Hospital Malnutrition in the Medicine and Neurology Departments: A Complex Challenge
Abstract
:1. Introduction
Study Objective
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Study Procedures
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Sample
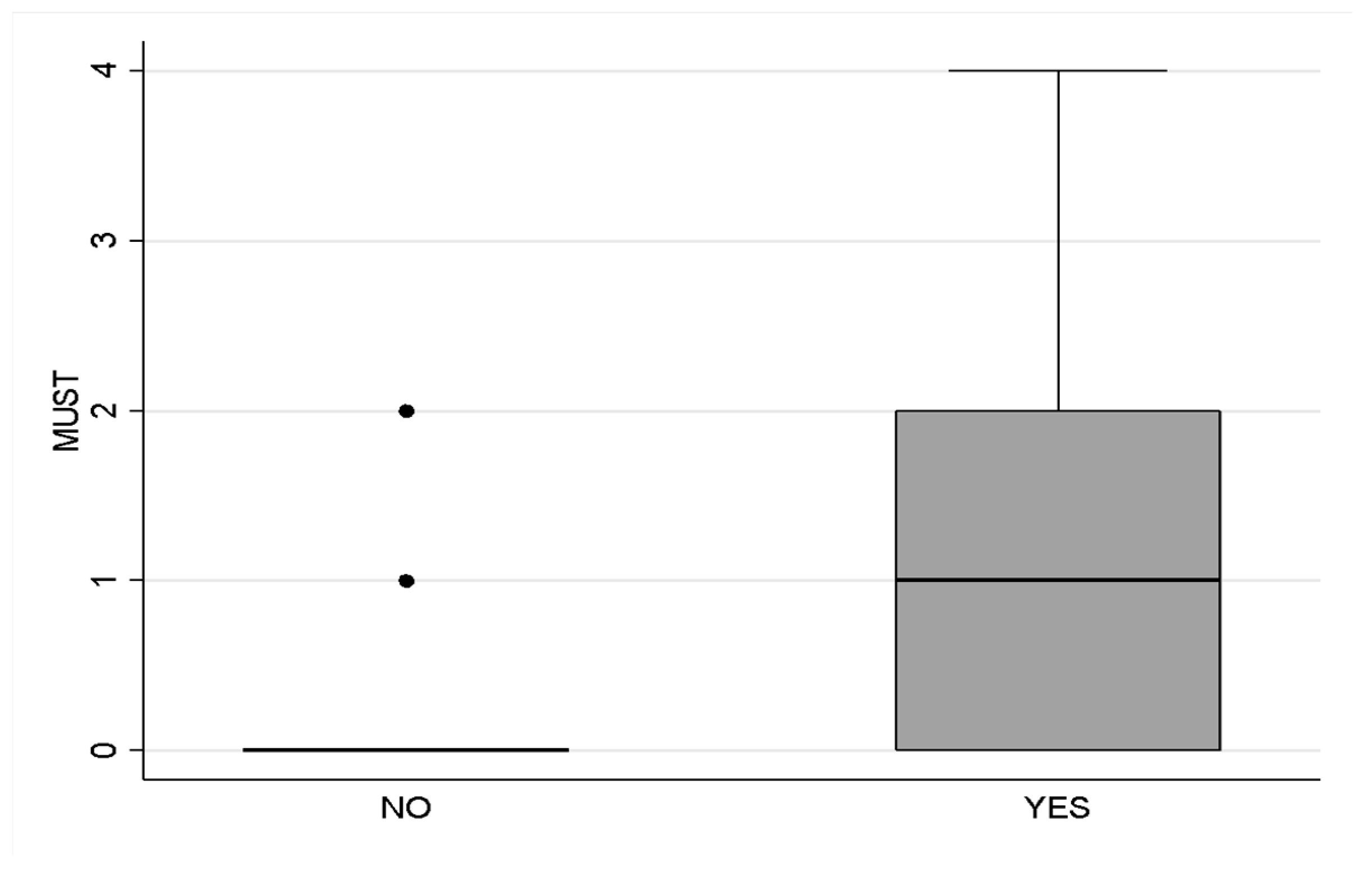
3.2. Nutritional Status and Dietary Autonomy
3.3. LOS, Clinical Variables, and Food and Water Intake
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Uhl, S.; Siddique, S.M.; Bloschichak, A.; McKeever, W.; D’Anci, K.; Leas, B.; Mull, N.K.; Compher, C.; Lewis, J.D.; Wu, G.D.; et al. Interventions for malnutrition in hospitalized adults: A systematic review and meta-analysis. J. Hosp. Med. 2022, 17, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. The Importance of Nutrition in Neurological Disorders and Nutrition Assessment Methods. Brain Neurorehabilit. 2022, 28, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wei, J.; Chen, W.; Yang, X.; Cui, H.; Zhu, S.; Ad hoc Working Group. Nutritional Risk and Nutritional Status at Admission and Discharge among Chinese Hospitalized Patients: A Prospective, Nationwide, Multicenter Study. J. Am. Coll. Nutr. 2017, 36, 357–363. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Herke, M.; Fink, A.; Langer, G.; Wustmann, T.; Watzke, S.; Hanff, A.M.; Burckhardt, M. Environmental and behavioural modifications for improving food and fluid intake in people with dementia. Cochrane Database Syst. Rev. 2018, 7, CD011542. [Google Scholar] [CrossRef]
- Panebianco, M.; Marchese-Ragona, R.; Masiero, S.; Restivo, D.A. Dysphagia in neurological diseases: A literature review. Neurol. Sci. 2020, 41, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yahiro, A.; Sakuragi, Y.; Iwamatsu, K.; Sakamoto, H.; Kaizuka, Y.; Tsuchihashi, T.; Kamouchi, M. Impaired Swallowing in Hospitalized Patients. Nurs. Res. 2016, 65, 389–396. [Google Scholar] [CrossRef]
- McCarty, E.B.; Chao, T.N. Dysphagia and Swallowing Disorders. Med. Clin. N. Am. 2021, 105, 939–954. [Google Scholar] [CrossRef]
- Nawaz, S.; Tulunay-Ugur, O.E. Dysphagia in the older patient. Otolaryngol. Clin. N. Am. 2018, 51, 769–777. [Google Scholar] [CrossRef]
- Spronk, P.E.; Spronk, L.E.J.; Lut, J.; Gnacke, E.; Mijnes, D.; Munster, B.; Kröner, A. Prevalence and characterization of dysphagia in hospitalized patients. Neurogastroenterol. Motil. 2019, 32, e13763. [Google Scholar] [CrossRef]
- Altman, K.W.; Yu, G.P.; Schaefer, S.D. Consequence of dysphagia in the hospitalized patient: Impact on prognosis and hospital resources. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Mancin, S.; Mazzoleni, B. Probiotics as adjuvant therapy in the treatment of Allergic Rhinitis. Res. J. Pharm. Technol. 2023, 16, 2393–2398. [Google Scholar] [CrossRef]
- Chen, B.; Liu, W.; Chen, Y.; She, Q.; Li, M.; Zhao, H.; Zhao, W.; Peng, Z.; Wu, J. Effect of poor nutritional status and comorbidities on the occurrence and outcome of pneumonia in elderly adults. Front. Med. 2021, 8, 719530. [Google Scholar] [CrossRef] [PubMed]
- Sabbouh, T.; Torbey, M.T. Malnutrition in Stroke Patients: Risk Factors, Assessment, and Management. Neurocritical Care 2018, 29, 374–384. [Google Scholar] [CrossRef]
- Cass, A.R.; Charlton, K.E. Prevalence of hospital-acquired malnutrition and modifiable determinants of nutritional deterioration during inpatient admissions: A systematic review of the evidence. J. Hum. Nutr. Diet 2022, 35, 1043–1058. [Google Scholar] [CrossRef]
- Wu, X.S.; Miles, A.; Braakhuis, A. Nutritional intake and meal composition of patients consuming texture modified diets and thickened fluids: A systematic review and meta-analysis. Healthcare 2020, 8, 579. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Sharma, Y.; Avina, P.; Ross, E.; Horwood, C.; Hakendorf, P.; Thompson, C. Validity of the Malnutrition Universal Screening Tool for Evaluation of Frailty Status in Older Hospitalised Patients. Gerontol. Geriatr. Med. 2022, 8, 23337214221107817. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Stalenhoef, J.E.; van der Starre, W.E.; Vollaard, A.M.; Steyerberg, E.W.; Delfos, N.M.; Leyten, E.M.S.; Koster, T.; Ablij, H.C.; Van’t Wout, J.W.; van Dissel, J.T.; et al. Hospitalization for community-acquired febrile urinary tract infection: Validation and impact assessment of a clinical prediction rule. BMC Infect. Dis. 2017, 17, 400. [Google Scholar] [CrossRef] [PubMed]
- Crippa, C.; Matteucci, S.; Pastore, M.; Morenghi, E.; Starace, E.; De Pasquale, G.; Pieri, G.; Soekeland, F.; Gibbi, S.M.; Lo Cricchio, G.; et al. A Comparative Evaluation of the Caloric Intake and Economic Efficiency of Two Types of Homogenized Diets in a Hospital Setting. Nutrients 2023, 15, 4731. [Google Scholar] [CrossRef] [PubMed]
| n | 82 |
|---|---|
| Sex (male) (n, %) | 38 (46.34%) |
| Age (years) (mean, SD) | 82.1 ± 10.5 |
| BMI (kg/m2) (mean, SD) | 22.7 ± 4.3 |
| Length of stay (days) (median, range) | 15 (5–86) |
| Reason for hospitalization | |
| Cancer | 9 (10.98%) |
| UTIs | 8 (9.76%) |
| Pneumonia | 21 (25.61%) |
| Neurodegenerative pathologies | 30 (36.59%) |
| Sepsis | 22 (26.83%) |
| Comorbidity (n, %) | 53 (64.63%) |
| DM (n, %) | 20 (37.74%) |
| Cardiopathy (n, %) | 27 (50.94%) |
| COPD (n, %) | 16 (30.19%) |
| CKD (n, %) | 16 (30.19%) |
| Reason for diet | |
| Edentulous (n, %) | 15 (18.29%) |
| Cognitive impairment (n, %) | 19 (23.17%) |
| Dysphagia (n, %) | 34 (41.46%) |
| Presbyphagia (n, %) | 26 (31.71%) |
| MUST | ||||
|---|---|---|---|---|
| 0 | 1 | >2 | p Value | |
| n | 44 | 14 | 24 | |
| Sex (male) | 19 (43.18%) | 9 (64.29%) | 10 (41.67%) | 0.394 |
| Age (years) | 82.0 ± 11.4 | 83.4 ± 8.0 | 81.4 ± 10.5 | 0.885 |
| BMI (kg/m2) | 24.2 ± 4.6 | 23.1 ± 2.6 | 19.6 ± 2.6 | <0.001 |
| Length of stay (days) | 13 (6–76) | 23.5 (6–59) | 16 (5–86) | 0.332 |
| Comorbidity | 27 (61.36%) | 10 (71.43%) | 16 (66.67%) | 0.820 |
| Reason for diet | ||||
| Edentulous | 8 (18.18%) | 4 (28.57%) | 3 (12.50%) | 0.469 |
| Cognitive decay | 8 (18.18%) | 4 (28.57%) | 7 (29.17%) | 0.519 |
| Dysphagia | 20 (45.45%) | 6 (42.86%) | 8 (33.33%) | 0.642 |
| Presbyphagia | 13 (29.55%) | 4 (28.57%) | 9 (37.50%) | 0.807 |
| Albuminemia (g/L) | 29.9 ± 5.6 | 30.2 ± 5.0 | 28.2 ± 5.9 | 0.426 |
| Weight loss in the previous 3–6 months | 0 (0–4.62) | 5.56 (0–9.76) | 10.32 (0–22.5) | <0.001 |
| Autonomy in food intake | 25 (56.82%) | 4 (28.57%) | 8 (33.33%) | 0.073 |
| GLIM | |||
|---|---|---|---|
| Yes | No | p Value | |
| n | 51 | 31 | |
| Sex (male) | 23 (45.10%) | 15 (48.39%) | 0.772 |
| Age (years) | 83.7 ± 9.0 | 79.5 ± 12.4 | 0.079 |
| BMI (kg/m2) | 21.1 ± 2.6 | 25.3 ± 5.2 | <0.001 |
| Length of stay (days) | 14 (6–86) | 18 (5–76) | 0.193 |
| Comorbidity | 35 (68.63%) | 18 (58.06%) | 0.332 |
| Reason for diet | |||
| Edentulous | 9 (17.65%) | 6 (19.35%) | 0.846 |
| Cognitive decay | 14 (27.45%) | 5 (16.13%) | 0.289 |
| Dysphagia | 20 (39.22%) | 14 (45.16%) | 0.596 |
| Presbyphagia | 15 (29.41%) | 11 (35.48%) | 0.597 |
| Albuminemia (g/L) | 28.9 ± 5.6 | 30.3 ± 5.5 | 0.254 |
| Weight loss in the previous 3–6 months | 5.17 (0–22.5) | 0 (0–15.31) | <0.001 |
| Autonomy in food intake | 22 (43.14%) | 15 (48.39%) | 0.643 |
| Slope (95%CI) | p Value | |
|---|---|---|
| Sex (male) | 2.25 (−5.66; 10.16) | 0.573 |
| Age (years) | −0.45 (−0.81; −0.08) | 0.016 |
| BMI | 0.73 (−0.18; 1.64) | 0.115 |
| Comorbidity | −5.79 (−13.96; 2.37) | 0.162 |
| Reason for diet | ||
| Edentulous | −8.28 (−18.34; 1.77) | 0.105 |
| Cognitive decay | −5.35 (−14.64; 3.94) | 0.256 |
| Dysphagia | 1.88 (−6.13; 9.89) | 0.642 |
| Presbyphagia | 1.32 (−7.16; 9.81) | 0.757 |
| Albuminemia (g/L) | 0.46 (−0.25; 1.16) | 0.199 |
| Weight loss in the previous 3–6 months | 1.21 (0.47; 1.94) | 0.002 |
| Acute pathology | −11.11 (−18.82; −3.39) | 0.005 |
| GLIM | −6.03 (−14.07; 2.01) | 0.139 |
| MUST | 3.43 (0.02; 6.84) | 0.049 |
| Autonomy in food intake | −22.28 (−28.49; −16.08) | <0.001 |
| Fluid intake (100 mL) | −1.16 (−2.06; −0.25) | 0.013 |
| Food intake (100 Kcal) | −0.43 (−2.38; 1.51) | 0.658 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starace, E.; De Pasquale, G.; Morenghi, E.; Crippa, C.; Matteucci, S.; Pieri, G.; Soekeland, F.; Gibbi, S.M.; Lo Cricchio, G.; Reggiani, F.; et al. Hospital Malnutrition in the Medicine and Neurology Departments: A Complex Challenge. Nutrients 2023, 15, 5061. https://doi.org/10.3390/nu15245061
Starace E, De Pasquale G, Morenghi E, Crippa C, Matteucci S, Pieri G, Soekeland F, Gibbi SM, Lo Cricchio G, Reggiani F, et al. Hospital Malnutrition in the Medicine and Neurology Departments: A Complex Challenge. Nutrients. 2023; 15(24):5061. https://doi.org/10.3390/nu15245061
Chicago/Turabian StyleStarace, Erica, Giulia De Pasquale, Emanuela Morenghi, Camilla Crippa, Sofia Matteucci, Gabriella Pieri, Fanny Soekeland, Stefano Maria Gibbi, Giuliana Lo Cricchio, Francesco Reggiani, and et al. 2023. "Hospital Malnutrition in the Medicine and Neurology Departments: A Complex Challenge" Nutrients 15, no. 24: 5061. https://doi.org/10.3390/nu15245061
APA StyleStarace, E., De Pasquale, G., Morenghi, E., Crippa, C., Matteucci, S., Pieri, G., Soekeland, F., Gibbi, S. M., Lo Cricchio, G., Reggiani, F., Calatroni, M., Pastore, M., Mazzoleni, B., & Mancin, S. (2023). Hospital Malnutrition in the Medicine and Neurology Departments: A Complex Challenge. Nutrients, 15(24), 5061. https://doi.org/10.3390/nu15245061






