Blood Glucose Response of a Low-Carbohydrate Oral Nutritional Supplement with Isomaltulose and Soluble Dietary Fiber in Individuals with Prediabetes: A Randomized, Single-Blind Crossover Trial
Abstract
:1. Introduction
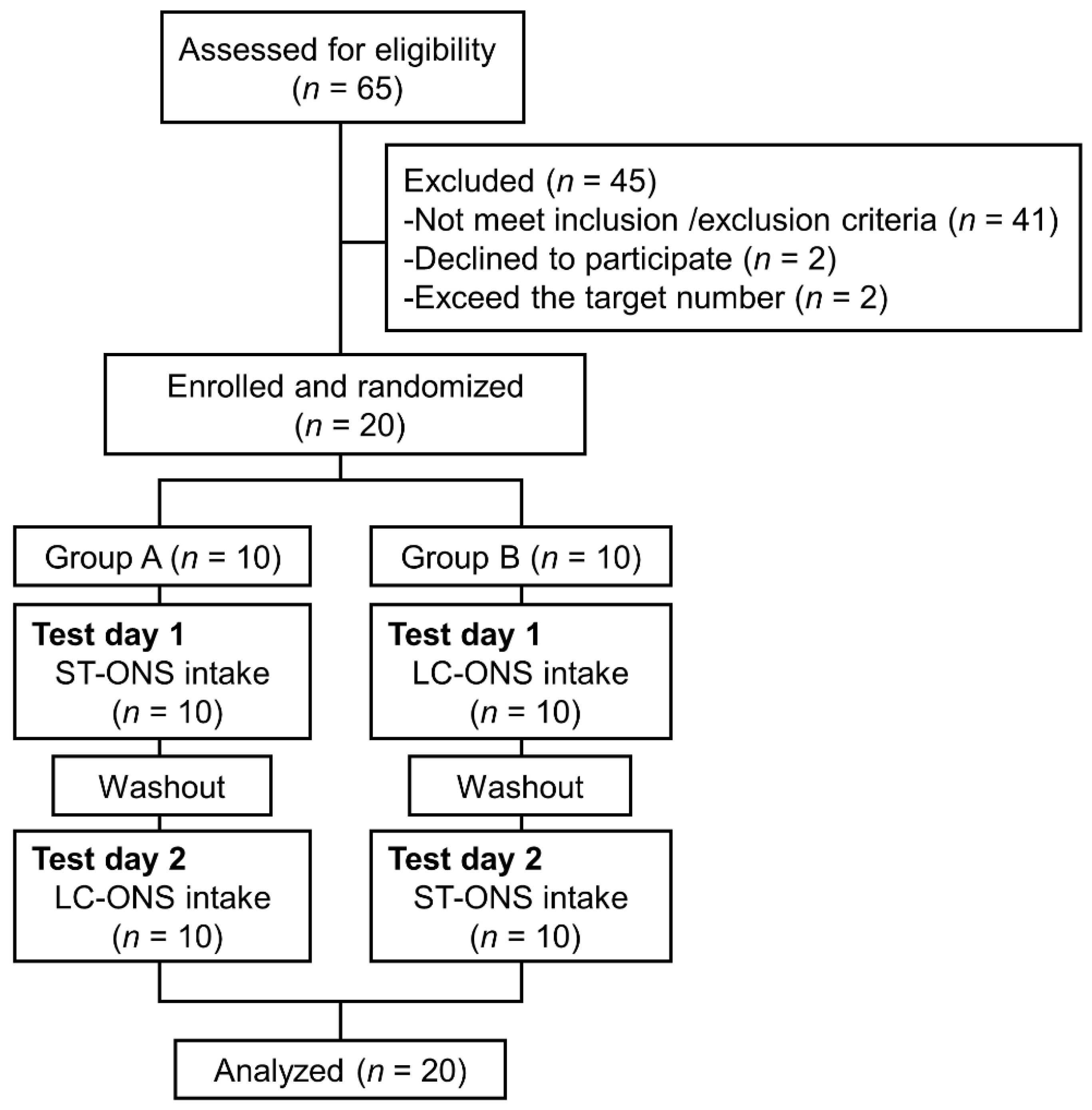
2. Materials and Methods
2.1. Trial Design and Ethics
2.2. Subjects
2.3. Test Products
2.4. Intervention
2.5. Laboratory Measurements
2.6. Statistical Analyses
3. Results
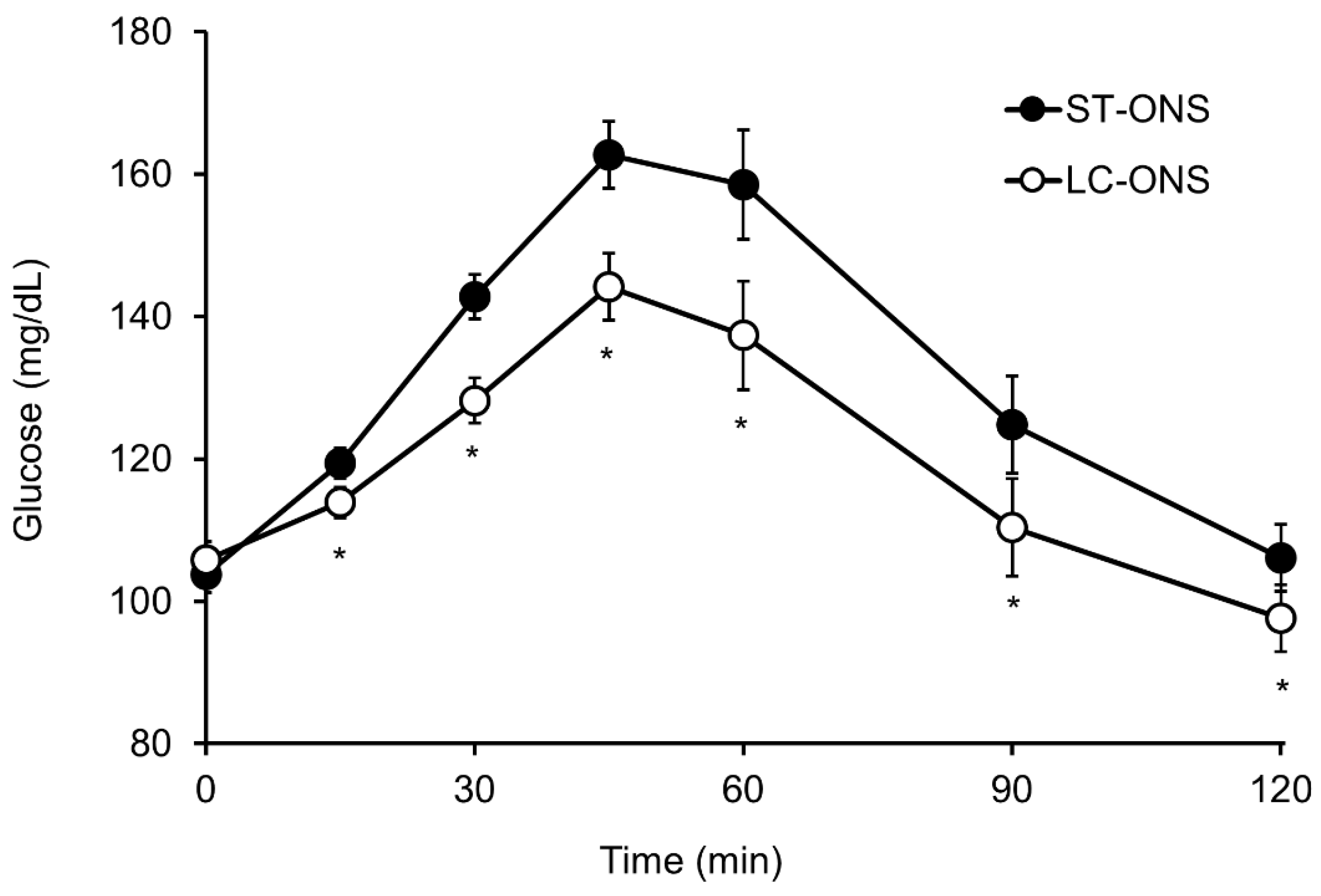
3.1. Blood Glucose Responses
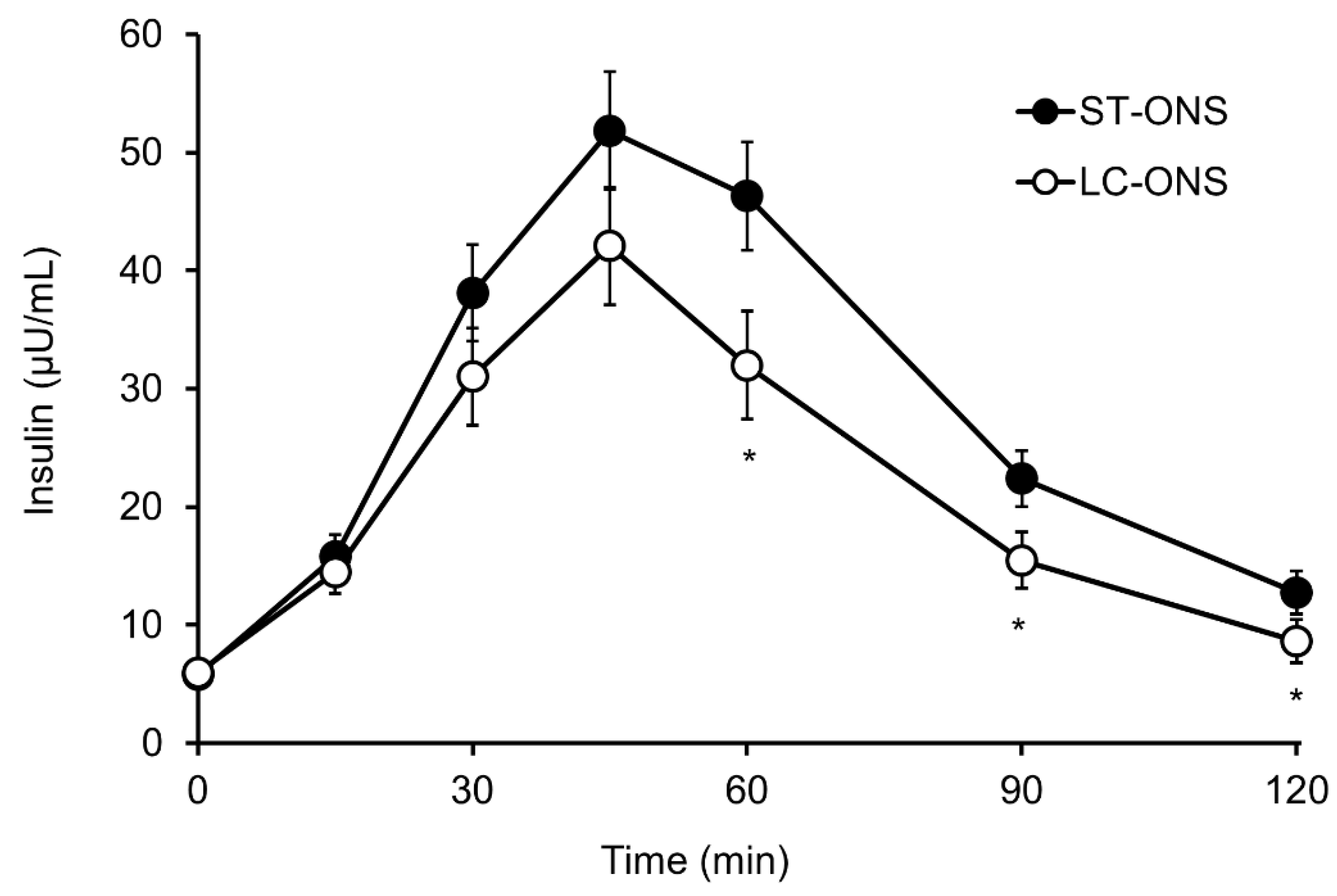
3.2. Insulin Responses
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clegg, M.E.; Williams, E.A. Optimizing Nutrition in Older People. Maturitas 2018, 112, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Milne, A.C.; Potter, J.; Vivanti, A.; Avenell, A. Protein and Energy Supplementation in Elderly People at Risk from Malnutrition. Cochrane Database Syst. Rev. 2009, 2, CD003288. [Google Scholar] [CrossRef] [PubMed]
- Care, D.; Suppl, S.S. Older Adults: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S168–S179. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Fang, L.; Guan, Q.; Guan, L.; Li, Q. Prevalence, Awareness, Treatment and Control of Diabetes Mellitus among Middle-Aged and Elderly People in a Rural Chinese Population: A Cross-Sectional Study. PLoS ONE 2018, 13, e0198343. [Google Scholar] [CrossRef]
- Xu, G.; Liu, B.; Sun, Y.; Du, Y.; Snetselaar, L.G.; Hu, F.B.; Bao, W. Prevalence of Diagnosed Type 1 and Type 2 Diabetes among US Adults in 2016 and 2017: Population Based Study. BMJ 2018, 361, k1497. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515–2523. [Google Scholar] [CrossRef]
- Vatcheva, K.P.; Fisher-Hoch, S.P.; Reininger, B.M.; McCormick, J.B. Sex and Age Differences in Prevalence and Risk Factors for Prediabetes in Mexican-Americans. Diabetes Res. Clin. Pract. 2020, 159, 107950. [Google Scholar] [CrossRef]
- Madsbad, S. Impact of Postprandial Glucose Control on Diabetes-Related Complications: How Is the Evidence Evolving? J. Diabetes Complicat. 2016, 30, 374–385. [Google Scholar] [CrossRef]
- Ceriello, A. Postprandial Hyperglycemia and Diabetes Complications: Is It Time to Treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [Green Version]
- García-Rodríguez, C.E.; Mesa, M.D.; Olza, J.; Buccianti, G.; Pérez, M.; Moreno-Torres, R.; de la Cruz, A.P.; Gil, A. Postprandial Glucose, Insulin and Gastrointestinal Hormones in Healthy and Diabetic Subjects Fed a Fructose-Free and Resistant Starch Type IV-Enriched Enteral Formula. Eur. J. Clin. Nutr. 2013, 52, 1569–1578. [Google Scholar] [CrossRef]
- Huhmann, M.B.; Smith, K.N.; Schwartz, S.L.; Haller, S.K.; Irvin, S.; Cohen, S.S. Plasma Glucose and Insulin Response to Two Oral Nutrition Supplements in Adults with Type 2 Diabetes Mellitus. BMJ Open Diabetes Res. Care 2016, 4, e000240. [Google Scholar] [CrossRef] [Green Version]
- Arai, H.; Mizuno, A.; Sakuma, M.; Fukaya, M.; Matsuo, K.; Muto, K.; Sasaki, H.; Matsuura, M.; Okumura, H.; Yamamoto, H.; et al. Effects of a Palatinose-Based Liquid Diet (Inslow) on Glycemic Control and the Second-Meal Effect in Healthy Men. Metabolism 2007, 56, 115–121. [Google Scholar] [CrossRef]
- Lansink, M.; van Laere, K.M.J.; Vendrig, L.; Rutten, G.E.H.M. Lower Postprandial Glucose Responses at Baseline and after 4 Weeks Use of a Diabetes-Specific Formula in Diabetes Type 2 Patients. Diabetes Res. Clin. Pract. 2011, 93, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Laksir, H.; Lansink, M.; Regueme, S.C.; de Vogel-van den Bosch, J.; Pfeiffer, A.F.H.; Bourdel-Marchasson, I. Glycaemic Response after Intake of a High Energy, High Protein, Diabetes-Specific Formula in Older Malnourished or at Risk of Malnutrition Type 2 Diabetes Patients. Clin. Nutr. 2018, 37, 2084–2090. [Google Scholar] [CrossRef] [Green Version]
- Devitt, A.A.; Williams, J.A.; Choe, Y.S.; Hustead, D.S.; Mustad, V.A. Glycemic Responses to Glycemia-Targeted Specialized-Nutrition Beverages with Varying Carbohydrates Compared to a Standard Nutritional Beverage in Adults with Type 2 Diabetes. Adv. Biosci. Biotechnol. 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kuzuya, T.; Nakagawa, S.; Satoh, J.; Kanazawa, Y.; Iwamoto, Y.; Kobayashi, M.; Nanjo, K.; Sasaki, A.; Seino, Y.; Ito, C.; et al. Report of the Committee of Japan Diabetes Society on the Classification and Diagnostic Criteria of Diabetes Mellitus. J. Jpn. Diabetes Soc. 1999, 42, 385–404. [Google Scholar]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef]
- Ginsberg, H.; Olefsky, J.M.; Kimmerling, G.; Crapo, P.; Reaven, G.M. Induction of Hypertriglyceridemia by a Low-Fat Diet. J. Clin. Endocrinol. Metab. 1976, 42, 729–735. [Google Scholar] [CrossRef]
- Kawai, K.; Okuda, Y.; Yamashita, K. Changes in Blood Glucose and Insulin after an Oral Palatinose Administration in Normal Subjects. Endocrinol. Jpn. 1985, 32, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Lina, B.A.R.; Jonker, D.; Kozianowski, G. Isomaltulose (Palatinose®): A Review of Biological and Toxicological Studies. Food Chem. Toxicol. 2002, 40, 1375–1381. [Google Scholar] [CrossRef]
- Wada, T.; Sugatani, J.; Terada, E.; Ohguchi, M.; Miwa, M. Physicochemical Characterization and Biological Effects of Inulin Enzymatically Synthesized from Sucrose. J. Agric. Food Chem. 2005, 53, 1246–1253. [Google Scholar] [CrossRef]
- Livesey, G.; Tagami, H. Interventions to Lower the Glycemic Response to Carbohydrate Foods with a Low-Viscosity Fiber (Resistant Maltodextrin): Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2009, 89, 114–125. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Food Based Dietary Guidelines in the WHO European Region; World Health Organization: Copenhagen, Denmark, 2003; Volume 79832. [Google Scholar]
- Overview of Dietary Reference Intakes for Japanese. 2020. Available online: https://www.mhlw.go.jp/content/10900000/000862500.pdf (accessed on 4 April 2022).
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Tappy, L. Fructose-Containing Caloric Sweeteners as a Cause of Obesity and Metabolic Disorders. J. Exp. Biol. 2018, 221, jeb164202. [Google Scholar] [CrossRef] [Green Version]
- Geidl-Flueck, B.; Hochuli, M.; Németh, Á.; Eberl, A.; Derron, N.; Köfeler, H.C.; Tappy, L.; Berneis, K.; Spinas, G.A.; Gerber, P.A. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 2021, 75, 46–54. [Google Scholar] [CrossRef]
- Brunner, S.; Wolf, P.; Holub, I.; Amann-Gassner, U.; Theis, S.; Scheppach, W.; Gostner, A.; Hauner, H.; Melcher, R. Metabolic Effects of Replacing Sucrose by Isomaltulose in Subjects with Type 2 Diabetes: A Randomized Double-Blind Trial. Diabetes Care 2012, 35, 1249–1251. [Google Scholar] [CrossRef] [Green Version]
- Arai, H.; Mizuno, A.; Matsuo, K.; Fukaya, M.; Sasaki, H.; Arima, H.; Matsuura, M.; Taketani, Y.; Doi, T.; Takeda, E. Effect of a Novel Palatinose-Based Liquid Balanced Formula (MHN-01) on Glucose and Lipid Metabolism in Male Sprague-Dawley Rats after Short- and Long-Term Ingestion. Metabolism 2004, 53, 977–983. [Google Scholar] [CrossRef]
- Astina, J.; Saphyakhajorn, W.; Borompichaichartkul, C.; Sapwarobol, S. Tapioca Resistant Maltodextrin as a Carbohydrate Source of Oral Nutrition Supplement (ONS) on Metabolic Indicators: A Clinical Trial. Nutrients 2022, 14, 916. [Google Scholar] [CrossRef]
- Buranapin, S.; Siangruangsang, S.; Chantapanich, V.; Hengjeerajarus, N. The Comparative Study of Diabetic Specific Formula and Standard Formula on Postprandial Plasma Glucose Control in Type 2 DM Patients. J. Med. Assoc. Thail. 2014, 97, 582–588. [Google Scholar]
- Galgani, J.; Aguirre, C.; Díaz, E. Acute Effect of Meal Glycemic Index and Glycemic Load on Blood Glucose and Insulin Responses in Humans. Nutr. J. 2006, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Lu, J.; Ma, X.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Zhou, J. Breakfast Replacement with a Liquid Formula Improves Glycaemic Variability in Patients with Type 2 Diabetes: A Randomised Clinical Trial. Br. J. Nutr. 2019, 121, 560–566. [Google Scholar] [CrossRef]
- Matia Martin, P.; Robles Agudo, F.; Lopez Medina, J.A.; Sanz Paris, A.; Tarazona Santabalbina, F.; Domenech Pascual, J.R.; Lopez Penabad, L.; Sanz Barriuso, R. Effectiveness of an Oral Diabetes-Specific Supplement on Nutritional Status, Metabolic Control, Quality or Life, and Functional Status in Elderly Patients. A Multicentre Study. Clin. Nutr. 2019, 38, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Keyhani-Nejad, F.; Kemper, M.; Schueler, R.; Pivovarova, O.; Rudovich, N.; Pfeiffer, A.F.H. Effects of Palatinose and Sucrose Intake on Glucose Metabolism and Incretin Secretion in Subjects with Type 2 Diabetes. Diabetes Care 2016, 39, e38–e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Ke, M.-Y.; Li, W.-H.; Zhang, S.-Q.; Fang, X.-C. The Impact of Soluble Dietary Fibre on Gastric Emptying, Postprandial Blood Glucose and Insulin in Patients with Type 2 Diabetes. Asia Pac. J. Clin. Nutr. 2014, 23, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, D.M.; Wu, D.J.; Peng, H.Y.; Chuang, L.M. Assessment of Blood Glucose Regulation and Safety of Resistant Starch Formula-Based Diet in Healthy Normal and Subjects with Type 2 Diabetes. Medicine 2015, 94, e1332. [Google Scholar] [CrossRef]
- De Vogel-van den Bosch, J.; van den Berg, S.A.A.; Bijland, S.; Voshol, P.J.; Havekes, L.M.; Romijn, H.A.; Hoeks, J.; van Beurden, D.; Hesselink, M.K.C.; Schrauwen, P.; et al. High-Fat Diets Rich in Medium- versus Long-Chain Fatty Acids Induce Distinct Patterns of Tissue Specific Insulin Resistance. J. Nutr. Biochem. 2011, 22, 366–371. [Google Scholar] [CrossRef]
- Eckel, R.H.; Hanson, A.S.; Chen, A.Y.; Berman, J.N.; Yost, T.J.; Brass, E.P. Dietary Substitution of Medium-Chain Triglycerides Improves Insulin-Mediated Glucose Metabolism in NIDDM Subjects. Diabetes 1992, 41, 641–647. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Law, B.Y.K.; Xu, Y. Effect of Inulin-Type Carbohydrates on Insulin Resistance in Patients with Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 5101423. [Google Scholar] [CrossRef] [Green Version]
| LC-ONS (125 mL) | ST-ONS (125 mL) | |
|---|---|---|
| Energy kcal | 200 | 200 |
| Protein g (En%) 1 | 7.5 (15) | 7.5 (15) |
| Fat g (En%) | 8.9 (40) | 6.7 (30) |
| Carbohydrate g (En%) | 27.1 (45) | 29.3 (55) |
| Available carbohydrate g (En%) | 20.1 (40) | 26.8 (54) |
| Isomaltulose g | 7.7 | 0 |
| Indigestible carbohydrate g (En%) | 7.0 (5) | 2.5 (1) |
| Lactulose g | 1.0 | 0 |
| Dietary fiber g | 6.0 | 2.5 |
| Resistant maltodextrin g | 4.5 | 2.5 |
| Inulin g | 1.5 | 0 |
| Parameter | Value 1 |
|---|---|
| Age (years) | 54.0 ± 5.6 |
| Sex (Male/Female) | 11/9 |
| Body mass index (kg/m2) | 23.1 ± 3.4 |
| HbA1c (%) | 5.69 ± 0.32 |
| Fasting blood glucose (mg/dL) | 106.0 ± 10.7 |
| Glucose at 1 hr (mg/dL) | 195.3 ± 31.7 |
| Glucose at 2 hr (mg/dL) | 153.8 ± 48.4 |
| Fasting plasma insulin (µU/mL) | 6.36 ± 3.62 |
| ST-ONS (n = 20) 1 | LC-ONS (n = 20) 1 | Difference (95% CI) 2 | p-Value | ||
|---|---|---|---|---|---|
| Glucose | iAUC (mg/dL∙min) | 3735 ± 391 | 2207 ± 391 | −1528 (−2150 to −905) | <0.001 |
| Cmax (mg/dL) | 173.9 ± 4.9 | 149.5 ± 4.9 | −24.4 (−33.7 to −15.2) | <0.001 | |
| iCmax (mg/dL) | 68.6 ± 4.9 | 45.2 ± 4.9 | −23.5 (−32.8 to −14.1) | <0.001 | |
| Insulin | iAUC (µU/mL∙min) | 2814 ± 317 | 2007 ± 317 | −807 (−1251 to −363) | 0.001 |
| Cmax (µU/mL) | 61.9 ± 5.0 | 45.4 ± 5.0 | −16.6 (−26.9 to −6.2) | 0.004 | |
| iCmax (µU/mL) | 55.6 ± 7.0 | 40.0 ± 7.0 | −15.6 (−24.8 to −6.3) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokubo, E.; Morita, S.; Nagashima, H.; Oshio, K.; Iwamoto, H.; Miyaji, K. Blood Glucose Response of a Low-Carbohydrate Oral Nutritional Supplement with Isomaltulose and Soluble Dietary Fiber in Individuals with Prediabetes: A Randomized, Single-Blind Crossover Trial. Nutrients 2022, 14, 2386. https://doi.org/10.3390/nu14122386
Kokubo E, Morita S, Nagashima H, Oshio K, Iwamoto H, Miyaji K. Blood Glucose Response of a Low-Carbohydrate Oral Nutritional Supplement with Isomaltulose and Soluble Dietary Fiber in Individuals with Prediabetes: A Randomized, Single-Blind Crossover Trial. Nutrients. 2022; 14(12):2386. https://doi.org/10.3390/nu14122386
Chicago/Turabian StyleKokubo, Eri, Shunsuke Morita, Hirotaka Nagashima, Kazutaka Oshio, Hiroshi Iwamoto, and Kazuhiro Miyaji. 2022. "Blood Glucose Response of a Low-Carbohydrate Oral Nutritional Supplement with Isomaltulose and Soluble Dietary Fiber in Individuals with Prediabetes: A Randomized, Single-Blind Crossover Trial" Nutrients 14, no. 12: 2386. https://doi.org/10.3390/nu14122386
APA StyleKokubo, E., Morita, S., Nagashima, H., Oshio, K., Iwamoto, H., & Miyaji, K. (2022). Blood Glucose Response of a Low-Carbohydrate Oral Nutritional Supplement with Isomaltulose and Soluble Dietary Fiber in Individuals with Prediabetes: A Randomized, Single-Blind Crossover Trial. Nutrients, 14(12), 2386. https://doi.org/10.3390/nu14122386






