Cohort Study of Maternal Gestational Weight Gain, Gestational Diabetes, and Childhood Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Asthma and Allergies
2.3. Prenatal Exposures
2.4. Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrante, G.; La Grutta, S. The Burden of Pediatric Asthma. Front. Pediatr. 2018, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2019 National Health Interview Survey (NHIS) Data. Available online: https://www.cdc.gov/asthma/nhis/2019/data.htm (accessed on 23 February 2022).
- Tai, A.; Tran, H.; Roberts, M.; Clarke, N.; Gibson, A.-M.; Vidmar, S.; Wilson, J.; Robertson, C.F. Outcomes of childhood asthma to the age of 50 years. J. Allergy Clin. Immunol. 2014, 133, 1572–1578.e3. [Google Scholar] [CrossRef] [PubMed]
- Bobolea, I.; Arismendi, E.; Valero, A.; Agustí, A. Early Life Origins of Asthma: A Review of Potential Effectors. J. Investig. Allergy Clin. Immunol. 2019, 29, 168–179. [Google Scholar] [CrossRef]
- Camargo, C.A. Gestational weight gain and offspring asthma: A novel opportunity for primary prevention research. Clin. Exp. Allergy 2015, 45, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Li, Z.; Ait-Hadad, W.; Camargo, C.A.; Leynaert, B.; Pison, C.; Dumas, O.; Varraso, R. The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. Int. J. Environ. Res. Public Health 2021, 18, 3013. [Google Scholar] [CrossRef]
- Melero, V.; Assaf-Balut, C.; De La Torre, N.G.; Jiménez, I.; Bordiú, E.; Del Valle, L.; Valerio, J.; Familiar, C.; Durán, A.; Runkle, I.; et al. Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study. J. Clin. Med. 2020, 9, 1454. [Google Scholar] [CrossRef]
- Forno, E.; Young, O.M.; Kumar, R.; Simhan, H.; Celedón, J.C. Maternal Obesity in Pregnancy, Gestational Weight Gain, and Risk of Childhood Asthma. Pediatrics 2014, 134, e535–e546. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, B.; Wang, Y.; Wang, K.; Zhang, Z.; Niu, W. Pre-pregnancy Maternal Weight and Gestational Weight Gain Increase the Risk for Childhood Asthma and Wheeze: An Updated Meta-Analysis. Front. Pediatr. 2020, 8, 134. [Google Scholar] [CrossRef]
- Li, Z.; Yu, M.; Wang, P.; Qian, H.; Fan, Y.; Li, X.; Xu, Q.; Wang, X.; Wang, X.; Lu, C. Association between maternal diabetes mellitus and allergic diseases in children—A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.M.; Der Voort, A.M.S.-V.; Gaillard, R.; Hofman, A.; De Jongste, J.C.; Jaddoe, V.W.; Duijts, L. Maternal weight, gestational weight gain and preschool wheezing: The Generation R Study. Eur. Respir. J. 2013, 42, 1234–1243. [Google Scholar] [CrossRef]
- Harpsøe, M.C.; Basit, S.; Bager, P.; Wohlfahrt, J.; Benn, C.S.; Nøhr, E.A.; Linneberg, A.; Jess, T. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: A study within the Danish National Birth Cohort. J. Allergy Clin. Immunol. 2012, 131, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Dumas, O.; Varraso, R.; Gillman, M.W.; Field, A.E.; Camargo, C.A. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 2016, 71, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Halonen, M.; Lohman, I.C.; Stern, D.A.; Ellis, W.L.; Rothers, J.; Wright, A.L. Perinatal Tumor Necrosis Factor-α Production, Influenced by Maternal Pregnancy Weight Gain, Predicts Childhood Asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Polinski, K.J.; Liu, J.; Boghossian, N.S.; McLain, A.C. Maternal Obesity, Gestational Weight Gain, and Asthma in Offspring. Prev. Chronic Dis. 2017, 14, 199–204.e3. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Lyu, J.; Xia, Y.; Ying, Y.; Hu, Y.; Qu, J.; Tong, S.; Li, S. Association of Maternal Prepregnancy Weight and Gestational Weight Gain With Children’s Allergic Diseases. JAMA Netw. Open 2020, 3, e2015643. [Google Scholar] [CrossRef]
- Srugo, S.A.; Fell, D.B.; Corsi, D.J.; Fakhraei, R.; Guo, Y.; Gaudet, L.M. Examining the role of pre-pregnancy weight and gestational weight gain in allergic disease development among offspring: A population-based cohort study in Ontario, Canada. Paediatr. Périnat. Epidemiol. 2021, 36, 144–155. [Google Scholar] [CrossRef]
- Drucker, A.M.; Pope, E.I.; Field, A.E.; Qureshi, A.A.; Dumas, O.; Camargo, C.A. Association Between Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain, and Offspring Atopic Dermatitis: A Prospective Cohort Study. J. Allergy Clin. Immunol. Pract. 2018, 7, 96–102.e2. [Google Scholar] [CrossRef] [PubMed]
- Deputy, N.; Sharma, A.; Kim, S.; Hinkle, S. Prevalence and Characteristics Associated With Gestational Weight Gain Ade-quacy. Obstet. Gynecol. 2015, 125, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Lavery, J.A.; Friedman, A.M.; Keyes, K.M.; Wright, J.D.; Ananth, C.V. Gestational diabetes in the United States: Temporal changes in prevalence rates between 1979 and 2010. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 804–813. [Google Scholar] [CrossRef]
- Rusconi, F.; Galassi, C.; Forastiere, F.; Bellasio, M.; De Sario, M.; Ciccone, G.; Brunetti, L.; Chellini, E.; Corbo, G.; La Grutta, S.; et al. Maternal Complications and Procedures in Pregnancy and at Birth and Wheezing Phenotypes in Children. Am. J. Respir. Crit. Care Med. 2007, 175, 16–21. [Google Scholar] [CrossRef]
- Nasreen, S.; Wilk, P.; Mullowney, T.; Karp, I. The effect of gestational diabetes mellitus on the risk of asthma in offspring. Ann. Epidemiol. 2021, 57, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Becker, A.B.; Kozyrskyj, A.L. Association of maternal diabetes and child asthma. Pediatr. Pulmonol. 2012, 48, 545–552. [Google Scholar] [CrossRef]
- Haataja, P.; Korhonen, P.; Ojala, R.; Hirvonen, M.; Paassilta, M.; Gissler, M.; Luukkaala, T.; Tammerla, O. Asthma and Atopic Dermatitis in Children Born Moderatly and Late Preterm. Eur. J. Pediatr. 2016, 175, 799–808. [Google Scholar] [CrossRef]
- Zugna, D.; Galassi, C.; Annesi-Maesano, I.; Baiz, N.; Barros, H.; Basterrechea, M.; Correia, S.; Duijts, L.; Esplugues, A.; Fantini, M.P.; et al. Maternal complications in pregnancy and wheezing in early childhood: A pooled analysis of 14 birth cohorts. Int. J. Epidemiol. 2015, 44, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zamstein, O.; Sheiner, E.; Wainstock, T.; Landau, D.; Walfisch, A. Maternal gestational diabetes and long-term respiratory related hospitalizations of the offspring. Diabetes Res. Clin. Pract. 2018, 140, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Adgent, M.A.; Gebretsadik, T.; Reedus, J.; Graves, C.; Garrison, E.; Bush, N.; Davis, R.; LeWinn, K.Z.; Tylavsky, F.; Carroll, K.N. Gestational diabetes and childhood asthma in a racially diverse US pregnancy cohort. Pediatr. Allergy Immunol. 2021, 32, 1190–1196. [Google Scholar] [CrossRef]
- Martinez, M.P.; Lin, J.; Chow, T.; Chung, J.; Wang, X.; Xiang, A.H. Maternal Gestational Diabetes and Type 2 Diabetes During Pregnancy and Risk of Childhood Asthma in Offspring. J. Pediatr. 2020, 219, 173–179.e1. [Google Scholar] [CrossRef]
- Westberg, A.P.; Salonen, M.K.; von Bonsdorff, M.; Osmond, C.; Kajantie, E.; Eriksson, J.G.; Von Bondsdorff, M. Maternal adiposity in pregnancy and offspring asthma in adulthood. Eur. Respir. J. 2018, 52, 1801152. [Google Scholar] [CrossRef]
- Lowe, A.J.; Ekéus, C.; Braback, L.; Rajaleid, K.; Forsberg, B.; Hjern, A. Impact of Maternal Obesity on Inhaled Corticosteroid Use in Childhood: A Registry Based Analysis of First Born Children and a Sibling Pair Analysis. PLoS ONE 2013, 8, e67368. [Google Scholar] [CrossRef]
- Conrad, L.A.; Cabana, M.D.; Rastogi, D. Defining pediatric asthma: Phenotypes to endotypes and beyond. Pediatr. Res. 2020, 90, 45–51. [Google Scholar] [CrossRef]
- Balekian, D.S.; Linnemann, R.W.; Hasegawa, K.; Thadhani, R.; Camargo, C.A. Cohort Study of Severe Bronchiolitis during Infancy and Risk of Asthma by Age 5 Years. J. Allergy Clin. Immunol. Pract. 2016, 5, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- Berggren, E.K.; Boggess, K.A.; Stuebe, A.M.; Funk, M.J. National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes. Am. J. Obstet. Gynecol. 2011, 205, 253.e1–253.e7. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Jemec, G.B.E.; Ulrik, C.S. Associations between maternal and environmental exposures on atopic disease in the offspring of mothers with asthma. Immun. Inflamm. Dis. 2021, 9, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, L.; Yao, H.; Dai, H.; Zheng, R.; Zhang, W. Prepregnancy BMI, gestational weight gain and risk of childhood atopic dermatitis: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Hivert, M.-F.; Udler, M.S. Defining Heterogeneity Among Women With Gestational Diabetes Mellitus. Diabetes 2020, 69, 2064–2074. [Google Scholar] [CrossRef]
- Liu, X.; Agerbo, E.; Li, J.; Dharmage, S.C.; Thomsen, R.W.; Olsen, J.; Munk-Olsen, T. Maternal pregestational or gestational diabetes and childhood wheezing: A population-based cohort study. Allergy 2018, 73, 2247–2250. [Google Scholar] [CrossRef]
- Headen, I.; Cohen, A.K.; Mujahid, M.; Abrams, B. The accuracy of self-reported pregnancy-related weight: A systematic review. Obes. Rev. 2017, 18, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Ekström, S.; Magnusson, J.; Kull, I.; Lind, T.; Almqvist, C.; Melén, E.; Bergström, A. Maternal BMI in Early Pregnancy and Offspring Asthma, Rhinitis and Eczema up to 16 Years of Age. Clin. Exp. Allergy 2015, 45, 283–291. [Google Scholar] [CrossRef] [PubMed]
| All (n = 16,351) | Child without Asthma (n = 14,045) | Child with Asthma (n = 2306) | p | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Early pregnancy BMI, kg/m2, % | <0.0001 | |||
| <20.0 | 8.2 | 8.3 | 7.4 | |
| 20.0–22.4 | 19.6 | 20.2 | 16.3 | |
| 22.5–24.9 | 22.6 | 22.7 | 21.8 | |
| 25–29.9 | 28.8 | 28.6 | 30.1 | |
| ≥30 | 20.8 | 20.2 | 24.4 | |
| GWG, lb, % | 0.02 | |||
| <15 | 13.8 | 13.5 | 16.0 | |
| 15–24 | 25.3 | 25.4 | 24.8 | |
| 25–34 | 34.0 | 34.1 | 33.0 | |
| 35–44 | 19.3 | 19.5 | 18.3 | |
| ≥45 | 7.6 | 7.5 | 7.9 | |
| GWG relative to recommendations, % | 0.29 | |||
| Under recommended weight gain | 23.8 | 23.8 | 24.3 | |
| Meets recommended weight gain | 36.4 | 36.6 | 34.9 | |
| Over recommended weight gain | 39.8 | 39.6 | 40.8 | |
| GDM, Carpenter–Coustan criteria (missing for 1180, 7%), % | 4.7 | 4.5 | 5.8 | 0.01 |
| GDM, National Diabetes Data Group criteria (missing for 1180, 7%), % | 2.9 | 2.7 | 4.1 | 0.0002 |
| Cesarean delivery, % | 28.3 | 27.7 | 32.2 | <0.0001 |
| Nulliparous, % | 0.24 | |||
| Yes | 47.3 | 47.4 | 46.7 | |
| No | 49.6 | 49.6 | 49.7 | |
| Missing | 3.1 | 3.0 | 3.6 | |
| Age at delivery, mean (SD) | 30.3 (6.4) | 30.4 (6.4) | 30.0 (6.4) | 0.01 |
| Maternal race/ethnicity, % | <0.0001 | |||
| White | 51.2 | 52.1 | 45.8 | |
| Black | 7.6 | 7.4 | 8.9 | |
| Hispanic | 20.2 | 19.9 | 22.2 | |
| Asian | 7.6 | 7.6 | 7.3 | |
| None of the above | 13.4 | 13.0 | 15.8 | |
| Maternal asthma, % | 8.0 | 7.2 | 12.8 | <0.0001 |
| Smoking status (smoker 3 months prior to pregnancy or during pregnancy), % | 7.8 | 7.7 | 8.3 | 0.30 |
| Insurance status at birth, % | <0.0001 | |||
| Private | 55.0 | 56.0 | 49.2 | |
| Public | 34.8 | 33.7 | 41.4 | |
| Limited | 6.6 | 6.6 | 6.3 | |
| Other | 3.6 | 3.7 | 3.0 | |
| Child’s characteristics | ||||
| Female, % | 47.9 | 49.2 | 39.9 | <0.0001 |
| Birth weight, lb, % | <0.0001 | |||
| <5.5 | 6.9 | 6.5 | 9.7 | |
| 5.5–6.9 | 26.7 | 26.8 | 25.6 | |
| 7.0–8.4 | 48.8 | 49.1 | 47.1 | |
| 8.5–9.9 | 16.1 | 16.2 | 15.6 | |
| ≥10 | 1.5 | 1.4 | 2.0 | |
| Gestational age at birth, weeks, % | <0.0001 | |||
| <32 | 1.1 | 0.8 | 2.7 | |
| 32–36 | 6.0 | 5.7 | 7.7 | |
| ≥37 | 92.9 | 93.5 | 89.6 | |
| Atopic dermatitis, % | 9.3 | 8.0 | 17.7 | <0.0001 |
| Allergic rhinitis, % | 10.4 | 8.2 | 24.3 | <0.0001 |
| All Asthma | Non-Allergic Asthma | Allergic Asthma | ||||
|---|---|---|---|---|---|---|
| No. of Cases | OR (95% CI) | No. of Cases | OR (95% CI) | No. of Cases | OR (95% CI) | |
| Maternal early pregnancy BMI, kg/m2 | ||||||
| <20.0 | 170 | 1.08 (0.89–1.32) | 97 | 0.89 (0.69–1.13) | 73 | 1.52 (1.12–2.06) |
| 20.0–22.4 (ref.) | 375 | 1 | 261 | 1 | 114 | 1 |
| 22.5–24.9 | 502 | 1.17 (1.01–1.35) | 328 | 1.08 (0.91–1.29) | 174 | 1.35 (1.06–1.72) |
| 25–29.9 | 695 | 1.21 (1.05–1.39) | 451 | 1.11 (0.94–1.31) | 244 | 1.44 (1.14–1.81) |
| ≥30 | 564 | 1.29 (1.12–1.50) | 388 | 1.24 (1.04–1.48) | 176 | 1.39 (1.09–1.79) |
| p-trend | 0.001 | 0.002 | 0.16 | |||
| Maternal GWG (lb) † | ||||||
| <15 | 369 | 1.04 (0.90–1.21) | 269 | 1.12 (0.94–1.32) | 100 | 0.88 (0.68–1.13) |
| 15–24 | 573 | 0.90 (0.84–1.06) | 363 | 0.89 (0.77–1.02) | 210 | 1.05 (0.87–1.28) |
| 25–34 (ref.) | 760 | 1 | 507 | 1 | 253 | 1 |
| 35–44 | 422 | 0.97 (0.85–1.10) | 281 | 0.95 (0.82–1.12) | 141 | 0.97 (0.78–1.20) |
| ≥45 | 182 | 1.01 (0.85–1.21) | 105 | 0.86 (0.69–1.08) | 77 | 1.31 (1.00–1.71) |
| p-trend | 0.84 | 0.20 | 0.14 | |||
| GWG relative to recommendations † | ||||||
| Under recommendations | 561 | 1.04 (0.92–1.17) | 380 | 1.04 (0.91–1.20) | 181 | 1.03 (0.84–1.25) |
| Meets recommendations | 805 | 1 | 540 | 1 | 265 | 1 |
| Over recommendations | 940 | 1.01 (0.91–1.13) | 605 | 0.96 (0.85–1.09) | 335 | 1.11 (0.94–1.32) |
| All Asthma | Non-Allergic Asthma | Allergic Asthma | ||||
|---|---|---|---|---|---|---|
| No. of Cases | OR (95% CI) | No. of Cases | OR (95% CI) | No. of Cases | OR (95% CI) | |
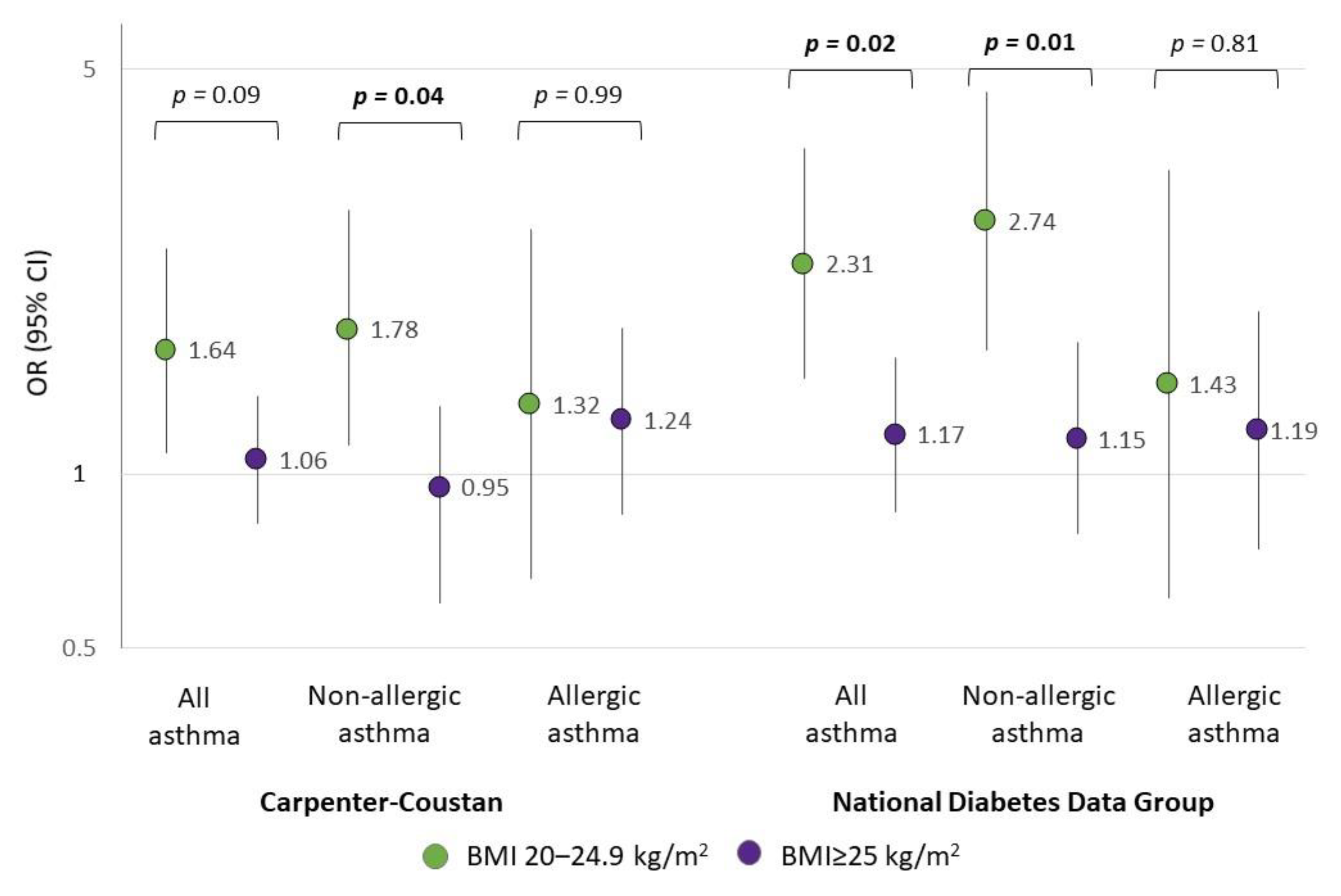
| GDM, Carpenter–Coustan criteria | ||||||
| No | 1980 | 1 | 1301 | 1 | 679 | 1 |
| Yes | 122 | 1.24 (1.01–1.53) | 78 | 1.21 (0.93–1.56) | 44 | 1.28 (0.93–1.76) |
| GDM, National Diabetes Data Group criteria | ||||||
| No | 2015 | 1 | 1318 | 1 | 697 | 1 |
| Yes | 87 | 1.46 (1.14–1.88) | 61 | 1.57 (1.17–2.11) | 26 | 1.23 (0.81–1.85) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumas, O.; Arroyo, A.C.; Faridi, M.K.; James, K.; Hsu, S.; Powe, C.; Camargo, C.A., Jr. Cohort Study of Maternal Gestational Weight Gain, Gestational Diabetes, and Childhood Asthma. Nutrients 2022, 14, 5188. https://doi.org/10.3390/nu14235188
Dumas O, Arroyo AC, Faridi MK, James K, Hsu S, Powe C, Camargo CA Jr. Cohort Study of Maternal Gestational Weight Gain, Gestational Diabetes, and Childhood Asthma. Nutrients. 2022; 14(23):5188. https://doi.org/10.3390/nu14235188
Chicago/Turabian StyleDumas, Orianne, Anna Chen Arroyo, Mohammad Kamal Faridi, Kaitlyn James, Sarah Hsu, Camille Powe, and Carlos A. Camargo, Jr. 2022. "Cohort Study of Maternal Gestational Weight Gain, Gestational Diabetes, and Childhood Asthma" Nutrients 14, no. 23: 5188. https://doi.org/10.3390/nu14235188
APA StyleDumas, O., Arroyo, A. C., Faridi, M. K., James, K., Hsu, S., Powe, C., & Camargo, C. A., Jr. (2022). Cohort Study of Maternal Gestational Weight Gain, Gestational Diabetes, and Childhood Asthma. Nutrients, 14(23), 5188. https://doi.org/10.3390/nu14235188






