Low Geriatric Nutritional Risk Index Is Associated with Poorer Prognosis in Elderly Diffuse Large B-Cell Lymphoma Patients Unfit for Intensive Anthracycline-Containing Therapy: A Real-World Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment of DLBCL
2.3. Geriatric Nutritional Risk Index
2.4. Clinical Outcomes and Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Treatment Strategies
3.2. Assessment of the Nutritional Status
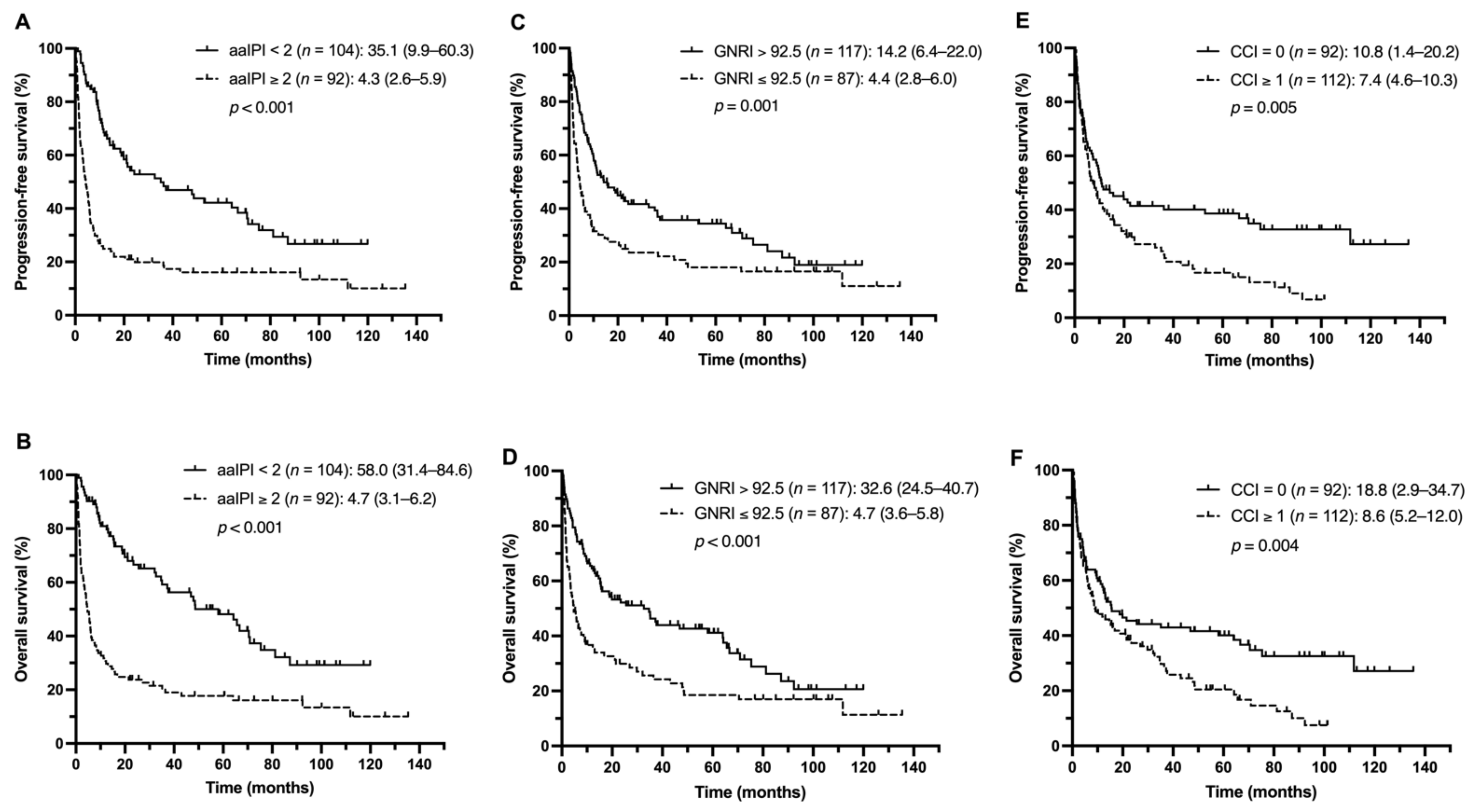
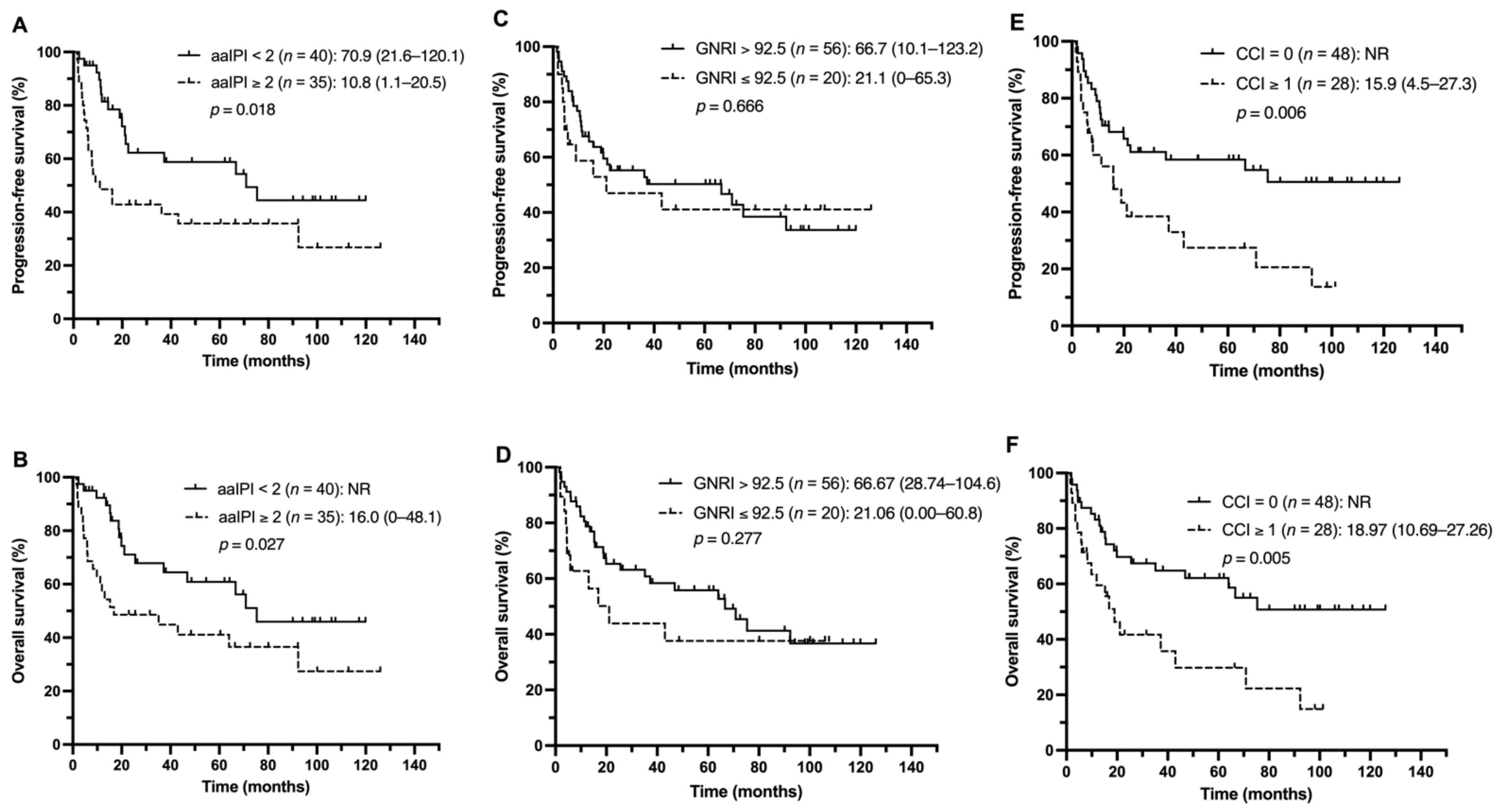
3.3. Outcome Analysis
3.4. Identification of Prognostic Factors for Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef]
- Dixon, D.O.; Neilan, B.; Jones, S.E.; Lipschitz, D.A.; Miller, T.P.; Grozea, P.N.; Wilson, H.E. Effect of age on therapeutic outcome in advanced diffuse histiocytic lymphoma: The Southwest Oncology Group experience. J. Clin. Oncol. 1986, 4, 295–305. [Google Scholar] [CrossRef]
- Vose, J.M.; Armitage, J.O.; Weisenburger, D.D.; Bierman, P.J.; Sorensen, S.; Hutchins, M.; Moravec, D.F.; Howe, D.; Dowling, M.D.; Mailliard, J.; et al. The importance of age in survival of patients treated with chemotherapy for aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 1988, 6, 1838–1844. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Briere, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pfreundschuh, M.; Trümper, L.; Osterborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.L.; et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391. [Google Scholar] [CrossRef]
- Coccaro, N.; Anelli, L.; Zagaria, A.; Perrone, T.; Specchia, G.; Albano, F. Molecular Complexity of Diffuse Large B-Cell Lymphoma: Can It Be a Roadmap for Precision Medicine? Cancers 2020, 12, 185. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.F.; Wu, W.H.; Yang, Y.H.; Liu, Y.C.; Hsiao, H.H.; Chang, C.S. Investigation of treatment pattern, medical resource utilization and demographic prognostic factors in older patients with non-Hodgkin lymphoma: A nationwide population-based study. J. Geriatr. Oncol. 2018, 9, 315–320. [Google Scholar] [CrossRef]
- Hershman, D.L.; McBride, R.B.; Eisenberger, A.; Tsai, W.Y.; Grann, V.R.; Jacobson, J.S. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 2008, 26, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Tsutsué, S.; Tobinai, K.; Yi, J.; Crawford, B. Nationwide claims database analysis of treatment patterns, costs and survival of Japanese patients with diffuse large B-cell lymphoma. PLoS ONE 2020, 15, e0237509. [Google Scholar] [CrossRef] [PubMed]
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Carson, K.R.; Bartlett, N.L.; McDonald, J.R.; Luo, S.; Zeringue, A.; Liu, J.; Fu, Q.; Chang, S.H.; Colditz, G.A. Increased body mass index is associated with improved survival in United States veterans with diffuse large B-cell lymphoma. J. Clin. Oncol. 2012, 30, 3217–3222. [Google Scholar] [CrossRef]
- Dalia, S.; Chavez, J.; Little, B.; Bello, C.; Fisher, K.; Lee, J.H.; Chervenick, P.; Sokol, L.; Sotomayor, E.; Shah, B. Serum albumin retains independent prognostic significance in diffuse large B-cell lymphoma in the post-rituximab era. Ann. Hematol. 2014, 93, 1305–1312. [Google Scholar] [CrossRef]
- Kanemasa, Y.; Shimoyama, T.; Sasaki, Y.; Hishima, T.; Omuro, Y. Geriatric nutritional risk index as a prognostic factor in patients with diffuse large B cell lymphoma. Ann. Hematol. 2018, 97, 999–1007. [Google Scholar] [CrossRef]
- Miura, K.; Konishi, J.; Miyake, T.; Makita, M.; Hojo, A.; Masaki, Y.; Uno, M.; Ozaki, J.; Yoshida, C.; Niiya, D.; et al. A Host-Dependent Prognostic Model for Elderly Patients with Diffuse Large B-Cell Lymphoma. Oncologist 2017, 22, 554–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saygin, C.; Jia, X.; Hill, B.; Dean, R.; Pohlman, B.; Smith, M.R.; Jagadeesh, D. Impact of comorbidities on outcomes of elderly patients with diffuse large B-cell lymphoma. Am. J. Hematol. 2017, 92, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Fujita, K.; Morishita, T.; Negoro, E.; Oiwa, K.; Tsukasaki, H.; Yamamura, O.; Ueda, T.; Yamauchi, T. Prognostic utility of a geriatric nutritional risk index in combination with a comorbidity index in elderly patients with diffuse large B cell lymphoma. Br. J. Haematol. 2021, 192, 100–109. [Google Scholar] [CrossRef]
- Matsukawa, T.; Suto, K.; Kanaya, M.; Izumiyama, K.; Minauchi, K.; Yoshida, S.; Oda, H.; Miyagishima, T.; Mori, A.; Ota, S.; et al. Validation and comparison of prognostic values of GNRI, PNI, and CONUT in newly diagnosed diffuse large B cell lymphoma. Ann. Hematol. 2020, 99, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Go, S.-I.; Kim, H.-G.; Kang, M.H.; Park, S.; Lee, G.-W. Prognostic model based on the geriatric nutritional risk index and sarcopenia in patients with diffuse large B-cell lymphoma. BMC Cancer 2020, 20, 439. [Google Scholar] [CrossRef]
- Boyle, T.; Connors, J.M.; Gascoyne, R.D.; Berry, B.R.; Sehn, L.H.; Bashash, M.; Spinelli, J.J. Physical activity, obesity and survival in diffuse large B-cell and follicular lymphoma cases. Br. J. Haematol. 2017, 178, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Habermann, T.M.; Gordon, L.I.; Hochster, H.; Gascoyne, R.D.; Morrison, V.A.; Fisher, R.I.; Bartlett, N.L.; Stiff, P.J.; Cheson, B.D.; et al. The role of body mass index in survival outcome for lymphoma patients: US intergroup experience. Ann. Oncol. 2014, 25, 669–674. [Google Scholar] [CrossRef]
- Bairey, O.; Shacham-Abulafia, A.; Shpilberg, O.; Gurion, R. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: An important simple prognostic factor. Hematol. Oncol. 2016, 34, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.; Hee, S.W.; Lim, L.C.; Tao, M.; Quek, R.; Yap, S.P.; Loong, E.L.; Sng, I.; Hwan-Cheong, T.L.; Ang, M.K.; et al. Prognostic factors in patients with diffuse large B cell lymphoma: Before and after the introduction of rituximab. Leuk. Lymphoma 2008, 49, 462–469. [Google Scholar] [CrossRef]
- Camus, V.; Lanic, H.; Kraut, J.; Modzelewski, R.; Clatot, F.; Picquenot, J.M.; Contentin, N.; Lenain, P.; Groza, L.; Lemasle, E.; et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur. J. Haematol. 2014, 93, 9–18. [Google Scholar] [CrossRef]
- Lanic, H.; Kraut-Tauzia, J.; Modzelewski, R.; Clatot, F.; Mareschal, S.; Picquenot, J.M.; Stamatoullas, A.; Leprêtre, S.; Tilly, H.; Jardin, F. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk. Lymphoma 2014, 55, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Marian, M.; August, D.A. Prevalence of malnutrition and current use of nutrition support in cancer patient study. JPEN J. Parenter. Enter. Nutr. 2014, 38, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am. J. Med. 2020, 133, 713–722.e717. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lidoriki, I.; Schizas, D.; Frountzas, M.; Machairas, N.; Prodromidou, A.; Kapelouzou, A.; Karavokyros, I.; Pikoulis, E.; Kales, S.N.; Liakakos, T. GNRI as a Prognostic Factor for Outcomes in Cancer Patients: A Systematic Review of the Literature. Nutr. Cancer 2021, 73, 391–403. [Google Scholar] [CrossRef]
- Konishi, T.; Doki, N.; Kishida, Y.; Nagata, A.; Yamada, Y.; Kaito, S.; Kurosawa, S.; Yoshifuji, K.; Shirane, S.; Uchida, T.; et al. Geriatric nutritional risk index (GNRI) just before allogeneic hematopoietic stem cell transplantation predicts transplant outcomes in patients older than 50 years with acute myeloid leukemia in complete remission. Ann. Hematol. 2019, 98, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Nakazato, T.; Ito, C.; Fujita, Y.; Ogura, S.; Kamiya, T.; Sakurai, A.; Aisa, Y.; Mori, T. The prognostic value of geriatric nutritional risk index in patients with follicular lymphoma. Ann. Hematol. 2019, 98, 1777–1779. [Google Scholar] [CrossRef]
- Kaito, S.; Wada, A.; Adachi, H.; Konuma, R.; Kishida, Y.; Nagata, A.; Konishi, T.; Yamada, Y.; Kumagai, T.; Yoshifuji, K.; et al. Geriatric nutritional risk index as a useful prognostic factor in second allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2020, 99, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.F.; Liu, Y.C.; Hsiao, H.H.; Huang, C.T.; Tsai, Y.F.; Wang, H.C.; Lin, S.F.; Liu, T.C. Investigation on treatment strategy, prognostic factors, and risk factors for early death in elderly Taiwanese patients with diffuse large B-cell lymphoma. Sci. Rep. 2017, 7, 44282. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lin, T.L.; Kuo, M.C.; Shih, L.Y.; Dunn, P.; Wang, P.N.; Wu, J.H.; Tang, T.C.; Chang, H.; Hung, Y.S. The impact of age, Charlson comorbidity index, and performance status on treatment of elderly patients with diffuse large B cell lymphoma. Ann. Hematol. 2012, 91, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Grossoeuvre, A.; Houot, R.; Broussais-Guillaumont, F.; Salles, G.; Traullé, C.; Espinouse, D.; Coiffier, B. Non-Hodgkin’s lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann. Oncol. 2008, 19, 774–779. [Google Scholar] [CrossRef]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-López, A.; Hagenbeek, A.; et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999, 17, 1244. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A.; Hamlin, P.; Soubeyran, P.; Stauder, R.; Wadhwa, P.; Aapro, M.; Lichtman, S.M. Approach to therapy of diffuse large B-cell lymphoma in the elderly: The International Society of Geriatric Oncology (SIOG) expert position commentary. Ann. Oncol. 2015, 26, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A.; Hamlin, P.; Soubeyran, P.; Stauder, R.; Wadhwa, P.; Aapro, M.; Lichtman, S. Diffuse large B-cell lymphoma in the elderly: Impact of prognosis, comorbidities, geriatric assessment, and supportive care on clinical practice. An International Society of Geriatric Oncology (SIOG) expert position paper. J. Geriatr. Oncol. 2015, 6, 141–152. [Google Scholar] [CrossRef]
- Chaganti, S.; Illidge, T.; Barrington, S.; McKay, P.; Linton, K.; Cwynarski, K.; McMillan, A.; Davies, A.; Stern, S.; Peggs, K. Guidelines for the management of diffuse large B-cell lymphoma. Br. J. Haematol. 2016, 174, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Merli, F.; Luminari, S.; Rossi, G.; Mammi, C.; Marcheselli, L.; Ferrari, A.; Spina, M.; Tucci, A.; Stelitano, C.; Capodanno, I.; et al. Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: Results from a study of the Fondazione Italiana Linfomi. Leuk. Lymphoma 2014, 55, 38–43. [Google Scholar] [CrossRef]
- Wieringa, A.; Boslooper, K.; Hoogendoorn, M.; Joosten, P.; Beerden, T.; Storm, H.; Kibbelaar, R.E.; Veldhuis, G.J.; van Kamp, H.; van Rees, B.; et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: A population-based cohort study. Br. J. Haematol. 2014, 165, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All Patients (n = 205) | R–CHOP (n = 76) | Non-R–CHOP (n = 129) | p | |
|---|---|---|---|---|
| Age | ||||
| Median, range (years) | 75 (65–96) | 71 (65–93) | 77 (65–96) | <0.001 |
| ≥80, n (%) | 64 (31.2) | 10 (13.2) | 54 (84.4) | <0.001 |
| 70–79, n (%) | 94 (45.9) | 34 (44.7) | 60 (46.3) | 0.495 |
| 65–69, n (%) | 47 (22.9) | 32 (42.1) | 15 (11.6) | <0.001 |
| Gender, n (%) | ||||
| Male | 107 (52.2) | 46 (60.5) | 61 (47.3) | 0.091 |
| Female | 98 (47.8) | 30 (39.5) | 68 (57.2) | |
| ECOG PS, n (%) | ||||
| ≤1 | 135 (65.9) | 66 (86.8) | 69 (53.5) | <0.001 |
| ≥2 | 70 (34.1) | 10 (13.2) | 60 (46.5) | |
| Ann Arbor stage, n (%) * | ||||
| I/II | 73 (36.3) | 34 (44.7) | 39 (31.2) | 0.074 |
| III/IV | 128 (63.7) | 42 (55.3) | 86 (68.8) | |
| aaIPI, n (%) * | ||||
| ≤1 | 93 (47.2) | 41 (53.9) | 52 (43.0) | 0.158 |
| ≥2 | 104 (52.8) | 35 (46.1) | 69 (57.0) | |
| Extranodal, n (%) | 157 (76.6) | 54 (71.1) | 103 (79.8) | 0.151 |
| BM involved, n (%) | 54 (29.3) | 17 (22.7) | 37 (33.9) | 0.137 |
| B symptoms, n (%) | 102 (50) | 33 (43.4) | 69 (53.9) | 0.148 |
| LDH | ||||
| median, range (IU/dL) | 240 (33–3595) | 222 (107–1482) | 250 (33–3592) | 0.139 |
| >ULN, n (%) | 126 (64) | 44 (58.7) | 82 (67.2) | 0.225 |
| Serum Alb, | ||||
| median, range (g/dL) | 3.62 (1.76–4.93) | 3.79 (1.88–4.93) | 3.5 (1.76–4.80) | <0.001 |
| <LLN, n (%) | 74 (37.8) | 15 (20.5) | 59 (48.0) | <0.001 |
| Serum Cr, | ||||
| median, range (mg/dL) | 0.91 (0.11–12.9) | 0.90 (0.35–2.9) | 0.94 (0.11–5.69) | 0.22 |
| CCI ≥ 1 | 112 (54.9) | 28 (36.8) | 84 (65.6) | <0.001 |
| CCI ≥ 3 | 31 (15.2) | 5 (6.6) | 26 (20.3) | 0.008 |
| GNRI score, median, range | 94 (40–115) | 97 (40–115) | 92 (42–113) | <0.001 |
| Low GNRI (≤92.5), n (%) | 87 (42.6) | 20 (26.3) | 67 (32.8) | <0.001 |
| Treatment Choices | Age (Years) # | ECOG PS # | |||
|---|---|---|---|---|---|
| ≥80 (n = 64) | 70–79 (n = 94) | 65–69 (n = 47) | 0–1 (n = 135) | 2–4 (n = 70) | |
| R–CHOP, n (%) | 10 (15.6) | 34 (36.2) | 32 (68.1) | 66 (48.9) | 10 (14.2) |
| R–COP, n (%) | 22 (34.4) | 41 (43.6) | 9 (19.1) | 51 (37.8) | 21 (30.0) |
| Other regimens *, n (%) | 14 (21.9) | 4 (4.2) | 1 (2.1) | 10 (7.4) | 9 (12.9) |
| Steroid alone, n (%) | 18 (28.1) | 15 (16.0) | 5 (10.7) | 8 (5.9) | 30 (42.9) |
| Variables | Univariate Analysis | Multivariate Analysis * | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age ≥ 80 | 4.75 (2.24–10.1) | <0.001 | 3.48 (1.49–8.13) | 0.004 |
| Male gender | 0.79 (0.53–1.24) | 0.168 | ||
| PS 2–4 | 5.74 (2.71–12.2) | <0.001 | 3.51 (1.44–8.57) | 0.006 |
| aaIPI 2–3 | 1.52 (0.85–2.71) | 0.159 | ||
| BM involved | 1.75 (0.90–3.43) | 0.101 | ||
| Stage III/IV | 1.79 (0.99–3.22) | 0.054 | ||
| Extranodal | 1.61 (0.84–3.11) | 0.153 | ||
| Abnormal LDH | 1.44 (0.80–2.62) | 0.226 | ||
| Abnormal B2M | 3.20 (1.74–5.89) | <0.001 | 1.02 (0.47–2.21) | 0.969 |
| Abnormal Cr | 3.53 (1.48–8.43) | 0.005 | 2.32 (0.78–6.89) | 0.130 |
| B symptoms | 1.52 (0.86–2.70) | 0.148 | ||
| Low BMI | 3.48 (0.75–16.1) | 0.111 | ||
| Low Alb | 3.57 (1.83–6.70) | <0.001 | 2.59 (1.16–5.79) | 0.020 |
| CCI (≥ 1) | 3.27 (1.81–5.92) | <0.001 | 3.08 (1.52–6.23) | 0.002 |
| High GNRI (n = 117) | Low GNRI (n = 87) | p | |
|---|---|---|---|
| Age | |||
| Median, range (years) | 75 (63–94) | 76 (65–95) | 0.03 |
| ≥80, n (%) | 30 (25.6) | 16 (18.4) | 0.058 |
| 70–79, n (%) | 58 (49.6) | 36 (41.4) | 0.132 |
| 65–69, n (%) | 29 (24.8) | 35 (40.2) | 0.059 |
| Gender, n (%) | |||
| Male | 60 (51.3) | 47 (54.0) | 0.689 |
| Female | 57 (48.7) | 40 (46.0) | |
| ECOG PS, n (%) | |||
| ≤1 | 92 (78.6) | 43 (49.4) | <0.001 |
| ≥2 | 25 (21.4) | 44 (50.6) | |
| Ann Arbor stage, n (%) * | |||
| I/II | 56 (47.9) | 17 (20.2) | <0.001 |
| III/IV | 61 (52.1) | 67 (79.8) | |
| aaIPI, n (%) * | |||
| ≤1 | 71 (60.7) | 22 (27.2) | <0.001 |
| ≥2 | 46 (39.3) | 58 (72.5) | |
| Extranodal, n (%) | 86 (73.5) | 70 (80.5) | 0.247 |
| BM involved, n (%) | 25 (22.5) | 29 (39.7) | 0.012 |
| B symptoms, n (%) | 46 (39.7) | 55 (63.2) | 0.001 |
| LDH | |||
| median, range (IU/dL) | 199 (33–2435) | 274 (82–3595) | <0.001 |
| >ULN, n (%) | 61 (53.0) | 64 (79.0) | <0.001 |
| Serum Alb, | |||
| median, range (g/dL) | 3.89 (3.47–4.93) | 3.05 (1.76–3.84) | <0.001 |
| <LLN, n (%) | 3 (2.6) | 70 (89.7) | <0.001 |
| Serum Cr, | |||
| median, range (mg/dL) | 0.91 (0.11–8.36) | 0.95 (0.53–12.9) | 0.207 |
| CCI ≥ 1 | 64 (54.7) | 48 (55.8) | 0.875 |
| CCI ≥ 3 | 18 (15.4) | 13 (15.1) | 0.958 |
| R–CHOP, n (%) | 56 (47.9) | 20 (23.0) | <0.001 |
| R–CHOP Group | Non-R–CHOP Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariate Analysis | Multivariate Analysis * | Univariate Analysis | Multivariate Analysis * | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 80 | 1.87 (0.86–4.06) | 0.114 | 1.21 (0.82–1.76) | 0.337 | ||||
| Male gender | 1.32 (0.70–2.50) | 0.389 | 1.41 (0.97–2.06) | 0.070 | ||||
| PS 2–4 | 20.2 (7.78–52.4) | <0.001 | 14.9 (4.77–46.4) | <0.001 | 2.76 (1.87–4.07) | <0.001 | 1.65 (0.92–2.95) | 0.092 |
| aaIPI 2–3 | 2.09 (1.12–3.93) | 0.021 | 1.17 (1.31–1.88) | 0.036 | 3.18 (2.10–4.83) | <0.001 | 2.70 (1.18–6.27) | 0.029 |
| BM involved | 1.43 (0.71–2.89) | 0.318 | 2.85 (1.83–4.43) | <0.001 | 2.50 (1.47–4.25) | 0.001 | ||
| Stage III/IV | 1.78 (0.94–3.36) | 0.076 | 2.70 (1.73–4.23) | <0.001 | 0.99 (0.48–2.04) | 0.977 | ||
| Extranodal | 0.84 (0.44–1.60) | 0.596 | 1.03 (0.64–1.63) | 0.916 | ||||
| Abnormal LDH | 1.86 (0.96–3.62) | 0.066 | 2.24 (1.46–3.43) | <0.001 | 0.92 (0.50–1.84) | 0.781 | ||
| Abnormal B2M | 1.90 (1.01–3.58) | 0.046 | 1.01 (0.47–2.19) | 0.927 | 2.68 (1.66–4.31) | <0.001 | 2.98 (1.61–5.53) | 0.001 |
| B symptoms | 1.43 (0.78–2.64) | 0.251 | 1.73 (1.18–2.53) | 0.005 | 0.96 (0.59–1.57) | 0.871 | ||
| Low Alb | 1.39 (0.68–2.86) | 0.366 | 1.75 (1.19–2.58) | 0.004 | 1.81 (0.66–4.97) | 0.252 | ||
| CCI (≥1) | 2.30 (1.24–4.27) | 0.008 | 1.58 (0.80–3.11) | 0.185 | 0.88 (0.59–1.32) | 0.536 | ||
| Low GNRI | 1.17 (0.58–2.33) | 0.667 | 1.47 (1.01–2.14) | 0.049 | 2.85 (1.05–7.72) | 0.039 | ||
| R–CHOP Group | Non-R–CHOP Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Univariate Analysis | Multivariate Analysis * | Univariate Analysis | Multivariate Analysis * | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 80 | 2.08 (0.95–4.56) | 0.066 | 1.30 (0.88–1.92) | 0.187 | ||||
| Male gender | 1.38 (0.71–2.65) | 0.341 | 1.48 (1.01–2.18) | 0.047 | 1.36 (0.84–2.19) | 0.212 | ||
| PS 2–4 | 21.7 (8.31–56.6) | <0.001 | 15.2 (4.82–47.7) | <0.001 | 3.22 (2.15–4.82) | <0.001 | 1.46 (0.78–2.74) | 0.243 |
| aaIPI 2–3 | 2.04 (1.07–3.89) | 0.030 | 1.39 (1.01–1.92) | 0.043 | 3.97 (2.55–6.18) | <0.001 | 3.08 (1.22–7.77) | 0.017 |
| BM involved | 1.22 (0.59–2.52) | 0.598 | 3.15 (2.00–4.98) | <0.001 | 2.69 (1.56–4.65) | <0.001 | ||
| Stage III/IV | 1.80 (0.93–3.46) | 0.080 | 3.18 (1.97–5.13) | <0.001 | 0.91 (0.42–1.98) | 0.819 | ||
| Extranodal | 0.98 (0.50–1.95) | 0.962 | 1.10 (0.68–1.78) | 0.710 | ||||
| Abnormal LDH | 1.90 (0.96–3.76) | 0.067 | 2.67 (1.70–4.20) | <0.001 | 0.99 (0.51–1.90) | 0.969 | ||
| Abnormal B2M | 2.23 (1.16–4.29) | 0.016 | 1.25 (0.57–2.74) | 0.585 | 2.67 (1.63–4.37) | <0.001 | 2.99 (1.54–5.80) | 0.001 |
| B symptoms | 1.40 (0.75–2.63) | 0.291 | 1.78 (1.20–2.64) | 0.004 | 0.93 (0.55–1.57) | 0.790 | ||
| Low Alb | 1.51 (0.73–3.12) | 0.263 | 2.00 (1.34–2.98) | 0.001 | 1.95 (0.66–5.82) | 0.229 | ||
| CCI (≥1) | 2.41 (1.28–4.54) | 0.007 | 1.58 (0.78–3.19) | 0.202 | 0.94 (0.62–1.42) | 0.750 | ||
| Low GNRI | 1.28 (0.63–2.57) | 0.495 | 1.62 (1.09–2.41) | 0.016 | 2.98 (1.02–8.90) | 0.045 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, T.-M.; Liu, Y.-C.; Hsiao, H.-H.; Wang, H.-C.; Du, J.-S.; Yeh, T.-J.; Gau, Y.-C.; Ke, Y.-L.; Yang, C.-I.; Lee, C.-P.; et al. Low Geriatric Nutritional Risk Index Is Associated with Poorer Prognosis in Elderly Diffuse Large B-Cell Lymphoma Patients Unfit for Intensive Anthracycline-Containing Therapy: A Real-World Study. Nutrients 2021, 13, 3243. https://doi.org/10.3390/nu13093243
Chuang T-M, Liu Y-C, Hsiao H-H, Wang H-C, Du J-S, Yeh T-J, Gau Y-C, Ke Y-L, Yang C-I, Lee C-P, et al. Low Geriatric Nutritional Risk Index Is Associated with Poorer Prognosis in Elderly Diffuse Large B-Cell Lymphoma Patients Unfit for Intensive Anthracycline-Containing Therapy: A Real-World Study. Nutrients. 2021; 13(9):3243. https://doi.org/10.3390/nu13093243
Chicago/Turabian StyleChuang, Tzer-Ming, Yi-Chang Liu, Hui-Hua Hsiao, Hui-Ching Wang, Jeng-Shiun Du, Tsung-Jang Yeh, Yuh-Ching Gau, Ya-Lun Ke, Ching-I Yang, Ching-Ping Lee, and et al. 2021. "Low Geriatric Nutritional Risk Index Is Associated with Poorer Prognosis in Elderly Diffuse Large B-Cell Lymphoma Patients Unfit for Intensive Anthracycline-Containing Therapy: A Real-World Study" Nutrients 13, no. 9: 3243. https://doi.org/10.3390/nu13093243
APA StyleChuang, T.-M., Liu, Y.-C., Hsiao, H.-H., Wang, H.-C., Du, J.-S., Yeh, T.-J., Gau, Y.-C., Ke, Y.-L., Yang, C.-I., Lee, C.-P., Hsu, C.-M., & Cho, S.-F. (2021). Low Geriatric Nutritional Risk Index Is Associated with Poorer Prognosis in Elderly Diffuse Large B-Cell Lymphoma Patients Unfit for Intensive Anthracycline-Containing Therapy: A Real-World Study. Nutrients, 13(9), 3243. https://doi.org/10.3390/nu13093243








