Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Cells
2.2. Dietary Supplement
2.3. In Vitro Studies
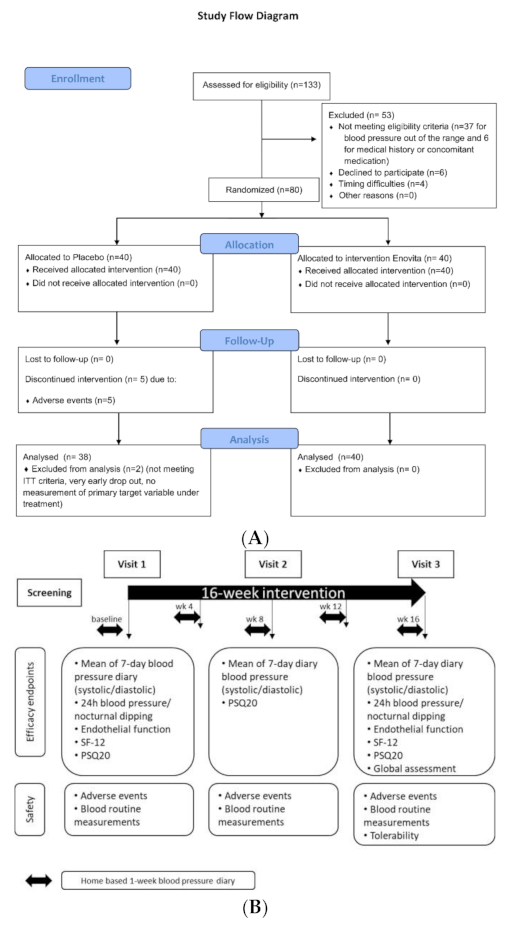
2.4. Human Study
2.4.1. Participants
2.4.2. Dietary Intervention
2.4.3. Objectives
2.4.4. Criteria for Objectives Measurements
2.4.5. Safety
2.4.6. Statistical Analysis
3. Results
3.1. In Vitro Cell Studies
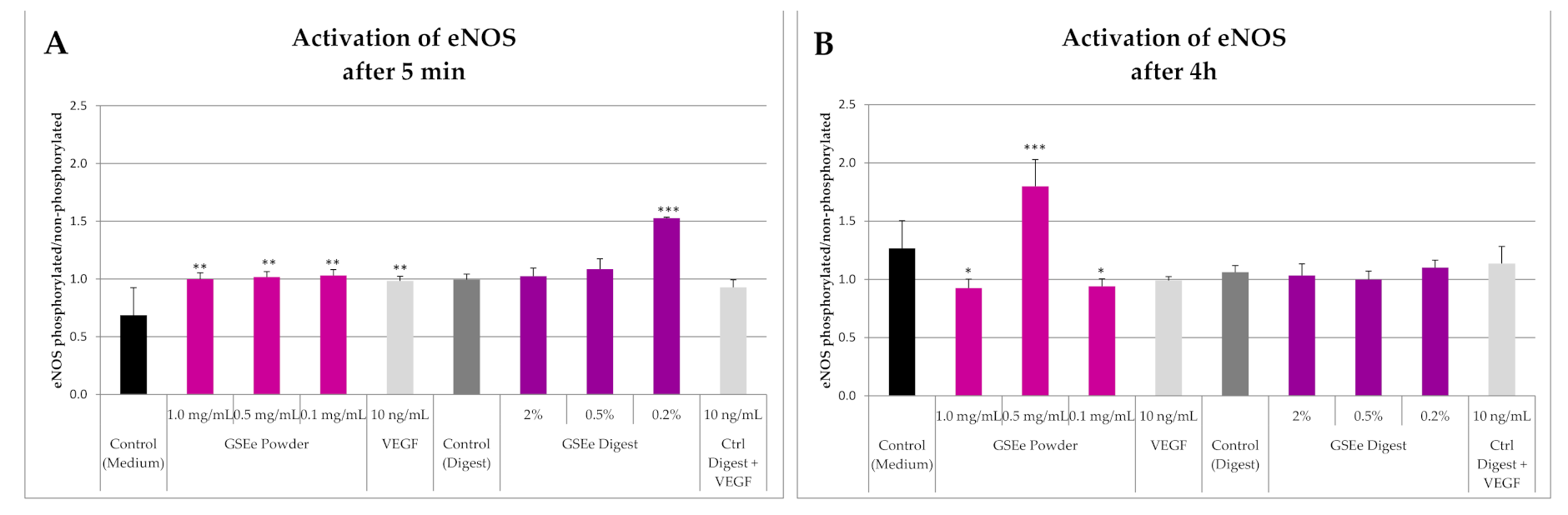
3.1.1. eNOS
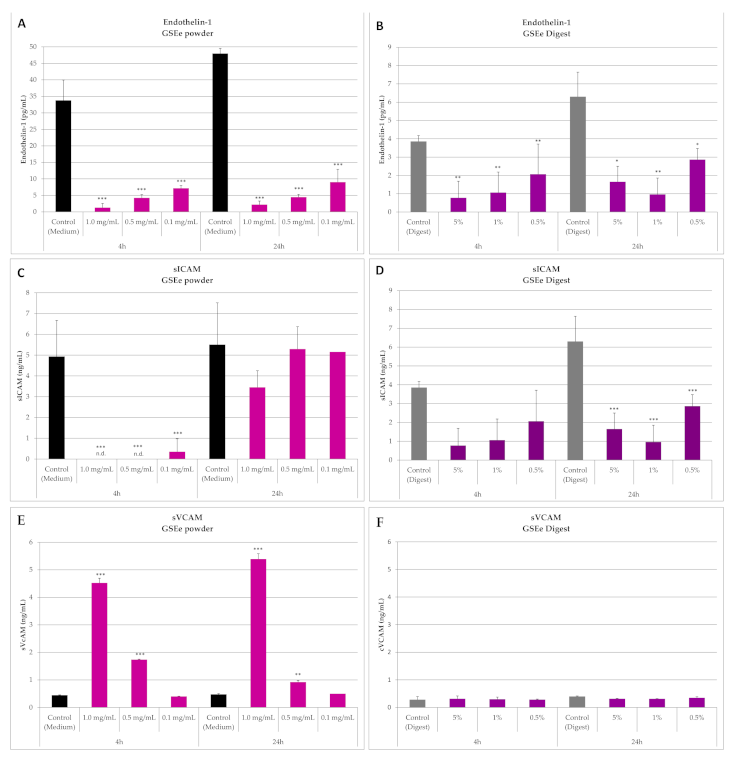
3.1.2. Endothelin-1, sICAM and sVCAM
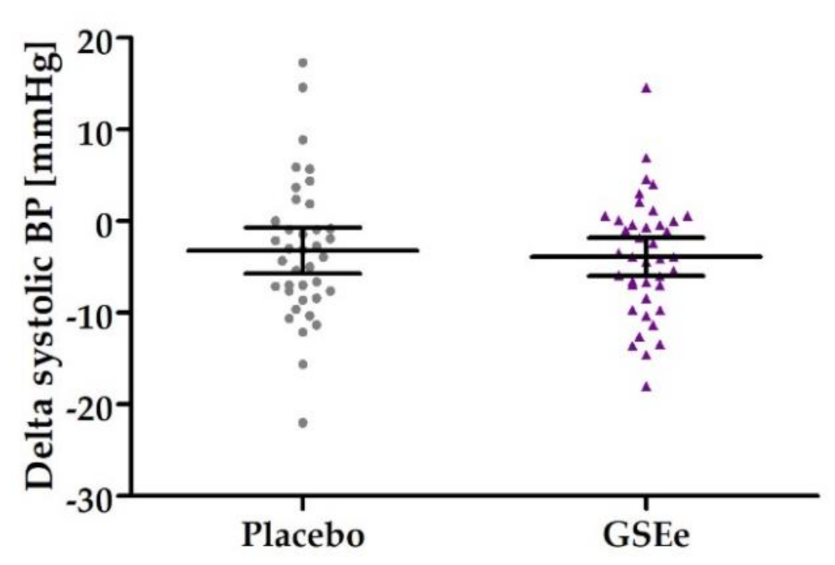
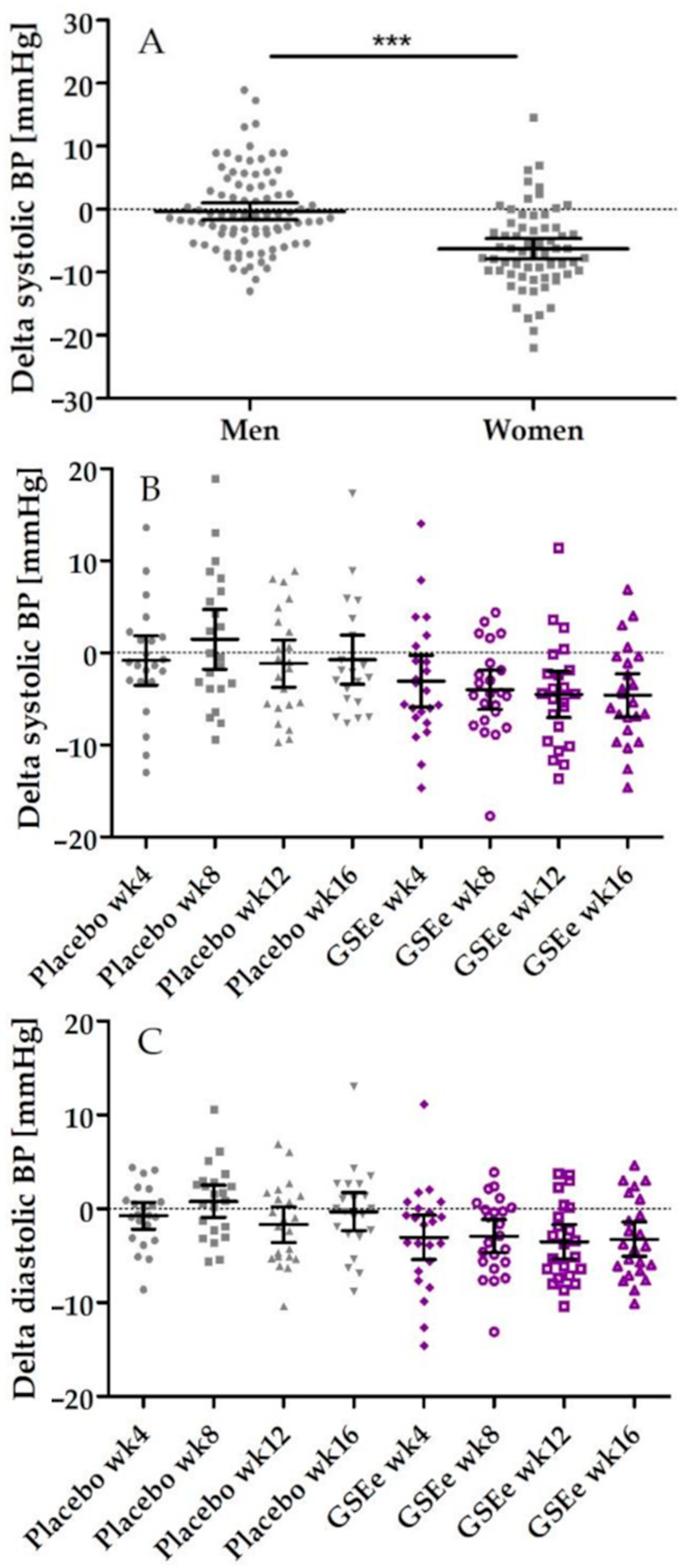
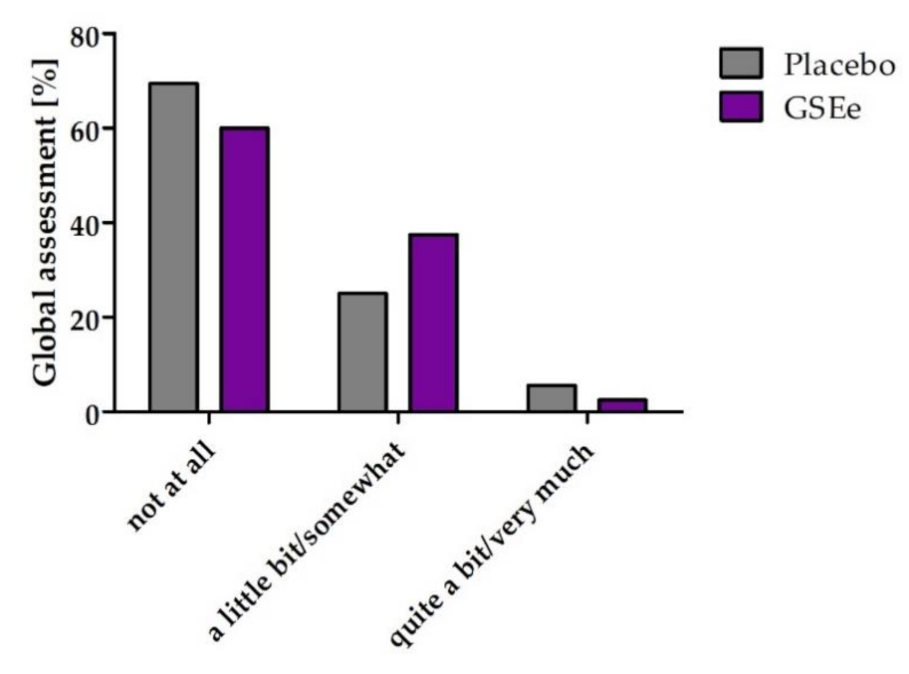
3.2. Human Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wani-Parekh, P.; Blanco-Garcia, C.; Mendez, M.; Mukherjee, D. Guide of Hypertensive Crisis Pharmacotherapy. Cardiovasc. Hematol. Disord. Drug Targets 2017, 17, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Chiu, H.-F.; Wang, C.K. Impact of functional foods and nutraceuticals on high blood pressure with a special focus on meta-analysis: Review from a public health perspective. Food Funct. 2020, 11, 2792–2804. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Haq, I.U.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Belcaro, G.; Ledda, A.; Hu, S.; Cesarone, M.R.; Feragalli, B.; Dugall, M. Grape Seed Procyanidins in Pre- and Mild Hypertension: A Registry Study. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Dirsch, V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric. Oxide 2009, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.F.; Cherng, J.Y. Reduction of Adhesion Molecule Production and Alteration of eNOS and Endothelin-1 mRNA Expression in Endothelium by Euphorbia hirta L. through Its Beneficial β-Amyrin Molecule. Molecule 2014, 19, 10534–10545. [Google Scholar] [CrossRef] [PubMed]
- Gerstgrasser, A.; Röchter, S.; Dressler, D.; Schön, C.; Reule, C.; Buchwald-Werner, S. In Vitro Activation of eNOS by Mangifera indica (Careless™) and Determination of an Effective Dosage in a Randomized, Double-Blind, Human Pilot Study on Microcirculation. Planta Medica 2015, 82, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols-A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Modha, K.; Whiteside, J.P.; Spier, R.E. The determination of cellular viability of hybridoma cells in microtitre plates: A colorimetric assay based on neutral red. Cytotechnology 1993, 13, 227–232. [Google Scholar] [CrossRef]
- Luzak, B.; Boncler, M.; Rywaniak, J.; Dudzińska, D.; Rozalski, M.; Krajewska, U.; Balcerczak, E.; Podsędek, A.; Redzynia, M.; Watala, C. Extract from Ribes nigrum leaves in vitro activates nitric oxide synthase (eNOS) and increases CD39 expression in human endothelial cells. J. Physiol. Biochem. 2014, 70, 1007–1019. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.-J.; Han, Z.-H.; Li, Y.-Q.; Xue, J.-H.; Gao, D.-F.; Wu, X.-S.; Wang, C.-X. PI3K/AKT signaling pathway plays a role in enhancement of eNOS activity by recombinant human angiotensin converting enzyme 2 in human umbilical vein endothelial cells. Int. J. Clin. Exp. Pathol. 2014, 7, 8112–8117. [Google Scholar]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.-Y.; Bach, F.H. Heme Oxygenase-1 Modulates the Expression of Adhesion Molecules Associated with Endothelial Cell Activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef]
- Ulrich, K.; Fitzgerald, A.P.; Gohlke, H.; Wellmann, J.; Hense, H. Risikoabschätzung tödlicher Herz-Kreislauf-Erkrankungen.Die neuen SCORE-Deutschland-Tabellen für die Primärprävention [Risk stratification of cardiovascular diseases in primary prevention—The new SCORE-Deutschland risk charts]. Dtsch. Ärzteblatt 2005, 102, A-1808/B-1526/C-1441. [Google Scholar]
- Muris, D.M.J.; Houben, A.J.H.M.; Schram, M.T.; Stehouwer, C.D.A. Microvascular dysfunction: An emerging pathway in the pathogenesis of obesity-related insulin resistance. Rev. Endocr. Metab. Disord. 2013, 14, 29–38. [Google Scholar] [CrossRef]
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-Sectional Relations of Digital Vascular Function to Cardiovascular Risk Factors in the Framingham Heart Study. Circ. 2008, 117, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Cioni, G.; Boddi, M.; Fatini, C.; Romagnuolo, I.; Casini, A.; Gensini, G.F.; Abbate, R.; Sofi, F. Peripheral-Arterial Tonometry for Assessing Endothelial Function in Relation to Dietary Habits. J. Investig. Med. 2013, 61, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Fliege, H.; Rose, M.; Arck, P.; Walter, O.B.; Kocalevent, R.-D.; Weber, C.; Klapp, B.F. The Perceived Stress Questionnaire (PSQ) Reconsidered: Validation and Reference Values From Different Clinical and Healthy Adult Samples. Psychosom. Med. 2005, 67, 78–88. [Google Scholar] [CrossRef]
- Von Morfeld, M.; Kircheberger, I.; Bullinger, M. Auflage Handbuch SF-36 Fragebogen zum Gesundheitszustand, 2nd ed; Hogrefe: Boston, MA, USA, 2011. [Google Scholar]
- University, U. Utah Health Status Update: Medical Outcomes Study SF-12. 2003. Available online: https://collections.lib.utah.edu/details?id=188302 (accessed on 31 October 2016).
- Imai, Y.; Kario, K.; Shimada, K.; Kawano, Y.; Hasebe, N.; Matsuura, H.; Tsuchihashi, T.; Ohkubo, T.; Kuwajima, I. The Japanese Society of Hypertension Guidelines for Self-monitoring of Blood Pressure at Home (Second Edition). Hypertens. Res. 2012, 35, 777–795. [Google Scholar] [CrossRef]
- Yano, Y.; Kario, K. Nocturnal blood pressure and cardiovascular disease: A review of recent advances. Hypertens. Res. 2012, 35, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat. Res. Mol. Mech. Mutagen. 2014, 768, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Barbe, A.; Ramé, C.; Mellouk, N.; Estienne, A.; Bongrani, A.; Brossaud, A.; Riva, A.; Guérif, F.; Froment, P.; Dupont, J. Effects of Grape Seed Extract and Proanthocyanidin B2 on In Vitro Proliferation, Viability, Steroidogenesis, Oxidative Stress, and Cell Signaling in Human Granulosa Cells. Int. J. Mol. Sci. 2019, 20, 4215. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Chatelain, K.; Farris, D.; Jensen, D.; Pickup, J.; Swapp, A.; O’Malley, S.; Kingsley, K. Oral squamous cell carcinoma proliferative phenotype is modulated by proanthocyanidins: A potential prevention and treatment alternative for oral cancer. BMC Complement. Altern. Med. 2007, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wang, H.; Du, Y.; Gao, L. Grape Seed Procyanidins Attenuates Cisplatin-induced Human Embryonic Renal Cell Cytotoxicity by Modulating Heme Oxygenase-1 in Vitro. Cell Biophys. 2019, 77, 367–377. [Google Scholar] [CrossRef]
- Wang, Y.J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 22, 1117–1120. [Google Scholar]
- Shao, Z.-H.; Becker, L.B.; Hoek, T.L.V.; Schumacker, P.T.; Li, C.-Q.; Zhao, D.; Wojcik, K.; Anderson, T.; Qin, Y.; Dey, L.; et al. Grape seed proanthocyanidin extract attenuates oxidant injury in cardiomyocytes. Pharmacol. Res. 2003, 47, 463–469. [Google Scholar] [CrossRef]
- Garcia, V.; Sessa, W.C. Endothelial NOS: Perspective and recent developments. Br. J. Pharmacol. 2019, 176, 189–196. [Google Scholar] [CrossRef]
- Fitzpatrick, D.F.; Bing, B.; Maggi, D.A.; Fleming, R.C.; O’Malley, R.M. Vasodilating Procyanidins Derived from Grape Seeds. Ann. New York Acad. Sci. 2002, 957, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Pasceri, V.; Wu, H.D.; Willerson, J.T.; Yeh, E.T.H. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation 2000, 101, 235–238. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2019, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Zhao, P.; Tian, H.-B.; Chen, L.-H.; Cui, L.-Q. Effect of Grape Polyphenols on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0137665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Li, L.; Liu, S.; Mi, J.; Tian, G. The impact of grape seed extract treatment on blood pressure changes: A meta-analysis of 16 randomized controlled trials. Medicine 2016, 95, e4247. [Google Scholar]
- Edirisinghe, I.; Burton-Freeman, B.; Kappagoda, C.T. Mechanism of the endothelium-dependent relaxation evoked by a grape seed extract. Clin. Sci. 2008, 114, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; FörstermannU. Resveratrol, a Polyphenolic Phytoalexin Present in Red Wine, Enhances Expression and Activity of Endothelial Nitric Oxide Synthase. Circ. 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Jakubowski, M.; Turek-Jakubowska, A.; Szahidewicz-Krupska, E.; Gawrys, K.; Gawrys, J.; Doroszko, A. Profiling the endothelial function using both peripheral artery tonometry (EndoPAT) and Laser Doppler Flowmetry (LD)—Complementary studies or waste of time? Microvasc. Res. 2020, 130, 104008. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Choy, Y.Y.; Waterhouse, A.; Burton-Freeman, B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: A randomised, double-blinded, two-arm, parallel, placebo-controlled trial. Br. J. Nutr. 2015, 115, 226–238. [Google Scholar] [CrossRef]
- American Heart Association. Managing stress to control high blood pressure. Available online: https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/managing-stress-to-control-high-blood-pressure. (accessed on 31 October 2016).
- Fuster, V.; Hall, J.E.; Granger, J.P.; Jones, D.W.; Hall, M.E. Pathophysiology of hypertension. Chapter 24. In Hurst’s the Heart, 14th ed.; McGraw-Hill Education: New York, NY, USA, 11 April 2017. [Google Scholar]
- Kellerman, R.D.; Rakel, D.P.; Kashif, A.; Abdul-Hussein, M.; Adam, R.D. Hypertension. Section 3 Cardiovascular System, Chapter 32. In Conn’s Current Therapy 2020, 1st ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Nguyen, T.T.J. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.M.; Scholey, A. Polyphenols for Brain and Cognitive Health. Recent Advances in Polyphenol Research 2016, 12, 259–288. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
| Placebo | GSEe | |
|---|---|---|
| N | 38 | 40 |
| Age [years] | 56.9 (54.6–59.3) | 56.4 (53.9–58.9) |
| BMI [kg/m2] | 26.1 (25.1–27.1) | 25.2 (24.1–26.3) |
| Known familiar incidence of elevated blood pressure | 45% | 43% |
| Frequency [%] men / women | 57.9% / 42.1% | 57.5% / 42.5% |
| 10-year risk Score according to SCORE Germany ≤5%; Frequency [%] | 87% | 85% |
| Systolic blood pressure [mmHg] | 134.1 (132.8–135.4) | 134.6 (133.2–136.0) |
| Diastolic blood pressure [mmHg] | 82.3 (80.3–84.4) | 83.7 (81.8–85.7) |
| Cholesterol [mg/dL] | 229 (217–241) | 231 (220–241) |
| LDL-cholesterol [mg/dL] | 142 (132–152) | 141 (132–150) |
| GPT [U/L] | 29 (25–33) | 26 (23–28) |
| GOT [U/L] | 22 (20–24) | 20 (19–22) |
| Glucose [mg/dL] | 94 (91–97) | 91 (88–94) |
| Men 1 | Women 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimated Mean Difference | 95% C.I. of Mean Difference Lower upper | p Value | Estimated Mean Difference | 95% C.I. of mean Difference Lower Upper | p Value | |||
| Systolic Pressure | ||||||||
| After 4 weeks | −2.19 | −5.97 | 1.59 | 0.2489 | 4.76 | 1.49 | 8.03 | 0.0057 * |
| After 8 weeks | −5.36 | −9.01 | −1.70 | 0.0051 * | 0.98 | −3.51 | 5.47 | 0.6599 |
| After 12 weeks | −3.27 | −6.71 | 0.17 | 0.0619 ° | 0.98 | −3.36 | 5.33 | 0.6469 |
| After 16 weeks | −3.76 | −7.16 | −0.37 | 0.0306 * | 3.73 | −2.22 | 9.67 | 0.2103 |
| Diastolic Pressure | ||||||||
| After 4 weeks | −2.15 | −4.90 | 0.61 | 0.1234 | 2.60 | −0.23 | 5.42 | 0.0702 ° |
| After 8 weeks | −3.59 | −6.04 | −1.14 | 0.0051 * | 0.56 | −2.82 | 3.95 | 0.7363 |
| After 12 weeks | −1.77 | −4.38 | 0.85 | 0.1795 | 0.22 | −3.33 | 3.77 | 0.8998 |
| After 16 weeks | −2.98 | −5.70 | −0.27 | 0.0320 * | 0.69 | −2.97 | 4.35 | 0.7046 |
| PSQ20 Worries Score | Placebo V1 | Placebo V2 | Placebo V3 | GSEe | GSEe V2 | GSEe V3 |
|---|---|---|---|---|---|---|
| Mean | 17.9 | 14.9 | 18.4 | 16.5 | 14.7 | 13.7 * |
| Std Deviation | 14.5 | 15.5 | 16.3 | 14.9 | 14.6 | 16.0 |
| Lower 95% C.I. | 13.1 | 9.8 | 13.1 | 11.7 | 10.0 | 8.6 |
| Upper 95% C.I. | 22.7 | 20.0 | 23.8 | 21.3 | 19.3 | 18.8 |
| SF-12 | Placebo PCS12 | GSEe PCS12 | Placebo MCS12 | GSEe MCS12 |
|---|---|---|---|---|
| Mean | −0.15 | 0.07 | −1.71 | 1.32 * |
| Std Deviation | 6.99 | 3.16 | 7.77 | 4.67 |
| Lower 95% C.I. | −2.44 | −0.94 | −4.27 | 0.18 |
| Upper 95% C.I. | 2.15 | 1.08 | 0.84 | 2.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schön, C.; Allegrini, P.; Engelhart-Jentzsch, K.; Riva, A.; Petrangolini, G. Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients 2021, 13, 654. https://doi.org/10.3390/nu13020654
Schön C, Allegrini P, Engelhart-Jentzsch K, Riva A, Petrangolini G. Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients. 2021; 13(2):654. https://doi.org/10.3390/nu13020654
Chicago/Turabian StyleSchön, Christiane, Pietro Allegrini, Karin Engelhart-Jentzsch, Antonella Riva, and Giovanna Petrangolini. 2021. "Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers" Nutrients 13, no. 2: 654. https://doi.org/10.3390/nu13020654
APA StyleSchön, C., Allegrini, P., Engelhart-Jentzsch, K., Riva, A., & Petrangolini, G. (2021). Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients, 13(2), 654. https://doi.org/10.3390/nu13020654






