Daily Energy Intake Distribution and Cognitive Performance in Non-Demented Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Cognitive Function Assessment
2.3. Dietary Intake Assessment
2.4. Statistical Analysis
- (1)
- Energy intake trends for different levels of cognition
- (2)
- Interaction between energy trends and global cognitive score
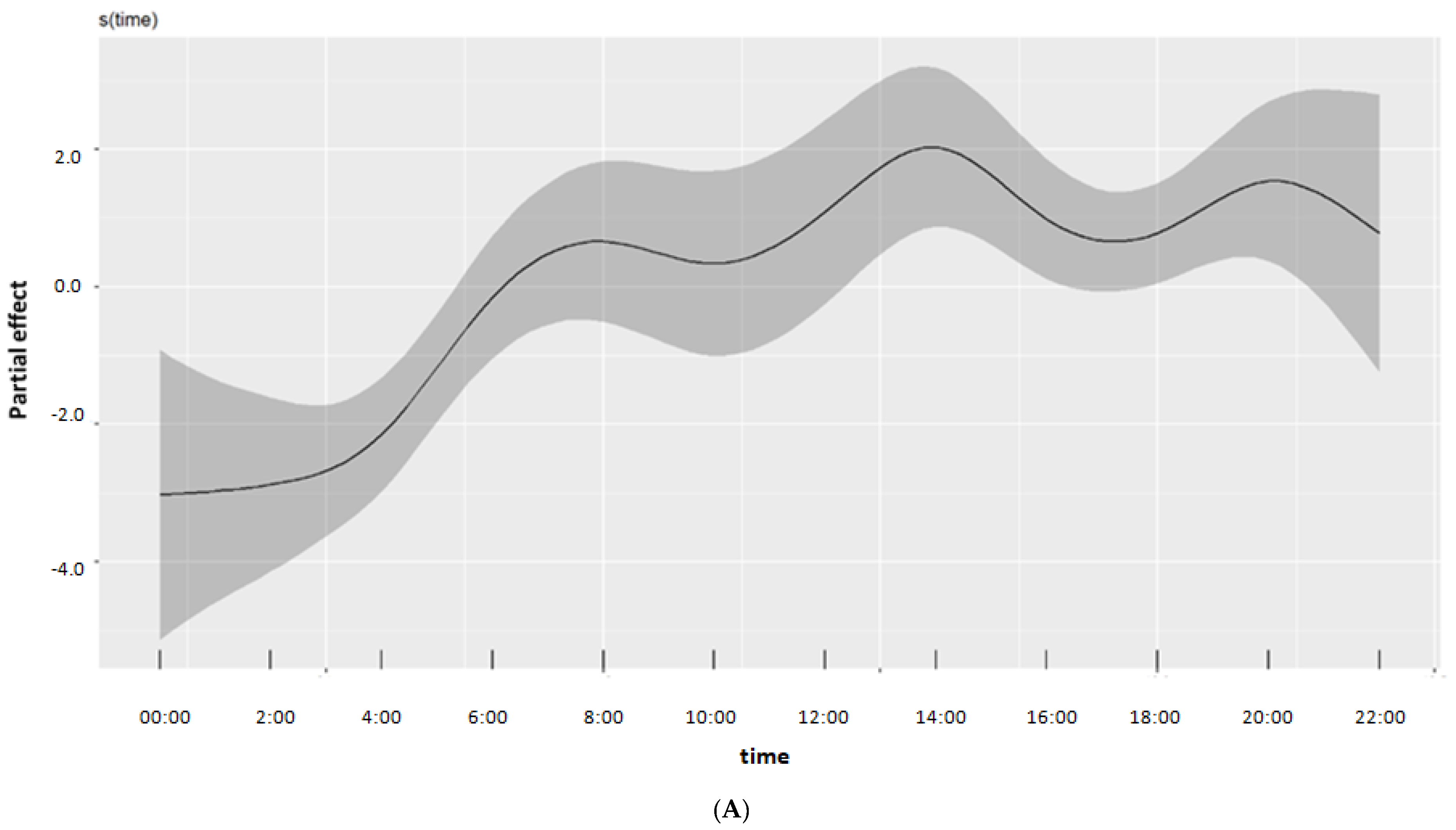
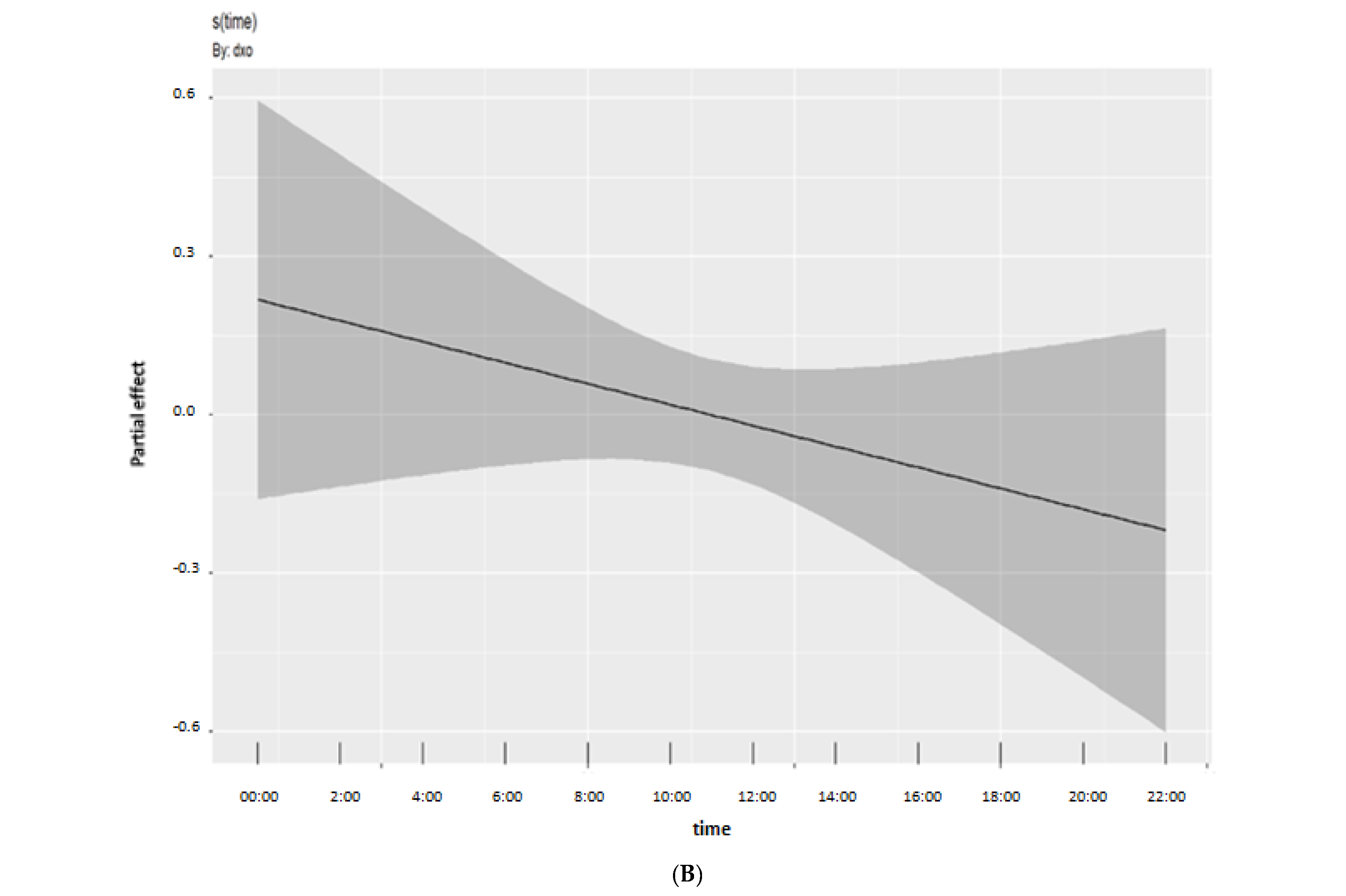
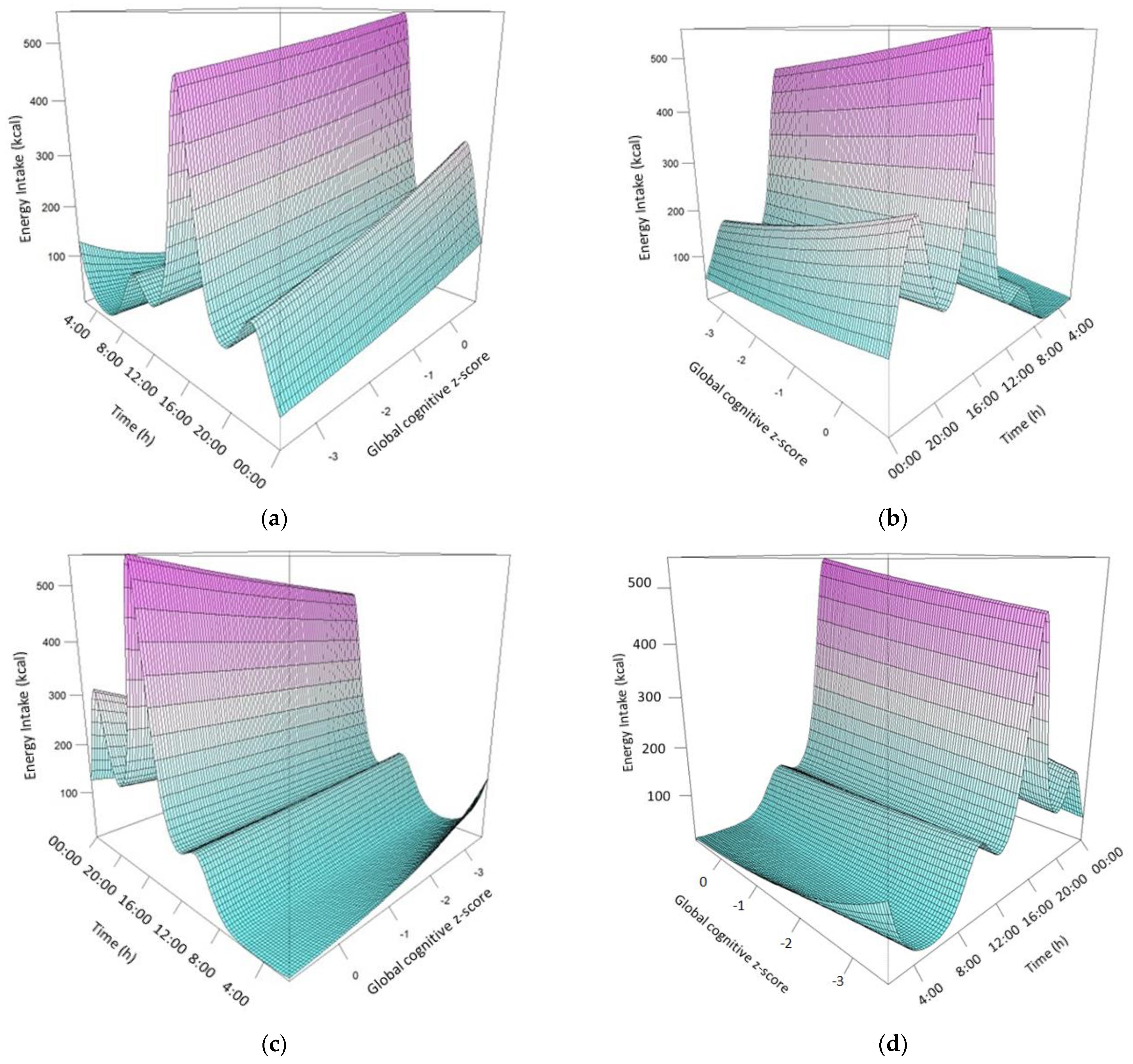
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Collaborators, G.B.D.D.F. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 2021, 17, 327–406. [CrossRef] [PubMed]
- Petersen, R.C. Aging, mild cognitive impairment, and Alzheimer’s disease. Neurol. Clin. 2000, 18, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Buckley, J.S.; Salpeter, S.R. A Risk-Benefit Assessment of Dementia Medications: Systematic Review of the Evidence. Drugs Aging 2015, 32, 453–467. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Zhao, C.; Noble, J.M.; Marder, K.; Hartman, J.S.; Gu, Y.; Scarmeas, N. Dietary Patterns, Physical Activity, Sleep, and Risk for Dementia and Cognitive Decline. Curr. Nutr. Rep. 2018, 7, 335–345. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Wieckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Buckinx, F.; Aubertin-Leheudre, M. Nutrition to Prevent or Treat Cognitive Impairment in Older Adults: A GRADE Recommendation. J. Prev. Alzheimers Dis. 2021, 8, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Doorduijn, A.S.; de van der Schueren, M.A.E.; van de Rest, O.; de Leeuw, F.A.; Hendriksen, H.M.A.; Teunissen, C.E.; Scheltens, P.; van der Flier, W.M.; Visser, M. Energy intake and expenditure in patients with Alzheimer’s disease and mild cognitive impairment: The NUDAD project. Alzheimers Res. 2020, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Basolo, A.; Bechi Genzano, S.; Piaggi, P.; Krakoff, J.; Santini, F. Energy Balance and Control of Body Weight: Possible Effects of Meal Timing and Circadian Rhythm Dysregulation. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gomez-Abellan, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef]
- Henry, C.J.; Kaur, B.; Quek, R.Y.C. Chrononutrition in the management of diabetes. Nutr. Diabetes 2020, 10, 6. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Association between Time Restricted Feeding and Cognitive Status in Older Italian Adults. Nutrients 2021, 13, 191. [Google Scholar] [CrossRef]
- Duan, H.; Sun, C.; Zhu, Y.; Liu, Q.; Du, Y.; Lin, H.; Jin, M.; Fu, J.; Ma, F.; Li, W.; et al. Association of Dietary Habits with Mild Cognitive Impairment among Elderly in Rural Area of North China. Curr. Alzheimer Res. 2021, 18, 256–264. [Google Scholar] [CrossRef]
- Wittig, F.; Hummel, E.; Wenzler, G.; Heuer, T. Energy and macronutrient intake over the course of the day of German adults: A DEDIPAC-study. Appetite 2017, 114, 125–136. [Google Scholar] [CrossRef]
- Johnson, G.H.; Anderson, G.H. Snacking definitions: Impact on interpretation of the literature and dietary recommendations. Crit. Rev. Food Sci. Nutr. 2010, 50, 848–871. [Google Scholar] [CrossRef]
- Kalligerou, F.; Ntanasi, E.; Voskou, P.; Velonakis, G.; Karavasilis, E.; Mamalaki, E.; Kyrozis, A.; Sigala, E.; Economou, N.T.; Patas, K.; et al. Aiginition Longitudinal Biomarker Investigation Of Neurodegeneration (ALBION): Study design, cohort description, and preliminary data. Postgrad. Med. 2019, 131, 501–508. [Google Scholar] [CrossRef]
- Scarmeas, N.; Daskalaki, A.; Kalligerou, F.; Ntanasi, E.; Mamalaki, E.; Gargalionis, A.N.; Patas, K.; Chatzipanagiotou, S.; Yannakoulia, M.; Constantinides, V.C. Initial Data and a Clinical Diagnosis Transition for the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Medicine 2022, 58. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef]
- Vlahou, C.; Kosmidis, M. The Greek Trail Making Test: Preliminary normative data for clinical and research use. Psychol. J. Hell. Psychol. Soc. 2002, 9, 336–352. [Google Scholar]
- Wechsler, D. Adult Intelligence Scale—Administration and Scoring Manual, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wuhr, P. A Stroop effect for spatial orientation. J. Gen. Psychol. 2007, 134, 285–294. [Google Scholar] [CrossRef]
- Vlahou, C.H.; Kosmidis, M.H.; Dardagani, A.; Tsotsi, S.; Giannakou, M.; Giazkoulidou, A.; Zervoudakis, E.; Pontikakis, N. Development of the Greek Verbal Learning Test: Reliability, construct validity, and normative standards. Arch. Clin. Neuropsychol. 2013, 28, 52–64. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Conway, J.M.; Ingwersen, L.A.; Vinyard, B.T.; Moshfegh, A.J. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am. J. Clin. Nutr. 2003, 77, 1171–1178. [Google Scholar] [CrossRef]
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. J. Am. Diet Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef]
- Tran, K.M.; Johnson, R.K.; Soultanakis, R.P.; Matthews, D.E. In-person vs telephone-administered multiple-pass 24-hour recalls in women: Validation with doubly labeled water. J. Am. Diet Assoc. 2000, 100, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Wood, S.N. Thin plate regression splines. J. R. Stat. Soc. Ser. B Stat. Methodol. 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Simpson, G.L. Modelling Palaeoecological Time Series Using Generalised Additive Models. Front. Ecol. Evol 2018, 6, 149. [Google Scholar] [CrossRef]
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing Parameter and Model Selection for General Smooth Models. J. Am. Stat. Assoc. 2016, 111, 1548–1563. [Google Scholar] [CrossRef]
- Valdez, P.; Reilly, T.; Waterhouse, J. Rhythms of mental performance. Mind Brain Educ. 2008, 2, 7–16. [Google Scholar] [CrossRef]
- Young, K.W.; Greenwood, C.E. Shift in diurnal feeding patterns in nursing home residents with Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M700–M706. [Google Scholar] [CrossRef]
- De Castro, J.M. Circadian rhythms of the spontaneous meal pattern, macronutrient intake, and mood of humans. Physiol. Behav. 1987, 40, 437–446. [Google Scholar] [CrossRef]
- Duffy, J.F.; Zeitzer, J.M.; Rimmer, D.W.; Klerman, E.B.; Dijk, D.J.; Czeisler, C.A. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E297–E303. [Google Scholar] [CrossRef]
- Manni, R.; Cremascoli, R.; Perretti, C.; De Icco, R.; Picascia, M.; Ghezzi, C.; Cerri, S.; Sinforiani, E.; Terzaghi, M. Evening melatonin timing secretion in real life conditions in patients with Alzheimer disease of mild to moderate severity. Sleep Med. 2019, 63, 122–126. [Google Scholar] [CrossRef]
- Flynn-Evans, E.E.; Shekleton, J.A.; Miller, B.; Epstein, L.J.; Kirsch, D.; Brogna, L.A.; Burke, L.M.; Bremer, E.; Murray, J.M.; Gehrman, P.; et al. Circadian Phase and Phase Angle Disorders in Primary Insomnia. Sleep 2017, 40, zsx163. [Google Scholar] [CrossRef]
- Stahelin, H.B.; Hofer, H.O.; Vogel, M.; Held, C.; Seiler, W.O. Energy and protein consumption in patients with senile dementia. Gerontology 1983, 29, 145–148. [Google Scholar] [CrossRef]
- Morley, J.E.; Silver, A.J. Anorexia in the elderly. Neurobiol. Aging 1988, 9, 9–16. [Google Scholar] [CrossRef]
- Nordberg, A. Neuroreceptor changes in Alzheimer disease. Cereb. Brain Metab. Rev. 1992, 4, 303–328. [Google Scholar]
| Variables | ALL | NCF (N = 73) | MCI (N = 31) | p-Value |
|---|---|---|---|---|
| Sex (% female) | 65.4 | 68.5 | 58.1 | 0.307 |
| Age (years) | 65± 9 (40, 79) | 64± 9 (40, 79) | 67± 7 (53, 79) | 0.094 |
| Education (years) | 13 ± 4 (6, 22) | 14 ± 4 (6, 22) | 12 ± 4 (6, 17) | 0.019 |
| BMI (kg/m2) | 27 ± 4 (15, 38) | 27 ± 4 (15, 38) | 27 ± 4 (21, 34) | 0.962 |
| Daily energy intake (kcal) | 1829 ± 530 (878, 3555) | 1889 ± 526 (993, 3554) | 1688 ± 520 (878, 2856) | 0.077 |
| CHO | ||||
| g/day | 189 ± 89 (76, 415) | 194 ± 61 (76, 415) | 178 ± 61 (92, 383) | 0.237 |
| % E | 42 ± 9 (23, 70) | 42 ± 9 (23,70) | 43 ± 8 (24, 59) | 0.508 |
| Lipids | ||||
| g/day | 89 ± 32 (35, 186) | 93 ± 32 (35, 186) | 81 ± 30 (36, 162) | 0.071 |
| % E | 44 ± 7 (21, 59) | 44 ± 8 (21, 59) | 43 ± 6 (28, 54) | 0.471 |
| Proteins | ||||
| g/day | 69 ± 23 (25, 143) | 72 ± 23 (36, 143) | 62 ± 23 (25, 132) | 0.039 |
| % E | 15 ± 3 (9.5, 24) | 15 ± 3 (10, 24) | 15 ± 3 (9, 22) | 0.275 |
| g/kg body weight | 0.94 ± 0.34 (0.35, 2.8) | 0.98 ± 0.35 (0.46, 2.8) | 0.85 ± 0.31 (0.35, 1.62) | 0.053 |
| Parametric Terms | |||
|---|---|---|---|
| Estimate | Standard Error | p-Value | |
| Intercept | 4.21612 | 0.09798 | <0.001 |
| MCI | 0.01036 | 0.07190 | 0.885 |
| Smooth Terms | |||
| Effective Degrees of Freedom | Reference Degrees of Freedom | p-Value | |
| Time | 9.720691 | 10.425 | <0.001 |
| Sex 1 | 5.854168 | 22.000 | <0.001 |
| Cognition 2 | 1.001234 | 1.002 | 0.242 |
| Education | 1.000490 | 1.001 | 0.066 |
| Age | 1.375659 | 1.672 | 0.807 |
| BMI | 1.000392 | 1.001 | 0.902 |
| Tensor Interaction Terms | |||
| Time, BMI | 19.378845 | 27.967 | 0.024 |
| Time, Age | 1.002003 | 1.004 | 0.203 |
| Time, Education | 11.343028 | 16.509 | 0.185 |
| Parametric Terms | |||
|---|---|---|---|
| Estimate | Standard Error | p-Value | |
| Intercept | 4.19738 | 0.09464 | <0.001 |
| Smooth terms | |||
| Effective Degrees of Freedom | Reference Degrees of Freedom | p-Value | |
| Time | 9.780887 | 10.499 | <0.001 |
| Sex 1 | 5.369277 | 22.000 | <0.001 |
| Education | 1.000849 | 1.002 | 0.040 |
| Age 2 | 1.003762 | 1.007 | 0.831 |
| Global cognitive z-score | 1.025011 | 1.049 | 0.262 |
| BMI | 1.000879 | 1.002 | 0.820 |
| Tensor Interaction Terms | |||
| Time, Global cognitive z-score | 2.360638 | 2.958 | 0.043 |
| Time, BMI | 19.219090 | 27.799 | 0.025 |
| Time, Age | 1.002322 | 1.005 | 0.080 |
| Time, Education | 11.79925 | 17.091 | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brikou, D.; Charisis, S.; Drouka, A.; Christodoulakou, S.M.; Ntanasi, E.; Mamalaki, E.; Constadinides, V.C.; Scarmeas, N.; Yannakoulia, M. Daily Energy Intake Distribution and Cognitive Performance in Non-Demented Individuals. Nutrients 2023, 15, 673. https://doi.org/10.3390/nu15030673
Brikou D, Charisis S, Drouka A, Christodoulakou SM, Ntanasi E, Mamalaki E, Constadinides VC, Scarmeas N, Yannakoulia M. Daily Energy Intake Distribution and Cognitive Performance in Non-Demented Individuals. Nutrients. 2023; 15(3):673. https://doi.org/10.3390/nu15030673
Chicago/Turabian StyleBrikou, Dora, Sokratis Charisis, Archontoula Drouka, Stavroula Myrto Christodoulakou, Eva Ntanasi, Eirini Mamalaki, Vasilios C. Constadinides, Nikolaos Scarmeas, and Mary Yannakoulia. 2023. "Daily Energy Intake Distribution and Cognitive Performance in Non-Demented Individuals" Nutrients 15, no. 3: 673. https://doi.org/10.3390/nu15030673
APA StyleBrikou, D., Charisis, S., Drouka, A., Christodoulakou, S. M., Ntanasi, E., Mamalaki, E., Constadinides, V. C., Scarmeas, N., & Yannakoulia, M. (2023). Daily Energy Intake Distribution and Cognitive Performance in Non-Demented Individuals. Nutrients, 15(3), 673. https://doi.org/10.3390/nu15030673







