Understanding the Elements of Maternal Protection from Systemic Bacterial Infections during Early Life
Abstract
1. Introduction
2. Breastfeeding and LOS
3. Enteric Origin of Pathogens
3.1. Pathogens in LOS
3.2. Modification of the Infant Gut Microbiota
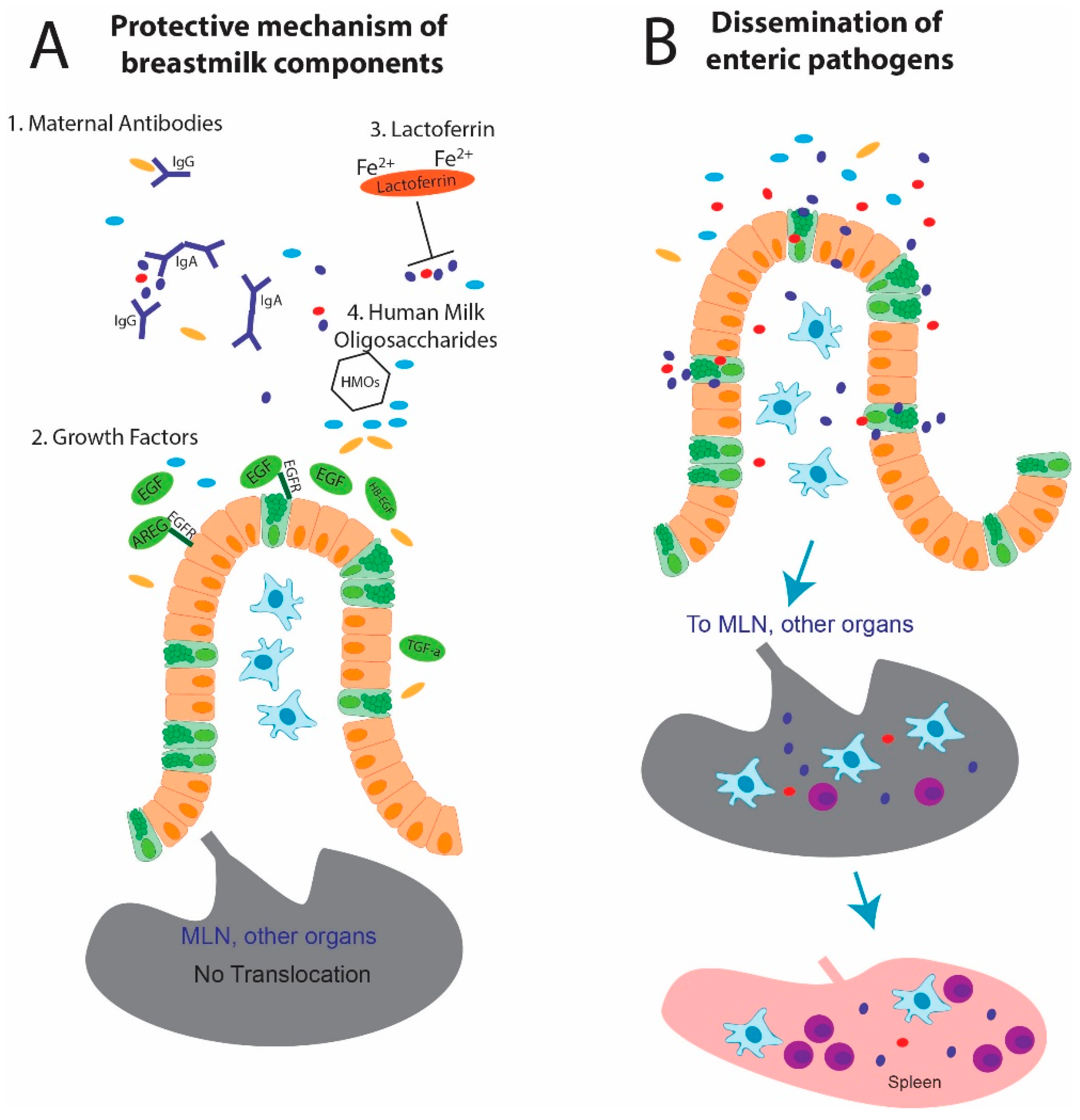
4. Components of Breastmilk
4.1. Antibodies
4.2. Growth Factors
4.3. Lactoferrin
4.4. Human Milk Oligosaccharides
5. Future Directions
5.1. Supplements and MOM Alternatives
5.2. Animal Modeling
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cortese, F.; Scicchitano, P.; Gesualdo, M.; Filaninno, A.; De Giorgi, E.; Schettini, F.; Laforgia, N.; Ciccone, M.M. Early and late infections in newborns: Where do we stand? A review. Pediatr. Neonatol. 2016, 57, 265–273. [Google Scholar] [CrossRef]
- Raymond, S.L.; Rincon, J.C.; Wynn, J.L.; Moldawer, L.L.; Larson, S.D. Impact of early-life exposures to infections, antibiotics, and vaccines on perinatal and long-term health and disease. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Stoll, B.J.; Gordon, T.; Korones, S.B.; Shankaran, S.; Tyson, J.E.; Bauer, C.R.; Fanaroff, A.A.; Lemons, J.A.; Donovan, E.F.; Oh, W.; et al. Late-onset sepsis in very low birth weight neonates: A report from the National Institute of Child Health and Human Development Neonatal Research Network. J. Pediatr. 1996, 129, 63–71. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD neonatal research network. Pediatrics 2002, 110, 285. [Google Scholar] [CrossRef]
- Cotten, C.M.; Taylor, S.; Stoll, B.; Goldberg, R.N.; Hansen, N.I.; Sánchez, P.J.; Ambalavanan, N.; Benjamin, D.K., Jr.; Network, N.N.R. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009, 123, 58–66. [Google Scholar] [CrossRef]
- Kuppala, V.S.; Meinzen-Derr, J.; Morrow, A.L.; Schibler, K.R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 2011, 159, 720–725. [Google Scholar] [CrossRef]
- Pharande, P.; Lindrea, K.B.; Smyth, J.; Evans, M.; Lui, K.; Bolisetty, S. Trends in late-onset sepsis in a neonatal intensive care unit following implementation of infection control bundle: A 15-year audit. J. Paediatr. Child Health 2018, 54, 1314–1320. [Google Scholar] [CrossRef]
- el Manouni el Hassani, S.; Berkhout, D.J.C.; Niemarkt, H.J.; Mann, S.; de Boode, W.P.; Cossey, V.; Hulzebos, C.V.; van Kaam, A.H.; Kramer, B.W.; van Lingen, R.A.; et al. Risk factors for late-onset sepsis in preterm infants: A multicenter case-control study. Neonatology 2019, 116, 42–51. [Google Scholar] [CrossRef]
- Kung, Y.-H.; Hsieh, Y.-F.; Weng, Y.-H.; Lien, R.-I.; Luo, J.; Wang, Y.; Huang, Y.-C.; Chen, C.-L.; Chen, C.-J. Risk factors of late-onset neonatal sepsis in Taiwan: A matched case-control study. J. Microbiol. Immunol. Infect. 2016, 49, 430–435. [Google Scholar] [CrossRef]
- Moro, G.E.; Arslanoglu, S.; Bertino, E.; Corvaglia, L.; Montirosso, R.; Picaud, J.-C.; Polberger, S.; Schanler, R.J.; Steel, C.; van Goudoever, J.; et al. XII. Human milk in feeding premature infants: Consensus statement. J. Pediatr. Gastroenterol. Nutr. 2015, 61, S16–S19. [Google Scholar] [CrossRef]
- Patel, A.L.; Johnson, T.J.; Engstrom, J.L.; Fogg, L.F.; Jegier, B.J.; Bigger, H.R.; Meier, P.P. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J. Perinatol. 2013, 33, 514–519. [Google Scholar] [CrossRef]
- Cortez, J.; Makker, K.; Kraemer, D.F.; Neu, J.; Sharma, R.; Hudak, M.L. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J. Perinatol. 2018, 38, 71–74. [Google Scholar] [CrossRef]
- Ashraf, R.N.; Jalil, F.; Zaman, S.; Karlberg, J.; Khan, S.R.; Lindblad, B.S.; Hanson, L.A. Breast feeding and protection against neonatal sepsis in a high risk population. Arch. Dis. Child. 1991, 66, 488–490. [Google Scholar] [CrossRef]
- Miller, J.; Tonkin, E.; Damarell, A.R.; McPhee, J.A.; Suganuma, M.; Suganuma, H.; Middleton, F.P.; Makrides, M.; Collins, T.C. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef]
- Meier, P.; Patel, A.; Esquerra-Zwiers, A. Donor human milk update: Evidence, mechanisms, and priorities for research and practice. J. Pediatr. 2017, 180, 15–21. [Google Scholar] [CrossRef]
- Widger, J.; O’Connell, N.H.; Stack, T. Breast milk causing neonatal sepsis and death. Clin. Microbiol. Infect. 2010, 16, 1796–1798. [Google Scholar] [CrossRef]
- Carl, M.A.; Ndao, I.M.; Springman, A.C.; Manning, S.D.; Johnson, J.R.; Johnston, B.D.; Burnham, C.A.; Weinstock, E.S.; Weinstock, G.M.; Wylie, T.N.; et al. Sepsis from the gut: The enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin. Infect. Dis. 2014, 58, 1211–1218. [Google Scholar] [CrossRef]
- Almuneef, M.A.; Baltimore, R.S.; Farrel, P.A.; Reagan-Cirincione, P.; Dembry, L.M. Molecular typing demonstrating transmission of gram-negative rods in a neonatal intensive care unit in the absence of a recognized epidemic. Clin. Infect. Dis. 2001, 32, 220–227. [Google Scholar] [CrossRef][Green Version]
- Graham, P.L., 3rd; Della-Latta, P.; Wu, F.; Zhou, J.; Saiman, L. The gastrointestinal tract serves as the reservoir for Gram-negative pathogens in very low birth weight infants. Pediatr. Infect. Dis. J. 2007, 26, 1153–1156. [Google Scholar] [CrossRef]
- Smith, A.; Saiman, L.; Zhou, J.; Della-Latta, P.; Jia, H.; Graham, P.L., 3rd. Concordance of gastrointestinal tract colonization and subsequent bloodstream infections with gram-negative bacilli in very low birth weight infants in the neonatal intensive care unit. Pediatr. Infect. Dis. J. 2010, 29, 831–835. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science (N. Y.) 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Persaud, R.R.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Sears, M.R.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: A prospective cohort study. BJOG 2016, 123, 983–993. [Google Scholar] [CrossRef]
- Knoop, K.A.; Gustafsson, J.K.; McDonald, K.G.; Kulkarni, D.H.; Coughlin, P.E.; McCrate, S.; Kim, D.; Hsieh, C.S.; Hogan, S.P.; Elson, C.O.; et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Foca, M.; Jakob, K.; Whittier, S.; Latta, P.D.; Factor, S.; Rubenstein, D.; Saiman, L. Endemic Pseudomonas aeruginosa Infection in a Neonatal Intensive Care Unit. N. Engl. J. Med. 2000, 343, 695–700. [Google Scholar] [CrossRef]
- Chen, H.N.; Lee, M.L.; Yu, W.K.; Lin, Y.W.; Tsao, L.Y. Late-onset Enterobacter cloacae sepsis in very-low-birth-weight neonates: Experience in a medical center. Pediatr. Neonatol. 2009, 50, 3–7. [Google Scholar] [CrossRef]
- Hornef, M. Pathogens, commensal symbionts, and pathobionts: discovery and functional effects on the host. ILAR J. 2015, 56, 159–162. [Google Scholar] [CrossRef]
- Kolter, J.; Henneke, P. Codevelopment of microbiota and innate immunity and the risk for Group B streptococcal disease. Front. Immunol. 2017, 8, 1497. [Google Scholar] [CrossRef]
- Singer, J.R.; Blosser, E.G.; Zindl, C.L.; Silberger, D.J.; Conlan, S.; Laufer, V.A.; DiToro, D.; Deming, C.; Kumar, R.; Morrow, C.D.; et al. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat. Med. 2019, 25, 1772–1782. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Zeng, M.Y. LOS in the dysbiotic gut. Cell Host Microbe 2020, 27, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, M.J.; Raskind, C.; Baltimore, R.S.; Gallagher, P.G. Seventy-Five years of neonatal sepsis at Yale: 1928–2003. Pediatrics 2005, 116, 595. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.K.; Scott, E.; Ilikj, M.; Bard, D.; Akins, D.R.; Dyer, D.W.; Chavez-Bueno, S. Route of infection alters virulence of neonatal septicemia Escherichia coli clinical isolates. PLoS ONE 2017, 12, e0189032. [Google Scholar] [CrossRef]
- Sekirov, I.; Tam, N.M.; Jogova, M.; Robertson, M.L.; Li, Y.; Lupp, C.; Finlay, B.B. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 2008, 76, 4726–4736. [Google Scholar] [CrossRef]
- Deshmukh, H.S.; Liu, Y.; Menkiti, O.R.; Mei, J.; Dai, N.; O’Leary, C.E.; Oliver, P.M.; Kolls, J.K.; Weiser, J.N.; Worthen, G.S. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 2014, 20, 524–530. [Google Scholar] [CrossRef]
- Vangay, P.; Ward, T.; Gerber, J.S.; Knights, D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 2015, 17, 553–564. [Google Scholar] [CrossRef]
- Bizzarro, M.J.; Dembry, L.-M.; Baltimore, R.S.; Gallagher, P.G. Changing patterns in neonatal Escherichia coli; sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics 2008, 121, 689. [Google Scholar] [CrossRef]
- Mohsen, L.; Ramy, N.; Saied, D.; Akmal, D.; Salama, N.; Abdel Haleim, M.M.; Aly, H. Emerging antimicrobial resistance in early and late-onset neonatal sepsis. Antimicrob. Resist. Infect. Control 2017, 6, 63. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407. [Google Scholar] [CrossRef]
- Garrido, D.; Barile, D.; Mills, D.A. A Molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv. Nutr. 2012, 3, 415S–421S. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Raffatellu, M. No vacancy: How beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 2015, 194, 4081. [Google Scholar] [CrossRef]
- Mugambi, M.N.; Musekiwa, A.; Lombard, M.; Young, T.; Blaauw, R. Probiotics, prebiotics infant formula use in preterm or low birth weight infants: A systematic review. Nutr. J. 2012, 11, 58. [Google Scholar] [CrossRef]
- Aceti, A.; Gori, D.; Barone, G.; Callegari, M.L.; Fantini, M.P.; Indrio, F.; Maggio, L.; Meneghin, F.; Morelli, L.; Zuccotti, G.; et al. Probiotics and time to achieve full enteral feeding in human milk-fed and formula-fed preterm infants: systematic review and meta-analysis. Nutrients 2016, 8, 471. [Google Scholar] [CrossRef]
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic supplementation and late-onset sepsis in preterm infants: A meta-analysis. Pediatrics 2016, 137, e20153684. [Google Scholar] [CrossRef]
- Kanic, Z.; Micetic Turk, D.; Burja, S.; Kanic, V.; Dinevski, D. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien. Klin. Wochenschr. 2015, 127, 210–215. [Google Scholar] [CrossRef]
- Sinha, A.; Gupta, S.S.; Chellani, H.; Maliye, C.; Kumari, V.; Arya, S.; Garg, B.S.; Gaur, S.D.; Gaind, R.; Deotale, V.; et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: A randomised controlled trial. BMJ Open 2015, 5, e006564. [Google Scholar] [CrossRef]
- Aceti, A.; Maggio, L.; Beghetti, I.; Gori, D.; Barone, G.; Callegari, M.L.; Fantini, M.P.; Indrio, F.; Meneghin, F.; Morelli, L.; et al. Probiotics prevent late-onset sepsis in human milk-fed, very low birth weight preterm infants: Systematic review and meta-analysis. Nutrients 2017, 9, 904. [Google Scholar] [CrossRef]
- Jakaitis, B.M.; Denning, P.W. Human breast milk and the gastrointestinal innate immune system. Clin. Perinatol. 2014, 41, 423–435. [Google Scholar] [CrossRef]
- Yeruva, L.; Spencer, N.E.; Saraf, M.K.; Hennings, L.; Bowlin, A.K.; Cleves, M.A.; Mercer, K.; Chintapalli, S.V.; Shankar, K.; Rank, R.G.; et al. Formula diet alters small intestine morphology, microbial abundance and reduces VE-cadherin and IL-10 expression in neonatal porcine model. BMC Gastroenterol. 2016, 16, 40. [Google Scholar] [CrossRef]
- Guaraldi, F.; Salvatori, G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef]
- Goldsmith, F.; O’Sullivan, A.; Smilowitz, J.T.; Freeman, S.L. Lactation and intestinal microbiota: How early diet shapes the infant gut. J. Mammary Gland Biol. Neoplasia 2015, 20, 149–158. [Google Scholar] [CrossRef]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and morbidity of gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef]
- Baumann-Dudenhoeffer, A.M.; D’Souza, A.W.; Tarr, P.I.; Warner, B.B.; Dantas, G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat. Med. 2018, 24, 1822–1829. [Google Scholar] [CrossRef]
- Taylor, S.N.; Basile, L.A.; Ebeling, M.; Wagner, C.L. Intestinal permeability in preterm infants by feeding type: Mother’s milk versus formula. Breastfeed. Med. 2009, 4, 11–15. [Google Scholar] [CrossRef]
- Saleem, B.; Okogbule-Wonodi, A.C.; Fasano, A.; Magder, L.S.; Ravel, J.; Kapoor, S.; Viscardi, R.M. Intestinal barrier maturation in very low birthweight infants: Relationship to feeding and antibiotic exposure. J. Pediatr. 2017, 183, 31–36.e31. [Google Scholar] [CrossRef]
- Go, L.L.; Albanese, C.T.; Watkins, S.C.; Simmons, R.L.; Rowe, M.I. Breast milk protects the neonate from bacterial translocation. J. Pediatr. Surg. 1994, 29, 1059–1064. [Google Scholar] [CrossRef]
- Yajima, M.; Nakayama, M.; Hatano, S.; Yamazaki, K.; Aoyama, Y.; Yajima, T.; Kuwata, T. Bacterial translocation in neonatal rats: The relation between intestinal flora, translocated bacteria, and influence of milk. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 592–601. [Google Scholar] [CrossRef]
- Nakayama, M.; Yajima, M.; Hatano, S.; Yajima, T.; Kuwata, T. Intestinal adherent bacteria and bacterial translocation in breast-fed and formula-fed rats in relation to susceptibility to infection. Pediatr. Res. 2003, 54, 364. [Google Scholar] [CrossRef]
- Goldsmith, S.J.; Dickson, J.S.; Barnhart, H.M.; Toledo, R.T.; Eiten-Miller, R.R. IgA, IgG, IgM and lactoferrin contents of human milk during early lactation and the effect of processing and storage. J. Food Prot. 1983, 46, 4–7. [Google Scholar] [CrossRef]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef]
- Bode, L.; Raman, A.S.; Murch, S.H.; Rollins, N.C.; Gordon, J.I. Understanding the mother-breastmilk-infant “triad”. Science 2020, 367, 1070. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, W.; Wu, M.; Song, X.; Caro, F.; Sun, X.; Gazzaniga, F.; Stefanetti, G.; Oh, S.; Mekalanos, J.J.; et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature 2020, 577, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, P.J.; Berger, J.E.; Meneses, J.; Phung, Y.; Pedersen, R.A.; Werb, Z.; Derynck, R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 1995, 376, 337–341. [Google Scholar] [CrossRef]
- Knoop, K.A.; Coughlin, P.E.; Floyd, A.N.; Ndao, I.M.; Hall-Moore, C.; Shaikh, N.; Gasparrini, A.J.; Rusconi, B.; Escobedo, M.; Good, M.; et al. Maternal activation of the EGFR prevents translocation of gut-residing pathogenic Escherichia coli in a model of late-onset neonatal sepsis. Proc. Natl. Acad. Sci. USA 2020. [Google Scholar] [CrossRef]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef]
- Van de Perre, P. Transfer of antibody via mother’s milk. Vaccine 2003, 21, 3374–3376. [Google Scholar] [CrossRef]
- Capasso, L.; Borrelli, A.; Cerullo, J.; Pisanti, R.; Figliuolo, C.; Izzo, F.; Paccone, M.; Ferrara, T.; Lama, S.; Raimondi, F. Role of immunoglobulins in neonatal sepsis. Transl. Med. Unisa 2014, 11, 28–33. [Google Scholar]
- Li, Y.; Yang, S.; Wang, G.; Liu, M.; Zhang, Z.; Liu, H.; Yu, K.; Wang, C. Effects of immunotherapy on mortality in neonates with suspected or proven sepsis: A systematic review and network meta-analysis. BMC Pediatr. 2019, 19, 270. [Google Scholar] [CrossRef]
- Koch Meghan, A.; Reiner Gabrielle, L.; Lugo Kyler, A.; Kreuk Lieselotte, S.M.; Stanbery Alison, G.; Ansaldo, E.; Seher Thaddeus, D.; Ludington William, B.; Barton Gregory, M. Maternal IgG and IgA antibodies dampen mucosal t helper cell responses in early life. Cell 2016, 165, 827–841. [Google Scholar] [CrossRef]
- Collins, A.; Weitkamp, J.-H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F391–F394. [Google Scholar] [CrossRef]
- Raymond, S.L.; López, M.C.; Baker, H.V.; Larson, S.D.; Efron, P.A.; Sweeney, T.E.; Khatri, P.; Moldawer, L.L.; Wynn, J.L. Unique transcriptomic response to sepsis is observed among patients of different age groups. PLoS ONE 2017, 12, e0184159. [Google Scholar] [CrossRef]
- Wynn, J.L.; Guthrie, S.O.; Wong, H.R.; Lahni, P.; Ungaro, R.; Lopez, M.C.; Baker, H.V.; Moldawer, L.L. Postnatal age is a critical determinant of the neonatal host response to sepsis. Mol. Med. 2015, 21, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Kobata, R.; Tsukahara, H.; Ohshima, Y.; Ohta, N.; Tokuriki, S.; Tamura, S.; Mayumi, M. High levels of growth factors in human breast milk. Early Hum. Dev. 2008, 84, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Michalsky, M.P.; Lara-Marquez, M.; Chun, L.; Besner, G.E. Heparin-binding EGF-like growth factor is present in human amniotic fluid and breast milk. J. Pediatr. Surg. 2002, 37, 1–6. [Google Scholar] [CrossRef]
- Nojiri, T.; Yoshizato, T.; Fukami, T.; Obama, H.; Yagi, H.; Yotsumoto, F.; Miyamoto, S. Clinical significance of amphiregulin and epidermal growth factor in colostrum. Arch. Gynecol. Obs. 2012, 286, 643–647. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Idota, T. The concentration of epidermal growth factor in Japanese mother’s milk. J. Nutr. Sci. Vitaminol. 1995, 41, 241–251. [Google Scholar] [CrossRef]
- Luetteke, N.C.; Qiu, T.H.; Fenton, S.E.; Troyer, K.L.; Riedel, R.F.; Chang, A.; Lee, D.C. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999, 126, 2739. [Google Scholar]
- Read, L.C.; Upton, F.M.; Francis, G.L.; Wallace, J.C.; Dahlenberg, G.W.; Ballard, F.J. Changes in the growth-promoting activity of human milk during lactation. Pediatr. Res. 1984, 18, 133–139. [Google Scholar] [CrossRef]
- Chang, C.-J.; Chao, J.C.-J. Effect of human milk and epidermal growth factor on growth of human intestinal Caco-2 cells. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, H.; Urao, M.; Lee, D.; Drongowski, R.A.; Coran, A.G. The effect of epidermal growth factor on bacterial translocation in newborn rabbits. J. Pediatr. Surg. 1998, 33, 225–228. [Google Scholar] [CrossRef]
- Good, M.; Sodhi, C.P.; Egan, C.E.; Afrazi, A.; Jia, H.; Yamaguchi, Y.; Lu, P.; Branca, M.F.; Ma, C.; Prindle, T., Jr.; et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, A.; Rueda, M.S.; Turin, C.G.; Ochoa, T.J. Factors affecting lactoferrin concentration in human milk: How much do we know? Biochem. Cell Biol. 2017, 95, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, R.; Chen, Q.; Wang, J.; Duan, Y.; Pang, X.; Jiang, S.; Bi, Y.; Zhang, H.; Lönnerdal, B.; et al. Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Czosnykowska-Łukacka, M.; Orczyk-Pawiłowicz, M.; Broers, B.; Królak-Olejnik, B. Lactoferrin in human milk of prolonged lactation. Nutrients 2019, 11, 2350. [Google Scholar] [CrossRef] [PubMed]
- Bullen, J.J.; Rogers, H.J.; Leigh, L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br. Med. J. 1972, 1, 69–75. [Google Scholar] [CrossRef]
- Sherman, M.P.; Miller, M.M.; Sherman, J.; Niklas, V. Lactoferrin and necrotizing enterocolitis. Curr. Opin. Pediatr. 2014, 26, 146–150. [Google Scholar] [CrossRef]
- Elass-Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-lipopolysaccharide interaction: Involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995, 312, 839–845. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Brouwer, C.P.; Bogaards, S.J.; Nemec, A.; Van Den Broek, P.J.; Nibbering, P.H. The synthetic N-terminal peptide of human lactoferrin, hLF (1-11), is highly effective against experimental infection caused by multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2004, 48, 4919–4921. [Google Scholar] [CrossRef]
- Sherman, M.P.; Adamkin, D.H.; Radmacher, P.G.; Sherman, J.; Niklas, V. Protective Proteins in mammalian milks. NeoReviews 2012, 13, e293. [Google Scholar] [CrossRef]
- He, Y.; Cao, L.; Yu, J. Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants: A PRISMA-compliant systematic review and meta-analysis. Med. (Baltim.) 2018, 97, e11976. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Sherman, J.; Arcinue, R.; Niklas, V. Randomized control trial of human recombinant lactoferrin: A substudy reveals effects on the fecal microbiome of very low birth weight infants. J. Pediatr. 2016, 173, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Zegarra, J.; Bellomo, S.; Carcamo, C.P.; Cam, L.; Castañeda, A.; Villavicencio, A.; Gonzales, J.; Rueda, M.S.; Turin, C.G.; et al. Randomized controlled trial of bovine lactoferrin for prevention of sepsis and neurodevelopment impairment in infants weighing less than 2000 grams. J. Pediatr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Militello, M.A.; Rizzollo, S.; Tavella, E.; Messina, A.; Pieretto, M.; Boano, E.; Carlino, M.; Tognato, E.; Spola, R.; et al. Is lactoferrin more effective in reducing late-onset sepsis in preterm neonates fed formula than in those receiving mother’s own milk? Secondary analyses of two multicenter randomized controlled trials. Am. J. Perinatol. 2019, 36, S120–S125. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Cheong, J.L.Y. Does bovine lactoferrin prevent late-onset neonatal sepsis? Lancet 2019, 393, 382–384. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Sakamoto, K.; Seo, S.-U.; Pickard, J.M.; Gillilland, M.G.; Pudlo, N.A.; Hoostal, M.; Li, X.; Wang, T.D.; Feehley, T.; et al. Neonatal acquisition of Clostridiaspecies protects against colonization by bacterial pathogens. Science 2017, 356, 315. [Google Scholar] [CrossRef]
- Feng, L.; Raman, A.S.; Hibberd, M.C.; Cheng, J.; Griffin, N.W.; Peng, Y.; Leyn, S.A.; Rodionov, D.A.; Osterman, A.L.; Gordon, J.I. Identifying determinants of bacterial fitness in a model of human gut microbial succession. Proc. Natl. Acad. Sci. USA 2020, 117, 2622. [Google Scholar] [CrossRef]
- Moossavi, S.; Atakora, F.; Miliku, K.; Sepehri, S.; Robertson, B.; Duan, Q.L.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Integrated analysis of human milk microbiota with oligosaccharides and fatty acids in the child cohort. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; García-Mantrana, I.; Bertua-Ríos, B.; Martínez-Costa, C.; Borsch, C.; Rudloff, S. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Engfer, M.B.; Stahl, B.; Finke, B.; Sawatzki, G.; Daniel, H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am. J. Clin. Nutr. 2000, 71, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Davis, J.C.C.; Kalanetra, K.M.; Gehlot, S.; Patole, S.; Tancredi, D.J.; Mills, D.A.; Lebrilla, C.B.; Simmer, K. Digestion of human milk oligosaccharides by bifidobacterium breve in the premature infant. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.L.; Doster, R.S.; Weitkamp, J.-H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. Human milk oligosaccharides exhibit antimicrobial and antibiofilm properties against group b streptococcus. ACS Infect. Dis. 2017, 3, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Li, B.; Koike, Y.; Määttänen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human milk oligosaccharides increase mucin expression in experimental necrotizing enterocolitis. Mol. Nutr. Food Res. 2019, 63, 1800658. [Google Scholar] [CrossRef]
- Cheng, L.; Kong, C.; Walvoort, M.T.C.; Faas, M.M.; de Vos, P. Human milk oligosaccharides differently modulate goblet cells under homeostatic, proinflammatory conditions and ER stress. Mol. Nutr. Food Res. 2020, 64, e1900976. [Google Scholar] [CrossRef]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb. Haemost. 2004, 92, 1402–1410. [Google Scholar] [CrossRef]
- Bode, L.; Rudloff, S.; Kunz, C.; Strobel, S.; Klein, N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J. Leukoc. Biol. 2004, 76, 820–826. [Google Scholar] [CrossRef]
- Noll, A.J.; Yu, Y.; Lasanajak, Y.; Duska-McEwen, G.; Buck, R.H.; Smith, D.F.; Cummings, R.D. Human DC-SIGN binds specific human milk glycans. Biochem. J. 2016, 473, 1343–1353. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 42–51. [Google Scholar] [CrossRef] [PubMed]
- Triantis, V.; Bode, L.; van Neerven, R.J.J. Immunological effects of human milk oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Meinzen-Derr, J.; Huang, P.; Schibler, K.R.; Cahill, T.; Keddache, M.; Kallapur, S.G.; Newburg, D.S.; Tabangin, M.; Warner, B.B.; et al. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J. Pediatr. 2011, 158, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Wejryd, E.; Martí, M.; Marchini, G.; Werme, A.; Jonsson, B.; Landberg, E.; Abrahamsson, T.R. Low diversity of human milk oligosaccharides is associated with necrotising enterocolitis in extremely low birth weight infants. Nutrients 2018, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Bering, S.B. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients 2018, 10, 1461. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Spooner, M.; Dallas, D.C. Differences in maternal immunoglobulins within mother’s own breast milk and donor breast milk and across digestion in preterm infants. Nutrients 2019, 11, 920. [Google Scholar] [CrossRef]
- Cossey, V.; Cossey, V.; Vanhole, C.; Eerdekens, A.; Rayyan, M.; Fieuws, S.; Schuermans, A. Pasteurization of mother’s own milk for preterm infants does not reduce the incidence of late-onset sepsis. Neonatology 2013, 103, 170–176. [Google Scholar] [CrossRef]
- Czank, C.; Prime, D.K.; Hartmann, B.; Simmer, K.; Hartmann, P.E. Retention of the immunological proteins of pasteurized human milk in relation to pasteurizer design and practice. Pediatr. Res. 2009, 66, 374–379. [Google Scholar] [CrossRef]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef]
- Baro, C.; Giribaldi, M.; Arslanoglu, S.; Giuffrida, M.G.; Dellavalle, G.; Conti, A.; Tonetto, P.; Biasini, A.; Coscia, A.; Fabris, C.; et al. Effect of two pasteurization methods on the protein content of human milk. Front. Biosci. (Elite Ed.) 2011, 3, 818–829. [Google Scholar] [CrossRef]
- Escuder-Vieco, D.; Espinosa-Martos, I.; Rodríguez, J.M.; Fernández, L.; Pallás-Alonso, C.R. Effect of HTST and holder pasteurization on the concentration of immunoglobulins, growth factors, and hormones in donor human milk. Front. Immunol. 2018, 9, 2222. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleist, S.A.; Knoop, K.A. Understanding the Elements of Maternal Protection from Systemic Bacterial Infections during Early Life. Nutrients 2020, 12, 1045. https://doi.org/10.3390/nu12041045
Kleist SA, Knoop KA. Understanding the Elements of Maternal Protection from Systemic Bacterial Infections during Early Life. Nutrients. 2020; 12(4):1045. https://doi.org/10.3390/nu12041045
Chicago/Turabian StyleKleist, Sierra A., and Kathryn A. Knoop. 2020. "Understanding the Elements of Maternal Protection from Systemic Bacterial Infections during Early Life" Nutrients 12, no. 4: 1045. https://doi.org/10.3390/nu12041045
APA StyleKleist, S. A., & Knoop, K. A. (2020). Understanding the Elements of Maternal Protection from Systemic Bacterial Infections during Early Life. Nutrients, 12(4), 1045. https://doi.org/10.3390/nu12041045





