekanayake, S. Effect of different cooking methods on glycaemic index of Indian and Pakistani basmati rice varieties. Ceylon Med. J. 2015, 60, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.T.; Stewart, M.L. Effect of variety and cooking method on resistant starch content of white rice and subsequent postprandial glucose response and appetite in humans. Asia Pac. J. Clin. Nutr. 2013, 22, 372–379. [Google Scholar] [CrossRef]
- Sonia, S.; Witjaksono, F.; Ridwan, R. Effect of cooling of cooked white rice on resistant starch content and glycemic response. Asia Pac. J. Clin. Nutr. 2015, 24, 620–625. [Google Scholar] [CrossRef]
- Li, M.; Piao, J.H.; Tian, Y.; Li, W.D.; Li, K.J.; Yang, X.G. Postprandial glycaemic and insulinaemic responses to GM-resistant starch-enriched rice and the production of fermentation-related H2 in healthy Chinese adults. Br. J. Nutr. 2010, 103, 1029–1034. [Google Scholar] [CrossRef]
- van Amelsvoort, J.M.; Weststrate, J.A. Amylose-amylopectin ratio in a meal affects postprandial variables in male volunteers. Am. J. Clin. Nutr. 1992, 55, 712–718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).