The Foetal Origins of Allergy and Potential Nutritional Interventions to Prevent Disease
Abstract
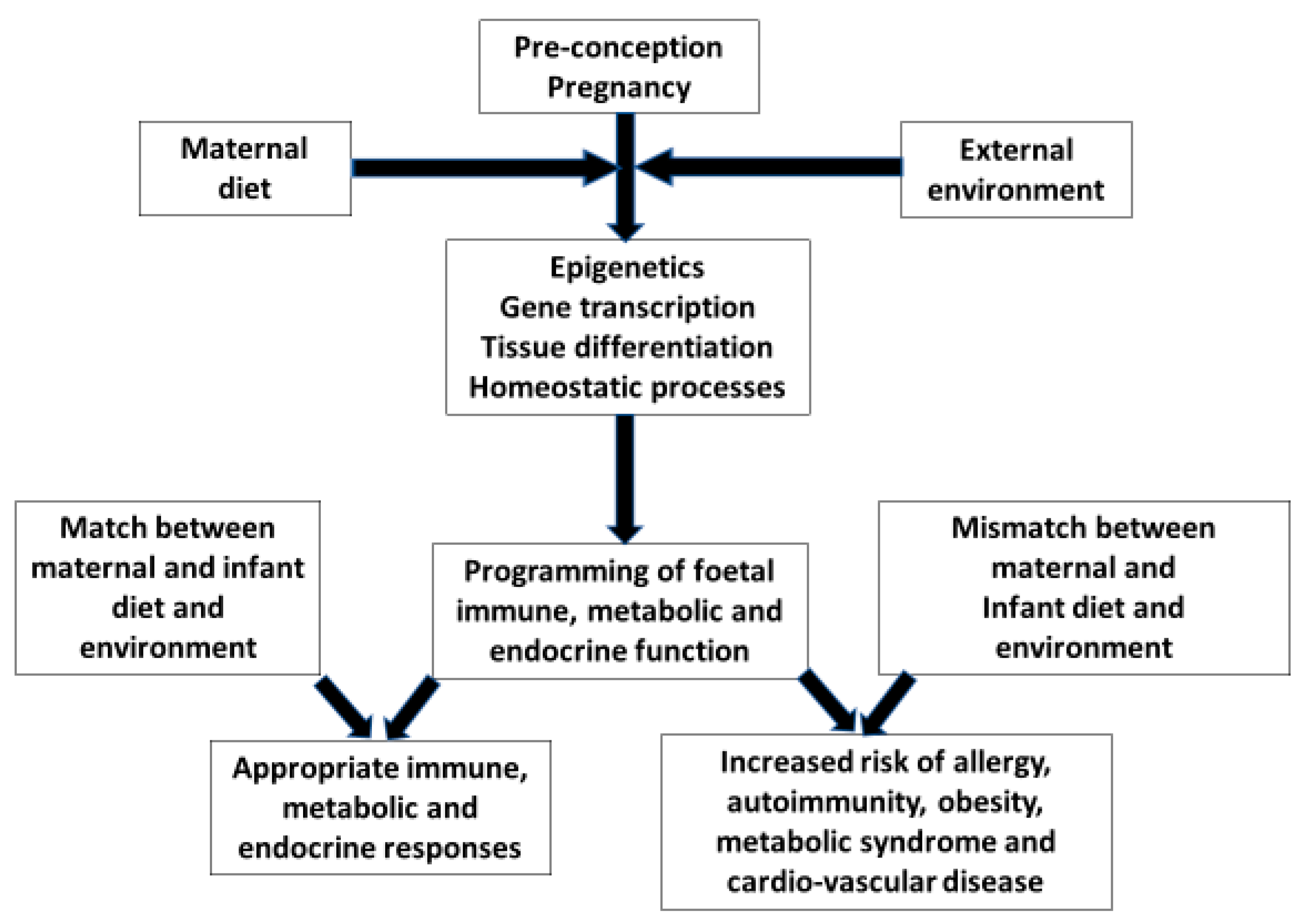
1. Introduction
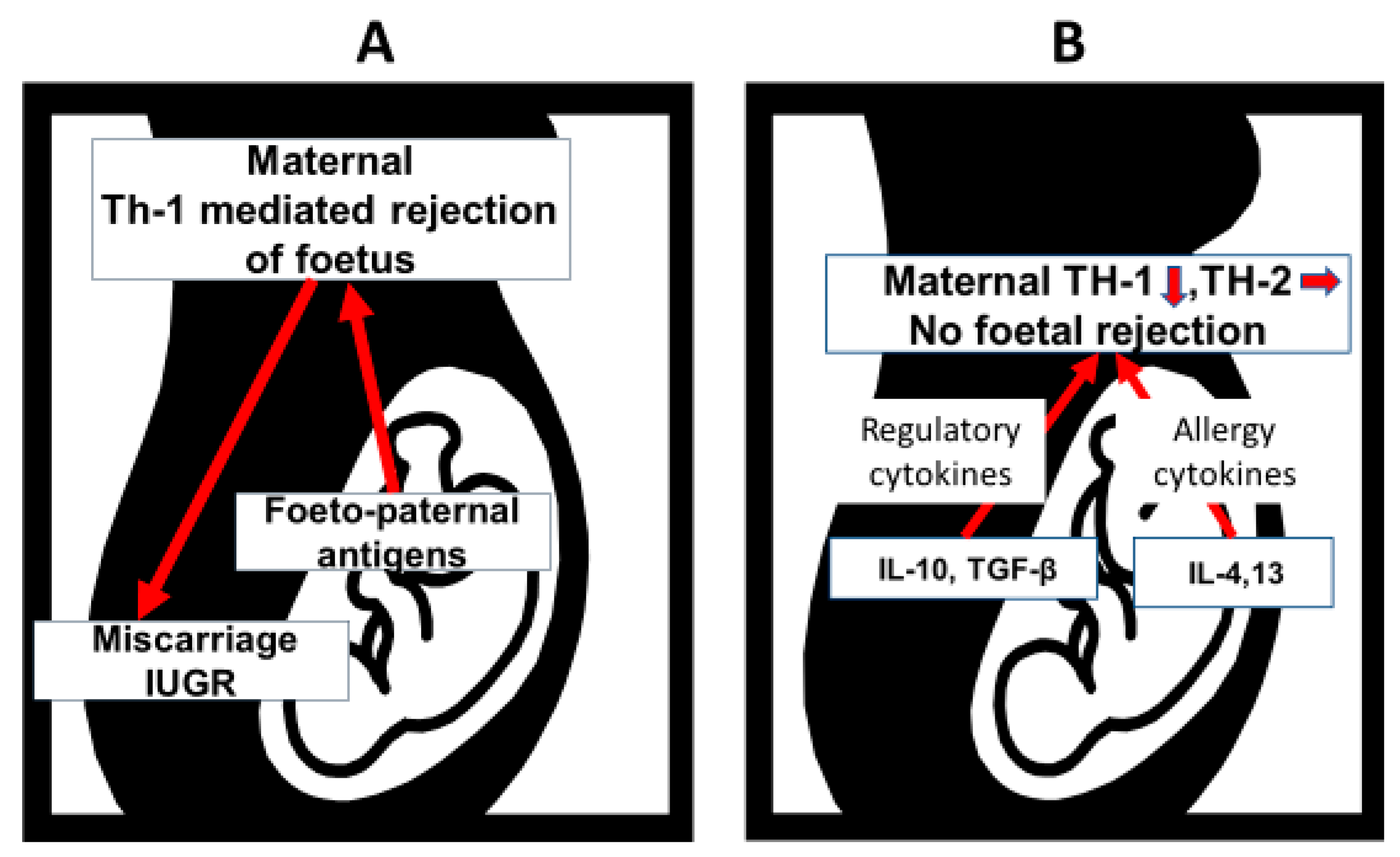
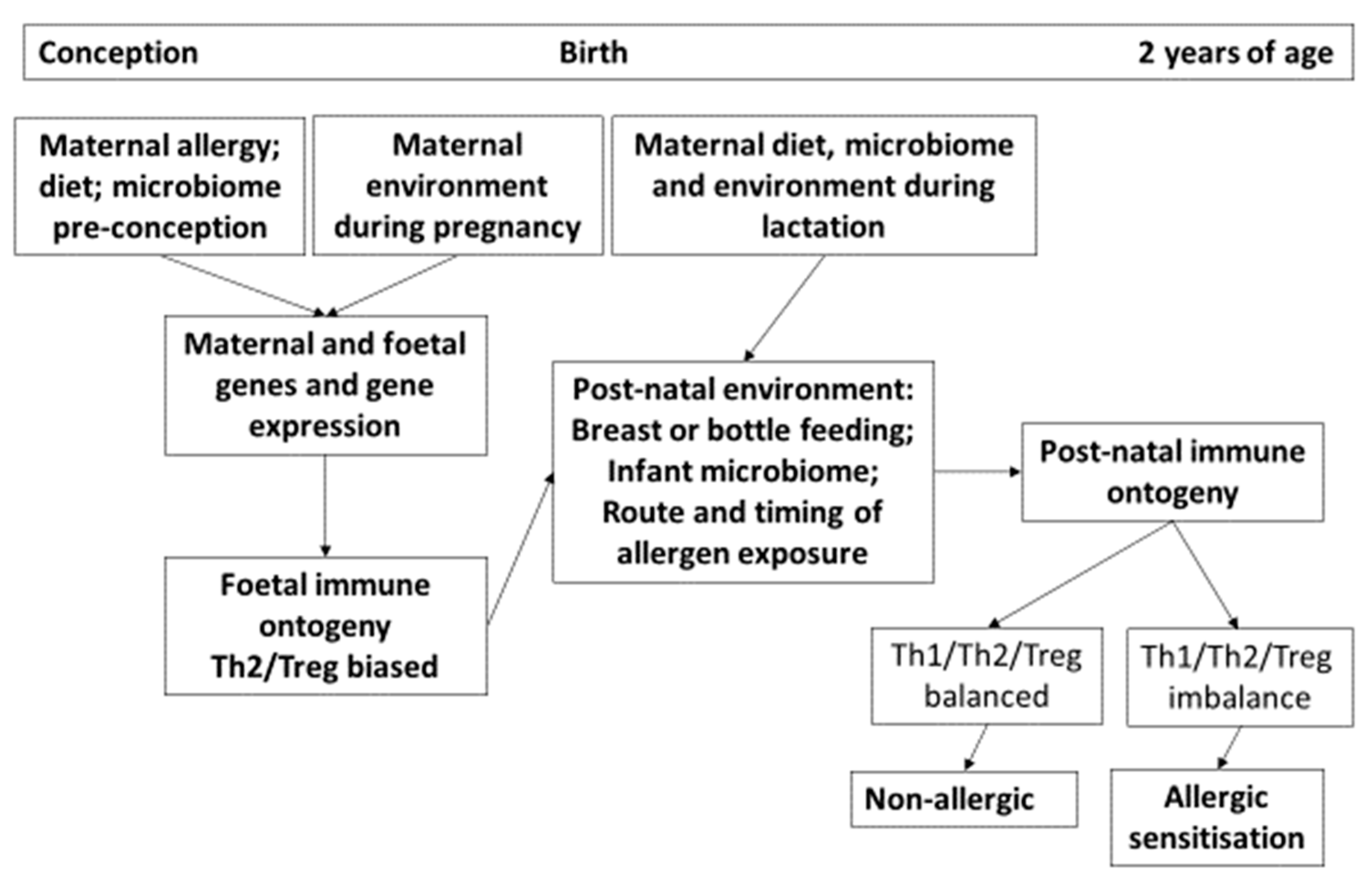
2. Immunology of Pregnancy and Neonatal Immune Responses
3. Foetal Sensitization to Allergens
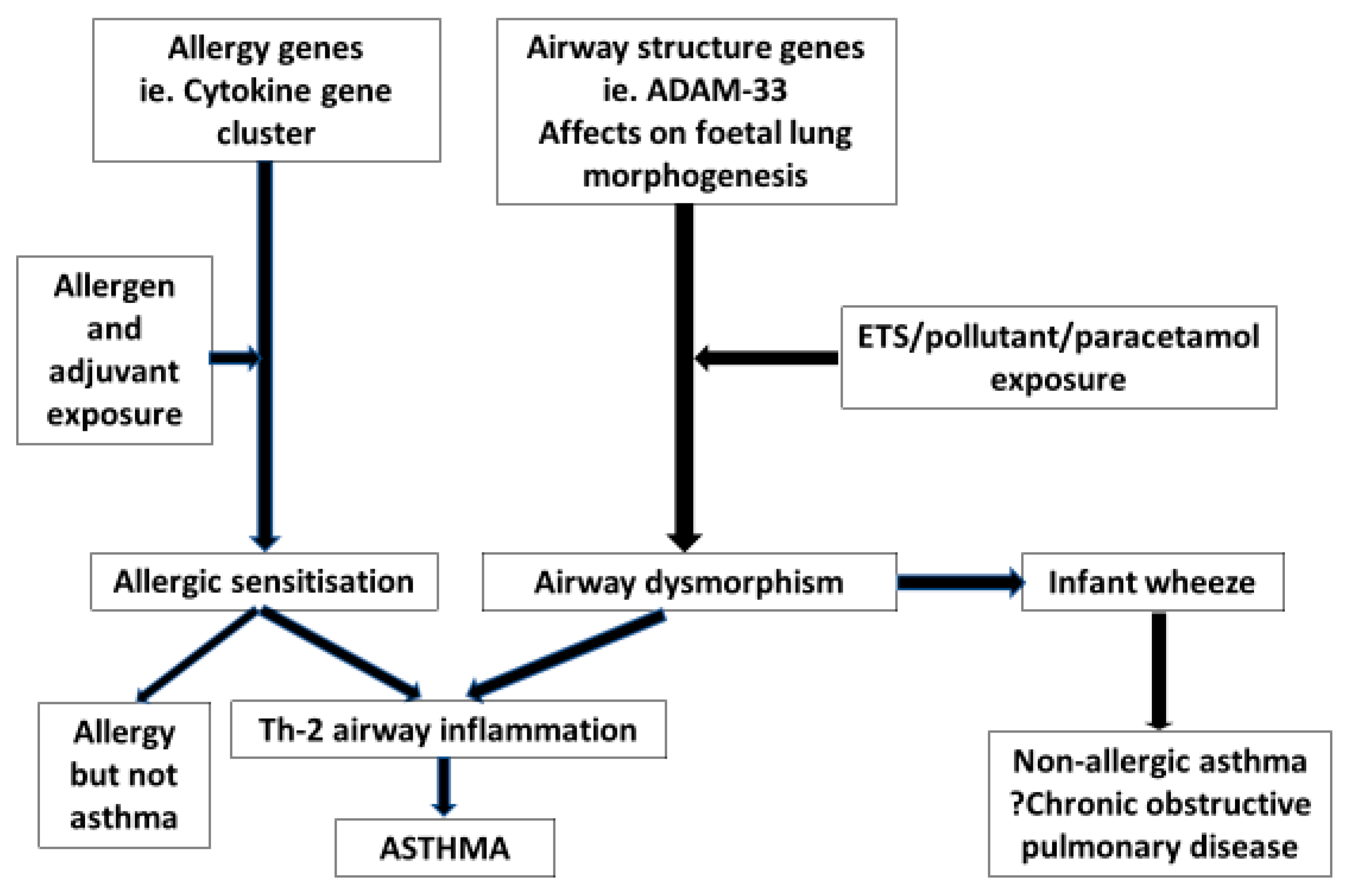
4. Foetal Gene/Environment Interactions
5. Foetal Nutrition and Allergic Disease
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Susuki, K. The developing world of DOHaD. J. Dev. Orig. Health Dis. 2018, 9, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Crespi, B.J. Why and How Imprinted Genes Drive Fetal Programming. Front. Endocrinol. 2020, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Tham, E.H.; Loo, E.X.L.; Zhu, Y.; Shek, L.P.C. Effects of migration on allergic diseases. Int. Arch. Allergy Immunol. 2019, 178, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Tsitoura, D.C.; Tassios, Y. Immunomodulation: The future cure for allergic diseases. Ann. N. Y. Acad. Sci. 2006, 1088, 100–115. [Google Scholar] [CrossRef]
- Breiteneder, H.; Peng, Y.Q.; Agache, I.; Diamant, Z.; Eiwegger, T.; Fokkens, W.J.; Traidl-Hoffmann, C.; Nadeau, K.; O’Hehir, R.E.; O’Mahony, L.; et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy 2020, 75, 3039–3068. [Google Scholar] [CrossRef]
- Peroni, D.G.; Nuzzi, G.; Trambusti, I.; Di Cicco, M.E.; Comberiati, P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol. 2020, 11, 700. [Google Scholar] [CrossRef]
- Garn, H.; Potaczek, D.P.; Pfefferle, P.I. The Hygiene Hypothesis and New Perspectives—Current Challenges Meeting an Old Postulate. Front. Immunol. 2021, 12, 847. [Google Scholar] [CrossRef]
- Gurram, R.K.; Zhu, J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell. Mol. Immunol. 2019, 16, 225–235. [Google Scholar] [CrossRef]
- Wambre, E.; Bajzik, V.; DeLong, J.H.; O’Brien, K.; Nguyen, Q.A.; Speake, C.; Gersuk, V.H.; DeBerg, H.A.; Whalen, E.; Ni, C.; et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017, 9, eaam9171. [Google Scholar] [CrossRef]
- Prescott, S.L.; Macaubas, C.; Holt, B.J.; Smallacombe, T.B.; Loh, R.; Sly, P.D.; Holt, P.G. Transplacental Priming of the Human Immune System to Environmental Allergens: Universal Skewing of Initial T Cell Responses Toward the Th2 Cytokine Profile. J. Immunol. 1998, 160, 4730–4737. [Google Scholar]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, Y.; Wei, H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front. Immunol. 2020, 11, 592010. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Vance, G.H.; Power, L.L.; Pender, S.L.; MacDonald, T.T.; Warner, J.O. Costimulatory molecules in the developing human gastrointestinal tract: A pathway for fetal allergen priming. J. Allergy Clin. Immunol. 2001, 108, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Belderbos, M.; Levy, O.; Bont, L. Neonatal innate immunity in allergy development. Curr. Opin. Pediatr. 2009, 21, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Ortega, B.; Goh, A.; Xepapadaki, P.; Sprikkelman, A.; Nicolaou, N.; Hernandez, R.E.H.; Latiff, A.H.A.; Yat, M.T.; Diab, M.; Hussaini, B.A.; et al. Strategies and Future Opportunities for the Prevention, Diagnosis, and Management of Cow Milk Allergy. Front. Immunol. 2021, 12, 1877. [Google Scholar] [CrossRef]
- Williams, T.J.; Jones, C.A.; Miles, E.A.; Warner, J.O.; Warner, J.A. Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J. Allergy Clin. Immunol. 2000, 105, 951–959. [Google Scholar] [CrossRef]
- Tsafaras, G.P.; Ntontsi, P.; Xanthou, G. Advantages and limitations of the neonatal immune system. Front. Pediatr. 2020, 8, 5. [Google Scholar] [CrossRef]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef]
- Jones, A.C.; Miles, E.A.; Warner, J.O.; Colwell, B.M.; Bryant, T.N.; Warner, J.A. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr. Allergy Immunol. 1996, 7, 109–116. [Google Scholar] [CrossRef]
- Holloway, J.A.; Warner, J.O.; Vance, G.H.; Diaper, N.D.; Warner, J.A.; Jones, C.A. Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet 2000, 356, 1900–1902. [Google Scholar] [CrossRef]
- Power, L.L.; Popplewell, E.J.; Holloway, J.A.; Diaper, N.D.; Warner, J.O.; Jones, C.A. Immunoregulatory molecules during pregnancy and at birth. J. Reprod. Immunol. 2002, 56, 19–28. [Google Scholar] [CrossRef]
- Zaghouani, H.; Hoeman, C.M.; Adkins, B. Neonatal immunity: Faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009, 30, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Semmes, E.C.; Chen, J.-L.; Goswami, R.; Burt, T.D.; Permar, S.R.; Fouda, G.G. Understanding Early-Life Adaptive Immunity to Guide Interventions for Pediatric Health. Front. Immunol. 2021, 11, 3544. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Bolte, G.; Holscher, B.; Douwes, J.; Lehmann, I.; Fahlbusch, B.; Bischof, W.; Weiss, M.; Borte, M.; Wichmann, H.-E. Allergens and endotoxin on mothers’ mattresses and total immunoglobulin E in cord blood of neonates. Eur. Respir. J. 2002, 20, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.O. The foetal origins of allergy. Curr. Allergy Clin. Immunol. 2017, 30, 60–68. [Google Scholar]
- Schaub, B.; Liu, J.; Höppler, S.; Schleich, I.; Huehn, J.; Olek, S.; Wieczorek, G.; Illi, S.; von Mutius, E. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J. Allergy Clin. Immunol. 2009, 123, 774–782. [Google Scholar] [CrossRef]
- Campbell, B.E.; Lodge, C.J.; Lowe, A.J.; Burgess, J.A.; Matheson, M.C.; Dharmage, S.C. Exposure to ‘farming’and objective markers of atopy: A systematic review and meta-analysis. Clin. Exp. Allergy 2015, 45, 744–757. [Google Scholar] [CrossRef]
- Perkin, M.R.; Strachan, D.P. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J. Allergy Clin. Immunol. 2006, 117, 1374–1781. [Google Scholar] [CrossRef]
- Richgels, P.K.; Yamani, A.; Chougnet, C.A.; Lewkowich, I.P. Maternal house dust mite exposure during pregnancy enhances severity of house dust mite–induced asthma in murine offspring. J. Allergy Clin. Immunol. 2017, 140, 1404–1415. [Google Scholar] [CrossRef]
- Torrent, M.; Sunyer, J.; Garcia, R.; Harris, J.; Iturriaga, M.V.; Puig, C.; Vall, O.; Antó, J.M.; Taylor, A.J.N.; Cullinan, P. Early-Life Allergen Exposure and Atopy, Asthma, and Wheeze up to 6 Years of Age. Am. J. Respir. Crit. Care Med. 2007, 176, 446–453. [Google Scholar] [CrossRef]
- Liccardi, G.; Cazzola, M.; Canonica, G.W.; Passalacqua, G.; D’Amato, G. New insights in allergen avoidance measures for mite and pet sensitized patients. A critical appraisal. Respir. Med. 2005, 99, 1363–1376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kramer, M.S.; Kakuma, R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid. Based Child Health A Cochrane Rev. J. 2014, 9, 447–483. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.A.; Holloway, J.A.; Popplewell, E.J.; Shute, J.K.; Boughton, J.; Warner, J.O. Fetal exposure to intact immunoglobulin E occurs via the gastrointestinal tract. Clin. Exp. Allergy 2003, 33, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Vance, G.H.S.; Grimshaw, K.E.C.; Briggs, R.; Lewis, S.A.; Mullee, M.A.; Thornton, C.A.; Warner, J.O. Serum ovalbumin-specific IgG responses during pregnancy reflects maternal intake of dietary egg and relate to the development of allergy in infancy. Clin. Exp. Allergy 2004, 34, 1855–1861. [Google Scholar] [CrossRef]
- Jenmalm, M.C.; Björkstén, B. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clin. Exp. Allergy 2000, 30, 34–40. [Google Scholar] [CrossRef]
- Glovsky, M.M.; Ghekiere, L.; Rejzek, E. Effect of maternal immunotherapy on immediate skin test reactivity, specific rye I IgG and IgE antibody, and total IgE of the children. Ann. Allergy 1991, 67, 21–24. [Google Scholar]
- Van Duren-Schmidt, K.; Pichler, J.; Ebner, C.; Bartmann, P.; Förster, E.; Urbanek, R.; Szépfalusi, Z. Prenatal Contact with Inhalant Allergens. Pediatr. Res. 1997, 41, 128–131. [Google Scholar] [CrossRef]
- Lowe, A.J.; Olsson, D.; Bråbäck, L.; Forsberg, B. Pollen exposure in pregnancy and infancy and risk of asthma hospitalisation-a register based cohort study. Allergy Asthma Clin. Immunol. 2012, 8, 17. [Google Scholar] [CrossRef]
- Collins, S.A.; Lockett, G.A.; Holloway, J.W. The genetics of allergic diseases and asthma. In Pediatric Allergy Principles and Practice, 3rd ed.; Leung, Y.M., Sampson, H.A., Geha, R.S., Szefler, S.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 18–30. [Google Scholar]
- Ortiz, R.A.; Barnes, K.C. Genetics of Allergic Diseases. Immunol. Allergy Clin. N. Am. 2015, 35, 19–44. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef]
- Davies, E.R.; Kelly, J.F.; Howarth, P.H.; Wilson, D.I.; Holgate, S.T.; Davies, D.E.; Whitsett, J.A.; Haitchi, H.M. Soluble ADAM33 initiates airway remodeling to promote susceptibility for allergic asthma in early life. JCI Insight. 2016, 1, e87632. [Google Scholar] [CrossRef] [PubMed]
- Perzanowski, M.S.; Miller, R.L.; Tang, D.; Ali, D.; Garfinkel, R.S.; Chew, G.L.; Goldstein, I.F.; Perera, F.P.; Barr, R.G. Prenatal acetaminophen exposure and risk of wheeze at age 5 years in an urban low-income cohort. Thorax 2010, 65, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Luthers, C.R.; Dunn, T.M.; Snow, A.L. ORMDL3 and Asthma: Linking Sphingolipid Regulation to Altered T Cell Function. Front. Immunol. 2020, 11, 3120. [Google Scholar] [CrossRef] [PubMed]
- McLean, W. Filaggrin failure-from ichthyosis vulgaris to atopic eczema and beyond. Br. J. Dermatol. 2016, 175, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.J.; Brabak, L.; Lowe, A.J.; Dharmage, S.C.; Olsson, D.; Forsberg, B. Grandmaternal smoking increases asthma risk in grandchildren: A nationwide Swedish cohort. Clin. Exp. Allergy 2018, 48, 167–174. [Google Scholar] [CrossRef]
- Barton, S.J.; Ngo, S.; Costello, P.; Garratt, E.; El-Heis, S.; Antoun, E.; Clarke-Harris, R.; Murray, R.; Bhatt, T.; Burdge, G.; et al. DNA methylation of Th2 lineage determination genes at birth is associated with allergic outcomes in childhood. Clin. Exp. Allergy 2017, 47, 1599–1608. [Google Scholar] [CrossRef]
- Veeranki, S.P.; Gebretsadik, T.; Mitchel, E.F.; Tylavsky, F.A.; Hartert, T.V.; Cooper, W.O.; Dupont, W.D.; Dorris, S.L.; Hartman, T.J.; Carroll, K.N. Maternal folic acid supplementation during pregnancy and early childhood asthma. Epidemiology 2015, 26, 934. [Google Scholar] [CrossRef]
- Godfrey, K.; Barker, D.J.P.; Osmond, C. Disproportionate fetal growth and raised IgE concentration in adult life. Clin. Exp. Allergy 1994, 24, 641–648. [Google Scholar] [CrossRef]
- Lucas, J.S.; Inskip, H.M.; Godfrey, K.M.; Foreman, C.T.; Warner, J.O.; Gregson, R.K.; Clough, J.B. Small size at birth and greater postnatal weight gain: Relationships to diminished infant lung function. Am. J. Respir. Crit. Care Med. 2004, 170, 534–540. [Google Scholar] [CrossRef]
- Tedner, S.G.; Örtqvist, A.K.; Almqvist, C. Fetal growth and risk of childhood asthma and allergic disease. Clin. Exp. Allergy 2012, 42, 1430–1447. [Google Scholar] [CrossRef]
- Pike, K.C.; Crozier, S.R.; Lucas, J.S.; Inskip, H.M.; Robinson, S.; Roberts, G.; Godfrey, K.M. Southampton Women’s Survey Study Group. Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax 2010, 65, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Erkkola, M.; Nwaru, B.I.; Kaila, M.; Kronberg-Kippilä, C.; Ilonen, J.; Simell, O.; Veijola, R.; Knip, M.; Virtanen, S. Risk of asthma and allergic outcomes in the offspring in relation to maternal food consumption during pregnancy: A Finnish birth cohort study. Pediatr. Allergy Immunol. 2012, 23, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Alessandri, C.; Sopo, S.M.; Panetta, V.; Pingitore, G.; Tripodi, S.; Zappala, D.; Zicari, A.M.; the Lazio Association of Pediatric Allergology (APAL) Study Group. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: Role of maternal atopy. Pediatr. Allergy Immunol. 2006, 17, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Northstone, K.; Henderson, A.J.; Shaheen, S.O. Maternal intake of sugar during pregnancy and childhood respiratory and atopic outcomes. Eur. Respir. J. 2017, 50, 1700073. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedon, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef]
- Bédard, A.; Li, Z.; Ait-hadad, W.; Camargo, C.A., Jr.; Leynaert, B.; Pison, C.; Dumas, O.; Varraso, R. The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. Int. J. Environ. Res. Public Health. 2021, 18, 3013. [Google Scholar] [CrossRef]
- Rizzo, G.S.; Sen, S. Maternal obesity and immune dysregulation in mother and infant: A review of the evidence. Paediatr. Respir. Rev. 2015, 16, 251–257. [Google Scholar] [CrossRef]
- Darabi, B.; Rahmati, S.; Hafeziahmadi, M.R.; Badfar, G.; Azami, M. The association between caesarean section and childhood asthma: An updated systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Mitselou, N.; Hallberg, J.; Stephansson, O.; Almqvist, C.; Melén, E.; Ludvigsson, J.F. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J. Allergy Clin. Immunol. 2018, 142, 1510–1514. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, Y.; Jiang, Y.; Sun, W.; Zhu, Q.; Wang, B.; Jiang, F.; Zhang, J. Cesarean section without medical indication and risks of childhood allergic disorder, attenuated by breastfeeding. Sci. Rep. 2017, 7, 9762. [Google Scholar] [CrossRef]
- Metzler, S.; Frei, R.; Schmaußer-Hechfellner, E.; von Mutius, E.; Pekkanen, J.; Karvonen, A.M.; Kirjavainen, P.V.; Dalphin, J.C.; Divaret-Chauveau, A.; Riedler, J.; et al. Association between antibiotic treatment during pregnancy and infancy and the development of allergic diseases. Pediatr. Allergy Immunol. 2019, 30, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.; Stråvik, M.; Broberg, K.; Sandin, A.; Wold, A.E.; Sandberg, A.-S. Proportions of Polyunsaturated Fatty Acids in Umbilical Cord Blood at Birth Are Related to Atopic Eczema Development in the First Year of Life. Nutrients 2021, 13, 3779. [Google Scholar] [CrossRef]
- Larsen, V.G.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Wu, S.; Li, C. Influence of Maternal Fish Oil Supplementation on the Risk of Asthma or Wheeze in Children: A Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 2022, 10, 817110. [Google Scholar] [CrossRef]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef]
- Tareke, A.A.; Hadgu, A.A.; Ayana, A.M.; Zerfu, T.A. Prenatal vitamin D supplementation and child respiratory health: A systematic review and meta-analysis of randomized controlled trials. World Allergy Organ. J. 2020, 13, 100486. [Google Scholar] [CrossRef]
- Hypponen, E.; Berry, D.J.; Wjst, M.; Power, C. Serum 25-hydroxyvitamin D and IgE-a significant but nonlinear relationship. Allergy 2009, 64, 613–620. [Google Scholar] [CrossRef]
- Chen, L.W.; Lyons, B.; Navarro, P.; Shivappa, N.; Mehegan, J.; Murrin, C.M.; Hébert, J.R.; Kelleher, C.C.; Phillips, C.M. Maternal dietary inflammatory potential and quality are associated with offspring asthma risk over 10-year follow-up: The Lifeways Cross-Generation Cohort Study. Am. J. Clin. Nutr. 2020, 111, 440–447. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, B.; Kroll, F.; Hanson, C.; Vicencio, A.; Coca, S.; Uribarri, J.; Bose, S. Increased advanced glycation end product and meat consumption is associated with childhood wheeze: Analysis of the National Health and Nutrition Examination Survey. Thorax 2021, 76, 292–294. [Google Scholar] [CrossRef]
- Venter, C.; Pickett, K.; Starling, A.; Maslin, K.; Smith, P.K.; Palumbo, M.P.; O’Mahony, L.; Ben Abdallah, M.; Dabelea, D. Advanced glycation end product intake during pregnancy and offspring allergy outcomes: A Prospective cohort study. Clin. Exp. Allergy 2021, 51, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L. What Are the Effects of a Mediterranean Diet on Allergies and Asthma in Children? Front. Pediatr. 2017, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Northstone, K.; Henderson, A.J.; Shaheen, S.O. Mediterranean diet during pregnancy and childhood respiratory and atopic outcomes: Birth cohort study. Eur. Respir. J. 2020, 55, 1901215. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns During Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, K.E.; Maskell, J.; Oliver, E.M.; Morris, R.C.; Foote, K.D.; Mills, E.C.; Margetts, B.M.; Roberts, G. Diet and food allergy development during infancy: Birth cohort study findings using prospective food diary data. J. Allergy Clin. Immunol. 2014, 133, 511–519. [Google Scholar] [CrossRef] [PubMed]
| Timing | Target | Intervention |
|---|---|---|
| Pre-conception | Maternal obesity [58] | Weight loss No maternal or grand-mother smoking [46] |
| Pre-conception | Maternal nutrition | Healthy balanced diet [76] |
| Pregnancy | Maternal nutrition | More fish less meat Fresh fruit and vegetables [75] Optimal vitamins D, E and zinc [67,68,69,70] No allergen avoidance [29,30,31,32] |
| Pregnancy | Medications to avoid if possible | Antibiotics [62] Paracetamol [43] |
| Pregnancy | Maternal microbiome [6] | Pre-/pro-/syn-biotics [6] |
| Delivery | Avoid if possible | Caesarean section [59,60,61] Bottle feeding [15] |
| Neonatal period | Infant microbiome [6] | Breast feeding [15] Pre-/pro-/syn-biotics [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warner, J.O.; Warner, J.A. The Foetal Origins of Allergy and Potential Nutritional Interventions to Prevent Disease. Nutrients 2022, 14, 1590. https://doi.org/10.3390/nu14081590
Warner JO, Warner JA. The Foetal Origins of Allergy and Potential Nutritional Interventions to Prevent Disease. Nutrients. 2022; 14(8):1590. https://doi.org/10.3390/nu14081590
Chicago/Turabian StyleWarner, John O., and Jill Amanda Warner. 2022. "The Foetal Origins of Allergy and Potential Nutritional Interventions to Prevent Disease" Nutrients 14, no. 8: 1590. https://doi.org/10.3390/nu14081590
APA StyleWarner, J. O., & Warner, J. A. (2022). The Foetal Origins of Allergy and Potential Nutritional Interventions to Prevent Disease. Nutrients, 14(8), 1590. https://doi.org/10.3390/nu14081590







