Abstract
Pre-diabetic or early-stage type 2 diabetes patients may develop an adverse diabetic progression, leading to several complications and increasing hospitalization rates. Mulberry leaves, which contain 1-deoxynojirimycin (DNJ), have been used as a complementary medicine for diabetes prevention and treatment. Our recent study demonstrated that mulberry leaf powder with 12 mg of DNJ improves postprandial hyperglycemia, fasting plasma glucose, and glycated hemoglobin. However, the detailed mechanisms are still unknown. This study investigates the effect of long-term (12-week) supplementation of mulberry leaves in obese people with prediabetes and patients with early-stage type 2 diabetes. Participants’ blood was collected before and after supplementation. The protein profile of the plasma was examined by proteomics. In addition, the mitochondrial function was evaluated by energetic and homeostatic markers using immunoelectron microscopy. The proteomics results showed that, from a total of 1291 proteins, 32 proteins were related to diabetes pathogenesis. Retinol-binding protein 4 and haptoglobin protein were downregulated, which are associated with insulin resistance and inflammation, respectively. For mitochondrial function, the haloacid dehalogenase-like hydrolase domain-containing protein 3 (HDHD-3) and dynamin-related protein 1 (Drp-1) displayed a significant increment in the after treatment group. In summary, administration of mulberry leaf powder extract in prediabetes and the early stage of diabetes can alleviate insulin resistance and inflammation and promote mitochondrial function in terms of energy production and fission.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder and the most common found, making up over 90% of diabetes cases [,]. It is characterized by insulin resistance and the dysfunction of pancreatic islet β-cells, which leads to persistent hyperglycemia and altered lipid metabolism [,]. T2DM-related complications cause various life-threatening diseases, such as cardiovascular disease, cerebrovascular disease, neuropathy, nephropathy, retinopathy, foot disease, and peripheral gangrene [,,]. In 2021, epidemiological data of the International Diabetes Federation (IDF) reported that 6.7 million people died due to diabetes and 537 million adults aged between 20 and 79 years had diabetes. In addition, the American Diabetes Association (ADA) reported that the economic burden associated with diabetes increased 60% from 2007 to 2017 []. The global medical expenditure on diabetes was evaluated at approximately USD 760 billion in 2019 []. Hence, preventing and alleviating the progression of diabetes is important to reduce the economic and health burden. Glycemic control is considered as the main therapeutic goal for the treatment of T2DM as it reduces the complications related to T2DM []. Furthermore, pharmacological treatment depends on comorbidities and patient-centered factors []. However, it has been reported that oral medication for diabetes treatment may cause adverse effects in some patients [,,,].
Currently, natural or herbal compounds have been shown to have antidiabetic activities [,]. Mulberry (Morus alba L.) leaves have been used in traditional Chinese medicines for several centuries [] and have effects on diabetes therapy and its complications []. Interestingly, 1-deoxynojirimycin (DNJ), which is a well-known anti-diabetic agent because of its α-glucosidase inhibition activity, is the most-abundant phytochemical and bioactive compound in mulberry leaves [,,]. Previous research regarding mulberry leaf extract suggested that postprandial glycemia was improved in individuals with impaired glucose metabolism in a dose-dependent manner []. The elevation of postprandial glucose and insulin secretion were significantly suppressed in healthy volunteers [] and type 2 diabetic patients []. The plasma lipid profile in humans following the administration of mulberry leaf extract revealed that serum triglyceride levels decreased and lipoprotein levels were positively altered []. Nevertheless, the study of mulberry leaves on plasma protein expression is still limited.
Our previous randomized controlled clinical study of mulberry DNJ to find the optimal dose and examine the effect of the long-term administration of mulberry leaves revealed that 12 mg of mulberry DNJ was an optimal dose to reduce postprandial hyperglycemia, and there were no side effects []. Moreover, long-term ingestion of mulberry leaves showed a decrease in fasting plasma glucose (FPG). According to the results from a previous clinical study, the objective of this study aimed to examine the effect of long-term supplementation with mulberry leaves for the improvement of HbA1c in obese persons with prediabetes and patients with early-stage T2DM based on specific mechanisms. Plasma protein expression and mitochondrial functions were investigated using proteomics and electron microscopic studies. The significance of this study is that DNJ in mulberry leaf extract can be an upcoming organic candidate for inhibiting the progression of diabetes and improving clinical outcomes.
2. Materials and Methods
2.1. Mulberry Leaf Powder Preparation and Determination of DNJ
The mulberry leaf powder production process and DNJ determination were described previously []. Briefly, it was made from 50–70-day-old fresh mulberry leaves (Chiang Mai, Thailand), which were dried using electromagnetic radiation in a microwave oven. The dried mulberry leaves were finely ground and sieved through an 80-mesh sieve. Then, the powder was sterilized by gamma radiation and packed in sachets. Liquid chromatography with tandem mass spectrometry (API 3000 LC-MS/MS) (Sciex, Framingham, MA, USA) was applied to determine the DNJ in the mulberry leaf powder. The minimum DNJ in the mulberry leaf powder used in this study was 2.6 mg/g of dried weight.
2.2. Study Design
2.2.1. Ethical Statement and Participant Inclusion
The study protocol was examined and approved by the Institutional Review Boards of the Royal Thai Army Medical Department, Phramonkutklao College of Medicine (No. Q038h/60). This study is a part of our previous clinical trial []. Recruitment criteria in our previous study were based on the ADA [,]. Briefly, participant recruitment involved obese persons aged between 20 and 65 years who had a BMI ≥ 25 kg/m2, a 75 g oral glucose tolerance test of 140–199 mg/dL, and an FPG of 100–140 mg/dL and/or 2 h postprandial glucose. The criteria for exclusion were as follows: persons who have been on antihyperglycemic drugs or had a record of diabetes treatment, high levels of aspartate transaminase and/or alanine transaminase ≥ three-times the upper limit of normal, diabetic complications and life-threatening conditions, creatinine ≥ 2.0 mg/dL, pregnancy or lactation, taking medication, supplements, or herbs affecting blood glucose levels within one month of study enrollment. Regarding our inclusion criteria, the overall population was 54 participants. They were randomly allocated into 2 groups: (i) 12 mg of DNJ as a treatment group (n = 28) and (ii) a non-treatment group (n = 26) over 12 weeks. According to the result from our previous trial, 12 mg of DNJ is an optimal dose to reduce postprandial hyperglycemia, improves HbA1C, and has no adverse effect. In addition, 12 participants in the treatment group had HbA1C reduction after 12-week supplementation of 12 mg of DNJ mulberry leaf powder. Thus, 12 participants from the treatment group were selected and used in the present study.
2.2.2. Nutritional and Diet Control
Nutritional consulting with a licensed dietitian was performed on all participants. They were given the education about how to improve eating habits including a review and discussion of usual eating habits and developing an individualized eating pattern. Besides, participants were assigned to record a food diary such as foods, cooking methods of foods, snacks, drinks, and portions that they consumed on a representative day. Total caloric intake and the percentage of carbohydrate, protein, and fat consumed were calculated by the Thai Nutrisurvey® software version 2.0 (developed by Faculty of Tropical Medicine, Mahidol university, Nakhon Pathom, Thailand). The assessment of the total caloric intake and macronutrients between the treatment and control groups at Weeks 0 and 12 is presented in Table 1. These determined that all participants had the same nutritional background before being subjected to the experiment.

Table 1.
The mean total calorie and macronutrient intake per day at Weeks 0 and 12 (n = 54).
2.2.3. Human Experimental Protocol
Participants received 4.6 g of mulberry leaf powder along with nutritional control over the 12 weeks of the study. A single-ration sachet contained 4.6 g of mulberry leaf powder contained 12 mg of DNJ. For the instructions, the mulberry leaf powder from the sachet was mixed with 150 mL of warm water and ingested three times per day before meals.
2.2.4. Specimen Collection and Plasma Preparation
Blood specimens of participants were collected at Week 0 and Week 12. The blood sample was centrifuged for blood fractionation, and the plasma was collected. According to the results of the previous study, 12 participants who had a reduction of HbA1c in the treatment group were selected for this study. The clinical characteristics of the participants are described in Table 2. These determined that all participants had the same clinical background before being subjected to the experiment. Their plasma specimens were used for proteomic analysis. Peripheral blood mononuclear cells (PBMCs) were collected from the buffy coat in a blood fractionation process for ultrastructure examination.

Table 2.
Clinical characteristic of the participants.
The plasma samples before treatment at Week 0 (n = 12) and after treatment at Week 12 (n = 12) were pooled within the same group, and each group was divided into three groups of four samples per group. Each of the pooled samples were considered as independent replicates and analyzed separately.
2.3. Label-Free Quantitative Proteomics of Plasma Samples from Mulberry Leaf Treatment
2.3.1. Plasma Sample Extraction and Protein Size Separation
To investigate the effect of mulberry leaves on protein expression in the plasma of people with impaired glucose metabolism, such as obese persons with prediabetes and patients with early-stage T2DM, the plasma specimens were used to quantify the concentration of total protein using a Bradford protein assay (Bio-Rad®, Hercules, CA, USA). For the SDS polyacrylamide gel electrophoresis (SDS-PAGE), a 4% stacking and 12% separating gel was loaded with 30 µg of each crude protein from the plasma samples and run with a constant voltage at 120 V for 80 min in the gel electrophoresis unit (Bio-Rad®, USA), until the bromophenol blue dye reached the bottom of the gel.
2.3.2. In-Gel Tryptic Digestion
The gel was stained using Coomassie Blue-R for 10 min and destained with destaining solution (30% methanol and 10% acetic acid) for 3 h. Afterwards, the destained gel was soaked in distilled water for 15 min before gel photography via a gel documentary system (Bio-Rad®, USA). Entire gel lanes were cut, and each lane was incised into 12 pieces, which were individually placed into 1.5 mL tubes. All gel pieces were dehydrated using 50% acetonitrile (Merck®, Kenilworth, NJ, USA) in HPLC-grade water (Merck®, Kenilworth, NJ, USA). They were incubated in 7 mM DTT in 50 mM ammonium bicarbonate (NH4HCO3) for 15 min at 60 °C. Proteins were alkylated in 250 mM iodoacetamide at room temperature in the dark for 30 min. The alkylation reaction was quenched by adding 7 mM DTT in 50 mM NH4HCO3. The gel pieces were dehydrated in 100% acetonitrile and dried at room temperature for 1 h. For trypsin digestion, trypsin in 50 mM NH4HCO3 was added to each gel piece at 37 °C for 16 h. Acetonitrile was added and incubated for 20 min at room temperature to collect the digested peptides. The samples were centrifuged at 10,000× g for 15 min at room temperature. The supernatant was collected and transferred into new 1.5 mL tubes. Then, the supernatant, which contained the digested peptides, was completely dried using a CentriVap Vacuum Concentrator (Labconco, Kansas City, MO, USA) at 40 °C.
2.3.3. Protein Identification
The dried peptides were resuspended in 0.1% formic acid for LC-MS/MS analysis. They were subjected to the UltiMate® 3000 Nano-LC system (Dionex, Altrincham, UK). The column was an Acclaim PepMap RSLC C18 75 μm × 15 cm (Thermo Scientific, Waltham, MA, USA) in stationary phase, at a flow rate of 300 nL/min. Mobile phase Solutions A and B consisted of 0.1% formic acid and 80% acetonitrile in 0.1% formic acid, respectively. The initial mobile phase step was performed using 4% of Solution B for 5 min. Gradient conditions were used to elute the peptides, from 4% to 50% of Solution B for 30 min, holding for 5 min, and then, returning to the initial phase for 10 min. Subsequently, the eluted peptides were subjected to a positive electrospray ionization system coupled with a microTOF-Q II (Bruker, Bremen, Germany). The MS and MS/MS spectra covered the mass range of m/z 400–2000 and m/z 50–1500, respectively. The raw data files were converted to mascot generic files using the DataAnalysis software version 3.4 (Bruker, Bremen, Germany) Mascot Daemon version 2.3.02 (Matrix Science, London, UK) was used for the identification and quantification of the proteins. The protein database was obtained from SwissProt specific to Homo sapiens (searching date: 2 November 2020). For the search parameter settings, the methionine oxidation and carbamidomethylation of cysteines were set as the fixed modification and variable modification, respectively. The identified proteins with a significant score (p < 0.05) that appeared in at least two samples in the group were reported. The semi-quantitative protein expression from peptide counts was determined by the Exponentially Modified Protein Abundance Index (emPAI) []. The change of protein expression was analyzed by comparing the emPAI count before and after treatment. The characterization of biological processes and proteins associated with organs was accomplished using the UniProt database (www.uniprot.org (accessed on 30 December 2020)). The STRING software was used to predict protein–protein interaction networks (https://sring-db.org/ (accessed on 30 December 2020)).
2.4. Extraction of Mitochondria from Peripheral Blood Mononuclear Cells before and after Mulberry Leaf Treatment
Mitochondria were extracted form PBMCs using a Mitochondria Isolation Kit (My BioSource, San Diego, CA, USA). In brief, mitochondria isolation buffer was added to the sample and vortexed for 5 s, followed by incubation on ice for 2 min. Then, the sample had added 10 µL of Reagent A and was vortexed for 5 s before incubation on ice for 5 min, vortexing every minute for 5 s. The sample was then centrifuged at 600× g for 10 min at 4 °C. The supernatant was transferred into a separate tube and centrifuged at 7000× g for 10 min at 4 °C. The supernatant was discarded, and the pellet was washed with mitochondria isolation buffer. Afterwards, the sample was repeatedly centrifuged, and the pellet was resuspended in storage buffer.
2.5. Mitochondrial Immunogold Labeling from PBMCs
To examine the specific markers that relate to PBMCs mitochondrial functions before and after mulberry leaf treatment, immunogold labeling was performed. The extracted mitochondria were pooled in each group, before and after treatment. They were fixed in 2.5% glutaraldehyde for 1 h and 1% osmium tetroxide for 1 h, respectively. They were dehydrated in serial-graded ethanol and infiltrated with a series of LR white resin (EMS®, Houston, TX, USA). The samples were embedded in pure LR white (EMS®, USA) and polymerized at 60 °C for 48 h. The mitochondrial samples were cut into 100 nm. Subsequently, they were incubated with primary antibodies, including the fusion marker antioptic atrophy 1 (OPA-1), the fission marker antidynamin-related protein 1 (Drp-1), the energy marker antihaloacid dehalogenase-like hydrolase-domain-containing protein 3 (HDHD-3), and nuclear factor erythroid-2-related factor 2 (Nrf-2) for 1 h and washed using 0.1% BSA in PBS. Then, goat anti-rabbit or mouse IgG conjugated with ultra-small (3–5 nm) or 10 nm gold particles (EMS®, USA) were applied to the sections and washed several times using 0.1% BSA in PBS. A silver enhancement (Aurion R-Gent SE-EM kit, EMS, USA) was used to improve the contrast of gold particle labelling after washing the mitochondrial sections with distilled water. They were stained with lead citrate and uranyl acetate prior to observation under a transmission electron microscope. The number of labelled gold particles was counted as immunolabeling per field.
2.6. Statistical Analysis
Statistical analysis was performed using GraphPad Prism® version 9 (GraphPad, San Diego, CA, USA). The immunogold labelling was analyzed using the paired t-test. The difference between the before and after treatment groups is presented as the mean ± the standard deviation (SD) and the range. The statistical significance was defined at p-values < 0.05.
3. Results
3.1. Identification of the Protein Profile in Plasma before and after Mulberry Leaf Treatment
To determine the effects of mulberry leaves and specific mechanisms that involve reduced the HbA1C in the plasma of people with impaired glucose metabolism, such as obese persons with prediabetes and patients with early-stage T2DM, label-free proteomics was performed to discover the plasma protein profile. The results were acquired from plasma specimens of the selected cases before (Week 0) and after (Week 12) mulberry leaf treatment. The crude protein of the plasma samples was extracted, and extracted proteins (30 µg) were separated using SDS-PAGE gels (Figure 1A). Each gel lane was excised into 12 pieces and individually digested in a tryptic digestion process prior to mass spectrometry. A total of 1291 proteins were identified from the plasma before and after mulberry leaf treatment. The protein expression in the before and after treatment groups found that 308 and 204 different proteins were presented only in the before and after treatment groups, respectively (Supplementary Material S1), while 779 proteins were expressed in both groups (Figure 1B). The overlapping 779 proteins were analyzed, and the result showed 7 up-expressed and 4 down-expressed proteins (Supplementary Material S1).
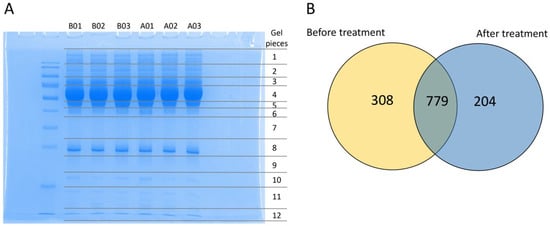
Figure 1.
The label-free quantitative proteomic analysis of plasma specimens from the mulberry leaf treatment study. (A) SDS-PAGE gel stained with Coomassie Blue-R of plasma proteins from the before and after treatment group. Each lane represents the plasma samples from before treatment with triplication (B01, B02, and B03, respectively) and after treatment with triplication (A01, A02, and A03, respectively). (B) The illustration of the total of 1291 proteins revealed 308 and 204 differentially expressed proteins expressed in the before and after treatment groups, respectively. The overlapping 779 proteins indicated the number of altered proteins shared between the before and after treatment groups.
3.2. Differentiation of Proteins, Their Function, and Associated Pathways
All of the proteins from the 308 proteins in the before treatment group, the 204 proteins in the after treatment group, and the 7 up-expressed and 4 down-expressed proteins in both groups were identified according to biological functions. Gene ontology enrichment analysis was performed to focus on the specific proteins related to metabolic disturbance in T2DM pathogenesis. The result revealed 20 unique proteins presented in the before treatment group, 10 proteins found in the after treatment group, and 2 down-expressed proteins in both groups. These specific proteins were closely associated with the pathogenesis and pathophysiology of metabolic disturbance in T2DM. The functional protein interaction and associated pathway of the different proteins were explored using the STRING database and KEGG pathway database, respectively. The associated pathways were then classified into potential 13 pathways and categorized into 4 major groups: (i) metabolic regulation (21.62%), (ii) inflammatory response (24.32%), (iii) immune response (27.03%), and (iv) ECM constituents and organization (27.03%) (Figure 2 and Figure 3). All differential proteins are illustrated in Table 3.
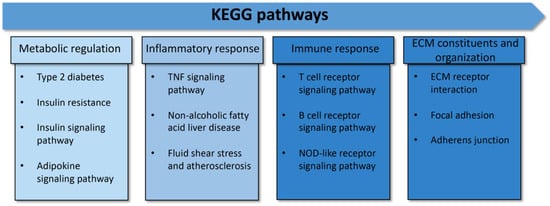
Figure 2.
Protein categorization by KEGG pathways. The 13 associated pathways in metabolic disturbance in type 2 diabetes were categorized into 4 major groups: 1. metabolic regulation, 2. inflammatory response, 3. immune response, and 4. ECM constituents and organization.
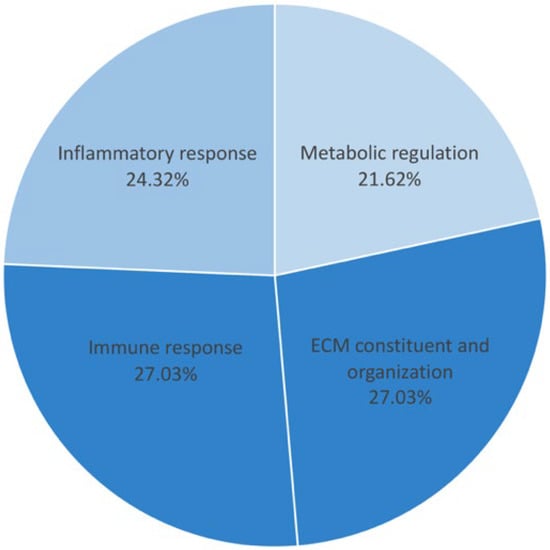
Figure 3.
The percentage of proteins in 4 major pathways. The metabolic regulation pathway was 21.62%; the inflammatory response pathway was 24.32%; the immune response and ECM constituents and organization pathway were 27.03%.

Table 3.
The list of differential proteins associated with metabolic disturbance in type 2 diabetes.
3.3. Immunogold Labeling of PBMCs’ Mitochondria from before and after Treatment
To investigate the effect of mulberry leaf treatment in term of PBMCs’ mitochondrial functions, immunogold labeling was performed. The specific markers related to mitochondrial functions were examined, including the fusion marker (OPA-1), fission marker (Drp-1), and energy production markers (HDHD-3 and Nrf-2). The result demonstrated that the expression of HDHD-3 (Figure 4A–C) and Drp-1 (Figure 4D–F) in the PBMCs’ mitochondria after treatment was significantly higher than in the PBMCs’ mitochondria before treatment. However, the expression of Nrf-2 (Figure 4G–I) and OPA-1 (Figure 4J–L) was not significantly different between the PBMCs’ mitochondria in the before and after treatment groups.
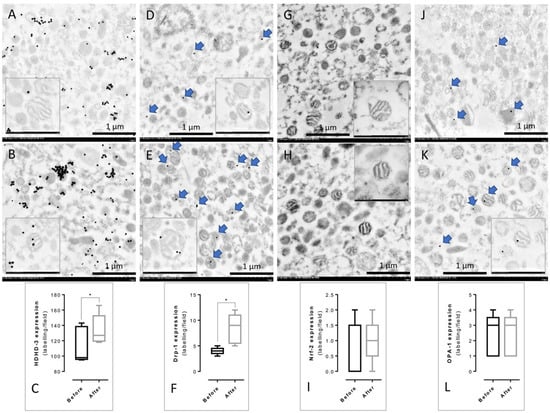
Figure 4.
Immunogold labeling of the PBMCs’ mitochondria from the before and after treatment groups. Before treatment (A,D,G,J) and after treatment (B,E,H,K) with gold labeling (arrow) were revealed. The presence of mitochondrial function markers was represented in HDHD-3 (A–C), Drp-1 (D–F), Nrf-2 (G–I), and OPA-1 (J–L). All data in the bar graph are presented as the mean ± SD, * p < 0.05.
4. Discussion
The present study investigated the effect of long-term mulberry leaf supplementation and specific mechanisms that improved the HbA1C in the plasma protein profile and mitochondrial functions in patients with early-stage T2DM and obese persons with prediabetes. The results of our experiment demonstrated that a total of 1291 plasma proteins were identified from the before and after treatment groups. We found 308 unique proteins present in the before treatment plasma, 204 unique proteins in the after treatment group, and 11 unique proteins in both groups (Figure 1). These proteins were then classified to determine the biological functions and specific proteins that relate to T2DM pathogenesis, identifying 20 proteins in the before treatment group, 10 proteins in the after treatment group, and 2 down-expressed proteins present in both groups (Table 3). These 32 proteins were identified with four associated pathways: (i) metabolic regulation, (ii) inflammatory response, (iii) immune response, and (iv) ECM constituents and organization (Figure 2). Moreover, the percentage of the 32 different proteins involved in the four pathways was 21.62% for the metabolic regulation pathway, 24.32% for the inflammatory response pathway, 27.03% for the immune response pathway, and 27.03% for the ECM constituents and organization pathway, respectively (Figure 3). Interestingly, proteins related to insulin resistance and inflammation-induced hyperglycemia (retinol-binding protein 4 and haptoglobin, respectively) were downregulated after treatment. In addition, it has been reported that mitochondrial dysfunction related to T2DM causes the lowering of ATP production and β-oxidation and a higher production of reactive oxygen species, leading to insulin resistance [,,,,]. Therefore, to determine the mitochondrial functions of the PBMCs in terms of homeostatic proteins (fusion and fission) and energy production, immunogold labeling was performed in this study. The results showed the significant upregulation of HDHD-3 and Drp-1 in the after treatment group (Figure 4). According to these results, mulberry leaf powder extract treatment contributes to an increase in the energy maintenance protein and mitochondrial fission, which facilitates the removal of damaged mitochondria [].
In the present study, protein expressions in the after mulberry leaf treatment group were discovered and categorized into metabolic regulation, ECM constituent and organization, and immune response pathways. Insulin receptor substrate 2 (IRS2) was found, which is a major insulin receptor substrate in the insulin signaling pathway and has been indicated to play a crucial role in glucose metabolism and adipose tissue []. The upregulated expression of IRS2 can prevent the progression of diabetes []. Oxysterol LXR-alpha receptor expression serves as an endogenous ligand in cholesterol metabolism and stimulates excess cholesterol flux via the reverse cholesterol transport by high-density lipoprotein []. This result indicated that long-term mulberry leaf supplementation containing 12 mg of DNJ improves the metabolic pathway by altering glucose and cholesterol metabolisms via the expression of IRS2 and oxysterol LXR-alpha proteins. Interestingly, we found the new proteins associated with immune response after mulberry leaf ingestion were pyrin and NACHT-, LRR-, and PYD-domain-containing protein 7. According to this finding, mulberry leaf supplementation may improve the immunological protein level. Nevertheless, the evidence and the function of these two proteins in relation to diabetes are still unclear. Furthermore, integrin β-6 was present in the after treatment group, which is a transmembrane receptor that mediates cell adhesion, migration, and proliferation. Jacobsen et al. suggested that integrin β-6 may prohibit re-epithelialization of diabetic wounds []. Dynamin 1-like protein (drp1) was also found in this group; its function is to regulate mitochondrial fission []. It was reported that metformin can suppress excessive mitochondrial fission by inhibiting drp1 protein activity and restoring mitochondrial homeostasis in diabetic mice []. Son of sevenless protein 1 (SOS1) is involved in the tyrosine phosphorylation of insulin receptor substrate 1 (IRS1) in the insulin signaling pathway []. CREB-binding protein acts as a transcriptional factor and is located in the nucleus. Depletion of CREB-binding protein in the vasculature may enhance atherosclerosis in a diabetes in vivo model []. Finally, the proteins in this group associated with metabolic regulation such as the insulin signaling pathway and cholesterol metabolism may enhance the metabolic pathway, leading to an improvement in insulin resistance and prohibit the progression of diabetes. Pyrin and NACHT-, LRR-, and PYD-domain-containing protein 7 were found in our study; these two proteins are involved in the immune response, and their function in diabetes is still ambiguous.
One protein discovered in both the before and after treatment groups, retinol-binding protein 4 (RBP4), was downregulated in our study. RBP4 is a protein secreted by adipocytes and is a useful biomarker for systemic insulin resistance []. Additionally, the increase in serum RBP4 levels correlated with insulin resistance among the subjects with T2DM, obesity, and impaired glucose tolerance [,]. The other downregulated protein was a haptoglobin (Hp), which is an acute-phase protein in inflammation. Elevated Hp is also influenced by inflammation, obesity, hypertension, and their polymorphism []. In agreement with this finding, this information could suggest that mulberry leaves reduce insulin resistance and inflammation by the downregulation of RBP4 and Hp proteins, respectively.
Before mulberry leaf treatment, the discovered proteins were mostly related to the immune, inflammatory response, and ECM constituent and organization pathways and found to be only slightly related to the metabolic regulation pathway (Table 3). The proteins in this group were mainly found to be various types of collagen, such as type 2, type 4, and type 6 collagen. However, we found a few proteins present in the ECM constituent and organization pathway related to T2DM in the after treatment group. Moreover, other proteins were predominantly present in the immune and inflammatory response pathways, such as proteins involving the activation of NF-κB, the acute phase reactant, and the TNF-α receptor (Table 3). The proteins shown in the metabolic regulation pathway included phosphatidylinositol 3-kinase regulatory subunit beta (PIK3R2) and the insulin receptor, an important enzyme and receptor in the insulin signaling pathway [], and putative hexokinase HKDC1, which is a hexokinase involved in glucose metabolism []. Although, we found some proteins that may play an important role in the insulin signaling pathway, the DNJ in the mulberry leaves could not maintain the prolonged action of those proteins after mulberry leaf supplementation. On the other hand, DNJ improved insulin signaling and insulin resistance through the expression of these proteins in the after treatment group.
The limitations of this study should be noted, regarding our limited sub-population of participants, who were selected from our previous trial using the reduction of HbA1C criterion []. However, our results provide preliminary data for further studies associated with the effect of DNJ on insulin resistance and inflammation.
5. Conclusions
The effect of the long-term supplementation of mulberry leaf powder and specific mechanisms that involve reducing the HbA1C in individuals with prediabetes and early-stage T2DM was examined using plasma via proteomic and mitochondrial immunogold labeling. The plasma protein profile demonstrated that 12 mg of DNJ in mulberry leaf powder could enhance metabolic regulation by modulating the expression of signaling proteins in the insulin signaling pathway and an endogenous ligand in cholesterol metabolism. The downregulation of secreted adipocyte and acute-phase proteins may reduce insulin resistance and inflammation. Mitochondrial energy homeostasis and fission proteins increased after mulberry leaf supplementation. Our study provides information regarding the effect of long-term mulberry leaf supplementation and specific mechanisms, which could improve clinical outcomes and be further developed as an alternative supplementation for diabetes patients.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14214538/s1, Supplementary Material S1: The differential proteins in the before and after treatment groups.
Author Contributions
Conceptualization, K.F., S.A., O.R. and P.A.; methodology, K.F., T.T., K.R., T.K. and O.R.; validation, P.A.; formal analysis, K.F., S.A., O.R. and P.A.; resources, P.A.; data curation, K.F., S.A., O.R. and P.A.; writing—original draft preparation, K.F.; writing—review and editing, K.F., S.A., O.R. and P.A.; visualization, P.A.; supervision, P.A.; funding acquisition, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Thailand Science Research and Innovation Fund Chulalongkorn University (CU_FRB65_hea(50)_059_33_03) and The National Research Council of Thailand.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of the Royal Thai Army Medical Department, Phramonkutklao College of Medicine (approval number: Q038h/60 and approval date: 12 February 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The additional support was provided by Faculty of Tropical Medicine, Mahidol University and Faculty of Pharmaceutical Sciences, Chulalongkorn University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Blaslov, K.; Naranda, F.S.; Kruljac, I.; Renar, I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes 2018, 9, 209–219. [Google Scholar] [CrossRef]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Clements, R.S., Jr.; Bell, D.S. Complications of diabetes. Prevalence, detection, current treatment, and prognosis. Am. J. Med. 1985, 79, 2–7. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, C.; Wang, W.; Xu, B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients—A cross-sectional hospital based survey in urban China. Health Qual. Life Outcomes 2010, 8, 62. [Google Scholar] [CrossRef]
- Carracher, A.M.; Marathe, P.H.; Close, K.L. International Diabetes Federation 2017. J. Diabetes 2018, 10, 353–356. [Google Scholar] [CrossRef]
- American Diabetes, A. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besancon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef]
- Afroz, A.; Ali, L.; Karim, M.N.; Alramadan, M.J.; Alam, K.; Magliano, D.J.; Billah, B. Glycaemic Control for People with Type 2 Diabetes Mellitus in Bangladesh—An urgent need for optimization of management plan. Sci. Rep. 2019, 9, 10248. [Google Scholar] [CrossRef]
- American Diabetes, A. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef]
- Frenchman, I.B. Treatment and treatment-related adverse events of type 2 diabetes mellitus in residents of long-term care facilities: A retrospective study. Curr. Ther. Res. Clin. Exp. 2003, 64, 1–9. [Google Scholar] [CrossRef][Green Version]
- Loke, Y.K.; Singh, S.; Furberg, C.D. Long-term use of thiazolidinediones and fractures in type 2 diabetes: A meta-analysis. Can. Med. Assoc. J. 2009, 180, 32. [Google Scholar] [CrossRef]
- Razavi-Nematollahi, L.; Ismail-Beigi, F. Adverse Effects of Glycemia-Lowering Medications in Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 132. [Google Scholar] [CrossRef]
- Seidu, S.; Cos, X.; Brunton, S.; Harris, S.B.; Jansson, S.P.O.; Mata-Cases, M.; Neijens, A.M.J.; Topsever, P.; Khunti, K. 2022 update to the position statement by Primary Care Diabetes Europe: A disease state approach to the pharmacological management of type 2 diabetes in primary care. Prim. Care Diabetes 2022, 16, 223–244. [Google Scholar] [CrossRef]
- Chang, C.L.; Lin, Y.; Bartolome, A.P.; Chen, Y.C.; Chiu, S.C.; Yang, W.C. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid. Based Complement. Altern. Med. 2013, 2013, 378657. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Lown, M.; Fuller, R.; Lightowler, H.; Fraser, A.; Gallagher, A.; Stuart, B.; Byrne, C.; Lewith, G. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: Results of a randomised double-blind placebo-controlled study. PLoS ONE 2017, 12, e0172239. [Google Scholar] [CrossRef]
- Zhang, L.; Su, S.; Zhu, Y.; Guo, J.; Guo, S.; Qian, D.; Ouyang, Z.; Duan, J.A. Mulberry leaf active components alleviate type 2 diabetes and its liver and kidney injury in db/db mice through insulin receptor and TGF-beta/Smads signaling pathway. Biomed. Pharmacother. 2019, 112, 108675. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, D.; Singh, P.; Kumar, U.; Singh, V. Mulberry 1-deoxynojirimycin (DNJ): An exemplary compound for therapeutics. J. Hortic. Sci. Biotechnol. 2020, 95, 679–686. [Google Scholar]
- Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules 2016, 21, 1600. [Google Scholar] [CrossRef]
- Oku, T.; Yamada, M.; Nakamura, M.; Sadamori, N.; Nakamura, S. Inhibitory effects of extractives from leaves of Morus alba on human and rat small intestinal disaccharidase activity. Br. J. Nutr. 2006, 95, 933–938. [Google Scholar] [CrossRef]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef]
- Banu, S.; Jabir, N.R.; Manjunath, N.C.; Khan, M.S.; Ashraf, G.M.; Kamal, M.A.; Tabrez, S. Reduction of post-prandial hyperglycemia by mulberry tea in type-2 diabetes patients. Saudi J. Biol. Sci. 2015, 22, 32–36. [Google Scholar] [CrossRef]
- Kojima, Y.; Kimura, T.; Nakagawa, K.; Asai, A.; Hasumi, K.; Oikawa, S.; Miyazawa, T. Effects of mulberry leaf extract rich in 1-deoxynojirimycin on blood lipid profiles in humans. J. Clin. Biochem. Nutr. 2010, 47, 155–161. [Google Scholar] [CrossRef]
- Thaipitakwong, T.; Supasyndh, O.; Rasmi, Y.; Aramwit, P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement. Ther. Med. 2020, 49, 102292. [Google Scholar] [CrossRef]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef]
- Krako Jakovljevic, N.; Pavlovic, K.; Jotic, A.; Lalic, K.; Stoiljkovic, M.; Lukic, L.; Milicic, T.; Macesic, M.; Stanarcic Gajovic, J.; Lalic, N.M. Targeting Mitochondria in Diabetes. Int. J. Mol. Sci. 2021, 22, 6642. [Google Scholar] [CrossRef]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S.; Lee, K.U.; Lee, H.K. Mitochondrial metabolism and diabetes. J. Diabetes Investig. 2010, 1, 161–169. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef]
- Sharma, K. Mitochondrial hormesis and diabetic complications. Diabetes 2015, 64, 663–672. [Google Scholar] [CrossRef]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Turaihi, A.H.; Bakker, W.; van Hinsbergh, V.W.M.; Serne, E.H.; Smulders, Y.M.; Niessen, H.W.M.; Eringa, E.C. Insulin Receptor Substrate 2 Controls Insulin-Mediated Vasoreactivity and Perivascular Adipose Tissue Function in Muscle. Front. Physiol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Hennige, A.M.; Burks, D.J.; Ozcan, U.; Kulkarni, R.N.; Ye, J.; Park, S.; Schubert, M.; Fisher, T.L.; Dow, M.A.; Leshan, R.; et al. Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J. Clin. Investig. 2003, 112, 1521–1532. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Murzilli, S.; Salvatore, L.; D’Errico, I.; Petruzzelli, M.; Conca, P.; Jiang, Z.Y.; Calabresi, L.; Parini, P.; Moschetta, A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010, 12, 187–193. [Google Scholar] [CrossRef]
- Jacobsen, J.N.; Steffensen, B.; Hakkinen, L.; Krogfelt, K.A.; Larjava, H.S. Skin wound healing in diabetic beta6 integrin-deficient mice. APMIS 2010, 118, 753–764. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Yang, Y.; Tang, H.; Si, Y.; Chen, Z.; Shi, Y.; Fang, H. Metformin Protects Against Diabetes-Induced Cognitive Dysfunction by Inhibiting Mitochondrial Fission Protein DRP1. Front. Pharmacol. 2022, 13, 832707. [Google Scholar] [CrossRef]
- Roth, R.A.; Liu, F.; Chin, J.E. Biochemical mechanisms of insulin resistance. Horm. Res. 1994, 41 (Suppl. S2), 51–55. [Google Scholar] [CrossRef]
- Watson, P.A.; Nesterova, A.; Burant, C.F.; Klemm, D.J.; Reusch, J.E. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J. Biol. Chem. 2001, 276, 46142–46150. [Google Scholar] [CrossRef]
- Park, S.E.; Park, C.Y.; Sweeney, G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit. Rev. Clin. Lab. Sci. 2015, 52, 180–190. [Google Scholar] [CrossRef]
- Graham, T.E.; Yang, Q.; Bluher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.A.; Smith, U.; et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Pietrani, N.T.; Carvalho, L.M.L.; Bosco, A.A.; Sandrim, V.C.; Ferreira, C.N.; Gomes, K.B. Haptoglobin levels are influenced by Hp1–Hp2 polymorphism, obesity, inflammation, and hypertension in type 2 diabetes mellitus. Endocrinol. Diabetes Y Nutr. 2019, 66, 99–107. [Google Scholar]
- Kushi, R.; Hirota, Y.; Ogawa, W. Insulin resistance and exaggerated insulin sensitivity triggered by single-gene mutations in the insulin signaling pathway. Diabetol. Int. 2021, 12, 62–67. [Google Scholar] [CrossRef]
- Khan, M.W.; Terry, A.R.; Priyadarshini, M.; Ilievski, V.; Farooq, Z.; Guzman, G.; Cordoba-Chacon, J.; Ben-Sahra, I.; Wicksteed, B.; Layden, B.T. The hexokinase “HKDC1” interaction with the mitochondria is essential for liver cancer progression. Cell Death Dis. 2022, 13, 660. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).