Abstract
Most studies confirm the beneficial effects of enteral nutrition on the quality of life, but some studies indicate an inverse association and its detrimental impacts. However, there are insufficient data on the effects of enteral nutrition on the quality of life of cancer patients. This systematic review aimed to describe the influence of applied enteral nutrition on the quality of life of cancer patients, based on the results of randomized controlled trials. It was registered in the PROSPERO database (CRD42021261226) and conducted based on the PRISMA guidelines. The searching procedure was conducted using the PubMed and Web of Science databases, as well as Cochrane Library, and it included studies published until June 2021. It was conducted to select randomized controlled trials assessing the influence of enteral nutrition (compared with the other model of nutrition) on the quality of life of cancer patients. A general number of 761 records were screened and a final number of 16 studies were included in the systematic review. The studies were included and assessed by two independent researchers, while the risk of bias was analyzed using the Newcastle–Ottawa Scale (NOS). Studies compared patients treated with and without enteral nutrition, patients treated with various methods of enteral nutrition or with enteral diets of various content, as well as patients treated with enteral and parenteral nutrition. Within the included studies, the majority were conducted in patients with cancers located in various parts of the body, or diverse areas within the gastrointestinal system, while some studies were conducted in specific populations of patients with a defined cancer location—esophagus, stomach, or ovary. The duration of applied enteral nutrition within the included studies was diversified—from two weeks or less to half a year or even more. The vast majority of studies used well-known and validated tools to assess the quality of life, either developed for a specific group of head/neck, esophagus/stomach, and ovary cancer patients or developed for more general patient populations. Most studies concerning patients treated with and without enteral nutrition supported applying enteral nutrition, which was concluded in seven studies out of ten (including four studies with a low risk of bias). The other important observations to be emphasized—formulated based on the studies with a low risk of bias—presented the role of oral supportive nutrition guided by a dietitian, as well as the beneficial role of enteral and parenteral nutrition, combined. In spite of a relatively low number of randomized controlled trials assessing the influence of enteral nutrition on the quality of life of cancer patients, which should be considered as a limitation, the results were promising. Most studies supported the positive influence of enteral nutrition on the quality of life, either assessed based on the psychological measures of the quality of life or by considering the other potential determinants (e.g., malnutrition, complications, etc.). Taking this into account, enteral nutrition should be applied whenever possible, both to prevent and treat malnutrition in cancer patients. However, considering the limited number of studies conducted so far, further research conducted in homogenic populations of patients is necessary.
Keywords:
cancer; diet; nutrition; enteral nutrition; oncology; quality of life; QoL; randomized controlled trials 1. Introduction
Cancer is a growing global problem, being the first or second leading cause of death of individuals aged under 70 years in 112 of 183 countries, according to the World Health Organization (WHO) [1]. The Global Cancer Observatory (GCO) by the International Agency for Research on Cancer (IARC) and the WHO, within their GLOBOCAN 2020 estimates of incidence and mortality, indicated nearly 19.3 million new cancer cases and almost 10 million cancer deaths registered worldwide in 2020 [2]. Taking this into account, the WHO emphasizes that cancer is one of the main challenges for public health within both areas of prevention and treatment [3].
The cancer treatment methods are classified by the National Cancer Institute (NCI) as biomarker testing, chemotherapy, hormone therapy, immunotherapy, radiation therapy, stem cell transplant, surgery, and targeted therapy [4]. As indicated in the systematic review by Shrestha et al. [5], while choosing the therapeutic option, the length of life and quality of life are taken into consideration—patients with better health value rather than length of life over quality of life, and those with poorer physical status value rather than the quality of life over the length of life. The quality of life is defined as a sense of well-being and includes physical, psychological, social, and spiritual aspects, which may be changed in cancer patients [6]. The quality of life of cancer patients is significantly reduced [7,8,9], which results from the disease process itself— its course, symptoms and complications, the applied treatment, and the disease duration [10].
Among cancer symptoms and complications, malnutrition is one of the most common, as it results from anorexia and metabolic dysregulation combined, both caused by the tumor itself or by its treatment and contributing to cachexia [11]. It may affect up to 80% of cancer patients, while its prevalence depends on the cancer type, disease setting, comorbidities, and type of treatment performed [12]. Although the problem of malnutrition and cancer-related cachexia have been known for a long time, effective prevention and treatment remain a challenge [13]. Prevention and treatment are especially important as malnutrition not only affects the effectiveness of cancer treatment, as well as the prognosis and hospital stay length [14], but also influences the quality of life [15,16,17].
Taking this into consideration, the European Society for Clinical Nutrition and Metabolism (ESPEN), within its guidelines, indicated that the most important action against cancer-related malnutrition is to provide early screening and to assure individualized nutritional interventions [18]. An effective, personalized nutrition plan should include not only an appropriate diet or oral nutrition support but also enteral or parenteral nutrition if needed [19]. However, the recommendations by ESPEN indicate the superiority of feeding by the gastrointestinal tract over parenteral nutrition, and enteral nutrition is recommended if possible [20]. Similarly, the systematic review by Chow et al. [21] indicated that, for cancer patients, parenteral nutrition may result in an increased risk of complications compared with enteral nutrition but would not prolong survival.
However, there are insufficient data on the effects of enteral nutrition on the quality of life of cancer patients. A recent systematic review by Ojo et al. [22] assessed the effect of enteral tube feeding on the quality of life of various patients, including not only cancer patients but also those with other diseases and conditions. Based on this review, it was stated that most studies confirm the beneficial effect of enteral nutrition, but some studies indicate inverse association and its detrimental effects on the quality of life [22]. Taking this into account, the present systematic review aimed to describe the influence of applied enteral nutrition on the quality of life of cancer patients, based on the results of randomized controlled trials.
2. Materials and Methods
2.1. The Systematic Review Design and Registration
The systematic review of the influence of applied enteral nutrition on the quality of life in cancer patients was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations for the literature search and screening, including studies and reporting results [23]. The literature search was based on the PubMed and Web of Science databases, as well as Cochrane Library, and included studies published until June 2021.
The systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42021261226).
2.2. The Inclusion and Exclusion Criteria
The studies that were assessed within the present systematic review were intended to be randomized controlled trials assessing the influence of enteral nutrition (compared with the other model of nutrition) on the quality of life of cancer patients.
The inclusion criteria comprised research articles, presenting randomized controlled trials with full texts published in peer-reviewed journals in English, as well as the studied populations of cancer individuals with any enteral nutrition applied and quality of life assessed in any way.
The exclusion criteria comprised studies conducted in an animal model, studies not comparing enteral nutrition with any other model of nutrition but assessing technical aspects of enteral nutrition (such as the study by Patel et al. [24]), and studied populations of participants with any eating disorder (influencing the effectiveness of enteral nutrition applied) or intellectual disability (influencing the declared quality of life assessed within the studies).
While including the studies, no additional criteria associated with the type of cancer, characteristics of the studied population, or country were taken into account.
The summarized inclusion and exclusion criteria for the patient, intervention/exposure, comparator, outcome, and study design (PICOS) are presented in Table 1.

Table 1.
The summarized inclusion and exclusion criteria for a patient, intervention/exposure, comparator, outcome, and study design (PICOS).
2.3. The Procedure of Systematic Review
The electronic search was conducted within the PubMed and Web of Science databases, as well as Cochrane Central Register of Controlled Trials, and the detailed electronic search strategy is presented in Table 2.

Table 2.
The detailed electronic search strategy applied within the systematic review for the PubMed and Web of Science databases, as well as Cochrane Central Register of Controlled Trials.
The procedure of identification, screening, and inclusion applied within the systematic review is presented in Figure 1. The identification of the eligible studies was performed independently by two researchers and conducted within three stages—based on the title, abstract, and full text of the study. The titles and abstracts were sourced from electronic databases, and full texts were also sourced from corresponding authors of studies if in electronic databases they were unavailable. If any disagreement appeared at any stage, it was consulted with the other researcher.
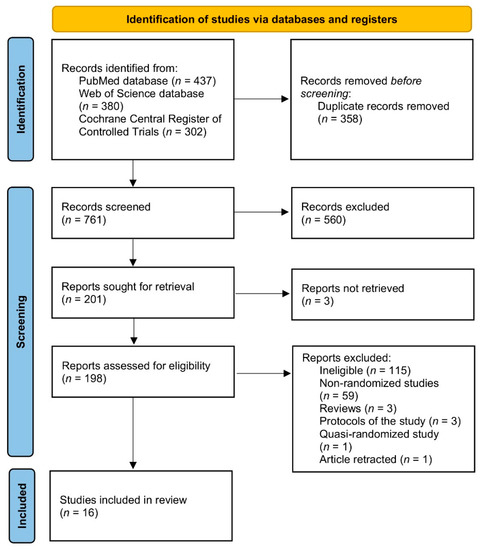
Figure 1.
The procedure of identification, screening, and inclusion applied within the systematic review.
2.4. The Procedure of Data Extraction
Data extraction was performed independently by two researchers and if any disagreement appeared, it was consulted with the other researcher. If any information was unavailable within the study, it was obtained from the corresponding author of the study and, in such cases, it is referred to as data provided on request.
The extracted data comprised basic characteristics of the study (study design as defined within the article, the country with detailed location, and the time when the study was conducted), basic characteristics of the studied influence (nature of the studied group as defined within the study, disease location, and psychological measure for the assessed quality of life), basic characteristics of the studied group (the number of studied participants and female participants, age of the studied group, inclusion criteria, and exclusion criteria), basic characteristics of the applied nutritional intervention associated with enteral nutrition (applied enteral nutrition, duration of applied nutritional intervention, and any other information about nutrition as defined within the article), and the results and conclusions (the effect of the applied nutritional intervention on the quality of life).
The assessment of the risk of bias was conducted to define the methodological quality of the included studies [25] and the Newcastle–Ottawa Scale (NOS) [26] was used. The selection, comparability, and exposure/outcome were scored as follows: 0–4, 0–2, and 0–3 and, afterward, the total score was attributed to the categories of very high risk of bias (total score of 0–3), high risk of bias (total score of 4–6), and low risk of bias (total score of 7–9) [27].
3. Results
The basic characteristics of the studies included in the systematic review [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] are presented in Table 3. Within the included studies, the majority were conducted in European countries—in Sweden [29,32,33,34], United Kingdom [35,39], Netherlands [28], Italy [38] and Poland [42]—but there were also some studies conducted in China [37,40,43] and Australia [36,41], which may have influenced various approaches applied to enteral nutrition. Most of the studies were conducted in the 2010s [31,35,36,37,38,39,40,41,43], but there were also some studies conducted in the 2000s [30,31,32,33,34,36,38] and even the 1990s [28].

Table 3.
The basic characteristics of the studies included in the systematic review.
The basic characteristics of the influence studied within the studies included in the systematic review are presented in Table 4. The majority of the included studies were conducted in patients with cancers in various locations [28,30,31,32,33,34,41,42] such as the esophagus [40,43], stomach [37], or ovary [36], or various locations within the gastrointestinal system [29,35,38,39]. Most of the studies presented populations recruited from a specific hospital/clinic/department [28,29,31,33,35,37,39,40,41,42,43] or a cancer registry [32,34,38], but for some studies, the specific clinic of origin was not defined [30,36]. The vast majority of studies used well-known and validated tools to assess the quality of life, either developed for a specific group of head/neck [31,32,33,34,41], esophagus/stomach [35,39], and ovarian cancer patients [36], or developed for more general populations of patients [28,29,32,34,36,37,38,39,40,41,42,43]. Only one study used a tool that was not previously validated and was developed based on other validated tools [30].

Table 4.
The basic characteristics of the influence studied within the studies included in the systematic review.
The basic characteristics of the groups studied within the studies included in the systematic review are presented in Table 5. The included studies presented the observations formulated in a samples of various sizes—small samples of less than 50 participants [28,30,35,39,42], medium-size samples of less than 100 participants [29,37,38,40,43], or large samples of 100 or more participants [31,32,33,34,36,41]. The age of the studied patients in most studies was about 60 years [28,29,30,32,33,34,35,36,37,39,41,42,43], but there were also some studies analyzing younger patients of less than 60 [40] or older patients of more than 60 years [38]. Within the inclusion criteria, the diagnosis of cancer was considered [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], but in some studies, it was accompanied by malnutrition [28,30,36,38].

Table 5.
The basic characteristics of the groups studied within the studies included in the systematic review.
The basic characteristics of the nutritional interventions associated with enteral nutrition applied within the studies included in the systematic review are presented in Table 6. The included studies compared patients treated with and without enteral nutrition [28,32,33,34,35,36,38,39,41,43], patients treated with various methods of enteral nutrition [30,31] or with enteral diets of various content [42], and patients treated with enteral and parenteral nutrition [29,37,40]. The duration of applied enteral nutrition within the included studies was diversified—from two weeks or less [28,40], through a few weeks or months [30,31,35,36,38,39,42,43], to half a year or even more [29,32,33,34].

Table 6.
The basic characteristics of the nutritional interventions associated with enteral nutrition applied within the studies included in the systematic review.
The results and conclusions associated with the effect of the applied nutritional intervention on the quality of life within the studies included in the systematic review are presented in Table 7. The results presented are based on the description formulated by authors of the studies within their abstracts.

Table 7.
The results and conclusions associated with the effect of the applied nutritional intervention on the quality of life within the studies included in the systematic review.
The summary of conclusions from the studies comparing patients treated with and without enteral nutrition included in the systematic review accompanied by the Newcastle-Ottawa Scale (NOS) score is presented in Table 8. It was stated that the majority of studies supported applying enteral nutrition, which was concluded in seven studies out of ten (including four studies of a low risk of bias). Although two of the supporting studies [32,34] were conducted within the same cohort, such observation is prominent.

Table 8.
The summary of conclusions from the studies comparing patients treated with and without enteral nutrition included to the systematic review accompanied by the Newcastle–Ottawa Scale (NOS) score.
The summary of conclusions from the studies comparing patients treated with enteral and parenteral nutrition, with various methods of enteral nutrition, and with enteral nutrition of various contents, included in the systematic review accompanied by the Newcastle–Ottawa Scale (NOS) score is presented in Table 9. The important conclusions that should be emphasized, formulated based on the studies of a low risk of bias, present the role of oral supportive nutrition guided by a dietitian [29], as well as the beneficial role of enteral and parenteral nutrition combined [40].

Table 9.
The summary of conclusions from the studies comparing patients treated with enteral and parenteral nutrition, with various methods of enteral nutrition, and with enteral nutrition of various contents, included in the systematic review accompanied by the Newcastle–Ottawa Scale (NOS) score.
4. Discussion
Due to an increase in the effectiveness of anti-cancer treatment [44] and an increase in life expectancy in cancer patients [45], the long-term complications will probably be observed more often, resulting in increasing role of the quality of life [46]. Taking this into account, it must be emphasized that the systematic review by Lis et al. [47], assessing the role of nutritional status in predicting quality of life in cancer individuals, indicated that correcting malnutrition may improve quality of life in cancer patients. Considering this, the presented systematic review is based on the assumption that enteral nutrition may promote a better nutritional status.
In agreement with the indicated association between nutritional status and quality of life, the ESPEN, within its recent practical guidelines [48] recommended applying nutritional support, including dietary advice, oral nutrition supplements, and enteral and parenteral nutrition as an effective way of improving nutritional status and malnutrition prevention. However, while choosing the method of nutritional support, it is indicated that, despite nutritional interventions, enteral nutrition should be recommended if oral nutrition remains inadequate, and parenteral nutrition should be recommended if enteral nutrition is not sufficient or feasible [48].
The results of the conducted systematic review of the randomized controlled trials confirmed the beneficial effects of enteral nutrition for cancer patients in the area of quality of life. While comparing patients treated with and without enteral nutrition, it was stated that enteral nutrition has a beneficial effect on the quality of life in a majority of studies, confirmed in groups of head and neck cancer patients [28,32,34], upper gastrointestinal tract cancer patients [38,39,43], and ovarian cancer patients [36]. At the same time, the results were not so consistent while comparing patients treated with enteral and parenteral nutrition; depending on the study, the various results were observed [29,37,40], but generally combined enteral and parenteral nutrition was stated to be superior to both enteral [40] and parenteral nutrition alone [37]. The indicated observations are in agreement with the recommendations by ESPEN [48], indicating the need to meet the energy requirements of patients, which must be considered the overall objective.
In spite of the fact that the majority of included studies concluded the beneficial role of enteral nutrition (especially while compared with no nutritional support), some disadvantages or contradictory results are also indicated. Such observations were formulated mainly within studies assessing the effect of prophylactic enteral nutrition, applied, not when necessary, but earlier, in order to limit the risk of malnutrition [33,35,41]. This may result from the fact that the enteral nutrition procedure itself can generate complications [49]. As such complications may indirectly affect the quality of life, each of them needs to be considered while choosing the best option for nutritional support.
While describing the results gathered within randomized controlled trials, it should be emphasized that only a small number of such studies have been conducted so far, while various methods of enteral nutrition and various nutritional values of diet have been studied. The various studies comparing patients treated with and without enteral nutrition and comparing patients treated with enteral and parenteral nutrition are incomparable due to various enteral nutrition procedures applied within the studies. At the same time, only two randomized controlled trials comparing patients treated with various methods of enteral nutrition [30,31], and one comparing patients treated with enteral nutrition of various contents [42], have been conducted so far, so no deeper conclusions can be made, especially if the results of the studies are contradictory [30,31].
The other problem while comparing the results of the included studies is associated with various cancer locations in the included studies, but also with the well-known diverse cancer course and intra-patient variability observed in the treatment effectiveness [50]. As a result, gathering and combining results observed within various studies may be impossible.
Despite the described difficulties in synthesizing results of the included studies, the most prominent observation formulated within the majority of studies remains consistent and is associated with the positive influence of enteral nutrition on the quality of life. While the quality of life is linked to the stage of cancer [51], the prognosis [52], malnutrition [53], and applied therapy [54], enteral nutrition must also be taken into account as a factor indirectly affecting it by improving the effectiveness of cancer therapy [55] and reducing the risk of malnutrition [56].
Considering the described results of gathered randomized controlled trials, and in agreement with the ESPEN guidelines, enteral nutrition should be applied whenever possible to improve the quality of life of cancer patients.
5. Conclusions
Most of the studies support the positive influence of enteral nutrition on the quality of life, either assessed based on the psychological measures of the quality of life or by considering the other potential determinants (e.g., malnutrition, complications, etc.). Taking this into account, enteral nutrition should be applied whenever possible, both to prevent and treat malnutrition in cancer patients. However, considering the limited number of studies conducted so far, further research conducted in homogenic populations of patients is necessary.
Author Contributions
Conceptualization, E.G., D.G. (Dominika Guzek), and D.G. (Dominika Głąbska); methodology, E.G., D.G. (Dominika Guzek), and D.G. (Dominika Głąbska); formal analysis, E.G., D.G. (Dominika Guzek), Z.P., and D.G. (Dominika Głąbska); investigation, E.G., D.G. (Dominika Guzek), Z.P., and D.G. (Dominika Głąbska); writing—original draft preparation, E.G., D.G. (Dominika Guzek), Z.P., J.S., and D.G. (Dominika Głąbska); writing—review and editing, E.G., D.G. (Dominika Guzek), Z.P., J.S., and D.G. (Dominika Głąbska). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Higher Education within funds of the Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS), for scientific research.
Institutional Review Board Statement
The literature search was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42021261226).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organizations (WHO). Cause-Specific Mortality, 2000–2019. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 5 November 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 1, 209–249. [Google Scholar] [CrossRef]
- World Health Organizations (WHO). Cancer. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 5 November 2021).
- National Institute of Health (NIH); National Cancer Institute (NIC). Types of Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 5 November 2021).
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Jitender, S.; Mahajan, R.; Rathore, V.; Choudhary, R. Quality of life of cancer patients. J. Exp. Ther. Oncol. 2018, 12, 217–221. [Google Scholar] [PubMed]
- Nayak, M.G.; George, A.; Vidyasagar, M.S.; Mathew, S.; Nayak, S.; Nayak, B.S.; Shashidhara, Y.N.; Kamath, A. Quality of Life among Cancer Patients. Indian J. Palliat. Care 2017, 23, 445–450. [Google Scholar] [CrossRef]
- Abdollahzadeh, F.; Sadat Aghahossini, S.; Rahmani, A.; Asvadi Kermani, I. Quality of life in cancer patients and its related factors. J. Caring. Sci. 2012, 1, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Binotto, M.; Reinert, T.; Werutsky, G.; Zaffaroni, F.; Schwartsmann, G. Health-related quality of life before and during chemotherapy in patients with early-stage breast cancer. Ecancermedicalscience 2020, 14, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Próchnicki, M.; Rudzki, S.; Laskowska, B.; Brudniak, J. Quality of Life of Cancer Patients Treated with Chemotherapy. Int. J. Environ. Res. Public Health 2020, 17, 6938. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Corsaro, E.; Molfino, A. Awareness of Cancer-Related Malnutrition and Its Management: Analysis of the Results From a Survey Conducted Among Medical Oncologists. Front. Oncol. 2021, 11, 682999. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E. Cancer-associated malnutrition. Eur. J. Clin. Nutr. 2018, 72, 1255–1259. [Google Scholar] [CrossRef]
- Kim, D.H. Nutritional issues in patients with cancer. Intest. Res. 2019, 17, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Polański, J.; Jankowska-Polańska, B.; Mazur, G. Relationship Between Nutritional Status and Quality of Life in Patients with Lung Cancer. Cancer Manag. Res. 2021, 13, 1407–1416. [Google Scholar] [CrossRef]
- Rios, T.C.; de Oliveira, L.P.M.; da Costa, M.L.V.; da Silva Baqueiro Boulhosa, R.S.; Roriz, A.K.C.; Ramos, L.B.; Bueno, A.A. A poorer nutritional status impacts quality of life in a sample population of elderly cancer patients. Health Qual. Life Outcomes 2021, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Sonneborn-Papakostopoulos, M.; Dubois, C.; Mathies, V.; Heß, M.; Erickson, N.; Ernst, T.; Huebner, J. Quality of life, symptoms and dietary habits in oncology outpatients with malnutrition: A cross-sectional study. Med. Oncol. 2021, 38, 20. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Cotogni, P. Enteral versus parenteral nutrition in cancer patients: Evidences and controversies. Ann. Palliat. Med. 2016, 5, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2020, 39, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Chow, R.; Bruera, E.; Chiu, L.; Chow, S.; Chiu, N.; Lam, H.; McDonald, R.; DeAngelis, C.; Vuong, S.; Ganesh, V.; et al. Enteral and parenteral nutrition in cancer patients: A systematic review and meta-analysis. Ann. Palliat. Med. 2016, 5, 30–41. [Google Scholar] [CrossRef]
- Ojo, O.; Keaveney, E.; Wang, X.-H.; Feng, P. The Effect of Enteral Tube Feeding on Patients’ Health-Related Quality of Life: A Systematic Review. Nutrients 2019, 11, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.R.; Bailey, S.; Tai, E.; Mirrahimi, A.; Mafeld, S.; Beecroft, J.R.; Tan, K.T.; Annamalai, G. Randomized Controlled Trial of Percutaneous Radiologic Gastrostomy Performed With and Without Gastropexy: Technical Success, Patient-Reported Outcomes and Safety. Cardiovasc. Intervent. Radiol. 2021, 44, 1081–1088. [Google Scholar] [CrossRef]
- Assessing Risk of Bias in Non-Randomized Studies. Chapter 13.5.2.3. Available online: http://handbook-5-1.cochrane.org/ (accessed on 16 November 2021).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 November 2021).
- You, S.; Kong, T.H.; Han, W. The Effects of short-term and long-term hearing changes on music exposure: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 2091. [Google Scholar] [CrossRef] [Green Version]
- Van Bokhorst-de Van der Schuer, M.A.; Langendoen, S.I.; Vondeling, H.; Kuik, D.J.; Quak, J.J.; Van Leeuwen, P.A. Perioperative enteral nutrition and quality of life of severely malnourished head and neck cancer patients: A randomized clinical trial. Clin. Nutr. 2000, 19, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hyltander, A.; Bosaeus, I.; Svedlund, J.; Liedman, B.; Hugosson, I.; Wallengren, O.; Olsson, U.; Johnsson, E.; Kostic, S.; Henningsson, A.; et al. Supportive nutrition on recovery of metabolism, nutritional state, health-related quality of life, and exercise capacity after major surgery: A randomized study. Clin. Gastroenterol. Hepatol. 2005, 3, 466–474. [Google Scholar] [CrossRef]
- Corry, J.; Poon, W.; McPhee, N.; Milner, A.D.; Cruickshank, D.; Porceddu, S.V.; Rischin, D.; Peters, L.J. Randomized study of percutaneous endoscopic gastrostomy versus nasogastric tubes for enteral feeding in head and neck cancer patients treated with (chemo)radiation. J. Med. Imaging. Radiat. Oncol. 2008, 52, 503–510. [Google Scholar] [CrossRef]
- Sadasivan, A.; Faizal, B.; Kumar, M. Nasogastric and percutaneous endoscopic gastrostomy tube use in advanced head and neck cancer patients: A comparative study. J. Pain Palliat. Care Pharmacother. 2012, 26, 226–232. [Google Scholar] [CrossRef]
- Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Larsson, S.; Hammerlid, E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: A randomized study. Head Neck 2012, 34, 1–9. [Google Scholar] [CrossRef]
- Silander, E.; Jacobsson, I.; Bertéus-Forslund, H.; Hammerlid, E. Energy intake and sources of nutritional support in patients with head and neck cancer—A randomised longitudinal study. Eur. J. Clin. Nutr. 2013, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L.; Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Hammerlid, E. Effect of prophylactic percutaneous endoscopic gastrostomy tube on swallowing in advanced head and neck cancer: A randomized controlled study. Head Neck 2017, 39, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Bowrey, D.J.; Baker, M.; Halliday, V.; Thomas, A.L.; Pulikottil-Jacob, R.; Smith, K.; Morris, T.; Ring, A. A randomised controlled trial of six weeks of home enteral nutrition versus standard care after oesophagectomy or total gastrectomy for cancer: Report on a pilot and feasibility study. Trials 2015, 21, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, J.; Janda, M.; Graves, N.; Bauer, J.; Banks, M.; Garrett, A.; Chetty, N.; Crandon, A.J.; Land, R.; Nascimento, M.; et al. Quality of life after early enteral feeding versus standard care for proven or suspected advanced epithelial ovarian cancer: Results from a randomised trial. Gynecol. Oncol. 2015, 137, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.H.; Han, L.; Du, T.P.; Guo, M.J. The effect of low-nitrogen and low-calorie parenteral nutrition combined with enteral nutrition on inflammatory cytokines and immune functions in patients with gastric cancer: A double blind placebo trial. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1345–1350. [Google Scholar] [PubMed]
- Gavazzi, C.; Colatruglio, S.; Valoriani, F.; Mazzaferro, V.; Sabbatini, A.; Biffi, R.; Mariani, L.; Miceli, R. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: A multicentre randomised clinical trial. Eur. J. Cancer 2016, 64, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Froghi, F.; Sanders, G.; Berrisford, R.; Wheatley, T.; Peyser, P.; Rahamim, J.; Lewis, S. A randomised trial of post-discharge enteral feeding following surgical resection of an upper gastrointestinal malignancy. Clin. Nutr. 2017, 36, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhong, M.; Zhu, D.M.; Song, J.Q.; Huang, J.F.; Wang, Q.; Tan, L.J. Effect of Early Full-Calorie Nutrition Support Following Esophagectomy: A Randomized Controlled Trial. JPEN J. Parenter. Enteral. Nutr. 2017, 41, 1146–1154. [Google Scholar] [CrossRef]
- Brown, T.E.; Banks, M.D.; Hughes, B.G.M.; Lin, C.Y.; Kenny, L.M.; Bauer, J.D. Randomised controlled trial of early prophylactic feeding vs standard care in patients with head and neck cancer. Br. J. Cancer 2017, 117, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaźmierczak-Siedlecka, K.; Folwarski, M.; Ruszkowski, J.; Skonieczna-Żydecka, K.; Szafrański, W.; Makarewicz, W. Effects of 4 weeks of Lactobacillus plantarum 299v supplementation on nutritional status, enteral nutrition tolerance, and quality of life in cancer patients receiving home enteral nutrition—A double-blind, randomized, and placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9684–9694. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ji, S.; Xu, Y.; Diao, Q.; Shao, C.; Luo, J.; Zhu, Y.; Jiang, Z.; Diao, Y.; Cong, Z.; et al. Safety, feasibility, and effect of an enhanced nutritional support pathway including extended preoperative and home enteral nutrition in patients undergoing enhanced recovery after esophagectomy: A pilot randomized clinical trial. Dis. Esophagus 2020, 33, doz030. [Google Scholar] [CrossRef]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.C.; Drefahl, S.; Ahlbom, A.; Lambe, M.; Modig, K. Trends in life expectancy: Did the gap between the healthy and the ill widen or close? BMC Med. 2020, 18, 41. [Google Scholar] [CrossRef]
- Gayatri, D.; Efremov, L.; Kantelhardt, E.J.; Mikolajczyk, R. Quality of life of cancer patients at palliative care units in developing countries: Systematic review of the published literature. Qual. Life Res. 2021, 30, 315–343. [Google Scholar] [CrossRef]
- Lis, C.G.; Gupta, D.; Lammersfeld, C.A.; Markman, M.; Vashi, P.G. Role of nutritional status in predicting quality of life outcomes in cancer—A systematic review of the epidemiological literature. Nutr. J. 2012, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Wanden-Berghe, C.; Patino-Alonso, M.C.; Galindo-Villardón, P.; Sanz-Valero, J. Complications Associated with Enteral Nutrition: CAFANE Study. Nutrients 2019, 11, 2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schell, R.F.; Sidone, B.J.; Caron, W.P.; Walsh, M.D.; White, T.F.; Zamboni, B.A.; Ramanathan, R.K.; Zamboni, W.C. Meta-analysis of inter-patient pharmacokinetic variability of liposomal and non-liposomal anticancer agents. Nanomedicine 2014, 10, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Singh, S.P.; Behere, P.B.; Roy, S.K.; Singh, S.; Shukla, V.K. Quality of life in patients with early and advanced carcinoma of the breast. Eur. J. Surg. Oncol. 2000, 26, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Ringdal, G.I.; Ringdal, K. A follow-up study of the quality of life in cancer patients with different prognoses. Qual. Life Res. 2000, 9, 65–73. [Google Scholar] [CrossRef]
- Salas, S.; Mercier, S.; Moheng, B.; Olivet, S.; Garcia, M.E.; Hamon, S.; Sibertin-Blanc, C.; Duffaud, F.; Auquier, P.; Baumstarck, K. Nutritional status and quality of life of cancer patients needing exclusive chemotherapy: A longitudinal study. Health Qual. Life Outcomes 2017, 15, 85. [Google Scholar] [CrossRef] [Green Version]
- Lakusta, C.M.; Atkinson, M.J.; Robinson, J.W.; Nation, J.; Taenzer, P.A.; Campo, M.G. Quality of life in ovarian cancer patients receiving chemotherapy. Gynecol. Oncol. 2001, 81, 490–495. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, X. The effectiveness of enteral nutrition for patients with primary liver cancer: A randomized controlled study protocol. Medicine 2021, 100, e23973. [Google Scholar] [CrossRef] [PubMed]
- Fietkau, R.; Lewitzki, V.; Kuhnt, T.; Hölscher, T.; Hess, C.F.; Berger, B.; Wiegel, T.; Rödel, C.; Niewald, M.; Hermann, R.M.; et al. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: Results of a randomized, controlled, multicenter trial. Cancer 2013, 119, 3343–3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).