Abstract
Depressive disorders have a major impact on occupational health and are costly to the economy and the healthcare system. Probiotics are live, non-pathogenic micro-organisms that, when ingested in adequate amounts, can colonize the intestinal tract and confer health benefits on the patient. In recent years, numerous studies have described the potential usefulness of certain probiotic strains in the treatment and prevention of depressive disorders, with differing results. In order to evaluate the possible efficacy and safety of these microorganisms in preventing or ameliorating these disorders, we systematically searched the bibliographic databases MEDLINE (via Pubmed), EMBASE, the Cochrane library, Scopus and Web of science, using the descriptors “Occupational health”, “Probiotics”, “Depressive Disorder” and “Depression” and filters “Humans” and “Clinical Trials”. After applying our inclusion and exclusion criteria, 18 studies were accepted for review and critical analysis. Our analysis suggests that a combination of different probiotic strains, most of them from the genus Bifidobacterium sp. and Lactobacillus sp., could be a good mixture as an adjuvant in the treatment of depressive disorders for the working population.
1. Introduction
The World Health Organization (WHO) assembly proposes to develop national policies and action plans and to create institutional competencies for occupational health, ensuring, in collaboration with other relevant national health programmes, mental health [1]. According to the International Labour Organization (ILO), work helps to maintain good health as long as the worker’s physical and mental capacities are not overtaxed [2].
Research shows that health interventions in the workplace can reduce sickness-related absenteeism by 27% and the cost of healthcare for companies by 26% [1]. Globally, 12,000 million working days are estimated to be lost each year due to depression and anxiety, at a cost of USD 1 billion per year in productivity loss. Related health problems in this area cause a loss of GDP (gross domestic product) of between 4 and 6% in most countries. In 2019, an estimated 15% of working-age adults suffered from a mental disorder [1], and the ILO has established a number of occupational illnesses, including mental and behavioural disorders [3].
1.1. Depressive Disorders
According to the International Classification of Diseases (ICD) [4], depressive disorders are characterised by a persistent mood of sadness, irritability and lack of interest in daily activities, with a loss of pleasure accompanied by other cognitive, behavioural or neurovegetative symptoms that significantly affect the individual’s ability to function. Depression can be long-lasting or recurring and considerably impair a person’s ability to work, study or to cope with everyday life. In its most severe form, depression can lead to suicide [5,6,7]. Depressive disorders represent one of the leading causes of disability in the world, affecting 6.8% of the adult population, with a higher prevalence in women than in men [8]. During the COVID-19 pandemic, 23% of frontline healthcare workers suffered from depression and anxiety, and 39% suffered from insomnia. These professions are prone to depressive disorders, with an increased risk of suicide worldwide [9].
Psychological treatment is the first line against depressive disorders. This can be combined with antidepressants in cases of moderate and severe disorders. Effective psychological treatment may include behavioural activation, cognitive behavioural therapy, interpersonal psychotherapy and problem resolution treatment. While the most commonly used pharmacological treatments are serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants [10,11,12,13], it should be borne in mind that all antidepressant drugs have a latency period of response, which can vary between two and four weeks, until the 5-HT1A receptor (serotonin receptor subtype located in presynaptic and postsynaptic regions) becomes desensitised. During this period, the patient, in addition to not noticing significant improvement, will perceive the possible side effects of the medication, which adds frustration, mistrust and possible non-adherence to the treatment [11,14]. Moreover, in most patients, the response to treatment is suboptimal, so that the dose has to be increased or combined with other antidepressants, further aggravating adverse symptomatology [15]. This insufficient response to antidepressants in a certain number of cases suggests that alternative or complementary medication, in addition to faster clinical response treatments, should be sought [16].
1.2. Microbiota
Microbiota are the set of microorganisms (bacteria, fungi, archaea, viruses and parasites) that reside in our body, which, in turn, can be differentiated into commensals, mutualists and pathogens [17,18]. Microbial communities can be found throughout the human body, the most complex and dense being the one inhabiting the digestive tract and, specifically, the large intestine (gut microbiota). It plays numerous roles in our organism, particularly the maturation and development of the central nervous system (CNS), as well as immune response development and modulation. It is referred to as the “second brain” of humans because of its regulatory effect on the central nervous system through neural, chemical and immune pathways [11,19,20]. Studies of the gut–brain axis (GBA) through the vagus nerve have provided essential insights into the healthy regulation of the hypothalamic–pituitary–adrenal axis (HPA), neuromodulation and neuronal plasticity [21]. This axis forms a network that includes the gastrointestinal tract, the enteric nervous system and the brain [20]. This gut microbiota play an essential role in the regulation of immune, endocrine and metabolic functions. Bacterial metabolites from this microbiota include the short chain fatty acids (SCFAs), made from fermentation of dietary fibre in the gut. Other enzymes and metabolites, such as tryptophan metabolites, gamma-aminobutyric acid (GABA), serotonin, dopamine, norepinephrine, acetylcholine and many neuropeptides, are produced by the microbiota present in the gut [16,19].
Given the ability of many probiotics to function as vehicles for the release of neuroactive compounds, such as classical neurotransmitters, they can be used as an adjunct therapy in the management of neurological disorders. For example, certain strains of Lactobacillus and Bifidobacterium secrete GABA, the main inhibitory neurotransmitter in the brain that regulates affective states, and increases levels of tryptophan, a precursor of serotonin, which suggests it is an antidepressant. The potential of some strains of Lactobacillus to produce acetylcholine, an essential neurotransmitter in several cognitive processes, such as learning and memory, means that they should be included as clear potential coadjuvant treatment, as psychobiotics [16].
Alterations in the gut microbiota, or dysbiosis, lead to a variety of diseases, such as inflammatory bowel disease, coeliac disease, metabolic syndrome, diabetes mellitus, colon cancer, as well as autism spectrum disorder, anxiety and neurodegenerative diseases [20,21]. This dysbiosis, due to increased permeability of the gut microbiota, induces decreased SCFA (short chain fatty acid) synthesis, HPA dysregulation and hypersensitivity of the vagus nerve, which predisposes one to depression [20,21] and, in some cases, to the progression or worsening of major depressive disorders (MDDs) [21].
1.3. Probiotics
Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits on the patient [22]. Recently, the International Scientific Association for Probiotics and Prebiotics [23] established a consensus document with a set of criteria for which microorganisms present in consumer products should be considered as probiotics:
- The microorganism in question must have been scientifically demonstrated to be a safe species that is supported by sufficient evidence of overall beneficial effect in humans.
- Evidence of its viability as a microorganism in human studies must be available.
Several studies [24,25] have proposed the concept of psychobiotics for probiotic bacteria, which, when consumed in adequate amounts, have a beneficial effect on mental health. The mechanisms of action by which bacteria exert their psychobiotic potential have not been fully elucidated. However, it has been found that these bacteria provide their benefits via the enteric nervous system or by stimulating the immune system. In addition, they affect psychophysiological markers of depression and anxiety. This can occur in three different ways: firstly, by affecting the stress response of the HPA axis and reducing systemic inflammation; secondly, through a direct effect on the immune system; and, thirdly, through the secretion of molecules, such as neurotransmitters, proteins and SCFAs [26,27].
Other studies link the intake of probiotics to the prevention and treatment of depression. The work of Bagga et al. [28] examined the clinical relevance of the gut–brain axis with the administration of a multi-species probiotic formulation for four weeks and associated it with changes in brain activation patterns in response to emotional memory tasks and emotional decision making, which were also accompanied by subtle changes in the gut microbiome profile. Akkasheh et al. [29] found that probiotic intake decreased depressive symptomatology as well as oxidative stress levels. In a study by Pirbaglou et al. 2016 [30], an improvement in immune function was observed in those taking probiotics, increasing the number of Natural Killer cells and lymphocytes. This said intake improved the bacterial composition of the gastrointestinal tract and, thus, behaviour and mental health. However, despite these previous works, a review by Vaghef-Mehrabany et al. [31] concluded that current studies are not sufficient to support or reject the antidepressant effects of probiotics.
The different studies on the subject and the differing results obtained only reinforce the belief that not all probiotics are valid, and that it is important to select probiotic strains that can influence the altered physiological processes of the illness targeted by the probiotic product in question [32]. What is important is that the selection of particular strains is based on objective criteria, preclinical studies or plausible hypotheses as to why a specific strain was selected instead of another. In this way, and with the support of clinical studies on the benefit of specific probiotics in specific pathologies, probiotic products can make a significant contribution to the health of the population and, thus, generate cost savings for health systems.
According to the WHO action plan, the loss of economic production due to mental disorders will amount to EUR 16.3 billion between 2011 and 2030. Depression alone accounts for 4.3% of the global burden of illnesses and is one of the leading causes of disability worldwide [33]. Therefore, new treatment options, such as probiotics, could help treat those affected by the illness and contribute to improving the mental health of the general population. Considering publications that demonstrate the benefits of certain probiotic strains in depressive disorders and their ability to stimulate the host’s immune response, we set out on this scoping review to identify and select probiotic strains that can prevent depressive disorders, decrease severity in those cases that eventually develop the illness and decrease the impact on occupational health.
2. Materials and Methods
2.1. Design
We conducted a descriptive study and critical analysis of papers retrieved through exploratory review according to the Preferred Reporting items for Systematic Reviews and Meta-Analyses extension for scoping reviews [34] (Supplementary Table S1).
2.2. Data Collection Sources
This review aims to carry out a critical and systematic study of works published on different databases by means of direct consultation and access via Internet to the following databases: MEDLINE (via PubMed), EMBASE, SCOPUS, Cochrane Library Plus and ISI-Web of Science (Institute for Scientific Information).
2.3. Information Processing
The document search was defined using the Thesaurus developed by the U.S. National Library of Medicine (Medical Subjects Headings—Mesh). Entry terms were also used. The terms “Occupational health”, “Probiotics”, “Depressive Disorder” and “Depression” were used as descriptors and free text in title and abstract. The final search equation was developed for use in the MEDLINE database, via PubMed, through the use of Boolean connectors and the filters “Humans” and “Clinical Trial”, with the following result: (“Depressive Disorder” (Mesh Terms) OR “Depression” (Title/Abstract) OR “Depressive Disorders” (Title/Abstract) OR “Depressive Symptoms” (Title/Abstract) OR “Emotional Depression” (Title/Abstract) OR (“occupational health” (Mesh Terms) OR “occupational health” (Title/Abstract) OR “Occupational Safety” (Title/Abstract) OR “industrial hygiene” (Title/Abstract) OR “employee health” (Title/Abstract))) AND (“Probiotics” (Mesh Terms) OR “Probiotics” (Title/Abstract) OR “Probiotics” (Title/Abstract) OR “Dietary Supplement” (Title/Abstract) OR “Synbiotic” (Title/Abstract) OR “Microbiota” (Title/Abstract) OR “Microbiotas” (Title/Abstract) OR “Dysbiosis” (Mesh Terms) OR “Dysbiosis” (Title/Abstract) OR “Dysbacteriosis” (Title/Abstract)).
The same strategy was adopted for the other databases mentioned above, taking into account their different characteristics. The search was carried out from the first available date until May 2023 (time of the last update). Additionally, as a secondary search and to reduce the number of papers not retrieved, the bibliographic lists for the articles selected in the main search were examined in order to identify previously undetected studies for the review.
2.4. Final Selection of Articles
The final selection of articles was made on the basis of the following inclusion criteria: papers had to be original clinical studies published in peer-reviewed journals, and there had to be a causal relationship between the intake of “probiotics” in “working-age subjects” and with “depressive disorders or “depression”, selecting those relevant with full text that could be retrieved, and which had to be written in English, Portuguese or Spanish. We excluded those that were not conducted on humans or that did not focus the intervention on probiotics in people of working age and that had no effect on depressive disorders or depression.
The selection of relevant articles was carried out independently by the authors of the present review (E.S.-S., J.A.P.-M., A.M.-O., A.R.T., A.H.-T., V.N.-L.). To validate the choice of articles for the review, it was established that the concordance assessment between these two authors (E.S.-S. and J.A.P.-M.) (Kappa index) needed to be greater than 0.80 (measure of very good concordance strength) [35]. Whenever this condition was met, possible discrepancies would be resolved through consultation with an expert in the field and subsequent consensus among the authors.
2.5. Methodological Quality Assessment
The quality of the selected articles was assessed on the basis of the CONSORT (Consolidated Standards of Reporting Trials) guidelines for reporting clinical trials [36], which contain a list of 25 essential items that should be described in the publication of these studies. For each selected article, one point was assigned for each item based on whether the article contained “1” or “0” with regards to the related information. If the evaluation of a particular item was not necessary, that point was not counted in the total (Not Applicable = NA). When an item was made up of several points, these were evaluated independently, giving the same value to each of them and then averaging (this being the final result for that item), so that in no case could the score of one point per item be exceeded.
2.6. Data Extraction
The control of the information extracted from the reviewed studies was carried out using double-entry tables that allowed for the detection of errors and correction by further consultation of the originals.
To determine how up to date the articles were, the Burton–Kebler half period (median age) and the Price Index (percentage of articles less than 5 years old) were calculated. The articles were grouped according to the variables under study in order to systematise and facilitate understanding of the results, coding the following data: first author of the bibliographical reference and year of publication, study design, country where the study was carried out, study population, period in which the work was carried out, what type of intervention took place and the results obtained.
3. Results
Using the inclusion and exclusion criteria described previously, a total of 25,511 references were found: 23,318 (96.85%) on the Web of Science, 932 (3.87%) on Embase, 806 (3.35%) on Scopus, 448 (1.86%) on Medline (via Pubmed) and 7 (0.029%) on the Cochrane Library. After filtering duplicates, applying the inclusion and exclusion criteria and consulting the bibliographic listings of the selected articles (Figure 1), it was possible to select 18 papers for review and critical analysis (Table 1).
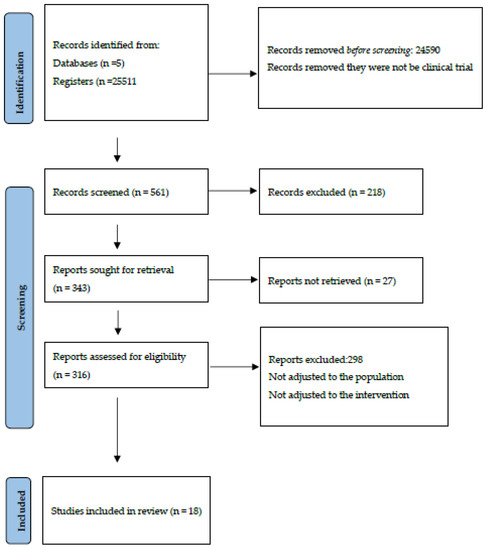
Figure 1.
Identification and selection of studies according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA statement) [37].
The agreement on the relevance of the selected studies among the authors, calculated using the Kappa index, was 85%. The selected articles presented, according to the Burton and Kebler semi-period or Burton–Kebler Index (BKI), a median of two years, with a Price Index (PI) of 88.88%. The papers were published between 2017 and 2023 [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. The years 2020 [43,47,48,52] and 2021 [39,41,44,45], with four articles each, had the highest number of publications, followed by 2019 [40,46,53] and 2022 [49,51,55] with three publications, respectively. There were also two publications [42,54] for the year 2023 and only one publication per year for 2017 [50] and 2018 [38].

Table 1.
Summary of reviewed studies on the relationship between probiotics and depressive disorders in healthy and ill subjects.
Table 1.
Summary of reviewed studies on the relationship between probiotics and depressive disorders in healthy and ill subjects.
| Author, Year | Design | Country | Patients | Population | Monitoring | Intervention Performed | Results |
|---|---|---|---|---|---|---|---|
| Bagga (2018) [38] | RCT | Austria | 45 M/F 23/22 Age: 20 to 40 years | Healthy adult | 4 weeks | 3 g/day OmniBiotic® Stress Repair (7.5 × 106) cfu | Positive role of a multi-strain probiotic administration in modulating the behavior, which is reflected as changes in the FC in healthy volunteers |
| Bloemendaal (2021) [39] | Exploratory analyses | Netherlands | F: 56 Median age: 21.8 years | Healthy female | 28 days | 2 g/day Ecologic ®Barrier (2.5 × 109) cfu | The increased relative abundance of the gut bacterial genus Ruminococcaccae_UCG-003 correlated significantly with the positive effects of probiotics on stress-induced working memory changes |
| Chahwan (2019 [40] | Randomized triple blinded placebo controlled clinical trial | Australia | 71 M/F: Age: years | Mild to severe level depression | 8 weeks | 4 g/day Ecologic®Barrier (2.5 × 109) cfu | Participants in the probiotic group demonstrated a significantly greater reduction in cognitive reactivity compared with the placebo group, particularly in the mild/moderate subgroup |
| Chen (2021) [41] | Open label trial | Taiwan | 11 M/F:3/8 Median age: 39.4 years | MDD | 8 weeks | 600 mg/day capsules PS128 (3 × 1010) cfu plus AM | Depressive severity in patients with MDD significantly ameliorated, but markers of inflammation, gut permeability, and the composition of gut microbiota did not significantly change. |
| Freijy (2023) [42] | RCT | Australia | 119 M/F: 11/108 Age: 18 to 65 years | Moderate psychological distress | 8 weeks follow up (week 20) | 2 capsules/day BioCeuticals © (12 × 109) cfu plus high-prebiotic diet or 5 g/day High-prebiotic or synbiotic. | High-prebiotic diet benefits patients with moderate depressive disorders. |
| Heidarzadeh-Rad (2020) [43] | Post hoc RCT | Iran | 78 M/F:23/55 Median age: 36 years | MDD | 8 weeks | 5 g/day CEREBIOME® (10 × 109)cfu and GOS (80%) | CEREBIOME® exerted a beneficial effect in improving depression scores compared to placebo, while the effects of prebiotic supplement were not major. |
| Ho (2021) [44] | RCT | Taiwan | 40 M/F 16/26 Age:20 to 40 years | Healthy adults with insomnia | 30 days | 850 mg/day capsules PS128 (3 × 1010) cfu | PS128 group showed significant decreases in BDI-II scores, fatigue levels, brainwave activity, and awakenings during the deep sleep stage. |
| Lee (2021) [45] | RCT | Seoul | 122 M/F:39/83 Median age: 38.3 years | Healthy adults with subclinical symptoms of depression, anxiety and insomnia | 8 weeks | 1000 mg/day capsules NVP-1704 (2.5 × 109) cfu | NVP-1704 group had a more significant reduction in depressive symptoms at four and eight weeks of treatment, and anxiety symptoms at four weeks compared to the placebo group |
| Nishida (2019) [46] | RCT | Japan | 60 M/F: 41/19 Median age: 25.3 years | Healthy young adults | 24 weeks | 2 tablets/day Heat-inactivated Lactobacillus gasseri CP2305 (1 × 1010) cfu | Lactobacillus gasseri CP2305 may be beneficial for young adults experiencing stressful conditions |
| Reininghaus (2020) [47] | RCT | Austria | 61 M/F: 14/47 Median Age: 41.5 years | Depressive disorders | 28 days | 3 g/day Omnibiotic Stress Repair® (7.5 × 109) cfu and biotin vitamin B7 125 mg plus in addition to AM. | Probiotic plus B7 plus AM improved microbiota composition in depressive subjects |
| Reiter (2020) [48] | Monocentric RCT | Austria | 61 M/F: 14/47 Median age: 41.5 | MDD | 4 weeks | 3 g/day Omnibiotic Stress Repair® drink (7.5 × 109) cfu and 125 mg biotin vitamin B7 plus in addition to AM | The probiotic group showed decreasing IL-6 gene expression Probiotics could be a useful additional treatment in MDD, due to their anti-inflammatory effects |
| Rode (2022) [49] | RCT | Sweden | 22 M/F: 6/16 Median age: 24.2 years | Healthy subjects | 4 weeks intervention periods | 3 g/day Puraflor® (3 × 109) cfu/day | The probiotic mixture had subtle effects on psychological health, such as rates of depression and sleep patterns, as well as on markers of gut-brain interactions, such as serum serotonin concentrations. |
| Romijn (2017) [50] | RCT | New Zealand | 79 M/F: 17/23 Median age: 35.5 years | Subjects Low Mood | 8 weeks | 1.5 g/day Cerebiome® (3 × 109) cfu/day | Probiotic group, those with high levels of vitamin D at baseline, experienced significant improvement in several psychological outcomes. |
| Schaub (2022) [51] | RCT | Switzerland | 47 M/F: 20/27 Median age: 38.6 | Depression | 4 weeks | Vivomixx® (9 × 109) cfu 4 times a day + AM | An add-on probiotic treatment improves depressive symptoms and maintains healthy enterotypes, species richness and increases specific health related bacterial taxa. |
| Siegel (2020) [52] | Pilot study RCT | USA | 79 M/F = 21/58 Median age: 19.5 years | Healthy undergraduate students | 7days | 800 mg/day capsules Bifidobacterium longum (4 × 1010) cfu | No significant reductions in stress, depression, or anxiety were observed after 1 week |
| Smith Ryan (2019) [53] | RCT | USA | 33 M/F:-/33 Median age: 30.5 years | Healthy female shift-workers | 6 weeks | 4 g/twice day Ecologic ®Barrier (2.5 × 109) cfu plus 10g prebiotic W117 | Reductions in anxiety and fatigue were greater in PRO than PLA |
| Yamanbaeva (2023) [54] | RCT | Switzerland | 32 M/F: 14/18 Median age: 37 | Depressive disorders (ICD-10) | 4 weeks | Vivomixx® (9 × 109) cfu 4 times a day plus AM | Probiotic is suggested to prevent neuronal degeneration along the uncinate fasciculus and alter fronto-limbic rsFC, effects that are partly related to the improvement of depressive symptoms. |
| Schneider (2022) [55] | RCT | Switzerland | 43 M/F: 17/26 Median age: 38.6 years | MDD | 4 weeks | Vivomixx® (9 × 109) cfu 4 times a day plus AM | Additional probiotic supplementation enhances verbal episodic memory and affects neural mechanisms underlying impaired cognition in MDD |
M/F: number males and females. ICD-11-10: International Classification of Diseases. CFU: Colony-forming units. MDD: Major depressive disorders OmniBiotic® Stress Repair: L. casei W56, Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Bifidobacterium lactis W51, Lactobacillus salivarius W24, Lactococcus lactis W19, Bifidobacterium lactis W52, L. plantarum W62, Bifidobacterium bifidum W23. Vivomixx®: Streptococcus thermophilus NCIMB 30438, Bifidobacterium breve NCIMB 30441, Bifidobacterium longum NCIMB 30435 (Re-classified as B. lactis), Bifidobacterium infantis NCIMB 30436 (Re-classified as B. lactis), Lactobacillus acidophilus NCIMB 30442, Lactobacillus plantarum NCIMB 30437 (re-classified Lactiplantibacillus plantarum), Lactobacillus paracasei NCIMB 30439, Lactobacillus delbrueckii subsp. bulgaricus NCIMB 30440 (Re-classified as L. helveticus). AM: Antidepressant or Antipsychotic medication. Ecologic®Barrier: Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58. Cerebiome®: Lactobacillus helveticus R0052 strain I-1722 and Bifidobacterium longum R0175 strain I-3470. GOS: Galactalogosaccharide. RCT: Double-blind randomized controlled trial. Bio Ceuticals©: Bifidobacterium bifidum (Bb-06), Bifidobacterium animalis subsp. lactis (HN019), Bifidobacterium longum (R0175), Lactobacillus acidophilus (La-14), Lactobacillus helveticus (R0052, Lactobacillus casei (Lc-11), Lactobacillus plantarum (Lp-115), Lactobacillus rhamnosus (HN001. FC: Functional connectivity. NVP-1704: mixture of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98. PRO: Ecological® barrier plus resistant maize starch. PLA: Placebo. W117: Resistant maize starch. Puraflor©:, Lactobacillus helveticus R0052 Lactiplantibacillus plantarum R1012 and Bifidobacterium longum R0175. BDI: Beck Depression Inventory. PS 128: Lactobacillus plantarum (Re-classified Lactiplantibacillus plantarum).
Using the CONSORT questionnaire [36], scores ranged from a minimum of five (out of 25 items) to a maximum of 19, with a median of 14.8 (Table 2).

Table 2.
Assessment of the methodological quality of the studies analyzed by means of the 25 items of the CONSORT 2010.
The majority of the studies were double-blind randomized controlled clinical trials (15.83%) [38,40,42,43,44,45,46,47,48,49,50,51,53]. The remainder were an open trial [41], an exploratory analysis [39] and a pilot study [52]. Out of the total, three studies were conducted in Austria [38,47,48] and Switzerland [51,54,55] each, with two in Australia [40,42], United States [52,53] and Taiwan [41,44], respectively. The remaining countries reviewed were featured in publications only once: South Korea [45], New Zealand [50], Netherlands [39], Iran [43], Japan [46] and Sweden [49]. All of the published papers reviewed were written in English.
The selected papers with the smallest sample sizes were those of Chen et al. [41] and Rode et al. [49], with 11 and 22 participants, respectively. In contrast, the papers by Freijy et al. [42] and Lee et al. [45], with 119 and 122 participants, respectively, presented the largest sample sizes.
The populations studied in the 18 selected studies consisted of people of both sexes. The age of the population included in the studies was between 18 and 65 years, with more or less severe depressive symptomatology [40,41,42,43,44,45,47,48,50,51,54,55], as well as healthy people [38,46,49,52]; of particular note, studies [39,53] focused exclusively on the female population. The follow-up period of the studies included in this scoping review ranged from a minimum of 7 days [52] to 24 weeks [46].
All the selected studies assessed the intake of different specific probiotic strains in the working population and their positive relationship with gut microbiota, biomarkers and clinical symptoms of depressive disorders (anxiety, insomnia, stress, depression, etc.). The species and strains used in the intervention were all Gram-positive, non-sporulating bacteria, and concentrations ranged from 106 to 1010 colony-forming units (CFUs). The studies by Nishida et al. [46], Siegel et al. [52], Freijy et al. [42], Ho et al. [44], Lee et al. [45] and Chen et al. [41] used a capsule as the dosage form, with different amounts between 600 and 1000 mg. In contrast, the pharmaceutical sachet format varying between 1.5 and 5 g was the most commonly used [38,40,43,47,48,50,51,54,55].
Most of the trials used different bacterial formulations containing specific probiotic strains, most of them including the genera Lactiplantibacillus, Ligilactobacillus and Limosilactobacillus (third one formerly Lactobacillus) [56]. The genera Bifidobacterium were used in 14 of the selected studies [38,39,40,42,43,45,47,48,49,50,51,53,54,55]. Among these genera are included Lactiplantibacillus plantarum (L. plantarum), Lacticaseibacillus casei (L. casei), Lacticaseibacillus rhamnosus (L. rhamnosus), Ligilactobacillus salivarius (L. salivarius) and Limosilactobacillus reuteri (L. reuteri). The specific mixtures included in the selected articles are:
OmniBiotic® Stress Repair: L. casei W56, Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Bifidobacterium lactis W51, Lactobacillus salivarius W24, Lactococcus lactis W19, Bifidobacterium lactis W52, L. plantarum W62 and Bifidobacterium bifidum W23.
Vivomixx®: Streptococcus thermophilus NCIMB 30438, Bifidobacterium breve NCIMB 30441, Bifidobacterium longum NCIMB 30435 (re-classified as B. lactis), Bifidobacterium infantis NCIMB 30436 (re-classified as B. lactis), Lactobacillus acidophilus NCIMB 30442, Lactobacillus plantarum NCIMB 30437, Lactobacillus paracasei NCIMB 30439 and Lactobacillus delbrueckii subsp. bulgaricus NCIMB 30440 (re-classified as L. helveticus).
Ecologic®Barrier: Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus. acidophilus W37, Lactobacillus brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58.
Cerebiome®: Lactobacillus helveticus R0052 (CNCM strain I-1722) and Bifidobacterium longum R0175 (CNCM strain I-3470).
Puraflor®: Lactobacillus helveticus R0052 (CNCM-I-1722, Lactiplantibacillus plantarum R1012 (CNCM-I-3736) and Bifidobacterium longum R0175 (CNCM-I-3470).
Bioceuticals®: Bifidobacterium bifidum (Bb-06), Bifidobacterium animalis subsp. lactis (HN019), Bifidobacterium longum (R0175), Lactobacillus acidophilus (La-14), Lactobacillus helveticus (R0052), L. casei (Lc-11), Lactobacillus plantarum (Lp-115) and L. rhamnosus (HN001).
NVP-1704®: Limosilactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98.
The remaining studies used a single strain, Lactiplantibacillus plantarum PS128 [41,44] and heat-inactivated Lactobacillus gasseri CP2305 [46], while the work of Siegel et al. [52] used a specific strain of Bifidobacterium longum without including further information on the strain. Some of the studies also combined probiotics with antidepressant medication [41,51,54,55] and vitamin B7 [47,48]. Others combined probiotics with prebiotics [42,43,53], and the rest used probiotic intervention alone.
The results of the probiotic intervention and its effect on depressive disorders in both healthy and ill subjects were varied. These results are summarized below.
3.1. Probiotic Intervention in Healthy Subjects
Bagga et al. [38] demonstrated, in their work, a close relationship between the effects of the intervention with nine probiotic strains and behaviour, reflected in improved functional connectivity (FC) and neuroimaging readings. However, they did not observe any difference in structural connectivity (SC). Bloemendaal et al. [39] observed an increase in the relative abundance of the genus Ruminococcaccae_UCG-003 in the gut microbiota, which correlated significantly with the positive effects of another nine probiotic strains on stress-induced working memory impairment in healthy women.
The work of Siegel et al. [52] and Rode et al. [49] presented similar negative results. In the former, the intervention with the B. longum species did not produce significant changes in improvements or reductions in stress, depression or anxiety in university students. In the latter, results with a mixture of three probiotic strains were subtle in terms of psychological health, depression rates and sleep patterns, as well as markers of gut–brain interactions, such as serum serotonin concentrations. In contrast, Nishida et al. [46] improved stress conditions in young adults with an inactivated probiotic CP2305 intervention, and Smith-Ryan et al. [53] observed a greater reduction in anxiety and fatigue with the intake of nine probiotic strains in female shift workers (nurses, emergency personnel, etc.), although without significant differences when compared with results in the control group.
3.2. Probiotics in Patients with Depressive Disorders
Reiter et al. [48] and Reininghaus et al. [47] reported positive results after ingestion of a mixture of nine probiotic strains together with vitamin B7 (biotin) in patients medicated with MDD. The probiotic mixture decreased interleukin 6 (IL-6) gene expression and improved the microbiota with an increase in potentially beneficial bacteria, such as Ruminococcus gauvreauii and Coprococcus 3.
The probiotic mixture of eight different strains together with antidepressant medication was used in the work of Yamanbaeva et al. [54], Schaub et al. [51] and Schneider et al. [55]. The results were prevention of neuronal degeneration along the uncinate fasciculus and alteration of the fronto-limbic rsFC, effects that are partly related to improvements in depressive symptoms, as measured by the Hamilton Rating Scale for Depression (HAM-D) and improvements in verbal episodic memory as well as modulation of neural mechanisms underlying impaired cognition with MDD. These clinical effects were accompanied by the maintenance of a healthy and diverse microbiota with an increase in specific health-related taxa.
With the same mixture of two different strains, the studies of Heidarzadeh-Rad et al. [43] and Romijn et al. [50] presented differing results. The former [43] showed a beneficial effect in improving depression scores compared to placebo. However, the latter [50] found no significant difference between the probiotic and placebo groups in any psychological improvement outcome.
The probiotic strain PS128 significantly improved the severity of depression in patients with MDD [41] and showed significant decreases in Beck’s Depression Inventory-II scores, fatigue levels, brain wave activity and awakenings during the deep sleep phase [44]. However, markers of inflammation, gut permeability and gut microbiota composition [41] and improved ANS functionality [44] did not change significantly.
Finally, the results of Chawan et al. [40] with the intervention of nine probiotic strains showed a significantly greater reduction in cognitive reactivity compared to the placebo group, especially in the mild to moderate subgroup, as did the results from the work of Lee et al. [45], where adults with subclinical symptoms of depression, anxiety and insomnia who consumed the NVP-1704 strain experienced a significant reduction in depressive symptoms after four and eight weeks of treatment, and in anxiety symptoms after four weeks, as well as a decrease in serum interleukin-6 (IL6) levels and an improvement in gut microbiota composition.
Other study by Freijy et al. [42], using a symbiotic combination of eight probiotic strains and prebiotics or only probiotics, showed no beneficial results on depressive symptoms. Only the prebiotic-rich diet was beneficial in patients with moderate depressive disorders.
4. Discussion
The study of the relevance/obsolescence of the chosen topic is quite pertinent and interesting. Out of the total amount of documents retrieved for this review, the majority were published in the last five years; the Burton–Kebler index (BKI) and the Price index (PI), respectively, presented values below the average and above the corresponding values in the area of health sciences. This data demonstrate the full relevance of the topic under study. In health sciences, an IBK of 9 to 12 years and a PI of 33.33% would be expected. [57].
Moreover, according to the degree of evidence and recommendation of the Scottish Intercollegiate Guidelines Network (SIGN) Grading Review Group [58], randomized controlled clinical trials are those that provide the most scientific evidence due to their consistent cause–effect relationship. The quality assessment of the studies included in this review using CONSORT was acceptable, with a mean of 14.8 out of 25. Therefore, the grade of recommendation was B (moderate evidence that the measure is effective and the benefits outweigh the harms).
Furthermore, English is the language of choice for the publication of most articles, which has become common in recent years since publishing in a different language is negative for the impact factor and citations of articles [59]. Moreover, the number of English-language journals available in the databases is currently very high [60].
The Comprehensive Mental Health Action Plan 2013–2030 presents, as main objectives, the implementation of promotion and prevention strategies, the strengthening of information systems, scientific evidence and research [33]. In this research study, we aimed to find out whether the use of probiotics could be an effective contributor to such a promotion and prevention strategy, as well as to improvements in depressive disorders. The 18 selected studies focused on various pathologies, such as anxiety, insomnia and depression. Most of these studies focus on the sick population and therapeutic intervention [40,41,42,43,44,45,47,48,50,51,54,55], while other studies, in a lesser amount, focus on prevention in healthy subjects [38,39,46,49,52,53] of working age.
The systematic review and meta-analysis by Huang et al. [61] confirmed that oral probiotic intake in workers under 60 years of age can effectively reduce scores for depression (Hamilton Rating Scale for Depression HAM-D). In addition, these scores were also improved in the different depressive stages of both healthy subjects and those with depressive symptoms. These data suggest, as concluded in the meta-analysis, that probiotics may be recommended for use in both non-depressed people and patients with depressive disorders. These results occur in the works of Bagga et al. [38], Nishida et al. [46], Smith-Ryan et al. [53] and Bloemendaal et al. [39]. However, the studies of Rode et al. [49], Romjin et al. [50], Siegel et al. [52] and Freijy et al. [42] did not show a significant reduction in improvements in depressive disorders.
The fact that, in the analyzed studies in this exploratory review, at least 28 different strains of bacteria were used successfully, either alone [41,44,46] or in different combinations [38,39,40,43,47,48,51,53,54,55], suggests that not only one specific strain or a single combination of probiotics will work but that many combinations may be a better treatment option. The use of different probiotic formulations, with no specific strain [52] or two [50] or three [49] probiotic strains alone or high doses of prebiotics [42] may account for the different results observed in these studies concerning improvements in depressive disorders. In selecting the appropriate combination of strains, important factors have to be taken into account, such as the fact that not all combinations are capable of obtaining positive results [32]. In this regard, the combination of Lactobacillus helveticus R0052 (CNCM-I-1722), Lactiplantibacillus plantarum R1012 (CNCM-I-3736) and Bifidobacterium longum R0175 (CNCM-I-3470) [49,50] had barely any effect on improving sleep patterns, depression or on serotonin concentration (biomarker of the gut–microbiota–brain axis). There were also no significant positive results in reducing stress, depression or anxiety with the Bifidobacterium longum intervention [52]. For the very young adult population included in this study, with an average age of 19.5 years, the use of a single species and the fact that the intervention lasted only seven days could be the reason for these weak results. In addition, the dosage used, lifestyle factors and the duration of the intervention could further influence the interpretation of the results [32].
Most of the studies included in this review used a combination of Lactobacillus, Bifidobacterium and/or S. thermophilus or Lactococcus lactis strains. The interventions with probiotic strains in healthy subjects [38,39,46,53] that showed positive results were L. casei W56, L. paracasei W20, L. salivarius W24, L. plantarum W62, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24 Lactococcus lactis W19, Lactococcus lactis W58 and heat-inactivated L. gasseri CP2305 and B. lactis W51, B. lactis W52 and B. bifidum W23. Although the results of the selected studies are not homogeneous, it is once again corroborated that communication between the gut microbiota and the brain is a dynamic process, which can be modulated by a specific (probiotic) intervention, leading to changes in behaviour and brain function [38], and is associated with changes in frontal brain regions during cognitive control and with a microbiota increase in beneficial bacteria such as Ruminococcaccae_UCG-003 [39]. These results are in line with those obtained in clinical studies by Steenbergen et al. [62] and Papalini et al. [63], where a positive correlation was observed between intervention with these probiotic strains and cognition in difficult situations induced by acute stress. These probiotic strains also showed improvements in anxiety and fatigue reduction in female shift workers when combined with the prebiotic W117 [53].
Of particular note is the intervention with the heat-inactivated strain L. gasseri CP2305 and its benefits on young students under stress, with a 24-week treatment period [46]. Similar results were obtained in another study with the same inactivated strain, also including young chronically stressed students and with an intervention period of only 12 weeks [64].
On the one hand, intervention with the combination of 10 probiotic strains together with vitamin B7 in subjects with MDD [47,48] and in people with moderate to severe levels of depressive disorders [40], as well as the L. reuteri NK33 and B. adolescentis NK98 in subjects with subclinical symptoms of depression, anxiety and insomnia [45], improved the plasma levels of proinflammatory cytokines such as IL-6. The latter plays an important role in the pathogenesis of depression, and higher levels were associated with a poor prognosis and worse illness outcome [65,66], as well as with an improvement in negative thought patterns (cognitive reactivity) [40].
Other studies provide results of probiotic intervention on gut microbiota. In particular, an increase in potentially beneficial bacteria, such as Ruminococcus gauvreauii and Coprococcus 3 [47,48], and a decrease in proteobacteria [45] are reported.
Similarly, a combination of eight probiotic strains (Streptococcus thermophilus NCIMB 30438, B. breve NCIMB 30441, B lactis NCIMB 3043, B.lactis NCIMB 30436, L. acidophilus NCIMB 30442, L. plantarum NCIMB 30437, L. paracasei NCIMB 30439 and L helveticus NCIMB 30440), together with antidepressant medication, presented positive results correlating neuronal protection with improvements in depression symptoms (HAM-D), together with changes in bacterial diversity and an increase in healthy bacterial taxa of the gut microbiota. Such probiotics could be a useful additional treatment in MDD or in subjects with symptoms of depression, anxiety and insomnia, due to their anti-inflammatory effects [15,30], and this reinforces the potential microbiota-related treatment approach as one of the accessible therapies in disorders within the sphere of depression.
The studies evaluating the effect of the probiotic composition L. helveticus R0052 (CNCM strain I-1722) and B. longum R0175 (CNCM strain I-3470) presented contradictory results. While in the study by Heidarzadeh-Rad et al. [43], this combination produced beneficial effects on Beck’s Depression Inventory (BDI) scores, in the work of Romjin et al. [50], no positive results were obtained with respect to depressive symptomatology. Similarly, the addition of the probiotic strain L. plantarum R1012 to this probiotic composition [49] in healthy subjects also showed no positive results on depression scores, sleep patterns or gut–brain interactions. These results may suggest that these probiotic strains have a greater effect in patients with MDD than in patients with mild symptomatology.
B. bifidum (Bb-06), B animalis subsp. lactis (HN019), B. longum (R0175), L. acidophilus (La-14), L. helveticus (R0052), L. casei (Lc-11), L. plantarum (Lp-115) and L. rhamnosus (HN001) were used as another composition in a study by Freijy et al. [42], where the results showed improvements in mood, anxiety and sleep disorders in adults without a clinical history with the intervention of a prebiotic-rich diet, while the symbiotic mixture (prebiotic plus probiotic) or probiotic alone did not improve mild to moderate psychological disorders in patients with depression. These results should be viewed with caution, as this was not a double-blind study and, therefore, the participants and researchers were not masked in the allocation of the dietary intervention, which could have influenced how participants perceived or reported symptoms, an important bias to consider when assessing the results and information collected in the different forms of Total Mood Disturbances (TMDs), Beck Anxiety Inventory (BAI), etc.
The results of the present review are limited by the shortcomings inherent to each reviewed study [67]. For example, the probiotics selected in the reference papers, as well as the dosage and duration of treatment, are not the same, and other interferences, such as diet and medication, could also have affected the results. Another possible limitation is the small number of articles [44,53] found that specifically assess the impact on occupational health of probiotic intervention on people under stress or with depressive disorders. The depression assessment forms chosen by the different selected studies are not always the same. Moreover, the population studied was not homogeneous (different countries, different diet and different microbiota, shift workers, full-time and part-time workers, students, with mild to moderate and severe depressive disorders, with and without medication, etc.) and, in some cases, the monitoring of the population does not allow the results to be interpreted as strongly as expected.
As a conclusion of this review, and because occupational health is responsible, among other objectives, for the prevention of occupational illnesses among workers, such as anxiety, depression, insomnia, etc., caused by their working conditions [68], these inconclusive results should reinforce the interest of future research on probiotics, and the existing knowledge can serve as a starting point for the selection of strains with functional properties in improvements in depressive disorders. The combination of some probiotic strains that have shown a benefit in mental health could be considered as an adjuvant therapy in the management of mental disorders.
5. Conclusions
Despite the heterogeneity of the results obtained in the studies included in this review, probiotics may provide an effective adjuvant therapeutic tool to improve mental disorders in adults within the framework of public health. There were virtually no adverse effects in the populations analyzed.
For all the reasons presented throughout this study, we suggest that a combination that includes some probiotic strains, Lacticaseibacillus casei W56 Lactobacillus acidophilus W22, Lactobacillus paracasei W20, Ligilactobacillus salivarius W24, Lactiplantibacillus plantarum W62, Lactobacillus acidophilus NCIMB 30442, Lactiplantibacillus plantarum NCIMB 30437, Lactobacillus paracasei NCIMB 30439, Lactobacillus helveticus NCIMB 30440, L. acidophilus W37, Lactobacillus brevis W63, Limosilactobacillus reuteri NK33, Lactiplantibacillus plantarum PS128, Lactococcus lactis W19, Lactococcus lactis W58, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Bifidobacterium bifidum W23, Bifidobacterium breve NCIMB 30441, Bifidobacterium adolescentis NK98, Bifidobacterium lactis NCIMB 30435 and Bifidobacterium lactis NCIMB 30436, should be tested on workers and people of working age with depressive symptomatology using large-scale clinical trials to assess their efficacy in improving their illness and to identify the best dosage for treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15163551/s1, Table S1: PRISMA checklist for scoping review.
Author Contributions
Conceptualization, E.S.-S., V.N.-L. and J.A.P.-M.; methodology, E.S.-S. and J.A.P.-M.; validation, J.A.P.-M. and V.N.-L.; investigation, E.S.-S. and J.A.P.-M.; writing—original draft preparation, E.S.-S. and J.A.P.-M.; writing—review and editing, E.S.-S., J.A.P.-M. and V.N.-L.; visualization, R.A.T., A.M.-O. and A.H.-T.; supervision, R.A.T., A.M.-O. and A.H.-T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to the Department of Pharmacology, Paediatrics and Organic Chemistry for letting me and supporting me in the realization of this research work. Thanks to the Mibiopath Research Group for their knowledge in the area of microbiota/probiotics and for allowing me to learn with them. Thanks to the MATCH R&D department for their review and comments on the achievement of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Occupational Health. Available online: https://www.who.int/health-topics/occupational-health (accessed on 17 June 2023).
- Occupational Health (Safety and Health at Work). Available online: https://www.ilo.org/global/topics/safety-and-health-at-work/areasofwork/occupational-health/lang--en/index.htm (accessed on 17 June 2023).
- Mental Health at Work. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-health-at-work (accessed on 17 June 2023).
- International Classification of Diseases (ICD). Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 17 June 2023).
- Salvagioni, D.A.J.; Melanda, F.N.; Mesas, A.E.; González, A.D.; Gabani, F.L.; de Andrade, S.M. Physical, Psychological and Occupational Consequences of Job Burnout: A Systematic Review of Prospective Studies. PLoS ONE 2017, 12, e0185781. [Google Scholar] [CrossRef] [PubMed]
- Depresión-OPS/OMS|Organización Panamericana de la Salud. Available online: https://www.paho.org/es/temas/depresion (accessed on 17 June 2023).
- Depresión. Available online: https://www.who.int/es/news-room/fact-sheets/detail/depression (accessed on 17 June 2023).
- GBD Results. Available online: https://vizhub.healthdata.org/gbd-results (accessed on 15 June 2023).
- Salud Ocupacional: Los Trabajadores de la Salud. Available online: https://www.who.int/es/news-room/fact-sheets/detail/occupational-health--health-workers (accessed on 17 June 2023).
- Heerlein, A. Tratamientos Farmacológicos Antidepresivos. Rev. Chil. Neuro-Psiquiatr. 2002, 40, 21–45. [Google Scholar] [CrossRef]
- Benedí, J.; Romero, C. Antidepresivos. Farm. Prof. 2005, 19, 76–81. [Google Scholar]
- Beck, A.T.; Haigh, E.A.P. Advances in Cognitive Theory and Therapy: The Generic Cognitive Model. Annu. Rev. Clin. Psychol. 2014, 10, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R. The Effects of Probiotics on Depressive Symptoms in Humans: A Systematic Review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, K.S. Treatment-Resistant Depression: Therapeutic Trends, Challenges, and Future Directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and Mental Health: A Review. Brain. Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- De la Fuente del Rey, M.; González-Pinto, A.; Pérez Miralles, F.C. Documento de Consenso Sobre la Microbiota y el Uso de Probióticos/Prebióticos en Patologías Neurológicas y Psiquiátricas; 1a.; Neuraxpharm: Barcelona, Spain, 2021; ISBN 978-84-17844-98-1. [Google Scholar]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Barrio, C.; Arias-Sánchez, S.; Martín-Monzón, I. The Gut Microbiota-Brain Axis, Psychobiotics and Its Influence on Brain and Behaviour: A Systematic Review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef]
- Rathour, D.; Shah, S.; Khan, S.; Singh, P.K.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Role of Gut Microbiota in Depression: Understanding Molecular Pathways, Recent Research, and Future Direction. Behav. Brain Res. 2023, 436, 114081. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization (Eds.) Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. In FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Zhou, L.; Foster, J.A. Psychobiotics and the Gut–Brain Axis: In the Pursuit of Happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics Drive Gut Microbiome Triggering Emotional Brain Signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic Supplementation Can Positively Affect Anxiety and Depressive Symptoms: A Systematic Review of Randomized Controlled Trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Maleki, V.; Behrooz, M.; Ranjbar, F.; Ebrahimi-Mameghani, M. Can Psychobiotics “Mood” Ify Gut? An Update Systematic Review of Randomized Controlled Trials in Healthy and Clinical Subjects, on Anti-Depressant Effects of Probiotics, Prebiotics, and Synbiotics. Clin. Nutr. Edinb. Scotl. 2020, 39, 1395–1410. [Google Scholar] [CrossRef]
- Grumet, L.; Tromp, Y.; Stiegelbauer, V. The Development of High-Quality Multispecies Probiotic Formulations: From Bench to Market. Nutrients 2020, 12, 2453. [Google Scholar] [CrossRef]
- WHO Comprehensive Mental Health Action Plan 2013–2020–2030. Available online: https://www.who.int/initiatives/mental-health-action-plan-2013-2030 (accessed on 17 June 2023).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Sanz-Valero, J. Systematic Reviews in Nutrition: Standardized Methodology. Br. J. Nutr. 2012, 107 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Aigner, C.S.; Reichert, J.L.; Cecchetto, C.; Fischmeister, F.P.S.; Holzer, P.; Moissl-Eichinger, C.; Schöpf, V. Influence of 4-Week Multi-Strain Probiotic Administration on Resting-State Functional Connectivity in Healthy Volunteers. Eur. J. Nutr. 2019, 58, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Bloemendaal, M.; Szopinska-Tokov, J.; Belzer, C.; Boverhoff, D.; Papalini, S.; Michels, F.; van Hemert, S.; Arias Vasquez, A.; Aarts, E. Probiotics-Induced Changes in Gut Microbial Composition and Its Effects on Cognitive Performance after Stress: Exploratory Analyses. Transl. Psychiatry 2021, 11, 300. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut Feelings: A Randomised, Triple-Blind, Placebo-Controlled Trial of Probiotics for Depressive Symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Chen, H.-M.; Kuo, P.-H.; Hsu, C.-Y.; Chiu, Y.-H.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H. Psychophysiological Effects of Lactobacillus Plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. [Google Scholar] [CrossRef] [PubMed]
- Freijy, T.M.; Cribb, L.; Oliver, G.; Metri, N.-J.; Opie, R.S.; Jacka, F.N.; Hawrelak, J.A.; Rucklidge, J.J.; Ng, C.H.; Sarris, J. Effects of a High-Prebiotic Diet versus Probiotic Supplements versus Synbiotics on Adult Mental Health: The “Gut Feelings” Randomised Controlled Trial. Front. Neurosci. 2023, 16, 2274. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-Derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef]
- Ho, Y.-T.; Tsai, Y.-C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus Plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.-K.; Kim, D.-H.; Jang, S.W.; Han, S.-W.; Yoon, I.-Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus Gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression-A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Reiter, A.; Bengesser, S.A.; Hauschild, A.-C.; Birkl-Töglhofer, A.-M.; Fellendorf, F.T.; Platzer, M.; Färber, T.; Seidl, M.; Mendel, L.-M.; Unterweger, R.; et al. Interleukin-6 Gene Expression Changes after a 4-Week Intake of a Multispecies Probiotic in Major Depressive Disorder—Preliminary Results of the PROVIT Study. Nutrients 2020, 12, 2575. [Google Scholar] [CrossRef]
- Rode, J.; Edebol Carlman, H.M.T.; König, J.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Brummer, R.J. Multi-Strain Probiotic Mixture Affects Brain Morphology and Resting State Brain Function in Healthy Subjects: An RCT. Cells 2022, 11, 2922. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A Double-Blind, Randomized, Placebo-Controlled Trial of Lactobacillus Helveticus and Bifidobacterium longum for the Symptoms of Depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Schaub, A.-C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, Gut Microbial and Neural Effects of a Probiotic Add-on Therapy in Depressed Patients: A Randomized Controlled Trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Conklin, S. Acute Intake of B. Longum Probiotic Does Not Reduce Stress, Anxiety, or Depression in Young Adults: A Pilot Study. Brain Behav. Immun.-Health 2020, 2, 100029. [Google Scholar] [CrossRef] [PubMed]
- Smith-Ryan, A.E.; Mock, M.G.; Trexler, E.T.; Hirsch, K.R.; Blue, M.N.M. Influence of a Multistrain Probiotic on Body Composition and Mood in Female Occupational Shift Workers. Appl. Physiol. Nutr. Metab. 2019, 44, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Yamanbaeva, G.; Schaub, A.-C.; Schneider, E.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Mählmann, L.; Brand, S.; Beglinger, C.; Borgwardt, S.; et al. Effects of a Probiotic Add-on Treatment on Fronto-Limbic Brain Structure, Function, and Perfusion in Depression: Secondary Neuroimaging Findings of a Randomized Controlled Trial. J. Affect. Disord. 2023, 324, 529–538. [Google Scholar] [CrossRef]
- Schneider, E.; Doll, J.P.K.; Schweinfurth, N.; Kettelhack, C.; Schaub, A.-C.; Yamanbaeva, G.; Varghese, N.; Mählmann, L.; Brand, S.; Eckert, A.; et al. Effect of Short-Term, High-Dose Probiotic Supplementation on Cognition, Related Brain Functions and BDNF in Patients with Depression: A Secondary Analysis of a Randomized Controlled Trial. J. Psychiatry Neurosci. JPN 2023, 48, E23–E33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Tomás-Casterá, V.; Sanz-Valero, J.; Juan-Quilis, V. Estudio Bibliométrico de La Producción Científica y de Consumo de Las Revistas Sobre Nutrición Indizadas En La Red SciELO. Nutr. Hosp. 2013, 28, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Elliott, H.; Hillside, C. (Eds.) A Guideline Developer’s Handbook, 2011th ed.; Scottish Intercollegiate Guidelines Network: Edinburgh, UK, 2011; Volume 1, ISBN 978-1-905813-25-4. [Google Scholar]
- Franco-López, A.; Sanz-Valero, J.; Culebras, J.M. El factor de impacto ya no es el patrón oro; la declaración de San Francisco sobre evaluación de la investigación. JONNPR 2017, 2, 173–176. [Google Scholar] [CrossRef]
- Sanz-Valero, J.; Wanden-Berghe, C. Análisis bibliométrico de la producción científica, indizada en MEDLINE, sobre los servicios de salud proporcionados por las unidades de hospitalización a domicilio. Hosp. Domic. 2017, 1, 21–34. [Google Scholar] [CrossRef][Green Version]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain. Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress Matters: Randomized Controlled Trial on the Effect of Probiotics on Neurocognition. Neurobiol. Stress 2019, 10, 100141. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily Administration of Paraprobiotic Lactobacillus Gasseri CP2305 Ameliorates Chronic Stress-Associated Symptoms in Japanese Medical Students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a Keystone Cytokine in Health and Disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Stewart, R.; Kim, J.-W.; Kang, H.-J.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; Yoon, J.-S. Changes in Pro-Inflammatory Cytokine Levels and Late-Life Depression: A Two Year Population Based Longitudinal Study. Psychoneuroendocrinology 2018, 90, 85–91. [Google Scholar] [CrossRef]
- Domingo-Pueyo, A.; Sanz-Valero, J.; Wanden-Berghe, C. Efectos Sobre La Salud de La Exposición Laboral al Cromo y Sus Compuestos: Revisión Sistemática—Effects of Occupational Exposure to Chromium and Its Compounds: A Systematic Review. Arch. Prev. Riesgos Laborales 2014, 17, 142–153. [Google Scholar] [CrossRef]
- Whole Person Health: What You Need to Know. Available online: https://www.nccih.nih.gov/health/whole-person-health-what-you-need-to-know (accessed on 2 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).