Increase in Vascular Function Parameters According to Lifestyles in a Spanish Population without Previous Cardiovascular Disease—EVA Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population
2.3. Variables and Measuring Instruments
2.3.1. Measurement of Arterial Stiffness
2.3.2. Lifestyles Assessment
2.3.3. Evaluation of Cardio-Vascular Risk Factors
2.4. Statistical Analysis
2.5. Ethical Principles
3. Results
3.1. Study Population
3.2. Correlation between Increased Stiffness Measures and Lifestyles
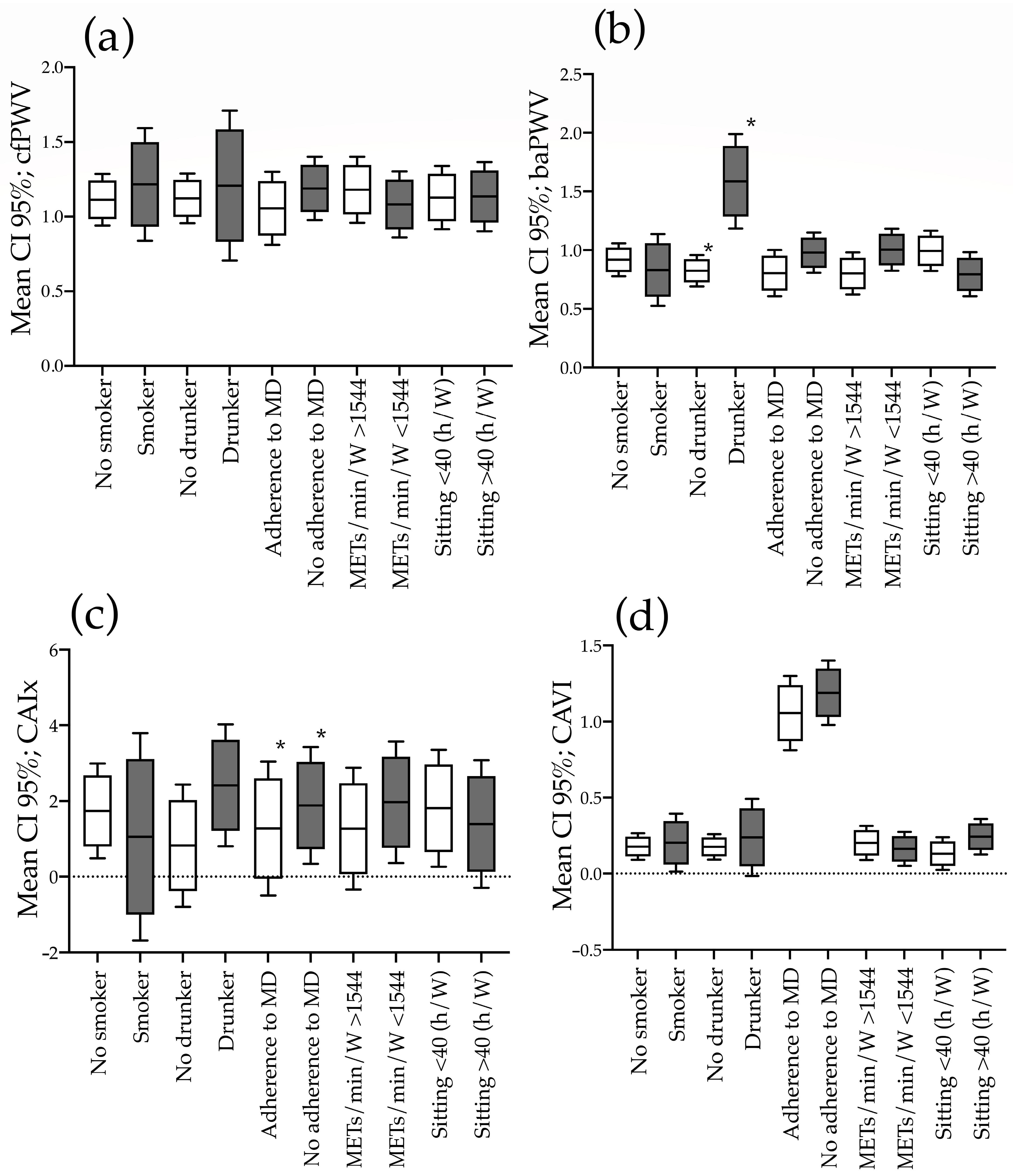
3.3. Increased Measures of Stiffness in Subjects with Unhealthy and Healthy Lifestyles Globally and by Gender
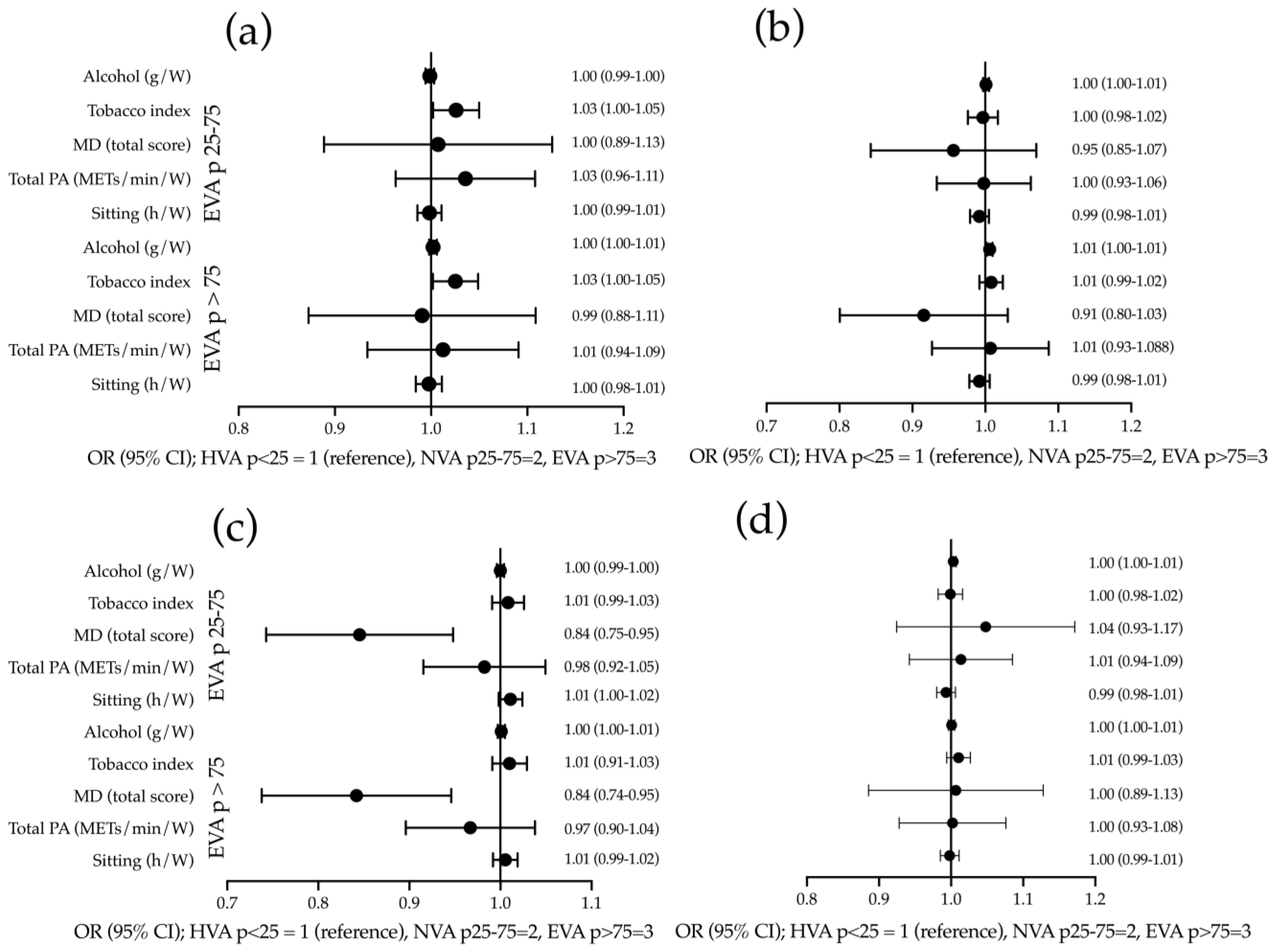
3.4. Association between Increased Measures of Stiffness and Lifestyles
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurent, S.; Boutouyrie, P.; Cunha, P.G.; Lacolley, P.; Nilsson, P.M. Concept of Extremes in Vascular Aging. Hypertension 2019, 74, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Matsushita, K.; Ding, N.; Kim, E.D.; Budoff, M.; Chirinos, J.A.; Fernhall, B.; Hamburg, N.M.; Kario, K.; Miyoshi, T.; Tanaka, H.; et al. Cardio-ankle vascular index and cardiovascular disease: Systematic review and meta-analysis of prospective and cross-sectional studies. J. Clin. Hypertens. 2019, 21, 16–24. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, H.; Shirai, K.; Horinaka, S.; Higaki, J.; Yamamura, S.; Saiki, A.; Takahashi, M.; Masaki, M.; Okura, T.; et al. Predictive Value of the Cardio-Ankle Vascular Index for Cardiovascular Events in Patients at Cardiovascular Risk. J. Am. Heart Assoc. 2021, 10, e020103. [Google Scholar] [CrossRef]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Yasuharu, T.; Setoh, K.; Kawaguchi, T.; Nakayama, T.; Matsuda, F. Brachial-ankle pulse wave velocity and cardio-ankle vascular index are associated with future cardiovascular events in a general population: The Nagahama Study. J. Clin. Hypertens. 2021, 23, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Hu, M.J.; Cui, Y.J.; Liang, L.; Zhou, M.M.; Yang, Y.W.; Huang, F. Carotid-Femoral Pulse Wave Velocity in the Prediction of Cardiovascular Events and Mortality: An Updated Systematic Review and Meta-Analysis. Angiology 2018, 69, 617–629. [Google Scholar] [CrossRef]
- Hwang, C.L.; Muchira, J.; Hibner, B.A.; Phillips, S.A.; Piano, M.R. Alcohol Consumption: A New Risk Factor for Arterial Stiffness? Cardiovasc. Toxicol. 2022, 22, 236–245. [Google Scholar] [CrossRef]
- Del Giorno, R.; Maddalena, A.; Bassetti, S.; Gabutti, L. Association between Alcohol Intake and Arterial Stiffness in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 1207. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, J.; Garcia-Ortiz, L.; Rodriguez-Sanchez, E.; Maderuelo-Fernandez, J.A.; Tamayo-Morales, O.; Lugones-Sanchez, C.; Recio-Rodriguez, J.I.; Gomez-Marcos, M.A. The Relationship Between Alcohol Consumption with Vascular Structure and Arterial Stiffness in the Spanish Population: EVA Study. Alcohol. Clin. Exp. Res. 2020, 44, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Saz-Lara, A.; Martínez-Vizcaíno, V.; Sequí-Domínguez, I.; Álvarez-Bueno, C.; Notario-Pacheco, B.; Cavero-Redondo, I. The effect of smoking and smoking cessation on arterial stiffness: A systematic review and meta-analysis. Eur. J. Cardiovasc. Nurs. 2022, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Kuntic, M.; Keaney, J.F.; Deanfield, J.E.; Daiber, A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020, 41, 4057–4070. [Google Scholar] [CrossRef]
- Vallée, A. Association between tobacco smoking and alcohol consumption with arterial stiffness. J. Clin. Hypertens. 2023, 25, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.D.; Couch, S.C.; The, N.S.; Crandell, J.L.; Lawrence, J.M.; Crume, T.L.; Mayer-Davis, E.J.; Zhong, V.W.; Urbina, E.M. Association between diet quality indices and arterial stiffness in youth with type 1 diabetes: SEARCH for Diabetes in Youth Nutrition Ancillary Study. J. Diabetes Complicat. 2020, 34, 107709. [Google Scholar] [CrossRef]
- Jennings, A.; Berendsen, A.M.; de Groot, L.; Feskens, E.J.M.; Brzozowska, A.; Sicinska, E.; Pietruszka, B.; Meunier, N.; Caumon, E.; Malpuech-Brugère, C.; et al. Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension 2019, 73, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, H.Y.; Lim, K.; Park, J. The role of habitual physical activity on arterial stiffness in elderly Individuals: A systematic review and meta-analysis. J. Exerc. Nutr. Biochem. 2017, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.J.; Beydoun, N.; Mehta, A.; Kim, J.H.; Ko, Y.A.; Jin, Q.; Baltrus, P.; Topel, M.L.; Liu, C.; Mujahid, M.S.; et al. Association of physical activity with arterial stiffness among Black adults. Vasc. Med. 2022, 27, 13–20. [Google Scholar] [CrossRef]
- Tanaka, H.; Palta, P.; Folsom, A.R.; Meyer, M.L.; Matsushita, K.; Evenson, K.R.; Aguilar, D.; Heiss, G. Habitual physical activity and central artery stiffening in older adults: The Atherosclerosis Risk in Communities study. J. Hypertens. 2018, 36, 1889–1894. [Google Scholar] [CrossRef]
- Vandercappellen, E.J.; Henry, R.M.A.; Savelberg, H.; van der Berg, J.D.; Reesink, K.D.; Schaper, N.C.; Eussen, S.; van Dongen, M.; Dagnelie, P.C.; Schram, M.T.; et al. Association of the Amount and Pattern of Physical Activity With Arterial Stiffness: The Maastricht Study. J. Am. Heart Assoc. 2020, 9, e017502. [Google Scholar] [CrossRef]
- Gomez-Marcos, M.A.; Martinez-Salgado, C.; Gonzalez-Sarmiento, R.; Hernandez-Rivas, J.M.; Sanchez-Fernandez, P.L.; Recio-Rodriguez, J.I.; Rodriguez-Sanchez, E.; García-Ortiz, L. Association between different risk factors and vascular accelerated ageing (EVA study): Study protocol for a cross-sectional, descriptive observational study. BMJ Open 2016, 6, e011031. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, R.V.f.A.S. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, A.; Tomiyama, H.; Takeda, K.; Tsuda, H.; Arai, T.; Hirose, K.; Koji, Y.; Hori, S.; Yamamoto, Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 2002, 25, 359–364. [Google Scholar] [CrossRef]
- The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): A major international collaboration. WHO MONICA Project Principal Investigators. J. Clin. Epidemiol. 1988, 41, 105–114. [CrossRef]
- Sanidad, M.d. Límites de Consumo de Bajo Riesgo de Alcohol. Actualización del Riesgo Relacionado con los Niveles de Consumo de Alcohol, el Patrón de Consumo y el Tipo de Bebida; Ministerio de Sanidad Madrid: Madrid, Spain, 2020. [Google Scholar]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Marshall, A.L.; Miller, Y.D.; Burton, N.W.; Brown, W.J. Measuring total and domain-specific sitting: A study of reliability and validity. Med. Sci. Sports Exerc. 2010, 42, 1094–1102. [Google Scholar] [CrossRef]
- Román Viñas, B.; Ribas Barba, L.; Ngo, J.; Serra Majem, L. Validity of the international physical activity questionnaire in the Catalan population (Spain). Gac. Sanit. 2013, 27, 254–257. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Uemura, H.; Katsuura-Kamano, S.; Yamaguchi, M.; Arisawa, K. Relationships of elevated levels of serum hepatic enzymes and alcohol intake with arterial stiffness in men. Atherosclerosis 2015, 238, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Yoshioka, E.; Saijo, Y.; Kita, T.; Okada, E.; Tamakoshi, A.; Kishi, R. Relation between alcohol consumption and arterial stiffness: A cross-sectional study of middle-aged Japanese women and men. Alcohol 2013, 47, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Hwang, I.C.; Kim, K.K.; Kang, W.C.; Cha, J.Y.; Moon, Y.A. Casual alcohol consumption is associated with less subclinical cardiovascular organ damage in Koreans: A cross-sectional study. BMC Public Health 2018, 18, 1091. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.; Britton, A.; Brunner, E.J.; Bell, S. Twenty-Five-Year Alcohol Consumption Trajectories and Their Association with Arterial Aging: A Prospective Cohort Study. J. Am. Heart Assoc. 2017, 6, e005288. [Google Scholar] [CrossRef]
- Kim, M.K.; Shin, J.; Kweon, S.S.; Shin, D.H.; Lee, Y.H.; Chun, B.Y.; Choi, B.Y. Harmful and beneficial relationships between alcohol consumption and subclinical atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 767–776. [Google Scholar] [CrossRef]
- Sluyter, J.D.; Hughes, A.D.; Thom, S.A.; Lowe, A.; Camargo, C.A., Jr.; Hametner, B.; Wassertheurer, S.; Parker, K.H.; Scragg, R.K. Arterial waveform parameters in a large, population-based sample of adults: Relationships with ethnicity and lifestyle factors. J. Hum. Hypertens. 2017, 31, 305–312. [Google Scholar] [CrossRef]
- Hwang, C.L.; Piano, M.R.; Thur, L.A.; Peters, T.A.; da Silva, A.L.G.; Phillips, S.A. The effects of repeated binge drinking on arterial stiffness and urinary norepinephrine levels in young adults. J. Hypertens. 2020, 38, 111–117. [Google Scholar] [CrossRef]
- Lin, Y.; Ying, Y.Y.; Li, S.X.; Wang, S.J.; Gong, Q.H.; Li, H. Association between alcohol consumption and metabolic syndrome among Chinese adults. Public Health Nutr. 2021, 24, 4582–4590. [Google Scholar] [CrossRef]
- Zilkens, R.R.; Burke, V.; Watts, G.; Beilin, L.J.; Puddey, I.B. The effect of alcohol intake on insulin sensitivity in men: A randomized controlled trial. Diabetes Care 2003, 26, 608–612. [Google Scholar] [CrossRef]
- Mouton, A.J.; El Hajj, E.C.; Ninh, V.K.; Siggins, R.W.; Gardner, J.D. Inflammatory cardiac fibroblast phenotype underlies chronic alcohol-induced cardiac atrophy and dysfunction. Life Sci. 2020, 245, 117330. [Google Scholar] [CrossRef]
- Alcohol use and burden for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [CrossRef] [PubMed]
- Rehill, N.; Beck, C.R.; Yeo, K.R.; Yeo, W.W. The effect of chronic tobacco smoking on arterial stiffness. Br. J. Clin. Pharmacol. 2006, 61, 767–773. [Google Scholar] [CrossRef]
- Jatoi, N.A.; Jerrard-Dunne, P.; Feely, J.; Mahmud, A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007, 49, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Doonan, R.J.; Hausvater, A.; Scallan, C.; Mikhailidis, D.P.; Pilote, L.; Daskalopoulou, S.S. The effect of smoking on arterial stiffness. Hypertens. Res. 2010, 33, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Schmitt, V.H.; Arnold, N.; Keller, K.; Prochaska, J.H.; Wild, P.S.; Schulz, A.; Lackner, K.J.; Pfeiffer, N.; Schmidtmann, I.; et al. Chronic cigarette smoking is associated with increased arterial stiffness in men and women: Evidence from a large population-based cohort. Clin. Res. Cardiol. 2023, 112, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Leone, A. Smoking and hypertension: Independent or additive effects to determining vascular damage? Curr. Vasc. Pharmacol. 2011, 9, 585–593. [Google Scholar] [CrossRef]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef]
- Marshall, Z.A.; Mackintosh, K.A.; McNarry, M.A. Investigating the influence of physical activity composition on arterial stiffness in youth. Eur. J. Sport Sci. 2022, 23, 617–624. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Lazaros, G.; Vogiatzi, G.; Mystakidi, V.C.; Goliopoulou, A.; Anastasiou, M.; Christoforatou, E.; Tousoulis, D. The Association of Physical Activity with Arterial Stiffness and Inflammation: Insight from the “Corinthia” Study. Angiology 2022, 73, 716–723. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, L.; Xu, L.; Sun, X.; Liu, W.; Zhou, S.; van de Vosse, F.; Greenwald, S.E. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: Systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0200829. [Google Scholar] [CrossRef] [PubMed]
- Oudegeest-Sander, M.H.; Thijssen, D.H.; Smits, P.; van Dijk, A.P.; Olde Rikkert, M.G.; Hopman, M.T. Association of Fitness Level With Cardiovascular Risk and Vascular Function in Older Nonexercising Individuals. J. Aging Phys. Act. 2015, 23, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Hernández-Quiñones, P.A.; Tordecilla-Sanders, A.; Álvarez, C.; Ramírez-Campillo, R.; Izquierdo, M.; Correa-Bautista, J.E.; Garcia-Hermoso, A.; Garcia, R.G. Effectiveness of HIIT compared to moderate continuous training in improving vascular parameters in inactive adults. Lipids Health Dis. 2019, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Suboc, T.B.; Knabel, D.; Strath, S.J.; Dharmashankar, K.; Coulliard, A.; Malik, M.; Haak, K.; Widlansky, M.E. Associations of Reducing Sedentary Time With Vascular Function and Insulin Sensitivity in Older Sedentary Adults. Am. J. Hypertens. 2016, 29, 46–53. [Google Scholar] [CrossRef]
- Ahmadi-Abhari, S.; Sabia, S.; Shipley, M.J.; Kivimäki, M.; Singh-Manoux, A.; Tabak, A.; McEniery, C.; Wilkinson, I.B.; Brunner, E.J. Physical Activity, Sedentary Behavior, and Long-Term Changes in Aortic Stiffness: The Whitehall II Study. J. Am. Heart Assoc. 2017, 6, e005974. [Google Scholar] [CrossRef]
| Global (n = 480) | Men (n = 237) | Women (n = 243) | p Value | |
|---|---|---|---|---|
| Lifestyles | ||||
| Alcohol (g/W) | 91.17 ± 85.79 | 113.21 ± 93.37 | 55.71 ± 65.43 | 0.010 |
| Appropriate consumption, n (%) | 431 (89.8) | 204 (42.5) | 227 (47.3) | 0,010 |
| Daily cigarettes (n/day) | 14.42 ± 10.65 | 14.79 ± 11.58 | 14.09 ± 10.20 | 0.646 |
| Tobacco index | 21.19 ± 22.08 | 21.61 ± 17.43 | 20.79 ± 25.70 | 0.797 |
| No Smoker, n (%) | 396 (82.5) | 192 (40.0) | 204 (42.5) | 0.403 |
| MD (total score) | 7.17 ± 2.08 | 6.71 ± 1.96 | 7.61 ± 2.09 | <0.001 |
| Adherence to MD, n (%) | 208 (43.3) | 86 (17.9) | 122 (25.4) | 0.002 |
| Total PA (METs/m/W) | 2536 ± 3307 | 3305 ± 3789 | 1786 ± 2549 | <0.001 |
| >1545 METs/m/W, n (%) | 240 (50) | 154 (32.1) | 86 (17.9) | <0.001 |
| Sitting (h/W) | 42.18 ± 17.80 | 47.81 ± 16.68 | 36.68 ± 17.178 | <0.001 |
| <40 h/W sitting, n (%) | 261 (54.4) | 98 (20.4) | 163 (34) | <0.001 |
| Conventional risk factors | ||||
| Age, (years) | 56 ± 14.20 | 55.97 ± 14.30 | 56.02 ± 14.127 | 0.968 |
| SBP (mmHg) | 120.80 ± 23.38 | 126.50 ± 19.87 | 115.24 ± 25.19 | <0.001 |
| DBP (mmHg) | 75.63 ± 9.94 | 77.55 ± 9.11 | 73.76 ± 10.37 | <0.001 |
| MBP (mmHg) | 90.69 ± 12.62 | 93.87 ± 11.17 | 87.59 ± 13.19 | <0.001 |
| Hypertension, n (%) | 142 (29.6) | 79 (16.5) | 63(13.1) | 0.089 |
| Antihypertensive drugs, n (%) | 92 (19.2) | 47 (9.8) | 45 (9.4) | 0.729 |
| Total cholesterol (mg/dL) | 195.10 ± 32.84 | 192.78 ± 32.79 | 197.35 ± 32.80 | 0.127 |
| LDL cholesterol (mg/dL) | 115.53 ± 29.48 | 117.38 ± 30.19 | 113.75 ± 28.72 | 0.180 |
| HDL cholesterol (mg/dL) | 58.94 ± 16.26 | 53.19 ± 14.17 | 64.52 ± 16.24 | <0.001 |
| Triglycerides (mg/dL) | 103.18 ± 53.84 | 112.49 ± 55.15 | 94.11 ± 51.03 | <0.001 |
| Dyslipidemia, n (%) | 185 (38.5) | 91 (19.09) | 94 (19.6) | 1.000 |
| Lipid–lowering drugs, n (%) | 100 (20.8) | 49.4 (10.0) | 52 (10.8) | 0.822 |
| FPG (mg/dL) | 88.03 ± 16.76 | 90.12 ± 18.62 | 85.99 ± 14.46 | 0.007 |
| HbA1c (%) | 5.49 ± 0.55 | 5.54 ± 0.61 | 5.43 ± 0.47 | 0.041 |
| Diabetes mellitus, n (%) | 36 (7.5) | 25 (5.2) | 11 (2.3) | 0.015 |
| Hypoglycemic drugs, n (%) | 33 (6.9) | 22 (4.6) | 11 (2.3) | 0.047 |
| Height, cm | 165,15 ± 9.77 | 171.80 ± 7.46 | 158.67 ± 7.024 | <0.001 |
| Weight, kg | 62.71 ± 13.78 | 79.72 ± 11.78 | 65.68 ± 11.96 | <0.001 |
| BMI (kg/m2) | 26.57 ± 4.25 | 27.01 ± 3.54 | 26.16 ± 4.82 | 0.029 |
| Obesity, n (%) | 91 (19.0) | 41 (8.5) | 50 (10.4) | 0.415 |
| CVR SCORE scale (%) | 4.21 ± 6.14 | 5.46 ± 7.20 | 2.98 ± 4.59 | <0.001 |
| SCORE > 5, n (%) | 134 (27.9) | 85 (17.7) | 49 (10.2) | <0.001 |
| Increasing in arterial stiffness values | ||||
| cfPWV (m/seg) | 1.11 ± 1.70 | 1.27 ± 1.70 | 0.95 ± 1.68 | 0.040 |
| baPWV (m/seg) | 0.90 ± 1.51 | 0.79 ± 1.53 | 1.01 ± 1.50 | 0.104 |
| CAVI | 0.19 ± 0.88 | 0.23 ± 0.86 | 0.14 ± 0.90 | 0.248 |
| CAIx | 1.94 ± 12.71 | 2.25 ± 13.97 | 1.63 ± 11.37 | 0.592 |
| Global | cfPWV | baPWV | CAVI | CAIx |
|---|---|---|---|---|
| Alcohol consumption (g/W) | 0.017 | 0.223 ** | 0.011 | −0.046 |
| Tobacco index | 0.086 | 0.145 * | 0.061 | 0.037 |
| MD (total score) | 0.003 | 0.029 | −0.096 * | 0.026 |
| Total PA (METs/min/W) | 0.019 | −0.075 | −0.055 | −0.001 |
| Sitting (h/W) | −0.025 | −0.098 * | 0.062 | 0.000 |
| Men | ||||
| Alcohol consumption (g/W)) | −0.001 | 0.240 ** | −0.011 | −0.015 |
| Tobacco index | 0.032 | 0.244 * | 0.069 | 0.016 |
| MD (total score) | 0.015 | 0.078 | −0.116 | −0.021 |
| Total PA (METs/min/W) | 0.042 | −0.037 | −0.044 | −0.016 |
| Sitting (h/W) | −0.053 | −0.107 | 0.094 | −0.018 |
| Women | ||||
| Alcohol consumption (g/W) | 0.018 | 0.256 * | 0.017 | −0.126 |
| Tobacco index | 0.128 | 0.090 | 0.060 | 0.060 |
| MD (total score) | 0.033 | −0.048 | −0.061 | −0.021 |
| Total PA (METs/min/W) | −0.069 | −0.096 | −0.108 | 0.010 |
| Sitting (h/W) | −0.061 | −0.051 | 0.007 | 0.003 |
| Variables | Global (n = 480) | Men (n = 237) | Women (n = 243) |
|---|---|---|---|
| cfPWV | |||
| Smoker/non-smoker | 0.051 (−0.349 to 0.452) | 0.057 (−0.498 to 0.613) | 0.011 (−0.569 to 0.591) |
| Drinkers/non-drinkers | −0.016(−0.519 to 0.487) | −0.195 (−0.823 to 0.433) | 0.135 (−0.724 to 0.994) |
| No adherence/adherence to MD | 0.007 (−0.299 to 0.315) | −0.099 (−0.552 to 0.353) | 0.017 (−0.409 to 0.444) |
| Sedentary/actives | −0.082 (−0.387 to 0.222) | −0.046 (−0.502 to 0.410) | 0.070 (−0.375 to 0.516) |
| Sitting > 40/sitting < 40 h/W | −0.060 (−0.366 to 0.245) | −0.181 (−0.638 to 0.275) | −0.123 (−0.576 to 0.330) |
| baPWV | |||
| Smoker/non-smoker | −0.231 (−0.589 to 0.126) | −0.222 (−0.722 to 0.277) | −0.218 (−0.735 to 0.296) |
| Drinkers/non-drinkers | 0.581 (0.134 to 1.028) | 0.713 (0.154 to 1.272) | 0.487 (−0.276 to 1.251) |
| No adherence/adherence to MD | −0.159 (−0.434 to 0.115) | −0.338 (−0.743 to 0.067) | 0.058 (−0.322 to 0.4389) |
| Sedentary/actives | 0.269 (−0.003 to 0.540) | 0.042 (−0369 to 0.453) | 0.395 (0.001 to 0.790) |
| Sitting > 40/sitting < 40 h/W | −0.317 (−0.589 to −0.045) | −0.349 (−0.744 to 0.047) | −0.200 (−0.604 to 0.204) |
| CAVI | |||
| Smoker/non-smoker | 0.067 (−0.140 to 0.274) | 0.079 (−0.200 to 0.360) | 0.043 (−0.266 to 0.352) |
| Drinkers/non-drinkers | 0.041 (−0.220 to 0.301) | 0.021 (−0.297 to 0.339) | 0.025(−0.433 to 0.484) |
| No adherence/adherence to MD | 0.102 (−0.057 to 0.261) | 0.117 (−0.111 to 0.345) | 0.067 (−0.160 to 0.294) |
| Sedentary/actives | −0.029 (−0.187 to 0.129) | −0.152 (−0.400 to 0.096) | 0.144 (−0.093 to 0.381) |
| Sitting > 40/sitting < 40 h/W | 0.083 (−0.075 to 0.241) | 0.148 (−0.074 to 0.371) | −0.027 (−0.269 to 0.215) |
| CAIx | |||
| Smoker/non-smoker | 0.736 (−2.267 to 3.739) | 2.549 (−2.007 to 7.105) | −1.348 (−5.267 to 2.572) |
| Drinkers/non-drinkers | 0.755 (−1.526 to 3.037) | −0.149 (−3.848 to 3.551) | 1.409 (−1.554 to 4.373) |
| No adherence/adherence to MD | 1.359 (−0.941 to 3.660) | 1.029 (−2.694 to 4.753) | 1.538 (−1.336 to 4.411) |
| Sedentary/actives | 0.665 (−1.617 to 2.947) | 2.307 (−1.437 to 6.051) | −0.406 (−3.417 to 2.605) |
| Sitting > 40/sitting < 40 h/W | −0.307 (−2.598 to 1.984) | −0.365 (−4.003 to 3.272) | −0.649 (−3.712 to 2.414) |
| Global | <P25 | Entre P25–75 | >P75 | p |
|---|---|---|---|---|
| cfPWV | ||||
| Alcohol (g/W) | 90.64 ± 71.58 | 86.72 ± 85.97 | 101.67 ± 97.73 | 0.551 |
| Tobacco index | 16.93 ± 16.28 | 21.53 ± 25.02 | 25.01 ± 19.66 | 0.210 |
| Mediterranean diet (total score) | 7.19 ± 2.04 | 7.03 ± 2.07 | 7.41 ± 2.12 | 0.266 |
| Total PA (METs/m/W) | 2517 ± 3700 | 2476 ± 2886 | 2677 ± 3697 | 0.861 |
| Sitting (h/W) | 43.36 ± 17.29 | 41.76 ± 18.30 | 41.85 ± 17.39 | 0.709 |
| baPWV | ||||
| Alcohol (g/W) | 92.84 ± 73.51 | 77.48 ± 73.50 | 106.57 ± 103.10 | 0.066 |
| Tobacco index | 17.35 ± 16.82 | 22.32 ± 26.51 | 22.27 ± 18.06 | 0.426 |
| Mediterranean diet (total score) | 7.20 ± 2.07 | 7.05 ± 2.13 | 7.28 ± 2.02 | 0.558 |
| Total PA (METs/m/W) | 2605 ± 3773 | 2429 ± 2846 | 2615 ± 3491 | 0.838 |
| Sitting (h/W) | 43.69 ± 17.13 | 42.30 ± 18.77 | 41.03 ± 17.11 | 0.468 |
| CAVI | ||||
| Alcohol (g/W) | 92.11 ± 88.11 | 88.35 ± 87.85 | 95.16 ± 80.79 | 0.876 |
| Tobacco index | 18.85 ± 13.73 | 20.65 ± 26.75 | 25.37 ± 20.44 | 0.312 |
| Mediterranean diet (total score) | 7.65 ± 2.06 | 7.04 ± 2.11 | 6.94 ± 1.95 | 0.012 |
| Total PA (METs/m/W) | 2785 ± 4166 | 2599 ± 3206 | 21868 ± 2426 | 0.322 |
| Sitting (h/W) | 39.58 ± 19.41 | 42.39 ± 17.02 | 44.31 ± 17.50 | 0.144 |
| CAIx | ||||
| Alcohol (g/W) | 97.42 ± 93.91 | 96.30 ± 87.46 | 74.92 ± 71.81 | 0.231 |
| Tobacco index | 20.99 ± 13.33 | 20.10 ± 18.78 | 23.35 ± 33.51 | 0.711 |
| Mediterranean diet (total score) | 7.34 ± 1.86 | 7.21 ± 2.20 | 6.89 ± 1.97 | 0.242 |
| Total PA (METs/m/W) | 2196 ± 2363 | 2704 ± 3793 | 2534 ± 3012 | 0.381 |
| Sitting (h/W) | 42.72 ± 19.36 | 41.51 ± 17.02 | 43.06 ± 17,86 | 0.694 |
| Men | ||||
| cfPWV | ||||
| Alcohol (g/W) | 117.58 ± 75.40 | 103.35 ± 92.77 | 132.43 ± 107.41 | 0.294 |
| Tobacco index | 19.38 ± 17.71 | 20.02 ± 14.64 | 26.76 ± 21.93 | 0.245 |
| Mediterranean diet (total score) | 6.73 ± 2.02 | 6.60 ± 1.84 | 6.91 ± 2.16 | 0.603 |
| Total PA (METs/m/W) | 3048 ± 3720 | 3103 ± 3282 | 3883 ± 4635 | 0.353 |
| Sitting (h/W) | 47.86 ± 15.49 | 48.63 ± 16.75 | 46.23 ± 17.56 | 0.649 |
| baPWV | ||||
| Alcohol (g/W) | 121.21 ± 76.40 | 93.36 ± 80.99 | 130.09 ± 109.39 | 0.087 |
| Tobacco index | 19.38 ± 17.71 | 20.92 ± 15.13 | 23.46 ± 19.66 | 0.680 |
| Mediterranean diet (total score) | 6.76 ± 2.04 | 6.54 ± 1.86 | 6.86 ± 2.03 | 0.532 |
| Total PA (METs/m/W) | 3099 ± 3787 | 3174 ± 3311 | 3544 ± 4240 | 0.731 |
| Sitting (h/W) | 48.37 ± 16.60 | 49.57 ± 17.10 | 45.75 ± 16.75 | 0.282 |
| CAVI | ||||
| Alcohol (g/W) | 118.16 ± 100.04 | 113.86 ± 95.80 | 107.95 ± 85.24 | 0.884 |
| Tobacco index | 17.72 ± 14.69 | 23.90 ± 19.46 | 22.86 ± 16.94 | 0.311 |
| Mediterranean diet (total score) | 7.24 ± 1.86 | 6.70 ± 2.03 | 6.32 ± 1.84 | 0.035 |
| Total PA (METs/m/W) | 3271 ± 4501 | 3658 ± 3857 | 2749 ± 2967 | 0.291 |
| Sitting (h/W) | 45.66 ± 18.15 | 46.94 ± 16.40 | 50.93 ± 15.72 | 0.164 |
| CAIx | ||||
| Alcohol (g/W) | 124.31 ± 109.00 | 114.54 ± 93.62 | 99.31 ± 74.97 | 0.519 |
| Tobacco index | 21.65 ± 13.18 | 22.34 ± 20.61 | 20.32 ± 16.63 | 0.905 |
| Mediterranean diet (total score) | 6.75 ± 1.68 | 6.87 ± 2.06 | 6.34 ± 1.98 | 0.241 |
| Total PA (METs/m/W) | 3116 ± 2930 | 3265 ± 4163 | 3572 ± 3717 | 0.805 |
| Sitting (h/W) | 48.35 ± 19.15 | 47.06 ± 15.89 | 48.89 ± 16.03 | 0.762 |
| Women | ||||
| cfPWV | ||||
| Alcohol (g/W) | 55.83 ± 48.67 | 57.07 ± 62.92 | 52.73 ± 52.02 | 0.958 |
| Tobacco index | 15.32 ± 15.39 | 22.99 ± 32.08 | 22.90 ± 16.86 | 0.404 |
| Mediterranean diet (total score) | 7.55 ± 1.98 | 7.46 ± 2.20 | 8.00 ± 1.93 | 0.278 |
| Total PA (METs/m/W) | 2112 ± 3661 | 1848 ± 2273 | 1251 ± 916 | 0.166 |
| Sitting (h/W) | 39.93 ± 17.91 | 34.89 ± 17.23 | 36.67 ± 15.83 | 0.157 |
| baPWV | ||||
| Alcohol consumption (g/W) | 55.45 ± 50.06 | 54.40 ± 52.74 | 57.86 ± 67.46 | 0.969 |
| Tobacco index | 15.79 ± 16.30 | 23.36 ± 32.63 | 20.57 ± 15.70 | 0.488 |
| Mediterranean diet (total score) | 7.54 ± 2.03 | 7.51 ± 2.24 | 7.80 ± 1.91 | 0.621 |
| Total PA (METs/m/W) | 2221 ± 3747 | 1755 ± 2151 | 1786 ± 2549 | 0.219 |
| Sitting (h/W) | 40.06 ± 17.49 | 35.72 ± 17.83 | 36.68 ± 17.18 | 0.190 |
| CAVI | ||||
| Alcohol (g/W) | 54.04 ± 47.03 | 51.74 ± 58.79 | 67.00 ± 63.09 | 0.596 |
| Tobacco index | 20.07 ± 12.79 | 18.10 ± 31.27 | 27.99 ± 23.68 | 0.318 |
| Mediterranean diet (total score) | 7.98 ± 2.17 | 7.35 ± 2.14 | 7.75 ± 1.79 | 0.113 |
| Total PA (METs/m/W) | 2387 ± 3860 | 1625 ± 2036 | 1412 ± 1075 | 0.070 |
| Sitting (h/W) | 34.61 ± 19.11 | 38.21 ± 16.56 | 35.69 ± 15.99 | 0.347 |
| CAIx | ||||
| Alcohol (g/W) | 62.86 ± 54.56 | 60.77 ± 60.73 | 39.80 ± 50.20 | 0.255 |
| Tobacco index | 20.38 ± 13.66 | 18.10 ± 16.97 | 26.27 ± 44.31 | 0.443 |
| Mediterranean diet (total score) | 7.82 ± 1.88 | 7.54 ± 2.30 | 7.50 ± 1.80 | 0.619 |
| Total PA (METs/m/W) | 1770 ± 1388 | 2143 ± 3305 | 1377 ± 1166 | 0.081 |
| Sitting (h/W) | 38.10 ± 18.41 | 35.96 ± 16.35 | 36.57 ± 17.68 | 0.713 |
| cfPWV (m/s) | β | IC 95% | p |
|---|---|---|---|
| Tobacco index | 0.007 | (−0.003 to 0.016) | 0.183 |
| Alcohol consumption (gr/W) | 0.001 | (−0.002 to 0.003) | 0.656 |
| Mediterranean diet (total score) | −0.025 | (−0.107 to 0.058) | 0.555 |
| Total PA (METs/min/W) | 0.003 | (−0.046 to 0.052) | 0.899 |
| Sitting (h/W) | −0.003 | (−0.012 to 0.006) | 0.523 |
| baPWV (m/s) | |||
| Tobacco index | 0.009 | (0.001 to 0.018) | 0.047 |
| Alcohol consumption (g/W) | 0.005 | (0.003 to 0.007) | <0.001 |
| Mediterranean diet (total score) | −0.064 | (−0.131 to 0.003) | 0.061 |
| Total PA (METs/min/W) | −0.010 | (−0.050 to 0.030) | 0.622 |
| Sitting (h/W) | −0.005 | (−0.013 to 0.002) | 0.165 |
| CAVI | |||
| Tobacco index | 0.003 | (−0.004 to 0.009) | 0.427 |
| Alcohol consumption (g/W) | 0.000 | (−0.001 to 0.002) | 0.711 |
| Mediterranean diet (total score) | −0.051 | (−0.092 to −0.010) | 0.016 |
| Total PA (METs/min/W) | −0.016 | (−0.040 to 0.009) | 0.215 |
| Sitting (h/W) | 0.003 | (−0.002 to 0.007) | 0.258 |
| CAIx | |||
| Tobacco index | 0.078 | (−0.009 to 0.165) | 0.079 |
| Alcohol consumption (g/W) | 0.011 | (−0.008 to 0.030) | 0.263 |
| Mediterranean diet (total score) | 0.242 | (−0.358 to 0.843) | 0.428 |
| Total PA (METs/min/W) | −0.024 | (−0.381 to 0.333) | 0.896 |
| Sitting (h/W) | −0.015 | (−0.082 to 0.052) | 0.667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro Cáceres, A.; Navarro-Matías, E.; Gómez-Sánchez, M.; Tamayo-Morales, O.; Lugones-Sánchez, C.; González-Sánchez, S.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Sánchez, L.; Gómez-Marcos, M.A.; et al. Increase in Vascular Function Parameters According to Lifestyles in a Spanish Population without Previous Cardiovascular Disease—EVA Follow-Up Study. Nutrients 2023, 15, 4614. https://doi.org/10.3390/nu15214614
Navarro Cáceres A, Navarro-Matías E, Gómez-Sánchez M, Tamayo-Morales O, Lugones-Sánchez C, González-Sánchez S, Rodríguez-Sánchez E, García-Ortiz L, Gómez-Sánchez L, Gómez-Marcos MA, et al. Increase in Vascular Function Parameters According to Lifestyles in a Spanish Population without Previous Cardiovascular Disease—EVA Follow-Up Study. Nutrients. 2023; 15(21):4614. https://doi.org/10.3390/nu15214614
Chicago/Turabian StyleNavarro Cáceres, Alicia, Elena Navarro-Matías, Marta Gómez-Sánchez, Olaya Tamayo-Morales, Cristina Lugones-Sánchez, Susana González-Sánchez, Emiliano Rodríguez-Sánchez, Luis García-Ortiz, Leticia Gómez-Sánchez, Manuel A. Gómez-Marcos, and et al. 2023. "Increase in Vascular Function Parameters According to Lifestyles in a Spanish Population without Previous Cardiovascular Disease—EVA Follow-Up Study" Nutrients 15, no. 21: 4614. https://doi.org/10.3390/nu15214614
APA StyleNavarro Cáceres, A., Navarro-Matías, E., Gómez-Sánchez, M., Tamayo-Morales, O., Lugones-Sánchez, C., González-Sánchez, S., Rodríguez-Sánchez, E., García-Ortiz, L., Gómez-Sánchez, L., Gómez-Marcos, M. A., & EVA-Follow-Up Investigators Group. (2023). Increase in Vascular Function Parameters According to Lifestyles in a Spanish Population without Previous Cardiovascular Disease—EVA Follow-Up Study. Nutrients, 15(21), 4614. https://doi.org/10.3390/nu15214614







