Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review
Abstract
1. Introduction
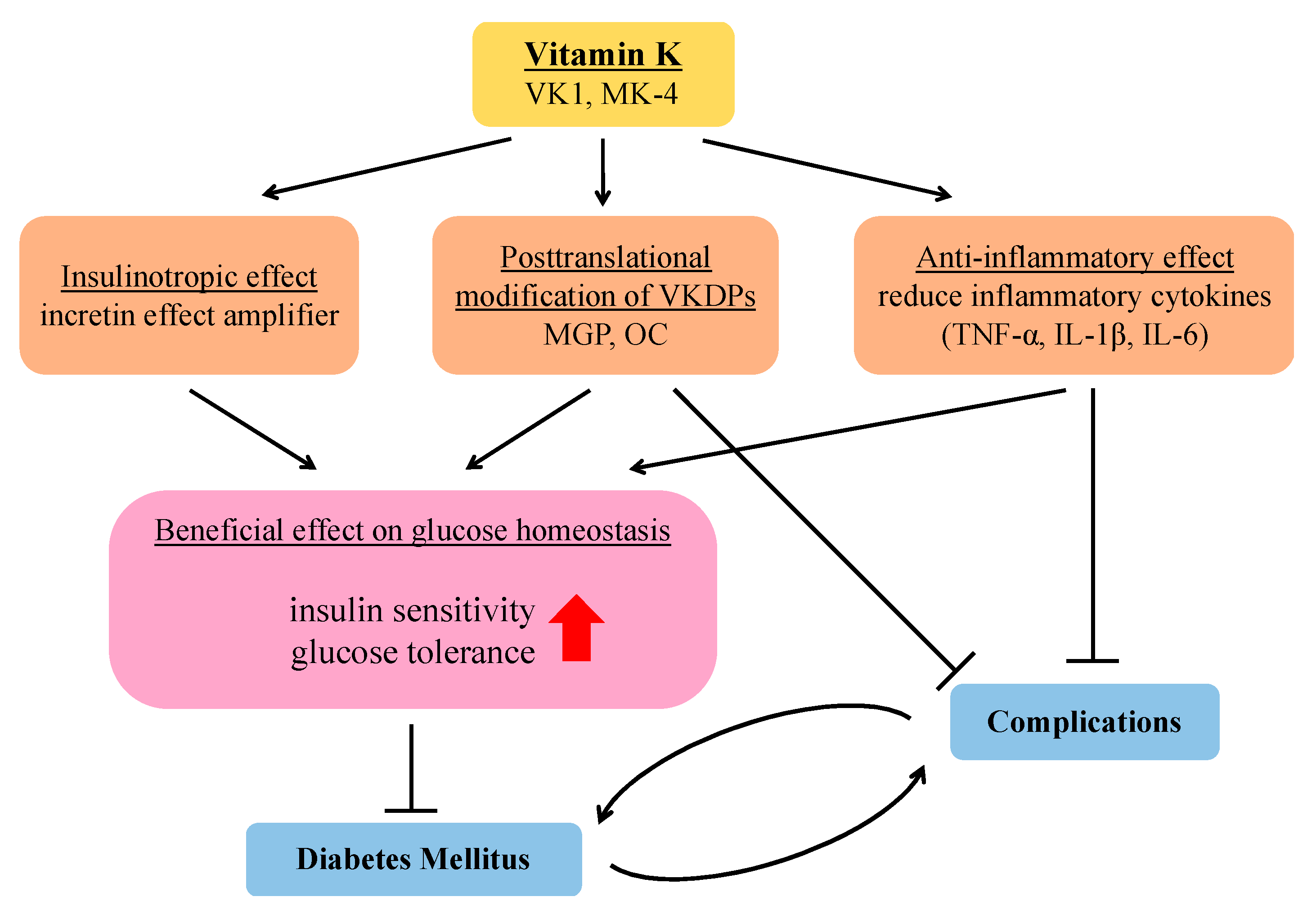
2. Improvement of Insulin Sensitivity and Glycemic Status
3. Possible Effect of VK Supplementation on Insulin Secretion and Glycemic Status
3.1. Insulinotropic Effect
3.2. Modulation of VK-Dependent Proteins
3.3. Prevention of Inflammation
4. Beneficial Effects of VK on Diabetes-Related Complications
4.1. Cataractogenesis
4.2. Diabetic Nephropathy
4.3. Diabetic Peripheral Neuropathy
4.4. Cardiovascular Disease
4.5. Osteopenia and Osteoporosis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. (Lausanne) 2017, 8, 6. [Google Scholar] [CrossRef]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Komatsu, R.; Matsuyama, T.; Namba, M.; Watanabe, N.; Itoh, H.; Kono, N.; Tarui, S. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7-36)-amide. Diabetes 1989, 38, 902–905. [Google Scholar] [CrossRef]
- Meier, J.J.; Nauck, M.A.; Schmidt, W.E.; Gallwitz, B. Gastric inhibitory polypeptide: The neglected incretin revisited. Regul. Pept. 2002, 107, 1–13. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul. Pept. 2003, 114, 115–121. [Google Scholar] [CrossRef]
- Prigeon, R.L.; Quddusi, S.; Paty, B.; D′Alessio, D.A. Suppression of glucose production by GLP-1 independent of islet hormones: A novel extrapancreatic effect. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E701–E707. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Herzberg-Schäfer, S.; Heni, M.; Stefan, N.; Häring, H.U.; Fritsche, A. Impairment of GLP1-induced insulin secretion: Role of genetic background, insulin resistance and hyperglycaemia. Diabetes Obes. Metab. 2012, 14 (Suppl. 3), 85–90. [Google Scholar] [CrossRef]
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Sanlioglu, S. Incretins: Their physiology and application in the treatment of diabetes mellitus. Diabetes Metab. Res. Rev. 2014, 30, 354–371. [Google Scholar] [CrossRef]
- Ghosh-Swaby, O.R.; Goodman, S.G.; Leiter, L.A.; Cheng, A.; Connelly, K.A.; Fitchett, D.; Jüni, P.; Farkouh, M.E.; Udell, J.A. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: An updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2020, 8, 418–435. [Google Scholar] [CrossRef]
- Langenberg, C.; Lotta, L.A. Genomic insights into the causes of type 2 diabetes. Lancet 2018, 391, 2463–2474. [Google Scholar] [CrossRef]
- Booth, S.L. Vitamin K: Food composition and dietary intakes. Food Nutr. Res. 2012, 56, 5505. [Google Scholar] [CrossRef]
- Fu, X.; Harshman, S.G.; Shen, X.; Haytowitz, D.B.; Karl, J.P.; Wolfe, B.E.; Booth, S.L. Multiple Vitamin K Forms Exist in Dairy Foods. Curr. Dev. Nutr. 2017, 1, e000638. [Google Scholar] [CrossRef]
- Martius, C.; Alvino, C. On the transformation of vitamin K-1 (phyllochinon) into vitamin K2(20) by the development of an embryo in a hen′s egg. Biochem. Z 1964, 340, 316–319. [Google Scholar]
- Thijssen, H.H.; Drittij-Reijnders, M.J. Vitamin K status in human tissues: Tissue-specific accumulation of phylloquinone and menaquinone-4. Br. J. Nutr. 1996, 75, 121–127. [Google Scholar] [CrossRef]
- Komai, M.; Shirakawa, H. Vitamin K metabolism. Menaquinone-4 (MK-4) formation from ingested VK analogues and its potent relation to bone function. Clin. Calcium 2007, 17, 1663–1672. [Google Scholar]
- Shirakawa, H.; Katsurai, T.; Komai, M. Conversion of menaquinone-4 in animal organs and it functions. Jpn. Oil Chem. Soc. 2014, 14, 547–553. [Google Scholar] [CrossRef]
- Sakamoto, N.; Nishiike, T.; Iguchi, H.; Sakamoto, K. Relationship between acute insulin response and vitamin K intake in healthy young male volunteers. Diabetes Nutr. Metab. 1999, 12, 37–41. [Google Scholar]
- Yoshida, M.; Booth, S.L.; Meigs, J.B.; Saltzman, E.; Jacques, P.F. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am. J. Clin. Nutr. 2008, 88, 210–215. [Google Scholar] [CrossRef]
- Beulens, J.W.; van der, A.D.; Grobbee, D.E.; Sluijs, I.; Spijkerman, A.M.; van der Schouw, Y.T. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care 2010, 33, 1699–1705. [Google Scholar] [CrossRef]
- Ibarrola-Jurado, N.; Salas-Salvadó, J.; Martínez-González, M.A.; Bulló, M. Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2012, 96, 1113–1118. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; Remmelzwaal, S.; Beulens, J.W.J.; Booth, S.L.; Burgess, S.; Dashti, H.S.; Imamura, F.; Feskens, E.J.M.; van der Schouw, Y.T.; Sluijs, I. Circulating Phylloquinone Concentrations and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes 2019, 68, 220–225. [Google Scholar] [CrossRef]
- Yoshida, M.; Jacques, P.F.; Meigs, J.B.; Saltzman, E.; Shea, M.K.; Gundberg, C.; Dawson-Hughes, B.; Dallal, G.; Booth, S.L. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 2008, 31, 2092–2096. [Google Scholar] [CrossRef]
- Rasekhi, H.; Karandish, M.; Jalali, M.T.; Mohammadshahi, M.; Zarei, M.; Saki, A.; Shahbazian, H. Phylloquinone supplementation improves glycemic status independent of the effects of adiponectin levels in premonopause women with prediabetes: A double-blind randomized controlled clinical trial. J. Diabetes Metab. Disord. 2015, 14, 1. [Google Scholar] [CrossRef]
- Rasekhi, H.; Karandish, M.; Jalali, M.T.; Mohammad-Shahi, M.; Zarei, M.; Saki, A.; Shahbazian, H. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: A double-blind randomized controlled clinical trial. Eur. J. Clin. Nutr. 2015, 69, 891–895. [Google Scholar] [CrossRef]
- Sakamoto, N.; Nishiike, T.; Iguchi, H.; Sakamoto, K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin. Nutr. 2000, 19, 259–263. [Google Scholar] [CrossRef]
- Choi, H.J.; Yu, J.; Choi, H.; An, J.H.; Kim, S.W.; Park, K.S.; Jang, H.C.; Kim, S.Y.; Shin, C.S. Vitamin K2 supplementation improves insulin sensitivity via osteocalcin metabolism: A placebo-controlled trial. Diabetes Care 2011, 34, e147. [Google Scholar] [CrossRef]
- Sakamoto, N.; Wakabayashi, I.; Sakamoto, K. Low vitamin K intake effects on glucose tolerance in rats. Int. J. Vitam. Nutr. Res. 1999, 69, 27–31. [Google Scholar] [CrossRef]
- Seyama, Y.; Kimoto, S.; Marukawa, Y.; Horiuchi, M.; Hayashi, M.; Usami, E. Comparative effects of vitamin K2 and estradiol on experimental arteriosclerosis with diabetes mellitus. Int. J. Vitam. Nutr. Res. 2000, 70, 301–304. [Google Scholar] [CrossRef]
- Barnett, A.H. The role of GLP-1 mimetics and basal insulin analogues in type 2 diabetes mellitus: Guidance from studies of liraglutide. Diabetes Obes. Metab. 2012, 14, 304–314. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H.; Hirahara, K.; Sone, H.; Kamiyama, S.; Komai, M. Menaquinone-4 Amplified Glucose-Stimulated Insulin Secretion in Isolated Mouse Pancreatic Islets and INS-1 Rat Insulinoma Cells. Int. J. Mol. Sci. 2019, 20, 1995. [Google Scholar] [CrossRef]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moysés, R.M.A.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K plasma levels determination in human health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef]
- El Asmar, M.S.; Naoum, J.J.; Arbid, E.J. Vitamin k dependent proteins and the role of vitamin k2 in the modulation of vascular calcification: A review. Oman Med. J. 2014, 29, 172–177. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Teebi, A.S.; Lambert, D.M.; Kaye, G.M.; Al-Fifi, S.; Tewfik, T.L.; Azouz, E.M. Keutel syndrome: Further characterization and review. Am. J. Med. Genet. 1998, 78, 182–187. [Google Scholar]
- Munroe, P.B.; Olgunturk, R.O.; Fryns, J.P.; Van Maldergem, L.; Ziereisen, F.; Yuksel, B.; Gardiner, R.M.; Chung, E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999, 21, 142–144. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewe, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. Int. J. Mol. Sci. 2019, 20, 628. [Google Scholar] [CrossRef]
- Basta, G.; Corciu, A.I.; Vianello, A.; Del Turco, S.; Foffa, I.; Navarra, T.; Chiappino, D.; Berti, S.; Mazzone, A. Circulating soluble receptor for advanced glycation end-product levels are decreased in patients with calcific aortic valve stenosis. Atherosclerosis 2010, 210, 614–618. [Google Scholar] [CrossRef]
- Olson, J.C.; Edmundowicz, D.; Becker, D.J.; Kuller, L.H.; Orchard, T.J. Coronary calcium in adults with type 1 diabetes: A stronger correlate of clinical coronary artery disease in men than in women. Diabetes 2000, 49, 1571–1578. [Google Scholar] [CrossRef][Green Version]
- Neubauer, B. A quantitative study of peripheral arterial calcification and glucose tolerance in elderly diabetics and non-diabetics. Diabetologia 1971, 7, 409–413. [Google Scholar] [CrossRef][Green Version]
- Conway, B.; Edmundowicz, D.; Matter, N.; Maynard, J.; Orchard, T. Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol. 2010, 12, 339–345. [Google Scholar] [CrossRef]
- Thomsen, S.B.; Rathcke, C.N.; Zerahn, B.; Vestergaard, H. Increased levels of the calcification marker matrix Gla Protein and the inflammatory markers YKL-40 and CRP in patients with type 2 diabetes and ischemic heart disease. Cardiovasc. Diabetol. 2010, 9, 86. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.J.; Vermeer, C.; Verschuren, W.M.; Boer, J.M.; Beulens, J.W. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care 2013, 36, 3766–3771. [Google Scholar] [CrossRef]
- Jeannin, A.C.; Salem, J.E.; Massy, Z.; Aubert, C.E.; Vemeer, C.; Amouyal, C.; Phan, F.; Halbron, M.; Funck-Brentano, C.; Hartemann, A.; et al. Inactive matrix gla protein plasma levels are associated with peripheral neuropathy in Type 2 diabetes. PLoS ONE 2020, 15, e0229145. [Google Scholar] [CrossRef]
- Parker, B.D.; Ix, J.H.; Cranenburg, E.C.; Vermeer, C.; Whooley, M.A.; Schurgers, L.J. Association of kidney function and uncarboxylated matrix Gla protein: Data from the Heart and Soul Study. Nephrol. Dial. Transpl. 2009, 24, 2095–2101. [Google Scholar] [CrossRef]
- Fulzele, K.; Riddle, R.C.; DiGirolamo, D.J.; Cao, X.; Wan, C.; Chen, D.; Faugere, M.C.; Aja, S.; Hussain, M.A.; Brüning, J.C.; et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 2010, 142, 309–319. [Google Scholar] [CrossRef]
- Faienza, M.F.; Luce, V.; Ventura, A.; Colaianni, G.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Skeleton and glucose metabolism: A bone-pancreas loop. Int. J. Endocrinol. 2015, 2015, 758148. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin-A Versatile Bone-Derived Hormone. Front. Endocrinol. (Lausanne) 2018, 9, 794. [Google Scholar] [CrossRef]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P.; et al. Endocrine regulation of male fertility by the skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef]
- Schwetz, V.; Pieber, T.; Obermayer-Pietsch, B. The endocrine role of the skeleton: Background and clinical evidence. Eur. J. Endocrinol. 2012, 166, 959–967. [Google Scholar] [CrossRef]
- Oury, F.; Ferron, M.; Huizhen, W.; Confavreux, C.; Xu, L.; Lacombe, J.; Srinivas, P.; Chamouni, A.; Lugani, F.; Lejeune, H.; et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J. Clin. Investig. 2013, 123, 2421–2433. [Google Scholar] [CrossRef]
- Karsenty, G.; Oury, F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol. Cell Endocrinol. 2014, 382, 521–526. [Google Scholar] [CrossRef]
- Wei, J.; Ferron, M.; Clarke, C.J.; Hannun, Y.A.; Jiang, H.; Blaner, W.S.; Karsenty, G. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J. Clin. Investig. 2014, 124, 1781–1793. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Ferron, M.; Hinoi, E.; Karsenty, G.; Ducy, P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5266–5270. [Google Scholar] [CrossRef]
- Reddi, K.; Henderson, B.; Meghji, S.; Wilson, M.; Poole, S.; Hopper, C.; Harris, M.; Hodges, S.J. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine 1995, 7, 287–290. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Hiwatashi, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. BioSci. Biotechnol. Biochem. 2006, 70, 926–932. [Google Scholar] [CrossRef]
- Shea, M.K.; Booth, S.L.; Massaro, J.M.; Jacques, P.F.; D′Agostino, R.B., Sr.; Dawson-Hughes, B.; Ordovas, J.M.; O′Donnell, C.J.; Kathiresan, S.; Keaney, J.F., Jr.; et al. Vitamin K and vitamin D status: Associations with inflammatory markers in the Framingham Offspring Study. Am. J. Epidemiol. 2008, 167, 313–320. [Google Scholar] [CrossRef]
- Varsha, M.K.; Thiagarajan, R.; Manikandan, R.; Dhanasekaran, G. Vitamin K1 alleviates streptozotocin-induced type 1 diabetes by mitigating free radical stress, as well as inhibiting NF-κB activation and iNOS expression in rat pancreas. Nutrition 2015, 31, 214–222. [Google Scholar] [CrossRef]
- Razny, U.; Fedak, D.; Kiec-Wilk, B.; Goralska, J.; Gruca, A.; Zdzienicka, A.; Kiec-Klimczak, M.; Solnica, B.; Hubalewska-Dydejczyk, A.; Malczewska-Malec, M. Carboxylated and undercarboxylated osteocalcin in metabolic complications of human obesity and prediabetes. Diabetes Metab. Res. Rev. 2017, 33, e2862. [Google Scholar] [CrossRef]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Estruch, R.; Portillo, M.P.; Casas, R.; Miranda, J.; Martínez-González, M.A.; Bulló, M. Association between dietary phylloquinone intake and peripheral metabolic risk markers related to insulin resistance and diabetes in elderly subjects at high cardiovascular risk. Cardiovasc. Diabetol. 2013, 12, 7. [Google Scholar] [CrossRef]
- Wieser, V.; Moschen, A.R.; Tilg, H. Inflammation, cytokines and insulin resistance: A clinical perspective. Arch. Immunol. Exp. (Warsz) 2013, 61, 119–125. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef]
- Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef]
- Kleinman, R.E.; Fracchia, M.S. Vitamin K and cystic fibrosis: Give me a double, please. Am. J. Clin. Nutr. 2010, 92, 469–470. [Google Scholar] [CrossRef][Green Version]
- Nakajima, S.; Iijima, H.; Egawa, S.; Shinzaki, S.; Kondo, J.; Inoue, T.; Hayashi, Y.; Ying, J.; Mukai, A.; Akasaka, T.; et al. Association of vitamin K deficiency with bone metabolism and clinical disease activity in inflammatory bowel disease. Nutrition 2011, 27, 1023–1028. [Google Scholar] [CrossRef]
- Sikkens, E.C.; Cahen, D.L.; Koch, A.D.; Braat, H.; Poley, J.W.; Kuipers, E.J.; Bruno, M.J. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology 2013, 13, 238–242. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Wei, F.F.; Huang, Q.F.; Zhang, Z.Y.; Van Keer, K.; Thijs, L.; Trenson, S.; Yang, W.Y.; Cauwenberghs, N.; Mujaj, B.; Kuznetsova, T.; et al. Inactive matrix Gla protein is a novel circulating biomarker predicting retinal arteriolar narrowing in humans. Sci. Rep. 2018, 8, 15088. [Google Scholar] [CrossRef]
- Sai Varsha, M.K.; Raman, T.; Manikandan, R. Inhibition of diabetic-cataract by vitamin K1 involves modulation of hyperglycemia-induced alterations to lens calcium homeostasis. Exp. Eye Res. 2014, 128, 73–82. [Google Scholar] [CrossRef]
- Thiagarajan, R.; Varsha, M.; Srinivasan, V.; Ravichandran, R.; Saraboji, K. Vitamin K1 prevents diabetic cataract by inhibiting lens aldose reductase 2 (ALR2) activity. Sci. Rep. 2019, 9, 14684. [Google Scholar] [CrossRef]
- Holden, R.M.; Morton, A.R.; Garland, J.S.; Pavlov, A.; Day, A.G.; Booth, S.L. Vitamins K and D status in stages 3–5 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 590–597. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef]
- Elliott, M.J.; Booth, S.L.; Hopman, W.M.; Holden, R.M. Assessment of potential biomarkers of subclinical vitamin K deficiency in patients with end-stage kidney disease. Can. J. Kidney Health Dis. 2014, 1, 13. [Google Scholar] [CrossRef]
- Epstein, M. Matrix Gla-Protein (MGP) Not Only Inhibits Calcification in Large Arteries but Also May Be Renoprotective: Connecting the Dots. EBioMedicine 2016, 4, 16–17. [Google Scholar] [CrossRef][Green Version]
- Puzantian, H.; Akers, S.R.; Oldland, G.; Javaid, K.; Miller, R.; Ge, Y.; Ansari, B.; Lee, J.; Suri, A.; Hasmath, Z.; et al. Circulating Dephospho-Uncarboxylated Matrix Gla-Protein Is Associated with Kidney Dysfunction and Arterial Stiffness. Am. J. Hypertens 2018, 31, 988–994. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.G.; Passadakis, P.; Tavridou, A. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Complicat. 2017, 31, 1527–1532. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef]
- Wei, F.F.; Trenson, S.; Thijs, L.; Huang, Q.F.; Zhang, Z.Y.; Yang, W.Y.; Moliterno, P.; Allegaert, K.; Boggia, J.; Janssens, S.; et al. Desphospho-uncarboxylated matrix Gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol. Dial. Transpl. 2018, 33, 1122–1128. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Plasma Desphospho-Uncarboxylated Matrix Gla Protein as a Marker of Kidney Damage and Cardiovascular Risk in Advanced Stage of Chronic Kidney Disease. Kidney Blood Press Res. 2016, 41, 231–239. [Google Scholar] [CrossRef]
- Aoun, M.; Makki, M.; Azar, H.; Matta, H.; Chelala, D.N. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: Risk factors and response to vitamin K2, a pre-post intervention clinical trial. BMC Nephrol. 2017, 18, 191. [Google Scholar] [CrossRef]
- Thamratnopkoon, S.; Susantitaphong, P.; Tumkosit, M.; Katavetin, P.; Tiranathanagul, K.; Praditpornsilpa, K.; Eiam-Ong, S. Correlations of Plasma Desphosphorylated Uncarboxylated Matrix Gla Protein with Vascular Calcification and Vascular Stiffness in Chronic Kidney Disease. Nephron 2017, 135, 167–172. [Google Scholar] [CrossRef]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Soderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef]
- Doi, Y.; Iwashima, Y.; Yoshihara, F.; Kamide, K.; Hayashi, S.; Kubota, Y.; Nakamura, S.; Horio, T.; Kawano, Y. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension 2012, 60, 770–777. [Google Scholar] [CrossRef]
- Jaques, D.A.; Pivin, E.; Pruijm, M.; Ackermann, D.; Guessous, I.; Ehret, G.; Wei, F.F.; Staessen, J.A.; Pechère-Bertschi, A.; Vermeer, C.; et al. Renal Resistive Index Is Associated with Inactive Matrix Gla (γ-Carboxyglutamate) Protein in an Adult Population-Based Study. J. Am. Heart Assoc. 2019, 8, e013558. [Google Scholar] [CrossRef]
- Miyata, K.N.; Nast, C.C.; Dai, T.; Dukkipati, R.; LaPage, J.A.; Troost, J.P.; Schurgers, L.J.; Kretzler, M.; Adler, S.G. Renal matrix Gla protein expression increases progressively with CKD and predicts renal outcome. Exp. Mol. Pathol. 2018, 105, 120–129. [Google Scholar] [CrossRef]
- Dyck, P.J. Detection, characterization, and staging of polyneuropathy: Assessed in diabetics. Muscle Nerve 1988, 11, 21–32. [Google Scholar] [CrossRef]
- Papanas, N.; Ziegler, D. Risk Factors and Comorbidities in Diabetic Neuropathy: An Update 2015. Rev. Diabet Stud. 2015, 12, 48–62. [Google Scholar] [CrossRef]
- Nakajima, M.; Furukawa, S.; Hayashi, K.; Yamada, A.; Kawashima, T.; Hayashi, Y. Age-dependent survival-promoting activity of vitamin K on cultured CNS neurons. Brain Res. Dev. Brain Res. 1993, 73, 17–23. [Google Scholar] [CrossRef]
- Goritz, C.; Thiebaut, R.; Tessier, L.H.; Nieweg, K.; Moehle, C.; Buard, I.; Dupont, J.L.; Schurgers, L.J.; Schmitz, G.; Pfrieger, F.W. Glia-induced neuronal differentiation by transcriptional regulation. Glia 2007, 55, 1108–1122. [Google Scholar] [CrossRef]
- Nishimoto, S.K.; Nishimoto, M. Matrix Gla protein C-terminal region binds to vitronectin. Co-localization suggests binding occurs during tissue development. Matrix Biol. 2005, 24, 353–361. [Google Scholar] [CrossRef]
- Moon, J.I.; Birren, S.J. Target-dependent inhibition of sympathetic neuron growth via modulation of a BMP signaling pathway. Dev. Biol. 2008, 315, 404–417. [Google Scholar] [CrossRef]
- Nishimoto, S.K.; Nishimoto, M. Matrix gla protein binds to fibronectin and enhances cell attachment and spreading on fibronectin. Int. J. Cell Biol. 2014, 2014, 807013. [Google Scholar] [CrossRef]
- Kendall, D.M.; Harmel, A.P. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: Understanding the role of insulin resistance. Am. J. Manag. Care 2002, 8, S635–S653. [Google Scholar]
- Schurgers, L.J.; Cranenburg, E.C.; Vermeer, C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar]
- Shea, M.K.; Holden, R.M. Vitamin K status and vascular calcification: Evidence from observational and clinical studies. Adv. Nutr. 2012, 3, 158–165. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Kruger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower Progress of Aortic Valve Calcification with Vitamin K Supplementation: Results from a Prospective Interventional Proof-of-Concept Study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef]
- Pivin, E.; Ponte, B.; Pruijm, M.; Ackermann, D.; Guessous, I.; Ehret, G.; Liu, Y.P.; Drummen, N.E.; Knapen, M.H.; Pechere-Bertschi, A.; et al. Inactive Matrix Gla-Protein Is Associated with Arterial Stiffness in an Adult Population-Based Study. Hypertension 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Harshman, S.G.; Shea, M.K. The Role of Vitamin K in Chronic Aging Diseases: Inflammation, Cardiovascular Disease, and Osteoarthritis. Curr. Nutr. Rep. 2016, 5, 90–98. [Google Scholar] [CrossRef]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef]
- Caluwe, R.; Pyfferoen, L.; De Boeck, K.; De Vriese, A.S. The effects of vitamin K supplementation and vitamin K antagonists on progression of vascular calcification: Ongoing randomized controlled trials. Clin. Kidney J. 2016, 9, 273–279. [Google Scholar] [CrossRef]
- Danziger, J.; Young, R.L.; Shea, K.M.; Duprez, D.A.; Jacobs, D.R.; Tracy, R.P.; Ix, J.H.; Jenny, N.S.; Mukamal, K.J. Circulating Des-gamma-carboxy prothrombin is not associated with cardiovascular calcification or stiffness: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2016, 252, 68–74. [Google Scholar] [CrossRef]
- Danziger, J.; Young, R.L.; Shea, M.K.; Tracy, R.P.; Ix, J.H.; Jenny, N.S.; Mukamal, K.J. Vitamin K-Dependent Protein Activity and Incident Ischemic Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. Arter. Thromb. Vasc. Biol. 2016, 36, 1037–1042. [Google Scholar] [CrossRef]
- Ishida, Y.; Kawai, S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. Am. J. Med. 2004, 117, 549–555. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Brueck, C.C.; Singh, S.K.; Dobnig, H. Osteoporosis in patients with diabetes mellitus. J. Bone Min. Res. 2007, 22, 1317–1328. [Google Scholar] [CrossRef]
- Shiraki, M.; Yamazaki, Y.; Shiraki, Y.; Hosoi, T.; Tsugawa, N.; Okano, T. High level of serum undercarboxylated osteocalcin in patients with incident fractures during bisphosphonate treatment. J. Bone Min. Metab. 2010, 28, 578–584. [Google Scholar] [CrossRef]
- Palermo, A.; Tuccinardi, D.; D′Onofrio, L.; Watanabe, M.; Maggi, D.; Maurizi, A.R.; Greto, V.; Buzzetti, R.; Napoli, N.; Pozzilli, P.; et al. Vitamin K and osteoporosis: Myth or reality? Metabolism 2017, 70, 57–71. [Google Scholar] [CrossRef]
- Iwamoto, J.; Seki, A.; Sato, Y.; Matsumoto, H.; Takeda, T.; Yeh, J.K. Vitamin K2 prevents hyperglycemia and cancellous osteopenia in rats with streptozotocin-induced type 1 diabetes. Calcif. Tissue Int. 2011, 88, 162–168. [Google Scholar] [CrossRef]
- Iwamoto, J.; Takeda, T.; Ichimura, S. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J. Orthop. Sci. 2000, 5, 546–551. [Google Scholar] [CrossRef]
- Shiraki, M.; Shiraki, Y.; Aoki, C.; Miura, M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J. Bone Min. Res. 2000, 15, 515–521. [Google Scholar] [CrossRef]
- Iwamoto, J.; Takeda, T.; Ichimura, S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: A comparison with the effect of etidronate. J. Orthop. Sci. 2001, 6, 487–492. [Google Scholar] [CrossRef]
- Inoue, T.; Fujita, T.; Kishimoto, H.; Makino, T.; Nakamura, T.; Nakamura, T.; Sato, T.; Yamazaki, K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): A phase IV clinical study of 15-mg menatetrenone capsules. J. Bone Min. Metab. 2009, 27, 66–75. [Google Scholar] [CrossRef]
- Iwamoto, J. Vitamin K2 therapy for postmenopausal osteoporosis. Nutrients 2014, 6, 1971–1980. [Google Scholar] [CrossRef]
- Beulens, J.W.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (vitamin K(2)) in human health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef]
- Vissers, L.E.; Dalmeijer, G.W.; Boer, J.M.; Monique Verschuren, W.M.; van der Schouw, Y.T.; Beulens, J.W. Intake of dietary phylloquinone and menaquinones and risk of stroke. J. Am. Heart Assoc. 2013, 2, e000455. [Google Scholar] [CrossRef]
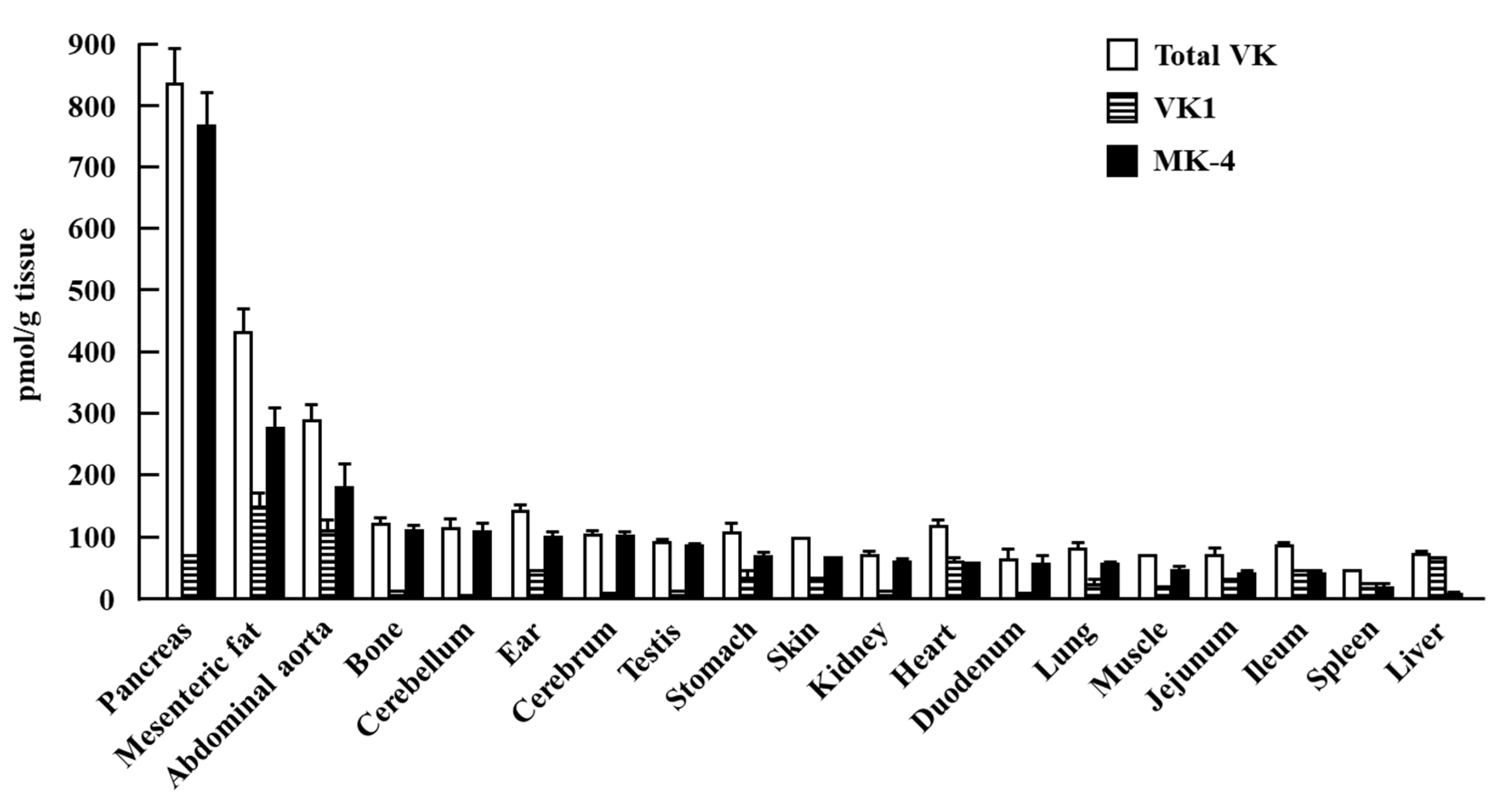
| Subjects (N) | VK dose/VK Status | Period | Outcome | Ref. |
|---|---|---|---|---|
| A. Human Studies | ||||
| (a) Observational Studies | ||||
| Healthy young men (16) | Usual dietary intake | Acute insulin response A 1-week food-frequency questionnaire to ascertain the daily VK intake | The participants with higher dietary VK intake showed a better insulin response and glucose tolerance. | [18] |
| Framingham offspring cohort study, adult men (1247) and women (1472) | Usual dietary intake | 12 months | In a cross-sectional analysis, higher dietary VK intake was associated with reduced insulin resistance in both adult men and women. | [19] |
| Adult men (9740) and women (28,354) | Usual dietary intake | 10.3 years | Dietary intake of both VK1 and VK2 were associated with a reduced risk of T2DM. | [20] |
| Elderly men (861) and women (1062) with high cardiovascular risk | Usual dietary intake | Median follow-up of 5.5 years | Dietary VK1 at the baseline was significantly lower in participants who developed T2DM during the study. Increased dietary VK1 intake was associated with a reduced risk of incident T2DM. | [21] |
| European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study, Diabetes Genetics Replication and Meta-analysis (DIAGRAM), and the UK Biobank (9400 case subjects and 12,182 sub-cohort participants) | Usual dietary intake | EPIC cohort: 1997–2007, DIAGRAM cohort: 2007 (included data from 23 studies), UK Biobank: 2006–2010 | Higher circulating VK1 may be causally related with lower risk of T2DM, highlighting the importance of sufficient phylloquinone intake in the human diet. | [22] |
| (b) Intervention Studies | ||||
| Elderly nondiabetic men (124) and women (165) | With or without 500 μg/day VK1 supplementation | 36 months | Dietary VK1 supplementation had a protective effect on the progression of insulin resistance in older men. | [23] |
| Prediabetic women (82) | With or without 1000 μg/day VK1 supplementation | 4 weeks | Dietary VK1 supplementation had beneficial effects on glycemic status and insulin sensitivity in premenopausal and prediabetic women. | [24,25] |
| Healthy young men (12) | 90 mg/day menaquinone-4 (MK-4) supplementation | 1 week | Short-term VK2 supplementation improved the insulin response after an oral glucose challenge in young men. | [26] |
| Healthy young men (42) | With or without 90 mg/day MK-4 supplementation | 4 weeks | Dietary VK2 supplementation improved insulin sensitivity in young men. | [27] |
| B. Animal Studies | ||||
| Rats (unknown) | Low-VK diet (<20% of the required VK1) | Unknown | Rats fed a low-VK diet had poor early insulin response and subsequently increased insulin secretion after a glucose load. | [28] |
| Arteriosclerotic rat model with DM (unknown) | 100 mg/day per kilogram body weight VK2 | 3 or 6 weeks | VK2 supplementation had a protective effect on arteriosclerosis, by decreasing the aortic Ca and P and the elastin fraction. Rats fed a VK2-rich diet had decreased serum glucose levels and increased serum insulin levels. | [29] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, H.-J.; Komai, M.; Shirakawa, H. Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review. Nutrients 2020, 12, 2485. https://doi.org/10.3390/nu12082485
Ho H-J, Komai M, Shirakawa H. Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review. Nutrients. 2020; 12(8):2485. https://doi.org/10.3390/nu12082485
Chicago/Turabian StyleHo, Hsin-Jung, Michio Komai, and Hitoshi Shirakawa. 2020. "Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review" Nutrients 12, no. 8: 2485. https://doi.org/10.3390/nu12082485
APA StyleHo, H.-J., Komai, M., & Shirakawa, H. (2020). Beneficial Effects of Vitamin K Status on Glycemic Regulation and Diabetes Mellitus: A Mini-Review. Nutrients, 12(8), 2485. https://doi.org/10.3390/nu12082485






