Abstract
The widespread use of plastics has caused severe environmental pollution, driving interest in biodegradable alternatives like polylactic acid (PLA). However, incomplete degradation of biodegradable plastics under natural conditions may generate micro/nanoplastics that could exacerbate ecological risks. This study investigated the combined effects of UV-aged microplastics from biodegradable PLA and conventional PET, along with sulfamethoxazole (SMX), on the conjugative transfer of antibiotic resistance genes (ARGs) between bacteria. Using UV aging to simulate environmental weathering, the microplastic morphology, adsorption behavior, and interaction with SMX were characterized. The study further evaluated the bacterial viability, ROS level, membrane permeability, and the expression of conjugative transfer-related genes to elucidate the underlying mechanisms. Results showed that aged PLA released significantly more nanoplastics and exhibited higher adsorption affinity for SMX than PET. Combined exposure to aged PLA and SMX significantly enhanced ARG transfer frequency by approximately 14.5-fold compared to the control. Mechanistic studies revealed that this promotion was associated with increased intracellular ROS levels, elevated membrane permeability, and upregulation of conjugative related genes. These findings underscore that biodegradable plastics, after environmental aging, may pose greater ecological risks than conventional plastics, and highlight the importance of considering environmental aging in the risk assessment of plastics.
1. Introduction
Owing to their favorable physicochemical properties and low cost, plastics have been widely used in various applications. Global plastic production is projected to exceed 1800 million tons per year by 2050 [1]. However, conventional petroleum-based plastics persist in the environment due to their resistance to degradation, contributing to severe plastic pollution [2]. Moreover, plastic production accounts for 7–9% of global oil consumption, significantly impacting energy resources worldwide [3]. In response, biodegradable plastics have emerged as a promising alternative. These polymers are synthesized from renewable resources such as starch, sugars, or other organic compounds [4] and can be fully broken down under specific conditions into end products like CO2 and water via biological metabolism [5]. Among them, polylactic acid (PLA)—produced primarily through the polymerization of lactic acid—is one of the most widely studied and used biodegradable plastics [6]. Owing to its excellent biocompatibility, transparency, and mechanical properties, PLA plays a significant role in traditional applications such as films, food packaging, medical devices, and textiles [7,8].
However, research indicated that the full degradation of biodegradable plastics demands specific environmental criteria that natural settings seldom provide [9], potentially leading to the generation of substantial microplastic particles [10], which could exacerbate pollution. After environmental degradation, the increased presence of oxygen-containing functional groups enhances the affinity and adsorption capacity of biodegradable plastics for contaminants such as antibiotics compared to conventional plastics [11,12].
Since the discovery of antibiotics by Fleming, they have been extensively used in healthcare and livestock farming. However, a significant proportion (40–90%) of antibiotics is not absorbed or fully metabolized and is excreted into the environment via feces and urine [13]. Sulfamethoxazole (SMX), known for its high stability and long half-life, is a frequently detected antibiotic in the environment, reaching concentrations of 0.16–11.07 mg/kg in animal manure [14]. Prolonged or high-level exposure to SMX can induce bacterial resistance through target enzyme mutations, efflux pump activation, or interference with the folate synthesis pathway [13].
The spread of antibiotic resistance genes (ARGs) is a fundamental mechanism of bacterial resistance [15]. Bacteria acquire resistance through two primary pathways: spontaneous mutation and horizontal gene transfer. Natural spontaneous mutation rates are low (approximately 10−6 to 10−8), and such resistance is often unstable, tending to diminish in the absence of antibiotic pressure; hence, mutation is not the major route of resistance acquisition [16]. Horizontal transfer, mainly via transformation, transduction, and conjugative transfer, is the dominant mechanism [17]. Conjugative transfer, mediated primarily by plasmids through direct cell contact or pilus connection, allows genetic material transfer between bacteria, even across species boundaries. As many plasmids have broad host ranges, conjugative transfer represents the most common form of horizontal gene transfer and plays a critical role in the dissemination of ARGs [18].
Current research has explored the effects of conventional plastics, biodegradable plastics, antibiotics, and their combinations with microplastics on the conjugative transfer of ARGs between bacteria [19,20,21]. However, all plastics undergo weathering processes in the environment, which alter their physicochemical properties and environmental behaviors [22]. Aging modifies the surface characteristics of microplastics, thereby influencing their interactions with antibiotics and subsequent environmental and biological effects. Based on these considerations, this study focused on widely used biodegradable PLA cups, with conventional polyethylene terephthalate (PET) cups- a ubiquitous, persistent plastic with similar packaging applications—selected as a reference [23,24]. This allows for a direct comparison of their environmental behaviors under aging, highlighting the potential risks specific to biodegradable plastics. Natural UV aging was simulated under laboratory conditions to investigate the combined effects of aged microplastics and SMX. This research aims to elucidate the influence of UV aging on the ability of microplastic-SMX combinations in facilitating the spread of ARGs between bacteria and uncovering the underlying mechanisms. The findings will enhance understanding of the long-term environmental behavior and ecotoxicological effects of aged biodegradable plastics, providing a scientific basis for assessing and mitigating the ecological and health risks posed by biodegradable microplastics and co-existing contaminants.
2. Materials and Methods
2.1. Plastics and UV Aging
The plastic cups used in the experiment were commercially available daily use products. Prior to UV aging, PLA and PET cups were cut into fragments with a side length of approximately 5 mm to serve as starting materials for accelerated UV aging. Ten grams of each type were washed, dried, and placed in quartz Petri dishes. Ultra-pure water was added to achieve a final concentration of 50 mg/mL [25]. The dishes were then exposed in a UV aging chamber equipped with UVA-340 lamps (0.76 W/m2, 25 °C) to simulate natural sunlight aging. To ensure uniform UV irradiation, the PLA and PET fragments were stirred every 6 h. A control group—consisting of ultra-pure water without any microplastics was placed under the same conditions. The plastics were irradiated for 3, 7, 15, 30, and 60 days, followed by characterization. This procedure closely mimics the photochemical aging behavior of most polymers in the environment [26].
2.2. Microplastics Characterization
The morphological changes in microplastics following 0, 15, 30, and 60 days of aging were analyzed using scanning electron microscopy (SEM, JEOL, Tokyo, Japan). To accurately determine the size and morphology of the micro/nanoparticles released into the solution after 60 days—a period sufficient for substantial fragmentation—the supernatants were analyzed by transmission electron microscopy (TEM, JEOL, Tokyo, Japan). Alterations in the specific surface area and porosity of the aged particles were quantified with a surface area and porosity analyzer (ASAP 2460, Micromeritics, Norcross, GA, USA). The concentration of nanoplastics in the supernatant was indirectly determined by measuring the total organic carbon (TOC) content using a TOC analyzer (multi N/C 3100, Analytik Jena, Jena, Germany), with calculations based on established TOC values [27]. Furthermore, the hydrodynamic diameter and zeta potential of the particles in suspension were assessed with a Zetasizer Nano ZS instrument (Malvern, Worcestershire, UK). The surface functional groups of the microplastics were characterized using Fourier Transform Infrared Spectroscopy (FTIR, FTS-6000, Bio-rad, Hercules, CA, USA).
2.3. Determination of Adsorption Capacity of Microplastics for SMX Before and After Aging
After aging, the PLA and PET microplastic fragments were collected, rinsed thoroughly, and dried. The supernatant was stored in amber glass vials at 4 °C for subsequent use. Microplastic fragments and corresponding supernatants from different aging periods were mixed with SMX to a final concentration of 1 mg/L. These mixtures were subjected to shaking at 130 rpm and maintained at 25 °C. Samples of the supernatant were collected at 0, 5, 10, 20, 40, 60, 90, 120, 150, and 180 min during the shaking adsorption process, then centrifuged and passed through a 0.22 μm sterile filter. The concentration of SMX in the adsorbed solutions was determined using high-performance liquid chromatography (HPLC, UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA). Based on the SMX standard curve (Figure S1) and the method for calculating the equilibrium adsorption capacity (qe) [28], the corresponding SMX concentrations were derived, and the adsorption saturation time was determined.
2.4. Effects of Aged Microplastics and SMX on the Conjugative Transfer of ARGs Between Bacteria
2.4.1. Bacterial Strains and Plasmids
Donor and recipient strains were selected to facilitate the study of plasmid-mediated conjugative transfer. The donor strain, E. coli DH5α, was engineered to harbor the RP4 plasmid, providing resistance to kanamycin, ampicillin, and tetracycline. Its counterpart, the recipient E. coli HB101 strain, lacks this plasmid but is intrinsically resistant to streptomycin due to a genomic marker [29].
2.4.2. Effects of Different Treatment Systems on the Conjugative Transfer of ARGs Between Bacteria
The donor and recipient strains, prepared in their respective antibiotic media, were mixed in equal volumes within the treatment systems (final density: 108 CFU/mL for each). The conjugation process was initiated with 1 h of shaking at 37 °C and continued for 8 h under static conditions at 37 °C. Subsequently, 500 μL aliquots of the mating culture were plated on selective agar containing ampicillin, kanamycin, tetracycline, and streptomycin to select for transconjugants. To eliminate false positives resulting from spontaneous mutation, 500 μL of each pure donor and recipient culture was separately plated on LB agar containing the same combination of four antibiotics.
The transfer of the RP4 plasmid to recipient cells was genetically confirmed by PCR. Specific primers RP4F and RP4R (Table S2), targeting the traG gene on the RP4 plasmid, were designed as previously described [30]. A 104 bp product was amplified from transconjugant DNA and visualized via gel electrophoresis, verifying the successful conjugative event.
2.5. Assessment of Bacterial Physiological Responses
To investigate the physiological responses of bacteria to various treatments, bacterial viability, intracellular reactive oxygen species (ROS) levels, and cell membrane permeability were evaluated. The detailed experimental procedures for these assays are provided in Texts S1–S3 of the Supplementary Materials.
2.6. Quantification of Conjugative Transfer Related Gene Expression
After conjugation, the expression levels of seven genes associated with conjugative transfer, ompA, ompC, trbB, trfA, korA, korB, and trbA were analyzed and 16S rDNA was used as the internal control. The analysis method for gene expression was based on previous studies [31]. See Text S4 for the specific method.
3. Results and Discussion
3.1. Characterization of Microplastics Before and After Aging
3.1.1. Surface Morphology Analysis
The morphology of two types of microplastics-PLA and PET under UV irradiation over a 60-day aging was examined using SEM. As shown in Figure 1, after 15 days of aging, a small quantity of nanoplastic particles began to appear on the surface of PLA. By day 60, substantial aggregation of micro- and nanoplastic particles had occurred on the PLA surface, accompanied by the development of a porous surface morphology. In contrast, under the same aging conditions, the traditional plastics PET primarily underwent surface fragmentation without noticeable accumulation of nanoplastic particles. This difference is likely attributable to the relatively lower yield of nanoplastic particles generated from PET under UV aging conditions. The observed changes in the surface morphology of microplastics following UV-induced aging are consistent with findings reported in previous studies [32]
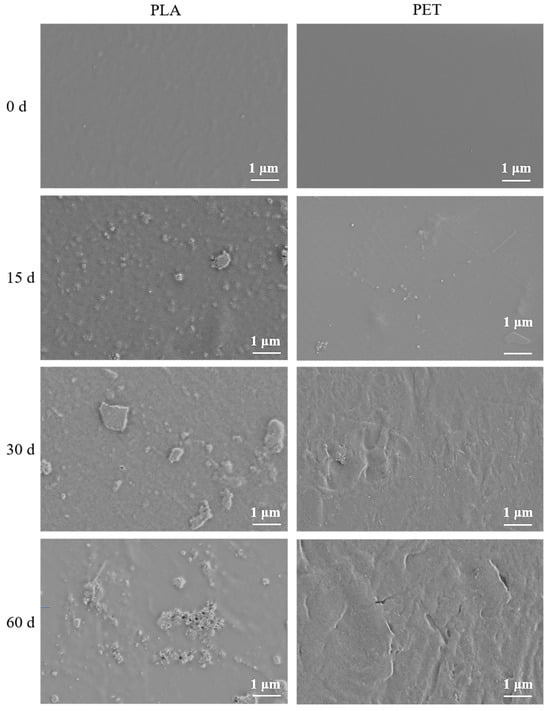
Figure 1.
SEM images of the surface morphology of two types of microplastics, PLA and PET, after different aging periods.
The aged nanoplastic suspension was subsequently subjected to morphological analysis by TEM. The results (Figure S2) showed that after 60 days of UV irradiation aging, there were a high abundance of particles smaller than 100 nm in the PLA suspension, and they were uniformly distributed, whereas the PET suspension contained markedly fewer. Additionally, the sizes of PET particles released into the solution varied significantly. This indicates that compared to the biodegradable plastic PLA, PET underwent relatively less aging within 60 days, a finding consistent with the SEM observations (Figure 1) and prior literature [25].
The specific surface area of PLA and PET microplastics before and after aging is shown in Table S1. The original PLA and PET had similar specific surface areas, indicating that both materials were initially relatively smooth. As the aging time increased, the specific surface area of both materials gradually increased. After 60 days of aging, increased in specific surface area by factors of 20.5 and 8.8 were observed for PLA and PET, respectively, after the aging process. The porous structure after aging can provide adsorption sites for microplastics to adsorb antibiotics and other pollutants [32].
3.1.2. Analysis of Micro/Nanoplastic Release Quantities
The concentration of micro/nanoplastic particles released from UV aging microplastics was determined using a TOC analyzer. Figure 2 shows that after 60 days of UV aging, there was a significant difference (p < 0.05) in the concentration of micro/nanoplastic particles released from PLA and PET. The concentration released from PET was significantly lower (38.04 ± 8.5 mg/L) than that from PLA (138.5 ± 10.9 mg/L). The TOC results are consistent with those from SEM and TEM, further indicating that under the same aging conditions, the release rate of micro/nanoplastic particles from the biodegradable plastic PLA is much faster than that from the conventional plastic PET. The aging behavior of biodegradable plastics in natural environments is influenced by various factors, such as environmental conditions and microbial communities [33]. Therefore, future studies should utilize actual samples (e.g., wastewater or surface water) to better simulate real environmental conditions.
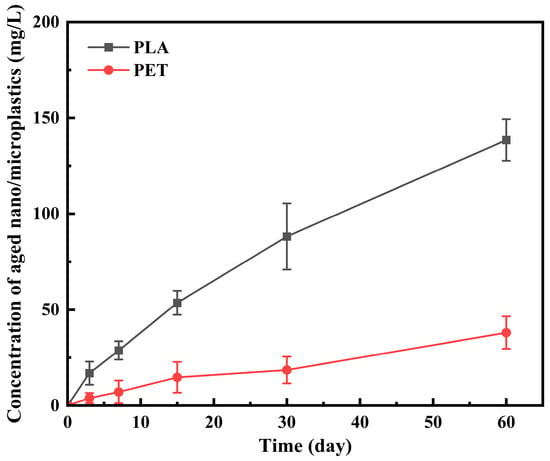
Figure 2.
The amounts of aged nano/microplastics released from PLA and PET microplastics during the 60 days UV aging.
3.1.3. Analysis of Surface Functional Groups
To validate the changes in surface functional groups of microplastics during UV aging, FTIR analysis was performed on PLA and PET samples after aging. The spectra for PLA (Figure S3a) showed a notable increase in the intensity of the carbonyl (C=O) stretching vibration peak at around 1650 cm−1 and the C-O stretching vibration peak around 1110 cm−1, along with a broadening of the hydroxyl (O-H) stretching band near 3400 cm−1 with prolonged aging [34]. This indicated the formation of additional oxygen-containing functional groups such as carboxylic acids and alcohols. In contrast, the FTIR spectra of PET (Figure S3b) revealed no significant changes in the characteristic peaks associated with oxygen-containing groups across the aging periods.
3.2. Combined Effects of Microplastics and SMX
3.2.1. Adsorption of SMX by Micro/Nanoplastics
After UV aging for 60 days, the fragments and suspensions of the two microplastics were separated, and their adsorption capacities for SMX were measured separately. Figure 3a shows that for both PLA and PET after aging, the aged fragments were the primary adsorbents for SMX, while the suspensions exhibited weaker adsorption capabilities. The adsorption of SMX by both fragments and suspensions showed a trend of rapid initial increase followed by stabilization. Compared to PLA, both the fragments and suspensions of PET had significantly lower adsorption capacities for SMX. At equilibrium, the adsorption capacities of PLA and PET fragments for SMX were 0.165 mg/g and 0.057 mg/g, respectively, while those of the suspensions were 0.028 mg/g and 0.005 mg/g, respectively.
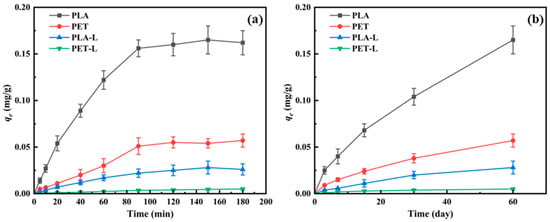
Figure 3.
Adsorption of SMX onto aged microplastics. (a) Adsorption kinetics of SMX onto microplastic fragments and their corresponding suspensions (L) after 60 days of UV aging. (b) Equilibrium adsorption capacity of SMX onto microplastic fragments and suspensions (L) at different UV aging time points. Note: PLA and PET denote aged microplastic fragments; PLA-L and PET-L denote the suspensions containing micro/nanoplastics released during the aging process.
Subsequently, the adsorption capacities of PLA and PET fragments and suspensions at different aging times were measured. As the aging time increased, the adsorption capacities of all systems for SMX gradually enhanced (Figure 3b). Similarly to Figure 3a, at each aging time point, the aged fragments of both plastics exhibited stronger adsorption capacities for SMX than the suspensions. Additionally, across all aging durations, PLA consistently exhibited a greater adsorption capacity for SMX than PET, a phenomenon that was attributed to several UV-induced factors, including a more pronounced formation of oxygen-containing functional groups and porous surfaces [35], the generation of a greater quantity of micro/nanoplastic particles (Figure 1 and Figure S2), and a substantially increased specific surface area (Table S1). These factors collectively provide PLA with more available adsorption sites for SMX.
3.2.2. Solution Stability of Micro/Nanoplastics Before and After Addition of SMX
The effects of pollutants on microorganisms in solution are influenced by their hydrodynamic diameter [36,37]. As shown in Table 1, the hydrodynamic diameters of both types of microplastics gradually decreased with prolonged aging time. After 60 days of aging, the hydrodynamic diameters of PLA and PET were 62.3 nm and 203.6 nm, respectively. These values significantly exceeded the sizes of nanoparticles in the suspension directly measured by TEM, indicating noticeable aggregation of particles in the solution [38]. Further characterization revealed that during the aging process, the zeta potential of PLA decreased from −7.6 mV to −13.6 mV, while that of PET decreased from −5.3 mV to −12.5 mV. Studies have confirmed that UV aging increases the negative charge of microplastics [32], and this study further supports this conclusion. Although the aging process increased the negative charge of the solution, the zeta potential values of the aged suspensions or their mixtures with SMX ranged from −5.3 mV to −13.6 mV, placing the solution in a metastable state [39] (Table 2). This is the direct cause of nanoparticle aggregation in the suspension. The addition of SMX further increased the hydrodynamic diameter of particles in the solution, which may potentially influence the transmission of ARGs among bacteria.

Table 1.
Changes in hydrodynamic diameter of aged microplastic suspensions before and after mixing with SMX.

Table 2.
Changes in zeta potential of aged microplastic suspensions before and after mixing with SMX.
3.3. Impact of Aged Microplastics and SMX on the Conjugative Transfer of ARGs Between Bacteria
In the absence of UV aging, the conjugative transfer frequency in the PLA and PET treatment groups showed no significant difference compared to the control group (Figure 4), which aligns with previous studies indicating that large microplastic particles exhibit no significant short-term effect on the frequency of bacterial conjugative transfer. The primary influence of large particles on the spread of ARGs lies in their role as carriers, enabling bacteria to gradually form biofilms on their surfaces and shortening the distance between bacteria during conjugation [40]. Additionally, at day 0, the combined effect of the two types of plastic particles and SMX on ARG transfer was not significantly different from that of SMX alone. This may be because the plastic particles had not yet degraded into smaller particles at day 0 and exhibited minimal adsorption of SMX (Figure 3a). Furthermore, changes in SMX concentration resulting from its interaction with microplastics did not significantly alter the conjugative transfer frequency (Figure S4). Although the adsorption capacity of aged microplastics for SMX increased with prolonged aging time (Figure 3b), except for the PLA and SMX treatment groups at 30 and 60 days, the adsorption of SMX by other microplastics did not significantly influence their conjugative transfer capability (Figure S4).
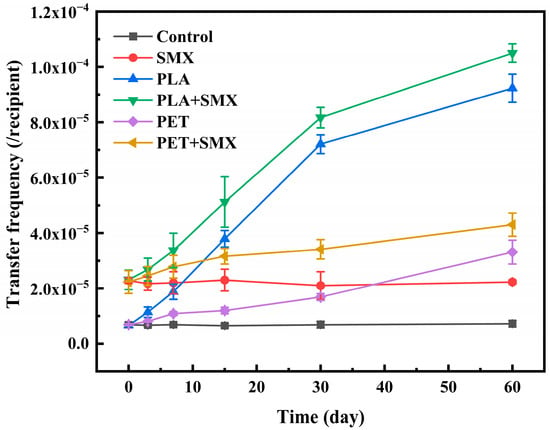
Figure 4.
Effects of PLA and PET alone or mixed with SMX on the conjugative transfer of ARGs in bacteria after different aging times.
Furthermore, as shown in Figure 4, aged PLA consistently induced a higher ARGs transfer frequency than aged PET. After 60 days, the frequency in the PLA-alone group reached 9.23 × 10−5, representing a 12.7-fold increase over the control. Moreover, co-exposure with SMX provided a notable enhancement in transfer frequency compared to treatments with microplastics alone. Among these, the combination of PLA and SMX exhibited the strongest promoting effect, reaching approximately 14.5 times that of the control.
However, it should be noted that according to DLS results (Table 1) the addition of SMX increased the size of nanoplastic particles in the solution, which may modulate the promotive effect of the mixed system on conjugative transfer. Previous studies have confirmed the significant impacts of nanoparticle size on conjugative transfer [18]. Nevertheless, the combined treatments consistently resulted in higher conjugative transfer frequencies compared to treatments with microplastics alone. These findings demonstrate that co-exposure of aged microplastics and SMX significantly facilitates ARG transfer, with a more pronounced effect observed for biodegradable PLA in combination with SMX than for conventional PET with SMX.
Confirmation of ARG transfer to the recipient E. coli HB101 was achieved by PCR amplification of the plasmid-borne traG gene. Electrophoretic analysis of the PCR products from transconjugant DNA revealed the expected 104 bp amplicon (e.g., Figure S5), verifying the successful acquisition of the RP4 plasmid.
3.4. Mechanisms Underlying the Effects of Aged Microplastics and SMX on the Transfer of ARGs Among Bacteria
3.4.1. Effects on Bacterial Viability
Previous studies have demonstrated that the survival rates of donor and recipient strains are critical factors influencing the transfer frequency of ARGs between bacteria [18,25]. Accordingly, the effects of different treatment groups on bacterial viability were assessed. As shown in Figure 5a, after exposure to the early aging stage (0–15 days) microplastics, neither of the aged microplastics significantly affected bacterial survival compared to the control. With prolonged aging, the release of micro/nanoplastic particles from both PLA and PET increased considerably (Figure 2), and this increased particle concentration correlated with a progressive reduction in bacterial viability. This can be attributed to the physical damage and oxidative stress induced by the micro/nanoplastics [27,41]. Compared to PET (96.3–98.5%), PLA released a greater amount of micro/nanoplastics, resulting in lower bacterial survival rates, approximately 93.1–96.3%. This is consistent with the characterization data, which showed that PLA released a significantly greater quantity of micro/nanoplastics (Figure 2) and developed a larger specific surface area (Table S1) after aging. These properties likely enhanced the direct physical interaction and overall bioavailability of the PLA particles towards bacterial cells, leading to greater toxicity [42].
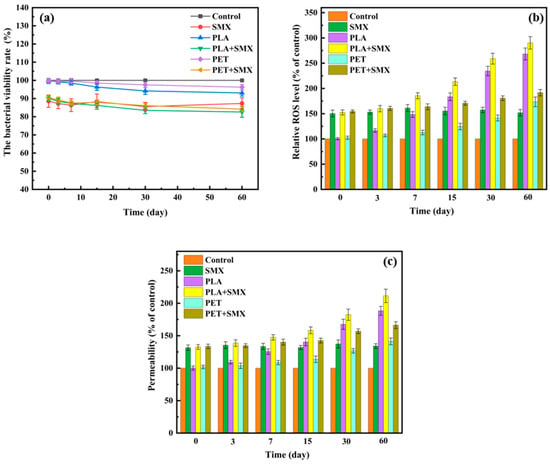
Figure 5.
Effects of PLA and PET alone or mixed with SMX on bacterial viability (a), ROS levels (b), and bacterial membrane permeability (c).
Treatment with SMX alone reduced bacterial survival to about 87.3%. When microplastics and SMX were combined, bacterial survival slightly decreased further. Specifically, co-exposure with PLA + SMX and PET + SMX resulted in survival rates of 82.6–90.1% and 84.2–90.5%, respectively. The combined effects were not simply additive, suggesting a potential interaction between the microplastics and SMX. In the initial aging phase, the limited release of micro/nanoplastics, coupled with the adsorption of SMX onto the larger plastic particles, may have slightly mitigated the toxicity of SMX to bacteria. In contrast, during later stages, the increasing amount of released plastic particles likely enhanced the combined toxicity with SMX. Notably, the observed changes in bacterial survival rates across treatments did not show a consistent correlation with the ARG transfer frequency. For instance, the group with the lowest viability (60-day aged PLA + SMX) also exhibited the highest transfer frequency. This indicates that while reduced viability may contribute to the overall cellular stress, it is not the primary driver of the enhanced conjugative transfer observed in this study. Instead, the sublethal stress responses, such as increased ROS generation and membrane permeability, appear to be the dominant mechanisms facilitating ARG transfer.
3.4.2. Effects on ROS Levels
Oxidative stress resulting from excessive ROS accumulation has been identified as a key factor influencing the horizontal transfer of ARGs [43]. To further investigate bacterial responses to microplastics and SMX exposure, intracellular ROS levels were measured. As shown in Figure 5b, SMX treatment alone significantly increased ROS levels in bacterial cells—approximately 1.5 times higher than those of control. For aged plastic particles, a dose-dependent relationship was observed between the duration of aging—which affects the release of micro/nanoplastics—and intracellular ROS levels. The PLA treated groups exhibited higher ROS levels compared to the PET groups, with a maximum increase of approximately 2.7 folds relative to the control. Combined exposure to aged PLA or PET along with SMX further enhanced intracellular ROS levels beyond those induced by either treatment alone. The highest ROS level was observed in the group exposed to the 60 days aged PLA and SMX, which showed 2.9 folds increase compared to the control. These findings suggest that the coexistence of aged microplastics and SMX induces oxidative stress in bacterial cells and facilitates the transfer of ARGs, consistent with the transfer frequency data shown in Figure 4.
To investigate the role of excessive ROS in the conjugative transfer process, the ROS scavenger thiourea was introduced into each conjugation system. Compared with the treatments without thiourea, the addition of thiourea significantly reduced ROS levels in all treatment groups, bringing them down to levels comparable to those of the control group (Figure S6a). Concurrently, the conjugative transfer frequencies of ARGs in these treatment groups showed no significant difference compared with the control (Figure S6b). These results confirm that the overaccumulation of ROS is a primary and direct driver behind the increase in ARG transfer frequency between bacteria.
To verify whether the ROS that caused stress in bacteria are formed solely under the influence of ultraviolet radiation or through interaction with micro/nanoplastics, an ultrafiltration centrifuge tube was used to separate the micro/nanoplastics from the filtrate. The ROS levels and conjugative transfer frequencies were then analyzed after treatment with the filtrate. As shown in Figure S7, when treated with the filtrate alone, neither the ROS levels nor the conjugative transfer frequencies in any treatment group showed a significant difference compared to the control. This indicated that the impacts of aged micro/nanoplastics on bacteria are primarily attributable to the particles.
3.4.3. Effects on Bacterial Membrane Permeability
Studies have demonstrated that excessive intracellular ROS could cause bacteria damage and alter cell membrane permeability, thereby influencing the transfer frequency of ARGs between bacteria [44]. As shown in Figure 5c, all treatment groups resulted in increased membrane permeability, with a trend consistent with that of relative ROS levels (Figure 5b). Similarly to the ROS level, the combined treatment of both microplastics with SMX significantly enhanced cell membrane permeability compared to treatments with microplastics alone. Notably, the combination of aged PLA and SMX induced the highest permeability, reaching approximately 2.09 times that of the control. The synergistic effect may be attributed to the following reasons. Firstly, the aged PLA, which released a greater abundance of nano-sized particles (Figure 1, Figure 2 and Figure S2) and possessed a larger specific surface area (Table S1), provided a greater total surface area for direct contact and potential disruption of the bacterial membranes [45]. Secondly, the high adsorption capacity of aged PLA for SMX (Figure 3) likely facilitated the co-transport and targeted delivery of both stressors to the bacterial cells [42]. This co-localization resulted in an intense, localized oxidative stress (as evidenced by the highest ROS level in Figure 5b), which synergistically damaged the lipid bilayer of the membrane, leading to the observed peak in permeability.
These findings indicated that co-exposure to aged microplastics and SMX enhanced membrane permeability and facilitated the horizontal transfer of ARGs, with the biodegradable PLA and SMX combination exhibiting the most pronounced effect.
3.4.4. Effects on the Expression of Conjugative Transfer Related Genes
To further elucidate the mechanism by which aged microplastics and SMX affect the conjugative transfer of ARGs between bacteria, the expression of eight genes related to this process was measured after exposure. Since the trends observed across groups with different aging periods were generally consistent, microplastics aged for 60 days and their mixtures with SMX were selected to analyze the effects of various treatments on gene expression. Among these, ompA and ompC are two important genes involved in bacterial pore formation and membrane transport [40,46]. As shown in Figure 6a, compared with the control, for all treatment groups, the expression levels of these two genes did not change significantly, which contrasts with previous studies. Some studies have reported that certain contaminants can significantly promote the expression of ompA and ompC [29]. This discrepancy may be attributed to the distinct properties and mechanisms of action of the pollutants used in the different studies. Integrating these results with the membrane permeability data (Figure 5c), it is likely that the increased permeability was not mediated by changes in ompA or ompC expression, but rather was a direct result of ROS-induced membrane damage.
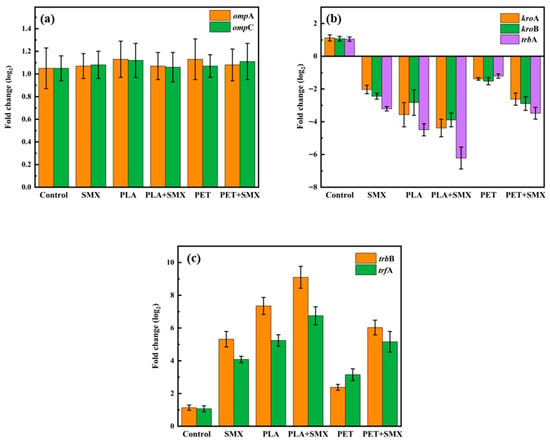
Figure 6.
Effects of PLA and PET alone or mixed with SMX on the expression of conjugative transfer related genes. (a) Effects on the expression of two outer membrane porin genes (ompA and ompC). (b) Effects on the expression of three major global regulatory genes (korA, korB and trbA). (c) Effects on the expression of mating-pair formation (Mpf) genes trbB and the DNA transfer and replication (Dtr) system genes trfA.
As shown in Figure 6b, all treatment groups led to a reduction in the expression of three major global regulatory genes (korA, korB, and trbA) compared with the control. Specifically, the inhibition of korB and trbA could activate the expression of mating-pair formation (Mpf) genes (e.g., trbB), while suppression of korA and korB promotes the expression of DNA transfer and replication (Dtr) system genes (e.g., trfA) [18,29]. The Mpf system contributes to the formation of membrane associated proteins and channels, thereby facilitating DNA transfer [47]. The Dtr system genes play a crucial role in initiating the replication process during conjugation [48]. After combined treatment with aged PLA and SMX, the expression of trbB and trfA was most significantly upregulated, increasing by approximately 9.1 folds and 6.7 folds, respectively, compared to the control. However, when the ROS scavenger thiourea was introduced, the expression levels of trbB and trfA decreased to levels comparable to the control (Figure S6c). Correspondingly, the ARGs transfer frequency showed no significant difference from the control (Figure S6b). These results demonstrated that the aged microplastics and SMX could enhance the expression of Mpf and Dtr system genes by inducing excessive ROS formation, which ultimately promoted the conjugative transfer of ARGs between bacteria.
3.5. Mechanism of Synergistic Enhancement by Aged PLA and SMX
The findings of this study indicate that the combination of UV-aged PLA and SMX exhibited the strongest promoting effect on the conjugative transfer of ARGs, reaching approximately 14.5 times that of the control (Figure 4). This remarkable effect is not a result of simple addition but of a synergistic interaction between the two stressors, which can be mechanistically explained by integrating the key results. The UV aging process endowed PLA with distinct physicochemical properties—notably, the formation of oxygen-containing functional groups, a significantly increased specific surface area (Table S1) and a high release rate of secondary nanoplastics (Figure 1, Figure 2, Figures S2 and S3). These properties underpinned its superior role as a carrier and concentrator for SMX, as evidenced by its high adsorption affinity for the antibiotic (Figure 3).
The co-localization and delivery of aged PLA particles and SMX to bacterial cells created a perfect storm of cellular stress. This was manifested as the highest level of intracellular ROS among all treatment groups (Figure 5b), which served as the primary trigger for the subsequent cascade. The intense oxidative stress, in turn, induced the most severe damage to the cell membrane [49], leading to the peak in membrane permeability (Figure 5c). Crucially, at the genetic level, this combined stress most effectively inhibited the expression of major global regulatory genes (Figure 6b), thereby derepressing and leading to the strongest upregulation of the key genes responsible for mating-pair formation gene trbB and DNA transfer/replication gene trfA (Figure 6c).
Therefore, the 14.5-fold increase in conjugative transfer is the ultimate consequence of a multi-faceted synergy; the uniquely potent, aged biodegradable plastic amplifies the effect of the antibiotic by enhancing its bioavailability and co-inducing severe oxidative stress, which collectively disrupts cellular integrity and maximally activates the genetic machinery required for horizontal gene transfer.
4. Conclusions
Based on the findings of this study, it is evident that ultraviolet-aged biodegradable PLA microplastics have a significantly greater potential to promote the conjugative transfer of ARGs compared to conventional PET, particularly under co-exposure with the antibiotic SMX. The aging process enhances the physicochemical properties of PLA, resulting in a 20.5-fold increase in specific surface area and the release of a high abundance of secondary nanoplastics (138.5 ± 10.9 mg/L), which collectively enhance its adsorption capacity for SMX. This combination induces substantial oxidative stress in bacterial cells (increasing intracellular ROS levels by up to 2.9-fold), leading to increased membrane permeability (approximately 2.09-fold higher than the control) and the upregulation of key conjugative transfer genes. Consequently, the conjugative transfer frequency was enhanced by approximately 14.5-fold in the system containing aged PLA and SMX. These results underscore that aged biodegradable plastics can act as important factors for ARG dissemination in antibiotic contaminated environments. This study provides critical insights for the environmental risk assessment of biodegradable plastics and highlights the need to consider their long-term ecological impacts, especially in the context of antibiotic resistance propagation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17229981/s1, Table S1: Changes in the specific surface area of microplastics before and after aging; Table S2: List of primers used in this work; Figure S1: The standard curve of Sulfamethoxazole; Figure S2: TEM images of micro/nanoparticles in the suspension after 60 days of aging. (A) PLA suspension; (B) PET suspension; Figure S3. FTIR spectra of (a) PLA and (b) PET microplastics after 0, 30, and 60 days of UV aging; Figure S4: The conjugative transfer frequency of ARGs after exposure to different concentration of sulfamethoxazole; Figure S5: PCR identification of transconjugants; Figure S6: The conjugative transfer frequency of ROS level (a), ARGs (b), and the expression of trbB and trfA (c) after the addition of thiourea. Figure S7: The ROS level (a) and the conjugative transfer frequency of ARGs after exposure to the filtrate of micro/nanoplastics. PLA-F denotes the filtrate of PLA micro/nanoplastics, and PET-F denotes the filtrate of PET micro/nanoplastics.
Author Contributions
Writing—original draft preparation, X.L. and S.S.; methodology, S.S.; writing—review and editing, X.L., M.Y., X.X. and S.H.; formal analysis, S.A.; resources, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Shandong Provincial Natural Science Foundation Youth Project, grant number ZR2024QD100; the research project of experimental technology of Qufu Normal University, grant number SJG202327.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ARGs | Antibiotic Resistance Genes |
| Dtr | DNA Transfer and Replication |
| HPLC | High-Performance Liquid Chromatography |
| LDH | Lactate Dehydrogenase |
| Mpf | Mating-Pair Formation |
| MPs | Microplastics |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| PET | Polyethylene Terephthalate |
| PLA | Polylactic Acid |
| qPCR | Quantitative Real-Time Polymerase Chain Reaction |
| ROS | Reactive Oxygen Species |
| SEM | Scanning Electron Microscopy |
| SMX | Sulfamethoxazole |
| TEM | Transmission Electron Microscopy |
| TOC | Total Organic Carbon |
| UV | Ultraviolet |
References
- Ahn, B.; Yun, S.; Yun, S.; Kim, Y.J.; Won, W. System-level analysis of strategies for biodegradable plastics production from microalgae. Sustain. Prod. Consum. 2025, 55, 340–352. [Google Scholar] [CrossRef]
- Liu, X.M.; Ahmad, S.; Ma, J.K.; Wang, D.; Tang, J.C. Comparative study on the toxic effects of secondary nanoplastics from biodegradable and conventional plastics on Streptomyces coelicolor M145. J. Hazard. Mater. 2023, 460, 132343. [Google Scholar] [CrossRef]
- Wang, K.F.; Guo, C.Y.; Li, J.; Wang, K.K.; Liang, S.Q.; Wang, W.; Wang, J.D. A critical review of the adsorption-desorption characteristics of antibiotics on microplastics and their combined toxic effects. Environ. Technol. Innov. 2024, 35, 103729. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.J.; Xia, S.Q.; Zhao, J.F. New insights into oxytetracycline (OTC) adsorption behavior on polylactic acid microplastics undergoing microbial adhesion and degradation. Chem. Eng. J. 2021, 416, 129085. [Google Scholar] [CrossRef]
- Hu, L.L.; Zhou, Y.H.; Chen, Z.; Zhang, D.Y.; Pan, X.L. Oligomers and Monomers from Biodegradable Plastics: An Important but Neglected Threat to Ecosystems. Environ. Sci. Technol. 2023, 57, 9895–9897. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Cornish, K.; Vodovotz, Y. Narrowing the Gap for Bioplastic Use in Food Packaging: An Update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Parolini, M.; De Felice, B.; Gazzotti, S.; Sugni, M.; Ortenzi, M.A. Comparison of the potential toxicity induced by microplastics made of polyethylene terephthalate (PET) and polylactic acid (PLA) on the earthworm Eisenia foetida. Environ. Pollut. 2024, 348, 123868. [Google Scholar] [CrossRef] [PubMed]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, H.; Chen, J.; Tan, F.; Cai, R.; Wang, Y. Photoaging Promotes Toxic Micro/Nanoplastics Release from PLA/PBAT Biodegradable Plastic in Gastrointestinal Condition. Environ. Health 2025, 3, 446–457. [Google Scholar] [CrossRef]
- Ni, N.; Shi, R.Y.; Meng, J.; Guo, X.Y.; Shi, M.L.; Zhang, X.H.; Yao, S.; Nkoh, J.N.; Wang, F.H.; Song, Y.; et al. Comparative analysis of the sorption behaviors and mechanisms of amide herbicides on biodegradable and nondegradable microplastics derived from agricultural plastic products. Environ. Pollut. 2023, 318, 120865. [Google Scholar] [CrossRef]
- Zheng, M.H.; Wu, P.W.; Li, L.Q.; Yu, F.; Ma, J. Adsorption/desorption behavior of ciprofloxacin on aged biodegradable plastic PLA under different exposure conditions. J. Environ. Chem. Eng. 2023, 11, 109256. [Google Scholar] [CrossRef]
- Ma, S.S.; Wang, L.; Ding, J.T.; Zhou, H.B.; Shen, Y.J.; Wang, J.; Chen, Y.X.; Yang, Y. Effects of high-concentration sulfamethoxazole on antibiotic resistance genes during swine manure aerobic composting. J. Environ. Chem. Eng. 2025, 13, 117216. [Google Scholar] [CrossRef]
- Osinski, Z.; Patyra, E.; Kwiatek, K. HPLC-FLD-Based Method for the Detection of Sulfonamides in Organic Fertilizers Collected from Poland. Molecules 2022, 27, 2031. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.S.; Mao, G.N.; Yin, X.L.; Ma, L.P.; Liu, L.; Bai, Y.H.; Zhang, T.; Qu, J.H. Identification and quantification of bacterial genomes carrying antibiotic resistance genes and virulence factor genes for aquatic microbiological risk assessment. Water Res. 2020, 168, 115160. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Igual, R.; Bernal-Bayard, J.; Rodriguez-Paton, A.; Ghigo, J.M.; Mazel, D. Engineered toxin-intein antimicrobials can selectively target and kill antibiotic-resistant bacteria in mixed populations. Nat. Biotechnol. 2019, 37, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Mendler, K.; Chen, H.; Parks, D.H.; Lobb, B.; Hug, L.A.; Doxey, A.C. AnnoTree: Visualization and exploration of a functionally annotated microbial tree of life. Nucleic Acids Res. 2019, 47, 4442–4448. [Google Scholar] [CrossRef]
- Qiu, Z.G.; Yu, Y.M.; Chen, Z.L.; Jin, M.; Yang, D.; Zhao, Z.G.; Wang, J.F.; Shen, Z.Q.; Wang, X.W.; Qian, D.; et al. Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proc. Natl. Acad. Sci. USA 2012, 109, 4944–4949. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, J.B.; Peng, S.; Wang, Y.M.; Lin, X.G.; Wang, S.S. Biodegradable microplastics exacerbate the risk of antibiotic resistance genes pollution in agricultural soils. J. Hazard. Mater. 2025, 496, 139490. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Zhang, M.; Wang, X.A.; Zhang, D.Y.; Pan, X.L. Persistent versus transient, and conventional plastic versus biodegradable plastic?-Two key questions about microplastic-water exchange of antibiotic resistance genes. Water Res. 2022, 222, 118899. [Google Scholar] [CrossRef]
- Rong, L.L.; Zhang, B.Y.; Qiu, H.M.; Zhang, H.L.; Yu, J.Y.; Yuan, Q.; Wu, L.G.; Chen, H.; Mo, Y.M.; Zou, X.M.; et al. Significant generational effects of tetracyclines upon the promoting plasmid-mediated conjugative transfer between typical wastewater bacteria and its mechanisms. Water Res. 2025, 287, 124290. [Google Scholar] [CrossRef]
- Chen, L.; Mao, C.K.; Yuan, S.N.; Pu, X.J.; Liang, H.H.; Chen, X.Y.; Shao, H.Y.; Wu, M.H. Comparison of aging behavior and adsorption processes of biodegradable and conventional microplastics. Chem. Eng. J. 2024, 502, 157915. [Google Scholar] [CrossRef]
- Yoo, J.W.; Park, J.S.; Lee, Y.H.; Choi, T.J.; Kim, C.B.; Jeong, T.Y.; Kim, C.H.; Kim, T.H.; Lee, Y.M. Toxic effects of fragmented polyethylene terephthalate particles on the marine rotifer Brachionus koreanus: Based on ingestion and egestion assay, in vivo toxicity test, and multi-omics analysis. J. Hazard. Mater. 2024, 472, 134448. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.X.; Wei, C.J.; Wang, W.; Chen, R.; Cui, L.W.; Wang, L.M.; Chen, D.L.; Yu, Y.L.; Li, B.; Li, Y.F. Screening the phytotoxicity of micro/nanoplastics through non-targeted metallomics with synchrotron radiation X-ray fluorescence and deep learning: Taking micro/nano polyethylene terephthalate as an example. J. Hazard. Mater. 2024, 463, 132886. [Google Scholar] [CrossRef]
- Liu, X.M.; Wang, X.L.; Wang, R.J.; Guo, S.S.; Ahmad, S.; Song, Y.H.; Gao, P.K.; Chen, J.F.; Liu, C.C.; Ding, N. Effects comparison between the secondary nanoplastics released from biodegradable and conventional plastics on the transfer of antibiotic resistance genes between bacteria. Environ. Pollut. 2023, 317, 120680. [Google Scholar] [CrossRef]
- Pu, Y.H.; Hao, Y.M.; Zeng, Q.Z.; Yang, Q.; Yang, B.W.; Wu, Y.X.; Yang, X.Y.; Sun, Y.; Wang, X.; Ma, Y.L.; et al. Effects of UV-aged tire wear particles (TWPs) on soil microorganisms: Microbial community, microbial metabolism, cell defense and repair, and transmission of ARGs. J. Environ. Chem. Eng. 2025, 13, 115624. [Google Scholar] [CrossRef]
- Gonzalez-Pleiter, M.; Tamayo-Belda, M.; Pulido-Reyes, G.; Amariei, G.; Leganes, F.; Rosal, R.; Fernandez-Pinas, F. Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environ. Sci. Nano 2019, 6, 1382–1392. [Google Scholar] [CrossRef]
- Razanajatovo, R.M.; Ding, J.N.; Zhang, S.S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, D.Q.; Luo, Y. Ionic Liquid Facilitates the Conjugative Transfer of Antibiotic Resistance Genes Mediated by Plasmid RP4. Environ. Sci. Technol. 2015, 49, 8731–8740. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, D.; Tang, J.; Liu, F.; Wang, L. Effect of dissolved biochar on the transfer of antibiotic resistance genes between bacteria. Environ. Pollut. 2021, 288, 117718. [Google Scholar] [CrossRef]
- Liu, X.M.; Tang, J.C.; Wang, L.; Giesy, J.P. Al2O3 nanoparticles promote secretion of antibiotics in Streptomyces coelicolor by regulating gene expression through the nano effect. Chemosphere 2019, 226, 687–695. [Google Scholar] [CrossRef]
- Fan, X.L.; Zou, Y.F.; Geng, N.; Liu, J.Q.; Hou, J.; Li, D.D.; Yang, C.F.; Li, Y. Investigation on the adsorption and desorption behaviors of antibiotics by degradable MPs with or without UV ageing process. J. Hazard. Mater. 2021, 401, 123363. [Google Scholar] [CrossRef]
- Chen, S.; Xu, G.H.L.; Chen, J.W.; Zhang, H.; Jiang, X.Z.; Liu, Z.W.; Lin, Z.W.; Zhang, C.Z.; Xu, L.; Zhang, J.B. Predicting the environmental fate of biodegradable mulch films: A machine learning approach for sustainable agriculture. J. Hazard. Mater. 2025, 492, 138277. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Zhang, X.; Xiao, S.Z.; Peng, B.Y.; Chen, J.B.; Yang, L.B.; Zhou, X.F.; Zhang, Y.L. Distinct exposure impact of non-degradable and biodegradable microplastics on freshwater microalgae (Chlorella pyrenoidosa): Implications for polylactic acid as a sustainable plastic alternative. J. Hazard. Mater. 2024, 480, 136265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Wan, S.; Xu, M.; Wu, G.P.; Wang, D.F.; Yi, C.; Cui, L.Z. Effect of aging on the properties of microplastics and their adsorption behavior of norfloxacin. J. Water Process Eng. 2025, 70, 107021. [Google Scholar] [CrossRef]
- Yang, X.X.; Zhou, Y.S.; Xia, R.; Liao, J.Q.; Liu, J.Q.; Yu, P.F. Microplastics and chemical leachates from plastic pipes are associated with increased virulence and antimicrobial resistance potential of drinking water microbial communities. J. Hazard. Mater. 2024, 463, 132900. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Q.; Jiang, F.S.; Geng, Y.X.; Zhang, L.F.; Li, C.; Li, J.; Li, Y.J.; Hu, X.W.; Huang, J.H.; et al. Interactions between inhalable aged microplastics and lung surfactant: Potential pulmonary health risks. Environ. Res. 2024, 245, 117803. [Google Scholar] [CrossRef]
- Villacorta, A.; Rubio, L.; Alaraby, M.; Lopez-Mesas, M.; Fuentes-Cebrian, V.; Moriones, O.H.; Marcos, R.; Hernandez, A. A new source of representative secondary PET nanoplastics. Obtention, characterization, and hazard evaluation. J. Hazard. Mater. 2022, 439, 129593. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lin, Z.H.; Ai, F.X.; Du, W.C.; Yin, Y.; Guo, H.Y. Effect of ultraviolet aged polytetrafluoroethylene microplastics on copper bioavailability and Microcystis aeruginosa growth. Aquat. Toxicol. 2024, 272, 106967. [Google Scholar] [CrossRef]
- Liu, X.M.; Wei, H.Y.; Ahmad, S.; Wang, R.J.; Gao, P.K.; Chen, J.F.; Song, Y.H.; Liu, C.C.; Ding, N.; Tang, J.C. Effects and mechanism of microplastics on abundance and transfer of antibiotic resistance genes in the environment—A critical review. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1852–1874. [Google Scholar] [CrossRef]
- Yang, W.F.; Gao, P.; Ma, G.Y.; Huang, J.Y.; Wu, Y.X.; Wan, L.; Ding, H.J.; Zhang, W.H. Transcriptome analysis of the toxic mechanism of nanoplastics on growth, photosynthesis and oxidative stress of microalga Chlorella pyrenoidosa during chronic exposure. Environ. Pollut. 2021, 284, 117413. [Google Scholar] [CrossRef]
- Wang, Y.H.; Shang, Y.J.; Guo, J.Y.; Xu, G.H.; Lu, G.H.; Zhou, X.; Liu, J.C. Aged biodegradable and non-biodegradable microplastics alter metolachlor toxicity in the gut and liver of crucian carp. J. Hazard. Mater. 2025, 496, 139212. [Google Scholar] [CrossRef]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal Antibiotic Treatment Leads to Multidrug Resistance via Radical-Induced Mutagenesis. Molecular Cell 2010, 37, 311–320. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Li, R.H. Aged Microplastics and Antibiotic Resistance Genes: A Review of Aging Effects on Their Interactions. Antibiotics 2024, 13, 941. [Google Scholar] [CrossRef]
- Shao, Y.T.; Hua, X.; Li, Y.H.; Wang, D.Y. Comparison of reproductive toxicity between pristine and aged polylactic acid microplastics in Caenorhabditis elegans. J. Hazard. Mater. 2024, 466, 133545. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, H.M.; Wu, F.; Liu, B.L.; Li, H.; Li, Y.W.; Cai, Q.Y.; Xiang, L.; Mo, C.H.; Li, Q.X. Perfluorooctane sulfonate (PFOS) promotes transformational transfer of antibiotic resistance genes and cross-resistance between antibiotics and PFOS. Water Res. 2025, 284, 123868. [Google Scholar] [CrossRef]
- Grahn, A.M.; Haase, J.; Bamford, D.H.; Lanka, E. Components of the RP4 conjugative transfer apparatus form an envelope structure bridging inner and outer membranes of donor cells: Implications for related macromolecule transport systems. J. Bacteriol. 2000, 182, 1564–1574. [Google Scholar] [CrossRef]
- Giusti, M.D.; Pistorio, M.; Lozano, M.J.; Tejerizo, G.A.T.; Salas, M.E.; Martini, M.C.; López, J.L.; Draghi, W.O.; Del Papa, M.F.; Pérez-Mendoza, D.; et al. Genetic and functional characterization of a yet-unclassified rhizobial Dtr (DNA-transfer-and-replication) region from a ubiquitous plasmid conjugal system present in Sinorhizobium meliloti, in Sinorhizobium medicae, and in other nonrhizobial Gram-negative bacteria. Plasmid 2012, 67, 199–210. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, X.H.; Li, Y.; Lin, X.H.; Li, Y.; Yang, Z.F. Photoaging of biodegradable nanoplastics regulates their toxicity to aquatic insects (Chironomus kiinensis) by impairing gut and disrupting intestinal microbiota. Environ. Int. 2024, 185, 108483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).