The Role of Berry Consumption on Blood Pressure Regulation and Hypertension: An Overview of the Clinical Evidence
Abstract
:1. Introduction
2. Berry Polyphenols and Blood Pressure
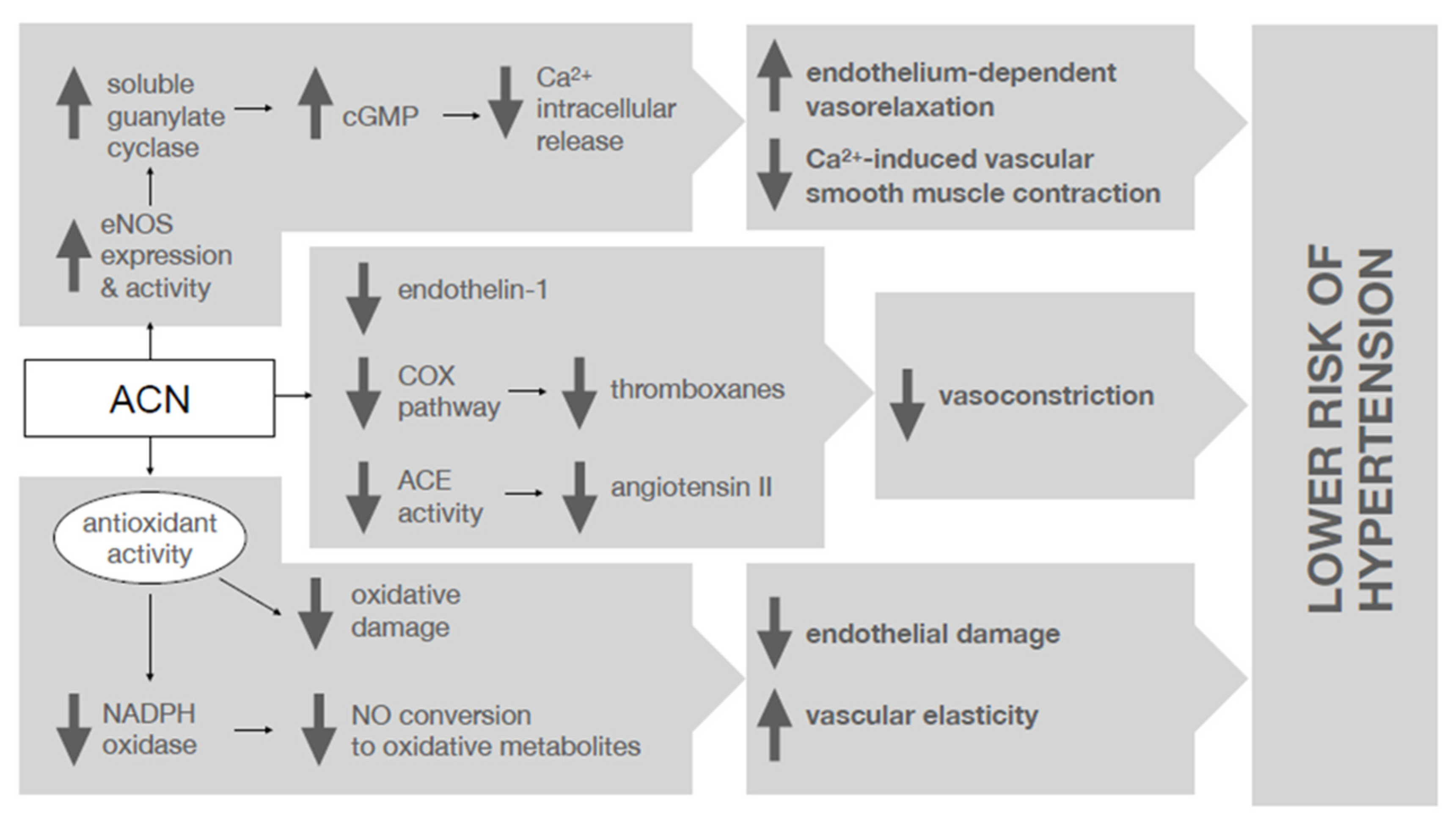
2.1. Anthocyanins
2.2. Condensed Tannins (Proanthocyanidins)
2.3. Ellagic Acid
3. Berry Consumption and Blood Pressure
3.1. Family Ericaceae
3.1.1. Highbush and Low Bush (Wild) Blueberry
3.1.2. Bilberry
3.1.3. Cranberry
3.2. Family Rosaceae
3.2.1. Red and Black Raspberry
3.2.2. Strawberry
3.2.3. Chokeberry
3.2.4. Sweet and Sour (Tart) Cherry
3.3. Family Grossulariaceae
Blackcurrant
3.4. Family Arecaceae
Açai Berry
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Savica, V.; Bellinghieri, G.; Kopple, J.D. The effect of nutrition on blood pressure. Annu. Rev. Nutr. 2010, 30, 365–401. [Google Scholar] [CrossRef] [Green Version]
- Juraschek, S.P.; Miller, E.R.; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef]
- Villa-Etchegoyen, C.; Lombarte, M.; Matamoros, N.; Belizán, J.M.; Cormick, G. Mechanisms Involved in the Relationship between Low Calcium Intake and High Blood Pressure. Nutrients 2019, 11, 1112. [Google Scholar] [CrossRef] [Green Version]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef]
- Aleixandre, A.; Miguel, M. Dietary Fiber and Blood Pressure Control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef]
- Elsahoryi, N.A.; Neville, C.E.; Patterson, C.C.; Linden, G.J.; Moitry, M.; Biasch, K.; Kee, F.; Amouyel, P.; Bongard, V.; Dallongeville, J.; et al. Association between Overall Fruit and Vegetable Intake, and Fruit and Vegetable Sub-Types and Blood Pressure: The PRIME Study (Prospective Epidemiological Study of Myocardial Infarction). Br. J. Nutr. 2020, 125, 557–567. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R. Effects of Vitamin c Supplementation on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. PTR 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Hügel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol Protection and Treatment of Hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Potential Factors Influencing the Effects of Anthocyanins on Blood Pressure Regulation in Humans: A Review. Nutrients 2019, 11, 1431. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Pinthong, D.; Mangmool, S. Blockade of the renin-angiotensin system with delphinidin, cyanin, and quercetin. Planta Med. 2012, 78, 1626–1632. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; O’Reilly, É.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual Intake of Flavonoid Subclasses and Incident Hypertension in Adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia; enhances antioxidant capacity; and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Du, Z.; Chen, Y.; Li, D.; Deng, S.; Liu, Z.; Yang, L. Effects of Anthocyanins on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef]
- Daneshzad, E.; Shab-Bidar, S.; Mohammadpour, Z.; Djafarian, K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1153–1165. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Wang, X.; Lu, W.; Wang, X.; Han, Z.; Qiu, C. The Effect of Anthocyanins on Blood Pressure: A PRISMA-Compliant Meta-Analysis of Randomized Clinical Trials. Medicine 2016, 95, e3380. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Krenn, L.; Steitz, M.; Schlicht, C.; Kurth, H.; Gaedcke, F. Anthocyanin- and Proanthocyanidin-Rich Extracts of Berries in Food Supplements-Analysis with Problems. Die Pharm. 2007, 62, 803–812. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844. [Google Scholar] [CrossRef] [Green Version]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of Grape Seed Proanthocyanidin Extract on Menopausal Symptoms, Body Composition, and Cardiovascular Parameters in Middle-Aged Women. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Valls, R.-M.; Llauradó, E.; Fernández-Castillejo, S.; Puiggrós, F.; Solà, R.; Arola, L.; Pedret, A. Effects of Low Molecular Weight Procyanidin Rich Extract from French Maritime Pine Bark on Cardiovascular Disease Risk Factors in Stage-1 Hypertensive Subjects: Randomized, Double-Blind, Crossover, Placebo-Controlled Intervention Trial. Phytomedicine 2016, 23, 1451–1461. [Google Scholar] [CrossRef]
- Belcaro, G.; Ledda, A.; Hu, S.; Cesarone, M.R.; Feragalli, B.; Dugall, M. Grape Seed Procyanidins in Pre- and Mild Hypertension: A Registry Study. Evid.-Based Complementary Altern. Med. 2013, 2013, 313142. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of Angiotensin Converting Enzyme (ACE) Activity by Flavan-3-Ols and Procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Sano, A.; Uchida, R.; Saito, M.; Shioya, N.; Komori, Y.; Tho, Y.; Hashizume, N. Beneficial Effects of Grape Seed Extract on Malondialdehyde-Modified LDL. J. Nutr. Sci. Vitaminol. 2007, 53, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Cao, A.H.; Wang, J.; Gao, H.Q.; Zhang, P.; Qiu, J. Beneficial clinical effects of grape seed proanthocyanidin extract on the progression of carotid atherosclerotic plaques. J. Geriatr. Cardiol. 2015, 12, 417–423. [Google Scholar] [CrossRef]
- DalBó, S.; Moreira, E.G.; Brandão, F.C.; Horst, H.; Pizzolatti, M.G.; Micke, G.A.; Ribeiro-do-Valle, R.M. Mechanisms Underlying the Vasorelaxant Effect Induced by Proanthocyanidin-Rich Fraction from Croton Celtidifolius in Rat Small Resistance Arteries. J. Pharmacol. Sci. 2008, 106, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, M.; Nakanishi, I.; Matsubayashi, S.; Imai, K.; Arai, T.; Matsumoto, K.; Fukuhara, K. Synthesis and Antioxidant Activity of a Procyanidin B3 Analogue. Bioorg. Med. Chem. Lett. 2017, 27, 1041–1044. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Kataoka, S.; Koga, T.; Ariga, T. Proanthocyanidin-Rich Extract from Grape Seeds Attenuates the Development of Aortic Atherosclerosis in Cholesterol-Fed Rabbits. Atherosclerosis 1999, 142, 139–149. [Google Scholar] [CrossRef]
- Shao, Z. Grape Seed Proanthocyanidin Extract Attenuates Oxidant Injury in Cardiomyocytes. Pharmacol. Res. 2003, 47, 463–469. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Suárez, M.; Bravo, F.I.; Muguerza, B. Changes in Arterial Blood Pressure Caused by Long-Term Administration of Grape Seed Proanthocyanidins in Rats with Established Hypertension. Food Funct. 2020, 11, 8735–8742. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, J.; Zhao, S.; You, B.; Ju, X.; Wang, Y.; Cui, X.; Wang, Q.; Gao, H. Grape Seed Proanthocyanidin Extract Alleviates Ouabain-Induced Vascular Remodeling through Regulation of Endothelial Function. Mol. Med. Rep. 2012, 6, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxidative Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, U.C.; Westfall, B.A. The Mechanism of Blood Pressure Depression by Ellagic Acid. Exp. Biol. Med. 1969, 132, 754–756. [Google Scholar] [CrossRef]
- Kannan, M.M.; Quine, S.D. Ellagic Acid Ameliorates Isoproterenol Induced Oxidative Stress: Evidence from Electrocardiological, Biochemical and Histological Study. Eur. J. Pharmacol. 2011, 659, 45–52. [Google Scholar] [CrossRef]
- Berkban, T.; Boonprom, P.; Bunbupha, S.; Welbat, J.; Kukongviriyapan, U.; Kukongviriyapan, V.; Pakdeechote, P.; Prachaney, P. Ellagic Acid Prevents L-NAME-Induced Hypertension via Restoration of ENOS and P47phox Expression in Rats. Nutrients 2015, 7, 5265–5280. [Google Scholar] [CrossRef]
- Tang, B.; Chen, G.; Liang, M.; Yao, J.; Wu, Z. Ellagic Acid Prevents Monocrotaline-Induced Pulmonary Artery Hypertension via Inhibiting NLRP3 Inflammasome Activation in Rats. Int. J. Cardiol. 2015, 180, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic Acid Attenuates High-Carbohydrate, High-Fat Diet-Induced Metabolic Syndrome in Rats. Eur. J. Nutr. 2012, 52, 559–568. [Google Scholar] [CrossRef]
- Looi, D.; Goh, B.H.; Khan, S.U.; Ahemad, N.; Palanisamy, U.D. Metabolites of the Ellagitannin, Geraniin Inhibit Human ACE; in vitro and in silico Evidence. Int. J. Food Sci. Nutr. 2020, 72, 470–477. [Google Scholar] [CrossRef]
- Jordão, J.; Porto, H.; Lopes, F.; Batista, A.; Rocha, M. Protective Effects of Ellagic Acid on Cardiovascular Injuries Caused by Hypertension in Rats. Planta Med. 2017, 83, 830–836. [Google Scholar] [CrossRef]
- Mari Kannan, M.; Darlin Quine, S. Ellagic Acid Protects Mitochondria from β-Adrenergic Agonist Induced Myocardial Damage in Rats; Evidence from in Vivo, in Vitro and Ultra Structural Study. Food Res. Int. 2012, 45, 1–8. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.J.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef]
- Tjelle, T.E.; Holtung, L.; Bøhn, S.K.; Aaby, K.; Thoresen, M.; Wiik, S.Å.; Paur, I.; Karlsen, A.S.; Retterstøl, K.; Iversen, P.O.; et al. Polyphenol-rich juices reduce blood pressure measures in a randomised controlled trial in high normal and hypertensive volunteers. Br. J. Nutr. 2015, 114, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, H.M.; Suomela, J.P.; Tahvonen, R.; Vaarno, J.; Venojärvi, M.; Viikari, J.; Kallio, H. Berry meals and risk factors associated with metabolic syndrome. Eur. J. Clin. Nutr. 2010, 64, 614–621. [Google Scholar] [CrossRef]
- Nilsson, A.; Salo, I.; Plaza, M.; Björck, I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PLoS ONE 2017, 12, e0188173. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and Cranberry Polyphenols Improve Insulin Sensitivity in Insulin-Resistant, Non-Diabetic Adults: A Parallel, Double-Blind, Controlled and Randomised Clinical Trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Puupponen-Pimiä, R.; Seppänen-Laakso, T.; Kankainen, M.; Maukonen, J.; Törrönen, R.; Kolehmainen, M.; Leppänen, T.; Moilanen, E.; Nohynek, L.; Aura, A.M.; et al. Effects of ellagitannin-rich berries on blood lipids; gut microbiota; and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function; blood pressure; and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Del Bo, C.; Porrini, M.; Fracassetti, D.; Campolo, J.; Klimis-Zacas, D.; Riso, P. A single serving of blueberry (V. corymbosum) modulates peripheral arterial dysfunction induced by acute cigarette smoking in young volunteers: A randomized-controlled trial. Food Funct. 2014, 5, 3107–3116. [Google Scholar] [CrossRef] [Green Version]
- Del Bo, C.; Deon, V.; Campolo, J.; Lanti, C.; Parolini, M.; Porrini, M.; Klimis-Zacas, D.; Riso, P. A serving of blueberry (V. corymbosum) acutely improves peripheral arterial dysfunction in young smokers and non-smokers: Two randomized, controlled, crossover pilot studies. Food Funct. 2017, 8, 4108–4117. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Del Pino-García, R.; George, T.W.; Vidal-Diez, A.; Heiss, C.; Spencer, J.P. Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol. Nutr. Food Res. 2014, 58, 1952–1961. [Google Scholar] [CrossRef]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [Green Version]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.-H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef] [Green Version]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlueTM) in Maintenance of Episodic and Working Memory in Older Adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef] [Green Version]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries Improve Biomarkers of Cardiometabolic Function in Participants with Metabolic Syndrome—Results from a 6-Month, Double-Blind, Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- McAnulty, S.R.; McAnulty, L.S.; Morrow, J.D.; Khardouni, D.; Shooter, L.; Monk, J.; Gross, S.; Brown, V. Effect of daily fruit ingestion on angiotensin converting enzyme activity, blood pressure, and oxidative stress in chronic smokers. Free Radic. Res. 2005, 39, 1241–1248. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in Blueberries Improve Insulin Sensitivity in Obese; Insulin-Resistant Men and Women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef]
- Riso, P.; Klimis-Zacas, D.; del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; de Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Stote, K.S.; Sweeney, M.I.; Kean, T.; Baer, D.J.; Novotny, J.A.; Shakerley, N.L.; Chandrasekaran, A.; Carrico, P.M.; Melendez, J.A.; Gottschall-Pass, K.T. The Effects of 100% Wild Blueberry (Vaccinium Angustifolium) Juice Consumption on Cardiometablic Biomarkers: A Randomized, Placebo-Controlled, Crossover Trial in Adults with Increased Risk for Type 2 Diabetes. BMC Nutr. 2017, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.; Chu, T.T.W.; Choi, S.W.; Benzie, I.F.F.; Tomlinson, B. Impact of Short-Term Bilberry Supplementation on Glycemic Control, Cardiovascular Disease Risk Factors, and Antioxidant Status in Chinese Patients with Type 2 Diabetes. Phytother. Res. 2021, 35, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habanova, M.; Saraiva, J.A.; Haban, M.; Schwarzova, M.; Chlebo, P.; Predna, L.; Gažo, J.; Wyka, J. Intake of bilberries (Vaccinium myrtillus L.) reduced risk factors for cardiovascular disease by inducing favorable changes in lipoprotein profiles. Nutr. Res. 2016, 36, 1415–1422. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Foster, M.; Scofield, R.H.; Basu, A. Cranberries Improve Postprandial Glucose Excursions in Type 2 Diabetes. Food Funct. 2017, 8, 3083–3090. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef]
- Basu, A.; Betts, N.M.; Ortiz, J.; Simmons, B.; Wu, M.; Lyons, T.J. Low-energy cranberry juice decreases lipid oxidation and increases plasma antioxidant capacity in women with metabolic syndrome. Nutr. Res. 2011, 31, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Hsia, D.S.; Zhang, D.J.; Beyl, R.S.; Greenway, F.L.; Khoo, C. Effect of Daily Consumption of Cranberry Beverage on Insulin Sensitivity and Modification of Cardiovascular Risk Factors in Adults with Obesity: A Pilot, Randomised, Placebo-Controlled Study. Br. J. Nutr. 2020, 124, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.T.; Chan, Y.C.; Lin, C.W.; Lee, W.J.; Sheu, W.H. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet. Med. 2008, 25, 1473–1477. [Google Scholar] [CrossRef]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry Juice Consumption Lowers Markers of Cardiometabolic Risk, Including Blood Pressure and Circulating C-Reactive Protein, Triglyceride, and Glucose Concentrations in Adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef] [Green Version]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Meily, S.; Petersen, K.S.; Kris-Etherton, P.M. Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial. Nutrients 2021, 13, 2618. [Google Scholar] [CrossRef] [PubMed]
- Ruel, G.; Lapointe, A.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Evidence that cranberry juice may improve augmentation index in overweight men. Nutr. Res. 2013, 33, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lamarche, B.; Couillard, C. Changes in plasma antioxidant capacity and oxidized low-density lipoprotein levels in men after short-term cranberry juice consumption. Metabolism 2005, 54, 856–861. [Google Scholar] [CrossRef]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br. J. Nutr. 2008, 99, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Franck, M.; de Toro-Martín, J.; Garneau, V.; Guay, V.; Kearney, M.; Pilon, G.; Roy, D.; Couture, P.; Couillard, C.; Marette, A.; et al. Effects of Daily Raspberry Consumption on Immune-Metabolic Health in Subjects at Risk of Metabolic Syndrome: A Randomized Controlled Trial. Nutrients 2020, 12, 3858. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, S.; Hong, S.J.; Choi, S.C.; Choi, J.H.; Kim, J.H.; Park, C.Y.; Cho, J.Y.; Lee, T.B.; Kwon, J.W.; et al. Black Raspberry Extract Increased Circulating Endothelial Progenitor Cells and Improved Arterial Stiffness in Patients with Metabolic Syndrome: A Randomized Controlled Trial. J. Med. Food 2016, 19, 346–352. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.J.; Cho, J.Y.; Lee, T.B.; Kwon, J.W.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.S. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: Randomized, double-blinded, placebo-controlled clinical trial. Nutrition 2016, 32, 461–467. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Lambert, J.D.; Proctor, D.N.; Kris-Etherton, P.M. Incorporating Freeze-Dried Strawberry Powder into a High-Fat Meal Does Not Alter Postprandial Vascular Function or Blood Markers of Cardiovascular Disease Risk: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2016, 105, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Chandra, P.; Kay, C.; Edirisinghe, I.; Burton-Freeman, B. Strawberry Consumption, Cardiometabolic Risk Factors, and Vascular Function: A Randomized Controlled Trial in Adults with Moderate Hypercholesterolemia. J. Nutr. 2021, 151, 1517–1526. [Google Scholar] [CrossRef]
- Amani, R.; Moazen, S.; Shahbazian, H.; Ahmadi, K.; Jalali, M.T. Flavonoid-rich beverage effects on lipid profile and blood pressure in diabetic patients. World J. Diabetes 2014, 5, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Wilkinson, M.; Penugonda, K.; Simmons, B.; Betts, N.M.; Lyons, T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009, 8, 43. [Google Scholar] [CrossRef]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Betts, N.M.; Nguyen, A.; Newman, E.D.; Fu, D.; Lyons, T.J. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J. Nutr. 2014, 144, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Izuora, K.; Betts, N.M.; Kinney, J.W.; Salazar, A.M.; Ebersole, J.L.; Scofield, R.H. Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial. Nutrients 2021, 13, 1421. [Google Scholar] [CrossRef] [PubMed]
- Feresin, R.G.; Johnson, S.A.; Pourafshar, S.; Campbell, J.C.; Jaime, S.J.; Navaei, N.; Elam, M.L.; Akhavan, N.S.; Alvarez-Alvarado, S.; Tenenbaum, G.; et al. Impact of Daily Strawberry Consumption on Blood Pressure and Arterial Stiffness in Pre- and Stage 1-Hypertensive Postmenopausal Women: A Randomized Controlled Trial. Food Funct. 2017, 8, 4139–4149. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Nguyen, T.H.; Kendall, C.W.; Faulkner, D.A.; Bashyam, B.; Kim, I.J.; Ireland, C.; Patel, D.; Vidgen, E.; Josse, A.R.; et al. The effect of strawberries in a cholesterol-lowering dietary portfolio. Metabolism 2008, 57, 1636–1644. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef]
- Zunino, S.J.; Parelman, M.A.; Freytag, T.L.; Stephensen, C.B.; Kelley, D.S.; Mackey, B.E.; Woodhouse, L.R.; Bonnel, E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012, 108, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Ahles, S.; Stevens, Y.R.; Joris, P.J.; Vauzour, D.; Adam, J.; de Groot, E.; Plat, J. The Effect of Long-Term Aronia Melanocarpa Extract Supplementation on Cognitive Performance, Mood, and Vascular Function: A Randomized Controlled Trial in Healthy, Middle-Aged Individuals. Nutrients 2020, 12, 2475. [Google Scholar] [CrossRef]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska- Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28–CR34. [Google Scholar] [PubMed]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia Berry (Poly)Phenols on Vascular Function and Gut Microbiota: A Double-Blind Randomized Controlled Trial in Adult Men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Petrović-Oggiano, G.; Takic, M.; Glibetić, N.; Zec, M.; Debeljak-Martacic, J.; Konić-Ristić, A. Effects of glucomannan-enriched, aronia juice-based supplement on cellular antioxidant enzymes and membrane lipid status in subjects with abdominal obesity. Sci. World J. 2014, 2014, 869250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kardum, N.; Konić-Ristić, A.; Savikin, K.; Spasić, S.; Stefanović, A.; Ivanišević, J.; Miljković, M. Effects of polyphenol-rich chokeberry juice on antioxidant/pro-oxidant status in healthy subjects. J. Med. Food 2014, 17, 869–874. [Google Scholar] [CrossRef]
- Kardum, N.; Milovanović, B.; Šavikin, K.; Zdunić, G.; Mutavdžin, S.; Gligorijević, T.; Spasić, S. Beneficial Effects of Polyphenol-Rich Chokeberry Juice Consumption on Blood Pressure Level and Lipid Status in Hypertensive Subjects. J. Med. Food 2015, 18, 1231–1238. [Google Scholar] [CrossRef]
- Loo, B.M.; Erlund, I.; Koli, R.; Puukka, P.; Hellström, J.; Wähälä, K.; Mattila, P.; Jula, A. Consumption of chokeberry (Aronia mitschurinii) products modestly lowered blood pressure and reduced low-grade inflammation in patients with mildly elevated blood pressure. Nutr. Res. 2016, 36, 1222–1230. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Laniewska, I.; Millo, B.; Dłuzniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef]
- Pokimica, B.; García-Conesa, M.-T.; Zec, M.; Debeljak-Martačić, J.; Ranković, S.; Vidović, N.; Petrović-Oggiano, G.; Konić-Ristić, A.; Glibetić, M. Chokeberry Juice Containing Polyphenols Does Not Affect Cholesterol or Blood Pressure but Modifies the Composition of Plasma Phospholipids Fatty Acids in Individuals at Cardiovascular Risk. Nutrients 2019, 11, 850. [Google Scholar] [CrossRef] [Green Version]
- Sikora, J.; Broncel, M.; Mikiciuk-Olasik, E. Aronia melanocarpa Elliot reduces the activity of angiotensin i-converting enzyme-in vitro and ex vivo studies. Oxid. Med. Cell. Longev. 2014, 2014, 739721. [Google Scholar] [CrossRef] [Green Version]
- Skoczynska, A.; Jedrychowska, I.; Poreba, R.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R.; Jedrychowska-Bianchi, I. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 59, 177–182. [Google Scholar]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black Chokeberry Aronia Melanocarpa Extract Reduces Blood Pressure, Glycemia and Lipid Profile in Patients with Metabolic Syndrome: A Prospective Controlled Trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.E.; Jenner, A.; Roodenrys, S. Acute reduction in blood pressure following consumption of anthocyanin-rich cherry juice may be dose-interval dependant: A pilot cross-over study. Int. J. Food Sci. Nutr. 2016, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, D.S.; Adkins, Y.; Reddy, A.; Woodhouse, L.R.; Mackey, B.E.; Erickson, K.L. Sweet bing cherries lower circulating concentrations of markers for chronic inflammatory diseases in healthy humans. J. Nutr. 2013, 143, 340–344. [Google Scholar] [CrossRef]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus Cerasus, L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Keane, K.M.; Haskell-Ramsay, C.F.; Veasey, R.C.; Howatson, G. Montmorency Tart cherries (Prunus cerasus L.) modulate vascular function acutely, in the absence of improvement in cognitive performance. Br. J. Nutr. 2016, 116, 1935–1944. [Google Scholar] [CrossRef] [Green Version]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of montmorency tart cherry (L. Prunus Cerasus) consumption on nitric oxide biomarkers and exercise performance. Scand. J. Med. Sci. Sports 2018, 28, 1746–1756. [Google Scholar] [CrossRef]
- Ataie-Jafari, A.; Hosseini, S.; Karimi, F.; Pajouhi, M. Effects of sour cherry juice on blood glucose and some cardiovascular risk factors improvements in diabetic women: A pilot study. Nutr. Food Sci. 2008, 38, 355–360. [Google Scholar] [CrossRef]
- Chai, S.C.; Davis, K.; Wright, R.S.; Kuczmarski, M.F.; Zhang, Z. Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: A randomized controlled trial. Food Funct. 2018, 9, 3185–3194. [Google Scholar] [CrossRef] [Green Version]
- Desai, T.; Bottoms, L.; Roberts, M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur. J. Appl. Physiol. 2018, 118, 2523–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, T.; Roberts, M.; Bottoms, L. Effects of Short-Term Continuous Montmorency Tart Cherry Juice Supplementation in Participants with Metabolic Syndrome. Eur. J. Nutr. 2020, 60, 1587–1603. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Navaei, N.; Pourafshar, S.; Jaime, S.J.; Akhavan, N.S.; Alvarez-Alvarado, S.; Proaño, G.V.; Litwin, N.S.; Clark, E.A.; Foley, E.M.; et al. Effects of Montmorency Tart Cherry Juice Consumption on Cardiometabolic Biomarkers in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Trial. J. Med. Food 2020, 23, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Murray, L.; Keane, K.M.; Haggerty, K.; Howatson, G.; Lodge, J.K. The Influence of Tart Cherries (Prunus Cerasus) on Vascular Function and the Urinary Metabolome: A Randomised Placebo-Controlled Pilot Study. J. Nutr. Sci. 2021, 10, e73. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. The Influence of Tart Cherry (Prunus Cerasus, Cv Montmorency) Concentrate Supplementation for 3 Months on Cardiometabolic Risk Factors in Middle-Aged Adults: A Randomised, Placebo-Controlled Trial. Nutrients 2021, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Mathew, S.; Moore, C.T.; Russell, J.; Robinson, E.; Soumpasi, V.; Barker, M.E. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum. Nutr. 2014, 69, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E. Cardiovascular function during supine rest in endurance-trained males with New Zealand blackcurrant: A dose-response study. Eur. J. Appl. Physiol. 2017, 117, 247–254. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Willems, M.E.T. Blackcurrant Alters Physiological Responses and Femoral Artery Diameter during Sustained Isometric Contraction. Nutrients 2017, 9, 556. [Google Scholar] [CrossRef]
- Cook, M.D.; Sandu, A.K.; Joyce, J.P. Effect of New Zealand Blackcurrant on Blood Pressure, Cognitive Function and Functional Performance in Older Adults. J. Nutr. Gerontol. Geriatr. 2020, 39, 99–113. [Google Scholar] [CrossRef]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol- rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Takenami, E.; Iwasaki-Kurashige, K.; Osada, T.; Katsumura, T.; Hamaoka, T. Effects of blackcurrant anthocyanin intake on peripheral muscle circulation during typing work in humans. Eur. J. Appl. Physiol. 2005, 94, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Hashimoto, Y.; Kobayashi, R.; Nakazato, K.; Willems, M.E.T. Effects of Blackcurrant Extract on Arterial Functions in Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Clin. Exp. Hypertens. 2020, 42, 640–647. [Google Scholar] [CrossRef]
- Alqurashi, R.M.; Galante, L.A.; Rowland, I.R.; Spencer, J.P.; Commane, D.M. Consumption of a flavonoid-rich açai meal is associated with acute improvements in vascular function and a reduction in total oxidative status in healthy overweight men. Am. J. Clin. Nutr. 2016, 104, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Aranha, L.N.; Silva, M.G.; Uehara, S.K.; Luiz, R.R.; Nogueira Neto, J.F.; Rosa, G.; Moraes de Oliveira, G.M. Effects of a Hypoenergetic Diet Associated with Açaí (Euterpe Oleracea Mart.) Pulp Consumption on Antioxidant Status, Oxidative Stress and Inflammatory Biomarkers in Overweight, Dyslipidemic Individuals. Clin. Nutr. 2020, 39, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Udani, J.K.; Singh, B.B.; Singh, V.; Barrett, M.L. Effects of Açai (Euterpe oleracea Mart.) berry preparation on metabolic parameters in a healthy overweight population: A pilot study. Nutr. J. 2011, 10, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Sun, J.; Lu, W.; Wang, X.; Wang, X.; Han, Z.; Qiu, C. Effects of blueberry supplementation on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2017, 31, 165–171. [Google Scholar] [CrossRef]
- Matta, F.V.; Xiong, J.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Chemical Composition and Bioactive Properties of Commercial and Non-Commercial Purple and White Açaí Berries. Foods 2020, 9, 1481. [Google Scholar] [CrossRef]
| Berry | Treatment Duration (*) | Study Design (**) | Number of Participants | Characteristics of Participants (***) | Treatment (****) | Effect on Blood Pressure (*****) | References |
|---|---|---|---|---|---|---|---|
| Blueberry (highbush) | SD | C, cross | 10 | Healthy, age 21 ± 2, BMI 23 ± 2 | Blueberry drink from FDP (348 mg ACN) | =SBP, =DBP | [56] |
| Blueberry (highbush) | SD | C, par | 16 | Smokers, age 24 ± 1, BMI 23 ± 1 | Blueberry drink from FDP (348 mg ACN), followed by smoking 1 cigarette | ↓SBP post smoke, = DBP | [56] |
| Blueberry (highbush) | SD | C, cross | 12 | M, healthy, age 24 ± 1, BMI 23 ± 1 | Drink from FDP (309 mg ACN) | =SBP, =DBP | [57] |
| Blueberry (highbush) | SD | C, cross | 12 | M, smokers, age 24 ± 1, BMI 23 ± 1 | Drink from FDP (309 mg ACN) followed by smoking 1 cigarette | =SBP, =DBP | [57] |
| Blueberry (highbush) | SD | DB, C, cross | 10 | M, healthy, age 27 ± 3, BMI 25 ± 3 | Drink from FDP (310 mg ACN) | =SBP, =DBP | [58] |
| Blueberry (highbush) | SD | DB, C, cross | 10 | Healthy, age 27 ± 1, BMI 25 ± 1 | Drink from FDP (330 mg ACN), or baked product with same amount of FDP (196 mg ACN) | =SBP, =DBP | [59] |
| Blueberry (highbush) | 8 W | DB, PC, par | 48 | W, postmenopausal, with pre- and stage-1 hypertension, age 59 ± 5, BMI 31 ± 6 | 480 mL drink from 22 g FDP (469 mg ACN) | ↓SBP, ↓DBP | [60] |
| Blueberry (highbush) | 8 W | SB, C, par | 48 | With MetS, age 50 ± 3, BMI 38 ± 2 | 480 mL drink from 50 g FDP (742 mg ACN) | ↓SBP, ↓DBP | [61] |
| Blueberry (highbush) | 6 W | PC, par | 25 | Healthy, age 43 ± 12, BMI 26 ± 4 | FDP [eq. 250 g fresh berries] | ↓aortic systolic pressures (ASPs), ↓SBP, =DBP, ↓DBP in subset of 9 pre-hypertensive subjects | [62] |
| Blueberry (highbush) | 18 W | C, par | 34 | W, in early pregnancy with history of gestational diabetes, age 27 ± 5, BMI 36 ± 4 | 280 g whole frozen blueberries (700 mg ACN) + 12 g soluble fiber supplement per day | =SBP, ↓DBP | [63] |
| Blueberry (highbush) | 4 M | DB, PC, par | 63 | With knee osteoarthritis, age 56 ± 1, BMI 32 ± 1 | 40 g FD whole blueberry powder daily | ↓SBP, =DBP | [64] |
| Blueberry (wild) | 6 M | DB, PC, par | 122 | Older adults, age 71 ± 4, weight 71 ± 4 kg | 1 or 2 g FDP, or 200 mg extract (2.7, 5.4 or 14 mg ACN) | ↓SBP with extract, but not with powders, at 3 and 6 M | [65] |
| Blueberry (highbush) | 6 M | DB, PC, par | 115 | With overweight/obesity and MetS, age 63 ± 7, BMI 31 ± 3 | 13 or 26 mg/day FDP (182 or 364 mg ACN) | =SBP, =DBP | [66] |
| Blueberry (highbush) | 3 W | C, par | 20 | Smokers, age 28 ± 4, BMI 29 ± 3 | 250 g fresh berries | =SBP, =DBP | [67] |
| Blueberry (highbush) | 6 W | DB, PC, par | 32 | With obesity and insulin resistance, age 52 ± 3, BMI 37 ± 1 | 45 g FDP added to smoothie and yogurt (580 mg ACN) | =SBP, =DBP | [68] |
| Blueberry (highbush) | 6 W | DB, PC, par | 44 | With MetS, age 57 ± 2, BMI 36 ± 1 | 45 g FDP added to smoothie and yogurt (580 mg ACN) | =SBP, =DBP | [69] |
| Blueberry (wild) | 6 W | PC, cross | 18 | M, with risk factors for CVD, age 48 ± 10, BMI 25 ± 3 | 250 mL drink from 25 g FDP (400 mg ACN) | =SBP, =DBP | [70] |
| Blueberry (wild) | 1 W | SB, PC, cross | 19 | W, with T2DM risk, age 39–64, BMI 27–37 | 240 mL juice | =SBP, =DBP | [71] |
| Bilberry | 4 W | DB, PC, cross | 20 | With T2DM, age 52. ± 3, BMI 27 ± 2 | 1.4 g/day extract | =SBP, =DBP | [72] |
| Bilberry | 8 W | C, par | 27 | With MetS, age 51 ± 6, BMI 32 ± 4 | 200 g puree + 40 g dried (1381 mg ACN) | =SBP, =DBP | [73] |
| Bilberry | 6 W | Pre-post | 36 | Healthy, age 48 ± 6, BMI 27 ± 4 | Frozen berries, 3 times a week (456 mg ACN) | =SBP, =DBP | [74] |
| Cranberry | SD | C, cross | 40 | With obesity and T2DM, age 56 ± 6, BMI 40 ± 7 | 40 g FDP following high fat meal, test after 1 h, 2 h and 4 h | =SBP, =DBP | [75] |
| Cranberry | SD | Pre-post | 15 | With overweight/obesity and coronary artery disease, age 62 ± 8, BMI ? | 480 mL juice following meal | =SBP, =DBP | [76] |
| Cranberry | 4 W | PC, cross | 44 | With overweight/obesity and coronary artery disease, age 62 ± 10, BMI 29 ± 4 | 480 mL/day juice | =SBP, =DBP | [76] |
| Cranberry | 8 W | DB, PC, par | 31 | W, with MetS, age 52 ± 8, BMI 40 ± 7 | 480 mL/day juice | =SBP, =DBP | [77] |
| Cranberry | 8 W | DB, PC, par | 53 | With obesity and elevated fasting glucose or impaired glucose tolerance, age 48 ± 14, BMI 37 ± 5 | 450 mL/day low-energy berry beverage (6.5 mg ACN) | =SBP, =DBP | [78] |
| Cranberry | 12 W | DB, PC, par | 30 | With T2DM, age 65.5 ± 2, BMI 26 ± 1 | 500 mg/capsule FDP | =SBP, =DBP | [79] |
| Cranberry | 8 W | PC, par | 56 | With overweight/obesity, age 50 ± 11, BMI 28 ± 4 | Twice daily juice (173 or 62 mg TP) | =SBP, ↓DBP | [80] |
| Cranberry | 8 W | PC, cross | 40 | With overweight/obesity and pre-hypertension, age 47 ± 12, BMI 29 ± 5 | 500 mL/day drink (27% cranberry juice) | =SBP, =DBP, but ↓ 24-h ambulatory DBP during daytime hours. | [81] |
| Cranberry | 4 W | DB, PC, cross | 35 | M, with abdominal obesity, with (13) or without MetS, age 45 ± 10, BMI 28 ± 2 | 500 mL/day of low-calorie drink (27% juice) | =SBP, =DBP | [82] |
| Cranberry | 2 W | Pre-post | 21 | M, with dyslipidemia and abdominal obesity, BMI 27 ± 4, age 38 ± 8 | 7 mL/kg BW (range 460–760 mL/day berry juice) | =SBP, =DBP | [83] |
| Cranberry | 12 W | Pre-post | 30 | M, with abdominal obesity, with (9) or without MetS, age 51 ± 10, BMI 28± 3 | 125 mL/day juice (4 W) + 250 mL/day (4 W) + 500 mL/day (4 W) | ↓SBP with highest dose, =DBP | [84] |
| Raspberry (red) | 4 W | C, cross | 22 | With obesity, age 54 ± 4, BMI 33 ± 2 | Frozen berries (225 mg ACN) | ↓SBP, =DBP | [85] |
| Raspberry (red) | SD | C, cross | 25 | With obesity and T2DM, age 54 ± 4, BMI 35 ± 2 | Post-prandial assessment with or without frozen berries (225 mg ACN) | =SBP, =DBP | [85] |
| Raspberry (red) | 8 W | C, par | 50 | With overweight/abdominal obesity and slight hyperinsulinemia or hypertriglyceridemia, BMI 30 ± 5, age 32 ± 9 | 280 g/day of frozen berries | =SBP, =DBP | [86] |
| Raspberry (black) | 8 W | DB, PC, par | 45 | With pre-hypertension, age 57 ± 12, BMI 25 ± 3 | 1500 mg or 2500 mg daily FDP | ↓SBP with high dose, =DBP | [87] |
| Raspberry (black) | 12 W | DB, PC, par | 51 | With MetS, age 59 ± 10, BMI 25 ± 4 | 750 mg daily FDP | =SBP, =DBP | [88] |
| Strawberry | SD | PC, cross | 30 | With overweight/obesity, age 28 ± 2, BMI 31 ± 1 | 40 g FDP + high-fat meal [eq. 450 g fresh berries] | =SBP | [89] |
| Strawberry | 4 W | DB, C, par | 34 | With overweight/obesity, age 53 ± 5, BMI 31 ± 5 | Twice daily drink from 25 g FDP each (total 142 mg ACN) | =SBP, =DBP | [90] |
| Strawberry | SD | DB, C, par | 34 | With overweight/obesity, age 53 ± 5, BMI 31 ± 5 | Drink from 50 g FDP (142 mg ACN total) | ↓SBP 1 h post treatment | [90] |
| Strawberry | 6 W | DB, PC, par | 36 | With T2DM, BMI 28± 4, age 51 ± 11 | 2 cups/day drink from 25 g FDP each (142 mg ACN total) | =SBP, ↓DBP | [91] |
| Strawberry | 4 W | Pre-post | 16 | W, with obesity and MetS, age 39–71, BMI 39 ± 2 | 2 cups/day drink from 25 g FDP each (142 mg ACN total) | =SBP, =DBP | [92] |
| Strawberry | 8 W | C, par | 27 | With obesity and MetS, age 47 ± 3, BMI 37 ± 2 | 4 cups/day drink from 25 g FDP each (284 mg ACN total) | =SBP, =DBP | [93] |
| Strawberry | 12 W | C, par | 60 | With CVD risk factors, age 49 ± 10; BMI 36 ± 5 | 2 cups/day drink with low dose (25 g) or high dose (50 g) FDP (142 or 284 mg ACN) | =SBP, =DBP | [94] |
| Strawberry | 4 W | PC, cross | 33 | With obesity and dyslipidemia, age 53 ± 13, BMI 33 ± 3 | 13 or 32 mg/day FDP (38 or 92 mg ACN) | =SBP, =DBP | [95] |
| Strawberry | 8 W | DB, PC, par | 60 | W, postmenopausal, with pre- or stage 1 hypertension, age 59 ± 8, BMI 32 ± 7 | 25 or 50 mg/day FDP (102 mg or 204 mg ACN) | ↓SBP with 25 mg, =DBP | [96] |
| Strawberry | 4 W | C, cross | 28 | With dyslipidemia, age 38–75, BMI 20–32 | 454 g/day fresh strawberries | =SBP, =DBP | [97] |
| Strawberry | 12 W | DB, PC, cross | 17 | With knee osteoarthritis, age 57 ± 7, BMI 39 ± 2 | Twice daily drink from 50 g FDP [eq. 500 g fresh berries] | =SBP, =DBP | [98] |
| Strawberry | 7 W | DB, C, cross | 20 | With obesity, age 20–50, BMI 30–40 | Two servings of FDP mixed as a milkshake, in yogurt, cream cheese, or water [eq. 320 g frozen berries] | =SBP, =DBP | [99] |
| Chokeberry | 24 W | DB, PC, par | 101 | With overweight/obesity, age 53 ± 10, BMI 29 ± 5 | 90 mg or 150 mg berry extract capsules (16 mg or 27 mg ACN) | ↓DBP with 150 compared to 90 mg | [100] |
| Chokeberry | 8 W | Pre-post | 25 | With MetS, age 42–65, BMI 31 ± 3 | Berry extract (300 mg ACN) | ↓SBP, ↓DBP | [101] |
| Chokeberry | 12 W | DB, PC, par | 66 | M, healthy, age 24 ± 5, BMI 23 ± 2 | Capsules of polyphenol-rich extract (30 mg ACN) or whole chokeberry powder (4 mg ACN) | =SBP, =DBP | [102] |
| Chokeberry | 4 W | Pre-post | 20 | W, postmenopausal, with abdominal obesity, age 53 ± 5, BMI 36 ± 4 | 100 mL/day glucomannan-enriched (2 g), berry juice (25 mg ACN) | ↓SBP, =DBP | [103] |
| Chokeberry | 12 W | Pre-post | 29 | W, healthy, age 35 ± 8, BMI 23 ± 4 | 100 mL/day glucomannan-enriched (2 g), berry juice (25 mg ACN) | =SBP, =DBP | [104] |
| Chokeberry | 4 W | Pre-post | 23 | With pre- or stage-1 hypertension, age 48 ± 10, weight 82 ± 20 | 200 mL/day of polyphenol-rich organic berry juice (358 mg ACN) | ↓SBP, ↓DBP, ↓ average 24 h BP | [105] |
| Chokeberry | 16 W | SB, PC, cross | 37 | With mild hypertension, age 40–70, BMI 26 ± 3 | Cold-pressed berry juice and oven-dried berry powder (1024 mg ACN total) | ↓daytime DBP (recorded over 15 h), =SBP | [106] |
| Chokeberry | 6 W | DB, PC, par | 44 | Post myocardial infarction patients, receiving statin therapy, age 66 ± 8, BMI 27 ± 3 | 255 mg/day berry polyphenol-rich extract (64 mg ACN) | ↓SBP, ↓DBP | [107] |
| Chokeberry | 4 W | DB, PC, par | 84 | With CVD risk factors, age 41 ± 8, BMI 27 ± 6 | 100 mL/day high-polyphenols or 100 mL/day low-polyphenols berry juice (113 mg or 28 mg ACN) | =SBP, =DBP | [108] |
| Chokeberry | 8 W | Pre-post | 23 | 23 with untreated MetS (BMI 31 ± 4), reference group with 25 treated MetS patients (BMI 29 ± 3) and 20 healthy controls (BMI 23 ± 1) | Berry extract (60 mg ACN), or ACE-inhibitors | ↓SBP, ↓DBP | [109] |
| Chokeberry | 6 W + 6 W | Pre-post | 58 | M, with mild hypercholesterolemia, age 54 ± 6, BMI 28 ± 3 | 250 mL/day berry juice (6-week intervention + 6-week wash-out + 6-week intervention) (90 mg ACN) | ↓SBP after 12 W, ↓DBP after 6 and 12 W | [110] |
| Chokeberry | 4 W | Pre-post | 143 | With MetS, BMI 32 ± 6, age 55 ± 15 | 30 mL/day berry extract (120 mg ACN) | ↓SBP, ↓DBP | [111] |
| Chokeberry | 12 W | PC, par | 49 | Healthy former smokers, age 35 ± 3, BMI 26 ± 1 | 500 mg berry extract (45 mg ACN) | =SBP, =DBP | [112] |
| Cherry (sweet) | SD | Cross | 13 | 6 young (age 22 ± 1, BMI 26 ± 4) and 7 older adults (age 78 ± 6, BMI 29 ± 4) | Juice (207 mg ACN), in single dose or split into three doses over 2 h | ↓SBP, ↓DBP at 2 h after consumption, if given in a single dose (but not if split in three doses given 1 h apart) | [113] |
| Cherry (sweet) | 12 W | C, par | 49 | Older adults, age 80 ± 6, BMI 26 ± 3 | Juice (138 mg) | ↓SBP, =DBP | [114] |
| Cherry (sweet) | 4 W | Pre-post | 18 | Healthy, age 50 ± 4, BMI 26 ± 4 | 280 g fresh fruit | =SBP, =DBP at the end of the trial and after 1 month | [115] |
| Cherry (tart) | SD | SB, PC, cross | 15 | M, with early hypertension, age 31 ± 9, BMI 27 ± 4 | Juice (74 mg ACN) | ↓SBP, ↓MAP, = DBP | [116] |
| Cherry (tart) | SD | DB, PC, cross | 27 | Healthy, age 50 ± 6, BMI 26 ± 5 | Concentrate (68 mg ACN) | ↓SBP | [117] |
| Cherry (tart) | SD | DB, PC, cross | 10 | Athletes, age 28 ± 7, weight 78 ± 9 kg | Juice (68 mg ACN) | ↓SBP, =DBP, =MAP | [118] |
| Cherry (tart) | 6 W | Pre-post | 19 | W, with T2DM, age 53 ± 9, BMI 30 ± 4 | Juice (720 mg ACN) | ↓SBP, ↓DBP | [119] |
| Cherry (tart) | 12 W | C, par | 34 | With overweight/obesity, age 70 ± 4, BMI 28 ± 4 | Juice [451 mg TP] | ↓SBP, =DBP | [120] |
| Cherry (tart) | 3 W | SB, PC, par | 11 | Healthy, age 30 ± 10, BMI 24 ± 3 | Juice (540 mg ACN) | =SBP, =DBP pre or post exercise | [121] |
| Cherry (tart) | 1 W | SB, PC, cross | 12 | With MetS, age 50 ± 10, BMI 31 ± 7 | Juice (270 mg ACN) | =SBP, =DBP, =MAP but ↓24-h ambulatory SBP, DBP and MAP. | [122] |
| Cherry (tart) | 12 W | SB, PC, par | 19 | With MetS, age 36 ± 11, BMI 34 ± 8 | 240 mL juice, twice daily (176 mg ACN) | =SBP, =DBP, =MAP (both central and peripheral) | [123] |
| Cherry (tart) | 4 W | DB, PC, par | 23 | Healthy, age 23 ± 3, BMI 25 ± 3 | Twice daily 30 mL juice (74 mg ACN) | =SBP, =DBP, =MAP | [124] |
| Cherry (tart) | 3 M | PC, par | 50 | With overweight/obesity, age 48 ± 6, BMI 28 ± 4 | Twice daily 30 mL juice (74 mg ACN) | =SBP, =DBP, =MAP | [125] |
| Cherry (tart) | 6 W | C, par | 47 | Healthy, age 38 ± 6, BMI 24 ± 3 | Concentrate (275 mg ACN) | =SBP, =DBP | [126] |
| Blackcurrant | SD | DB, cross | 23 | Healthy, age 46 ± 14, BMI 26 ± 3.8 | Drink from extract at different doses (150, 300 or 600 mg ACN), following high-carbohydrate meal | =SBP, =DBP after 2 h | [127] |
| Blackcurrant | 1 W | C, cross | 15 | Athletes, age 38 ± 12, weight 76 ± 10 kg | Extract at different doses (105, 210 or 315 mg ACN) | = SBP, =DBP, ↓MAP with 210 and 315 mg | [128] |
| Blackcurrant | 1 W | DB, PC, cross | 13 | M, healthy, age 26 ± 4, BMI 25 ± 3 | Extract (315 mg ACN) | = SBP, =DBP, =MAP at rest; ↓SBP, ↓DBP, ↓MAP during isomeric contraction | [129] |
| Blackcurrant | 1 W | DB, PC, cross | 14 | Older adults, age 69 ± 4, weight 85 ± 12 kg | 600 mg/day extract (210 mg ACN) | ↓SBP, ↓DBP | [130] |
| Blackcurrant | 6 W | PC, par | 66 | Healthy or overweight, age 52 ± 10, BMI 29 ± 6 | Juice, low or high dose (40 mg or 143 mg ACN) | =SBP, =DBP | [131] |
| Blackcurrant | 2 W | DB, PC, cross | 11 | Healthy, age 39 ± 12, BMI ? | ACN-rich extract (7.7 mg ACN/kg of body weight) | =SBP, =DBP after 30 min typing workload | [132] |
| Blackcurrant | 1 W | DB, PC, cross | 14 | Older adults, age 73 ± 6, BMI 22 ± 3 | Two 300 mg capsules extract/day (35% blackcurrant extract) (210 mg ACN) | ↓central SBP, ↓brachial SBP, DBP and MAP | [133] |
| Açai | SD | DB, C, cross | 23 | M, healthy, age 46 ± 9, BMI 28 ± 2 | Berry smoothie following high-fat meal (493 mg ACN) | =SBP, =DBP at 2 and 6 h | [134] |
| Açai | 2 M | DB PC, par | 69 | With overweight and dyslipidemia, age 41 ± 10, BMI 35 ± 6 | 200 g pulp with hypoenergetic diet [684 mg TP] | =SBP, =DBP | [135] |
| Açai | 4 W | Pre-post | 10 | With overweight, age 28±?, BMI 27 ± 2 | 100 g pulp [0.77 mg/mL ACN in the pulp, density unknown] | =SBP, =DBP | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendrame, S.; Adekeye, T.E.; Klimis-Zacas, D. The Role of Berry Consumption on Blood Pressure Regulation and Hypertension: An Overview of the Clinical Evidence. Nutrients 2022, 14, 2701. https://doi.org/10.3390/nu14132701
Vendrame S, Adekeye TE, Klimis-Zacas D. The Role of Berry Consumption on Blood Pressure Regulation and Hypertension: An Overview of the Clinical Evidence. Nutrients. 2022; 14(13):2701. https://doi.org/10.3390/nu14132701
Chicago/Turabian StyleVendrame, Stefano, Tolu Esther Adekeye, and Dorothy Klimis-Zacas. 2022. "The Role of Berry Consumption on Blood Pressure Regulation and Hypertension: An Overview of the Clinical Evidence" Nutrients 14, no. 13: 2701. https://doi.org/10.3390/nu14132701
APA StyleVendrame, S., Adekeye, T. E., & Klimis-Zacas, D. (2022). The Role of Berry Consumption on Blood Pressure Regulation and Hypertension: An Overview of the Clinical Evidence. Nutrients, 14(13), 2701. https://doi.org/10.3390/nu14132701








