Abstract
(1) Background: The effect of cinnamon on the regulation of glycolipid levels in type 2 diabetic patients is still controversial, and there is a lack of research on the dose–response relationship between cinnamon and glycolipid indicators in type 2 diabetic patients. (2) Methods: This dose–response meta-analysis was performed to explore the effect of the cinnamon intervention on glycolipid metabolism. We conducted a comprehensive database search for literature published before November 2022. Nonlinear models were used for dose–response relationship analysis. (3) Results: We identified that a cinnamon intervention was effective in controlling triglyceride (TG) levels (mean difference = −7.31; 95%CI: −12.37, −2.25, p = 0.005) and low-density lipoprotein cholesterol (LDL-C) levels (mean difference = −6.78; 95%CI: −11.35, −2.22, p = 0.004) in type 2 diabetic patients; however, it also was able to increase high-density lipoprotein cholesterol (HDL-C) levels in patients with type 2 diabetes (mean difference = 1.53; 95%CI: 1.01, 2.05, p < 0.001). However, the cinnamon intervention had no significant effect on the level of fasting blood glucose, glycated hemoglobin (HbA1c), or total cholesterol (TC) levels. We found a significant effect of the cinnamon intervention dose on the TG level (p-nonlinearity = 0.016) and LDL-C (p-nonlinearity = 0.019) in the nonlinear dose–response analysis. In the subgroup analysis, we found a hypoglycemic effect with the cinnamon dose ≤1200 mg (mean difference = −11.1, 95%CI: −14.64, −7.58, p < 0.001). (4) Conclusion: Cinnamon intervention may be beneficial in lowering TG and LDL-C levels while enhancing HDL-C levels, and the dosage of the intervention was an important factor in influencing the TG and LDL-C levels.
1. Introduction
Type 2 diabetes is defined as an ongoing decrease in beta-cell insulin production in the context of insulin resistance that develops over time and accounts for 90% to 95% of all diabetes cases [1]. The chronic hyperglycemia of diabetes causes a gradual progression from chronic damage to failure of many vital organs in the body, including serious damage to the eyes, kidneys, heart, and other organs [2]. It was estimated in 2019 that diabetes adversely affects the health status and quality of life of approximately 9.3% of the global population and is anticipated to climb to 10.2% (578 million people) and 10.9% (700 million people) by 2030 and 2045, respectively [3]. Current treatment options for diabetes include non-insulin antidiabetic drug therapy, insulin and insulin analogues therapy, and a combination of insulin and oral hypoglycemic drugs [4]. Although most anti-diabetic drugs show beneficial effects either used alone or in combination, they also have many negative effects; therefore, one of the primary priorities in fighting against this disease is discovering the ideal treatment.
Cinnamon bark is one of the most widely used spices globally, not only in cooking but also as a traditional herbal. Cinnamaldehyde and trans-cinnamaldehyde (Cin) are major important components of cinnamon, both of which are present in cinnamon essential oil [5]. It was found that cinnamaldehyde exerts its anti-hyperglycemic effect by stimulating insulin release, and the mechanisms of action might be similar to that of glibenclamide [6]. Notably, cinnamon-derived water-soluble polyphenol polymers could enhance insulin-dependent in vitro glucose metabolism by approximately 20-fold. Such an effect was thought to be related to the antioxidant activity of the polymers [7].
Diabetes is closely associated with cardiovascular diseases. On the other hand, factors contributing to cardiovascular risks, such as obesity, hypertension, and dyslipidemia, are common in diabetes, and cardiovascular disease is still the primary cause of death in diabetics [8]. Although cinnamon has been reported to have some positive impacts on glucose and lipid metabolism in diabetic individuals, as well as the potential to be employed in the treatment of cardiovascular illnesses, more research is needed to confirm these findings [9]. Especially when used as a therapeutic or complementary medicine [10,11,12], the potential metabolic benefits of cinnamon are still debatable [13]. Collectively, this study systematically reviewed the effects of cinnamon intervention on glucolipid metabolic indexes in type 2 diabetics and added the analysis of dose–response relationships, which sought to provide a more comprehensive understanding of the role of cinnamon in regulating glucolipid metabolism in type 2 diabetics.
2. Materials and Methods
This study was planned in conformity with the Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 recommended guidance.
2.1. Literature Search Strategy
The initial literature search was systematic and comprehensive, all retrieved literature was published before November 2022, and the types of studies covered by the literature were randomized controlled trials. Databases searched included Web of Science, PubMed, the Cochrane Library, and Embase. To conduct the search, terms from titles or abstracts were combined with MeSH. The terms included “Type 2 diabetes OR diabetes mellitus/typeIIOR type 2 diabetes mellitus OR type 2 diabetes OR Diabetes” for the population, and “Cinnamomum zeylanicum OR Cinnamomum verum OR Cinnamon OR Cinnamons” was used for interventions. The references listed in the retrieved publications were also evaluated to locate more relevant studies. Endnote X9 was used for document management.
2.2. Inclusion and Exclusion Criteria
Clinical trials that match the criteria given below were included: (1) The study was developed as a randomized clinical trial to look into the effects of cinnamon on blood glucose and lipid metabolism in type 2 diabetic patients; (2) At least one of the following outcome variables should be included in the study: glycosylated hemoglobin (HbA1c), fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG); and (3) Insulin was not used during the clinical trial. Any clinical trial that fit one of the following descriptions was excluded: (1) the study looked at the biological benefits of cinnamon in addition to its hypoglycemic and lipid-lowering effects; (2) previous consumption of cinnamon in any form; (3) interventions other than cinnamon were used; and (4) the cinnamon intervention was combined with other food supplements.
2.3. Data Extraction
Papers that matched the criteria were eventually included, and two researchers independently assessed and retrieved data from them. The extracted information includes the first author’s last name, publication year, the sample size for each group, country, age range, dosage (mg/d), follow-up period, and type of medication taken. In addition, the standard deviation, and net mean change of fasting blood glucose, glycosylated hemoglobin, total cholesterol, triglycerides, and high-density lipoprotein low-density lipoprotein in the literature were also extracted. For the trial evaluating two different doses of cinnamon, the results were analyzed as two separate trials.
2.4. Quality Assessment
The following criteria were used to gauge the quality of the included literature using the Cochrane Collaborative Risk of Bias Tool: (1) creation of random sequences, (2) hiding of allocation, (3) application of blinding to participants and staff, (4) blinding of the result evaluation, (5) inadequate outcome data, (6) selectively reporting outcomes, and (7) additional potential risk biases.
2.5. Data Analysis
The International System of Units (IFCC) of glycosylated hemoglobin (mmol/mol) was converted to traditional units (NGSP) (%) using the equation: NGSP = 0.0915 × IFCC + 2.15%. Different units of blood glucose levels and lipid levels were translated to mg/dL (1 mmol/L of TC, LDL-C, and HDL-C was translated to 38.7 mg/dL; 1 mmol/L of TG was translated to 88.5 mg/dL;1 mmol/L FBG was translated to 18 mg/dL). We extracted and organized the data from the literature and then imported them into Stata SE 15.0 software for processing and analysis.
Mean changes from baseline in lipid parameters, blood glucose levels, and glycated hemoglobin levels were considered continuous variables. If changes were not directly reported, calculations were performed using baseline and endpoint data to extrapolate the mean change in outcome from the test.
The threshold for statistical significance was fixed at 0.05. I2 statistics measured statistical heterogeneity. In general, fixed effects models are used for analysis, when I2 > 50%, indicating high heterogeneity, the random effects model was adopted. To identify sources of heterogeneity, we analyzed subgroups based on dosage and period of cinnamon intervention.
Sensitivity analysis was utilized to evaluate how specific trials affected the outcome of the study as a whole. To avoid publication bias affecting the authenticity and reliability of the study results, funnel plots were drawn and Egger tests were performed. Wherever the bias existed, the “trim and fill” procedure was used to repair it. In a nonlinear dose–response analysis, fractional polynomial models were used to assess the unique quantitative-effect relationships of cinnamon intervention dose (mg/day) and intervention duration (weeks) in relation to intervention effects [14].
3. Results
3.1. Literature Selection Process
After searching four databases, 2129 relevant papers were retrieved, of which, 529 duplicated literatures were excluded. Based on the titles and abstracts, 1567 irrelevant papers were excluded. The qualification review covered a total of 33 pieces of literature, of which, 20 were excluded for the following reasons: (1) incomplete data; (2) full text unavailable; (3) cinnamon was used in combination with other drugs; and (4) placebo was not used in the control group. In the end, a total of 14 pieces of literature with 15 trials were used for the analysis. Figure 1 depicts the complete flow diagram of the selection process.
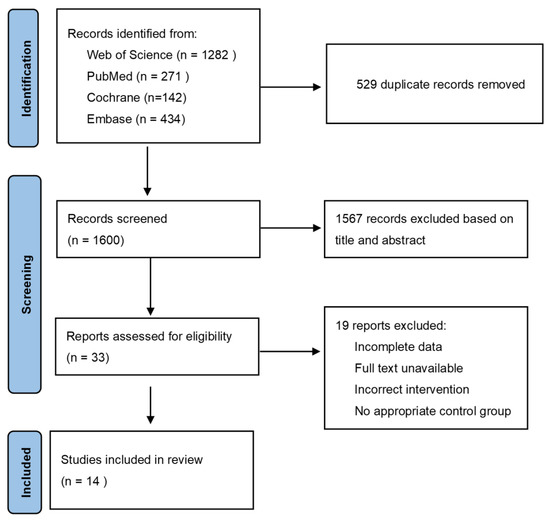
Figure 1.
PRISAM flaw chart of selected trails.
3.2. Study Characteristics
The essential features of the studies examined are summarized in the studies involved in this meta-analysis were published between 2006 and 2021 and involved 965 participants in China, Thailand, the United States, the United Kingdom, Germany, the Netherlands, Israel, and Iran. The mean age of subjects in each trial varied, with the vast majority being in the middle-aged to older age group of 52.1 to 64.4 years. Intervention periods of cinnamon ranged from 6 weeks to 16 weeks, with doses ranging from 120 mg/d to 3000 mg/d (Table 1).

Table 1.
Baseline characteristics and interventions of the studies included.
3.3. Results of Meta-Analysis
3.3.1. Effects of Cinnamon on the Related Indexes of Glucose Metabolism
The study of fasting blood glucose levels comprised 12 eligible studies with 13 effect sizes. The effect of the cinnamon intervention on FBG levels was not statistically significant (mean difference = −4.95; 95%CI: −11.27, 1.36, p = 0.124, I2 = 76.1%, Figure 2A). Furthermore, the nonlinear dose–response relationship analysis revealed that the dosage (p-nonlinearity = 0.180, Figure 3A) and duration (p-nonlinearity = 0.420, Figure 4A) of cinnamon supplementation showed no significant effect on the FBG level.
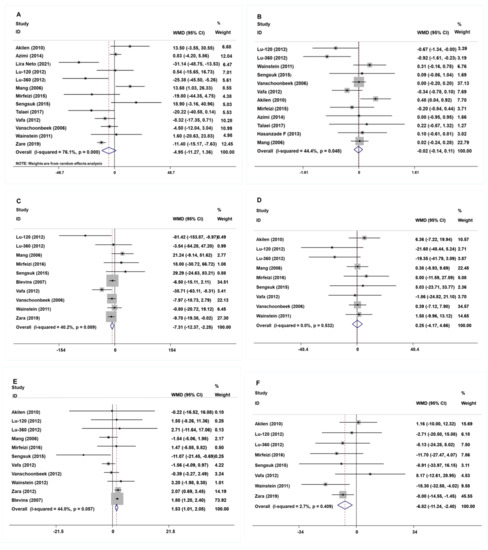
Figure 2.
The effects of cinnamon on (A) FBG, (B) HbA1c, (C) TG, (D) TC, (E) HDL-C, and (F) LDL-C. FBG = fasting blood glucose; HbA1c = Glycosylated hemoglobin; TG = Triglyceride; TC = Total cholesterol; HDL-C = High-density lipoprotein cholesterol; LDL-C = Low-density lipoprotein cholesterol [15,16,17,18,19,20,21,22,23,24,25,26,27,28].
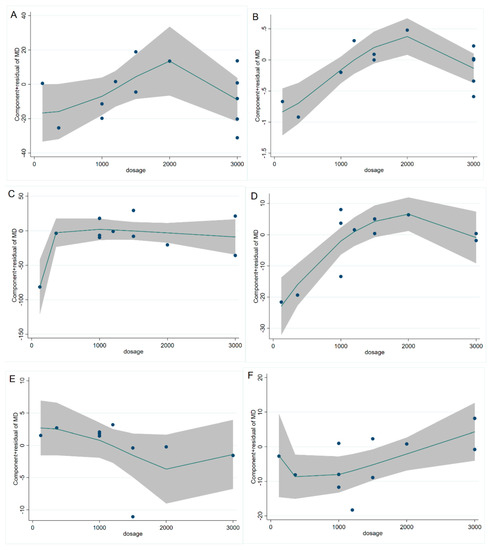
Figure 3.
Cinnamon dosage (mg) and unstandardized mean difference (mg/dL) in (A) FBG, (B) HbA1c, (C) TG, (D) TC, (E) HDL-C, and (F) LDL-C. FBG = fasting blood glucose; HbA1c = Glycosylated hemoglobin; TG = Triglyceride; TC = Total cholesterol; HDL-C = High-density lipoprotein cholesterol; LDL-C = Low-density lipoprotein cholesterol.
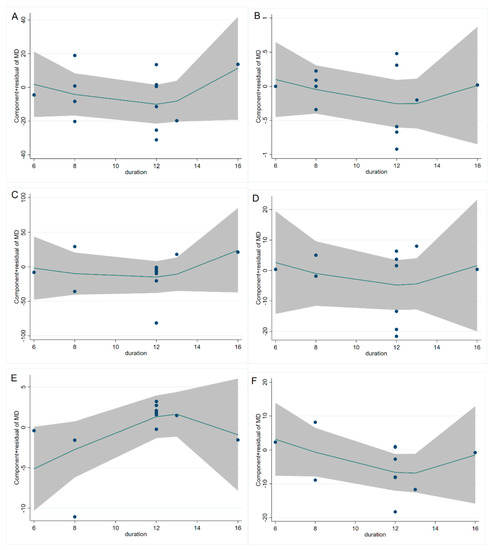
Figure 4.
Cinnamon duration (week) and unstandardized mean difference (mg/dL) in (A) FBG, (B) HbA1c, (C) TG, (D) TC, (E) HDL-C, and (F) LDL-C. FBG = fasting blood glucose; HbA1c = Glycosylated hemoglobin; TG = Triglyceride; TC = Total cholesterol; HDL-C = High-density lipoprotein cholesterol; LDL-C = Low-density lipoprotein cholesterol.
Overall, the level of HbA1c was studied using 11 trials with 12 effect sizes; the results of the fixed effects model revealed that cinnamon intervention had no significant influence on HbA1c level (mean difference = −0.02; 95%CI: −0.14, 0.11, p = 0.801, I2 = 44.4%, Figure 2B). We found that cinnamon intake had no discernible impact on the level of HbA1c in the nonlinear dose–response study (p-nonlinearity = 0.159, Figure 3B), and found no discernible effect on the duration of cinnamon intervention (p-nonlinearity = 0.616, Figure 4B).
3.3.2. Effects of Cinnamon on Lipid Metabolism-Related Indexes
For the analysis of triglycerides, we included 10 effect sizes from 9 studies, and we found that cinnamon intervention caused a significant reduction in triglyceride levels (mean difference = −7.31; 95%CI: −12.37, −2.25, p = 0.005, I2 = 40.2%, Figure 2C). We noticed a significant non-linear relationship between cinnamon dose and triglycerides levels (p-nonlinearity = 0.016, Figure 3C); however, there was no significant non-linear relationship between the supplementation duration and TG level (p-nonlinearity = 0.536, Figure 4C).
The effect of cinnamon supplementation on the level of HDL-C was studied in 10 studies with 11 effect sizes, and we found that cinnamon supplementation had significant effects on HDL-C levels (mean difference = 1.53; 95%CI: 1.01, 2.05, p < 0.001, I2 = 44.0%, Figure 2E). Additionally, there was no effect of cinnamon dosage (p-nonlinearity = 0.336, Figure 3E) or duration (p-nonlinearity = 0.183, Figure 4E) on HDL-C levels.
To summarize the effect of cinnamon on the level of LDL-C, seven randomized controlled trials (eight effect sizes) were pooled and the results indicated a significant effect of cinnamon supplements on LDL-C concentration (mean difference = −6.78; 95%CI: −11.35, −2.22, p = 0.004, I2 = 2.7%, Figure 2F). In addition, we found a non-linear correlation between the dose of cinnamon intervention and LDL-C levels (p-nonlinearity = 0.019, Figure 3F); however, the non-linear correlation between duration and LDL-C levels was insignificant (p-nonlinearity = 0.527, Figure 4F).
To investigate whether cinnamon interventions improve the level of total cholesterol, eight eligible studies (nine effect sizes) were selected for analysis. The results suggested that cinnamon interventions were not effective in reducing the level of TC level (mean difference = 0.25; 95%CI: −4.17, 4.66, p = 0.913, I2 = 0.0%, Figure 2D). TC based on intervention dosage (p-nonlinearity = 0.002, Figure 3D) is affected in a non-linear manner. However, no significant correlation was found for intervention duration (p-nonlinearity = 0.788, Figure 4D).
3.4. Subgroup Analysis
When the heterogeneity is higher than 50%, the combined results may be unreliable, or the combined results themselves may be inappropriate, at which point we need to determine the source of heterogeneity by subgroup analysis, as indicated in Table 2. With respect to the effect of the cinnamon intervention on FBG, the heterogeneity among studies disappeared when we grouped them according to intervention dosage (I2 = 29.6%, p = 0.224). In these trials, cinnamon intervention ≤1200mg/d significantly reduced FBG levels (mean difference = −11.1; 95%CI: −14.64, −7.58, p < 0.001).

Table 2.
The results of subgroup analysis.
3.5. Publication Bias and Sensitivity Analysis
The Egger test and visual funnel plot test were utilized to determine if the research contained any publication bias. As illustrated in Figure 5, the visual funnel plot is roughly symmetrical, suggesting that no publication bias exists, and the Egger test result further confirms that there is no publication bias in FBG (p = 0.700), HbA1c (p = 0.537), TG (p = 0.842), TC (p = 0.355), HDL-C (p = 0.103), and LDL-C (p = 0.759). To ensure that the individual study did not significantly alter the overall study results, we eliminated each paper step-by-step. In this process, no single study was found to have a significant impact on the overall effect.
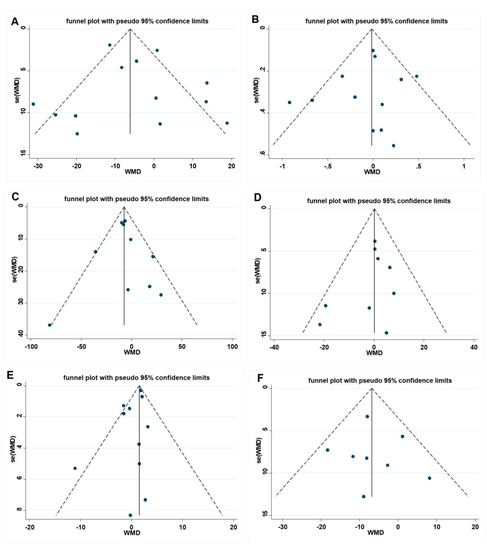
Figure 5.
Funnel plot and Egger test for assessing publication bias, (A) FBG Egger’s test (p = 0.699), (B) HbA1c Egger’s test (p = 0.576), (C) TG Egger’s test (p = 0.842), (D) TC Egger’s test (p = 0.233), (E) HDL-C Egger’s test (p = 0.103), and (F) LDL-C Egger’s test (p = 0.092). A = FBG, B = HbA1c, C = TG, D= TC, E = HDL-C, F = LDL-C. FBG= fasting blood glucose; HbA1c = Glycosylated hemoglobin; TG = Triglyceride; TC = Total cholesterol; HDL-C = High-density lipoprotein cholesterol; LDL-C = Low-density lipoprotein cholesterol.
4. Discussion
In this meta-analysis, we discovered that cinnamon intervention contributed to lower triglyceride and LDL-C levels while increasing HDL-C levels. In addition, we noted a decline in fasting blood glucose levels when the cinnamon dose was less than 1200 mg. The cinnamon intervention, however, had no effect on HbA1c or TC. In a non-linear dose–response relationship analysis, we found that the dose of cinnamon intervention had a significant influence on triglyceride and LDL-C levels.
Diabetes is manifested by chronic hyperglycemia caused by insufficient insulin secretion and/or insulin dysfunction. Previous studies have indicated that cinnamon and its extracts can increase insulin sensitivity and ameliorate insulin resistance [29,30]. It has been reported that aqueous extracts of cinnamon could act as dual activators of PPARγ/α and might serve as a substitute for PPARγ agonists in the control of obesity-related diabetes and hyperlipidemia [31]. B-type procyanidin C1 in the cinnamon extract also induces preadipocyte differentiation and functions as a possible enhancer of insulin action in mature adipocytes via the AKT-eNOS pathway [32]. In our study results, the use of cinnamon supplements did not improve the FBG of the subjects, which is different from the results of previous meta-analyses [10,33,34]. However, we found a noticeable drop in the level of FBG at an intervention dose of cinnamon ≤1200 mg in the sub-group analysis. Previous research found a paradoxical effect of the low-dose cinnamon interventions (doses ≤ 1200 mg) on the level of blood glucose, with some studies showing that low-dose cinnamon significantly reduced the level of FBG [20,22,28], while others showed no statistically significant reduction in FBG [27]. Clearly, the results of our analysis support the hypoglycemic effect of low-dose cinnamon, and the exact range of low doses needs to be explored in more well-designed trials in the future.
It has been shown that cinnamaldehyde, the main active ingredient in cinnamon, could improve glycated hemoglobin levels in STZ-induced diabetic rats despite the unclear mechanism [6]. However, cinnamaldehyde is unstable in the human body and may be metabolized to cinnamic acid and converted to cinnamyl alcohol and lose its effect; therefore, the stability of cinnamaldehyde is an important factor affecting its biological activity [35]. We found no significant change in glycosylated hemoglobin levels in the present study. Although some meta-analyses have shown that cinnamon interventions can reduce glycated hemoglobin levels [33,36], which might be due to different inclusion criteria in the literature, such as the inclusion of insulin-using populations, which may confound the actual effects of cinnamon due to the effects of insulin. In addition, the source and form of the cinnamon used can also make a difference in the results.
Type 2 diabetes is associated with a number of interconnected plasma lipid and lipoprotein abnormalities, including low-HDL cholesterol, a predominance of tiny LDL-C particles, and high triglycerides [37]. We found that cinnamon supplementation was effective in lowering LDL cholesterol and raising HDL cholesterol. Previous studies have shown that cinnamic acid could inhibit lipase activity in vitro [38]. The inhibitory effect of lipase activity also restricted the hydrolysis of dietary triglycerides from being absorbed by monoglycerides, whereas free fatty acids were absorbed in the intestine. This effect may help improve the lipid profile of the rats, ultimately resulting in a decrease in LDL-C levels and an increase in HDL-C levels [39].
Cinnamon supplementation was found to be beneficial in decreasing the level of TG in this investigation, but the effect on the total cholesterol level was not found. S-(+)-Linalool is the main component of the leaf essential oil of C. osmophloeum ct. Linalool, which significantly reduced blood TG levels and inhibited lipid accumulation by down-regulating 3T3-L1 lipids [40].
In addition, there was a non-linear dose–response association between cinnamon supplementation dosage and changes in TG and LDL-C levels. This suggests that the reduction in TG and LDL-C levels by cinnamon may rely on the dose of the intervention. Interestingly, our analysis yielded results that the cinnamon intervention did not change total cholesterol levels; yet, we observed a non-linear correlation between the dose of cinnamon intervention and the total cholesterol level. We speculate that this phenomenon is caused by the presence of covariance with other factors.
As of now, our study is the first dose–response analysis to explore the effect of the cinnamon intervention on glycolipid levels in type 2 diabetic patients. The findings of our study have a strong causal inference because only RCTs were used in it. In addition, we kept the heterogeneity of the literature included in the study at a low level by sensitivity analysis. However, we could not control the confounding factors among different studies. For example, the species of cinnamon, the form of cinnamon used, the type of medication used by the subjects, dietary habits, and dietary patterns.
According to our meta-analysis, we believe that a daily intake of cinnamon of less than 1200 mg can effectively reduce fasting blood glucose levels, which is an appropriate intervention dose, and the results of related studies also indicate that cinnamon intervention doses of less than 2 g per day or less than 1.5 g per day could exert a more significant effect on blood lipids and blood pressure [41,42]. This result provides new ideas for the design of future clinical trials in this direction, with the aim of finding effective and safe doses of cinnamon for improving glycolipid levels in diabetic patients. Although cinnamon is considered safe by the FDA, adverse reactions, such as gastric upset and rash, as well as itching, have been reported in previous studies [43,44]. Therefore, the dose should be controlled within the safe range when using cinnamon for population interventions.
Although the benefits of cinnamon for diabetic patients are undeniable, and it is inexpensive and has few adverse side effects, cinnamon is not yet sufficient to be a stand-alone treatment for controlling glucose and lipid levels in type 2 diabetic patients, not only because of the conflicting results shown in various clinical trials so far but also due to the unclear mechanisms. In addition, the hypoglycemic and hypolipidemic effects exhibited by cinnamon are not yet well linked to the underlying mechanisms. Future studies may consider exploring the mechanism of action of cinnamon in improving the glycolipid index of patients.
5. Conclusions
In conclusion, cinnamon supplementation was shown to improve some glycolipid indicators in diabetic patients. It significantly reduced the levels of TG and LDL-C, but elevated the level of HDL-C and reduced the level of FBG only at doses below 1200 mg in type 2 diabetic patients. In addition, we identified a dose–response relationship between cinnamon intervention and triglycerides and LDL-C, which may provide a reference for future trials.
Author Contributions
T.Y., X.C. and W.L. contributed to the conception and design of the systematic review and meta-analysis. T.Y., W.L. and H.X. were involved in the acquisition and analysis of the data. T.Y., K.L. and W.L. drafted this manuscript. H.X., S.W., G.S. and L.C. revised and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported by the National Natural Science Foundation of China (82103834) and the Fundamental Research Funds for the Central Universities. Wang Liao is the recipient of the Elite Scientists Sponsorship Program by CAST (2021QNRC001) and the Zhishan Young Scholar Award of the Southeast University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elliott, T.L.; Pfotenhauer, K.M. Classification and Diagnosis of Diabetes. Prim. Care 2022, 49, 191–200. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2012, 35, S64–S71. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.F.; Luo, C.Y.; Lin, C.Y.; Cheng, S.S.; Hsu, Y.R.; Chang, S.T. Methods for thermal stability enhancement of leaf essential oils and their main constituents from indigenous cinnamon (Cinnamomum osmophloeum). J. Agric. Food Chem. 2013, 61, 6293–6298. [Google Scholar] [CrossRef] [PubMed]
- Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde—A potential antidiabetic agent. Phytomedicine Int. J. Phytother. Phytopharm. 2007, 14, 15–22. [Google Scholar] [CrossRef]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D.J. Isolation and characterization of polyphenol type—A polymers from cinnamon with insulin-like biological activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Bornfeldt, K.E.; Goldberg, I.J. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021, 33, 1519–1545. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid.-Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Deyno, S.; Eneyew, K.; Seyfe, S.; Tuyiringire, N.; Peter, E.L.; Muluye, R.A.; Tolo, C.U.; Ogwang, P.E. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A meta-analysis and meta-regression. Diabetes Res. Clin. Pract. 2019, 156, 107815. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.R.; de Vas Gunawardana, N.; Katulanda, P. Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis. Diabet. Med. A J. Br. Diabet. Assoc. 2012, 29, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Bernardo, M.A.; Singh, J.; de Mesquita, M.F. Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes Obes. Metab. 2012, 14, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liao, W.; Xia, H.; Wang, S.; Sun, G. The Effect of Resveratrol on Blood Lipid Profile: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 3755. [Google Scholar] [CrossRef]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: A randomized, placebo-controlled, double-blind clinical trial. Diabet. Med. 2010, 27, 1159–1167. [Google Scholar] [CrossRef]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hariri, M.; Abbasi, B. Effects of Cinnamon, Cardamom, Saffron, and Ginger Consumption on Markers of Glycemic Control, Lipid Profile, Oxidative Stress, and Inflammation in Type 2 Diabetes Patients. Rev. Diabet. Stud. RDS 2014, 11, 258–266. [Google Scholar] [CrossRef]
- Blevins, S.M. Effect of Cinnamon on Glucose and Lipid Levels in Non-Insulin Dependent Type 2 Diabetes Mellitus. Diabetes Care 2007, 30, 2236–2237. Available online: https://clinicaltrials.gov/show/NCT00237640 (accessed on 16 January 2007). [CrossRef]
- Hasanzade, F.; Toliat, M.; Emami, S.A.; Emamimoghaadam, Z. The Effect of Cinnamon on Glucose of Type II Diabetes Patients. J. Tradit. Complement. Med. 2013, 3, 171–174. [Google Scholar] [CrossRef]
- Lira Neto, J.C.G.; Damasceno, M.M.C.; Ciol, M.A.; de Freitas, R.; de Araújo, M.F.M.; Teixeira, C.R.S.; Carvalho, G.C.N.; Lisboa, K.; Marques, R.L.L.; Alencar, A.; et al. Efficacy of Cinnamon as an Adjuvant in Reducing the Glycemic Biomarkers of Type 2 Diabetes Mellitus: A Three-Month, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Am. Nutr. Assoc. 2022, 41, 266–274. [Google Scholar] [CrossRef]
- Lu, T.; Sheng, H.; Wu, J.; Cheng, Y.; Zhu, J.; Chen, Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr. Res. 2012, 32, 408–412. [Google Scholar] [CrossRef]
- Mang, B.; Wolters, M.; Schmitt, B.; Kelb, K.; Lichtinghagen, R.; Stichtenoth, D.O.; Hahn, A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur. J. Clin. Investig. 2006, 36, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Mirfeizi, M.; Mehdizadeh Tourzani, Z.; Mirfeizi, S.Z.; Asghari Jafarabadi, M.; Rezvani, H.R.; Afzali, M. Controlling type 2 diabetes mellitus with herbal medicines: A triple-blind randomized clinical trial of efficacy and safety. J. Diabetes 2016, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Sengsuk, C.; Sanguanwong, S.; Tangvarasittichai, O.; Tangvarasittichai, S. Effect of cinnamon supplementation on glucose, lipids levels, glomerular filtration rate, and blood pressure of subjects with type 2 diabetes mellitus. Diabetol. Int. 2016, 7, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Talaei, B.; Amouzegar, A.; Sahranavard, S.; Hedayati, M.; Mirmiran, P.; Azizi, F. Effects of Cinnamon Consumption on Glycemic Indicators, Advanced Glycation End Products, and Antioxidant Status in Type 2 Diabetic Patients. Nutrients 2017, 9, 991. [Google Scholar] [CrossRef]
- Vafa, M.; Mohammadi, F.; Shidfar, F.; Sormaghi, M.S.; Heidari, I.; Golestan, B.; Amiri, F. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int. J. Prev. Med. 2012, 3, 531–536. [Google Scholar]
- Vanschoonbeek, K.; Thomassen, B.J.; Senden, J.M.; Wodzig, W.K.; van Loon, L.J. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J. Nutr. 2006, 136, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Wainstein, J.; Stern, N.; Heller, S.; Boaz, M. Dietary Cinnamon Supplementation and Changes in Systolic Blood Pressure in Subjects with Type 2 Diabetes. J. Med. Food 2011, 14, 1505–1510. [Google Scholar] [CrossRef]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef]
- Couturier, K.; Batandier, C.; Awada, M.; Hininger-Favier, I.; Canini, F.; Anderson, R.A.; Leverve, X.; Roussel, A.M. Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Arch. Biochem. Biophys. 2010, 501, 158–161. [Google Scholar] [CrossRef]
- Sheng, X.Y.; Zhang, Y.B.; Gong, Z.W.; Huang, C.; Zang, Y.Q. Improved Insulin Resistance and Lipid Metabolism by Cinnamon Extract through Activation of Peroxisome Proliferator-Activated Receptors. PPAR Res. 2008, 2008, 581348. [Google Scholar] [CrossRef]
- Shang, C.; Lin, H.; Fang, X.; Wang, Y.; Jiang, Z.; Qu, Y.; Xiang, M.; Shen, Z.; Xin, L.; Lu, Y.; et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021, 12, 12194–12220. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Wang, T.; Ji, J.; Wang, Y.; Chen, K.X.; Jia, Q.; Li, Y.M.; Wang, H.Y. Procyanidin C1, a Component of Cinnamon Extracts, Is a Potential Insulin Sensitizer That Targets Adipocytes. J. Agric. Food Chem. 2019, 67, 8839–8846. [Google Scholar] [CrossRef] [PubMed]
- Medagama, A.B. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr. J. 2015, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; da Silva, G.A.R. To what extent does cinnamon administration improve the glycemic and lipid profiles? Clin. Nutr. ESPEN 2018, 27, 1–9. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, X.; Fu, S.; Li, Z.; Chen, Y.; Long, C.; Li, S.; Chen, Q. Efficacy of cinnamon supplementation on glycolipid metabolism in T2DM diabetes: A meta-analysis and systematic review. Front. Physiol. 2022, 13, 960580. [Google Scholar] [CrossRef]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef]
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Elfeki, A.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet-induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef]
- Subramaniam, S.; Subramaniam, R.; Rajapandian, S.; Uthrapathi, S.; Gnanamanickam, V.R.; Dubey, G.P. Anti-Atherogenic Activity of Ethanolic Fraction of Terminalia arjuna Bark on Hypercholesterolemic Rabbits. Evid.-Based Complement. Altern. Med. eCAM 2011, 2011, 487916. [Google Scholar] [CrossRef]
- Cheng, B.H.; Sheen, L.Y.; Chang, S.T. Hypolipidemic effects of S-(+)-linalool and essential oil from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2018, 8, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kutbi, E.H.; Sohouli, M.H.; Fatahi, S.; Lari, A.; Shidfar, F.; Aljhdali, M.M.; Alhoshan, F.M.; Elahi, S.S.; Almusa, H.A.; Abu-Zaid, A. The beneficial effects of cinnamon among patients with metabolic diseases: A systematic review and dose-response meta-analysis of randomized-controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 6113–6131. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Karimi, E.; Hajishafiee, M.; Milajerdi, A.; Amini, M.R.; Esmaillzadeh, A. Anti-hypertensive effects of cinnamon supplementation in adults: A systematic review and dose-response Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Hajimonfarednejad, M.; Nimrouzi, M.; Heydari, M.; Zarshenas, M.M.; Raee, M.J.; Jahromi, B.N. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: A randomized double-blind placebo controlled clinical trial. Phytother. Res. 2018, 32, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Hajimonfarednejad, M.; Ostovar, M.; Raee, M.J.; Hashempur, M.H.; Mayer, J.G.; Heydari, M. Cinnamon: A systematic review of adverse events. Clin. Nutr. 2019, 38, 594–602. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).