Inflammatory Bowel Disease and Reproductive Health: From Fertility to Pregnancy—A Narrative Review
Abstract
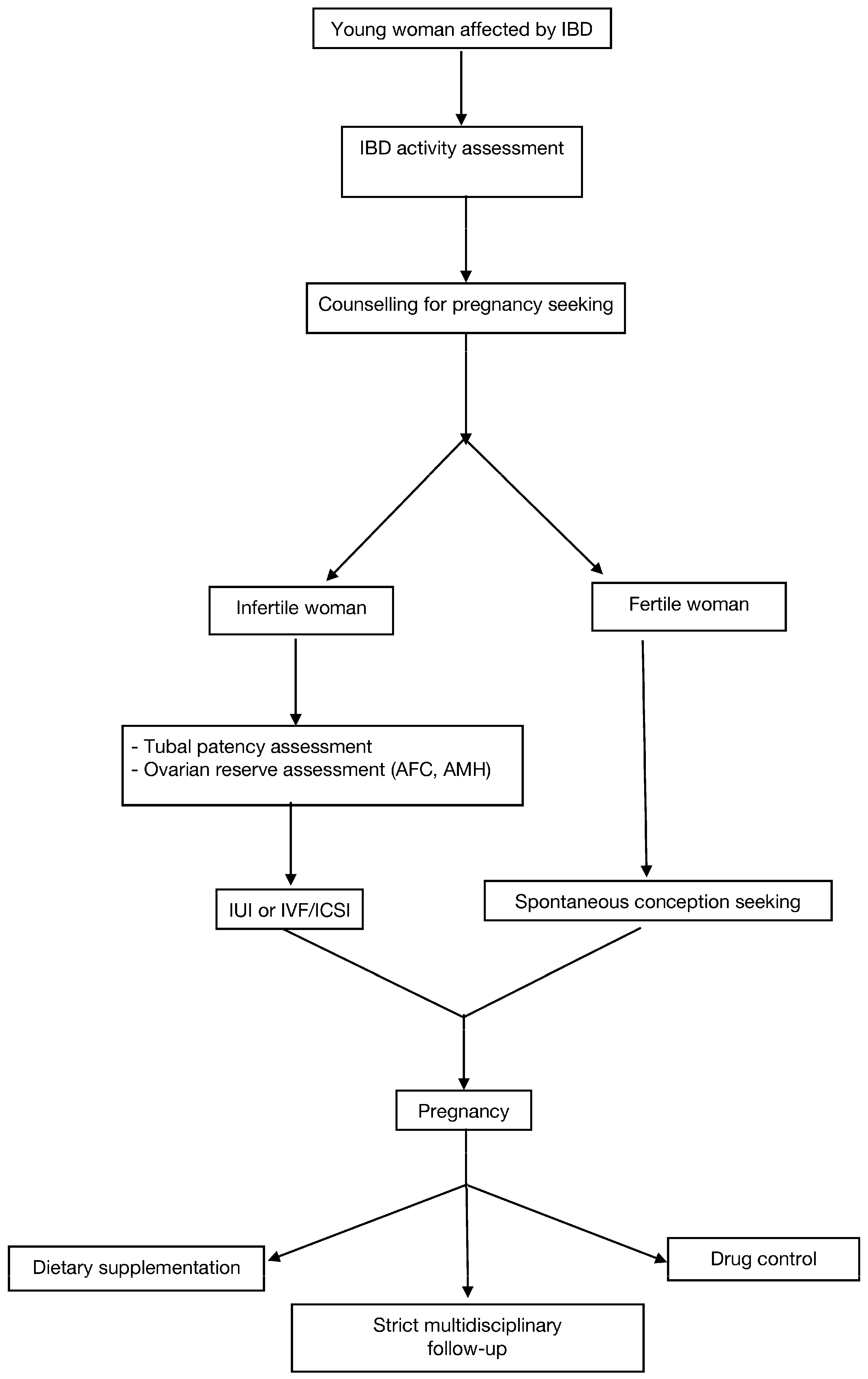
:1. Introduction
2. IBD and Fertility
2.1. Active CD and Infertility
2.2. IPAA: Surgical Approaches and Effects
2.3. IBD and ART Efficacy
3. IBD and Pregnancy
3.1. Medical Therapy
3.2. The Role of Diet during Pregnancy in IBD Patients
3.3. The Role of Microbiome
4. IBD and Delivery
5. IBD and Lactation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Heetun, Z.S.; Byrnes, C.; Neary, P.; O’Morain, C. Review article: Reproduction in the patient with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2007, 26, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, P. Crohn’s disease—Occurrence, course and prognosis. An epidemiologic cohort-study. Dan. Med. Bull. 1997, 44, 287–302. [Google Scholar] [PubMed]
- Manosa, M.; Navarro-Llavat, M.; Marin, L.; Zabana, Y.; Cabre, E.; Domenech, E. Fecundity, pregnancy outcomes, and breastfeeding in patients with inflammatory bowel disease: A large cohort survey. Scand. J. Gastroenterol. 2013, 48, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, R.; Riviere, P.; Papazian, P.; Nion-Larmurier, I.; Girard, G.; Laharie, D.; Marteau, P. Sexual health and fertility for individuals with inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 5423–5433. [Google Scholar] [CrossRef]
- van der Woude, C.J.; Ardizzone, S.; Bengtson, M.B.; Fiorino, G.; Fraser, G.; Katsanos, K.; Kolacek, S.; Juillerat, P.; Mulders, A.G.; Pedersen, N.; et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J. Crohns Colitis 2015, 9, 107–124. [Google Scholar] [CrossRef]
- Waljee, A.; Waljee, J.; Morris, A.M.; Higgins, P.D. Threefold increased risk of infertility: A meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut 2006, 55, 1575–1580. [Google Scholar] [CrossRef]
- Rajaratnam, S.G.; Eglinton, T.W.; Hider, P.; Fearnhead, N.S. Impact of ileal pouch-anal anastomosis on female fertility: Meta-analysis and systematic review. Int. J. Colorectal. Dis. 2011, 26, 1365–1374. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, B.; He, Y.; Zhang, S.; Qiu, Y.; Feng, R.; Yang, H.; Zeng, Z.; Ben-Horin, S.; Chen, M.; et al. Risk Factors Associated with Impaired Ovarian Reserve in Young Women of Reproductive Age with Crohn’s Disease. Intest. Res. 2020, 18, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Koller, T.; Kollerova, J.; Hlavaty, T.; Kadleckova, B.; Payer, J. Ovarian Reserve Assessed by the Anti-Mullerian Hormone and Reproductive Health Parameters in Women With Crohn s Disease, a Case-Control Study. Physiol. Res. 2021, 70, S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.M.; Folan, A.M.; Lee, M.J.; Jones, G.L.; Brown, S.R.; Lobo, A.J. A systematic review and meta-analysis of outcomes after elective surgery for ulcerative colitis. Colorectal Dis. 2021, 23, 18–33. [Google Scholar] [CrossRef]
- Chang, S.; Shen, B.; Remzi, F. When Not to Pouch: Important Considerations for Patient Selection for Ileal Pouch-Anal Anastomosis. Gastroenterol. Hepatol. 2017, 13, 466–475. [Google Scholar]
- Bemelman, W.A.; Warusavitarne, J.; Sampietro, G.M.; Serclova, Z.; Zmora, O.; Luglio, G.; de Buck van Overstraeten, A.; Burke, J.P.; Buskens, C.J.; Colombo, F.; et al. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J. Crohns Colitis 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Lee, S.; Crowe, M.; Seow, C.H.; Kotze, P.G.; Kaplan, G.G.; Metcalfe, A.; Ricciuto, A.; Benchimol, E.I.; Kuenzig, M.E. The impact of surgical therapies for inflammatory bowel disease on female fertility. Cochrane Database Syst. Rev. 2019, 7, CD012711. [Google Scholar] [CrossRef]
- Norgard, B.M.; Larsen, P.V.; Fedder, J.; de Silva, P.S.; Larsen, M.D.; Friedman, S. Live birth and adverse birth outcomes in women with ulcerative colitis and Crohn’s disease receiving assisted reproduction: A 20-year nationwide cohort study. Gut 2016, 65, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Norgard, B.M.; Wod, M.; Larsen, M.D.; Friedman, S.; Jolving, L.R.; Fedder, J. The impact of medical therapies and factors related to treatment procedures in women with rheumatoid arthritis and inflammatory bowel disease receiving assisted reproduction: A nationwide cohort study. Fertil. Steril. 2021, 116, 1492–1500. [Google Scholar] [CrossRef]
- Oza, S.S.; Pabby, V.; Dodge, L.E.; Moragianni, V.A.; Hacker, M.R.; Fox, J.H.; Correia, K.; Missmer, S.A.; Ibrahim, Y.; Penzias, A.S.; et al. In Vitro Fertilization in Women With Inflammatory Bowel Disease Is as Successful as in Women From the General Infertility Population. Clin. Gastroenterol. Hepatol. 2015, 13, 1641–1646.e3. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Nieto, C.; Sekhon, L.; Lee, J.; Gounko, D.; Copperman, A.; Sandler, B. Infertile patients with inflammatory bowel disease have comparable in vitro fertilization clinical outcomes to the general infertile population. Gynecol. Endocrinol. 2020, 36, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Lavie, I.; Lavie, M.; Doyev, R.; Fouks, Y.; Azem, F.; Yogev, Y. Pregnancy outcomes in women with inflammatory bowel disease who successfully conceived via assisted reproduction technique. Arch. Gynecol. Obstet. 2020, 302, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Doria, A.; Cutolo, M.; Ghirardello, A.; Zen, M.; Villalta, D.; Tincani, A.; Punzi, L.; Iaccarino, L.; Petri, M. Effect of pregnancy on serum cytokines in SLE patients. Arthritis Res. Ther. 2012, 14, R66. [Google Scholar] [CrossRef] [Green Version]
- Neuteboom, R.F.; Verbraak, E.; Voerman, J.S.; van Meurs, M.; Steegers, E.A.; de Groot, C.J.; Laman, J.D.; Hintzen, R.Q. First trimester interleukin 8 levels are associated with postpartum relapse in multiple sclerosis. Mult. Scler. 2009, 15, 1356–1358. [Google Scholar] [CrossRef]
- Christian, L.M.; Porter, K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine 2014, 70, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Airas, L.; Saraste, M.; Rinta, S.; Elovaara, I.; Huang, Y.H.; Wiendl, H.; Finnish Multiple, S.; Pregnancy Study, G. Immunoregulatory factors in multiple sclerosis patients during and after pregnancy: Relevance of natural killer cells. Clin. Exp. Immunol. 2008, 151, 235–243. [Google Scholar] [CrossRef]
- Castiglione, F.; Pignata, S.; Morace, F.; Sarubbi, A.; Baratta, M.A.; D’Agostino, L.; D’Arienzo, A.; Mazzacca, G. Effect of pregnancy on the clinical course of a cohort of women with inflammatory bowel disease. Ital. J. Gastroenterol. 1996, 28, 199–204. [Google Scholar]
- van der Giessen, J.; Binyamin, D.; Belogolovski, A.; Frishman, S.; Tenenbaum-Gavish, K.; Hadar, E.; Louzoun, Y.; Peppelenbosch, M.P.; van der Woude, C.J.; Koren, O.; et al. Modulation of cytokine patterns and microbiome during pregnancy in IBD. Gut 2020, 69, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Tarassishin, L.; Eisele, C.; Barre, A.; Nair, N.; Rendon, A.; Hawkins, K.; Debebe, A.; White, S.; Thjomoe, A.; et al. Longitudinal Changes in Fecal Calprotectin Levels Among Pregnant Women With and Without Inflammatory Bowel Disease and Their Babies. Gastroenterology 2021, 160, 1118–1130.e3. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Rezaie, A.; Abdollahi, M. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: A meta-analysis. Reprod. Toxicol. 2008, 25, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, S.E.; Erlich, J.M.; Peppercorn, M.A. Insights into the treatment of inflammatory bowel disease in pregnancy. Therap. Adv. Gastroenterol. 2019, 12, 1756284819852231. [Google Scholar] [CrossRef] [Green Version]
- Hviid, A.; Molgaard-Nielsen, D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ 2011, 183, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Czeizel, A.E.; Rockenbauer, M. Population-based case-control study of teratogenic potential of corticosteroids. Teratology 1997, 56, 335–340. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Seow, C.H.; Maxwell, C.; Huang, V.; Leung, Y.; Jones, J.; Leontiadis, G.I.; Tse, F.; Mahadevan, U.; van der Woude, C.J.; et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016, 150, 734–757.e1. [Google Scholar] [CrossRef] [Green Version]
- Broms, G.; Granath, F.; Linder, M.; Stephansson, O.; Elmberg, M.; Kieler, H. Birth outcomes in women with inflammatory bowel disease: Effects of disease activity and drug exposure. Inflamm. Bowel Dis. 2014, 20, 1091–1098. [Google Scholar] [CrossRef]
- McDonald, J.W.; Wang, Y.; Tsoulis, D.J.; MacDonald, J.K.; Feagan, B.G. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst. Rev. 2014, CD003459. [Google Scholar] [CrossRef] [Green Version]
- The Italian Group for the Study of Inflammatory Bowel Disease Working Group; Armuzzi, A.; Bortoli, A.; Castiglione, F.; Contaldo, A.; Daperno, M.; D’Inca, R.; Labarile, N.; Mazzuoli, S.; Onali, S.; et al. Female reproductive health and inflammatory bowel disease: A practice-based review. Dig. Liver Dis. 2022, 54, 19–29. [Google Scholar] [CrossRef]
- Knudsen, V.K.; Orozova-Bekkevold, I.M.; Mikkelsen, T.B.; Wolff, S.; Olsen, S.F. Major dietary patterns in pregnancy and fetal growth. Eur. J. Clin. Nutr 2008, 62, 463–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, Z.; Mansourian, M.; Kelishadi, R. Relationship of the intake of different food groups by pregnant mothers with the birth weight and gestational age: Need for public and individual educational programs. J. Educ. Health Promot. 2015, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.; Guldner, L.; Costet, N.; Kadhel, P.; Rouget, F.; Monfort, C.; Thome, J.P.; Multigner, L.; Cordier, S. Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: Results from a French Caribbean Mother-Child Cohort Study (TIMOUN). Paediatr. Perinat. Epidemiol. 2014, 28, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasil Lopes, M.; Rocha, R.; Castro Lyra, A.; Rosa Oliveira, V.; Gomes Coqueiro, F.; Silveira Almeida, N.; Santos Valois, S.; Oliveira Santana, G. Restriction of dairy products; a reality in inflammatory bowel disease patients. Nutr. Hosp. 2014, 29, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.B.; Hebuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef]
- Sousa Guerreiro, C.; Cravo, M.; Costa, A.R.; Miranda, A.; Tavares, L.; Moura-Santos, P.; MarquesVidal, P.; Nobre Leitao, C. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: A case-control study. Am. J. Gastroenterol. 2007, 102, 2551–2556. [Google Scholar] [CrossRef]
- Villar, J.; Rivera, J. Nutritional supplementation during two consecutive pregnancies and the interim lactation period: Effect on birth weight. Pediatrics 1988, 81, 51–57. [Google Scholar]
- Nafee, T.M.; Farrell, W.E.; Carroll, W.D.; Fryer, A.A.; Ismail, K.M. Epigenetic control of fetal gene expression. BJOG 2008, 115, 158–168. [Google Scholar] [CrossRef]
- Bengtson, M.B.; Aamodt, G.; Mahadevan, U.; Vatn, M.H. Inadequate Gestational Weight Gain, the Hidden Link Between Maternal IBD and Adverse Pregnancy Outcomes: Results from the Norwegian Mother and Child Cohort Study. Inflamm. Bowel Dis. 2017, 23, 1225–1233. [Google Scholar] [CrossRef]
- Bengtson, M.B.; Haugen, M.; Brantsaeter, A.L.; Aamodt, G.; Vatn, M.H. Intake of dairy protein during pregnancy in IBD and risk of SGA in a Norwegian population-based mother and child cohort. BMC Gastroenterol. 2020, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Dzakpasu, S.; Fahey, J.; Kirby, R.S.; Tough, S.C.; Chalmers, B.; Heaman, M.I.; Bartholomew, S.; Biringer, A.; Darling, E.K.; Lee, L.S.; et al. Contribution of prepregnancy body mass index and gestational weight gain to adverse neonatal outcomes: Population attributable fractions for Canada. BMC Pregnancy Childbirth 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, T.; Lv, Y. Inadequate gestational weight gain and adverse pregnancy outcomes among normal weight women in China. Int. J. Clin. Exp. Med. 2015, 8, 2881–2886. [Google Scholar] [PubMed]
- Myklebust-Hansen, T.; Aamodt, G.; Haugen, M.; Brantsaeter, A.L.; Vatn, M.H.; Bengtson, M.B. Dietary Patterns in women with Inflammatory Bowel Disease and Risk of Adverse Pregnancy Outcomes: Results from The Norwegian Mother and Child Cohort Study (MoBa). Inflamm. Bowel Dis. 2017, 24, 12–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englund-Ogge, L.; Brantsaeter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, g1446. [Google Scholar] [CrossRef] [Green Version]
- Goh, J.; O’Morain, C.A. Review article: Nutrition and adult inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 17, 307–320. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, C.N.; Ament, M.; Artinian, L.; Ridgeway, J.; Shanahan, F. Milk tolerance in adults with ulcerative colitis. Am. J. Gastroenterol. 1994, 89, 872–877. [Google Scholar]
- Gupta, R.; Makharia, G.; Khadgawat, R.; Yadav, R.K. Evaluation of lactose and milk intolerance, and bone mineral density in Indian patients with inflammatory bowel disease. Natl. Med. J. India 2012, 25, 327–331. [Google Scholar]
- Cohen-Mekelburg, S.; Tafesh, Z.; Coburn, E.; Weg, R.; Malik, N.; Webb, C.; Hammad, H.; Scherl, E.; Bosworth, B.P. Testing and Treating Small Intestinal Bacterial Overgrowth Reduces Symptoms in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 2439–2444. [Google Scholar] [CrossRef]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teruel, C.; Garrido, E.; Mesonero, F. Diagnosis and management of functional symptoms in inflammatory bowel disease in remission. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Mishkin, S. Dairy sensitivity, lactose malabsorption, and elimination diets in inflammatory bowel disease. Am. J. Clin. Nutr. 1997, 65, 564–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lederberg, J.; McCray, A.T. Ome SweetOmics—A Genealogical Treasury of Words. Scientist 2001, 15, 8. [Google Scholar]
- Karaduta, O.; Dvanajscak, Z.; Zybailov, B. Metaproteomics-An Advantageous Option in Studies of Host-Microbiota Interaction. Microorganisms 2021, 9, 980. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Rizk, G.; Cattoir, V.; Sassi, M.; Thibault, V.; Del Giudice, J.; Gangneux, J.-P. Efficient and Quality-Optimized Metagenomic Pipeline Designed for Taxonomic Classification in Routine Microbiological Clinical Tests. Microorganisms 2022, 10, 711. [Google Scholar] [CrossRef]
- Valle-Gough, R.E.; Samaniego-Gámez, B.Y.; Apodaca-Hernández, J.E.; Chiappa-Carrara, F.X.; Rodríguez-Dorantes, M.; Arena-Ortiz, M.L. RNA-Seq Analysis on the Microbiota Associated with the White Shrimp (Litopenaeus vannamei) in Different Stages of Development. Appl. Sci. 2022, 12, 2483. [Google Scholar] [CrossRef]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.; Relman, D.A. The application of ecological theory toward an understanding of the human microbiome. Science 2012, 336, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Tye, H.; Yu, C.H.; Simms, L.A.; de Zoete, M.R.; Kim, M.L.; Zakrzewski, M.; Penington, J.S.; Harapas, C.R.; Souza-Fonseca-Guimaraes, F.; Wockner, L.F.; et al. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat. Commun. 2018, 9, 3728. [Google Scholar] [CrossRef] [Green Version]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Backhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spor, A.; Koren, O.; Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial Changes during Pregnancy, Birth, and Infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Mandar, R.; Mikelsaar, M. Transmission of mother’s microflora to the newborn at birth. Biol. Neonate 1996, 69, 30–35. [Google Scholar] [CrossRef]
- Kuitunen, M.; Kukkonen, K.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Haahtela, T.; Savilahti, E. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 2009, 123, 335–341. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Penders, J.; Stobberingh, E.E.; Postma, D.S.; Koppelman, G.H.; Kerkhof, M.; Reijmerink, N.E.; Dompeling, E.; van den Brandt, P.A.; Ferreira, I.; et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 2011, 128, 948–955.e3. [Google Scholar] [CrossRef] [PubMed]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr 1999, 69, 1035S–1045S. [Google Scholar] [CrossRef] [PubMed]
- Murgas Torrazza, R.; Neu, J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J. Perinatol. 2011, 31 (Suppl. 1), S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Bjorksten, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Costello, E.K.; Berg-Lyons, D.; Gonzalez, A.; Stombaugh, J.; Knights, D.; Gajer, P.; Ravel, J.; Fierer, N.; et al. Moving pictures of the human microbiome. Genome Biol. 2011, 12, R50. [Google Scholar] [CrossRef] [Green Version]
- Torres, J.; Hu, J.; Seki, A.; Eisele, C.; Nair, N.; Huang, R.; Tarassishin, L.; Jharap, B.; Cote-Daigneault, J.; Mao, Q.; et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut 2020, 69, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [Green Version]
- Peter, I.; Maldonado-Contreras, A.; Eisele, C.; Frisard, C.; Simpson, S.; Nair, N.; Rendon, A.; Hawkins, K.; Cawley, C.; Debebe, A.; et al. A dietary intervention to improve the microbiome composition of pregnant women with Crohn’s disease and their offspring: The MELODY (Modulating Early Life Microbiome through Dietary Intervention in Pregnancy) trial design. Contemp. Clin. Trials Commun. 2020, 18, 100573. [Google Scholar] [CrossRef]
- Hatch, Q.; Champagne, B.J.; Maykel, J.A.; Davis, B.R.; Johnson, E.K.; Bleier, J.S.; Francone, T.D.; Steele, S.R. Crohn’s disease and pregnancy: The impact of perianal disease on delivery methods and complications. Dis. Colon Rectum 2014, 57, 174–178. [Google Scholar] [CrossRef]
- Spring, A.; Lee, M.; Patchett, S.; Deasy, J.; Wilson, I.; Cahill, R.A. Ileostomy obstruction in the third trimester of pregnancy. Colorectal Dis. 2012, 14, e631–e632. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.R.; Russell, R.K.; Wilson, M.L.; Gilmour, W.H.; Satsangi, J.; Wilson, D.C. Systematic review: The role of breastfeeding in the development of pediatric inflammatory bowel disease. J. Pediatr. 2009, 155, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Esbjorner, E.; Jarnerot, G.; Wranne, L. Sulphasalazine and sulphapyridine serum levels in children to mothers treated with sulphasalazine during pregnancy and lactation. Acta Paediatr. Scand. 1987, 76, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Nelis, G.F. Diarrhoea due to 5-aminosalicylic acid in breast milk. Lancet 1989, 1, 383. [Google Scholar] [CrossRef]
- Angelberger, S.; Reinisch, W.; Messerschmidt, A.; Miehsler, W.; Novacek, G.; Vogelsang, H.; Dejaco, C. Long-term follow-up of babies exposed to azathioprine in utero and via breastfeeding. J. Crohns Colitis 2011, 5, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ost, L.; Wettrell, G.; Bjorkhem, I.; Rane, A. Prednisolone excretion in human milk. J. Pediatr. 1985, 106, 1008–1011. [Google Scholar] [CrossRef]
- Gardner, D.K.; Gabbe, S.G.; Harter, C. Simultaneous concentrations of ciprofloxacin in breast milk and in serum in mother and breast-fed infant. Clin. Pharm. 1992, 11, 352–354. [Google Scholar]
- Nielsen, O.H.; Maxwell, C.; Hendel, J. IBD medications during pregnancy and lactation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 116–127. [Google Scholar] [CrossRef]
- Sachs, H.C.; Committee On, D. The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics 2013, 132, e796–e809. [Google Scholar] [CrossRef] [Green Version]
- Grosen, A.; Julsgaard, M.; Kelsen, J.; Christensen, L.A. Infliximab concentrations in the milk of nursing mothers with inflammatory bowel disease. J. Crohns Colitis 2014, 8, 175–176. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M.; Mountifield, R.E.; Van Langenberg, D.R.; Bampton, P.A.; Holtmann, G.J. Un-promoted issues in inflammatory bowel disease: Opportunities to optimize care. Intern. Med. J. 2010, 40, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, P. Pregnancy, fertility, and disease course in patients with Crohn’s disease and ulcerative colitis. Eur. J. Intern. Med. 2000, 11, 215–221. [Google Scholar] [CrossRef]
| Medical Treatment | Safety and Recommendations in Pregnancy | Safety and Recommendations in Breastfeeding |
|---|---|---|
| Aminosalicylates (mesalazine, sulfasalazine, balsalazide, olsalazide) | No increased obstetrical risk. Always recommended (formulation without dibutylphthalate are preferable and, if sulfasalazine is used, suggestion to supplement with folate). | Safe and must be discontinued only in case of neonatal severe bloody diarrhea. |
| Corticosteroids | Concerns about teratogenic effects, such as cleft lip or palate. Recommended only in case of active flares. | Recommended to breastfeed babies 4 h after taking corticosteroids. |
| Antibiotics (metronidazole and ciprofloxacin) | Concerns about teratogenic effects, such as cleft lip or palate. Recommended only after the first trimester of gestation. | Recommended to breastfeed babies 12–24 h after metronidazole and 48 h after ciprofloxacin intake. A short-term antibiotic regimen must be preferred. |
| Thiopurines (azathioprine or 6- mercaptopurine) | Slight increase in preterm deliveries. Recommended as monotherapy. | Advisable, no a higher risk of physical or developmental anomalies in newborns. |
| Methotrexate | Strong teratogenic and abortive effects. Never recommended in pregnancy. | Contraindicated. |
| Cyclosporine | No data on pregnant women available, only recommended as rescue therapy for acute severe steroid-refractory ulcerative colitis. | Contraindicated. |
| Anti-TNFα agents (infliximab, adalimumab, golimumab and certolizumab) | Evidence of crossing the placenta, except of certolizumab. Recommended stopping around the 24th week of gestation, if the case permits. | Safe due to their transmission in breast milk only in small amounts and deactivation by neonatal digestion enzymes. |
| Vedolizumab and ustekinumab | Should be avoided due to their transmission across the placenta and partial lack of data in pregnancy. Can eventually be prescribed only as an ultimate alternative. | Safety data are still missing, so their use is not recommended. |
| Tofacitinib, filgotinib and upadacitinib | Contraindicated due to the complete lack of data in pregnancy. | Safety data are still missing, so their use is not recommended. |
| Clinical Scenario | Practical Take Home Messages |
|---|---|
| IBD and female fertility | Ulcerative colitis without previous pelvic surgery and inactive Crohn’s disease (CD) do not impair fertility. On the other hand, active CD may impair fertility via multiple factors such as fallopian tube inflammation and a lowering of the ovarian reserve. Ileal pouch anal anastomosis (IPAA) seems to increase the risk of infertility by approximately threefold, mainly because of a tubal dysfunction caused by adhesions. |
| IBD and male fertility | Men with IBD may suffer from infertility due to two iatrogenic pathways: either the use of sulphasalazine producing reversible oligospermia or possible complications of IPAA (e.g., retrograde ejaculation or erectile dysfunction). |
| IBD and Assisted Reproductive Technologies (ART) efficacy | IBD patients may be addressed to ART earlier than the general population even after only six months of attempts. It is still not clear if the ART success rate in IBD patients differs from the general population. |
| IBD and pregnancy | IBD pregnant patients show an improvement in the modulation of cytokine patterns during pregnancy and a gradual decrease of inflammation marker levels during gestation. As for babies born to mothers with IBD, they may have a lower ability to achieve a balanced mucosal immunity or establish an optimal intestinal barrier function, a fact that may lead to a higher risk of IBD recurrence in the offspring. |
| IBD and diet in pregnancy | Diet influences the evolution of the disease and IBD determines food preferences in patients in order to reduce disease activity. Fruits and vegetables have a protective effect; on the contrary, carbohydrates, fats, and dairy products should be avoided in these patients. Malnutrition is the result of the complications of the disease, but also of self-reduction of food intake in order to minimize the symptoms. Malnutrition determines nutritional deficiencies, leading to an increase of the probability of inadequate gestational weight gain, with a consequent higher risk of preterm birth or small for gestational age babies. |
| IBD and microbiome in pregnancy | IBD patients seem to have reduced fecal bacterial diversity with a low concentration of commensal bacteria producing butyrate, and an abundance of Proteobacteria and Actinobacteria, which also happens in healthy pregnancies. In IBD patients, as immunological parameters improve in pregnancy, microbial diversity normalizes to that seen in healthy pregnant women. On the other hand, children born to IBD mothers showed an altered gut microbiome that is correlated to abnormal adaptive immune systems and a future risk of IBD. |
| IBD and delivery | Although there are no guidelines on the mode of delivery in IBD patients, vaginal delivery is commonly suggested, especially if the disease is quiescent or mild. On the contrary, in case of IPAA, a cesarean section should be advised due to the higher risk of the alteration of sphincter pressure that could be more frequently damaged by vaginal deliveries. Similarly, in case of active perianal disease, a higher risk of severe sphincter injuries has been reported, and cesarean delivery should be preferred. |
| IBD and lactation | Physicians have a key-role in a proper counselling to support breastfeeding, with a special emphasis on possible benefits for both mothers and newborns, and to educate women not to discontinue the therapy, to avoid smoking, and to keep the disease monitored. Indeed, during breastfeeding, many therapies are considered safe for the newborn, and maternal milk is shown to protect babies from the develop of an early-onset IBD. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronchetti, C.; Cirillo, F.; Di Segni, N.; Cristodoro, M.; Busnelli, A.; Levi-Setti, P.E. Inflammatory Bowel Disease and Reproductive Health: From Fertility to Pregnancy—A Narrative Review. Nutrients 2022, 14, 1591. https://doi.org/10.3390/nu14081591
Ronchetti C, Cirillo F, Di Segni N, Cristodoro M, Busnelli A, Levi-Setti PE. Inflammatory Bowel Disease and Reproductive Health: From Fertility to Pregnancy—A Narrative Review. Nutrients. 2022; 14(8):1591. https://doi.org/10.3390/nu14081591
Chicago/Turabian StyleRonchetti, Camilla, Federico Cirillo, Noemi Di Segni, Martina Cristodoro, Andrea Busnelli, and Paolo Emanuele Levi-Setti. 2022. "Inflammatory Bowel Disease and Reproductive Health: From Fertility to Pregnancy—A Narrative Review" Nutrients 14, no. 8: 1591. https://doi.org/10.3390/nu14081591
APA StyleRonchetti, C., Cirillo, F., Di Segni, N., Cristodoro, M., Busnelli, A., & Levi-Setti, P. E. (2022). Inflammatory Bowel Disease and Reproductive Health: From Fertility to Pregnancy—A Narrative Review. Nutrients, 14(8), 1591. https://doi.org/10.3390/nu14081591






