Abstract
Background: Gastrointestinal symptoms (GISs) can affect the performance of endurance athletes (EAs). This study aims to analyze the efficacy of carbohydrate (CHO), gluten-free, and low-mono-saccharide and polyol (FODMAP) diets in preventing GISs in adult EAs of both sexes. Methods: A systematic search was conducted prior to 30 June 2024 in accordance with the PRISMA statement. We searched for original studies from the last eight years, in English or Spanish, that looked at the effect of CHO, gluten-free, or FODMAP diets on the GISs of EAs. In PubMed, the MeSH (medical subject heading) categories were used. The search was repeated in EBSCO, Google Scholar, and Web of Science. The inclusion criteria were determined using the PICOS framework and the risk of bias in each paper was assessed using the PEDro scale quality criteria checklist (systematic review registration: INPLASY202490080). Results: Of 289 articles identified, only 3.5% met the eligibility criteria. All studies found that GISs are common in EAs. We found that 60% of the articles used an experimental method; moreover, based on 80% of the articles, following a bowel training diet, like CHO, reduced fiber and dairy products, or a low-FODMAP diet, has the potential to reduce gastrointestinal symptoms and improve the athletic performance of EA. Conclusions: We found that low-FODMAP diets, gut training with CHO intake, and decreased fiber and dairy intake may have favorable effects in preventing GISs. No studies support a gluten-free diet in reducing GISs in EAs.
1. Introduction
Endurance athletes (EAs) often experience gastrointestinal symptoms (GISs) [1,2] that affect their performance and health [3,4]. Previous findings suggest that up to 70% of EAs, during rest and moderate and vigorous exercise, can have a high frequency and intensity of symptoms [5,6]. These multifactorial symptoms involve mechanical, physiological, and nutritional factors [2,7]. They may occur before, during, or after exercise and manifest as upper symptoms (including nausea, vomiting, and reflux) or lower symptoms (including abdominal pain, bloating, flatulence, diarrhea, and rectal bleeding) [8].
Appropriate nutrition could help with managing the symptomology of GISs. The implementation of popular diets has increased rapidly in recent years due to their perceived ergogenic and health benefits [9,10,11]. A recently published exploratory study found that the self-reported nutritional strategies used more commonly by EAs are those related to different types of carbohydrates (CHOs) (dietary fiber reduction, dairy avoidance, FODMAP diet) [12]. In addition, a recent review [1] showed that the FODMAP diet and employing repetitive gut training using carbohydrates are the most used approaches by Ultra-EAs to alleviate exercise-induced GISs. Interestingly, over recent years, adherence to a gluten-free diet in non-celiac athletes has become increasingly popular [13]. Surprisingly, forty percent of non-celiac athletes report following this diet at least half the time, and 60% have a self-reported “gluten intolerance” [10,14].
CHO is a fuel source oxidized by skeletal muscle tissue during prolonged exercise [15]. The consumption of CHOs during exercise can improve endurance and performance during prolonged exercise (>2 h) [16]. Additionally, previous studies have reported that efficient oxidation is associated with a lower accumulation of CHO in the gastrointestinal tract, reducing the risk of developing GIS discomfort [17,18]. Gastrointestinal training with a high amount of CHO is a strategy that can help athletes improve their tolerance to CHO intake and reduce GISs during exercise [19,20]. This involves the consumption of CHO during training to enhance absorption and utilization during prolonged exercise [16].
Gluten is a complex mixture of proteins present in foods such as wheat, rye, barley, and oats that are incompletely digested by intestinal enzymes [21]. A gluten-free diet is essential for managing symptoms in people diagnosed with celiac disease or gluten sensitivity [22]. This diet has also been popularized among non-celiac athletes [10] due to the belief that it is a healthy and balanced diet [13]. However, there is no scientific evidence that this diet supports improved mental or physical performance in healthy people [23].
FODMAPs are CHOs that are poorly absorbed and highly fermentable by the intestinal flora, and are present in fruits, vegetables, cereals, milk, dairy products, legumes, and sweeteners [24]. The main types of FODMAPs are fructose, lactose, oligosaccharides, and polyols, each with a distinct action mechanism [25]. The low-FODMAP diet [24,26,27] involves the reduced consumption of fermentable short-chain CHOs and is used in people with non-specific digestive symptoms such as irritable bowel syndrome [28,29]. In athletes who perform strenuous exercise, undigested molecules can increase the osmotic load in the small intestine, leading to increased stool volume or diarrhea [30]. The low-FODMAP diet is associated with improved GISs in 50–80% of patients. However, following this diet is not often easy due to an unintuitive and very restrictive list of foods that can increase the risk of deficiencies and imbalances in the microbiota [31].
The relationship between nutritional interventions and the maintenance or alteration of intestinal integrity is still unclear. Even though the intake of CHOs could benefit the performance of athletes, their impact on GISs is yet unknown, making it difficult to develop recommendations [32]. In addition, gluten-free and low-FODMAP diets are popularly suggested to improve gastrointestinal health [33]. Based on previous reviews, it is evident that a substantial number of athletes are not diagnosed with a clinical condition necessitating a gluten-free diet to prevent gastrointestinal issues [34]. Additionally, athletes who adhere to a gluten-free diet inadvertently reduce their intake of high-FODMAP foods, effectively reducing gastrointestinal symptoms [11,35]. This approach depends on the athletes’ characteristics and the severity of gastrointestinal issues [36]. Given this context, the following question arises: Are CHO, gluten-free, and low-FODMAP diets effective in mitigating GISs in EAs? Consequently, this review aims to analyze the efficacy of CHO, gluten-free, and low-FODMAP diets in preventing GISs in adult EAs of both sexes.
2. Materials and Methods
2.1. Search Strategy
A systematic search was carried out for articles published before June 30, 2024, following the criteria of the PRISMA declaration [37]. We used generic terms to identify all studies addressing the efficacy of CHO, gluten-free, and low-FODMAP diets in preventing GISs in adult EAs of both sexes. The search criteria were (((“Diet, Carbohydrate-Restricted”))) OR (“Diet, Gluten-Free” AND (“Gastrointestinal Diseases”)) AND (“Athletes”). In PubMed, the MeSH (Medical Subject Heading) terms were used. The same search strategy and combination of terms was repeated in EBSCO, Google Scholar, and Web of Science. A PRISMA flow diagram was used to show the search strategy steps for this systematic review.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were determined using the PICOS (population, intervention, comparators, outcomes, study design) model (Table 1).

Table 1.
PICOS model for determination of inclusion criteria.
A researcher (KNMC) reviewed, in detail, whether the articles met the inclusion criteria established in two phases: (a) reading the title and abstract and (b) reading the full text of the articles included in the previous phase.
The exclusion criteria were (a) narrative or systematic reviews; (b) studies on athletes diagnosed with celiac disease; (c) articles related to non-athletes or other sports disciplines; and (d) studies that included results of the use or intake of CHO, gluten, and FODMAP diets not related to GISs.
2.3. Quality Assessment of Studies
The risk of bias and quality in each paper was assessed by KNMC using the (Table 2) PEDro Scale (Physiotherapy Evidence Database) checklist [38] containing 11 criteria (eligibility, random allocation, concealed allocation, baseline comparability, blind subjects, blind therapies, blind assessors, adequate follow-up, intention-to-treat analyses, between-group comparations, and point estimates and variability). A score > 6 was considered acceptable to be considered in this review. Further details regarding the PEDro Scale methodology can be found elsewhere [38].

Table 2.
Scientific quality of studies according to PEDro Scale [38].
2.4. Process of Extraction of Information
After the inclusion criteria and PEDro scale checklist were applied, information on the author and year of publication, objective, type of diet, methodology, results, and conclusion were extracted by two authors (KNMC, MJAT), and the systematic review was registered at https://inplasy.com/inplasy-2024-9-0080/ (accessed on 18 September 2024), identifier INPLASY202490080.
3. Results
A total of 651 articles were identified, of which 55% (n = 362) were duplicated in the different databases. In this phase, 289 articles were selected by reading the title and abstract. Moreover, 93% (n = 271) were excluded because they were systematic reviews or did not include results that would allow for an analysis of the efficacy of CHO, gluten-free, and low-FODMAP diets in preventing GISs in adult EAs of both sexes. The remaining 18 articles were evaluated using the PEDro Scale [38]. Of these, eight articles were excluded because they did not score ≥6 on the PEDro Scale. The PRISMA flow diagram (Figure 1) shows the steps of the search strategy and the 10 articles considered for this systematic review.
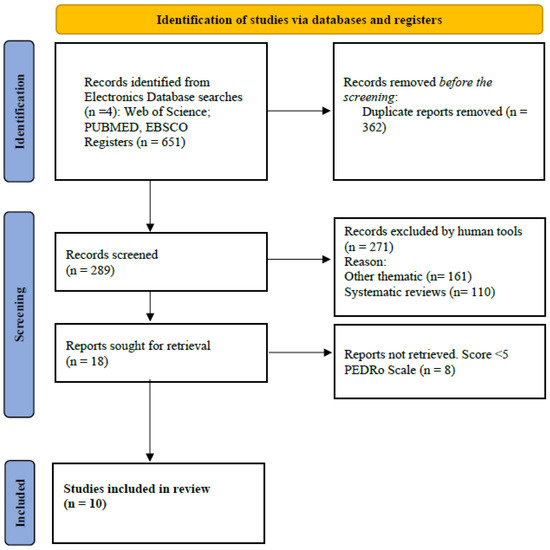
Figure 1.
Flow diagram according to PRISMA 2020.
Table 3 summarizes the selected articles. Of the studies included in this review, 60% included males and females, and 90% involved trained or recreational adult runners. One study incorporated EAs from a discipline other than running [47].

Table 3.
Studies examining the efficacy of gluten-free, FODMAP, and CHO-free diets in the prevention of GISs in adult endurance athletes of one or both sexes.
Of the 10 articles, 60% used an experimental method to search for results that supported the benefit of dietary interventions based on a CHO diet, gluten-free diet, or low-FODMAP diet. In contrast, the other 40% implemented validated questionnaires or diary records to collect background information on the strategies used to mitigate gastrointestinal discomfort in EAs. It is worth mentioning that two of the studies used dietary records as a tool that was complementary to the experimental intervention. In addition, for determining the incidence and severity of GISs related to exercise, scales or questionnaires were used. The instruments used by the authors were (i) a 10-point Likert-type rating scale (n = 3), (ii) the Syndrome Severity Scoring System (n = 1), (iii) web-based questionnaires (n = 1), (iv) the visual analog scale (n = 3) [48], (v) a questionnaire to assess GI symptoms exercise-induced (without Likert-scale items) (n = 1), and (vi) a gut comfort questionnaire (validate by prior authors) (n = 1).
Finally, 80% of the articles concluded that an endurance athlete’s daily intake of a bowel training diet involving CHO (n = 3), a decrease in fiber and dairy products (n = 1), or low-FODMAP foods (n = 4) could reduce GISs and improve sports performance. Studies on using a gluten-free diet to mitigate GISs, which also met our selection criteria, were not found.
4. Discussion
The results of the present systematic review show that the most successful strategies to reduce GISs are gastrointestinal training using CHO and the introduction of a low-FODMAP diet. However, no scientific evidence supports using gluten-free dietary strategies to mitigate such symptoms in EAs. We found a result [12] that suggested mitigating GISs by avoiding dairy and fiber. This finding is not a widely used solution, given that no other reviews or studies were found for or against it. The elimination of dairy and fiber and the reduction of gastrointestinal symptoms may not solely be attributed to lactose-containing foods and increased intestinal transit. These effects can also be concealed by symptoms caused by other FODMAPs. Due to the development of recommendations that simultaneously improve athletic performance by attenuating gastrointestinal symptoms, which is necessary for trainers and sports nutritionists, our results emphasize the need to increase the number of controlled clinical trials that compare the efficacy of CHO and FODMAP diets on managing the symptomology of GISs in endurance disciplines.
Previous research has suggested that consuming carbohydrates during exercise can lead to gastrointestinal distress and diarrhea in athletes [49]. As a result, CHO training during exercise could serve as a preventive nutritional strategy. However, in this review, we only found two studies that concluded that CHO training reduces intestinal malabsorption and GISs in exercise-associated gastrointestinal distress among EAs. A higher intake of CHO during exercise could increase the severity of GISs and lead to intolerance, particularly during high-intensity exercise [45]. A linear relationship between CHO intake and GISs based on the distance (kilometers) run has yet to be observed [42]. Consequently, sports nutritionists must take this evidence into consideration when they plan to address GISs in EAs using CHO training, and more studies are also necessary to clarify the effect of CHO training in different endurance disciplines and develop recommendations grams per weight before and during exercise for GIS management.
Our findings align with the results previously shown by Devrim-Lanpir et al. [11], who investigated the impact of five different dietary approaches (i.e., vegetarian, high-fat, intermittent fasting, gluten-free, and low-FODMAP) on the performance and health aspects of EAs. They concluded that a low-FODMAP diet may be more beneficial than a gluten-free diet in athletes without gluten intolerance. Nevertheless, due to the recommendation for a low-FOD dairy intake (g/d) not being determined in EAs, the results do not propose this diet as a definitive strategy. Interestingly, a recent study [14] showed that up to 80% of athletes frequently remove sources of lactose compared to other high-FODMAP foods to improve their GISs. This finding indicates that a low-FODMAP diet may not be sustainable over the entire life course and that restricting foods could inadvertently cause more problems (for example, low intake of essential nutrients such as calcium and imbalance in the microbiota) [50]. However, it could be considered a strategy for the following competencies and highlights the need to evaluate groups of foods on exercise-induced GI symptoms in athletes to confirm low-FODMAP diets as a solution to EAs’ intestinal disorders. Low-FODMAP could also be considered a strategy to reduce GISs in EAs.
The increasing popularity of gluten-free diets in EAs without celiac disease has been discussed extensively [10,13]. Surprisingly, almost 40% (more females than males) of EAs have declared gluten elimination from their daily food intake with the objective of GIS reduction [13,34]. In agreement with our findings, Lis and Cols [51] assert that such a diet would not have a beneficial effect on performance, gastrointestinal health, or well-being; the reduced integrity of the GI barrier in non-celiac athletes is a consequence of exercise intensity and splanchnic hypoperfusion, and not of damage to the intestinal barrier (celiac disease). Interestingly, reducing FODMAPs, rather than gluten, may improve symptoms [36,51], which could be explained by the decrease in the fructans and galactic-oligosaccharides (FODMAPs) present in wheat [52]. Adopting a gluten-free diet when not medically necessary for non-celiac athletes may lead to unintended consequences such as reduced energy levels and a lack of important nutrients like B vitamins, fiber, and iron, which are crucial for optimal sports nutrition. Additionally, it can also result in a higher financial burden and psychosocial implications [34,51]. Adequate and personalized advice is necessary before adopting a gluten-free diet. When planning a gluten-free diet for EAs, it is important for sports nutritionists to consider this evidence.
A strength of this study is the application of the PEDro Scale [38] when selecting high-quality articles for this review. However, a limitation of this study may be the restriction of the search period to only the last 8 years, which could explain the absence of studies on using a gluten-free diet to treat GISs.
5. Conclusions
In summary, a personalized gastrointestinal training plan can benefit EAs, helping them achieve their nutritional and athletic goals more quickly. Dietary strategies such as CHO training during exercise and reducing dietary FODMAP intake could help mitigate GISs in adult EAs of both sexes. However, these strategies need more scientific evidence to support their effectiveness. In conclusion, current nutritional recommendations for athletes do not include adequate plans to reduce GISs [10,53]. Thus, randomized clinical trials are required to determine the efficacy of CHO and FODMAP diets and to determine the guidelines for sports nutrition and GIS management.
Funding
This research received no external funding and the APC was funded by Universidad Mayor, Chile.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ryan, T.; Daly, E.; Ryan, L. Exploring the Nutrition Strategies Employed by Ultra-Endurance Athletes to Alleviate Exercise-Induced Gastrointestinal Symptoms-A Systematic Review. Nutrients 2023, 15, 4330. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44 (Suppl. S1), S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Triadafilopoulos, G. Exercise and gastrointestinal function and disease: An evidence-based review of risks and benefits. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2003, 1, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Lira, C.A.B.; Viana, R.B.; Mesquista, K.P.; Santos, D.A.T.; Campos, M.H.; Andrade, M.S.; Vancini, R.L. Frequency and intensity of gastrointestinal symptoms in exercisers individuals at rest and during physical exercise: An internet-based survey. Intest. Res. 2019, 17, 537–545. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C. The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 533–538. [Google Scholar] [CrossRef]
- Pugh, J.N.; Fearn, R.; Morton, J.P.; Close, G.L. Gastrointestinal symptoms in elite athletes: Time to recognise the problem? Br. J. Sports Med. 2018, 52, 487–488. [Google Scholar] [CrossRef]
- Papantoniou, K.; Michailides, C.; Bali, M.; Papantoniou, P.; Thomopoulos, K. Gastrointestinal bleeding in athletes. Ann. Gastroenterol. 2023, 36, 267–274. [Google Scholar] [CrossRef]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef]
- Lis, D.M.; Kings, D.; Larson-Meyer, D.E. Dietary Practices Adopted by Track-and-Field Athletes: Gluten-Free, Low FODMAP, Vegetarian, and Fasting. Int. J. Sport. Nutr. Exerc. Metab. 2019, 29, 236–245. [Google Scholar] [CrossRef]
- Devrim-Lanpir, A.; Hill, L.; Knechtle, B. Efficacy of Popular Diets Applied by Endurance Athletes on Sports Performance: Beneficial or Detrimental? A Narrative Review. Nutrients 2021, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Scrivin, R.; Costa, R.J.S.; Pelly, F.; Lis, D.; Slater, G. An exploratory study of the management strategies reported by endurance athletes with exercise-associated gastrointestinal symptoms. Front. Nutr. 2022, 9, 1003445. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M.; Stellingwerff, T.; Shing, C.M.; Ahuja, K.D.; Fell, J.W. Exploring the popularity, experiences, and beliefs surrounding gluten-free diets in nonceliac athletes. Int. J. Sport. Nutr. Exerc. Metab. 2015, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.; Ahuja, K.D.; Stellingwerff, T.; Kitic, C.M.; Fell, J. Food avoidance in athletes: FODMAP foods on the list. Appl. Physiol. Nutr. Metab.=Physiol. Appl. Nutr. Et. Metab. 2016, 41, 1002–1004. [Google Scholar] [CrossRef]
- Cermak, N.M.; van Loon, L.J. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef]
- Rehrer, N.J.; van Kemenade, M.; Meester, W.; Brouns, F.; Saris, W.H. Gastrointestinal complaints in relation to dietary intake in triathletes. Int. J. Sport. Nutr. 1992, 2, 48–59. [Google Scholar] [CrossRef]
- Brouns, F.; Beckers, E. Is the gut an athletic organ? Digestion, absorption and exercise. Sports Med. 1993, 15, 242–257. [Google Scholar] [CrossRef]
- Pfeiffer, B.; Stellingwerff, T.; Hodgson, A.B.; Randell, R.; Pöttgen, K.; Res, P.; Jeukendrup, A.E. Nutritional intake and gastrointestinal problems during competitive endurance events. Med. Sci. Sports Exerc. 2012, 44, 344–351. [Google Scholar] [CrossRef]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional considerations for single-stage ultra-marathon training and racing. J. Int. Soc. Sports Nutr. 2019, 16, 50. [Google Scholar] [CrossRef]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-Free Diet in Celiac Disease-Forever and for All? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R. History of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 5–7. [Google Scholar] [CrossRef]
- Wiffin, M.; Smith, L.; Antonio, J.; Johnstone, J.; Beasley, L.; Roberts, J. Effect of a short-term low fermentable oligiosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diet on exercise-related gastrointestinal symptoms. J. Int. Soc. Sports Nutr. 2019, 16, 1. [Google Scholar] [CrossRef]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef]
- Killian, L.A.; Muir, J.G.; Barrett, J.S.; Burd, N.A.; Lee, S.Y. High Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) Consumption Among Endurance Athletes and Relationship to Gastrointestinal Symptoms. Front. Nutr. 2021, 8, 637160. [Google Scholar] [CrossRef]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef]
- Diduch, B.K. Gastrointestinal Conditions in the Female Athlete. Clin. Sports Med. 2017, 36, 655–669. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Arribalzaga, S.; Viribay, A.; Calleja-González, J.; Fernández-Lázaro, D.; Castañeda-Babarro, A.; Mielgo-Ayuso, J. Relationship of Carbohydrate Intake during a Single-Stage One-Day Ultra-Trail Race with Fatigue Outcomes and Gastrointestinal Problems: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 5737. [Google Scholar] [CrossRef] [PubMed]
- Ajamian, M.; Rosella, G.; Newnham, E.D.; Biesiekierski, J.R.; Muir, J.G.; Gibson, P.R. Effect of Gluten Ingestion and FODMAP Restriction on Intestinal Epithelial Integrity in Patients with Irritable Bowel Syndrome and Self-Reported Non-Coeliac Gluten Sensitivity. Mol. Nutr. Food Res. 2021, 65, e1901275. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M.; Fell, J.W.; Ahuja, K.D.; Kitic, C.M.; Stellingwerff, T. Commercial Hype Versus Reality: Our Current Scientific Understanding of Gluten and Athletic Performance. Curr. Sports Med. Rep. 2016, 15, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Ribichini, E.; Scalese, G.; Cesarini, A.; Mocci, C.; Pallotta, N.; Severi, C.; Corazziari, E.S. Exercise-Induced Gastrointestinal Symptoms in Endurance Sports: A Review of Pathophysiology, Symptoms, and Nutritional Management. Dietetics 2023, 2, 289–307. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.; Camões-Costa, V.; Gibson, P. Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl. Physiol. Nutr. Metab.=Physiol. Appl. Nutr. Et. Metab. 2017, 42, 547–557. [Google Scholar] [CrossRef]
- Lis, D.M.; Stellingwerff, T.; Kitic, C.M.; Fell, J.W.; Ahuja, K.D.K. Low FODMAP: A Preliminary Strategy to Reduce Gastrointestinal Distress in Athletes. Med. Sci. Sports Exerc. 2018, 50, 116–123. [Google Scholar] [CrossRef]
- Miall, A.; Khoo, A.; Rauch, C.; Snipe, R.M.J.; Camões-Costa, V.L.; Gibson, P.R.; Costa, R.J.S. Two weeks of repetitive gut-challenge reduce exercise-associated gastrointestinal symptoms and malabsorption. Scand. J. Med. Sci. Sports 2018, 28, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Hoogervorst, D.; van der Burg, N.; Versteegen, J.J.; Lambrechtse, K.J.; Redegeld, M.I.; Cornelissen, L.A.J.; Wardenaar, F.C. Gastrointestinal Complaints and Correlations with Self-Reported Macronutrient Intake in Independent Groups of (Ultra)Marathon Runners Competing at Different Distances. Sports 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Wagner-Jones, K.; Madden, R.F.; Erdman, K.A. Dietary restrictions in endurance runners to mitigate exercise-induced gastrointestinal symptoms. J. Int. Soc. Sports Nutr. 2020, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, S.K.; Taylor, B.; Muir, J.; Costa, R.J.S. Impact of 24-h high and low fermentable oligo-, di-, monosaccharide, and polyol diets on markers of exercise-induced gastrointestinal syndrome in response to exertional heat stress. Appl. Physiol. Nutr. Metab.=Physiol. Appl. Nutr. Et. Metab. 2020, 45, 569–580. [Google Scholar] [CrossRef]
- Rauch, C.E.; McCubbin, A.J.; Gaskell, S.K.; Costa, R.J.S. Feeding Tolerance, Glucose Availability, and Whole-Body Total Carbohydrate and Fat Oxidation in Male Endurance and Ultra-Endurance Runners in Response to Prolonged Exercise, Consuming a Habitual Mixed Macronutrient Diet and Carbohydrate Feeding During Exercise. Front. Physiol. 2021, 12, 773054. [Google Scholar] [CrossRef]
- Etxebarria, N.; Beard, N.A.; Gleeson, M.; Wallett, A.; McDonald, W.A.; Pumpa, K.L.; Pyne, D.B. Dietary Intake and Gastrointestinal Integrity in Runners Undertaking High-Intensity Exercise in the Heat. Int. J. Sport. Nutr. Exerc. Metab. 2021, 31, 314–320. [Google Scholar] [CrossRef]
- Scrivin, R.; Costa, R.J.; Pelly, F.; Lis, D.; Slater, G. Development and validation of a questionnaire investigating endurance athletes practices to manage gastrointestinal symptoms around exercise. Nutr. Diet. J. Dietit. Assoc. Aust. 2021, 78, 286–295. [Google Scholar] [CrossRef]
- Gaskell, S.K.; Snipe, R.M.J.; Costa, R.J.S. Test-Retest Reliability of a Modified Visual Analog Scale Assessment Tool for Determining Incidence and Severity of Gastrointestinal Symptoms in Response to Exercise Stress. Int. J. Sport. Nutr. Exerc. Metab. 2019, 29, 411–419. [Google Scholar] [CrossRef]
- De Oliveira, E.P. Runner’s diarrhea: What is it, what causes it, and how can it be prevented? Curr. Opin. Gastroenterol. 2017, 33, 41–46. [Google Scholar] [CrossRef]
- Eskici, G. Low FODMAP diet approach in exercise-related gastrointestinal problems. Türkiye Klin. Spor Bilim. 2020, 12, 233–240. [Google Scholar]
- Lis, D. From Celiac Disease, Gluten-Sensitivity vs. Gluten Sensationalism, to Fodmap Reduction as a Tool to Manage Gastrointestinal Symptoms in Athletes. Sports Sci. Exch. 2018, 31, 1–6. [Google Scholar]
- Dieterich, W.; Zopf, Y. Gluten and FODMAPS-Sense of a Restriction/When Is Restriction Necessary? Nutrients 2019, 11, 1957. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Nutrition for endurance sports: Marathon, triathlon, and road cycling. J. Sports Sci. 2011, 29 (Suppl. S1), S91–S99. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

