Abstract
The effectiveness of biofeedback and neurofeedback has been investigated in a range of psychiatric disorders. However, to date, there are few studies on the clinical usefulness of feedback-based techniques for eating disorders (EDs) and EDs-related symptoms (e.g., food craving). A systematic search of PubMed, Scopus and PsychINFO identified 162 articles. Among these, thirteen studies exploring the therapeutic use of biofeedback and neurofeedback in EDs or EDs-related symptoms were included. Biofeedback and neurofeedback were implemented respectively in five and eight of all reviewed articles. No studies incorporated different feedback modalities or both biofeedback and neurofeedback. The considered studies provide preliminary data of the usefulness of feedback-based techniques in the treatment of several dysfunctional eating behaviors (e.g., food craving, rumination). Although no significant effect has been reported for other important EDs-related symptoms (i.e., body image disturbance), feedback-based techniques are also associated with significant modifications of both sympathetic reaction to food-related stimuli and brain activity in several regions of the reward system (e.g., insula). Taken together the results of the present review suggest that feedback-based treatments may be useful in the treatment of several dysfunctional eating behaviors operating both on top-down and bottom-up individual coping strategies. Methodological and clinical issues are also discussed.
1. Introduction
Eating disorders (EDs) are severe and disabling conditions caused by multiple factors (e.g., genetic and psychosocial) [1], which are associated with significant functional impairments [2], high mortality risk [3] and treatment difficulties [4]. The last edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [5] includes a substantially revised section on EDs [6,7], now named “Feeding and Eating Disorders”. This section includes the following diagnoses: pica, rumination disorder (RD), avoidant/restrictive food intake disorder (ARFID), anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder (BED), and other specified feeding or eating disorder (OSFED).
EDs and related symptoms are common in both children and adolescents [8,9]. In adults, the lifetime prevalence is about 0.6% for AN, 1% for BN, and 3% for BED [2,3]. Furthermore, it has been recently reported that, following the transition to DSM-5, the prevalence of OSFED has decreased, and that AN, BN and BED have increased in non-clinical samples [10]. Similarly, EDs-related symptoms, such as food craving (i.e., an intense desire to consume a specific food that is difficult to resist); Refs. [11,12,13] or loss of control over eating, are widely reported amongst the general population. For example, in a non-clinical sample it has been reported [14] that 28% of women and 13% of men reported food craving at least once a week during the past 6 months. This symptom is also commonly experienced in AN [15,16], BN [16,17,18,19], and BED [20,21]. Moreover, it has been reported that food craving severity is positively associated with both body mass index (BMI) [13,22,23,24,25], drop-out from weight loss programs [25,26,27], and a meta-analysis [28] on 3292 individuals showed that it significantly contributes to dysfunctional eating behaviours and weight gain.
Similarly, loss of control over eating is a relatively frequent experience among both adolescents from the general population (i.e., about 17%) [29], adults with obesity (i.e., BMI ≥ 30 kg/m2) and patients with EDs [30]. Additionally, it was positively associated with other psychopathological symptoms (e.g., non-suicidal self-injury, body dissatisfaction, higher depressive symptoms) [31].
Multidisciplinary approaches, combining medical, dietetic and psychological interventions are generally recommended in the treatment of EDs [32]. However, it is known that current treatments for EDs are often related to treatment difficulties [4] and poor long-term efficacy [1,33,34]. For example, it has been reported that several treatments, such as nutritional rehabilitation and psychological approaches (e.g., cognitive-behavioral therapy, family-based therapy), are associated with high relapse rates in adolescents with AN [33]. Similarly, binge eating remission rates at the end of treatments are only found in 30–40% of patients with BN [1]. Therefore, the necessity of additional treatment modalities for these mental disorders has been proposed [35].
Feedback-based treatments (i.e., biofeedback and neurofeedback) have been suggested as additional treatment modalities for EDs [36]. Feedback is considered a crucial component of mental health interventions, increasing motivation, facilitating the learning process and modifying dysfunctional thoughts and behaviors [37]. Over the last thirty years there has been an increasing number of disorders, including EDs, for which biofeedback and neurofeedback have been investigated with more or less empirically supported results [38,39].
Biofeedback and neurofeedback are operant-conditioning based trainings that allow individuals to learn how to regulate neurophysiological activity in response to real-time feedback, in order to improve health and performance [38]. Biofeedback refers to an intervention that helps individuals to control or change their physiologic functioning (e.g., heart rate, electrodermal activity, respiratory rate, muscle tension and peripheral temperature) [40]. Neurofeedback refers to a form of biofeedback involving different modalities, such as electroencephalography (EEG) or real-time functional magnetic resonance imaging (rt-fMRI), that trains individuals to control or modify their brain activity [41]. Compared to other neuro-stimulation techniques (i.e., transcranial direct current stimulation), which may be associated with several mild and transient adverse effects [42], feedback-based treatments are not related with side effects [43] and seems to be easy and affordable techniques for general practices and clinicians [44,45].
During a typical feedback-based session, neurophysiological activity (e.g., brain and or electrodermal activity) is “fed back” to the individual using a brain-computer interface, providing continuously updated information about their success in regulating their neurophysiological parameters [36]. The successful self-regulation of the individuals’ physiology represents an immediate and effective positive reinforcement, creating a positive loop between the machine’s feedback and the patient’s successful self-regulation [40]. Therefore the three necessary components for a feedback-based session are: (i) a therapist explaining the equipment and its use, (ii) a patient and (iii) a monitoring machine that provides accurate neurophysiological information [38]. Although training sessions and modalities may be different according to the individual needs and/or the diagnosis [40], general protocol guidelines are provided [38].
2. Study Rationale
The clinical efficacy of biofeedback and neurofeedback has been investigated in a range of psychiatric disorders [38,39]. However, to date, there are few studies on the clinical usefulness of feedback-based techniques for EDs or EDs-related symptoms. Therefore, the present systematic review (PRISMA checklist is reported in Table S1) was carried out to explore the current therapeutic use of biofeedback and neurofeedback in EDs and EDs-related symptoms (i.e., food craving, binge eating, loss of control over eating). Specifically, in this study we investigated: (i) the type of feedback-based protocol applied, and (ii) the usefulness of feedback-based techniques assessed through clinical scales and/or neurophysiological measures.
3. Methods
3.1. Inclusion/Exclusion Criteria
We used the P.I.C.O.S. (Population, Interventions, Comparators, Outcomes, and Study Design) criteria to identify relevant studies. This review focused on feedback-based interventions for EDs and related symptoms. Therefore, original articles reporting data from studies investigating the potential therapeutic effects of different biofeedback (i.e., heart rate, electrodermal activity, respiratory rate, muscle tension and peripheral temperature) and neurofeedback modalities (i.e., EEG, fMRI) in this field were initially considered. Studies involving children and adolescents were also taken into consideration. Book chapters, conference papers, reviews, dissertations, and case reports were not included. Articles in which either feedback-based treatments for EDs or EDs-related symptoms were not the focal point were also excluded. Moreover, feedback-based studies investigating EDs-related symptoms (i.e., food craving, binge eating, overeating) in non-clinical or subthreshold sample were initially considered. Both randomized and non-randomized controlled studies, as well as pre-/post-intervention comparison reports were considered.
According to guidelines for the evaluation of the clinical efficacy of psychophysiological interventions [46], as well as with previous systematic reviews focused of feedback-based treatments in psychiatric disorders [39,47], articles that provided the following information were included in the study: (i) feedback modality type, (ii) sample (i.e., including age, sex, medication status, BMI, recruitment), (iii) study design (including type, number and duration of protocol sessions, as well as description of study conditions), (iv) collection and analysis of neurophysiological (e.g., EEG power spectrum, electrodermal activity etc.), behavioral (e.g., number of binge episodes) and/or psychological outcomes measure (i.e., clinical scales). Furthermore, according to Schoenberg and David [39], studies that did not report 2 or more components of points (ii) and (iii), and/or articles that did not specify points (i), (iv) and (v) were excluded.
3.2. Search Strategy
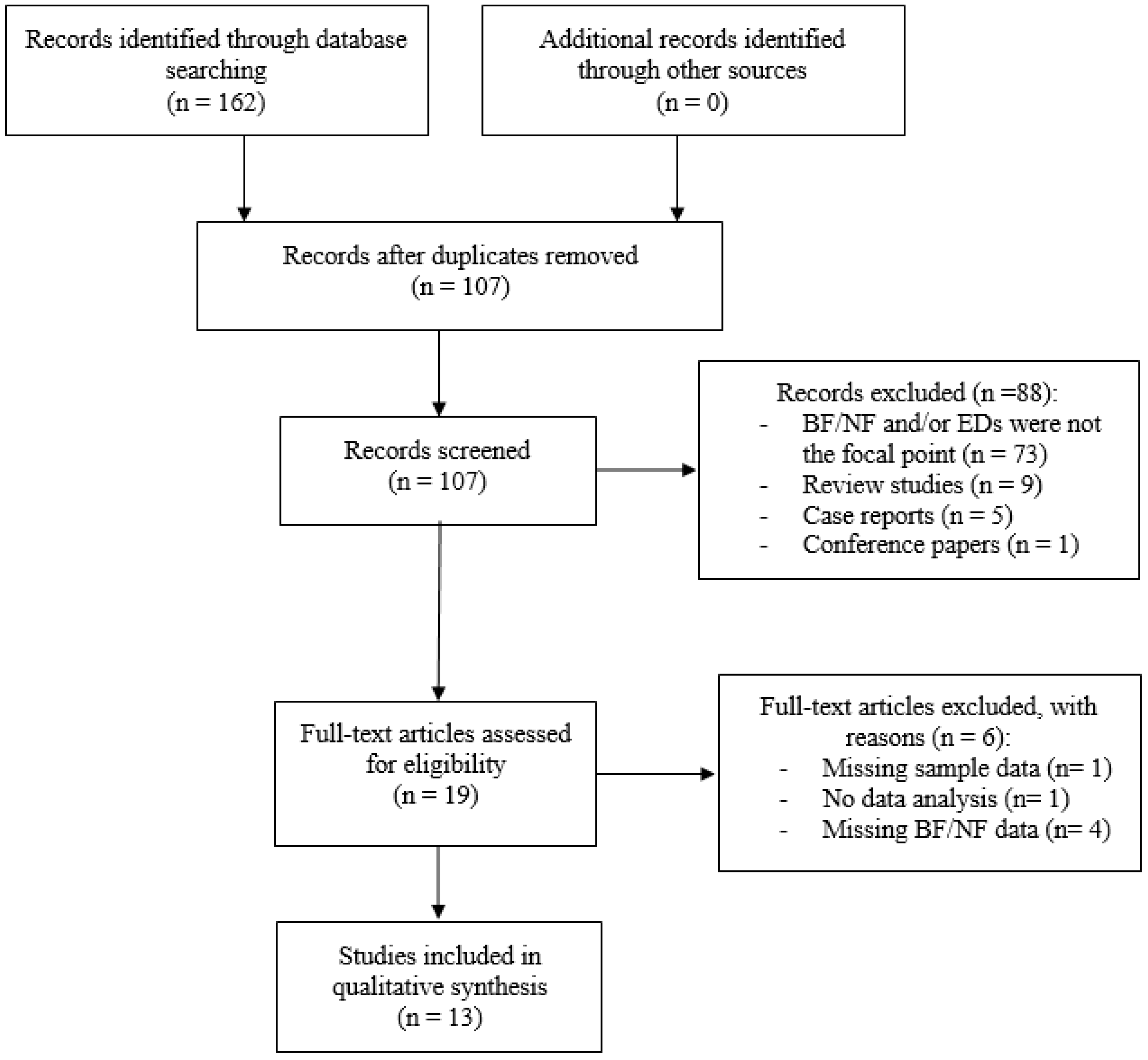
Potentially relevant studies were initially identified by searching publications from the year 1970 to 5 August 2018 through the following databases: PubMed, Scopus and PsychInfo. Only English articles were taken into account. The following search terms were entered into the databases: “biofeedback” OR “neurofeedback” AND “anorexia nervosa”, “bulimia nervosa”, “binge eating disorder”, “pica”, “rumination disorder”, “avoidant/restrictive food intake disorder”, “food intake disorder”, “food craving”, “binge eating”, “overeating”, “eating psychopathology”. Articles resulting from the search strategy were examined for relevance by screening titles and abstracts. Then, articles that appeared to meet inclusion criteria were further evaluated by two independent researchers (C.I. and M.M.) in order to assess all inclusion/exclusion criteria. In case of disagreement, a senior researcher (B.F.) resolved any discrepancies and decided whether or not the study was going to be included. A detailed flow diagram of selection of studies is reported in Figure 1.
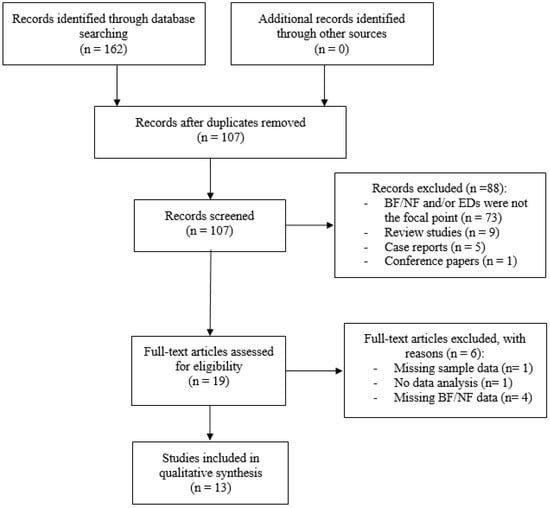
Figure 1.
Flow diagram of selection of studies. Abbreviation: BF = Biofeedback; NF = Neurofeedback; EDs = Eating Disorders.
4. Results
The initial search resulted in 162 articles. Duplicate articles were eliminated, leaving 107 screened studies. Of these articles, 19 met initial inclusion criteria and were assessed for eligibility. Six articles were subsequently excluded with reasons (e.g., missing information pertaining to methods/outcome measures, no description of biofeedback modality, no statistical analysis etc.).
Thirteen articles fulfilled the quality assessment and are considered in this review (Table 1).

Table 1.
Characteristics of the included studies (n = 13).
Biofeedback and neurofeedback were implemented respectively in five and eight of all reviewed articles. No studies incorporated different feedback modalities (e.g., electrodermal and electromyography biofeedback) or both biofeedback and neurofeedback. None of the studies investigated in this review reported relevant side effects for both biofeedback and neurofeedback training.
Mean number of sessions per study was 7.42 (range 1–12). Among reviewed article, three studies were focused on non-clinical samples [55,56,57], two on subthreshold samples [58,59], two on adolescents with AN [60,61], two on patients with RD [62,63] and 5 on overweight/obese individuals [61,64,65,66,67].
Eight studies were randomized [56,57,58,59,60,64,65,67], but only one was a double-blind trial [65]. Four studies did not have a control condition [55,61,63,66]. No treatment/waiting list was the most frequently (n = 7) control condition used [56,57,58,59,62,64,67], and only one study implemented a placebo condition (i.e., simulation protocol) [65] or an alternative treatment [59] (i.e., mental imagery).
Due to the high heterogeneity of samples, outcome measures and feedback modality, a meta-analysis was not performed.
4.1. Biofeedback Studies
Biofeedback was investigated in five of all reviewed articles. Of these, two studies implemented electrodermal biofeedback (ED-BF) [61,67], one heart rate variability biofeedback (HRV-BF) [57], one electromyography biofeedback (EMG-BF) [62], and one diaphragmatic breathing biofeedback (DB-BF) [63].
Barba et al. [62] and Halland et al., [63], investigated the usefulness of EMG-BF and DB-BF in patients with RD respectively. Both studies reported significant changes in clinical (e.g., decrease in regurgitation and rumination episodes) and physiological (e.g., decrease of intragastric pressure) outcomes.
Two studies examined the potential therapeutic effects of ED-BF in obese patients with overeating. Pop-Jordanova [61], in a pre-post study design, showed that 5 sessions of ED-BF were associated with a decrease of electrodermal activity in a sample of girls with obesity (n = 76) and AN (n = 27). In a sample of obese women (n = 30), Teufel et al. [67], through a randomized controlled study, reported that compared to a waiting list, both ED-BF focused on food stimuli and ED-BF focused on unspecific stimuli were associated with an increased ability to tolerate food-related stress (e.g., decrease of electrodermal activity and increase self-efficacy in dealing with food). Although these results remained stable after 6-months follow-up, no significant changes were observed in BMI and in ED-related symptoms (e.g., tendency to lose control of food intake).
Finally, in a study on non-clinical sample of cravers (n = 56), Meule et al., [57], reported that, compared to a waiting-list, 12 sessions of HRV-BF were associated with a decrease of food craving as well as with a decrease of eating and weight concerns. No significant changes were observed in physiological measures (e.g., HRV) and in other EDs-related symptoms (e.g., shape concern, binge frequency).
4.2. Neurofeedback Studies
Neurofeedback was investigated in eight of all reviewed articles. Of these, six studies implemented EEG [56,58,59,60,64,65] and two rt-fMRI [55,66].
Two randomized controlled studies investigated the usefulness of EEG beta training neurofeedback (i.e., decrease beta activity at Cz electrode) in female subthreshold samples of restrained eaters (n = 27) [58] and binge eaters (n = 57) [59]. Both studies reported that, compared to a waiting-list, 10 sessions of neurofeedback were associated with a decrease in overeating episodes and related distress. These results remained stable after 3-months follow-up.
Two randomized controlled studies investigated the effectiveness of EEG alpha/theta training neurofeedback (i.e., raise posterior theta over alpha amplitude with eyes closed without falling asleep) in reducing food craving, in a non-clinical sample (n = 50) [56] and in a sample of overweight women (n = 30) respectively [64]. It has been observed that, compared to a waiting-list, 10 sessions of neurofeedback were associated with a decrease of food craving severity [56,64] as well as with an improvement of mental health [64]. A significant increase of resting EEG alpha power in several brain areas involved in food craving (e.g., insula) and food cue reactivity (e.g., parahippocampal gyrus) was also documented [56]. Although changes in food craving persisted after 4-months follow-up, no significant modifications were reported in other EDs-related symptoms (e.g., weight and shape concerns) and in the general level of psychopathology [56].
Lackner et al. [60] examined the potential therapeutic effects of EEG alpha training neurofeedback (i.e., raise posterior alpha activity) in female adolescents with AN (n = 22). It has been observed that, compared to the standard treatment, 10 sessions of neurofeedback were associated with an improvement in several ED-related symptoms (e.g., decrease of restriction and dieting behavior) and with an increase of emotional competence. Although modifications of resting EEG power were also documented (i.e., increase of theta power), no significant modifications were reported in psychopathological symptoms (with the exception of interpersonal sensitivity), in BMI and in body image related symptoms.
Two studies investigated the usefulness of rt-fMRI neurofeedback during the exposure to appetitive food pictures, respectively in a non-clinical sample of women (n = 10) [55] and in overweight/obese men (n = 8) [66]. Both studies showed that rt-fMRI neurofeedback could modify brain activity [55] and brain connectivity [66] in crucial areas involved in the reward system (e.g., amygdala and prefrontal cortex). Although no significant improvement was reported in food craving or in calorie intake assessment, a decrease of subjective hunger [55] and negative mood [66] related to food stimuli was reported.
Finally, in the only double-blind, placebo-controlled neurofeedback study, Leong et al. [65], investigated the effectiveness of infraslow EEG neurofeedback (i.e., the modulation of slow wave activity (0–0.1 Hz)) in obese women with food addiction symptoms. Compared to placebo condition, it has been reported that 6 sessions of neurofeedback were associated with an increase of infraslow activity in the posterior cingulate cortex as well as with a decrease of state food craving.
4.3. Risk of Bias
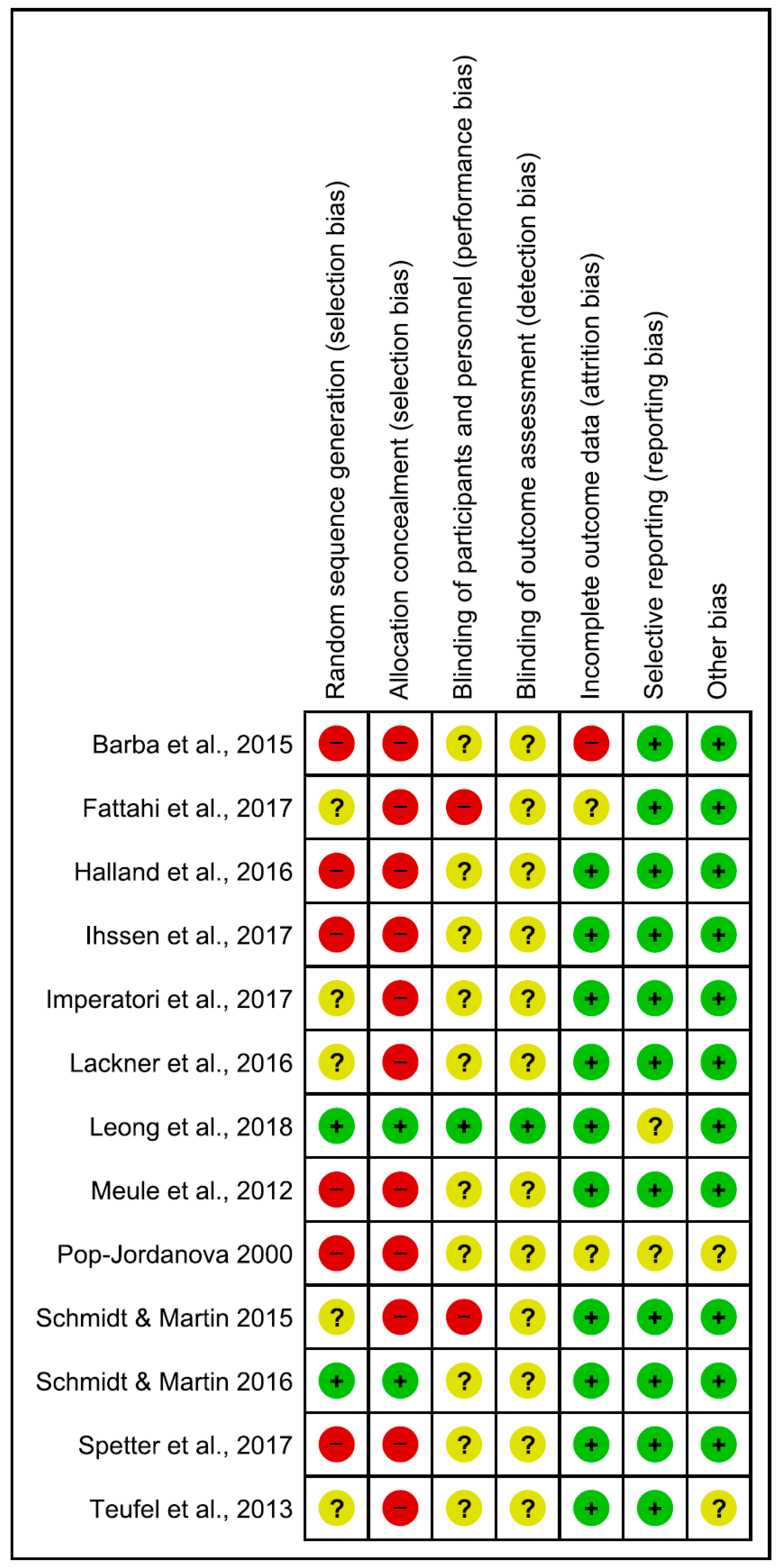
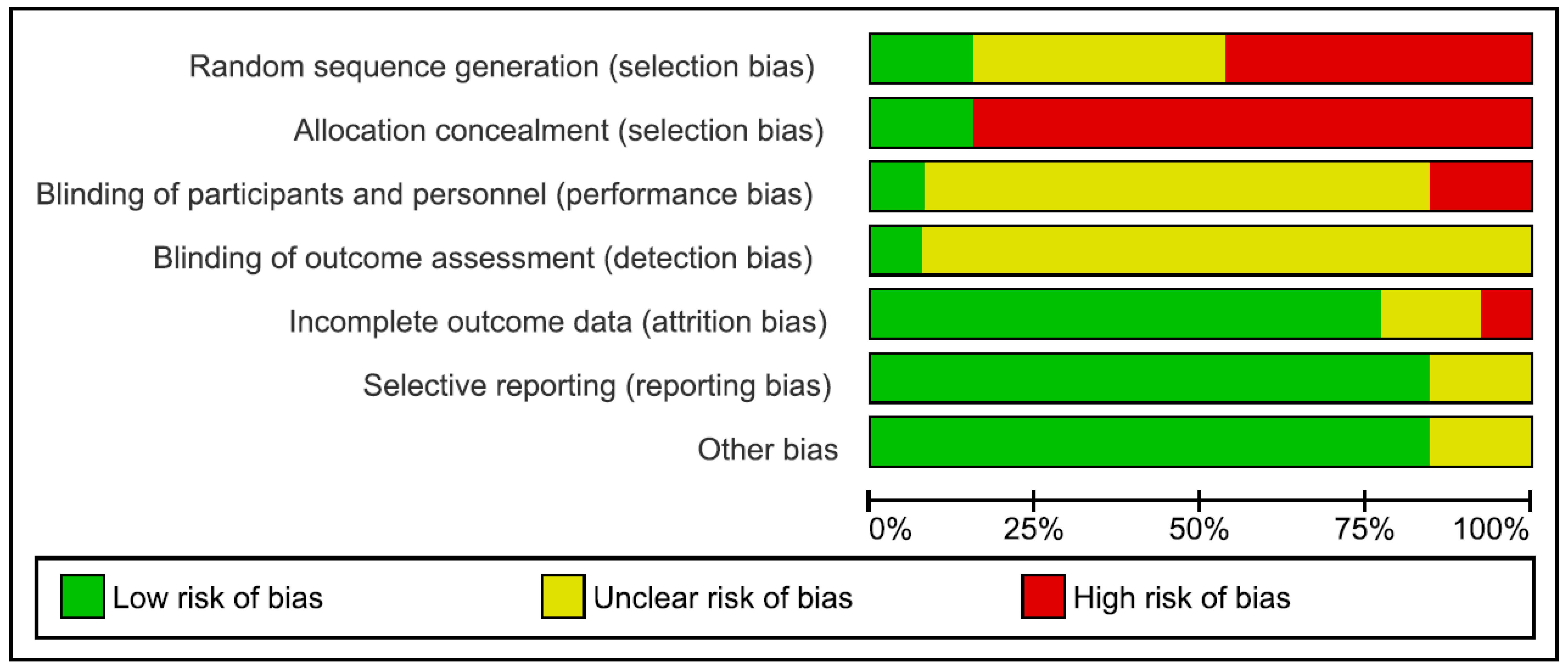
Risk of bias in the reviewed articles was performed according to Cochrane standards of practice [68]. Two reviewers (CI and GDM) independently assessed the risk for bias. In case of disagreement, a senior researcher (BF) resolved any discrepancies. Risk for bias was mainly related to the lack of both blinding and control conditions. Assessment of bias in the included studies are reported in Figure 2 and Figure 3.
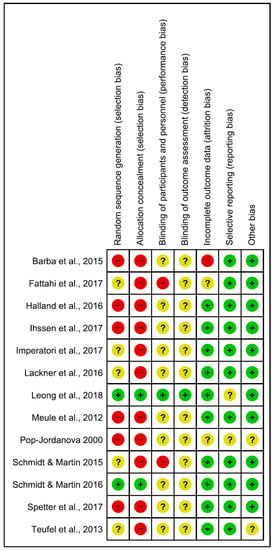
Figure 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. Green, yellow and red circles represent low, unclear and high risk of bias respectively.
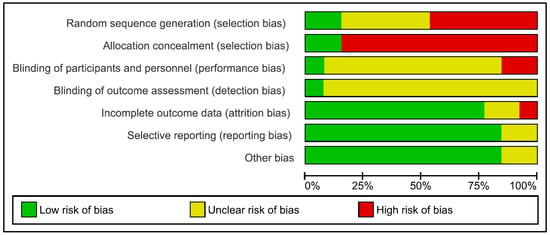
Figure 3.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
5. Discussion
The main aim of the present systematic review was to report how feedback-based treatment (i.e., biofeedback and neurofeedback) have been used in the treatment of EDs and EDs-related symptoms. To the best of our knowledge, this is the first systematic review that investigates both biofeedback and neurofeedback applications in this field.
The review identified 13 articles, five focused on biofeedback and eight focused on neurofeedback, providing preliminary data of the usefulness of feedback-based techniques in the treatment of several dysfunctional eating behaviors (Table 2). Specifically, it has been reported that both neurofeedback and biofeedback training may decrease food craving severity [56,57,58,64,65], overeating episodes [58,59], regurgitation [62] and rumination [63] episodes, restricting behavior [60], eating and weight concerns [57]. Furthermore, this review showed that feedback-based techniques are associated with significant modifications of both sympathetic reaction to food-related stimuli [61,62,63,67] and brain activity in several regions of the reward system (e.g., prefrontal cortex, amygdala, insula) [55,56,60,65,66].

Table 2.
Usefulness of feedback-based techniques for the treatment of dysfunctional eating behaviors.
These results seem to suggest that both biofeedback and neurofeedback increased the ability to better tolerate stress and the ability to cope with situations involving food. Indeed, the most common goal of feedback-based treatments implemented for EDs and EDs-related symptoms is the reduction of stressful arousal and the increase of top-down control abilities. For example, the aim of EEG alpha training [60] is to enhance individual alpha frequency, which is usually associated with alert relaxation [69], in the parietal area (i.e., Pz electrode). Similarly, the goal of EEG beta training [58,59] is to down-regulate beta activity, which is positively associated with ruminative states of stressful arousal [70]. Consistently, the aim of alpha/theta training is to raise posterior (i.e., Pz electrode) theta over alpha amplitude in order to produce a state of deep relaxation, enhancing top-down mental functions [71], such as mentalization [72]. Finally, an rt-fMRI neurofeedback has been implemented to increase functional connectivity between brain areas (i.e., dorsolateral prefrontal cortex and ventromedial prefrontal cortex) that regulate the top-down control of appetite for high-calorie foods [66].
Interestingly, it has also been suggested that the target of neurofeedback is to increase bottom-up strategies in order to decrease the salience attached to food [65], or down-regulate brain regions activation (i.e., amygdala) during exposure to food cues [55]. Therefore, taken together these data suggest that feedback-based treatments implemented for EDs and EDs-related symptoms can operate both on top-down and bottom-up strategies in order to foster the neural mechanisms underlying successful coping during stressful food-related situations.
On the other hand, this review showed no significant effect of feedback-based techniques in improving other important EDs-related symptoms, such as body image disturbance, a crucial core of AN and BN [5], which seems to affect also BED [73]. No significant modifications associated with both biofeedback and neurofeedback were also reported for BMI in both patients with AN and overweigh/obesity. These results confirm the need of multidisciplinary approaches, combining medical, dietetic and different kind of psychological interventions in the treatment of EDs and ED-related symptoms [32]. For example, combining EEG alpha training neurofeedback [60] with cognitive-behavioral exposure-based body image therapy [74] may be useful in the treatment of EDs.
It is also interesting to note that, compared to feedback-based interventions in other psychiatric disorders [39], no studies considered in the present review incorporated different biofeedback modalities or both biofeedback and neurofeedback. Therefore, it is possible that combining HRV-biofeedback [57] and EEG alpha/theta neurofeedback [56,64] may be more effective in reducing food craving severity in both clinical and non-clinical samples. Similarly, the combination of two neurofeedback training methods, such as EEG alpha/theta training and EEG beta training, or the combination of feedback-based techniques with other cognitive-behavioral techniques (e.g., cognitive restructuring, exposure therapy and response prevention) may be more effective in improving a wider range of ED-related symptoms (i.e., binge eating episodes, food craving).
Although the present review provided preliminary results of the usefulness of feedback-based techniques in the treatment of EDs and EDs-related symptoms, several methodological issue should be considered.
First, up to date, there are few randomized, double-blind, placebo-controlled studies investigating the effectiveness of both biofeedback and neurofeedback in large clinical samples. Indeed, among reviewed randomized trials (n = 8) the most frequent control condition implemented was the no treatment/waiting list, and only one study was double-blind. Consequently, most of the studies included in the present review had a high risk of bias, which was mostly related to the lack of both blinding and control conditions (e.g., mock feedback). This is in line with a recent systematic review investigating the potential therapeutic effects of neurofeedback training in psychiatric disorders associated with criminal offending [47]. Therefore, future studies should compare biofeedback and neurofeedback with sham procedures in order to rule out the placebo effect. Secondly, compared to feedback-based interventions in other psychiatric disorders [39], the mean number of sessions was relatively lower (i.e., 7.42). Although it has been reported that patients may benefit by undergoing 8 to 12 sessions [40], the possibility that a greater number of sessions (i.e., from 20 to 30) may maximize both biofeedback and neurofeedback results should be assessed. Furthermore, the stability of psychological/behavioral and neurophysiological (e.g., EEG power) outcomes should also be investigated taking into account long-term follow-up (i.e., at least one year). Finally, although the present review did not provide relevant differences associated with both age and gender, future studies should also investigate the relationship between feedback-based interventions outcomes (e.g., adherence to treatment, symptoms improvement) and several socio-demographic data (e.g. adolescent vs adults with EDs, and/or men vs women with EDs).
Study Limitations and Conclusions
There are several limitations of the present review that should be considered. Firstly, the search strategy was limited to articles published in English. Secondly, due to the high heterogeneity of samples, outcome measures and feedback modalities, a meta-analysis in order to quantify the effectiveness of both biofeedback and neurofeedback was not performed.
Despite these limitations, to the best of our knowledge, this is the first systematic review that investigates both biofeedback and neurofeedback applications in the treatment of EDs and EDs-related symptoms. In conclusion, the results of the present review suggest that, although future studies are needed in order to draw definitive conclusions, feedback-based techniques may be useful in the treatment of several dysfunctional eating behaviors (e.g., food craving, binge eating) operating both on top-down and bottom-up individual coping strategies.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/2072-6643/10/11/1806/s1, Table S1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Author Contributions
C.I.: study design, literature searches, data extraction, manuscript writing; M.M.: literature searches, data extraction, manuscript editing; G.D.M.: supervision, manuscript writing; E.M.V.: data extraction; B.F.: study design, supervision, manuscript writing.
Funding
This research was funded by Atena Onlus Foundation.
Acknowledgments
We thank the Atena Onlus Foundation for assistance and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Treasure, J.; Claudino, A.M.; Zucker, N. Eating disorders. Lancet 2010, 375, 583–593. [Google Scholar] [CrossRef]
- Hudson, J.I.; Hiripi, E.; Pope, H.G., Jr.; Kessler, R.C. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol. Psychiatry 2007, 61, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Smink, F.R.; Van Hoeken, D.; Hoek, H.W. Epidemiology of eating disorders: Incidence, prevalence and mortality rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.S.; Garfinkel, P.E. Difficulties in treating patients with eating disorders: A review of patient and clinician variables. Can. J. Psychiatry 1999, 44, 665–670. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-DSM-5, 5th ed.; American Psychiatric Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- Erzegovesi, S.; Bellodi, L. Eating disorders. CNS Spectr. 2016, 21, 304–309. [Google Scholar] [PubMed]
- Hoek, H.W. Classification, epidemiology and treatment of dsm-5 feeding and eating disorders. Curr. Opin. Psychiatry 2013, 26, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Benjasuwantep, B.; Chaithirayanon, S.; Eiamudomkan, M. Feeding problems in healthy young children: Prevalence, related factors and feeding practices. Pediatr. Rep. 2013, 5, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.A.; Crow, S.J.; Le Grange, D.; Swendsen, J.; Merikangas, K.R. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch. Gen. Psychiatry 2011, 68, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Lindvall Dahlgren, C.; Wisting, L.; Ro, O. Feeding and eating disorders in the dsm-5 era: A systematic review of prevalence rates in non-clinical male and female samples. J. Eat. Disord. 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, H.P.; Elston, D. The phenomenology of food cravings. Appetite 1990, 15, 231–246. [Google Scholar] [CrossRef]
- Weingarten, H.P.; Elston, D. Food cravings in a college population. Appetite 1991, 17, 167–175. [Google Scholar] [CrossRef]
- White, M.A.; Whisenhunt, B.L.; Williamson, D.A.; Greenway, F.L.; Netemeyer, R.G. Development and validation of the food-craving inventory. Obes. Res. 2002, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lafay, L.; Thomas, F.; Mennen, L.; Charles, M.A.; Eschwege, E.; Borys, J.M.; Basdevant, A. Gender differences in the relation between food cravings and mood in an adult community: Results from the fleurbaix laventie ville sante study. Int. J. Eat. Disord. 2001, 29, 195–204. [Google Scholar] [CrossRef]
- Gendall, K.A.; Sullivan, P.F.; Joyce, P.R.; Bulik, C.M. Food cravings in women with a history of anorexia nervosa. Int. J. Eat. Disord. 1997, 22, 403–409. [Google Scholar] [CrossRef]
- Moreno, S.; Rodriguez, S.; Fernandez, M.C.; Tamez, J.; Cepeda-Benito, A. Clinical validation of the trait and state versions of the food craving questionnaire. Assessment 2008, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Van der Ster Wallin, G.; Norring, C.; Holmgren, S. Binge eating versus nonpurged eating in bulimics: Is there a carbohydrate craving after all? Acta Psychiatr. Scand. 1994, 89, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.; Hill, A.; Waller, G. Bulimics’ responses to food cravings: Is binge-eating a product of hunger or emotional state? Behav. Res. Ther. 2001, 39, 877–886. [Google Scholar] [CrossRef]
- Moreno, S.; Warren, C.S.; Rodriguez, S.; Fernandez, M.C.; Cepeda-Benito, A. Food cravings discriminate between anorexia and bulimia nervosa. Implications for “success” versus “failure” in dietary restriction. Appetite 2009, 52, 588–594. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Grilo, C.M. Psychometric properties of the food craving inventory among obese patients with binge eating disorder. Eat. Behav. 2005, 6, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Fabbricatore, M.; Imperatori, C.; Pecchioli, C.; Micarelli, T.; Contardi, A.; Tamburello, S.; Innamorati, M.; Tamburello, A. Binge eating and bis/bas activity in obese patients with intense food craving who attend weight control programs. Obes. Metab. 2011, 7, e21–e27. [Google Scholar]
- Delahanty, L.M.; Meigs, J.B.; Hayden, D.; Williamson, D.A.; Nathan, D.M. Diabetes Prevenion Program Research Group. Psychological and behavioral correlates of baseline bmi in the diabetes prevention program (DPP). Diabetes Care 2002, 25, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Franken, I.H.; Muris, P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite 2005, 45, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Hermann, T.; Kubler, A. A short version of the food cravings questionnaire-trait: The fcq-t-reduced. Front. Psychol. 2014, 5, 190. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Lutz, A.; Vogele, C.; Kubler, A. Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the food cravings questionnaires in german. Appetite 2012, 58, 88–97. [Google Scholar] [PubMed]
- Meule, A.; Westenhofer, J.; Kubler, A. Food cravings mediate the relationship between rigid, but not flexible control of eating behavior and dieting success. Appetite 2011, 57, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Sitton, S.C. Role of craving for carbohydrates upon completion of a protein-sparing fast. Psychol. Rep. 1991, 69, 683–686. [Google Scholar] [PubMed]
- Boswell, R.G.; Kober, H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes. Rev. 2016, 17, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Goossens, L.; Soenens, B.; Braet, C. Prevalence and characteristics of binge eating in an adolescent community sample. J. Clin. Child Adolesc. Psychol. 2009, 38, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Heriseanu, A.I.; Hay, P.; Corbit, L.; Touyz, S. Grazing in adults with obesity and eating disorders: A systematic review of associated clinical features and meta-analysis of prevalence. Clin. Psychol. Rev. 2017, 58, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, A.B.; Loth, K.A.; MacLehose, R.F.; Pisetsky, E.M.; Berge, J.M.; Neumark-Sztainer, D. Overeating with and without loss of control: Associations with weight status, weight-related characteristics, and psychosocial health. Int. J. Eat. Disord. 2015, 48, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.; Chinn, D.; Forbes, D.; Madden, S.; Newton, R.; Sugenor, L.; Touyz, S.; Ward, W. Royal australian and new zealand college of psychiatrists clinical practice guidelines for the treatment of eating disorders. Aust. N. Z. J. Psychiatry 2014, 48, 977–1008. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B. Treatment of eating disorders in child and adolescent psychiatry. Curr. Opin. Psychiatry 2017, 30, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Amianto, F.; Ottone, L.; Abbate Daga, G.; Fassino, S. Binge-eating disorder diagnosis and treatment: A recap in front of dsm-5. BMC Psychiatry 2015, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Elias, G.J.B.; Lozano, A.M. Neuromodulation for the treatment of eating disorders and obesity. Ther. Adv. Psychopharmacol. 2018, 8, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Bartholdy, S.; Musiat, P.; Campbell, I.C.; Schmidt, U. The potential of neurofeedback in the treatment of eating disorders: A review of the literature. Eur. Eat. Disord. Rev. 2013, 21, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Musiat, P.; Hoffmann, L.; Schmidt, U. Personalised computerised feedback in e-mental health. J. Ment. Health 2012, 21, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Yucha, C.; Montgomery, D. Evidence-Based Practice in Biofeedback and Neurofeedback; Association for Applied Psychophysiology and Biofeedback: Wheat Ridge, CO, USA, 2008. [Google Scholar]
- Schoenberg, P.L.; David, A.S. Biofeedback for psychiatric disorders: A. systematic review. Appl. Psychophysiol. Biofeedback 2014, 39, 109–135. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.G. Biofeedback: An overview in the context of heart-brain medicine. Clevel. Clin. J. Med. 2008, 75, S31–S34. [Google Scholar] [CrossRef]
- Weiskopf, N. Real-time fmri and its application to neurofeedback. Neuroimage 2012, 62, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Lansbergen, M.M.; Van Dongen-Boomsma, M.; Buitelaar, J.K.; Slaats-Willemse, D. Adhd and eeg-neurofeedback: A double-blind randomized placebo-controlled feasibility study. J. Neural Transm. (Vienna) 2011, 118, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R.W. Advances in assessment and treatment of ADHD using network analyses. Biofeedback 2014, 42, 58–67. [Google Scholar] [CrossRef]
- Sherlin, L.H.; Arns, M.; Lubar, J.; Heinrich, H.; Kerson, C.; Strehl, U.; Sterman, M.B. Neurofeedback and basic learning theory: Implications for research and practice. J. Neurother. 2011, 15, 292–304. [Google Scholar] [CrossRef]
- La Vaque, T.J.; Hammond, D.C.; Trudeau, D.; Monastra, V.; Perry, J.; Lehrer, P.; Matheson, D.; Sherman, R. Template for developing guidelines for the evaluation of the clinical efficacy of psychophysiological interventions. Appl. Psychophysiol. Biofeedback 2002, 27, 273–281. [Google Scholar] [CrossRef]
- Fielenbach, S.; Donkers, F.C.L.; Spreen, M.; Visser, H.A.; Bogaerts, S. Neurofeedback training for psychiatric disorders associated with criminal offending: A. review. Front. Psychiatry 2017, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Cepeda-Benito, A.; Gleaves, D.H.; Williams, T.L.; Erath, S.A. The development and validation of the state and trait food-cravings questionnaires. Behav. Ther. 2000, 31, 151–173. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Beglin, S.J. Assessment of eating disorders: Interview or self-report questionnaire? Int. J. Eat. Disord. 1994, 16, 363–370. [Google Scholar] [PubMed]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale food addiction scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Legenbauer, T.; Vocks, S.; Schütt-Strömel, S. Dysfunctional cognitions in eating disorders: Which contents areas can be discriminated? J. Clin. Psychol. Psychother. 2007, 36, 207–215. [Google Scholar]
- Rosen, J.C.; Srebnik, D.; Saltzberg, E.; Wendt, S. Development of a body image avoidance questionnaire. Psychol. Assess. 1991, 3, 32–37. [Google Scholar] [CrossRef]
- Nijs, I.M.; Franken, I.H.; Muris, P. The modified trait and state food-cravings questionnaires: Development and validation of a general index of food craving. Appetite 2007, 49, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, N.; Sokunbi, M.O.; Lawrence, A.D.; Lawrence, N.S.; Linden, D.E.J. Neurofeedback of visual food cue reactivity: A potential avenue to alter incentive sensitization and craving. Brain Imaging Behav. 2017, 11, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, C.; Valenti, E.M.; Della Marca, G.; Amoroso, N.; Massullo, C.; Carbone, G.A.; Maestoso, G.; Quintiliani, M.I.; Contardi, A.; Farina, B. Coping food craving with neurofeedback. Evaluation of the usefulness of alpha/theta training in a non-clinical sample. Int. J. Psychophysiol. 2017, 112, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Freund, R.; Skirde, A.K.; Vogele, C.; Kubler, A. Heart rate variability biofeedback reduces food cravings in high food cravers. Appl. Psychophysiol. Biofeedback 2012, 37, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Martin, A. Neurofeedback reduces overeating episodes in female restrained eaters: A randomized controlled pilot-study. Appl. Psychophysiol. Biofeedback 2015, 40, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Martin, A. Neurofeedback against binge eating: A randomized controlled trial in a female subclinical threshold sample. Eur. Eat. Disord. Rev. 2016, 24, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Lackner, N.; Unterrainer, H.F.; Skliris, D.; Shaheen, S.; Dunitz-Scheer, M.; Wood, G.; Scheer, P.J.; Wallner-Liebmann, S.J.; Neuper, C. Eeg neurofeedback effects in the treatment of adolescent anorexia nervosa. Eat. Disord. 2016, 24, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Pop-Jordanova, N. Psychological characteristics and biofeedback mitigation in preadolescents with eating disorders. Pediatr. Int. 2000, 42, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Barba, E.; Burri, E.; Accarino, A.; Malagelada, C.; Rodriguez-Urrutia, A.; Soldevilla, A.; Malagelada, J.R.; Azpiroz, F. Biofeedback-guided control of abdominothoracic muscular activity reduces regurgitation episodes in patients with rumination. Clin. Gastroenterol. Hepatol. 2015, 13, 100–106.e1. [Google Scholar] [CrossRef] [PubMed]
- Halland, M.; Parthasarathy, G.; Bharucha, A.E.; Katzka, D.A. Diaphragmatic breathing for rumination syndrome: Efficacy and mechanisms of action. Neurogastroenterol. Motil. 2016, 28, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Naderi, F.; Asgari, P.; Ahadi, H. Neuro-feedback training for overweight women: Improvement of food craving and mental health. NeuroQuantology 2017, 15, 232–238. [Google Scholar] [CrossRef]
- Leong, S.L.; Vanneste, S.; Lim, J.; Smith, M.; Manning, P.; De Ridder, D.A. Randomised, double-blind, placebo-controlled parallel trial of closed-loop infraslow brain training in food addiction. Sci. Rep. 2018, 8, 11659. [Google Scholar] [CrossRef] [PubMed]
- Spetter, M.S.; Malekshahi, R.; Birbaumer, N.; Luhrs, M.; Van der Veer, A.H.; Scheffler, K.; Spuckti, S.; Preissl, H.; Veit, R.; Hallschmid, M. Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real-time fmri feedback study. Appetite 2017, 112, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Teufel, M.; Stephan, K.; Kowalski, A.; Kasberger, S.; Enck, P.; Zipfel, S.; Giel, K.E. Impact of biofeedback on self-efficacy and stress reduction in obesity: A randomized controlled pilot study. Appl. Psychophysiol. Biofeedback 2013, 38, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Marzbani, H.; Marateb, H.R.; Mansourian, M. Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 2016, 7, 143–158. [Google Scholar] [PubMed]
- Andersen, S.B.; Moore, R.A.; Venables, L.; Corr, P.J. Electrophysiological correlates of anxious rumination. Int. J. Psychophysiol. 2009, 71, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Gruzelier, J.H. Eeg-neurofeedback for optimising performance. Ii: Creativity, the performing arts and ecological validity. Neurosci. Biobehav. Rev. 2014, 44, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, C.; Della Marca, G.; Amoroso, N.; Maestoso, G.; Valenti, E.M.; Massullo, C.; Carbone, G.A.; Contardi, A.; Farina, B. Alpha/theta neurofeedback increases mentalization and default mode network connectivity in a non-clinical sample. Brain Topogr. 2017, 30, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Lewer, M.; Bauer, A.; Hartmann, A.S.; Vocks, S. Different facets of body image disturbance in binge eating disorder: A review. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Lewer, M.; Kosfelder, J.; Michalak, J.; Schroeder, D.; Nasrawi, N.; Vocks, S. Effects of a cognitive-behavioral exposure-based body image therapy for overweight females with binge eating disorder: A pilot study. J. Eat. Disord. 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).