Highlights
What are the main findings?
- Six vegetation indices were optimized, and a cross-scale monitoring method for wheat stripe rust was proposed.
- By integrating an unmanned aerial vehicle (UAV) hyperspectral system with the optimized indices, the impact of spectral fluctuations was effectively controlled, improving monitoring accuracy.
What are the implications of the main findings?
- This study provides key technical support for the practical application of hyperspectral technology in field monitoring of wheat stripe rust, addressing limitations of traditional methods such as low efficiency and significant spectral interference
- The proposed indicator optimization strategy and cross-scale framework can be extended to monitoring other crop diseases, promoting the development of precision agriculture.
Abstract
Wheat stripe rust is an important fungal disease that threatens global wheat production, and precise monitoring in field environments is crucial for disease prevention and control. This study proposes a cross-scale monitoring method based on optimized hyperspectral vegetation index to address the issues of low efficiency of traditional monitoring methods and susceptibility of spectral signals to interference in field environments. Through comparative studies between experimental fields (n = 68) and large fields (n = 155), the performance of six vegetation indices was systematically evaluated, and optimized versions were designed. The study mainly found that the Yellow Rust Severity Index optimized (YRSIO) index exhibited the best monitoring performance, with a field determination coefficient R2 of 0.5713 (experimental field R2 = 0.6118). The unmanned aerial vehicle (UAV) hyperspectral system combined with optimized vegetation index can effectively control spectral reflectance fluctuations, with a recognition accuracy of up to 85.2% in severely infected areas. This study also elucidated the three-stage physiological response mechanism of optimizing indicators on disease progression. This study provides key technical support for the practical application of hyperspectral technology in field monitoring of wheat stripe rust, and the proposed research method can be extended to other fields of crop disease monitoring.
1. Introduction
Crop diseases and pests, as a predominant biotic constraint in agricultural production, exhibit high biodiversity, substantial economic impacts, and epidemic-prone characteristics. These pathogenic and entomological stressors not only compromise crop yield and quality but also constitute a critical bottleneck for achieving sustainable agricultural intensification. In severe outbreak years, wheat stripe rust can reduce wheat yield by more than 40% [,]. With climate change and the continuous emergence of new strains of pathogens, the prevalence of this disease continues to expand, posing a serious threat to global food security []. Traditional disease monitoring mainly relies on manual field investigations, which have shortcomings such as low efficiency, strong subjectivity, and limited coverage []. In this context, the development of rapid, accurate, and non-destructive disease monitoring technology has become a research hotspot in the field of agricultural remote sensing.
Recent advancements in hyperspectral remote sensing have revolutionized agricultural pest and disease monitoring, effectively addressing the persistent challenges of delayed detection and suboptimal accuracy in crop protection management. Hyperspectral remote sensing technology demonstrates unique advantages in early disease detection through its integrated mapping capability, which precisely records subtle spectral variations within the 400–2500 nm range induced by pathogen infection in crops []. Compared with multispectral remote sensing, hyperspectral data contains more continuous and refined spectral information, which can identify the absorption and reflection characteristics unique to diseases []. Especially with the rapid development of unmanned aerial vehicle (UAV) hyperspectral platforms (with spatial resolution up to centimeter level), accurate disease monitoring at the field scale has become possible [].
Building upon these hyperspectral capabilities, controlled-environment studies have systematically cataloged spectral signatures corresponding to progressive wheat stripe rust infection stages. Early research focused on the visible near-infrared region (400–1000 nm) and found that diseases can cause a decrease in chlorophyll absorption characteristics (around 680 nm) and a blue shift phenomenon in the red edge (700–750 nm) []. As the spectral range expanded to short wave infrared, researchers further identified characteristic bands related to pathogen spore production []. In previous studies, vegetation indices performed well in monitoring wheat stripe rust. For example, Zheng et al. used the newly established photochemical reflectance index (PRI) at 570 nm, 525 nm, and 705 nm, as well as the anthocyanin reflectance index (ARI) based on 860 nm, to identify yellow rust at 790 nm and 750 nm []. Huang et al. developed a new vegetation index specifically for wheat yellow rust disease called the yellow rust spore index (YRSI), the results showed that the proposed indicators were very suitable for the identification and differentiation of yellow rust []. In addition, scholars have proposed vegetation indices for various specific diseases, such as the Rust Index (RI) [] and Modified Chlorophyll Absorption Ratio Index (MCARI) []. Despite the utility of disease-specific indices like RI and MCARI, these single-variable metrics often fail to capture complex spectral patterns associated with overlapping disease symptoms or subtle physiological changes in plants. To address this limitation, machine learning methods have emerged as powerful tools for integrating multi-dimensional spectral, temporal, or phenotypic data, enabling more robust pattern recognition and reducing reliance on handcrafted indices []. Among these, ensemble methods like random forest (RF) offer superior performance in handling non-linear relationships and high-dimensional agricultural data while providing feature importance insights. Consistent with these advances, the introduction of machine learning methods significantly improved classification accuracy, with RF achieving a recognition accuracy of 92% in the experimental field []. Especially for wheat stripe rust, RF has been proven to be useful for precise remote monitoring and management of the disease [,]. In addition, multiple studies have demonstrated the robustness of RF to multicollinearity in hyperspectral datasets and its ability to prioritize disease sensitive bands [].
However, when these methods were transplanted from experimental fields to real field environments, the accuracy generally decreased by 30–50% []. Three primary interference factors were identified: (1) soil background heterogeneity, particularly under low vegetation coverage where soil signals may contribute up to 40% of the total spectral response []; (2) meteorological variability, furthermore, induces up to 15% spectral variation due to illumination changes from dynamic cloud conditions []; (3) notably, inter-cultivar differences in healthy wheat leaves can exceed disease-induced spectral variations []. Currently, most research is still in the stage of single factor correction and lacks systematic solutions []. This critical knowledge gap highlights the necessity to develop adaptive spectral compensation frameworks capable of dynamically addressing soil-background interference, meteorological variability, and cultivar-specific spectral responses, thereby achieving reliable transfer of wheat stripe rust monitoring from controlled experiments to operational field applications through hyperspectral remote sensing.
Recently, research has begun to focus on multi-source data fusion and new index design [,,,,,]. The collaborative use of UAV and satellite platforms can accommodate high spatiotemporal resolution [], while dynamic calibration models combined with meteorological data can reduce environmental noise []. For spectral index optimization, both conventional methods (e.g., successive projections algorithm, SPA) and deep learning-based feature extraction demonstrate superior performance in identifying disease-sensitive spectral features []. It is particularly noteworthy that indices targeting specific physiological processes of diseases, such as spore production related indices, are more specific than general vegetation indices []. These integrated approaches collectively represent a transformative leap in hyperspectral monitoring systems, achieving the crucial balance between scientific precision and field applicability necessary to convert experimental insights into practical agricultural intelligence.
This study aims to bridge the translational gap between experimental plots and commercial-scale fields by addressing three critical challenges: (1) developing a physiology-informed vegetation index that accounts for pathogen behavior and environmental resilience; (2) systematically quantifying the interference mechanisms of soil background and phenological stages on spectral signatures; (3) establishing a transferable monitoring model adaptable to diverse agronomic conditions. The research results will promote the practical application of hyperspectral technology from the laboratory, providing core technical support for disease early warning systems in smart agriculture []. From a broader perspective, the methodological framework developed in this study can be extended to other crop disease monitoring, which has important practical value for achieving sustainable agricultural production in line with the United Nations Sustainable Development Goals [,].
2. Materials and Methods
2.1. Experimental Sites and Experimental Design
The study comprised two parallel experiments: a controlled trial in experimental wheat plots (Experiment 1) and a validation campaign in commercial wheat fields (Experiment 2). High spectral data of wheat canopy, severity of wheat stripe rust disease, and visible light data from UAV was collected in May 2022.
Experiment 1 was conducted at the experimental base of Shandong Academy of Agricultural Sciences, located in Jinan City, Shandong Province, China (116°58′43″E 36°59′1″N). The annual average temperature in the area was 14.2 °C, the average annual precipitation in the past five years was 685 mm, the average annual relative humidity was 66%, and the frequency of spring drought exceeds 43%. During the 2022 wheat growing season, we performed spore inoculation on wheat cultivar Luyuan 502 to simulate natural infection of stripe rust, adhering to the Chinese agricultural standard NY/T 1443.1–2007 [] for wheat disease resistance evaluation.
Experiment 2 was conducted after discovering natural infection of stripe rust in wheat in real fields. The experimental site was located in Heze City, Shandong Province, China (116°3′12″E 35°27′53″N). The annual average temperature in the area was 13.5 °C, the average precipitation in the past five years was 659 mm, the average relative humidity was 68%, and the frequency of spring drought exceeds 40%. The straight-line distance between the two experimental sites was 182 km, and both sites were located in the temperate monsoon climate zone. The distribution of two experimental sites is shown in Figure 1, where Figure 1a shows the visible light orthophoto of the UAV in the wheat inoculation experimental field in Experiment 1, and Figure 1b shows the visible light orthophoto of the UAV in the real field area in Experiment 2.
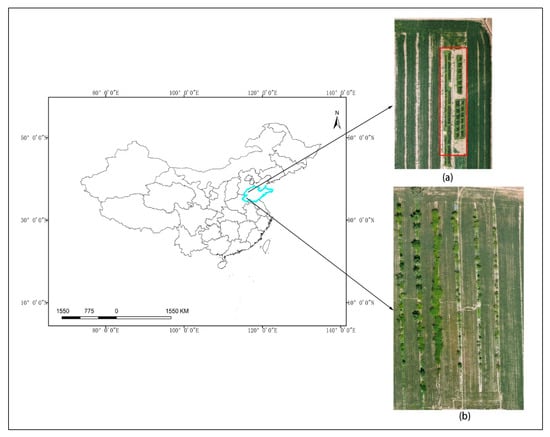
Figure 1.
Distribution of experimental sites. (a) The visible light orthophoto of the UAV in the area where Experiment 1 is located, with the red box indicating the wheat inoculation test area; (b) Visible light orthophoto of the UAV in the area where Experiment 2 is located.
2.2. Spectral Data Acquisition
2.2.1. Experimental Field Ground Spectral Acquisition (Experiment 1)
The experiment used an ASD FieldSpec spectrometer (Analytical Spectral Devices, Inc., Boulder, CO, USA) to collect ground spectral data of wheat canopy. The band range was 350–2500 nm, with a spectral resolution of 3 nm/8 nm and a sampling interval of 1.4 nm (350–1000 nm)/1.1 nm (1001–2500 nm). The measurement speed was fixed and the scanning time was set to 3 s. The data collection for Experiment 1 was conducted on 6, 16, and 23 May 2022, while Experiment 2 was conducted on 19–20 May of the same year. All observations were completed between 10:30 and 14:00 p.m. (Beijing time). During observation, meet the meteorological conditions of no cumulus clouds/dense clouds and wind force <3 levels. The operator wore dark clothing and kept the probe vertically downward. Each spectral data is obtained through 10 samplings, synchronized with 20 dark current corrections, and repeated 10 times for each observation target to ensure data reliability.
2.2.2. Field UAV Hyperspectral Acquisition (Experiment 2)
Unlike Experiment 1, Experiment 2 collected near ground hyperspectral information of wheat canopy in the real field experimental area. The equipment used was the DJI M600 multi rotor UAV (DJI Innovations, Shenzhen, China) equipped with the hyperspectral imaging instrument Pika L (Resonon Inc., Bozeman, MT, USA) (Figure 2), with a band range of 400–1000 nm, a band number of 332, and a spectral resolution of 2.1 nm. By comparison, the ASD FieldSpec spectrometer (Analytical Spectral Devices, Inc., Boulder, CO, USA) has a wider band range (350–2500 nm), but there is too much noise within the band. In this study, the effective band range of 350–1800 nm was captured, while Pika L covered a narrower range (400–1000 nm), with higher uniform resolution and number of bands. The hyperspectral data collection of Experiment 2’s UAV was conducted on 19–20 May 2022, with a fixed flight altitude of 50 m, and the route planning ensured full coverage of the target area. The data collection period is from 10:30 to 14:00 Beijing time, with stable weather conditions, no clouds, and wind speeds below level 3, meeting the requirements of hyperspectral imaging. The raw data was preprocessed using SBGcenter software of version 3.5.498 (SBG Systems, La Ciotat, France). Radiometric calibration was completed using AirlineDivision software of version 1.7 (Beijing Airline Technology Co., Ltd., Beijing, China). Finally, image registration and data format conversion were performed in ArcGIS software of version 10.5 (Environmental Systems Research Institute, Redlands, CA, USA). Throughout all processing steps, sensor standard parameters were maintained without additional band filtering.

Figure 2.
UAV hyperspectral data acquisition system.
2.3. Select Vegetation Index
The selection of appropriate vegetation indices is critical for accurate wheat stripe rust monitoring, as different indices vary in their sensitivity to specific physiological changes induced by pathogen infection (e.g., chlorophyll degradation, photosynthetic inhibition, canopy structure disruption). To address the research gap between experimental field and real field, we systematically selected six vegetation indices with well-documented biophysical significance and prior validation in crop disease monitoring. This selection was guided by three key criteria: (1) coverage of diverse disease progression stages; (2) responsiveness to distinct physiological alterations caused by stripe rust; (3) integration of widely accepted benchmark indices and disease-specific indices to ensure comparability and specificity.
- Normalized Difference Vegetation Index (NDVI)
NDVI is a globally recognized vegetation monitoring benchmark index, and the calculation formula is shown in Table 1 []. It quantifies vegetation coverage and biomass by comparing chlorophyll absorption (red band) and canopy scattering (near-infrared band). Previous studies have shown that NDVI is practical in distinguishing severely infected crops from healthy crops, but its limitation lies in its low sensitivity to early stripe rust disease, as mild chlorophyll loss is often masked by canopy structure []. We use NDVI as a baseline for comparison with more specialized indicators.
- 2.
- Structure Insensitive Pigment Index (SIPI)
SIPI is another national standard index designed to minimize the influence of canopy structure, focusing instead on leaf pigment content []. It is responsive to early pigment degradation caused by stripe rust, but its performance declines in dense canopies or high soil background interference []. We selected SIPI to complement NDVI’s weakness in early disease detection.
- 3.
- Photochemical Reflectance Index (PRI)
PRI targets photosynthetic efficiency by capturing changes in the xanthophyll cycle, a rapid physiological response to biotic stress. Previous research has validated PRI’s stability across leaf and canopy scales for wheat stripe rust monitoring, as photosynthetic inhibition often precedes visible leaf necrosis [,,]. Its inclusion aimed to capture early-stage stress before significant chlorophyll loss.
- 4.
- Plant Senescence Reflectance Index (PSRI)
PSRI is sensitive to leaf senescence and chlorophyll degradation, with a strong correlation to late-stage disease progression []. It leverages the red band for chlorophyll absorption and green/near-infrared bands for background correction. We included PSRI to cover the senescence-related spectral signals of severe stripe rust infection.
- 5.
- Modified Simple Ratio (MSR)
MSR improves upon simple ratio indices by reducing saturation effects in dense canopies []. It reflects leaf area and canopy density, which are disrupted by severe stripe rust. While MSR is less disease-specific than other indices, its prior use in crop stress monitoring made it a valuable comparative tool for evaluating structural vs. pigment-related spectral changes [].
- 6.
- Yellow Rust Severity Index (YRSI)
YRSI is a disease specific index for wheat stripe rust, developed using laboratory- based spectral data of stripe rust spores and infected leaf tissues. Its band combination is tailored to the unique physiological characteristics of stripe rust, enabling it to distinguish stripe rust from general stress. Previous studies have demonstrated that YRSI performs well in controlled experiments, but its robustness in complex real-world domains has not been tested, making it the core focus of this study [].
All six indicators have been validated in field trials and have a high correlation with the severity of stripe rust, ensuring that they form a comprehensive benchmark system covering early to late disease stages, pigment to structural changes, and general to specific stress responses. Table 1 provides a detailed list of their spectral characteristics and application scenarios.

Table 1.
Vegetation index used for monitoring wheat yellow rust in this study.
Table 1.
Vegetation index used for monitoring wheat yellow rust in this study.
| Index | Name | Formula | Apply to | References |
|---|---|---|---|---|
| NDVI | Normalized difference vegetation index | (R830 − R675)/(R830 + R675) | Vegetation coverage or biomass | [] |
| SIPI | Structural Independent Pigment Index | (R800 − R445)/(R800 + R680) | Pigment content | [] |
| PRI | Photochemical Reflectance Index | (R570 − R531)/(R570 + R531) | Photosynthetic radiation | [] |
| PSRI | Plant senescence reflectance index | (R680 − R500)/R750 | Leaf senescence and ripening | [] |
| MSR | Modified Simple Ratio | (R800/R670 − 1)/sprt(R800/R670 + 1) | Leaf area | [] |
| YRSI | Yellow Rust Spore Index | (R682 − R452)/(R682 + R452) | Yellow rust | [] |
2.4. Disease Investigation
Following spectral data collection at both experimental sites, we performed in situ disease assessments at each sampling interval. Disease severity was quantified using the Disease Index (DI), calculated in accordance with China’s National Standard GB/T 15795-2011 [] “Technical Specification for Monitoring and Forecasting of Wheat Stripe Rust”. The disease investigation covers an area of approximately 1 square meter and primarily adopts the five—point sampling method. Each investigation area selects five symmetrical points, with 20 wheat plants sampled at each point. Disease incidence is investigated separately, and disease severity is divided into 9 gradients: 0%, 1%, 10%, 20%, 30%, 45%, 60%, 80%, and 100%. The number of leaves for each severity is recorded, and then the DI is calculated using Formula (1):
x is the level value of each gradient, n is the highest gradient value of 9, and f is the number of leaves in each gradient.
2.5. Partial Least Squares Regression (PLSR)
To develop a wheat stripe rust monitoring model, this study employs partial least squares regression (PLSR), a method proven effective in crop growth monitoring and physiological-biochemical parameter estimation [,,,,,]. It combines the characteristics of principal component analysis (PCA), canonical correlation analysis, and multiple linear regression analysis, and is a classic modeling method. The core principle is to use component projection to clarify the potential structure of variables, project the original variables into a new space, and thereby transform the original variables with high data redundancy into a small number of variables by screening the optimal latent variables. From a mathematical perspective, PLSR can be presented as a linear model that describes the relationship between predictor variables and observed variables, in the form of Formula (2):
β0 represents the intercept term, βn is the regression coefficient, Xn is the independent variable, N is the number of independent variables. In this study, different hyperspectral vegetation index sensitive bands and their combinations were selected as independent variables; the dependent variable is the DI of wheat stripe rust, which enables quantitative inversion from spectral information to disease severity. We conducted a comprehensive statistical analysis of the experimental data and performed outlier detection. To ensure the robustness of the model, the dataset is divided into a training set (n = 156) and a validation set (n = 67), and stratified sampling is used to maintain the distribution of disease severity in both subsets. The model training involves 1000 iterations using the leaf one cross validation (LOOCV) method. The number of 5 components was determined through root mean square error of LOOCV and validation through permutation testing, which confirmed statistical significance.
Y = β0 + β1 × 1 + β2X2 + β3X3 + … + βnXn
2.6. Accuracy Evaluation
To evaluate the performance of PLSR models at different spatial scales and features, this study used the coefficient of determination R2 and root mean square error (RMSE) for accuracy assessment. RMSE is used to reflect the degree of deviation between the estimated values of the model and the actual observed values. The calculation formula is shown in (3):
Pi and Qi are the estimated values of the model and the actual observed values, respectively, and n is the sample size. Meanwhile, the model training and validation were carried out using LOOCV method. This method selects one sample for validation each time, and the remaining k-1 samples (k is the total number of samples) are used for model training. After k cycles, the average accuracy is taken as the final model accuracy. The advantage of LOOCV lies in using almost all samples to participate in model training, which can effectively reduce the risk of overfitting and more accurately and reliably evaluate the predictive ability of the model. It has been widely validated in crop disease monitoring modeling and crop parameter inversion modeling [,,,,,]. This study used a collaborative workflow between Python (v3.9.12, Python Software Foundation, Beaverton, OR, USA) and Excel (Microsoft 365v2308, Microsoft Corporation, Redmond, OR, USA). Data preprocessing was completed through Excel, while model training and validation were automated using Python’s scikit learn library (v1.2.2, INRIA, Rocquencourt, France) to achieve the LOOCV process, balancing the intuitiveness of spreadsheets with the computational efficiency of programming tools. It should be noted that the application details of LOOCV are different in experiments 1 and 2: in experiment 1, the sample size was 68; in Experiment 2, the sample size was 155.
3. Results
3.1. Spectral Characteristics of Wheat Yellow Rust (Experiment 1)
Calculate the average spectra of sampling points for health (n = 20) and stripe rust infection (n = 20), with a confidence interval (CI) of 95%. Figure 3 shows the average spectral reflectance curves and 95% CI of healthy wheat (blue line, orange band) and diseased wheat (red line, yellow band) within the wavelength range of 350–1300 nm. The blue and red lines represent the average reflectance of the healthy and diseased groups, respectively, while the orange and yellow bands represent the 95% confidence interval, indicating the variability of each spectral dataset. There is a significant spectral difference between the two groups: for example, healthy wheat has a higher reflectance in the near-infrared region (such as 700–1300 nm) than diseased wheat, which is consistent with physiological changes caused by diseases (such as chlorophyll degradation). The 95% CI band provides insights into data variability, thereby enhancing the reliability of spectral comparisons between healthy and diseased wheat.
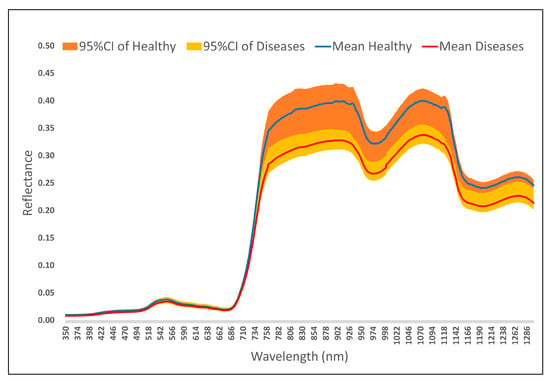
Figure 3.
Spectral characteristics of wheat yellow rust (350–1300 nm).
3.2. Evaluation of Spectral Index Disease Monitoring Capability Based on Experimental Field Data (Experiment 1)
In Experiment 1, 68 wheat canopy hyperspectral measurements acquired with an ASD spectrometer were paired with synchronous disease index assessments to evaluate the predictive performance of six vegetation indices (NDVI, SIPI, PRI, PSRI, MSR, YRSI) against stripe rust severity through LOOCV. The results are presented in Figure 4.
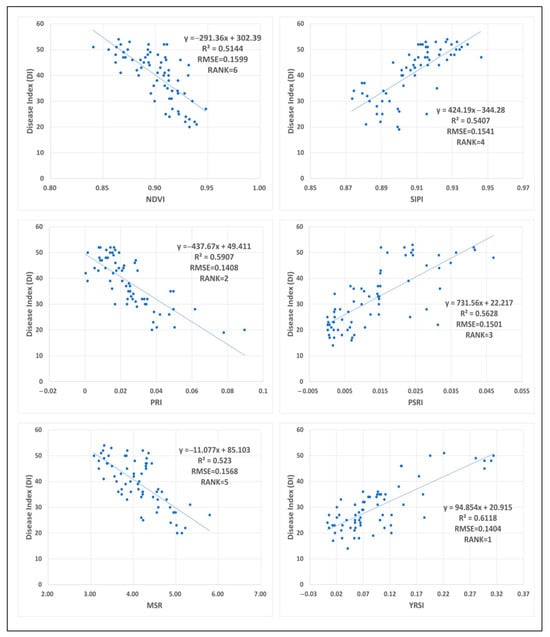
Figure 4.
Relationship between canopy spectral vegetation index and DI of wheat in experimental fields (n = 68).
The YRSI demonstrated superior predictive accuracy for disease severity (R2 = 0.6118, RMSE = 0.1404), exhibiting significantly lower dispersion in scatter point distribution compared to other indices. This performance advantage stems from its specific design targeting the characteristic spectral bands associated with leaf yellowing in stripe rust infection. PRI and PSRI ranked second and third, respectively (R2 = 0.5907 and 0.5628), confirming the relevance of photosynthetic efficiency parameters and pigment alteration metrics in disease detection.
While the traditional chlorophyll index SIPI achieved moderate accuracy (R2 = 0.5407), its reliance on chlorophyll degradation features limited its effectiveness to late-stage infections, resulting in inadequate early warning capability. MSR (R2 = 0.5230) and NDVI (R2 = 0.5144) showed comparable but weaker performance in stripe rust identification, which may be attributed to their shared spectral bands despite differing mathematical formulations. NDVI, PRI, and MSR are negatively correlated with DI, mainly due to the degradation of chlorophyll in leaves caused by stripe rust, and these three index values decrease with the aggravation of the disease.
LOOCV revealed fluctuations in RMSE values across iterations (range: 0.0195), suggesting potential instability in the analytical framework. Notably, YRSI exhibited the smallest coefficient of determination dispersion, with a standard deviation significantly lower than other indices, a statistical characteristic that supports its reliability for field applications.
3.3. Vegetation Index Optimization
The YRSI demonstrated superior performance in stripe rust detection. To further optimize vegetation indices for UAV-based monitoring, we first analyzed the spectral sensitivity to disease severity. In Experiment 1, in order to avoid the influence of spectral noise, this study analyzed the correlation between 68 ground measured disease severity samples and canopy spectral reflectance data in the 350–1149 nm band range. As shown in Figure 5, the color bars indicate a correlation coefficient range of 0.5 (blue–green) to 0.7 (red–yellow), and the red–yellow gradient represents moderate to severe positive correlation. The spectral ranges of 440–600 nm and 665–895 nm are visually distinguished by the color bar legend, highlighting the strong correlation between these spectral regions and DI. These regions correspond to chlorophyll absorption (440–600 nm) and leaf structure scattering (665–895 nm), which are key physiological responses to stripe rust infection. We can conclude that a strong correlation is observed in the wavelength range of 440–600 nm and 665–895 nm, and these regions are identified as sensitive spectral bands for wheat stripe rust detection.
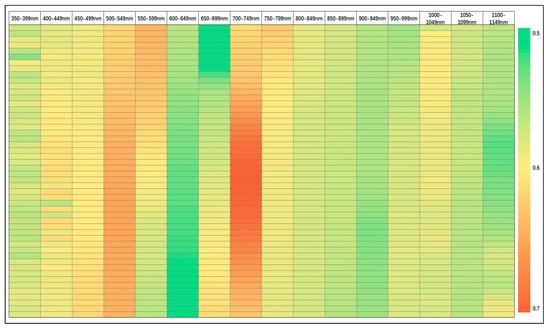
Figure 5.
Thermal map of correlation coefficient between DI and canopy spectral reflectance (350–1149 nm).
Hyperspectral data acquisition using UAV-mounted sensors in field conditions typically relies on selected sensitive bands for single band combination indices to construct disease detection models. However, spectral distortion caused by noise interference in UAV imagery may compromise the reliability of such models when using individual bands. To address this limitation, six optimized vegetation indices (NDVIO, SIPIO, PRIO, PSRIO, MSRO, YRSIO) were developed by integrating the spectral characteristics of the original indices (NDVI, SIPI, PRI, PSRI, MSR, YRSI) with the UAV sensor’s spectral resolution. The mean reflectance values within ±4.2 nm ranges of each index’s core bands were employed as independent variables to enhance disease information representation. It should be noted that Figure 5 was validated using measurements of canopy reflectance under 68 field conditions from Experiment 1. Although the hyperspectral UAV sensor was mainly deployed in Experiment 2, considering the practical application of using hyperspectral data from the field to the real field, the six optimized vegetation indices were developed using the spectral resolution of the UAV hyperspectral data.
3.4. Spectral Index Disease Assessment Based on Field Data (Experiment 2)
Experiment 2 obtained 155 sets of UAV hyperspectral data and corresponding disease index datasets of wheat infected with stripe rust in the field. The quantitative monitoring ability of six optimized vegetation indices on the degree of stripe rust infection was also evaluated using a LOOCV system, and the results are shown in Figure 6.
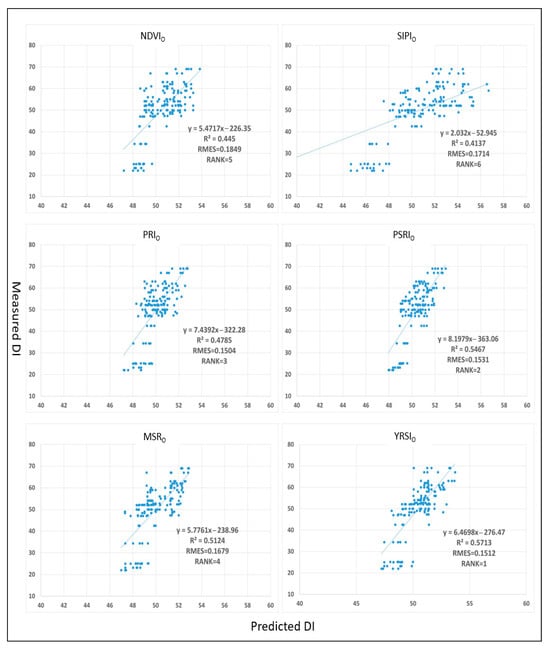
Figure 6.
Scatter plot of measured DI using field data (n = 155) and predicted DI.
The comparative analysis revealed distinct predictive performance among the six vegetation indices for stripe rust severity estimation. YRSIO demonstrated superior performance (R2 = 0.5713, RMSE = 0.1512), effectively capturing wheat canopy spectral variations induced by stripe rust and exhibiting the strongest disease severity quantification capacity. PSRIO ranked second (R2 = 0.5467, RMSE = 0.1531), with its sensitivity to plant senescence enabling effective physiological status monitoring at canopy scale. PRIO achieved third position (R2 = 0.4785, RMSE = 0.1504), showing consistent accuracy across both leaf and canopy scales, corroborating previous findings its stable disease response [].
Whereas MSRO (R2 = 0.5124, RMSE = 0.1679) showed moderate correlation, its explanatory power for disease severity remained limited compared to the top-performing indices. The classic NDVIO (R2 = 0.4450, RMSE = 0.1849) primarily reflected vegetation coverage changes but proved inadequate for precise rust severity characterization. SIPIO (R2 = 0.4137, RMSE = 0.1714) exhibited the weakest performance at canopy scale, being more suitable for leaf-level yellow rust monitoring as documented in prior studies [].
In Figure 4, NDVI, PRI, and MSR are negatively correlated with DI, mainly due to the degradation of chlorophyll in leaves caused by stripe rust, and these three index values decrease with the aggravation of the disease. All optimization indices in Figure 6 are positively correlated with DI, which is attributed to the fact that in Experiment 2, the optimization indices shifted the response target from chlorophyll loss to stripe rust spore and lesion characteristics by calculating the average reflectance of the core band ±4.2 nm. The predicted DI is concentrated at 48–52%, due to the positive response of the optimization index to stripe rust specific signals and the distribution characteristics of DI in field samples. The observed differences in model performance indicators (higher R2 and lower RMSE compared to the real field) are mainly attributed to the greater environmental homogeneity and controlled management practices in the experimental field, which is consistent with the initial optimization conditions of vegetation index. Experimental fields typically have the characteristics of minimal changes in soil properties, uniform crop management, and reduced external interference, which enhances the consistency of spectral signals and improves model fitting. In contrast, the real field environment introduces heterogeneity factors, increases spectral variability, and introduces noise, resulting in slightly lower R2 and slightly higher RMSE.
3.5. Spectral Index Disease Monitoring Based on Field Data (Experiment 2)
This section presents the field-scale spatial distribution inversion of stripe rust disease using six optimized vegetation indices (NDVIO, SIPIO, PRIO, PSRIO, MSRO, YRSIO), following their quantitative response evaluation. Concurrently collected UAV hyperspectral imagery and ground-measured DI data underwent standard preprocessing, including radiometric calibration, geometric correction, and atmospheric correction. Subsequently, each pixel’s vegetation index was optimized, and the empirical model derived from cross-validation in Section 3.3 was applied to predict DI values at pixel resolution, generating a comprehensive severity distribution map.
Correspondingly, the agricultural management classification system was adopted, categorizing disease severity as: healthy (DI ≤ 5%), mild infection (5 < DI ≤ 20%), moderate infection (20 < DI ≤ 50%), and severe infection (DI > 50%). Figure 7 illustrates the spatial distribution patterns obtained from the six indices, employing a standardized color scheme: green for healthy wheat, yellow-green for mild infection, yellow for moderate infection, and red for severe infection. The inversion accuracy was validated using 30 independent ground samples, with comparative results between predicted and measured DI presented in Table 2.
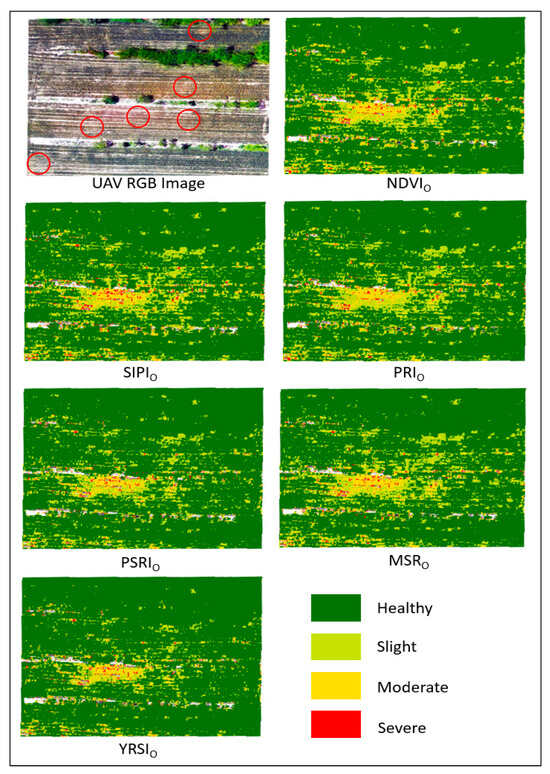
Figure 7.
Spatial distribution map of stripe rust severity obtained by optimizing index inversion. The obvious areas of rust occurrence discovered through ground visual investigation have been marked with red circles on the UAV RGB image.

Table 2.
Accuracy of optimized vegetation index disease inversion (comparison of predicted DI and measured DI for different disease severity) (%).
In the field environment, the characteristics of wheat stripe rust disease inverted by six optimized vegetation indices are as follows:
- NDVIO: The moderate and severe patches in the result graph are scattered and have a wide range, and the matching degree between the distribution of healthy areas and actual healthy wheat is moderate. Field verification shows that its ability to distinguish the boundaries of healthy/slight diseases is weak, and there is a large deviation between predicted DI and measured DI. The overall degree of disease differentiation is moderate.
- SIPIO: The proportion of healthy areas has increased, but moderate and severe patches are fragmented, and the continuity of disease spatial distribution is poor. In ground verification, the inversion of slight diseases is prone to misidentifying healthy plants as mild infections, resulting in slightly higher predicted DI values in slight areas than measured values.
- PRIO: The fit between healthy areas and the actual healthy range in RGB images is better, with increased clustering of moderate plaques and clearer boundaries. Ground verification shows that it has good mapping accuracy on moderate diseases, with high consistency between predicted DI and measured DI. However, in severe areas, some pixel predicted values are slightly lower than measured values, and the characterization of severe extreme values requires further refinement.
- PSRIO: The transition between healthy and slight areas is more natural, and the morphology of moderate patches matches the actual affected areas in the field more closely. Ground verification shows that the gradient inversion of “slight moderate” diseases is more accurate and can reflect the trend of gradually worsening diseases in the field. However, the pixel coverage in severe areas is insufficient, and the sensitivity to response to severe diseases is limited.
- MSRO: The proportion of healthy areas is large, but yellow and red patches are scattered and generally match with the actual core area of the disease. Ground verification revealed a tendency to underestimating the degree of disease, with many actual “slight/moderate” plants being misclassified as healthy/slight, and the overall predicted DI being low, indicating a conservative identification of disease severity.
- YRSIO: The distribution of healthy wheat in the healthy area is highly consistent with the RGB image, with the strongest clustering of yellow and red patches. The “severe moderate” patch morphology in the central disease core area is basically consistent with the actual field conditions. In ground validation, the consistency between predicted DI and measured DI is the best among all indices, and the differentiation of the severity of various diseases such as healthy, slight, moderate, and severe is relatively accurate. It can effectively cover the actual severely affected pixels, and the spatial inversion accuracy and disease degree differentiation ability are the highest.
4. Discussion
This study systematically explored the quantitative monitoring method of wheat stripe rust at the field scale from experimental fields to real fields by optimizing the hyperspectral vegetation index. The research results not only validated the effectiveness of the optimization index in disease monitoring but also revealed the responsiveness and application potential of spectral indices at different scales.
4.1. The Disease Response of Vegetation Index in Experimental Field Environment
In this study, the index YRSI performed the best in stripe rust monitoring, which is closely related to the specific band combination design of this index. The highest R2 achieved by YRSI is 0.6118, indicating its strong ability to quantify the severity of stripe rust disease. This performance is superior to traditional indicators such as NDVI (R2 = 0.5144) and comparable to the most advanced disease-specific indicators in wheat [,,], confirming the effectiveness of its band combination in capturing changes in the lutein cycle and photosynthetic inhibition. Although the R2 value may seem moderate, it is worth noting that on-site disease monitoring essentially involves variability in environmental factors, and the stability of YRSI on these variables (Section 3.2) makes it more suitable for practical use than other hyperspectral models. The integration of future and multi temporal data can further improve accuracy by considering disease progression dynamics. YRSI adopts a combination of 682 nm and 452 nm, where the 452 nm band is sensitive to changes in chlorophyll content and the 682 nm band reflects photosynthetic efficiency. This combination accurately captures the physiological response chain of “chlorophyll degradation photosynthesis inhibition canopy sparsity” in wheat under stripe rust stress and is more disease specific than traditional indices. In the field environment, the band combination designed for the characteristics of stripe rust spores effectively reduces the interference of non-biological factors such as soil background.
In contrast, NDVI relies too much on the simple ratio of near-infrared and red bands, which can easily confuse spectral signals of “healthy but thin canopy” and “slight disease”, resulting in lower inversion accuracy. PRI showed cross scale stability in this study (with a difference in less than 5% in leaf and canopy scale R2), which is consistent with the findings of Huang et al. []. The mechanism is that the 531 nm band can sensitively reflect changes in the lutein cycle, while the 571 nm band effectively eliminates interference from changes in lighting conditions. This design enables it to stably characterize the photosynthetic physiological damage caused by stripe rust at different scales, making it one of the few indices with both leaf and canopy monitoring capabilities. PSRI uses a combination of 750 nm, 550 nm, and 680 nm to better capture leaf senescence signals caused by stripe rust, with particularly outstanding recognition accuracy in moderately affected areas.
This study validated the monitoring ability of six vegetation indices (NDVI, SIPI, PRI, PSRI, MSR, YRSI) for wheat stripe rust through experimental and field comparisons. The results indicate that the YRSI exhibits the best disease monitoring ability, which is closely related to its specialized design of leaf yellowing characteristic bands caused by stripe rust. The performance of PRI and PSRI perform secondarily, confirming the sensitivity of photosynthetic efficiency parameters and pigment changes to disease response. Notably, while the traditional chlorophyll index SIPI achieves usable accuracy, the chlorophyll degradation characteristics it relies on only become significantly apparent in the later stages of the disease, resulting in insufficient early warning capabilities. This discovery provides important insights for the design of future vegetation indices: indices designed for specific physiological processes of diseases, such as stripe rust spore production, are more specific than general vegetation indices.
4.2. Optimization Index and Disease Response Mechanism in Field Environment
This study revealed the disease response mechanism of optimizing vegetation index in complex field environments through systematic analysis, mainly reflected in three key dimensions: firstly, in terms of soil background interference suppression, an index constructed using the average reflectance within the sensitive band ±4.2 nm range was used to achieve effective spectral separation of soil background and disease signals through dynamic combination of sensitive bands, this has been supported in the research work of B Somers et al. []; Secondly, in response to meteorological fluctuations, the UAV hyperspectral system (50 m altitude, 2.1 nm resolution) combines radiometric calibration technology to control reflectance fluctuations under a stable mechanism, this method of mitigating meteorological fluctuations through radiometric calibration is in line with the principles established by C Wang et al. []; Finally, at the physiological process response level, the optimization index demonstrated a hierarchical capture ability for the unique physiological processes of stripe rust disease, YRSIO identified wax layer damage caused by spores through the 682 nm band, PRIO enhanced the sensitivity of 531 nm/570 nm band to photosynthetic inhibition, and PSRIO quantified the chlorophyll degradation rate reflected by the ratio of 680–500 nm to 750 nm. The validation of 155 field samples shows that this technical framework significantly improves the accuracy of the model compared to traditional single band vegetation index methods, confirming the superiority of optimized index design based on spectral features in field environments.
4.3. Limitations and Future Research
This study has several limitations that warrant consideration. First, the data collection was geographically constrained to two locations in Shandong Province, China, necessitating expanded regional validation to ensure broader applicability. Second, the UAV hyperspectral system’s spectral coverage (400–1000 nm) excludes the shortwave infrared region, potentially omitting disease-specific spectral features. Third, the modeling approach did not account for cultivar resistance level variations, which may influence spectral responses. These aspects require targeted investigation in future research.
It should be pointed out that addressing data distribution and outliers is crucial for scientific rigor. Although the distribution of the original data is unknown, normality testing and outlier removal improve the robustness of the model. We acknowledge the existence of residual uncertainty and recommend using larger datasets in the future to validate distribution hypotheses. Suggestions for future work should focus on promoting research in the following four areas: firstly, integrating multi-source remote sensing data from satellites and UAV to establish a collaborative monitoring system []; Secondly, the spectral detection range will be extended to 2500 nm to enhance the ability of disease feature recognition []; Thirdly, develop specific spectral calibration models for different wheat varieties []; Finally, build an intelligent cloud monitoring platform to achieve real-time dynamic monitoring and early warning of field diseases [].
5. Conclusions
This study systematically compared the performance of hyperspectral vegetation indices in experimental and field environments, and drew the following main conclusions:
- The YRSI showed the best ability to monitor stripe rust in both the experimental and large fields (R2 of 0.6118 and 0.5713, respectively), and its optimized combination of 682 ± 4.2 nm and 452 ± 4.2 nm sensitive bands can effectively capture disease characteristics.
- The optimization index has achieved consistency in multi-scale monitoring. The difference in inversion accuracy between YRSI and PRI at leaf, canopy, and field scales is less than 8%, which solves the pain point of significant scale effects in traditional indices. Through band combination optimization, these indices successfully reduced the impact of interference factors such as soil background and lighting conditions in field environments.
- The combination of UAV hyperspectral imaging system and optimized vegetation index can achieve centimeter level accuracy in disease spatial inversion, with an accuracy rate of 85.2% in identifying severely infected areas.
The transitional method framework constructed from experimental fields to large fields can be extended to other crop disease monitoring, providing a reliable technical solution for crop disease warning. This research provides key technical support for the precise prevention and control of wheat stripe rust and takes an important step towards the practical application of hyperspectral technology in the field.
Author Contributions
Conceptualization, M.W. and D.H.; methodology, M.W.; software, Z.Z.; validation, T.L. and R.G.; formal analysis, D.H.; investigation, W.F.; resources, M.W.; data curation, Z.Z.; writing—original draft preparation, M.W. and R.G.; writing—review and editing, M.W., F.W. and R.G.; visualization, J.Z. and F.W.; supervision, J.Z.; project administration, J.Z.; funding acquisition, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Key R&D Program of Shandong Province, China (2025CXGC011117), the National Key Research and Development Program Project (No. 2021YFB3901303), the Research Startup Grant (CXGC2025G03) and Shandong Provincial Natural Science Foundation (ZR2022QD081/ZR2024MD080).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Key Research and Development Program of Shandong Province: Development of a Precision Monitoring and Control System for Major Diseases in Staple Crops.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, L.; Zhao, W.; Xia, C.; Zhao, N.; Li, H.; Sun, Z.; Yang, L.; Li, M.; Chen, W.; Yang, F.; et al. Wheat Stripe Rust Inoculum from the Southwest Dispersed to the East Huang-Huai-Hai Region Through Southern Anhui in China. Plant Dis. 2025, 109, 138–148. [Google Scholar] [CrossRef]
- Chen, X.M. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Chen, W.; Yao, L.; Zeng, Q.; Chen, X.; Zhao, J.; Wu, S.; Kang, Z. Spatiotemporal relationship of wheat stripe rust occurrence revealing the key inoculum origin area and migration route of Puccinia striiformisf. sp. tritici in the Yunnan-Guizhou epidemiological region. Phytopathol. Res. 2025, 7, 73. [Google Scholar] [CrossRef]
- Azzimonti, G.; Garcia, R.; González, N.; Domeniguini, V.; Germán, S. Field-Based Phenotyping for Wheat Diseases Within a New Multiple Diseases Platform in Uruguay: Promoting Germplasm Sharing to Increase Resistance Diversity; CABI Digital Library: Online, 2017; Available online: https://inia.uy/field-based-phenotyping-wheat-diseases-within-new-multiple-diseases-platform-uruguay-promoting (accessed on 28 September 2025).
- Yao, Z.; Lei, Y.; He, D. Early Visual Detection of Wheat Stripe Rust Using Visible/Near-Infrared Hyperspectral Imaging. Sensors 2019, 19, 952. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Niu, B.; Feng, Q.; Chen, B.; Ou, C.; Liu, Y.; Yang, J. HSI-TransUNet: A transformer based semantic segmentation model for crop mapping from UAV hyperspectral imagery. Comput. Electron. Agric. 2022, 201, 107297. [Google Scholar] [CrossRef]
- Azadbakht, M.; Ashourloo, D.; Aghighi, H.; Radiom, S.; Alimohammadi, A. Wheat leaf rust detection at canopy scale under different LAI levels using machine learning techniques. Comput. Electron. Agric. 2019, 156, 10. [Google Scholar] [CrossRef]
- Lv, X.; Jiang, J.; Yang, Z.; Lan, S.; Ma, Y.; Deng, J.; Zhou, C.; Wang, Z.; Li, Y.; Ma, Z. Rapid detection of Puccinia striiformis f. sp. tritici from wheat stripe rust samples using recombinase polymerase amplification combined with multiple visualization methods. Int. J. Biol. Macromol. 2024, 283, 137634. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Huang, W.; Cui, X.; Dong, Y.; Shi, Y.; Ma, H.; Liu, L. Identification of Wheat Yellow Rust Using Optimal Three-Band Spectral Indices in Different Growth Stages. Sensors 2019, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ye, H.; Huang, W.; Ma, H.; Guo, A.; Ruan, C.; Liu, L.; Qian, B. A new spectral index for the quantitative identification of yellow rust using fungal spore information. Big Earth Data 2021, 5, 201–216. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Ye, H.; Dong, Y.; Ma, H.; Ren, Y.; Ruan, C. Identification of Wheat Yellow Rust using Spectral and Texture Features of Hyperspectral Images. Remote Sens. 2020, 12, 1419. [Google Scholar] [CrossRef]
- Ahmad, W.; Azhar, E.; Anwar, M.; Ahmed, S.; Noor, T. Machine Learning Approaches for Detecting Vine Diseases: A Comparative Analysis. J. Inform. Web Eng. 2025, 4, 99–110. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Gao, R.; Zhang, J.; Feng, W. Unmanned Aerial Vehicle (UAV) Imagery for Plant Communities: Optimizing Visible Light Vegetation Index to Extract Multi-Species Coverage. Plants 2025, 14, 1677. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.; Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I. Effects of Chlorophyll Concentration on Green LAI prediction in Crop Canopies: Modelling and Assessment. In Proceedings of the International Sysmposium on Recent Advances in Quantitative Remote Sensing, Valencia, Spain, 16–20 September 2002. [Google Scholar]
- Cross, J.F.; Cobo, N.; Drewry, D.T. Non-invasive diagnosis of wheat stripe rust progression using hyperspectral reflectance. Front. Plant Sci. 2024, 15, 1429879. [Google Scholar] [CrossRef]
- Yashu; Kukreja, V.; Singh, A. AI-Driven Diagnosis of Wheat Leaf Diseases: Integrating CNN and Random Forest. In Proceedings of the 2025 International Conference on Advanced Computing Technologies (ICoACT), Sivalasi, India, 14–15 March 2025. [Google Scholar]
- Wang, Y.; Kootstra, G.; Yang, Z.; Khan, H.A. UAV multispectral remote sensing for agriculture: A comparative study of radiometric correction methods under varying illumination conditions. Biosyst. Eng. 2024, 248, 240–254. [Google Scholar] [CrossRef]
- Zhang, K.; Deng, J.; Zhou, C.; Liu, J.; Lv, X.; Wang, Y.; Sun, E.; Liu, Y.; Ma, Z.; Shang, J. Using UAV hyperspectral imagery and deep learning for Object-Based quantitative inversion of Zanthoxylum rust disease index. Int. J. Appl. Earth Obs. Geoinf. 2024, 135, 104262. [Google Scholar] [CrossRef]
- Minařík, R.; Langhammer, J.; Hanuš, J. Radiometric and Atmospheric Corrections of Multispectral μMCA Camera for UAV Spectroscopy. Remote Sens. 2019, 11, 2428. [Google Scholar] [CrossRef]
- Garca Bravo, M. The State of Food Security and Nutrition in the World 2023 [Book review]. Agroaliment. J. Rev. Agroaliment. 2024, 30, 192–198. [Google Scholar]
- van Thang, D.; Volkov, A.; Muthanna, A.; Elgendy, I.A.; Alkanhel, R.; Jayakody, D.N.K.; Koucheryavy, A. A Framework Integrating Federated Learning and Fog Computing Based on Client Sampling and Dynamic Thresholding Techniques. IEEE Access 2025, 13, 95019–95033. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Ren, B.; Huang, L.; Zheng, Q.; Ma, H. A Disease Index for Efficiently Detecting Wheat Fusarium Head Blight Using Sentinel-2 Multispectral Imagery. IEEE Access 2020, 8, 52181–52191. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, W.; Ye, H.; Zhou, X.; Ma, H.; Dong, Y.; Shi, Y.; Geng, Y.; Huang, Y.; Jiao, Q.; et al. Quantitative identification of yellow rust in winter wheat with a new spectral index: Development and validation using simulated and experimental data. Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102384. [Google Scholar] [CrossRef]
- Han, D.; Cai, H.; Yang, X.; Xu, X. Multi-Source Data Modeling of the Spatial Distribution of Winter Wheat Yield in China from 2000 to 2015. Sustainability 2020, 12, 5436. [Google Scholar] [CrossRef]
- Han, D.; Zhang, Z.; Gao, R.; Wang, F.; Zhang, J.; Yang, J.; Jia, K.; Wang, M. Winter Wheat Phenology Dynamics Based on MODIS Time-Series Imagery and Time Lag Effects to Climate. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2025, 18, 23816–23827. [Google Scholar] [CrossRef]
- Wang, M.; Liu, C.; Han, D.; Wang, F.; Hou, X.; Liang, S.; Sui, X. Assessment of GF3 Full-Polarimetric SAR Data for Dryland Crop Classification with Different Polarimetric Decomposition Methods. Sensors 2022, 22, 11. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, H.; Chen, Z.; Fei, S.; Zhou, J.; Ghamisi, P.; Zhang, B. Adaptive multi-stage fusion of hyperspectral and LiDAR data via selective state space models. Inf. Fusion 2025, 125, 103488. [Google Scholar] [CrossRef]
- Genze, N.; Ajekwe, R.; Güreli, Z.; Haselbeck, F.; Grieb, M.; Grimm, D.G. Deep learning-based early weed segmentation using motion blurred UAV images of sorghum fields. Comput. Electron. Agric. 2022, 202, 168. [Google Scholar] [CrossRef]
- Su, B.; Liu, D.; Chen, Q.; Han, D.; Wu, J. Method for the identification of wheat stripe rust resistance grade using time series vegetation index. Trans. Chin. Soc. Agric. Eng. 2024, 40, 155–165. [Google Scholar]
- Chen, J.; Chen, J.; Zhang, D.; Sun, Y.; Nanehkaran, Y.A. Using deep transfer learning for image-based plant disease identification. Comput. Electron. Agric. 2020, 173, 105393. [Google Scholar] [CrossRef]
- Ren, Y.; Meng, Y.; Huang, W.; Ye, H.; Han, Y.; Kong, W.; Zhou, X.; Cui, B.; Xing, N.; Guo, A.; et al. Novel Vegetation Indices for Cotton Boll Opening Status Estimation Using Sentinel-2 Data. Remote Sens. 2020, 12, 1712. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Third NASA Earth Resources Technology Satellite Symposium; NASA: Washington, DC, USA, 1974. [Google Scholar]
- Devadas, R.; Lamb, D.W.; Simpfendorfer, S.; Backhouse, D. Evaluating ten spectral vegetation indices for identifying rust infection in individual wheat leaves. Precis. Agric. 2009, 10, 459–470. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Dong, Y.; Ye, H.; Ma, H.; Liu, B.; Wu, W.; Ren, Y.; Ruan, C.; Geng, Y. Wheat Yellow Rust Detection Using UAV-Based Hyperspectral Technology. Remote Sens. 2021, 13, 123. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of Vegetation Indices and a Modified Simple Ratio for Boreal Applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Shi, X.-Z.; Aspandiar, M.; Oldmeadow, D. Using hyperspectral data and PLSR modelling to assess acid sulphate soil in subsurface. J. Soils Sediments 2014, 14, 904–916. [Google Scholar] [CrossRef]
- Axelsson, C.; Skidmore, A.K.; Schlerf, M.; Fauzi, A.; Verhoef, W. Hyperspectral analysis of mangrove foliar chemistry using PLSR and support vector regression. Int. J. Remote Sens. 2013, 34, 1724–1743. [Google Scholar] [CrossRef]
- Chen, P.; Jing, Q. A comparison of two adaptive multivariate analysis methods (PLSR and ANN) for winter wheat yield forecasting using Landsat-8 OLI images. Adv. Space Res. 2017, 59, 987–995. [Google Scholar] [CrossRef]
- Asante, E.A.; Du, Z.; Lu, Y.; Hu, Y. Detection and assessment of nitrogen effect on cold tolerance for tea by hyperspectral reflectance with PLSR, PCR, and LM models. Inf. Process. Agric. 2021, 8, 9. [Google Scholar] [CrossRef]
- Meacham-Hensold, K.; Montes, C.M.; Wu, J.; Guan, K.; Fu, P.; Ainsworth, E.A.; Pederson, T.; Moore, C.E.; Brown, K.L.; Raines, C.; et al. High-throughput field phenotyping using hyperspectral reflectance and partial least squares regression (PLSR) reveals genetic modifications to photosynthetic capacity. Remote Sens. Environ. 2019, 231, 111176. [Google Scholar] [CrossRef]
- Burnett, A.C.; Jeremiah, A.; Davidson, K.J.; Ely, K.S.; Julien, L.; Qianyu, L.; Morrison, B.D.; Dedi, Y.; Alistair, R.; Serbin, S.P. A best-practice guide to predicting plant traits from leaf-level hyperspectral data using partial least squares regression. J. Exp. Bot. 2021, 18, 6175–6189. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.; Wan, H.; Liu, L.; Wang, J. Monitoring of wheat yellow rust with dynamic hyperspectral data. In Proceedings of the IGARSS 2004. 2004 IEEE International Geoscience and Remote Sensing Symposium, Anchorage, AK, USA, 20–24 September 2004. [Google Scholar]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Maimaitiyiming, M.; Hartling, S.; Peterson, K.T.; Maw, M.J.W.; Shakoor, N.; Mockler, T.; Fritschi, F.B. Vegetation Index Weighted Canopy Volume Model (CVM VI) for soybean biomass estimation from Unmanned Aerial System-based RGB imagery. ISPRS J. Photogramm. Remote Sens. 2019, 151, 27–41. [Google Scholar] [CrossRef]
- Jay, S.; Gorretta, N.; Morel, J.; Maupas, F.; Bendoula, R.; Rabatel, G.; Dutartre, D.; Comar, A.; Baret, F. Estimating leaf chlorophyll content in sugar beet canopies using millimeter- to centimeter-scale reflectance imagery. Remote Sens. Environ. 2017, 198, 173–186. [Google Scholar] [CrossRef]
- Zhang, J.C.; Pu, R.L.; Wang, J.H.; Huang, W.J.; Yuan, L.; Luo, J.H. Detecting powdery mildew of winter wheat using leaf level hyperspectral measurements. Comput. Electron. Agric. 2012, 85, 13–23. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Ma, H. Monitoring Wheat Fusarium Head Blight Using Unmanned Aerial Vehicle Hyperspectral Imagery. Remote Sens. 2020, 12, 3811. [Google Scholar] [CrossRef]
- Deng, J.; Wang, R.; Yang, L.; Lv, X.; Yang, Z.; Zhang, K.; Zhou, C.; Pengju, L.; Wang, Z.; Abdullah, A.; et al. Quantitative Estimation of Wheat Stripe Rust Disease Index Using Unmanned Aerial Vehicle Hyperspectral Imagery and Innovative Vegetation Indices. IEEE Trans. Geosci. Remote Sens. 2023, 61, 4406111. [Google Scholar] [CrossRef]
- Bohnenkamp, D.; Behmann, J.; Mahlein, A.-K. remote sensing in-field detection of yellow rust in wheat on the ground canopy and uav scale. Remote Sens. 2019, 11, 2495. [Google Scholar] [CrossRef]
- Somers, B.; Tits, L.; Verstraeten, W.W.; Coppin, P. Soil reflectance modeling & hyperspectral mixture analysis: Towards vegetation spectra minimizing the soil background contamination. In Proceedings of the Workshop on Hyperspectral Image & Signal Processing: Evolution in Remote Sensing, Reykjavik, Iceland, 14–16 June 2010. [Google Scholar]
- Wang, C.; Myint, S.W. A Simplified Empirical Line Method of Radiometric Calibration for Small Unmanned Aircraft Systems-Based Remote Sensing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 1876–1885. [Google Scholar] [CrossRef]
- Fu, B.; Wu, Y.; Zhang, S.; Sun, W.; Jia, M.; Deng, T.; He, H.; Yuan, B.; Fan, D.; Wang, Y. Synergistic retrieval of mangrove vital functional traits using field hyperspectral and satellite data. Int. J. Appl. Earth Obs. Geoinf. 2024, 131, 103963. [Google Scholar] [CrossRef]
- Bao, S.; Cao, C.; Chen, W.; Yang, T.; Wu, C. Towards a subtropical forest spectral library: Spectra consistency and spectral separability. Geocarto Int. 2019, 36, 226–240. [Google Scholar] [CrossRef]
- Laabassi, K.; Belarbi, M.A.; Mahmoudi, S.; Mahmoudi, S.A.; Ferhat, K. Wheat varieties identification based on a Deep Learning approach. J. Saudi Soc. Agric. Sci. 2021, 20, 281–289. [Google Scholar] [CrossRef]
- Babu, A.R.; Lalitha, T.; Anjali, B.; Sree, U.C. Real-Time Crop Growth Tracking and Disease Detection using Machine Learning. In Proceedings of the 2024 IEEE 16th International Conference on Computational Intelligence and Communication Networks (CICN), Indore, India, 22–23 December 2024; pp. 457–461. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).