Abstract
Food safety is a critical global health concern, as the consumption of unsafe food can lead to various acute and chronic diseases. While various preservation methods are employed to prevent food spoilage, it remains a significant issue for the food industry, resulting not only in food waste but also significant economic losses for manufacturers and consumers alike. Furthermore, there is growing consumer concern regarding food quality and safety, leading to the rejection of chemical additives due to their associated health risks. Organic acids, naturally occurring compounds of plants and animals, and produced by various beneficial microorganisms, play an important role in enhancing food flavor, preserving nutritional quality, and extending the shelf life of food products. Recognized for their antimicrobial potential, organic acids are commonly utilized as food preservatives, thus contributing to food safety. This review focuses on organic acids as natural preservatives within the food industry. It delves into their chemical structures, mode of action in cells, the types commonly used in preservation along with their general properties, and their antimicrobial activity against bacteria, yeasts, and fungi. These insights are drawn from the published literature, providing comprehensive understanding of the role organic acids play in ensuring food safety and maintaining food quality.
1. Introduction
The preservation of food is a paramount concern for the food industry and consumers worldwide. Microbiological deterioration stands as one of the leading causes of food quality and safety loss, posing a significant challenge to the food industry. Various types of method are used to maintain preservation of food such as thermal processing, drying, freezing, refrigeration, irradiation, fermentation, modified atmosphere packaging, and the addition of antimicrobial agents, such as salts or other chemical or biological preservatives [1].
One of the most effective approaches to combat this deterioration is using natural antimicrobial agents, such as organic acids. Organic acids are chemical compounds found in a variety of natural foods such as fruits, vegetables, and fermented food products such vinegar, among others. They play a crucial role in preserving foods by inhibiting the growth of unwanted microorganisms such as bacteria, fungi, and yeasts. The antimicrobial capability of organic acids has been recognized and utilized for centuries in traditional food preservation techniques such as fermentation and acidification [2].
The utilization of organic acids as antimicrobial agents in food preservation has gained increasing interest due to their proven effectiveness, as well as consumer demand for natural ingredients and more sustainable production processes. Furthermore, rising concerns about food safety and antimicrobial resistance have driven the search for safe and effective alternatives to synthetic chemical preservatives including sorbates (E200–E203), nitrates (E251 and E252), nitrites (E249 and E250), and sulfites (E220–E228) [2,3]. In contrast to synthetic preservatives, which may pose health and environmental risks, organic acids such as lactic, acetic, and citric acids are naturally biosynthesized through microbial fermentation, resulting in a lower carbon footprint and reduced environmental impact compared to their chemically synthesized counterparts [4]. Of all the biological preserving agents, organic acids have the third largest production market in the world, and they are widely used as antimicrobial agents in the food industry [5]. Many organic acids and their salts are approved or listed as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) regulations. In addition, they are used in preservation, such as acidulants, antioxidants, flavoring agents, pH adjusters, and even nutrients as constituents of food through different processes such as biochemical metabolism, hydrolysis, and microbial activity [6,7].
This review provides a comprehensive overview of recognized and industrially utilized organic acids, focusing on their structural characteristics, antimicrobial properties, and diverse applications in food preservation. It focuses on their mechanisms of action at the cellular level and highlights their individual and combined efficacy as natural preservatives, drawing upon a wealth of recent and past studies. By synthesizing current knowledge and identifying gaps in the literature, this review aims to enrich scientific discourse and catalyze research on innovative applications of organic acids. It seeks to inspire advancements that support their effective integration into modern food preservation strategies, meeting the growing demand for safe, high-quality, and eco-friendly solutions.
2. General Characteristics of the Organic Acids
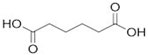
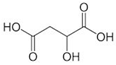
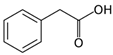
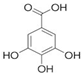
Organic acids typically occur naturally as constituents of various food products or are produced as fermented by-products by ubiquitous organisms such as bacteria, fungi and yeasts [8]. They are characterized as organic carboxylic acids with the general structure R-COOH, which includes fatty acids and amino acids. Organic acids are classified based on the nature of their carbon chain (aromatic, aliphatic, alicyclic, and heterocyclic) and functional groups, categorizing them into monocarboxylic, dicarboxylic, alpha-hydroxyl, and sugar acids [9]. Monocarboxylic acids, such as acetic, propionic, and sorbic acid, are examples of this classification. Dicarboxylic acids include adipic, fumaric, and succinic acid. Alpha hydroxyl acids encompass citric, lactic, and malic acid, while sugar acids consist of ascorbic, gluconic, and tartaric acid [10].
Short-chain organic acids, those with ten or fewer carbon atoms, are often associated with antimicrobial activity. Additionally, the antimicrobial effectiveness of organic acids relies on several key properties, including their ionic form, pKa value, molecular weight, minimum inhibitory concentration, the specific microorganism targeted, the buffering properties of the food matrix, and the duration of exposure to acid [11]. It is notable that most organic acids with antimicrobial properties possess a pKa between 3 and 5, making them well suited for use in food preservation [12].
Organic acids are only partially dissociated in aqueous solutions. Those with low molecular masses, such as lactic acid, are highly soluble in water, whereas acids with higher molecular masses, such as benzoic acid, tend to remain insoluble in their molecular form [13]. The use of organic acids as preservatives offers significant advantages due to their simple molecular structure and low molecular size, which enable them to penetrate microbial cells efficiently. Once inside, they disrupt intracellular activities, ultimately causing cell death [4]. The characteristics of organic acids are commonly utilized in the food industry, including their chemical formula, molecular structure, and additional critical properties, such as pKa, solubility, and GRAS status. These will influence their application and function, as presented in Table 1.

Table 1.
Chemical structure formulas, molecular structure, and additional critical properties of various organic acids that are widely employed as food preservatives.
3. Mode of Action of the Organic Acids on Cells
Organic acids are recognized for their impact on microbial activity through two distinct mechanisms: cytoplasmic acidification and the accumulation of the dissociated acid anion to toxic levels [25]. The primary mode of action of these lipid-soluble weak acids is through direct extracellular pH acidification, which releases protons into the surrounding medium. The extent of pH reduction is influenced by factors such as the acid constant (pKa) and dissociation constant (Ka) of the organic acid [26]. The antimicrobial efficacy of organic acids is highly dependent on the food’s pH. When the pH is lowered below that of the organic acids, their polarity diminishes, enhancing acid diffusion across microbial membranes and into the cytoplasm [9]. Once inside the cytoplasm, organic acids dissociate, releasing acid anions and protons (Figure 1). As H+ ions accumulate, the cytoplasmic pH drops, disrupting normal cellular functions [27].

Figure 1.
Action mechanism of organic acid on microbial cells. The undissociated acid form diffuses through the microbial membrane when the intracellular pH is higher than the surrounding environment of the cell. Once inside, the intracellular pH of acid causes cell damage.
Lowering the cytoplasmic pH reduces the energy available for microbial growth and disrupts the electrochemical gradient needed for active nutrient transport. To counteract this acidification, microbial cells actively pump out excess protons via H+-ATPase, a process that consumes large amounts of ATP, further depleting cellular energy stores [9]. Excess H+ ions also cause osmotic stress, leading to cell damage and disrupting macromolecule synthesis (nucleic acids, proteins, lipids, carbohydrates). This inhibits key enzymes such as pyruvate decarboxylase, which is essential for energy metabolism [28]. According to the weak acid theory, organic acids in their undissociated state exhibit higher lipophilicity, allowing them to penetrate microbial membranes more effectively at lower pH levels, which enhances their antimicrobial potency. Once inside, they dissociate in the neutral or slightly alkaline cytoplasmic environment, releasing protons and acid anions that disrupt cellular metabolism [13]. Notably, propionic acid remains undissociated at low pH, allowing it to penetrate fungal cell membranes more effectively and disrupt essential cellular processes, making it more potent than acetic and lactic acids in antifungal activity [29]. Additionally, some organic acids regulate oxidative stress; for example, gallic acid exhibits strong radical-scavenging activity, protecting cells from oxidative damage [23]. Similarly, phenyllactic acid interferes with bacterial quorum sensing and biofilm formation, preventing microbial adhesion [30].
4. Antimicrobial Activities of Organic Acids and Their Interactions
4.1. Acetic Acid
Acetic acid (ethanoic acid), also known as vinegar, is a weak organic acid widely utilized in the food industry. It serves as a significant microbial growth inhibitor in the food industry, employed as a preservative, an efficient solvent, or an intermediate ingredient for various commercial-grade chemicals. Acetic acid finds application as an antimicrobial agent, a food enhancer, an acidulant, a component in food packaging materials, and even as a ripening agent [14].
Several studies have highlighted the potential of acetic acid as a preservative agent, capable of inhibiting the growth of food spoilage bacteria, yeast, and fungi. Research conducted by [31] demonstrated the antimicrobial efficacy of a 1% acetic acid and 8 mM ZnO treatment evaluated on sheep meat inoculated with L. monocytogenes PTCC1163, E. coli PTCC1394, and S. aureus PTCC1431 at a concentration of 7 log CFU/g. After a 12-day incubation at 4 °C, the treatment resulted in a reduction in L. monocytogenes by 4.09–4.72 log CFU/g, E. coli by 0.84–1.24 log CFU/g, and S. aureus by 2.12–2.75 log CFU/g. The matrix, with its higher moisture and fat content, may facilitate acetic acid penetration, lowering its MIC compared to dry food matrices. In contrast, the study by [32] evaluated the antimicrobial effect of acetic acid, cinnamic aldehyde, and thymol on alfalfa seeds and sprouts, with a treatment of 1000 mg/L of air for 1, 3, and 7 h at 60 °C. The treatment resulted in a >3 log10 CFU/g reduction in Salmonella spp. S. Montevideo (G4639), S. Infantis (H3517), S. Anatum (H3536), S. Cubana (H7976), and S. Stanley H1256 after 7 h at 60 °C, and a >1.7 log CFU/g reduction after 12 h at 50 °C. Additionally, pH was found to significantly influence the antimicrobial activity of acetic acid. The study by [33] observed that acetic acid, at concentrations of 0.125% w/v and 0.25%, exhibited the highest antimicrobial activity against most bovine mastitis pathogens, including Staphylococcus spp. (S. agalactiae, S. aureus, S. uberis, S. epidermidis, S. xylosus, S. hyicus, S. haemolyticus) and Klebsiella spp. However, acetic acid showed reduced efficacy against E. coli in comparison to lauric and caprylic acids. The synergic effect of organic acid and other preservatives is influenced by pH and the targeted compound. For example, the study by [34] demonstrated that chitosan nanoparticles (ChNPs) at concentrations of 30 mg/mL and 40 mg/mL exhibited significant antimicrobial activity. At 30 mg/mL, ChNPs inhibited 92% of S. aureus (MTCC 734), producing a zone of inhibition of 12 mm, and 85% of E. coli (MTCC 723), with a zone of inhibition of 14 mm. At 40 mg/mL, ChNPs inhibited 85% of both E. coli and K. pneumoniae (MTCC 109), with a zone of inhibition of 12 mm for both pathogens.
Several studies have explored the antimicrobial properties of acetic acid, particularly its effectiveness against fungal pathogens. In the study by [35], the synergistic effects of formic acid and acetic acid at a concentration of 0.25% were investigated against Candida albicans, C. guilliermondii, and C. lusitaniae. Inoculum concentrations of 105–106 CFU/mL were exposed to acetic acid, which induced apoptosis in approximately 82% of the cells after 4 h of exposure. This supports earlier findings, highlighting acetic acid’s ability to both inhibit growth and induce apoptosis, especially in fungal pathogens. Further supporting these findings, a study on Kombucha tea revealed that higher acetic acid concentrations enhanced its antimicrobial effects, particularly against Candida krusei CCM 8271, Candida glabrata CCM 8270, Candida albicans CCM 8186, Candida tropicalis CCM 8223, and two Gram-negative bacteria (E. coli CCM 3954 and Haemophilus influenzae CCM 4454). Inhibition zones of 16 mm for C. glabrata and 15.81 mm for C. krusei were observed, highlighting its potent antimicrobial activity [36].
The antifungal effects of acetic acid were assessed, with 50 mM acetic acid completely inhibiting the growth of C. gloeosporioides, while 30 mM acetic acid resulted in partial inhibition. The antifungal activity was enhanced at pH 4.0. Furthermore, a combination of 30 mM acetic acid and 0.5% hydrogen peroxide also inhibited the growth of C. gloeosporioides KACC 40804, C. coccodes KACC 40010, and C. dematium KACC 40014. The antifungal activity of acetic acid is enhanced at pH 4.0, due to increased lipophilicity, which improves its penetration into microbial membranes [37]. Another investigation by [38] demonstrated that 5% acetic acid inhibited Aspergillus flavus by approximately 0.03 log CFU/mL, P. purpurogenum by 0.40 log CFU/mL, R. nigricans by 0.09 log CFU/mL, and F. oxysporum by 0.10 log CFU/mL. The 10% acetic acid treatment inhibited A. flavus by approximately 0.75 log CFU/mL and P. purpurogenum by 0.65 log CFU/mL, along with a 0.25–0.40 log CFU/mL reduction in aflatoxin B1 production after 8 days of incubation at 30 °C. Additionally, acetic acid (pH 4.4) significantly extended the shelf life of sourdough bread by 6 days for A. niger (FUA5001) with an MIC of 8.2 ± 3.4 mM, and by 5 days for P. roqueforti (FUA5005) with an MIC of 25.0 ± 5.5 mM [39]. In contrast, the study by [40] showed that 0.5% acetic acid completely inhibited aflatoxin B1 production by A. flavus, while concentrations of 0.01% and 0.05% were ineffective, demonstrating a dose-dependent relationship between acetic acid concentration and its antifungal activity (Supplementary Table S1).
4.2. Adipic Acid
Adipic acid is a water-soluble dicarboxylic acid commonly found in natural sources such as beets and sugarcane. It is used as a nontoxic food-grade additive for flavoring, acidity regulation, and as a gelling aid [41].
Studies on the antimicrobial effects of adipic acid and its derivatives are limited. One derivative, Poly (butylene adipate-co-terephthalate) (PBAT), is classified as a copolymer synthesized through the copolymerization of adipic acid, 1,4-butanediol, and terephthalic acid. These films align with the broader trend in active packaging, where antimicrobial agents like chitosan, essential oils, and biodegradable polymers are incorporated to extend shelf life and enhance food safety. The investigation in [42] indicates that PBAT combined with 3 wt% nisin inhibited the growth of S. aureus (ATCC 25923), Clostridium perfringens (ATCC 3624), and B. cereus (ATCC 9634), resulting in inhibition zones around 10 mm after 24 h of incubation at 37 °C. It was particularly effective against L. monocytogenes, highlighting adipic acid’s potential as an antimicrobial and antifungal agent for food safety applications. PBAT-based films demonstrate antimicrobial activity, with enhanced efficacy upon incorporation of ZnO or CNPs. Further supporting this trend, PBAT/ZnO films exhibited antimicrobial activity: inhibition zones ranged from 11 to 14.1 mm against E. coli and 11 to 15.1 mm against S. aureus, with the zone size increasing proportionally to ZnO concentrations (1–10 wt%) [43]. Notably, the antimicrobial efficacy of PBAT/ZnO films is influenced by ZnO concentration, with increased concentrations resulting in larger inhibition zones, highlighting the importance of formulation. In acidic environments, antimicrobial agent effectiveness increases due to better membrane penetration, while in neutral or alkaline conditions, buffering limits its antimicrobial action [44]. Further studies on PBAT/tannic acid/CNP films at 1.0 wt% exhibited inhibition zones of 14.0 mm against S. aureus and 12.1 mm against E. coli, which further increased to 17.8 mm and 15.3 mm at 5.0 wt% CNPs, respectively [45].
The antifungal activity of adipic acid derivatives has also been explored. AAME (adipic acid monoethyl ester) demonstrated significant antifungal activity against B. cinerea (CECT2100) on tomato leaves and fruits. Treatment with 3 mM AAME reduced the growth and hyphal development of B. cinerea on tomato leaves and fruits. Furthermore, AAME at 16 mM completely inhibited fruit infection, even after washing [46]. Similarly, treatment with adipic acid and 5-carbamoyl ethyl pentanoate in Capsicum annuum plants infected with A. solani resulted in a 61% reduction in necrosis, enhancing plant resistance to the pathogen [47]. A PBAT/PLA film containing carvacrol concentrations (0, 2, and 5%) was applied to bread-and-butter cake and tested against Penicillium sp., A. niger, and Rhizopus sp. The films with 2% and 5% carvacrol effectively extended the shelf life of the cakes by 2–4 days [48]. The comparative analysis of these studies is provided in Supplementary Table S2. These findings reinforce PBAT’s potential as a versatile platform for developing next-generation antimicrobial packaging materials, aligning with ongoing advancements in biodegradable and functional food packaging technologies.
4.3. Butyric Acid
Butyric acid, also known as butanoic acid or 1-Propanecarboxylic acid, is a short-chain volatile fatty acid with applications as a flavoring agent, particularly its esters, such as methyl, ethyl, and amyl butyrate, which are used in the food, beverage, and cosmetics industries [49].
The study in [50] evaluated the antimicrobial efficacy of butyric acid at various concentrations in milk. The results indicated that butyric acid concentrations of 10 mM and 20 mM at pH 5.0, and 40 mM at pH 5.5 and 6.0, effectively inhibited the growth of E. faecium, E. faecalis, E. casseliflavus (ATCC 12817), and K. pneumoniae (ATCC 13883). Furthermore, the antimicrobial activity was more pronounced at lower pH values (4.5–5.0), with a concentration of 16 mM being sufficient to prevent bacterial growth at stomach pH, demonstrating enhanced antimicrobial efficacy at lower pH levels. The study in [51] on broilers tested butyric acid (pH 6.4) concentrations from 250 to 7000 mg/kg, finding 90% inhibition of S. Typhimurium at 1500 mg/kg. Butyric acid showed increased inhibition of C. perfringens at a 10⁵ inoculation. Additionally, 50% mono butyrin (pH 7.4) inhibited C. perfringens at 3000 mg/kg anaerobically and reduced S. Typhimurium in cecal and C. perfringens in intestinal contents. Butyric acid demonstrates a concentration-dependent impact on the growth of A. flavus (ATCC 22546) and aflatoxin production. At higher concentrations (0.5% and 0.1%), butyric acid completely inhibited fungal growth and aflatoxin B1 production [40]. In contrast, at the lower concentration of 0.01%, while fungal growth was less markedly reduced, aflatoxin production remained unaffected. In contrast, butyric acid exhibited significant antifungal activity at concentrations of 1000 and 2000 μmol/L, reducing mycelial growth by 50–61% for A. solani, 48–59% for C. lagenarium, 17–33% for F. oxysporum f. sp. cucumerinum, and 16–19% for F. oxysporum f. sp. lycopersici. At a concentration of 100 μmol/L, only A. solani mycelial growth was reduced. Furthermore, at 2000 μmol/L, spore germination was inhibited in three fungi in PDA medium at 40–50 °C and pH 6.50 (Supplementary Table S3) [52]. The studies demonstrate that butyric acid inhibits fungal growth and disrupts key morphological processes essential for pathogenicity, including biofilm formation and filamentation.
4.4. Caprylic Acid
Caprylic acid is also known as octanoic acid, which is naturally present in coconut oil and goat’s milk. It is widely used in the food industry, particularly in confectionery and dairy products, as an antimicrobial agent [3].
The antimicrobial activity of caprylic acid (CA) encapsulated in mesoporous silica nanoparticles (MSPs) was reported by [53]. They demonstrated that CA-loaded MSPs maintained their antimicrobial efficacy while enabling controlled release. Free CA inhibited S. aureus (CECT 240) and L. monocytogenes (CECT 936) at 15–18.5 mM, E. coli (CECT 1103) and S. enterica CECT 915 at 15–18.5 mM, and E. coli and S. enterica at 18.5–20 mM, while CA-loaded MSPs exhibited activity against S. aureus and L. monocytogenes at 20–22.5 Mm, emphasizing the role of formulation in optimizing antimicrobial efficacy through sustained release and stability. The study by [54] demonstrated that caprylic acid and monocaprylin (MC) effectively reduced E. coli O157:H7 (E6, E10, E22, E137, and E 7927) and L. monocytogenes (ATCC 19115, Scott A, LM24, LM25, and LM116) populations in milk. At 50 mM, both compounds decreased microbial populations below the detectable limit after 48 h at 4 °C. At 25 mM, the compounds reduced microbial populations by 5.0 log CFU/mL within 6 h at 37 °C, showing that temperature and concentration significantly influence antimicrobial effectiveness, with lower temperatures requiring prolonged exposure for complete microbial reduction. Another investigation [55] reported that monocaprylin (1.28 mg/mL) effectively reduced E. coli (ATCC 25922) and S. aureus (ATCC 25923) populations by >5.5 log CFU/mL within 6 h at 25 °C. After 12 h, E. coli showed a reduction of 5.0 log CFU/mL and S. aureus decreased by 2.9 log CFU/mL. Another derivative of caprylic acid, monocaprin, with an MIC of 0.32 mg/mL, was effective against S. aureus (AS1.89), B. subtilis (AS1.1849), P. aeruginosa (AS1.10452), and E. coli (AS1.90) at pH 7.0–9.0. However, its antimicrobial activity was significantly reduced at weakly acidic pH 6.0, demonstrating pH-dependent effectiveness [56]. Caprylic acid and its derivatives exhibit antimicrobial efficacy primarily within neutral to slightly alkaline pH ranges.
Caprylic acid was also studied for its effects on C. albicans (ATCC 90028), showing biofilm inhibition, reduced adhesion, and impaired hyphal development. It effectively inhibited virulence factors at concentrations between 0.25 and 2 mg/mL. At 0.25 mg/mL, caprylic acid caused 5% hemolysis, while capric acid caused 90% at the same concentration [57]. Another study by [58] demonstrated the synergistic antimicrobial effect of caprylic acid (pH 4.8) at MICs of 0.01 to 0.03% and polygalacturonic acid at 0.25 to 1%. The combination effectively eradicated biofilms within 30 to 60 min for MRSA, P. aeruginosa, C. albicans, and E. coli, highlighting synergistic effects. Another recent study by [52] also shows that caprylic acid inhibits C. albicans with a zone of inhibition diameter of 4.0 mm and an MIC of 40 μg/mL. Beyond its potential anticandidal activity, caprylic acid has also shown efficacy in inhibiting the growth of spore-forming fungi. In a study performed by [59], caprylic acid (pH 6.5) at 2000 μmol/L exhibited antifungal activity by inhibiting the mycelial growth of A. solani, C. lagenarium, F. oxysporum f. sp. cucumerinum, and F. oxysporum f. sp. lycopersici. At 1000 μmol/L, it demonstrated selective inhibition against F. oxysporum f. sp. cucumerinum and F. oxysporum f. sp. lycopersici; it was also effective in inhibiting spore germination, except in the case of A. solani. A bioactive subfraction from Vitex mollis fruit, sHF3, containing methyl 4-decenoate, caprylic acid (MIC: 0.125 mg/mL), and 24-methylencycloartanol, exhibited strong antifungal activity against Colletotrichum gloeosporioides. At 0.50 mg/mL, sHF3 inhibited spore germination to 5% within 8 h, in contrast to 80% germination in control and thiabendazole treatments. The antifungal action was associated with membrane disruption in both spores and mycelia [60]. These findings further emphasize that caprylic acid’s antimicrobial activity varies based on pH, concentration, formulation, and microbial species, highlighting the importance of optimizing conditions for its effective application (Supplementary Table S4).
4.5. Citric Acid
Citric acid, also known as a tricarboxylic acid, is naturally found in citrus fruits such as limes, lemons, oranges, pineapples, and grapefruits. It is in high demand globally due to its various applications as an antimicrobial, flavor enhancer, pH regulator, firming agent, and pharmaceutical reagent. In the food industry, it is widely utilized for its antioxidant properties to preserve food and as an acidifier to enhance the flavors and aromas of fruit juices, ice creams, and marmalades [17].
Numerous studies have been published in the literature about the antimicrobial effects of citric acid. In an early study, it was reported that, on a molar basis, citric acid possesses stronger inhibitory properties compared to lactic and acetic acids [61]. The investigation by [62] on the inhibitory capacities of hydrochloric, citric, acetic, lactic, propionic, and phosphoric acids against Yersinia enterocolitica in tryptic soy broth after 24 h at 25 °C showed that citric acid exhibited the strongest antimicrobial activity on an equimolar basis. Citric acid’s activity against Shigella species in plant-based foods, such as tomatoes, lettuce, onions, and parsley, was examined by [63]. Citric acid (pH 4) at 300 ppm exhibited limited inhibitory effects on S. flexneri, achieved 5 log reduction in S. dysenteriae after 10 h of treatment at 37 °C in TSB medium. S. sonnei (KCCM 40949) was reduced by approximately 3 logs after 1 h, and S. boydii (KCCM 41649) and S. flexneri (KCCM 40948) showed reductions of approximately 2 and 1 log, respectively. In lettuce, citric acid for 30 s reduced S. boydii (KCCM 41649), S. flexneri (KCCM 40948), and S. sonnei (KCCM 40949) by 0.7, 1.6, and 1.2 log CFU/g, respectively. Some bacterial species may exhibit greater resistance, particularly in the presence of protective biofilms. Furthermore, many articles have been published on the antimicrobial activity of citric acid, particularly its role as a co-antimicrobial agent, demonstrating synergistic effects when combined with other bio-preserving agents against bacteria. For instance, LAB (lactic acid bacteria) combined with 1% citric acid (pH 3) reduced E. coli O157:H7 (ATCC 43889) to 5.45 log CFU/mL and S. Typhimurium (ATCC 41374) to 3.61 log CFU/mL in 60 min. At 2% citric acid, reductions of 1.96 log CFU/mL for E. coli O157:H7 (ATCC 43889) and 6.24 log CFU/mL for S. Typhimurium (ATCC 41374) were observed [64]. Similarly, ref. [65] evaluated the antimicrobial efficacy of citric acid and acetic acid (1.4% + 0.3%, 1.0% + 0.4%) on E. coli O157:H7, S. aureus (ATCC 25923), and S. Typhimurium (ATCC 14028) in salads. The study demonstrated that E. coli O157:H7 and S. Typhimurium were reduced to undetectable levels within 3 days at 21 °C, while S. aureus showed a 2 log CFU/g reduction at 4 °C after 7 days. These findings indicate that citric acid is particularly effective against Gram-negative bacteria like E. coli and Salmonella, whereas Gram-positive bacteria like S. aureus may exhibit slightly greater resistance. These findings are consistent with [66], demonstrating the synergistic antimicrobial effects of nisin and citric acid. The combination of nisin (16 μg/mL) and citric acid (0.25 mg/mL) exhibited a substantial reduction in bacterial counts in pasteurized milk, with a ≥2 log CFU/mL decrease in L. monocytogenes and S. aureus after 24 h of treatment. The MICs for S. aureus ranged from 0.25 to 0.375 mg/mL, while for L. monocytogenes, the MICs ranged from 0.19 to 0.375 mg/mL. Furthermore, a significant 3.8 log CFU/mL reduction in L. monocytogenes was observed after just 10h of exposure. The synergistic use of bacteriocins and organic acids offers an effective approach to improving antimicrobial activity in food preservation, particularly in controlling pathogenic bacteria in dairy products. This strategy allows for the reduction in organic acid concentrations while retaining their antimicrobial effectiveness, optimizing food safety.
The antifungal activity of citric acid, in combination with tartaric acid, was investigated by [67] against T. mentagrophytes, C. albicans, A. fumigatus, and M. furfur. The MIC and MFC values were found to be 5% and 2.5%, respectively, for T. mentagrophytes and C. albicans; 5% and 1.25% for A. fumigatus; and 10% and 5% for M. furfur. The combination demonstrated stronger antifungal activity compared to tartaric acid alone, exhibiting similar effects to citric acid. The effect of citric acid at pH values of 3.0, 4.0, and 4.5 on S. cerevisiae and Z. bailii was investigated by [68]. At pH 3.0, citric acid reduced the maximum specific growth rate (μmax) of both yeasts by approximately 0.57 log CFU/mL, with no impact on biomass yield (Ysx). At pH 4.0, μmax decreased by 0.82 log CFU/mL, and Ysx by 0.23 log CFU/mL. At pH 4.5, μmax was reduced by 0.84 log CFU/mL, and Ysx by 0.46 log CFU/mL. The citric acid exhibits pH-dependent antifungal activity, inducing greater metabolic disruption at higher pH levels. A study by [38] found that citric acid at 5% inhibited the growth of A. flavus, P. purpurogenum, R. nigricans, and F. oxysporum by approximately 0.02, 0.40, 0.86, and 0.24 log CFU/mL, respectively, after 8 days of incubation at 30 °C. When the concentration increased to 10%, the reduction increased to approximately 0.75, 0.88, 1.04, and 0.93 log CFU/mL, respectively, for the same incubation period. These studies collectively emphasize citric acid’s effectiveness as an antimicrobial and antifungal agent, particularly at lower pH and higher concentrations (Supplementary Table S5).
4.6. Fumaric Acid
Fumaric acid, also known as (E)-2-butenedioic acid or trans-1,2-ethylenedicarboxylic acid, is recognized as one of the relatively strongest weak organic acids. It is widely applied across industries, including the food, chemical, agriculture, and biomedical fields. Additionally, it serves as a food acidulant and beverage ingredient owing to its non-toxic nature [17].
Studies on fumaric acid consistently demonstrate strong antibacterial activity against E. coli O157:H7 (NCTC 12079), S. typhimurium (ATCC 14028), and L. monocytogenes (ATCC 19111). Published research has shown that fumaric acid, both alone and in combination with other antimicrobial agents like chlorine dioxide and nisin, effectively reduces microbial populations in food systems. For example, ref. [69] showed that the combined treatment of 50 ppm chlorine dioxide (ClO2) and 0.5% fumaric acid effectively reduced microbial populations in broccoli sprouts during storage. It achieved a 2.39 log CFU/g reduction in E. coli O157:H7 (NCTC 12079), 2.74 log CFU/g in S. typhimurium (ATCC 14028), and 2.65 log CFU/g in L. monocytogenes (ATCC 19111). Additionally, it reduced total aerobic bacteria and yeasts/molds by 2.70 log CFU/g and 2.46 log CFU/g, respectively, compared to the control. This treatment offers a viable alternative to chlorine-based methods for enhancing microbial safety. Another study [70] demonstrated that fumaric acid (0.25%, pH 2.4; 0.5%, pH 2.3) effectively inactivated E. coli (ATCC 43894), L. monocytogenes (ATCC 19115), and S. Typhimurium (ATCC 14028) after 1 min of dipping at temperatures ranging from 25 °C to 60 °C, achieving complete inactivation of these pathogens at all tested temperatures. S. aureus (ATCC 12598) was reduced by 3.95–5.76 log CFU/mL, with no significant increase in reduction observed when the temperature was increased from 40 °C to 60 °C. At the lower concentration (0.125%, pH 2.6), reductions in E. coli (ATCC 43894), L. monocytogenes (ATCC 19115), and S. Typhimurium (ATCC 14028) ranged from 3.20 to 4.97 log CFU/mL at 25 °C and 4.05 to 5.44 log CFU/mL at 40 °C. The effectiveness of fumaric acid increased with both concentration and temperature. In the study by [71], the treatment involving the addition of 0.2% calcium oxide (CaO) and 0.5% fumaric acid, followed by exposure to slightly acidic electrolyzed water (SAEW) for 3 min and incubation at 4 °C, resulted in the highest microbial reduction among all tested groups. Total aerobic bacteria reduced by 4.08 log CFU/g, coliforms by 4.29 log CFU/g, and yeast and mold by 4.27 log CFU/g, with apples exhibiting the greatest reduction. Additionally, significant reductions were observed for E. coli O157:H7 (4.06–4.80 log CFU/g) and L. monocytogenes (4.06–5.35 log CFU/g), with mandarins showing the highest pathogen inactivation. Furthermore, the authors of [72] support fumaric acid’s rapid antimicrobial action, as 15 s treatment with 3% fumaric acid on chicken meat reduced Salmonella (S. enterica subsp. enterica serotypes Typhimurium, Enteritidis, S. Thompson, S. Heidelberg, and S. Schwarzengrund) by approximately 2.22 log CFU/g at 4 °C. The application of fumaric acid in combination with ultraviolet-A (UV-A) irradiation has proven effective in reducing bacterial contamination in liquid food systems. For example, ref. [73] found that a combination of 0.1% fumaric acid and UV-A for 30 min at 25 °C resulted in reductions of 3.15 log CFU/mL in E. coli O157:H7 (ATCC 35150, ATCC 43889, and ATCC 43890), 2.21 log CFU/mL in S. Typhimurium (ATCC 19585, ATCC 43971, and DT 104), and 3.43 log CFU/mL in L. monocytogenes (ATCC 15313, ATCC 19111, and ATCC 19115) in apple juice, without significantly altering the pH (remaining at pH 3.0). Furthermore, ref. [74] demonstrated that nisin-loaded chitosan-monomethyl fumaric acid nanoparticles (CM-Ns) in orange juice (at 2.7 mg/mL) led to significant reductions in S. aureus (4.09 log CFU/mL), L. monocytogenes (3.65 log CFU/mL), and E. coli O157:H7 (3.0 log CFU/mL) over a 48 h period at 4 °C. In an antifungal study of fumaric acid, the effect of various organic acids combined with thermal treatment on Talaromyces flavus FRR-1265 and FRR-2386 ascospores was evaluated by [75]. The results indicated that in the case of 0.5% (w/v) fumaric acid, complete inactivation of T. flavus ascospores occurred within 20 min at 80 °C at pH 2.5–3.0. At pH 3.5, full activation was achieved within 20 min, followed by inactivation after 40 min. At pH 4.0, activation occurred within 20 min, but spores remained viable throughout the 100 min treatment. At pH 5.0, inactivation was not achieved even after 100 min. At 1.0% (w/v), inactivation was enhanced, but effectiveness decreased with increasing pH. Additionally, concentrations as high as 2.5% of malic, citric, and tartaric acids failed to affect the ascospores (Supplementary Table S6).
4.7. Lactic Acid
Lactic acid (2-hydroxypropanoic acid) is a natural substance found in various fruits and fermented products. It is recognized for its multifunctional roles, including antimicrobial activity against foodborne pathogens, flavor enhancement, antioxidant properties, acidulant functions, flavoring, and as a pH buffering agent [19].
Lactic acid has been widely recognized as an effective antimicrobial agent in food preservation, particularly in controlling E. coli, Salmonella, and L. monocytogenes across different food products. For instance, the study by [76] on beef treated with 1.2% acid for 120 s at 1–2 °C showed a 2.0 log CFU/g reduction in E. coli. Although the antimicrobial effect decreased over time, inhibition persisted for up to 9 days. In comparison, another study by [77] evaluated the application of a 3% lactic acid and 3% acetic acid combination to pork meat surfaces, demonstrating significant antimicrobial activity. Psychrotrophs reduced to 2.87 log CFU/cm2 after 24 h, with a subsequent moderate increase to 5.04 log CFU/cm2 by day 14. Enterobacteriaceae decreased to 0.51 log CFU/cm2 within the first 24 h but increased to 2.75 log CFU/cm2 by day 14. Aeromonas spp. was completely inhibited by day 5, while Yersinia spp. was completely inhibited by day 5. The combination of lactic and acetic acids on pork provides a broader range of microbial inhibition; however, its effectiveness decreases over time. In poultry, the study in [78] demonstrated that a spray (5 s) exposure to 2.5% lactic acid and citric acid reduced Salmonella (Typhimurium ATCC 14028, Heidelberg Sheldon 3347-1, and Enteritidis phage type 13) by 1.3 log CFU/mL, while dip treatments (5–20 s) achieved a 2.3 log CFU/mL reduction. In beef trim inoculated with E. coli O157:H7 and Salmonella (104 CFU/mL), lactic acid alone reduced E. coli by 1.4 log CFU/mL and Salmonella by 1 log CFU/mL. These reductions, while significant, often diminish over time, suggesting a transient antimicrobial effect that requires optimization for long-term efficacy. Lower pH enhances bacterial inhibition, as seen in its ability to disrupt E. coli and S. aureus, with inhibition zones ranging from 24 mm (E. coli) to 38.3 mm (E. faecalis), and MIC of ≥1.25 mg/mL, as demonstrated by the study in [19]. However, the buffering capacity of complex food matrices can minimize acidification effects, leading to variability in efficacy. For instance, the study in [79] reported that a 1.0% lactic acid treatment alone achieved only a 0.5 log reduction in L. monocytogenes on lettuce after 10 min at 22 °C, while the combination of 1.0% lactic acid and 100 ppm chlorine enhanced the reduction to 1.1 log CFU/g, highlighting the benefits of combination treatments. This suggests that food composition, including protein, fat, and carbohydrate content, may influence lactic acid’s penetration and long-term stability. Additionally, lactic acid has been shown to inhibit biofilm formation and quorum sensing, as demonstrated in the study in [80], which found that a 2% lactic acid treatment on cucumbers resulted in a 1 log reduction in E. coli (MTCC 727) and S. typhimurium (ATCC 14028) over six days at 15 °C. Moreover, it reduced E. coli biofilm formation by 0.4 log CFU/g and extracellular polymeric substance (EPS) production by 0.1 log CFU/g, indicating anti-quorum sensing activity. However, despite its initial effectiveness, lactic acid’s antimicrobial activity diminishes over time, suggesting a need for optimization to prolong its effects in food preservation.
The study by [81] found that lactic acid and n-butyl lactate inhibited various yeasts and molds, such as S. cerevisiae, A. niger, T. viride, P. cyclopium, and P. fluorescens, by 100% after 72 h. This suggests a broad-spectrum antifungal activity, a finding that aligns with the study by [82], which examined the in vitro antifungal activity of lactic acid (20–500 mM) against yeast (Pichia anomala J121, Rhodotorula mucilaginosa J350 (CFSQE 63), and Kluyveromyces marxianus J367 (CBS 1555)) and fungi (Aspergillus fumigatus, Aspergillus nidulans J283 (FSGC A4wt), Penicillium commune J238 (IBT 12400), P. roqueforti J268 (IBT 6754), and Fusarium sporotrichioides J304 (ITEM 168) at varying pH levels. For yeasts, lactic acid is effective at 20 mM to >500 mM at pH 3, 60 mM to >500 mM at pH 5, and 500 mM at pH 7. In fungi, the range varied from 8 mM to 500 mM at pH 3, 20 mM to 120 mM at pH 5, and 50 mM to >500 mM at pH 7. The research by [83] assessed the antifungal properties of lactic acid and acetic acid against A. flavus. The MICs for lactic acid and acetic acid were 357.7 mM and 41.6 mM. A synergistic effect was demonstrated at 78.0 mM lactic acid and 31.2 mM acetic acid, at pH 3.38, that effectively inhibited fungal growth. Furthermore, the effect of lactic acid on antifungal activity and toxin production was investigated by [38]. The application of 10% lactic acid concentration demonstrated significant antifungal activity, particularly against F. oxysporum growth, resulting in an approximately 1.37 log CFU/mL reduction and a 0.19 log CFU/mL decrease in aflatoxin B1 production. Similarly, inhibition rates of 0.046 log CFU/mL, 0.08 log CFU/mL, and 0.11 log CFU/mL were observed for A. flavus, P. purpurogenum, and R. nigricans, respectively. It highlights lactic acid’s effect against fungi toxin production, which is particularly relevant for food safety applications. The antifungal activity study revealed that the MICs of lactic acid for the yeasts Rhodotorula sp., C. albicans, and S. cerevisiae were 12.50, 18.75, and 37.50 mg/mL, respectively. The fungicidal concentrations for these species were 25, 37, and 75 mg/mL, respectively. Notably, S. cerevisiae required high lactic acid concentrations due to its lower sensitivity. These findings reveal that high concentrations of lactic acid required for effective antifungal activity present challenges in food applications, primarily due to sensory impacts and cost. Similarly, acetic and propionic acids also require specific concentrations to achieve efficacy. Synergistic combinations of lactic acid with these acids, or the use of controlled-release technologies, could enhance antifungal effectiveness while reducing the necessary concentrations and minimizing adverse effects on food quality (Supplementary Table S7).
4.8. Malic Acid
Malic acid is a dicarboxylic acid and, along with citric acid, is one of the two most prevalent organic acids found in fruits. It serves as the predominant acid in many fruits, including apples, bananas, litchis, and plums. Currently, the primary application of malic acid is in the food and beverage industry, where about 85–90% of the currently produced malic acid is utilized. It is widely used as an acidulant and flavor enhancer in products such as candies, soft drinks, bakery items, and jellies [84].
According to the published literature, malic acid exhibits stronger antimicrobial activity against bacteria compared to yeast and fungi. The antimicrobial activity of malic acid against S. Enteritidis 1.82 (NCTC 9001), L. monocytogenes 1.131 (CECT 932), and E. coli O157:H7 (CECT 4267) in fruit juices (apple, pear, and melon) was investigated by [85]. Results showed that higher concentrations, approximately 1.5–2.5% v/v of malic acid, were efficacious in reducing and inactivating microorganisms by more than 5 log cycles in 24 h at 5 °C. The effective concentrations at 20–35 °C were 0.2% (apple), 0.4% (pear), and 0.6% (melon) for L. monocytogenes and S. Enteritidis, while E. coli required 0.6% (apple), 1% (pear), and 2% (melon). Lower malic acid concentrations were required for inactivation of microorganisms at high temperature. Similarly, the study by [86] demonstrated that a 2.6% concentration of malic acid (pH 3.35) reduced the populations of L. monocytogenes, S. gaminara, and E. coli O157:H7 from 8.3, 9.0, and 8.9 log CFU/mL to 5.5, 3.0, and 6.8 log CFU/mL at 25 °C, respectively. The extent of inactivation depended on the concentration of malic acid, storage temperature, and time. Studies by [85,86] highlight that malic acid’s antimicrobial activity increases with concentration and is temperature-dependent. The study by [87] evaluated the synergistic antimicrobial effect of malic acid and acetic acid on Salmonella spp. in chicken. The treatment, using 5 mg/mL of both acids, led to the complete elimination of Salmonella (S. Heidelberg 271, S. Typhimurium 02:8423, S. Copenhagen PT 99, S. Enteritidis CRIFS 1016, and S. Kentucky 64701) at both 4 °C and 21 °C after 21 days of storage. In another study, the 8 min ultrasound treatment with a concentration of 1% malic acid in lemon juice reduced the initial bacterial count from between 7.2 and 7.6 log CFU/mL to 5, 4.6, 3.5, and 3.8 log CFU/mL for S. Typhi (PTCC 1609), S. dysenteriae (PTCC 1188), L. monocytogenes (PTCC 1298), and S. flexneri (PTCC 1865), respectively [88]. In a recent investigation by [89], the antibacterial activity of malic acid derived from natural extracts of Dillenia indica was explored. The MICs for S. aureus, P. vulgaris, and P. aeruginosa were 150 µg/mL, 200 µg/mL, and 100 µg/mL, and the corresponding MBCs were 450 µg/mL, 600 µg/mL, and 500 µg/mL, respectively. A 0.6% malic acid concentration demonstrated maximum inhibition, with zone diameters of 12 ± 0.2 mm for S. aureus, 9 ± 0.1 mm for P. vulgaris, and 18 ± 0.2 mm for P. aeruginosa.
The inhibitory effects of malic acid (3.0% w/v), nisin (50 IU/mL), and natamycin (0.005 g/mL) film coating (pH 3) on cheese surfaces were investigated by [90]. Results revealed that L. monocytogenes, P. aeruginosa, Yarrowia lipolytica, and P. roqueforti produced zones of inhibition with diameters of 3.3 mm, 1.0 mm, 8.4 mm, and 12.4 mm, respectively. Another study examined the antifungal activity and toxin production, conducted by [38]. The study found that treatment with 5% malic acid inhibited the growth of A. flavus, P. purpurogenum, R. nigricans, and F. oxysporum by approximately 0.09, 0.91, 0.32, and 0.29 log CFU/mL, respectively. Increasing the malic acid concentration to 10% enhanced the inhibition, resulting in reductions of 0.34, 1.25, 0.69, and 0.79 log CFU/mL for A. flavus, P. purpurogenum, R. nigricans, and F. oxysporum, respectively. The antimicrobial effect of maleic acid-capped silver nanoparticles was investigated by [91]. The MICs for Gram-negative and Gram-positive bacteria were 5 ppm and 25 ppm, respectively. At a concentration of 100 ppm (100 µg/mL), the maleic acid-capped AgNPs exhibited distinct inhibition zones as follows: M. luteus (ATCC 10240) (1.25 ± 0.9 cm), S. aureus (ATCC 6538) (1.21 ± 0.75 cm), B. subtilis (ATCC 6633) (1.31 ± 0.23 cm), A. tumefaciens (AT-10) (3.1 ± 1.33 cm), S. Setubal (ATCC 19196) (1.66 ± 0.5 cm), and E. aerogenes (ATCC 13048) (3.23 ± 0.34 cm). Furthermore, at a higher concentration of 500 ppm, maleic acid-capped AgNPs exhibited a growth inhibition of 50.56% against A. flavus and 25.72% against A. niger. Based on these findings, the food matrix might reduce the effectiveness of malic acid due to factors like reduced acid availability and binding to food components. Thus, further studies are needed to evaluate how the food matrix influences malic acid’s antimicrobial activity, particularly in complex food environments (Supplementary Table S8).
4.9. Phenyllactic Acid
Phenyllactic acid, also known as 2-hydroxy-3-phenylpropanoic acid, is a naturally occurring compound found in various plants and food products, including Chinese herbs, peanut skins, rye, honey, and fermented milk. It finds extensive use in food safety, as well as in the feed, pharmaceutical, and cosmetic industries [21].
The antimicrobial activity of phenyllactic acid was studied by [21], revealing that the MICs for L. monocytogenes, E. coli were 1.25 mg/mL and 2.5 mg/mL, respectively. Based on the MIC, the growth of L. monocytogenes was inhibited after 2 h of exposure to PLA, whereas E. coli required 12 h for inhibition. A subsequent study by [92] on beef demonstrated that 1.5% phenyllactic acid reduced E. coli O157:H7 and S. Typhimurium by 0.38 log CFU/g and 0.86 log CFU/g within 5 min at 21 °C. Additionally, in treated beef stored at −20 °C for 7 days, the inactivation of E. coli O157:H7 and S. Typhimurium was enhanced, with reductions of 1.06 and 1.46 log CFU/g, respectively. This highlights the effectiveness of phenyllactic acid in reducing bacterial populations on beef and its enhanced inactivation potential when combined with freezing, suggesting that these methods may help optimize phenyllactic acid’s use in food preservation and pathogen control. The study by [30] demonstrated that phenyllactic acid exhibits significant antibiofilm activity, resulting in a reduction in Enterobacter cloacae populations by approximately 6 log CFU/mL, while biofilm formation was inhibited by approximately 2.83 log CFU/mL after a 10 min exposure. Phenyllactic acid (10 mg/mL) in combination with bacteriocin XJS01 (2.0 mg/mL) was evaluated by [93] for its antibacterial and biofilm-inhibitory effects against Shigella flexneri. The MICs of phenyllactic acid and XJS01 were 2.45 mg/mL and 18.75 μg/mL, respectively. Treatment with 1/2MIC significantly reduced S. flexneri, achieving a reduction to 2.88 ± 0.17 log10 CFU/mL, compared to the control (8.06 ± 0.31 log10 CFU/mL). Additionally, this combination was effective in inhibiting biofilm formation.
An investigation by [94] demonstrated that phenyllactic acid, produced by Bacillus licheniformis isolated from fermented soybeans, exhibited inhibition at concentrations of 250 µg and 500 µg. At a concentration of 500 µg, the inhibition zones were observed to increase as follows: S. aureus (KCTC 1928): 13.2 mm; S. epidermidis (KCTC 1917): 12.8 mm; B. subtilis (KCTC 1928): 13.3 mm; L. plantarum (KCTC 3104): 11.2 mm; L. mesenteroides (KCTC 3100): 9.2 mm; M. luteus (KCTC 3523): 12.7 mm; S. pyogenes (KCTC 3096): 8.2 mm; E. coli (ATCC 10536): 13.7 mm; P. aeruginosa (KCTC 2513): 8.8 mm; C. albicans (KCTC 7965): 8.2 mm; and S. cerevisiae (KCTC 7904): 7.8 mm. In addition, phenyllactic acid demonstrated fungicidal activity, with MIC and MFC ranging from 3.7 to 7.5 mg/mL and 3.7 to 10 mg/mL, respectively. At concentrations of 5 and 7.5 mg/mL, phenylacetic acid caused significant inhibition, reducing fungal growth by 1.5–2 log CFU/mL across various fungal strains, including P. roqueforti (IBT1868), A. niger (FTDC322), A. flavus (FTDC3226), P. citrinum, P. verrucosum (FR22625), Fusarium spp. (ITEM515), P. chrysogenum (ITEM5151), P. commune (ITEM5150), and P. solitum (ITEM5149) after 72 h of incubation [95]. Furthermore, phenyllactic acid exhibited significant antifungal activity against Penicillium digitatum (INTA 2) at a concentration of 0.8 mM, with growth inhibition assessed after 72 h at pH 5. The antifungal potential of phenyllactic acid was found to be about 600-fold more effective compared to lactic acid [96]. Another study by [97] determined the MICs of phenyllactic acid as 25 mM for P. roqueforti, 60 mM for A. niger, and 30 mM for C. cladosporioides. At pH 4.4, A. niger exhibited growth similar to the control, while at pH 2.9, growth was significantly inhibited, demonstrating that phenyllactic acid’s inhibitory effect is more pronounced under acidic conditions. Additionally, increasing the concentrations resulted in a dose-dependent reduction in both growth and sporulation of A. niger. In comparison, the study by [98] identified energy metabolism as the target of phenyllactic acid against R. oryzae, with an MIC of 8 mg/mL, where it inhibits glycolytic and TCA cycle enzymes, reducing ATP production and inducing ROS-mediated apoptosis, emphasizing the potent effect on fungal growth and metabolic disruption under different experimental conditions. Phenyllactic acid exhibits potent antimicrobial and antibiofilm activity, yet its use in food preservation is limited by uncertainties in stability, synergy, and regulatory approval (Supplementary Table S9). Future research should assess its efficacy in diverse food matrices and clarify its mechanisms.
4.10. Propionic Acid
Propionic acid, also known as ethane carboxylic acid, is naturally present in low quantities in milk and higher concentrations in dairy products such as yogurt and cheese [99]. It has gained significant attention for its diverse applications, serving as an antimicrobial and anti-inflammatory agent, herbicide, food preservative, and artificial flavoring [100].
The potential antimicrobial activity of propionic acid on various bacteria, yeasts, and fungi makes it an effective alternative to synthetic preservatives. It has shown potential in reducing Salmonella spp. and also other bacteria (such as E. coli, E. freundii, Proteus vulgaris, P. aeruginosa, P. fluorescens, and Serratia marcescens) in poultry, as investigated by [101]. Concentrations ranging from 0.2% to 0.4% have been observed to inhibit microbial growth. In another investigation by [102], the antimicrobial properties of propionic acid were assessed across various microorganisms. The study demonstrated that for bacteria (A. aceti (KCTC12290), A. calcoaceticus (NCCP16013), A. salmonicida (KCCM40239), C. coli (ATCC33559), C. jejuni (ATCC33560), E. coli (NCCP16186, 16185), and S. Typhimurium (NCCP12441, 12219), MICs ranged from 400 ppm to 1600 ppm, indicating a strong bacteriostatic effect. Yeasts (C. lipolytica (NCCP32688), S. cerevisiae (KCTC7296), B. bruxellensis (KCCM11490), D. hansenii (KCCM50192), and M. guilliermondii (KCTC27416)) required higher concentrations, ranging from 1600 ppm to 6400 ppm, to effectively inhibit their growth. Fungal species (A. flavus (KCCM60330), A. niger (NCCP32627), A. oryzae (NCCP32629), A. versicolor (KCCM60336), C. cladosporioides (KCTC26745), C. sphaerospermum (KCTC26739), G. capitatum (NCCP32601), M. plumbeus (KCCM60265), P. roqueforti (KCTC6080), and R. oryzae (KCTC46312) exhibited moderate resistance with MICs between 800 ppm and 3200 ppm. Additionally, the antimicrobial effectiveness of propionic acid was influenced by the type of food matrix, with unprocessed animal products demonstrating lower MIC values compared to processed foods, due to differences in pH and composition. Propionic acid (1.25–2000 mM) also inhibits the growth of bacteria and yeast, as noted by [103]. For instance, it reduces the growth of S. aureus ATCC 6538P by 1 log CFU/mL at concentrations above 25 mM and completely suppresses it at concentrations of 100 mM or higher. It inhibits the growth of C. albicans and E. coli ATCC 8739 at concentrations exceeding 10 mM, with complete elimination observed at 25 mM and 50 mM, respectively. Propionic acid was examined at the range between 3.6 and 14.0 mM, with MICs of 7.0 ± 0.2 mM for E. coli, 6.1 ± 0.1 mM for K. pneumoniae (MSCL535), 5.7 ± 0.4 mM for P. aeruginosa (ATCC 9027), 3.6 ± 0.3 mM for B. subtilis (ATCC 6633), and 10.2 ± 0.9 mM for S. aureus (ATCC 6538P). Propionic acid showed the strongest inhibition against B. subtilis, with similar activity to acetic acid against E. coli, and comparable effects to acetic and formic acids against K. pneumoniae, P. aeruginosa, and S. aureus [104].
The antifungal activity and toxin production inhibition properties of propionic acid were explored by [38]. Treatment with 5% propionic acid inhibited A. flavus, P. pur-purogenum, R. nigricans, and F. oxysporum by approximately 0.133, 0.092, 0.085, and 0.084 log CFU/mL, respectively. At a 10% concentration, the inhibition percentages increased to 0.225, 0.166, 0.134, and 0.119 log CFU/mL, respectively. The synergistic antifungal effects of propionic acid with lactic and acetic acids were examined on mold isolated from bakery products by [29]. The MICs were as follows: A. niger (UBOCC-A-112064) (18.8 mmol/L), P. corylophilum (UBOCC-A-112081) (19.4 mmol/L), and E. repens (UBOCC-A-112075) (8.7 mmol/L). The combination of acetic acid (5 mmol/L) and propionic acid (15 mmol/L) led to complete inhibition of growth in A. niger and P. corylophilum after 35 days at pH 3.8 and 25 °C. In another study on the fungicidal effects of propionic acid, MIC values ranged from 6.1 to 31 mM. A. chevalieri exhibited an 80% reduction in germination rate, while A. proliferans showed a 49% reduction, resulting in 62% cell death. P. lanosocoeruleum exhibited a 28% decrease in conidia germination and extensive biofilm cell death (85%) after a 30 min treatment with 31 mM propionic acid (pH 3.9) [105]. The observed variability in MIC values and inhibition highlights the differential susceptibility of fungal species to propionic acid, with its activity being influenced by both concentration and environmental factors, particularly pH (Supplementary Table S10).
4.11. Gallic Acid
Gallic acid (3,4,5-trihydroxybenzoic acid) is a phenolic acid naturally found in beverages such as red wines and green tea, as well as in fruits including strawberries, berries, and grapes (especially in grape seeds). It is widely utilized in the food industry as an antioxidant, antimicrobial agent, stabilizer, and material for food packaging. Specifically, it serves as an antimicrobial agent, food preservative, oil stabilizer, active ingredient in food packaging materials, and stabilizer in food processing [23].
Antimicrobial studies of gallic acid against Pseudomonas strains to prevent the spoilage of black truffles were investigated by [106]. The results showed an MIC of 2.5 mg/mL for P. fragi (DSMZ 3456) and P. putida (DSMZ 291), while P. fluorescens (DSMZ 50090) had an MIC value of 5 mg/mL. The log reductions observed in the microbial populations of black truffles treated with 2.5 mg/mL gallic acid for 10 min and stored at 4 °C for 28 days were as follows: 1.2 log for Total Mesophilic Count (TMC), 1.1 log for Pseudomonas spp., 0.25 log for Enterobacteriaceae, and 0.87 log for Eumycetes. Antimicrobial films aimed at inhibiting C. jejuni NCTC12145 growth by incorporating gallic acid into zein-based films were investigated by [107]. The results showed that gallic acid concentrations between 2.5 and 10 mg/cm2 were effective against C. jejuni strains. The appropriate concentration of gallic acid in films is crucial for modulating the release rate and enhancing antimicrobial efficacy, with higher gallic acid content demonstrating a more rapid antimicrobial effect. In another study, gallic acid demonstrated antimicrobial activity against various spoilage bacteria with MIC and MBC values as follows: E. coli: 1500 µg/mL and 5000 µg/mL; P. aeruginosa: 500 µg/mL and 500 µg/mL; S. aureus: 1750 µg/mL and 5250 µg/mL; and L. monocytogenes: 2000 µg/mL and 5500 µg/mL [108]. Research by [109] demonstrated that gallic acid at a concentration of 8 mg/mL, applied to vegetables, exhibited significant antimicrobial activity, with inhibition zones of 15.6 mm and 13.2 mm for E. coli and S. mutans, respectively. In addition, 48 h of treatment at 25 °C resulted in a 0.206 log CFU/g reduction in E. coli in cabbage and 0.215 log CFU/g reduction in spinach. Furthermore, S. mutans in spinach exhibited a 0.215 log CFU/g reduction, indicating the substantial antimicrobial efficacy of gallic acid in reducing microbial populations in vegetables. However, further research is required to assess its stability and interactions with complex food matrices.
Gallic acid exhibited potential antifungal activity, demonstrating significant inhibition of Candida species C. albicans (ATCC 90028), C. glabrata (ATCC 2001), C. parapsilosis (ATCC 22019), and C. tropicalis (ATCC 750) and biofilms at concentrations as low as 0.156 mg/mL at 37 °C for 48 h. Gallic acid displayed considerable efficacy against Candida biofilms, resulting in a 2 log reduction in cell viability at a concentration of 5 mg/mL [110]. The antifungal activity of octyl gallate against S. cerevisiae ATCC 7754 and Z. bailii ATCC 60483 was assessed by [111]. The MIC and MFC values were 50 μg/mL for Z. bailii, and 12.5 μg/mL and 25 μg/mL for S. cerevisiae. Time-kill kinetics highlighted a 2 h reduction in cell viability at MFC at 30 °C, and complete elimination within 1 h at 2 × MFC, indicating rapid fungicidal activity. Another study assessed chitosan with gallic acid conjugate (Ch28GA) at a concentration of 2 g/L applied to artificially inoculated tomatoes with B. cinerea (VKM F-2712) and stored at 25 °C for 14 days. The treatment led to a significant reduction in fruit rot, achieving a 0.77 log CFU/g decrease in fungal growth, demonstrating the effective antifungal properties of Ch28GA in controlling post-harvest spoilage caused by B. cinerea [112] (Supplementary Table S11).
4.12. Succinic Acid
Succinic acid, also known as butanedioic acid or 1,2-ethanedicarboxylic acid, is utilized as an additive in various industries, including the food industry [10].
There are limited studies on the antimicrobial effect of succinic acid in the literature. The antimicrobial effect of succinic acid treatments (1%, 3%, and 5%) on Pseudomonas reduction and shelf life extension was evaluated in broiler chicken legs subjected to 60 °C for 1–3 min of dipping. The 3% treatment at 24 °C resulted in a log reduction from 1.12 to 1.61. At 60 °C, the reduction increased to 1.21 to 1.98 and shelf life extended from 2 to 3.5 days. In comparison, 5% treatment at 24 °C resulted in a log reduction of 1.04 to 1.61, while at 60 °C, the reduction increased to 1.13 to 1.98 log with a shelf life extension of 4.5 to 5 days. But it also caused whitening of the skin and discoloration in the treated poultry [113]. Another study investigated the antimicrobial activity of succinic acid with MICs in Japanese apricot fruits, conducted by [114]. They found that the MICs for E. coli ATCC35218, B. subtilis ATCC6633, and Streptococcus suis were 1.667, 6.667, and 32 mg/mL, respectively. The antimicrobial activities of zein films cross-linked using succinic anhydride were investigated by [115]. The antimicrobial activity of nisin-loaded zein films against L. monocytogenes (ATCC 19116) was evaluated by measuring the zone of inhibition. The results showed a concentration-dependent increase in the zone of inhibition: 10 mm at 2000 AU/mL, 14 mm at 3000 AU/mL, and 18 mm at 4000 AU/mL. The films also exhibited varying antimicrobial activity against other bacterial strains, with inhibition zones of 11–15 mm for B. cereus (ATCC 49064), 11–15 mm for S. enterica (ATCC 25566), 5–10 mm for Y. enterocolitica (ATCC 23715), and 1–4 mm for E. coli. Further research by [116] revealed that a 0.25% succinic acid concentration in agar medium completely inhibited the growth of several Salmonella strains (S. Enteritidis ATCC 13076, S. Typhimurium, S. Hadar, S. Infantis, and S. Virchow). Moreover, when applied to broiler chicken meat, succinic acid at a concentration of 2% potentially reduced bacterial counts by 1.42, 1.48, 1.22, 1.47, and 1.43 log CFU/g, while a 5% concentration reduced bacterial growth by 2.18, 2.31, 2.00, 3.20, and 2.17 log CFU/g for S. Enteritidis ATCC 13076, S. Typhimurium, S. Hadar, S. Infantis, and S. Virchow, respectively (Supplementary Table S12).
4.13. Tartaric Acid
L-Tartaric acid, also known as (2,3-Dihydroxybutanedioic acid), is naturally found in high quantities in grapes and tamarind, and it is one of the major organic acids found in wine. It is used in the food industry as a taste enhancer, preservative, pH regulator, chelating and firming agent, as well as an emulsifier [10].
There are limited studies on the antimicrobial effect of tartaric acid in the literature. A recent study [117] evaluated tartaric acid derivatives as antimicrobial agents for food packaging, demonstrating their efficacy against P. syringae, P. aeruginosa, X. beticola, and S. maltophilia. Inhibition was observed at 37 °C over time points of 12, 24, 48, 76, and 96 h. These results highlight the potential of tartaric acid derivatives as viable alternatives to traditional antimicrobials in food preservation. Tartaric acid’s antifungal activity against Trichophyton mentagrophytes var. mentagrophytes, Candida albicans, Aspergillus fumigatus, and Malassezia furfur was investigated by [67]. The MIC90 and MFC values for tartaric acid are as follows: for T. mentagrophytes, 5% and 2.5%; for A. fumigatus, 15% and 10%; for M. furfur, 15% and 10%; and for C. albicans, 20% and 15%. For the combination of tartaric and citric acid, the values are, for T. mentagrophytes, 5% and 2.5%; for A. fumigatus, 5% and 1.25%; for C. albicans, 5% and 2.5%; and for M. furfur, 10% and 5%. The combination of tartaric acid with citric acid showed the highest inhibition of fungi. The treatment of 0.75% tartaric acid combined with Plasma-Activated Water (PAW) treatment on lettuce, activated for 50 to 60 min, resulted in significant antimicrobial activity against S. aureus (ATCC 6538), achieving reductions of 5.5–6.0 log CFU/mL after a 10 min exposure. These results highlight the potential of TA and PAW treatment as effective antimicrobial strategies for enhancing food safety [118]. In another study by [119], the antimicrobial effect of 1% tartaric acid on L. monocytogenes (ATCC13932) was evaluated in cheese over 42 days. The study reported a reduction in L. monocytogenes counts from 6.67 log CFU/g to 4.95 log CFU/g on day 0, which further decreased to 4.17 log CFU/g by day 42 at 4 °C. Tartaric acid derived from plants exhibits significant antimicrobial activity, with in vitro MICs recorded as follows: S. aureus (5 mg/mL), L. monocytogenes (2.5 mg/mL), E. coli (1.25–2.5 mg/mL), S. enterica Typhimurium (2.5 mg/mL), G. candidum (20–50 mg/mL), and P. candidum (100 mg/mL). These results demonstrate strong antibacterial effects, while the compound shows comparatively weaker antifungal properties [120]. Another study investigating the antifungal activity and toxin production of tartaric acid was conducted by [38]. The results indicated that treatment with 5% tartaric acid resulted in the lowest inhibition rates for A. flavus, P. purpurogenum, R. nigricans, and F. oxysporum of about 0.0018 log CFU/mL, 0.0287 log CFU/mL, 0.0403 log CFU/mL, and 0.0074 log CFU/mL, respectively. The concentration of 10% tartaric showed inhibition rates of 0.0792 log CFU/mL, 0.0829 log CFU/mL, 0.1165 log CFU/mL, and 0.0090 log CFU/mL, respectively (Supplementary Table S13). More studies are needed to explore the effectiveness of tartaric acid across different food types with varying pH levels, moisture contents, and nutrient profiles. This would help determine the optimal concentrations of tartaric acid for various applications.
5. Application of Organic Acids in Combination with Other Bioactive Compounds in Food Preservation
Recent research highlights that combining organic acids with bioactive compounds, such as essential oils and plant extracts, enhances antimicrobial activity, improving food safety and quality. This synergistic combination not only inhibits microbial growth but also prevents oxidation and enzymatic degradation, preserving nutritional content and sensory attributes [121]. In addition, the nanoencapsulation method improves the stability and controlled release of bioactive compounds, extending their antimicrobial activity. This technology protects sensitive ingredients from degradation, enhancing their efficacy in complex food systems and promoting a sustainable preservation method [122]. Their dual role as functional additives and safety enhancers underlines their value in modern food preservation. Importantly, organic acids and their derivatives are GRAS by regulatory bodies, provided they adhere to prescribed usage limits and guidelines such as those established by EU legislation [13]. Table 2 compiles a comprehensive range of applications reported across various food products, showcasing the breadth of their efficacy and adaptability.

Table 2.
Antimicrobial applications of organic acids and synergic combination with other natural antimicrobial compounds in inhibiting foodborne pathogens across diverse food products.
In summary, this table highlights the antimicrobial effectiveness of organic acids combined with essential oils and plant extracts against various microorganisms.
The study by [123] demonstrated that fermented spinach (0.08%) with ascorbic acid, malic acid, citric acid, and tartaric acid (0.06%) enhanced redness, reduced residual nitrite, and inhibited oxidation in meat. Ascorbic acid (0.06%) was the most effective, highlighting the potential of natural nitrite sources and organic acids to improve cured meat quality. Similarly, the study published by [124] investigated the inhibition of S. Typhimurium growth with a combination of succinic acid, pyruvic acid, and oregano essential oil. The results indicated that these acids, when combined with OEO, exhibited strong inhibition of S. Typhimurium. The maximum inhibition observed was 1.0 log CFU/g with the combination of 6% succinic acid and 0.08% of OEO. Furthermore, 5 min exposure to a mixture of 1.5% succinic acid and 0.02% OEO resulted in a 6 log CFU reduction after 48 h.
These findings align with a recent study investigating the synergistic effect of OEO and citric acid in thermal inactivation during cooking of salmon [125]. The combination of 1% OEO and 0.5% citric acid resulted in a significant reduction in the D-values of L. monocytogenes (ATCC 7644), with the D-values decreasing from 5.50 °C in the control group to 6.92 °C in the combined treatment. These results indicate that the combination of oregano oil and citric acid increases the thermal sensitivity of L. monocytogenes (ATCC 7644), thereby shortening the time required for inactivation.
The combination of oregano and cranberry extracts (0.1 mg/mL total phenolic, 50:50, wt/wt) with lactic acid (pH 6.0) exhibited a synergistic antimicrobial effect, leading to the complete inhibition of V. parahaemolyticus in cod fillets and shrimp stored at 4 °C for 8 days. In contrast, treatments with oregano and cranberry extracts only achieved a 4.6 log CFU/g reduction [126]. In comparison, a study on the synergistic activity of malic acid and grapefruit seed extract on lettuce conducted in [127] demonstrated the antimicrobial effects of a combination treatment using 1% malic acid and 0.5% grapefruit seed extract. The treatment resulted in reductions of 4.96 log CFU/g for E. coli O157:H7 (ATCC 43895, ATCC 35150, and ATCC 43894), 4.80 log CFU/g for S. Typhimurium (ATCC 19585, ATCC 6994, and ATCC 14028), and 3.95 log CFU/g for L. monocytogenes (ATCC 7644, ATCC 19111, and ATCC 19115) over a 14-day storage period at 5 °C. The combination of malic acid and grapefruit seed extract provides a longer-lasting preservative effect, offering extended shelf life during storage.
Another study tested the combination of organic acids (acetic acid, butyric acid, propionic acid) and cinnamon oil, OEO, and lemongrass oil [128]. However, the synergistic effects between OEO (8000 µL/L air) and lemongrass oil (8000 µL/L air) with organic acids exhibited limited efficacy, as they did not fully inhibit A. flavus (NRRL3357) growth on corn kernels. In contrast, the combination of organic acids (acetic acid at 1500 mg/kg, butyric acid at 1760 mg/kg, and propionic acid at 2222 mg/kg) with cinnamon oil (8000 µL/L air) resulted in complete inhibition of A. flavus growth. This synergistic interaction was observed at 1/2 MIC of the organic acids (750 mg/kg AA, 880 mg/kg BA, 1111 mg/kg PA) combined with 1/4 MIC of cinnamon oil (2000 µL/L air), demonstrating a significant antimicrobial effect. Additionally, other treatments significantly reduced the yeast and mold count at p < 0.05.
Chitosan-based edible coatings incorporating acetic acid (1% w/v), ascorbic acid (1% w/v), and sea buckthorn essential oil (7.5%), along with 1% chitosan, were applied to strawberries and apple slices [129]. These coatings resulted in a significant reduction in microbial load (yeasts and molds) during cold storage at 4 °C and 8 °C, while effectively preserving ascorbic acid, total polyphenol content, and antioxidant activity. The coated samples exhibited reduced water activity (ranging from 0.96 to 0.98), which contributed to the inhibition of microbial growth. Further supporting the trend, chitosan-based coatings, formulated with 1% chitosan and 2% organic acids (acetic, lactic, and levulinic acids), effectively preserved the microbial quality of grape tomatoes during 21 days of storage at 10 °C. This treatment significantly reduced S. enterica (S. Panama 19,454, S. Poona 953, and S. Stanley H0558) populations from 3.65 to 1.28 log CFU/mL tomato within 24 h. Furthermore, the incorporation of 2% allyl isothiocyanate (AIT) in the chitosan–acid formulation enhanced its antimicrobial activity, reducing S. enterica populations to below 0.70 log CFU/tomato. Both treatments achieved complete elimination of S. enterica by day 2, whereas no reduction was observed in the control group. Moreover, the treated samples exhibited complete inhibition of yeasts and molds within 24 h, while fungal proliferation was observed in the control group by day 21. These findings demonstrate that chitosan–acid coatings, with or without AIT, serve as an effective intervention strategy for improving the microbial safety and shelf life of tomatoes during storage [130].
In addition, edible cellulose nanofiber coatings incorporated with 2% ginger essential oil and 1% citric acid on ready-to-cook barbecue chicken demonstrated significant reductions in microbial contamination and extended shelf life by up to 6 days [131]. Specifically, the treatment resulted in a reduction of 4.87 log CFU/g in TVC, 4.75 log CFU/g in Enterobacteriaceae spp., 4.91 log CFU/g in Pseudomonas spp., and 4.93 log CFU/g in yeasts and molds. Additionally, other treatments significantly reduced the yeast and mold count at p < 0.05. Therefore, based on what has been reported in this extensive body of literature, the integration of organic acids with other antimicrobial agents, such as plant extracts and essential oils, presents significant potential in improving food safety, quality, and preservation, offering promising alternatives for traditional preservatives.
6. Sustainability Considerations in the Use of Organic Acids as Food Preservatives
The sustainability of organic acids as food preservatives requires a more thorough evaluation, particularly regarding lifecycle assessments (LCAs) and the environmental trade-offs between efficacy and impact. While GRAS status and consumer demand are important, the environmental consequences of organic acid production, specifically energy use, raw material sourcing, and waste management, remain underexplored for various organic acids.
Organic acids are often produced by fermentation processes, which can have a variable environmental impact depending on the feedstock and production method. For example, citric acid is mainly obtained by fermenting sugars using the fungus A. niger and the environmental impact of its fermentation varies based on the recovery methods, with ion exchange being more sustainable than solvent extraction [132]. However, the sustainability of this process can be improved by using alternative substrates, such as waste glycerol from biodiesel production, reducing the need for environmentally costly feedstocks such as sugarcane [133]. Similarly, acetic acid, produced predominantly via ethanol fermentation, can have a high environmental impact, but alternative fermentation pathways from renewable biomass reduce energy consumption and carbon emissions [134]. Another example is lactic acid production that benefits from the use of lignocellulosic biomass, significantly lowering its fossil energy demand and global warming potential compared to conventional feedstocks [135].
On the other hand, synthetic preservatives, such as sodium benzoate, potassium sorbate, and sodium nitrite, are produced by chemical processes that involve the synthesis of compounds from petroleum and other non-renewable inputs, resulting in greater energy consumption and generation of chemical waste [136]. The organic acids still have several advantages, such as biodegradability, being easily metabolized in the environment and reducing chemical pollution, and lower toxicological risk, since, unlike synthetic preservatives, organic acids are not associated with the formation of potentially harmful substances, such as nitrosamines present in nitrites [4].
Therefore, replacing synthetic preservatives with organic acids can represent a significant advance for the sustainability of the food industry. However, it is essential that the production processes of these acids are constantly improved to minimize their environmental impact. The use of renewable raw materials, the optimization of fermentation processes, and the valorization of by-products are fundamental strategies to ensure that organic acids are, in fact, a sustainable and viable alternative for food preservation.
7. Conclusions and Future Perspectives
This review underscores the significant role of organic acids as antimicrobial agents in food preservation, drawing upon extensive research. Organic acids from natural sources effectively preserve a wide variety of foods, including meat, fish, vegetables, fruits, beverages, and confectionery. Their antimicrobial efficacy targets a broad spectrum of microorganisms, such as bacteria, fungi, and yeasts, which are primary contributors to food spoilage. These compounds also offer additional benefits, including flavor enhancement, nutritional preservation, and shelf life extension, aligning well with consumer preferences for safer, healthier, and high-quality food products. Moreover, the synergistic combination of organic acids with other organic acids and other bioactive compounds, such as plant extracts or essential oils, has demonstrated superior efficacy compared to individual applications. For instance, lactic and citric acids or acetic and ascorbic acids have shown enhanced microbial inhibition, extended shelf life, and better sensory preservation in various foods, highlighting the potential of tailored formulations leveraging complementary antimicrobial properties.
The use of organic acids presents distinct advantages, such as their versatility across diverse food matrices, their appeal as natural and safe ingredients, and their multifunctionality in improving sensory characteristics while preventing oxidation. However, challenges remain, including optimizing concentrations to balance sensory and antimicrobial effects, addressing food-specific preservation requirements, mitigating environmental impacts through sustainable sourcing, and navigating global regulatory frameworks while managing consumer perceptions.
Despite these benefits, several research gaps warrant attention. Deeper insights into the molecular mechanisms by which organic acids disrupt microbial growth are needed to inform targeted applications. The synergy between organic acids and other preservation techniques, such as natural preservatives, high-pressure processing, or innovative packaging, requires further exploration to optimize and standardize their use. Additionally, the efficacy of organic acids against emerging foodborne pathogens and spoilage organisms needs to be investigated. Identifying novel organic acids from diverse natural sources could further diversify available food preservatives, while their application in complex multicomponent foods remains underexplored. A deeper understanding of how combinations of organic acids interact chemically and biologically with diverse food systems would further enhance their effectiveness and adaptability.
By advancing research in this area and prioritizing sustainable sourcing, organic acids have the potential to drive significant progress toward more eco-friendly food preservation solutions, reducing waste, lowering environmental impact, and supporting a more sustainable global food system.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17083434/s1, Table S1. Antimicrobial activity of acetic acid (AA) against various microorganisms and in different matrices; Table S2. Antimicrobial activity of adipic acid (ADA) against various microorganisms and in different matrices; Table S3. Antimicrobial activity of butyric acid (BA) against various microorganisms and in different matrices; Table S4. Antimicrobial activity of caprylic acid (CAP) against various microorganisms and in different matrices; Table S5. Antimicrobial activity of citric acid (CA) against various microorganisms and in different matrices; Table S6. Antimicrobial activity of fumaric acid (FA) against various microorganisms and in different matrices; Table S7. Antimicrobial activity of lactic acid (LA) against various microorganisms and in different matrices; Table S8. Antimicrobial activity of malic acid (MA) against various microorganisms and in different matrices; Table S9. Antimicrobial activity of Phenyllactic acid (PLA) against various microorganisms and in different matrices; Table S10. Antimicrobial activity of Propionic acid (PA) against various microorganisms and in different matrices; Table S11. Antimicrobial activity of gallic acid (GA) against various microorganisms and in different matrices; Table S12. Antimicrobial activity of succinic acid (SA) against various microorganisms and in different matrices; Table S13. Antimicrobial activity of tartaric acid (TA) against various microorganisms and in different matrices.
Author Contributions
Conceptualization, K.B.S. and A.M.; methodology, K.B.S. and A.M.; investigation, K.B.S., A.M., M.C.H. and M.P.; writing—original draft preparation, K.B.S. and A.M.; writing—review and edit, K.B.S., A.M., M.C.H. and M.P.; visualization, K.B.S., A.M., M.C.H. and M.P.; supervision, A.M., M.C.H. and M.P.; project administration, M.P.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PRR (Programa de Recuperação e Resiliência) agenda VIIAFOOD—Platform for Valorization, Industrialization and Commercial Innovation for Agro-Food (C644929456-00000040). Additionally, this research was also funded by the Fundação para a Ciência e a Tecnologia through grant number UIDB/50016/2020.
Acknowledgments
Author Kavita Bhavin Sorathiya would like to acknowledge her individual PhD grant through PRR (Programa de Recuperação e Resiliência) agenda VIIAFOOD—Platform for Valorization, Industrialization and Commercial Innovation for Agro-Food.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GRAS | Generally recognized as safe |
| FDA | Food and Drug Administration |
| TLA | Acid constant |
| LD | Adenosine Triphosphate |
| ZnO | Zinc Oxide |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| PBAT | Poly (butylene adipate-co-terephthalate) |
| CNPs | Porous Carbon Nanoparticles |
| AAME | Adipic Acid Monoethyl Ester |
| ClO2 | Chlorine dioxide |
| UV-A | Ultraviolet-A |
| AgNPs | Nano-sized silver particles |
| ACP | Atmospheric Cold Plasma |
| TVC | Total viable count |
| EPS | Extracellular polymeric substances |
| OEO | Oregano essential oil |
| GSE | Grape seed extract |
References
- Aaliya, B.; Sunooj, K.V.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Theingi Hlaing, M.; George, J. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Coban, H.B. Organic acids as antimicrobial food agents: Applications and microbial productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic Acids Production from Lactic Acid Bacteria: A Preservation Approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Ali, H.; Zulkali, M. Utilization of agro-residual ligno-cellulosic substances by using solid state fermentation: A Review. Hrvat. Časopis Prehrambenu Tehnol. Biotehnol. Nutr. 2011, 6, 5–12. [Google Scholar]
- Theron, M.M.; Lues, J.F. Organic acids and meat preservation: A review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis; Academic Press: Cambridge, MA, USA, 2020; pp. 307–334. [Google Scholar]
- Bonetti, A.; Tugnoli, B.; Rossi, B.; Giovagnoni, G.; Piva, A.; Grilli, E. Nature-identical compounds and organic acids reduce E. coli K88 growth and virulence gene expression in vitro. Toxins 2020, 12, 468. [Google Scholar] [CrossRef]
- Anyasi, T.; Jideani, A.; Edokpayi, J.; Anokwuru, C. Application of organic acids in food preservation. In Organic Acids, Characteristics, Properties and Synthesis; Vargas, C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 1–47. [Google Scholar]
- Chahardoli, A.; Jalilian, F.; Farzaei, H.; Shokoohinia, Y. Chapter 26-Analysis of organic acids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 767–823. [Google Scholar]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Khan, S.H.; Iqbal, J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Ben Braïek, O.; Smaoui, S. Chemistry, safety, and challenges of the use of organic acids and their derivative salts in meat preservation. J. Food Qual. 2021, 2021, 6653190. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R. Collagen: From Waste to Gold. In Biotechnological Applications of Biomass; Chapter 12; Intech Open: London, UK, 2020; pp. 1–36. [Google Scholar]
- Deng, Y.; Ma, L.; Mao, Y. Biological production of adipic acid from renewable substrates: Current and future methods. Biochem. Eng. J. 2016, 105, 16–26. [Google Scholar] [CrossRef]
- Makowski, Z.; Lipiński, K.; Mazur-Kuśnirek, M. The Effects of sodium butyrate, coated sodium butyrate, and butyric acid glycerides on nutrient digestibility, gastrointestinal function, and fecal microbiota in turkeys. Animals 2022, 12, 1836. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Guo, F.; Wu, M.; Dai, Z.; Zhang, S.; Zhang, W.; Dong, W.; Zhou, J.; Jiang, M.; Xin, F. Current advances on biological production of fumaric acid. Biochem. Eng. J. 2020, 153, 107397. [Google Scholar] [CrossRef]
- Stanojevic-Nikolic, S.; Dimic, G.; Mojovic, L.; Pejin, J.; Djukic-Vukovic, A.; Kocic-Tanackov, S. Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J. Food Process. Preserv. 2015, 40, 990–998. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Z.P.; Wang, G.Y.; Khan, I.; Chi, Z.M. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Eş, I.; Khaneghah, A.M.; Hashemi, S.M.; Koubaa, M. Current advances in biological production of propionic acid. Biotechnol. Lett. 2017, 39, 635–645. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic acid: Technology development and commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Lianou, A.; Koutsoumanis, K.P.; Sofos, J.N. Organic acids and other chemical treatments for microbial decontamination of food. In Microbial Decontamination in the Food Industry: Novel Methods and Applications; Demirci, A., Ngadi, M.O., Eds.; Woodhead Publishing Inc.: Oxford, UK, 2012; pp. 592–664. ISBN 978-0-85709-575-6. [Google Scholar]
- Abdelhamid, A.G.; El-Dougdoug, N.K. Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies. Heliyon 2020, 6, e05020. [Google Scholar] [CrossRef] [PubMed]
- Dagnas, S.; Gauvry, E.; Onno, B.; Membré, J.M. Quantifying effect of lactic, acetic, and propionic acids on growth of molds isolated from spoiled bakery products. J. Food Prot. 2015, 78, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, F.; Du, L.; Zhao, T.; Doyle, M.P.; Wang, D.; Zhang, X.; Sun, Z.; Xu, W. Antibacterial and antibiofilm activity of phenyllactic acid against Enterobacter cloacae. Food Control 2018, 84, 442–448. [Google Scholar] [CrossRef]
- Ng, W.K.; Koh, C.B. The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquac. 2017, 9, 342–368. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Arjmand, V. Reducing pathogens by using zinc oxide nanoparticles and acetic acid in sheep meat. J. Food Prot. 2014, 77, 1599–1604. [Google Scholar] [CrossRef]
- Weissinger, W.R.; Mcwatters, K.H.; Beuchat, L.R. Evaluation of volatile chemical treatments for lethality to Salmonella on alfalfa seeds and sprouts. J. Food Prot. 2001, 64, 442–450. [Google Scholar] [CrossRef]
- Pangprasit, N.; Srithanasuwan, A.; Suriyasathaporn, W.; Pikulkaew, S.; Bernard, J.K.; Chaisri, W. Antibacterial activities of acetic acid against major and minor pathogens isolated from mastitis in dairy cows. Pathogens 2020, 9, 961. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Lastauskienė, E.; Zinkevičienė, A.; Girkontaitė, I.; Kaunietis, A.; Kvedarienė, V. Formic acid and acetic acid induce a programmed cell death in pathogenic Candida species. Curr. Microbiol. 2014, 69, 303–310. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The evaluation of chemical, antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Kang, H.; Park, Y.; Go, S. Growth inhibition of a phytopathogenic fungus, Colletotrichum species by acetic acid. Microbiol. Res. 2003, 158, 321–326. [Google Scholar] [CrossRef]
- Hassan, R.A.; Sand, M.I.; El-Kadi, S.M. Effect of some organic acids on fungal growth and their toxins production. J. Agric. Chem. Biotechnol. 2012, 3, 391–397. [Google Scholar] [CrossRef]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting synergies of sourdough and antifungal organic acids to delay fungal spoilage of bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Kim, H.M.; Chun, H.S.; Lee, S.E. Organic acids suppress aflatoxin production via lowering expression of aflatoxin biosynthesis-related genes in Aspergillus flavus. Food Control 2018, 88, 207–216. [Google Scholar] [CrossRef]
- Adili, L.; Roufegarinejad, L.; Tabibiazar, M.; Hamishehkar, H.; Alizadeh, A. Development and characterization of reinforced ethyl cellulose based oleogel with adipic acid: Its application in cake and beef burger. LWT Food Sci. Technol. 2020, 126, 109277. [Google Scholar] [CrossRef]
- Zehetmeyer, G.; Meira, S.M.M.M.; Scheibel, J.M.; de Oliveira, R.V.B.; Brandelli, A.; Soares, R.M.D. Influence of melt processing on biodegradable nisin-PBAT films intended for active food packaging applications. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Adv. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Moraes Filho, L.E.P.T.; Andrade, M.F.; Freitas, L.F.; Palha, M.D.L.A.P.F.; Vinhas, G.M. Development and Characterization of Poly(Butylene Adipate-Co-terephthalate) (PBAT) Antimicrobial Films with Clove and Cinnamon Essential Oils. Food Process. Preserv. 2022, 46, e16489. [Google Scholar] [CrossRef]
- Venkatesan, R.; Sivaprakash, P.; Kim, I.; Eldesoky, G.; Kim, S. Tannic acid as a crosslinking agent in poly (butylene adipate-co-terephthalate) composite films enhanced with carbon nanoparticles: Processing, characterization, and antimicrobial activities for food packaging. J. Environ. Chem. Eng. 2023, 11, 110194. [Google Scholar] [CrossRef]
- Vicedo, B.; de la O Leyva, M.; Flors, V.; Finiti, I.; Del Amo, G.; Walters, D.; Real, M.D.; García-Agustín, P.; González-Bosch, C. Control of the phytopathogen Botrytis cinerea using adipic acid monoethyl ester. Arch. Microbiol. 2006, 184, 316–326. [Google Scholar] [CrossRef]
- Flors, V.; Miralles, C.; González-Bosch, C.; Carda, M.; García-Agustín, P. Three novel synthetic amides of adipic acid protect Capsicum anuum plants against the necrotrophic pathogen Alternaria solani. Physiol. Mol. Plant Pathol. 2003, 63, 151–158. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly (lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.-M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A glimpse of the world of volatile fatty acids production and application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Sun, C.Q.; O’Connor, C.J.; Turner, S.J.; Lewis, G.D.; Stanley, R.A.; Roberton, A.M. The effect of pH on the inhibition of bacterial growth by physiological concentrations of butyric acid: Implications for neonates fed on suckled milk. Chem. Interact. 1998, 113, 117–131. [Google Scholar] [CrossRef]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial activity of butyrate glycerides toward Salmonella typhimurium and Clostridium perfringens. Poult. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Fuentes, C.; Pérez-Esteve, É.; Jiménez-Belenguer, A.I.; Quiles, A.; Marcos, M.D.; Martínez-Máñez, R.; Barat, J.M. Bactericidal activity of caprylic acid entrapped in mesoporous silica nanoparticles. Food Control 2015, 56, 77–85. [Google Scholar] [CrossRef]
- Nair, M.K.M.; Vasudevan, P.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes in milk by caprylic acid and monocaprylin. Food Microbiol. 2004, 21, 611–616. [Google Scholar] [CrossRef]
- Wang, J.; Ma, M.; Yang, J.; Chen, L.; Yu, P.; Wang, J. In vitro antibacterial activity and mechanism of monocaprylin against Escherichia coli and Staphylococcus aureus. J. Food Prot. 2018, 81, 1988–1996. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Zhang, G.; Chen, F.; Xu, B. In vitro antibacterial activities and mechanisms of action of fatty acid monoglycerides against four foodborne bacteria. J. Food Prot. 2020, 83, 331–337. [Google Scholar] [CrossRef]
- Jadhav, A.; Mortale, S.; Halbandge, S.; Jangid, P.; Patil, R.; Gade, W.; Kharat, K.; Karuppayil, S.M. The dietary food components capric acid and caprylic acid inhibit virulence factors in Candida albicans through multitargeting. J. Med. Food 2017, 20, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Reitzel, R.A.; Vargas-Cruz, N.; Chaftari, A.M.; Hachem, R.; Raad, I. Caprylic and polygalacturonic acid combinations for eradication of microbial organisms embedded in biofilm. Front. Microbiol. 2017, 8, 1999. [Google Scholar] [CrossRef] [PubMed]
- Akula, S.T.; Nagaraja, A.; Ravikanth, M.; Kumar, N.G.R.; Kalyan, Y.; Divya, D. Antifungal efficacy of LA and caprylic acid—Derivatives of virgin coconut oil against Candida albicans. Biomed. Biotechnol. Res. J. 2021, 5, 229–234. [Google Scholar] [CrossRef]
- López-Velázquez, J.G.; Ayón-Reyna, L.E.; Vega-García, M.O.; López-Angulo, G.; López-López, M.E.; López-Zazueta, B.A.; Delgado-Vargas, F. Caprylic acid in Vitex mollis fruit and its inhibitory activity against a thiabendazole-resistant Colletotrichum gloeosporioides strain. Pest. Manag. Sci. 2022, 78, 5271–5280. [Google Scholar] [CrossRef]
- Sorrells, K.M.; Enigl, D.C.; Hatfield, J.R. Effect of pH, acidulant, time, and temperature on the growth and survival of Listeria monocytogenes. J. Food Prot. 1989, 52, 571–573. [Google Scholar] [CrossRef]
- Brackett, R.E. Effects of various acids on growth and survival of Yersinia Enterocolitica. J. Food Prot. 1987, 50, 598–602. [Google Scholar] [CrossRef]
- In, Y.W.; Kim, J.J.; Kim, H.J.; Oh, S.W. Antimicrobial activities of acetic acid, citric acid and lactic acid against Shigella species. J. Food Saf. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Seo, S.; Jung, D.; Wang, X.; Seo, D.J.; Lee, M.H.; Lee, B.-H.; Choi, C. Combined effect of lactic acid bacteria and citric acid on Escherichia coli O157:H7 and Salmonella typhimurium. Food Sci. Biotechnol. 2013, 22, 1171–1174. [Google Scholar] [CrossRef]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of acetic and citric acids to inhibit Escherichia coli O157:H7, Salmonella typhimurium and Staphylococcus aureus in tabbouleh salad. Food Microb. 2018, 73, 61–66. [Google Scholar] [CrossRef]
- Zhao, X.; Zhen, Z.; Wang, X.; Guo, N. Synergy of a combination of nisin and citric acid against Staphylococcus aureus and Listeria monocytogenes. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk Assess 2017, 34, 2058–2068. [Google Scholar] [CrossRef]
- Shokri, H. Evaluation of inhibitory effects of citric and tartaric acids and their combination on the growth of Trichophyton mentagrophytes, Aspergillus fumigatus, Candida albicans, and Malassezia furfur. Comp. Clin. Path. 2011, 20, 543–545. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Arneborg, N. The effect of citric acid and pH on growth and metabolism of anaerobic Saccharomyces cerevisiae and Zygosaccharomyces bailii cultures. Food Microbiol. 2007, 24, 101–105. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.H.; Song, K.B. Efficacy of aqueous chlorine dioxide and fumaric acid for inactivating pre-existing microorganisms and Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on broccoli sprouts. Food Control 2009, 20, 1002–1005. [Google Scholar] [CrossRef]
- Tango, C.N.; Mansur, A.R.; Oh, D.-H. Fumaric Acid and slightly acidic electrolyzed water inactivate gram-positive and gram-negative foodborne pathogens. Microorganisms 2015, 3, 34–46. [Google Scholar] [CrossRef]
- Chen, X.; Tango, C.N.; Daliri, E.B.; Oh, S.Y.; Oh, D.H. Disinfection efficacy of slightly acidic electrolyzed water combined with chemical treatments on fresh fruits at the industrial scale. Foods 2019, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Rodriguez, A.; Fulco, M.; Soteras, T.; Mozgovoj, M.; Cap, M. Effects of lactic, malic and fumaric acids on Salmonella spp. counts and on chicken meat quality and sensory characteristics. J. Food Sci. Technol. 2021, 58, 3817–3824. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Ha, J.-W. Inactivating Foodborne Pathogens in Apple Juice by Combined Treatment with Fumaric Acid and Ultraviolet-A Light, and Mechanisms of Their Synergistic Bactericidal Action. Food Microbiol. 2020, 87, 103387. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Oh, D.-H. Evaluation of Nisin-Loaded Chitosan-Monomethyl Fumaric Acid Nanoparticles as a Direct Food Additive. Carbohydr. Polym 2018, 184, 100–107. [Google Scholar] [CrossRef]
- Beuchat, L.R. Influence of Organic Acids on Heat Resistance Characteristics of Talaromyces flavus Ascospores. Int. J. Food Microbiol. 1988, 6, 97–105. [Google Scholar] [CrossRef]
- Kotula, K.L.; Thelappurate, R. Microbiological and Sensory Attributes of Retail Cuts of Beef Treated with Acetic and Lactic Acid Solutions. JFP 1994, 57, 665–670. [Google Scholar] [CrossRef]
- Dan, S.D.; Mihaiu, M.; Reget, O.; Tăbăran, A. Residual Antimicrobial Effect of Weak Organic Acids on Spoilage Psychrotrophs at Pig Carcasses. Lucr. Științifice USAMV Iași Ser. Med. Vet. 2017, 60, 258–264. [Google Scholar]
- Laury, A.M.; Alvarado, M.V.; Nace, G.; Alvarado, C.Z.; Brooks, J.C.; Echeverry, A.; Brashears, M.M. Validation of a Lactic Acid– and Citric Acid–Based Antimicrobial Product for the Reduction of Escherichia coli O157:H7 and Salmonella on Beef Tips and Whole Chicken Carcasses. JFP 2009, 72, 2208–2211. [Google Scholar] [CrossRef]
- Zhang, S. The Effects of Various Disinfectants against Listeria monocytogenes Fresh-Cut Vegetables. Food Microbiol. 1996, 13, 311–321. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of Organic Acids on Biofilm Formation and Quorum Signaling of Pathogens from Fresh Fruits and Vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kavčič, S.; Knez, Ž.; Leitgeb, M. Antimicrobial Activity of N-Butyl Lactate Obtained via Enzymatic Esterification of Lactic Acid with n-Butanol in Supercritical Trifluoromethane. J. Supercrit. Fluids 2014, 85, 143–150. [Google Scholar] [CrossRef]
- Lind, H.; Jonsson, H.; Schnürer, J. Antifungal effect of dairy propionibacteria–contribution of organic acids. Int. J. Food Microbiol. 2003, 98, 157–165. [Google Scholar] [CrossRef]
- Peláez, A.L.; Cataño, C.S.; Yepes, E.Q.; Villarroel, R.G.; de Antoni, G.; Giannuzzi, L. Inhibitory activity of lactic and acetic acid on Aspergillus Flavus growth for food preservation. Food Control 2012, 24, 177–183. [Google Scholar] [CrossRef]
- Kövilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2020, 95, 513–526. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control 2009, 2, 105–112. [Google Scholar] [CrossRef]
- Eswaranandam, S.; Hettiarachchy, N.S.; Johnson, M.G. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J. Food Sci. 2006, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Ghoush, M.H.A.; Al-Nabulsi, A.A.; Qatatsheh, A.A.; Shahbaz, H.M.; Osaili, T.M.; Holley, R.A. The use of malic and acetic acids in washing solution to control Salmonella spp. on chicken breast. J. Food Sci. 2018, 83, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Jafarpour, D. Ultrasound and malic acid treatment of sweet lemon juice: Microbial inactivation and quality changes. J. Food Process. Preserv. 2020, 44, 14866. [Google Scholar] [CrossRef]
- Borah, H.J.; Borah, A.; Yadav, A.; Hazarika, S. Extraction of malic acid from Dillenia Indica in organic solvents and its antimicrobial activity. Sep. Sci. Technol. 2022, 58, 314–325. [Google Scholar] [CrossRef]
- Pintado, C.M.B.S.; Ferreira, M.A.S.S.; Sousa, I. Control of pathogenic and spoilage microorganisms from cheese surface by whey protein films containing malic acid, nisin and natamycin. Food Control 2010, 21, 240–246. [Google Scholar] [CrossRef]
- Dilshad, E.; Bibi, M.; Sheikh, N.A.; Tamrin, K.F.; Mansoor, Q.; Maqbool, Q.; Nawaz, M. Synthesis of functional silver nanoparticles and microparticles with modifiers and evaluation of their antimicrobial, anticancer, and antioxidant activity. J. Funct. Biomater. 2020, 11, 76. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, T.; Hung, Y.C.; Adhikari, K. Evaluation of bactericidal effects of phenyllactic acid on Escherichia coli O157:H7 and Salmonella typhimurium on beef meat. J. Food Prot. 2019, 82, 2016–2022. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Yang, L.-Y.; Xin, W.-G.; Zhang, Q.-L. Combined antibacterial and antibiofilm activity of phenyllactic acid and bacteriocin XJS01 against Shigella flexneri. Food Biosci. 2022, 45, 101512. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.-Y.; Kuk, J.-H.; Moon, J.-H.; Cho, J.-I.; Kim, Y.-C.; Park, K.-H. Identification and antimicrobial activity of phenylacetic acid produced by Bacillus licheniformis isolated from fermented soybean, chungkook-jang. Curr. Microbiol. 2004, 48, 312–317. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef]
- Gerez, C.L.; Carbajo, M.S.; Rollan, G.; Torres Leal, G.; Font de Valdez, G. Inhibition of citrus fungal pathogens by using lactic acid bacteria. J. Food Sci. 2010, 75, M354–M359. [Google Scholar] [CrossRef]
- Svanström, Å.; Boveri, S.; Boström, E.; Melin, P. The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Res. Notes 2013, 6, 464. [Google Scholar] [CrossRef]
- Fan, W.; Li, B.; Du, N.; Hui, T.; Cao, Y.; Li, X.; Ren, H. Energy metabolism as the target of 3-phenyllactic acid against Rhizopus oryzae. Int. J. Food Microbiol. 2022, 369, 109606. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Pilevar, Z.; Mousavi Khaneghah, A.; Hosseini, H.; Ranaei, V. Propionic Acid: Method of production, current state and perspectives. Food Technol. Biotechnol. 2020, 58, 115–127. [Google Scholar]
- Haque, M.N.; Chowdhury, R.; Islam, K.; Akbar, M.A. Propionic acid is an alternative to antibiotics in poultry diet. Bangladesh J. Anim. Sci. 1970, 38, 115–122. [Google Scholar] [CrossRef]
- Seo, Y.; Sung, M.; Hwang, J.; Yoon, Y. Minimum Inhibitory Concentration (MIC) of Propionic Acid, Sorbic Acid, and Benzoic Acid against Food Spoilage Microorganisms in Animal Products to Use MIC as Threshold for Natural Preservative Production. Food Sci. Anim. Resour. 2023, 43, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, A.; Huang, S.; Kuo, S.; Shu, M.; Tapia, C.P.; Yu, J.; Two, A.; Zhang, H.; Gallo, R.L.; et al. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Benef. Microbes 2014, 5, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Antone, U.; Ciprovica, I.; Zolovs, M.; Scerbaka, R.; Liepins, J. Propionic acid fermentation—Study of substrates, strains, and antimicrobial properties. Fermentation 2022, 9, 26. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Meijer, M.; van Doorn, T.; Houbraken, J.; Bruinenberg, P. The preservative propionic acid differentially affects survival of conidia and germ tubes of feed spoilage fungi. Int. J. Food Microbiol. 2019, 306, 108258. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Alkan, D.; Aydemir, L.Y.; Arcan, I.; Yavuzdurmaz, H.; Atabay, H.I.; Ceylan, C.; Yemenicioglu, A. Development of flexible antimicrobial packaging materials against Campylobacter jejuni by incorporation of gallic acid into zein-based films. J. Agric. Food. Chem. 2011, 59, 11003–11010. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015, 80, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.T.; Ferreira, I.C.; Barros, L.; Silva, S.; Azeredo, J.; Henriques, M. Antifungal activity of phenolic compounds identified in flowers from Northeastern Portugal against Candida species. Future Microbiol. 2014, 9, 139–146. [Google Scholar] [CrossRef]
- Fujita, K.; Kubo, I. Antifungal activity of octyl gallate. Int. J. Food Microbiol. 2002, 79, 193–201. [Google Scholar] [CrossRef]
- Karpova, N.; Shagdarova, B.; Lunkov, A.; Ilina, A.; Varlamov, V. Antifungal action of chitosan in combination with fungicides in vitro and chitosan conjugate with gallic acid on tomatoes against Botrytis cinerea. Biotechnol. Lett. 2021, 43, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.A.; Mercuri, A.J.; Juven, B.J.; Thomson, J.E.; Chew, V. Evaluation of succinic acid and heat to improve the microbiological quality of poultry meat. J. Food Sci. 1974, 39, 985–987. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, J.; Sun, H.; Zhong, W.; Zhuang, W.; Zhang, Z. Evaluation of different kinds of organic acids and their antibacterial activity in Japanese Apricot fruits. Afr. J. Agric. Res. 2012, 7, 4911–4918. [Google Scholar] [CrossRef]
- Khalil, A.A.; Deraz, S.F.; Elrahman, S.A.; El-Fawal, G. Enhancement of mechanical properties, microstructure, and antimicrobial activities of zein films cross-linked using succinic anhydride, eugenol, and citric Acid. Prep. Biochem. Biotechnol. 2015, 45, 551–567. [Google Scholar] [CrossRef]
- Radkowski, M.; Zdrodowska, B.; Gomółka-Pawlicka, M. Effect of succinic acid on elimination of Salmonella in chicken meat. J. Food Prot. 2018, 81, 1491–1495. [Google Scholar] [CrossRef]
- Mikaelyan, A.R.; Babayan, B.G.; Grigogerez, A.L.; Grigoryan, A.M.; Asatryan, N.L.; Melkumyan, M.A. Tartaric acid new derivatives as prospective and safe alternative to antimicrobials for food products Packing. FFHD 2024, 14, 33. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Li, T.; Hu, Z.; Huang, S.; Lin, M.; Xie, Y.; Yu, Z. Antimicrobial Effect of Plasma-Activated Water Combined with Tartaric Acid against Staphylococcus Aureus and Its Application on Fresh-Cut Asparagus Lettuce. Food Biosci. 2024, 60, 104293. [Google Scholar] [CrossRef]
- Tavsanli, H.; Irkin, R.; Kisadere, I. Effects of Addition of Different Organic Acids to White Cheese Brine on Microflora and Pathogen Listeria Monocytogenes. Kafkas Univ. Vet. Fak. Derg. 2018, 25, 201–207. [Google Scholar]
- Karpiński, T.M.; Ożarowski, M. Plant Organic Acids as Natural Inhibitors of Foodborne Pathogens. Appl. Sci. 2024, 14, 6340. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Han, A.; Deng, Z.; Qi, Z.; Long, H.; Wang, J.; Yao, W.; et al. Advances in the Role and Mechanisms of Essential Oils and Plant Extracts as Natural Preservatives to Extend the Postharvest Shelf Life of Edible Mushrooms. Foods 2023, 12, 801. [Google Scholar] [CrossRef]
- Chowdhury, M.A.K.; Song, H.; Liu, Y.; Bunod, J.-D.; Dong, X.-H. Effects of Microencapsulated Organic Acid and Their Salts on Growth Performance, Immunity, and Disease Resistance of Pacific White Shrimp Litopenaeus Vannamei. Sustainability 2021, 13, 7791. [Google Scholar] [CrossRef]
- Kim, T.-K.; Hwang, K.-E.; Lee, M.-A.; Paik, H.-D.; Kim, Y.-B.; Choi, Y.-S. Quality Characteristics of Pork Loin Cured with Green Nitrite Source and Some Organic Acids. Meat Sci. 2019, 152, 141–145. [Google Scholar] [CrossRef]
- Mohan, A.; Purohit, A.S. Anti-Salmonella Activity of Pyruvic and Succinic Acid in Combination with Oregano Essential Oil. Food Control 2020, 110, 106960. [Google Scholar] [CrossRef]
- Dogruyol, H.; Mol, S.; Cosansu, S. Increased Thermal Sensitivity of Listeria monocytogenes in Sous-Vide Salmon by Oregano Essential Oil and Citric Acid. Food Microbiol. 2020, 90, 103496. [Google Scholar] [CrossRef]
- Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Vibrio parahaemolyticus in Seafood Systems Using Oregano and Cranberry Phytochemical Synergies and Lactic Acid. IFSET 2005, 6, 453–458. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kwon, K.-H.; Oh, S.-W. Effects of Malic Acid or/and Grapefruit Seed Extract for the Inactivation of Common Food Pathogens on Fresh-Cut Lettuce. Food Sci. Biotechnol. 2016, 25, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Satterlee, T.; McDonough, C.M.; Gold, S.E.; Chen, C.; Glenn, A.E.; Pokoo-Aikins, A. Synergistic Effects of Essential Oils and Organic Acids against Aspergillus flavus Contamination in Poultry Feed. Toxins 2023, 15, 635. [Google Scholar] [CrossRef] [PubMed]
- Popescu, P.-A.; Palade, L.M.; Nicolae, I.-C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.Z.; Fan, X.; Mukhopadhyay, S. Antimicrobial Coating with Organic Acids and Essential Oil for the Enhancement of Safety and Shelf Life of Grape Tomatoes. Int. J. Food Microbiol. 2022, 378, 109827. [Google Scholar] [CrossRef]
- Khaledian, Y.; Pajohi-Alamoti, M.; Bazargani-Gilani, B. Development of Cellulose Nanofibers Coating Incorporated with Ginger Essential Oil and Citric Acid to Extend the Shelf Life of Ready-to-cook Barbecue Chicken. J. Food Process. Preserv. 2019, 43, e14114. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Z.; Li, Y.; Cao, L.; Lu, Z. Techno-Economic Analysis and Environmental Impact Assessment of Citric Acid Production through Different Recovery Methods. J. Clean. Prod. 2020, 249, 119315. [Google Scholar] [CrossRef]
- Becker, M.; Kohlheb, N.; Hunger, S.; Eschrich, S.; Müller, R.; Aurich, A. Early-stage Sustainability Assessment of Biotechnological Processes: A Case Study of Citric Acid Production. ELS 2020, 20, 90–103. [Google Scholar] [CrossRef]
- Budsberg, E.; Morales-Vera, R.; Crawford, J.T.; Bura, R.; Gustafson, R. Production Routes to Bio-Acetic Acid: Life Cycle Assessment. Biotechnol. Biofuels 2020, 13, 154. [Google Scholar] [CrossRef]
- Li, Y.; Bhagwat, S.S.; Cortés-Peña, Y.R.; Ki, D.; Rao, C.V.; Jin, Y.-S.; Guest, J.S. Sustainable Lactic Acid Production from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 1341–1351. [Google Scholar] [CrossRef]
- Kung, L.; Smith, M.L.; Benjamim Da Silva, E.; Windle, M.C.; Da Silva, T.C.; Polukis, S.A. An Evaluation of the Effectiveness of a Chemical Additive Based on Sodium Benzoate, Potassium Sorbate, and Sodium Nitrite on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2018, 101, 5949–5960. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).