Abstract
Armolipid Plus® is a multi-constituent nutraceutical that claims to improve lipid profiles. The aim of this PRISMA compliant systematic review and meta-analysis was to globally evaluate the efficacy and safety of Armolipid Plus® on the basis of the available randomized, blinded, controlled clinical trials (RCTs). A systematic literature search in several databases was conducted in order to identify RCTs assessing the efficacy and safety of dietary supplementation with Armolipid Plus®. Two review authors independently identified 12 eligible studies (1050 included subjects overall) and extracted data on study characteristics, methods, and outcomes. Meta-analysis of the data suggested that dietary supplementation with Armolipid Plus® exerted a significant effect on body mass index (mean difference (MD) = −0.25 kg/m2, p = 0.008) and serum levels of total cholesterol (MD = −25.07 mg/dL, p < 0.001), triglycerides (MD = −11.47 mg/dL, p < 0.001), high-density lipoprotein cholesterol (MD = 1.84 mg/dL, p < 0.001), low-density lipoprotein cholesterol (MD = −26.67 mg/dL, p < 0.001), high sensitivity C reactive protein (hs-CRP, MD = −0.61 mg/L, p = 0.022), and fasting glucose (MD = −3.52 mg/dL, p < 0.001). Armolipid Plus® was well tolerated. This meta-analysis demonstrates that dietary supplementation with Armolipid Plus® is associated with clinically meaningful improvements in serum lipids, glucose, and hs-CRP. These changes are consistent with improved cardiometabolic health.
1. Introduction
Atherosclerosis cardiovascular diseases (ASCVD) are the leading cause of mortality worldwide, and the main cause of death in persons under 75 years old in Western countries, with a huge social and economic impact [1]. Pooling data from 204 countries, the Global Burden of Disease (GBD) Study recently showed that prevalent cases of total CVD nearly doubled from 271 million in 1990 to 523 million in 2019, and the number of CVD deaths steadily increased from 12.1 million in 1990, reaching 18.6 million in 2019 [2].
High serum levels of low-density lipoprotein cholesterol (LDL-C) are the most important risk factor for the development of ASCVD [3]. The American Heart Association (AHA) 2016 update on heart disease and stroke statistics verified that only 75.7% of US children and 46.6% of US adults have total cholesterol (TC) within the advised ranges (< 170 mg/dL for untreated children and < 200 mg/dL for untreated adults), with comparable rates for other Western countries [4,5].
To reach the LDL-C target, the international guidelines recommend lifestyle changes and lipid-lowering therapy depending on the severity of dyslipidemia and global CV risk [6,7]. Specific lifestyle interventions for hypercholesterolemia include a diet low in saturated fat, moderate to high-intensity physical activity, smoking cessation, as well as weight loss for overweight and obese patients [8,9]. If maintained over the long term, these lifestyle modifications can reduce LDL-C by 5% to 15% and improve ASCVD risk [10]. However, patients unable to reach their target LDL-C goals through lifestyle interventions can consider using lipid-lowering nutraceuticals [11], as also suggested by the International Lipid Expert Panel [12].
Nutraceuticals with a detectable lipid-lowering effect can be divided into natural inhibitors of hepatic cholesterol synthesis, inhibitors of intestinal cholesterol absorption, and enhancers of the excretion of LDL-C on the basis of their mechanisms of action [12]. However, the lipid-lowering effect of most nutraceuticals occurs through multiple mechanisms. The possibility that they act synergistically on multiple stages of lipid-induced vascular damage makes them potential candidates for improving the lipid-lowering effects when used in combination with diet, medications, or other nutraceuticals [13].
Armolipid Plus® is a widely tested and used proprietary formulation of six naturally occurring substances containing red yeast extract (200 mg, corresponding to 3 mg of monacolin K), policosanols (10 mg), and berberine (500 mg), in addition to folic acid (0.2 mg), astaxanthin (0.5 mg), and coenzyme Q10 (2 mg), with a detectable effect on serum lipids, blood pressure (BP), fasting plasma glucose (FPG), and several markers of insulin resistance with a good safety profile [14].
Given the increasing number of good quality studies on this nutraceutical combination, the aim of our systematic review and meta-analysis was to evaluate the efficacy and safety of Armolipid Plus® on the basis of the available randomized, blinded, controlled clinical trials.
2. Materials and Methods
The study was designed according to guidelines inthe 2009 preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement [15], and was registered in the PROSPERO database (Registration number CRD42020212600). Due to the study design (meta-analysis), neither institutional review board (IRB) approval nor patient informed consent were required.
2.1. Search Strategy
PubMed, EMBASE, SCOPUS, Google Scholar, Web of Science by Clarivate, and ClinicaTrial.gov (accessed on 1 February 2021) databases were searched, with no language restriction, using the following search terms: “Armolipid Plus®” AND (“Cholesterol” OR “LDL” OR “Triglycerides” OR “Body mass index” OR “BMI” OR “Plasma Glucose” OR “Glycemia” OR “Insulin”). The wild-card term “*” was used to increase the sensitivity of the search strategy, which was limited to studies in humans. The reference list of identified papers was manually checked for additional relevant articles. In particular, additional searches for potential trials included the references of review articles on the topic of the meta-analysis and relevant abstracts from selected congresses. The literature was searched from inception to 3 February 2021.
All paper abstracts were screened by two reviewers (F.F. and A.F.G.C.) in an initial process to remove ineligible articles. The remaining articles were obtained in full text and assessed again by the same two researchers, who evaluated each article independently and carried out data extraction and quality assessment. Disagreements were resolved by discussion with a third party.
2.2. Study Selection Criteria
Original studies were included if they met the following criteria: (i) being a clinical trial with either a multicenter or single-center design, (ii) having an appropriate controlled design for Armolipid Plus®, (iii) investigating the effect of Armolipid Plus® on plasma lipids, (iv) testing the safety of Armolipid Plus®, and (v) reporting all the adverse events that occurred during the supplementation.
Exclusion criteria included the following: (i) lack of a control group for Armolipid Plus® administration, (ii) lack of blinding, (iii) lack of sufficient information about plasma lipids at baseline or follow-up, and (iv) lack of sufficient information about the prevalence and specification of adverse events. Studies were also excluded if they contained overlapping subjects with other studies.
2.3. Data Extraction
Data abstracted from the eligible studies were: (i) first author’s name; (ii) year of publication; (iii) study design; (iv) main inclusion criteria and underlying disease; (v) treatment duration; (vi) study groups; (vii) number of participants in the active and control group; (viii) background lipid-lowering treatment; (ix) age and sex of study participants; (x) weight, body mass index (BMI), waist circumference, systolic BP (SBP), diastolic BP (DBP), TC, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CPK), FPG, fasting plasma insulin (FPI), homeostatic model assessment for insulin resistance (HOMA-IR) and high sensitivity C reactive protein (hs-CRP) at baseline; and (xi) discontinuation of treatment and adverse events occurred during the trials. All data extraction and database typing were reviewed by the principal investigator (A.F.G.C.) before the final analysis, and doubts were resolved by mutual agreement among the authors.
2.4. Quality Assessment
A systematic assessment of risk of bias in the included studies was performed using the Cochrane criteria [16]. The following items were used: adequacy of sequence generation, allocation concealment, blind addressing of dropouts (incomplete outcome data), selective outcome reporting, and other probable sources of bias [17]. Risk-of-bias assessment was performed independently by 2 reviewers (F.F. and A.F.G.C.); disagreements were resolved by a consensus-based discussion.
2.5. Data Synthesis
Meta-analysis was entirely conducted using Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ) [18].
Net changes in the investigated parameters (change scores) were calculated by subtracting the value at baseline from the one after intervention, in the active-treated group and in the control one. All values were collated as mean change from baseline. Standard deviations (SDs) of the mean difference were obtained as follows, as reported by Follman et al.: SD = square root [(SDpre-treatment)2 + (SDpost-treatment)2 − (2R × SDpre-treatment × SDpost-treatment)], assuming a correlation coefficient (R) = 0.5 [19]. If the outcome measures were reported in median and range (or 95% confidence interval (CI)), mean and SD values were estimated using the method described by Wan et al. [20]. The findings of the included studies were combined using a fixed-effect model or a random-effect model (using the DerSimonian–Laird method) and the generic inverse variance method based on the level of inter-study heterogeneity, which was quantitatively assessed using the Higgins index (I2) [21]. For continuous parameters, effect sizes were expressed as absolute mean differences (MD) and 95%CI, standardized by the change score in SD. For treatment emergent adverse events, odd ratios (OR) and 95%CI intervals were calculated using the Mantel–Haenszel method [22]. A safety analysis was performed by excluding studies with zero events in both arms. If one or more outcomes could not be extracted from a study, the study was removed only from the analysis involving those outcomes. Adverse events were considered for the analysis only if they occurred in at least two of the included clinical trials.
In order to evaluate the influence of each study on the overall effect size, a sensitivity analysis was conducted using the leave-one-out method (i.e., removing one study at a time and repeating the analysis) [23]. Two-sided p-values ≤ 0.05 were considered statistically significant for all tests.
2.6. Publication Biases
Potential publication biases were explored using a visual inspection of Begg’s funnel plot asymmetry, Begg’s rank correlation test, and Egger’s weighted regression test [24]. The Duval and Tweedie “trim and fill” method was used to adjust the analysis for the effects of publication biases [25]. Two-sided p-values < 0.05 were considered statistically significant.
3. Results
3.1. Flow and Characteristics of the Included Studies
After database searches were performed according to inclusion and exclusion criteria, 445 published articles were identified, and the abstracts were reviewed. Of these, 112 were excluded because they were not original articles. Another 314 were eliminated because they did not meet the inclusion criteria. Thus, 19 articles were carefully assessed and reviewed. An additional 7 studies were excluded because of a lack of a controlled design for Armolipid Plus® administration (n = 3) or lack of blinding (n = 4). Finally, 12 studies were eligible and included in the meta-analysis [26,27,28,29,30,31,32,33,34,35,36,37]. The study selection process is shown in Figure 1.
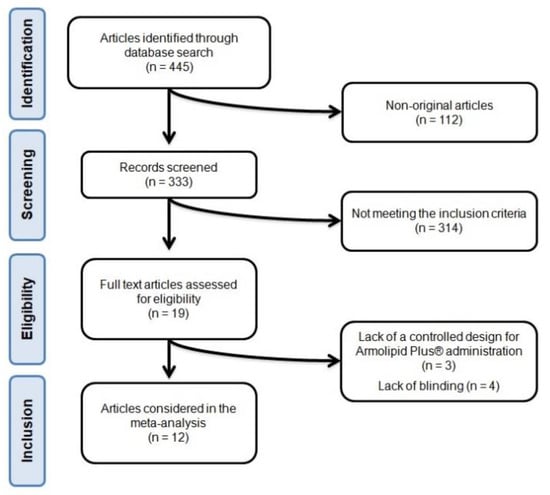
Figure 1.
Flow chart of the number of studies identified and included in the systematic review.
Data were pooled from 12 clinical trials comprising 24 treatment arms, which included 1050 subjects, with 544 in the actively treated arm and 536 in the control one.
Eligible studies were published between 2010 and 2020. Follow-up periods ranged between 4 weeks and 12 months. All selected trials were designed with parallel groups and were multicenter [29,35,37] or single-center [26,27,28,30,31,32,33,34,36] clinical studies. Enrolled subjects were patients in primary prevention for CVD [28,29,30,32,36], patients with documented coronary artery disease (CAD) [33], with a metabolic syndrome [26,30,34,35,36], or with a good status of health [28,37]. The baseline characteristics of the evaluated studies are summarized in Table 1 and Table 2.

Table 1.
Main characteristics of the included clinical studies and baseline hemodynamic parameters of enrolled patients.

Table 2.
Baseline lipids, fasting plasma glucose, and markers of insulin resistance.
3.2. Risk of Bias Assessment
Almost all of the included studies were characterized by sufficient information regarding sequence generation, allocation concealment, and personal and outcome assessments. All showed low risk of bias because of incomplete outcome data and selective outcome reporting. Details of the quality of bias assessment are reported in Table 3.

Table 3.
Quality of bias assessment of the included studies according to the Cochrane guidelines.
3.3. Effect of Armolipid Plus® on Anthropometric Measures, Blood Pressure, Serum Lipids, and Other Metabolic Parameters
Meta-analysis of the data suggested that Armolipid Plus® supplementation exerted a significant effect on BMI (MD = −0.25 kg/m2, 95%CI(−0.43,−0.06) Kg/m2, p = 0.008; I2 = 0%) (Figure 2) and serum levels of TC (MD = −25.07 mg/dL, 95%CI(−33.17,−16.97) mg/dL, p < 0.001; I2 = 87%), TG (MD = −11.47 mg/dL, 95%CI(−17.85,−5.08) mg/dL, p < 0.001; I2 = 34%), HDL-C (MD = 1.84 mg/dL, 95%CI(0.92,2.77) mg/dL, p < 0.001; I2 = 0%), LDL-C (MD = −26.67 mg/dL, 95%CI(−33.76,−19.58) mg/dL, p < 0.001; I2 = 82%) (Figure 3), hs-CRP (MD = −0.61 mg/L, 95%CI(−1.13,−0.09) mg/L, p = 0.022; I2 = 47%) (Figure 4), FPG (MD = −3.52 mg/dL, 95%CI(−5.1,−1.94) mg/dL, p < 0.001; I2 = 49%) (Figure 5), without affecting weight (MD = −0.89 kg, 95%CI(−4.60,2.82) kg, p = 0.638; I2 = 0%), waist circumference (MD = −0.5 cm, 95%CI(−3.17,2.17) cm, p = 0.714; I2 = 0%) (Figure S1), SBP (MD =− 0.57 mmHg, 95%CI(−3.2,2.06) mmHg, p = 0.670; I2 = 12%), DBP (MD = −0.89 mmHg, 95%CI(−2.61,0.83) mmHg, p = 0.312; I2 = 0%) (Figure S2), FPI (MD = −0.58 mU/L, 95%CI(−1.24,0.09), p = 0.091; I2 = 30%), and HOMA-IR (MD = −0.09, 95%CI(−0.44,0.26), p = 0.599; I2 = 61%) (Figure S3).
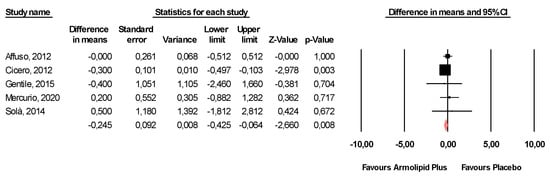
Figure 2.
Forest plot displaying mean differences and 95% confidence intervals for the impact of the supplementation with Armolipid Plus® on BMI.
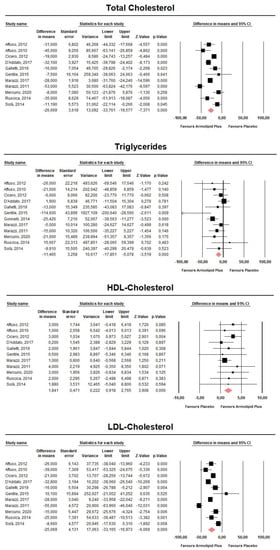
Figure 3.
Forest plot displaying mean differences and 95% confidence intervals for the impact of the supplementation with Armolipid Plus® on serum levels of TC, TG, HDL-C, and LDL-C.
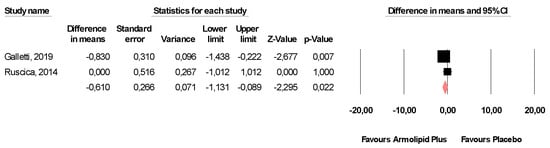
Figure 4.
Forest plot displaying mean differences and 95% confidence intervals for the impact of the supplementation with Armolipid Plus® on serum levels of hs-CRP.
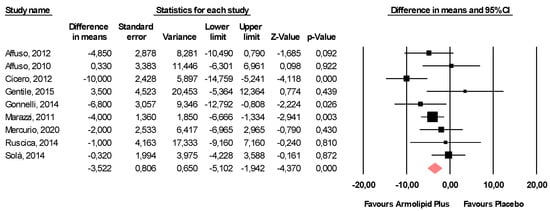
Figure 5.
Forest plot displaying mean differences and 95% confidence intervals for the impact of the supplementation with Armolipid Plus® on FPG.
The effect sizes were robust in the leave-one-out sensitivity analysis and not mainly driven by a single study (data not shown).
A visual inspection of Begg’s funnel plots did not show significant asymmetry, suggesting no potential publication bias for the effect of Armolipid Plus® on the efficacy outcomes (Figures S4–S7). This finding was confirmed by the results of Begg’s rank correlation test and Egger’s linear regression (Table 4).

Table 4.
Assessment of publication bias on efficacy outcomes.
The Duval and Tweedie trim-and-fill method identified three potentially missing studies on the left side of the funnel plot that resulted in the pooled effect size for DBP reaching statistical significance (Table 4).
3.4. Safety Analysis
Supplementation with Armolipid Plus® exerted a slight, though clinically insignificant, increase in serum levels of ALT (MD = 2.16 U/L, 95%CI(0.68,3.64) U/L, p = 0.004; I2 = 0%) (Figure 6), without affecting AST (MD = 0.63 U/L, 95%CI(−0.96,2.21) U/L, p = 0.437; I2 = 0%) or CPK (MD = 7.37 U/L, 95%CI(−1.20,15.93) U/L, p = 0.092; I2 = 39%) (Figure S8).
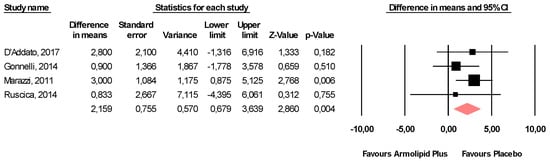
Figure 6.
Forest plot displaying mean differences and 95% confidence intervals for the impact of the supplementation with Armolipid Plus® on serum levels of ALT.
Moreover, supplementation with Armolipid Plus® was not associated with increased risk of either musculoskeletal disorders (OR = 0.78, 95%CI(0.29,2.11), p = 0.618; I2 = 0%) or gastrointestinal disorders (OR = 1.19, 95%CI(0.35,4.06), P = 0.786; I2 = 0%) (Figure S9).
The effect sizes were robust in the leave-one-out sensitivity analysis and not mainly driven by a single study (data not shown).
A visual inspection of Begg’s funnel plots did not show significant asymmetry, suggesting no potential publication bias for the effect of Armolipid Plus® on the safety outcomes (Figures S9–S13). This finding was confirmed by the results of Begg’s rank correlation test and Egger’s linear regression (Table 5).

Table 5.
Assessment of publication bias on safety outcomes.
The Duval and Tweedie trim-and-fill method yielded one potentially missing study on the right side of the funnel plot, increasing the pooled effect size for ALT, and one potentially missing study on the right side of the funnel plot, increasing the pooled effect size for CPK. In addition, Duval and Tweedie’s trim-and-fill method yielded one potentially missing study on the left side of the funnel plot, decreasing the pooled effect size for AST, and one potentially missing study on the left side of the funnel plot, decreasing the estimated risk of gastrointestinal disorders (Table 5).
4. Discussion
According to our findings, dietary supplementation with Armolipid Plus® exerts a significant effect on BMI and serum levels of TC, TG, HDL-C, LDL-C, hs-CRP, and FPG. Importantly, it is not associated with an increased risk of musculoskeletal symptoms and gastrointestinal disorders, though it results in a slight, though clinically insignificant, increase in ALT serum levels.
To our knowledge, this is the first systematic review and meta-analysis to comprehensively and critically evaluate the existing body of evidence for the use of Armolipid Plus® in daily clinical practice. As a matter of fact, previous meta-analyses on this topic are outdated and not PRISMA compliant [38,39]. Moreover, they included clinical trials that were not adequately controlled for Armolipid Plus® supplementation and clinical studies with an observational design that finally led to not fully reliable results [40,41,42,43,44].
Armolipid Plus® is a dietary supplement widely used in clinical practice and the only combined lipid-lowering nutraceutical recommended by the International Lipid Expert Panel (ILEP) for the management of hypercholesterolemia in statin-intolerant patients [45]. In effect, red yeast rice at the dosage contained in Armolipid Plus® has been shown to be safe also following a recent large meta-analysis of 53 randomized controlled clinical trials enrolling 8535 participants overall [46]. Dietary supplementation with Armolipid Plus® in statin-intolerant patients previously treated with ezetimibe resulted in reductions of approximately 35% in LDL-C and 25% in TG [47], which was similar to results reported for moderate-intensity statins, according to the latest guidelines from the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) [48].
The lipid-lowering effect of red yeast rice (alone or combined with other lipid-lowering nutraceuticals) is well known, as it has been verified by several meta-analyses of randomized controlled clinical trials [49,50]. The interaction between red yeast rice and other natural products with different mechanisms of action, such as the other components of Armolipid Plus®, may have additive or synergistic lipid-lowering effects [51]. As a matter of fact, the inhibition of HMG-CoA reductase by monacolins contained in red yeast rice might be advantageously coupled with other nutraceuticals to enhance the hepatic uptake of cholesterol (berberine, soybean proteins), increase lipid excretion in the bowel (soluble fibers, plant sterols, glucomannan, probiotics), or induce LDL-C excretion (berberine, soy proteins, chlorogenic acid) [52,53]. Furthermore, several studies evaluated the efficacy and safety of red yeast rice in combination with policosanols, a mixture of aliphatic alcohols derived from purified sugar cane, even though the mechanism underlying their lipid-lowering effect is still being discussed [54,55]. Policosanols, together with berberine, may also be responsible for the reduction in FPG levels observed after dietary supplementation with Armolipid Plus® [56].
Although there are no trials showing that Armolipid Plus® reduces the risk of ASCVD events, some studies have shown benefits in terms of improved vascular function, as demonstrated by flow-mediated dilation [27] and carotid-femoral pulse wave velocity [57]. Absolute and relative risk reduction (RRR) in CV events with Armolipid Plus® is challenging to estimate based on the available short-term data. There is a linear association between LDL-C reduction and a decrease in ASCVD events, as reported originally by the CTT’s(Cholesterol Treatment Trialists’) meta-analyses of the statin trials where a 1 mmol/L (~39 mg/dL) LDL-C reduction was associated with a 21–23% RRR in CV events over five years [58]. Robust and growing evidence highlights that this linear association is observed regardless of the LDL lowering approach adopted, i.e., low-fat diet, anion exchange resins, ezetimibe, etc. [59]. On the basis of our findings, it is therefore plausible to expect a 14–15% ASCVD event reduction after long-term dietary supplementation with Armolipid Plus®.
Despite its strengths, this systematic review and meta-analysis has some limitations. One limitation is the heterogeneity for the effect size on TC and LDL-C, which was moderately high, proving that additional evidence is needed to establish the extent of cholesterol reduction that can be achieved following supplementation with Armolipid Plus®. In addition, we had to exclude a relatively large number of clinical trials not compliant with the inclusion criteria for this meta-analysis. The sample size on some laboratory and clinical outcomes was consequently reduced. In particular, some non-significant results (e.g., changes in weight and waist circumference) might be related to a low statistical power.
Nonetheless, the observed results are in line also with a large trial carried out in a setting of the general population involving 1751 volunteers but not included in the meta-analysis, as it did not meet our pre-specified inclusion criteria [42].
Other lipid-lowering nutraceutical combinations could exert a relevant lipid-lowering effect, but the data on Armolipid Plus® are currently most robust.
5. Conclusions
Pooling data from the available randomized controlled clinical studies, the current systematic review and meta-analysis provides data in support of the use of Armolipid Plus® in clinical practice as add-on treatment to lifestyle modifications for hypercholesterolemia in order to promote improved cardiometabolic health. Further studies to identify a benefit in terms of CV outcomes are required.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/13/2/638/s1, Figure S1: Forest plot displaying mean differences and 95% confidence intervals for the impact of supplementation with Armolipid Plus® on weight and WC, Figure S2: Forest plot displaying mean differences and 95% confidence intervals for the impact of supplementation with Armolipid Plus® on SBP and DBP, Figure S3: Forest plot displaying mean differences and 95% confidence intervals for the impact of supplementation with Armolipid Plus® on FPI and HOMA-IR, Figure S4: Funnel plots detailing publication bias for the effect of supplementation with Armolipid Plus® on weight, BMI, and waist circumference, Figure S5: Funnel plots detailing publication bias for the effect of supplementation with Armolipid Plus® on blood pressure, Figure S6: Funnel plots detailing publication bias for the effect of supplementation with Armolipid Plus® on serum lipid concentrations, Figure S7: Funnel plots detailing publication bias for the effect of supplementation with Armolipid Plus® on glycemia and markers of insulin resistance, Figure S8: Forest plot displaying mean differences and 95% confidence intervals for the impact of supplementation with Armolipid Plus® on AST and CPK, Figure S9: Forest plots displaying the risk of treatment-emergent adverse events during supplementation with Armolipid Plus®, Figure S10: Funnel plot detailing publication bias for the effect of supplementation with Armolipid Plus® on serum concentrations of ALT, Figure S11: Funnel plot detailing publication bias for the effect of supplementation with Armolipid Plus® on serum concentrations of AST, Figure S12: Funnel plot detailing publication bias for the effect of supplementation with Armolipid Plus® on serum concentrations of CPK, Figure S13: Funnel plot detailing publication bias for risk of treatment-emergent adverse events during supplementation with Armolipid Plus®.
Author Contributions
Conceptualization, F.F. and A.F.G.C.; methodology, F.F. and A.F.G.C.; software, F.F.; validation, F.F. and A.F.G.C.; formal analysis, F.F.; investigation, F.F., M.B., P.P.T., and A.F.G.C.; data curation, F.F., M.B., and A.F.G.C.; writing—original draft preparation, F.F. and A.F.G.C.; writing—review and editing, C.K., T.K., M.B., C.M.G.G., A.Š., and P.P.T.; supervision, A.F.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the study design (meta-analysis).
Informed Consent Statement
Patient consent was waived due to the study design (meta-analysis).
Data Availability Statement
Data supporting findings of this analysis are available from the Corresponding Authors upon reasonable request.
Conflicts of Interest
A.F.G.C. served as consultant to Meda-Mylan and Sharper; F.F. served as consultant to Meda-Mylan and Neopharmed Gentili s.p.a. The other authors declare no conflict of interest.
References
- World Health Organization (WHO). Cardiovascular Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 26 December 2020).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Colantonio, L.D.; Bittner, V.; Reynolds, K.; Levitan, E.B.; Rosenson, R.S.; Banach, M.; Kent, S.T.; Derose, S.F.; Zhou, H.; Safford, M.M.; et al. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation 2016, 133, 256–264. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics—2016 Update: A Report from the American Heart Association. American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC); Taddei, C.; Jackson, R.; Zhou, B.; Bixby, H.; Danaei, G.; Di Cesare, M.; Kuulasmaa, K.; Hajifathalian, K.; Bentham, J.; et al. National trends in total cholesterol obscure heterogeneous changes in HDL and non-HDL cholesterol and total-to-HDL cholesterol ratio: A pooled analysis of 458 population-based studies in Asian and Western countries. Int. J. Epidemiol. 2019, 49, 173–192. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Polonsky, T.S.; Miedema, M.D.; Khera, A.; Kosinski, A.S.; Kuvin, J.T. Systematic Review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1144–e1161. [Google Scholar] [CrossRef]
- Zeitouni, M.; Sabouret, P.; Kerneis, M.; Silvain, J.; Collet, J.-P.; Bruckert, E.; Montalescot, G. 2019 ESC/EAS Guidelines for management of dyslipidaemia: Strengths and limitations. Eur. Hear. J. Cardiovasc. Pharmacother. 2020, 77. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Atherosclerosis 2016, 252, 207–274. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Toth, P.P.; Giglio, R.V.; Banach, M.; Noto, M.; Nikolic, D.; Montalto, G.; Rizzo, M. Nutraceuticals as an Important Part of Combination Therapy in Dyslipidaemia. Curr. Pharm. Des. 2017, 23, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Escobar, C.; Cicero, A.F.G.; Burke, D.; Fasching, P.; Banach, M.; Bruckert, E. A nutraceutical approach (Armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: Review of the clinical evidence. Atheroscler. Suppl. 2017, 24, 1–15. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. For the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Report Version 5.0.2.2009; John Wiley and Sons Ltd.: Chichester, UK, 2010. [Google Scholar]
- Fogacci, F.; Ferri, N.; Toth, P.P.; Ruscica, M.; Corsini, A.; Cicero, A.F.G. Efficacy and Safety of Mipomersen: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Drugs 2019, 79, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Version 3; Biostat: Englewood, NJ, USA, 2005; Volume 104. [Google Scholar]
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1992, 45, 769–773. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Melsen, W.G.; Bootsma, M.C.J.; Rovers, M.M.; Bonten, M.J.M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014, 20, 123–129. [Google Scholar] [CrossRef]
- Haenszel, W.; Hon, N.B. Statistical approaches to the study of cancer with particular reference to case registers. J. Chronic Dis. 1956, 4, 589–599. [Google Scholar] [CrossRef]
- Fogacci, F.; Banach, M.; Cicero, A.F.G. Resveratrol effect on patients with non-alcoholic fatty liver disease: A matter of dose and treatment length. Diabetes Obes. Metab. 2018, 20, 1798–1799. [Google Scholar] [CrossRef]
- Fogacci, F.; Rizzo, M.; Krogager, C.; Kennedy, C.; Georges, C.M.; Knežević, T.; Liberopoulos, E.; Vallée, A.; Pérez-Martínez, P.; Wenstedt, E.F.; et al. Safety Evaluation of α-Lipoic Acid Supplementation: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Clinical Studies. Antioxidants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Affuso, F. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J. Cardiol. 2012, 4, 77–83. [Google Scholar] [CrossRef]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Saccà, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; De Sando, V.; Benedetto, D.; Cevenini, M.; Grandi, E.; Borghi, C. Long-term efficacy and tolerability of a multicomponent lipid-lowering nutraceutical in overweight and normoweight patients. Nutrafoods 2012, 11, 55–61. [Google Scholar] [CrossRef]
- D’Addato, S.; Scandiani, L.; Mombelli, G.; Focanti, F.; Pelacchi, F.; Salvatori, E.; Di Loreto, G.; Comandini, A.; Maffioli, P.; DeRosa, G. Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q10 on lipid levels: A randomized, double-blind, placebo controlled study. Drug Des. Dev. Ther. 2017, 11, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Galletti, F.; Fazio, V.; Gentile, M.; Schillaci, G.; Pucci, G.; Battista, F.; Mercurio, V.; Bosso, G.; Bonaduce, D.; Brambilla, N.; et al. Efficacy of a nutraceutical combination on lipid metabolism in patients with metabolic syndrome: A multicenter, double blind, randomized, placebo controlled trial. Lipids Heal. Dis. 2019, 18, 66. [Google Scholar] [CrossRef]
- Gentile, M.; Calcaterra, I.; Strazzullo, A.; Pagano, C.; Pacioni, D.; Speranza, E.; Rubba, P.; Marotta, G. Effects of Armolipid Plus on small dense LDL particles in a sample of patients affected by familial combined hyperlipidemia. Clin. Lipidol. 2015, 10, 475–480. [Google Scholar] [CrossRef][Green Version]
- Gonnelli, S.; Caffarelli, C.; Stolakis, K.; Cuda, C.; Giordano, N.; Nuti, R. Efficacy and Tolerability of a Nutraceutical Combination (Red Yeast Rice, Policosanols, and Berberine) in Patients with Low-Moderate Risk Hypercholesterolemia: A Double-Blind, Placebo-Controlled Study. Curr. Ther. Res. 2015, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, G.; Campolongo, G.; Pelliccia, F.; Quattrino, S.; Vitale, C.; Cacciotti, L.; Massaro, R.; Volterrani, M.; Rosano, G. Comparison of Low-Dose Statin Versus Low-Dose Statin + Armolipid Plus in High-Intensity Statin-Intolerant Patients with a Previous Coronary Event and Percutaneous Coronary Intervention (ADHERENCE Trial). Am. J. Cardiol. 2017, 120, 893–897. [Google Scholar] [CrossRef]
- Marazzi, G.; Cacciotti, L.; Pelliccia, F.; Iaia, L.; Volterrani, M.; Caminiti, G.; Sposato, B.; Massaro, R.; Grieco, F.; Rosano, G. Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients. Adv. Ther. 2011, 28, 1105–1113. [Google Scholar] [CrossRef]
- Mercurio, V.; Pucci, G.; Bosso, G.; Fazio, V.; Battista, F.; Iannuzzi, A.; Brambilla, N.; Vitalini, C.; D’Amato, M.; Giacovelli, G.; et al. A nutraceutical combination reduces left ventricular mass in subjects with metabolic syndrome and left ventricular hypertrophy: A multicenter, randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2020, 39, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef]
- Sola, R.; Valls, R.-M.; Puzo, J.; Calabuig, J.-R.; Brea, A.; Pedret, A.; Moriña, D.; Villar, J.; Millán, J.; Anguera, A. Effects of Poly-Bioactive Compounds on Lipid Profile and Body Weight in a Moderately Hypercholesterolemic Population with Low Cardiovascular Disease Risk: A Multicenter Randomized Trial. PLoS ONE 2014, 9, e101978. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef]
- Millán, J.; Cicero, A.F.; Torres, F.; Anguera, A. Effects of a nutraceutical combination containing berberine (BRB), policosanol, and red yeast rice (RYR), on lipid profile in hypercholesterolemic patients: A meta-analysis of randomised controlled trials. Clín. Investig. Arterioscler. 2016, 28, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Lupattelli, G.; Del Giorno, R.; Schillaci, G.; Berisha, S.; Mannarino, M.R.; Bagaglia, F.; Melis, F.; Mannarino, E. Nutraceutical combination (red yeast rice, berberine and policosanols) improves aortic stiffness in low-moderate risk hypercholesterolemic patients. PharmaNutrition 2013, 1, 73–77. [Google Scholar] [CrossRef]
- Izzo, R.; De Simone, G.; Giudice, R.; Chinali, M.; Trimarco, V.; De Luca, N.; Trimarco, B. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J. Hypertens. 2010, 28, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Trimarco, B.; Benvenuti, C.; Rozza, F.; Cimmino, C.S.; Giudice, R.; Crispo, S. Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet. Med. J. Nutrition. Metab. 2011, 4, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Rovati, L.C.; Setnikar, I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents: A single-blind clinical investigation. Arzneimittelforschung 2007, 57, 26–30. [Google Scholar] [CrossRef]
- Pisciotta, L.; Bellocchio, A.; Bertolini, S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Heal. Dis. 2012, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Ž.; Mancini, J.; Rizzo, M.; Mitchenko, O.; et al. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef]
- Marazzi, G.; Campolongo, G.; Pelliccia, F.; Calabrò, P.; Cacciotti, L.; Vitale, C.; Massaro, R.; Volterrani, M.; Rosano, G. Usefulness of Low-Dose Statin Plus Ezetimibe and/or Nutraceuticals in Patients with Coronary Artery Disease Intolerant to High-Dose Statin Treatment. Am. J. Cardiol. 2019, 123, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.; Gerdes, V. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.P.; Liu, L.; Cheng, Y.C.; Shishehbor, M.H.; Liu, M.H.; Peng, D.Q.; Li, Y.L. Xuezhikang, an Extract of Cholestin, Protects Endothelial Function Through Antiinflammatory and Lipid-Lowering Mechanisms in Patients with Coronary Heart Disease. Circulation 2004, 110, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercholesterolemia. Methodist Debakey Cardiovasc. J. 2019, 15, 192–199. [Google Scholar] [PubMed]
- Cicero, A.F.; DeRosa, G.; Borghi, C. Red Yeast Rice and Statin-Intolerant Patients. Am. J. Cardiol. 2010, 105, 1504. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Zambon, A. Red Yeast Rice for Hypercholesterolemia. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef]
- Varady, K.A.; Wang, Y.; Jones, P.J. Role of policosanols in the prevention and treatment of cardiovascular disease. Nutr. Rev. 2003, 61, 376–383. [Google Scholar] [CrossRef]
- Guardamagna, O.; Abelló, F.; Baracco, V.; Stasiowska, B.; Martino, F. The treatment of hypercholesterolemic children: Efficacy and safety of a combination of red yeast rice extract and policosanols. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 424–429. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef]
- Pirro, M.; Francisci, D.; Bianconi, V.; Schiaroli, E.; Mannarino, M.R.; Barsotti, F.; Spinozzi, A.; Bagaglia, F.; Sahebkar, A.; Baldelli, F. NUtraceutical TReatment for hYpercholesterolemia in HIV-infected patients: The NU-TRY(HIV) randomized cross-over trial. Atherosclerosis 2019, 280, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.C.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).