The Use of Hydrogen in the Automotive Diesel Engine—An Efficient Solution to Control Its Operation with Reduced Carbon Emissions
Abstract
1. Introduction
Actual Context of the Research
2. Experimental Investigations
2.1. Scope of the Experimental Research
2.2. Methodology of the Experimental Investigations
3. Study Results and Discussion
Results of the Experimental Study
4. Conclusions
- -
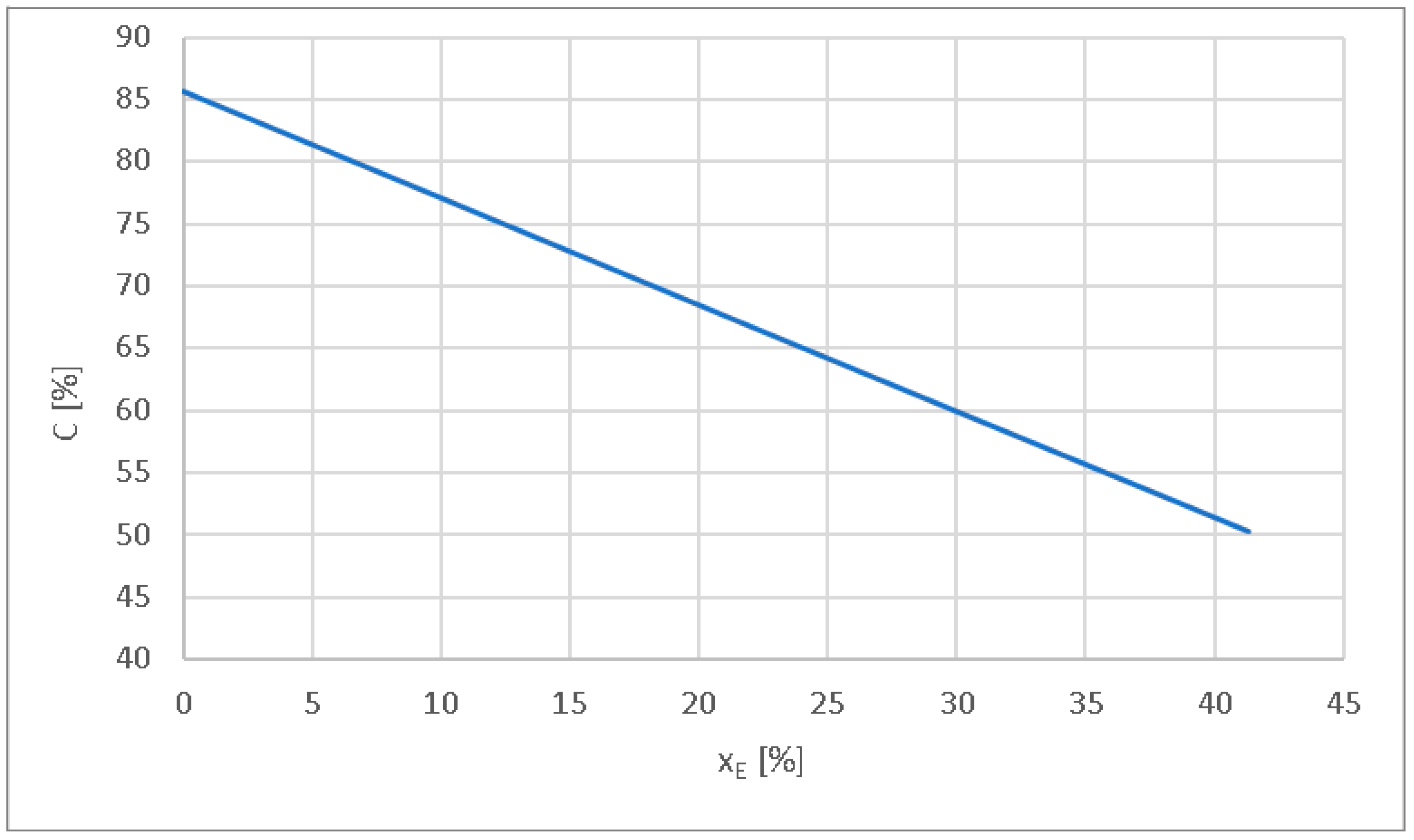
- The carbon content of the in-cylinder mixture decreases to 78.5% at xE = 8.4%, to 65.5% at xE = 23.5% and to 50.3% at xE = 41.3%, from 85.7% carbon with diesel fuelling. When using hydrogen, the mixture’s carbon content decreases by 8% for xE = 8.4%, by 23% for xE = 23.5%, and by 41% for xE = 41.3%.
- -
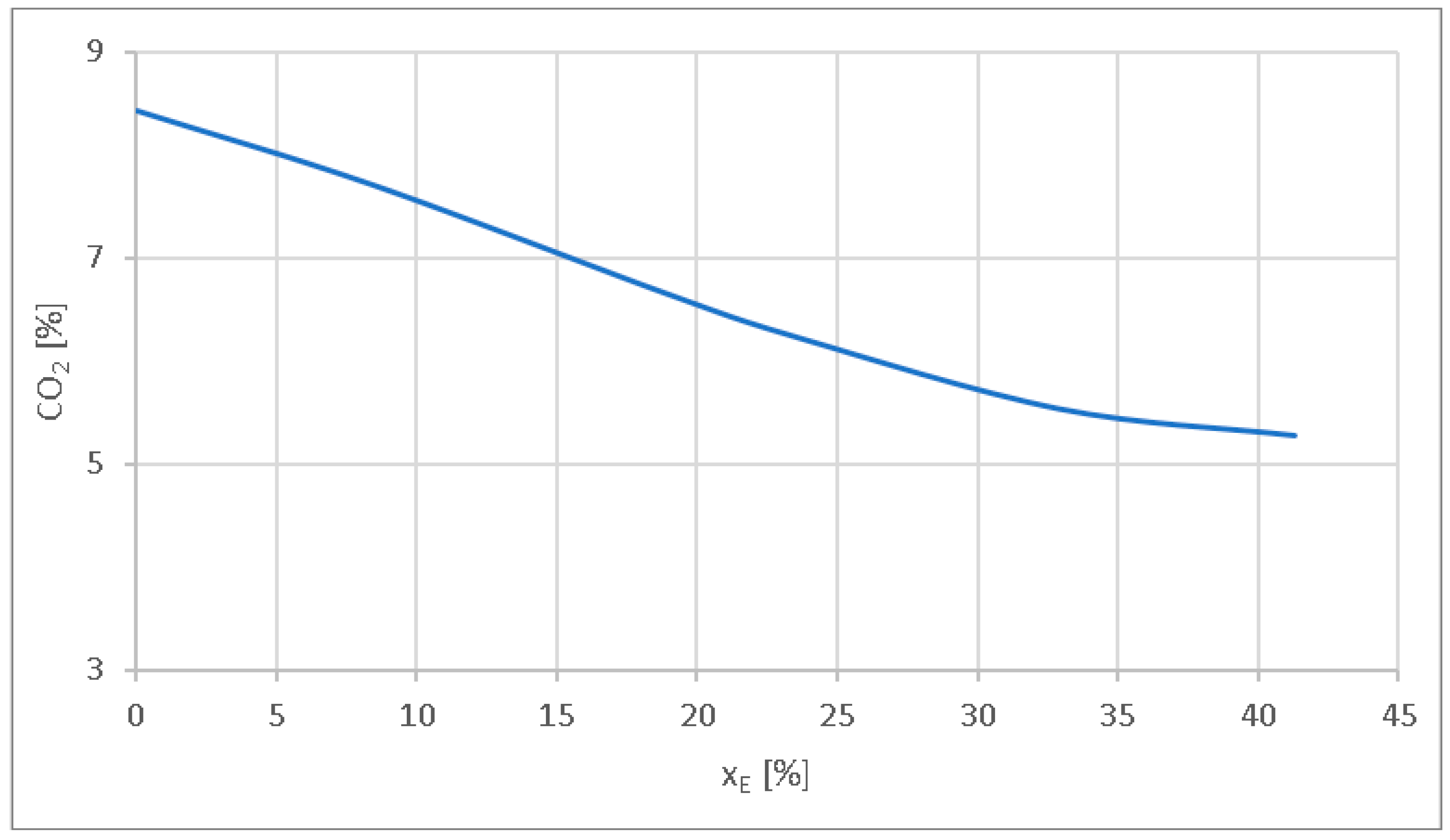
- The CO2 emission level is reduced by 11.13% at xE = 8.4%, by 26% at xE = 23.5%, and by 37.44% at xE = 41.3%. The reduction in the CO2 emission level is related to the reduction in the carbon content when using hydrogen.
- -
- The HC emission level decreases by 4% at xE = 8.4%, by 14% at xE = 23.5%, and by 18% at xE = 41.3%, due to high-speed combustion of homogeneous air–hydrogen mixtures.
- -
- Due to a reduction in the carbon content, the smoke emission level, which is assessed by the opacity value of the exhaust gases, decreases by 52.02% for xE = 8.4%, by 70.27% for xE = 23.5%, and by 45.27% for xE = 41.3%. At maximum xE, the reduction in smoke opacity is less pronounced, probably due to the reduction in the amount of air available in the cylinder. Limiting the tendency for smoke emissions to increase can be achieved by increasing the boost pressure, which will ensure an increase in the amount of air per cycle.
- -
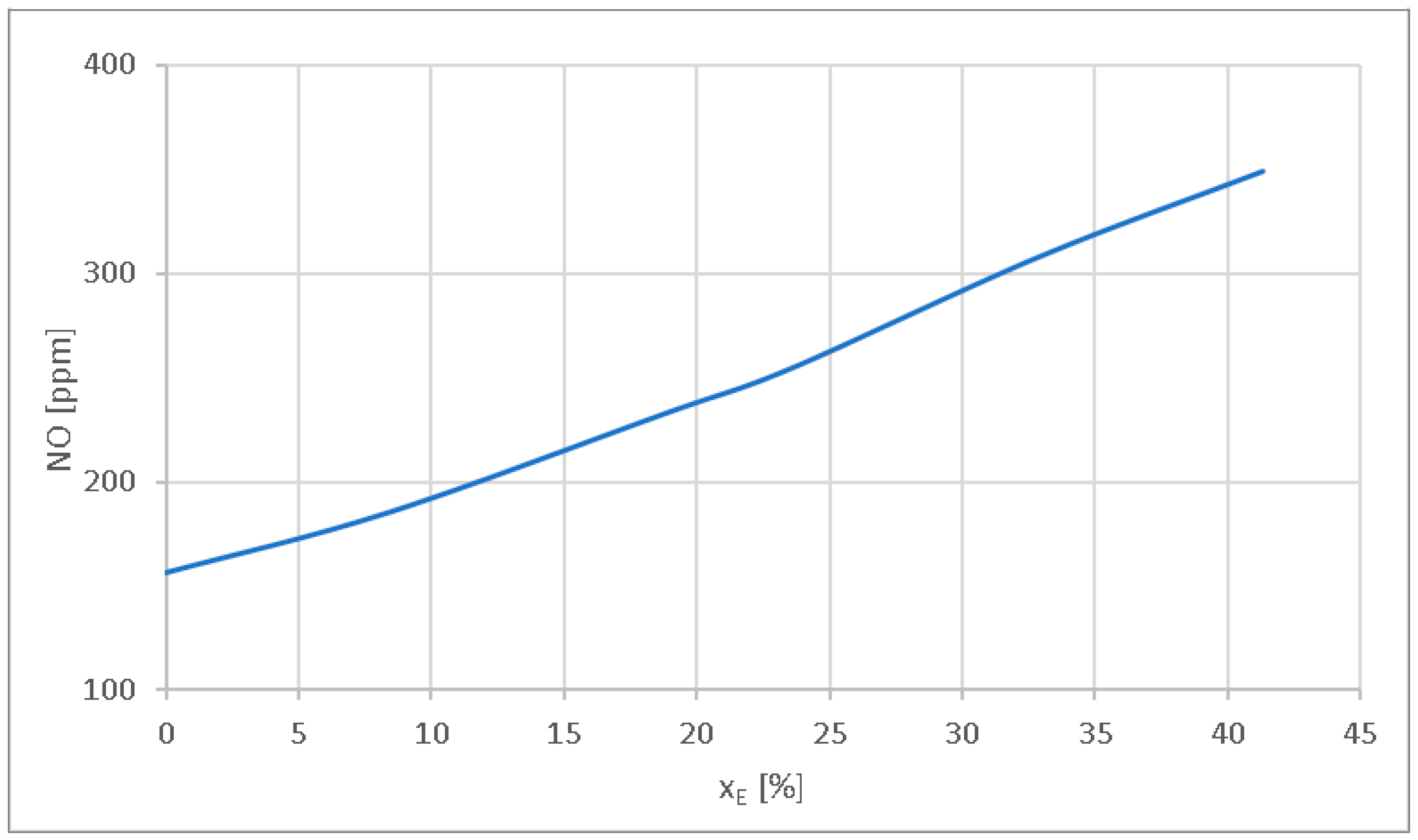
- The NO emission level increases because of temperature rise during combustion. For xE = 8.4%, the NO emission level increases by 18.58%. For xE = 23.5% and xE = 41.3%, the NO level increases by 62.82% and 123.71%, respectively. If solutions such as tuning the injection advance, EGR rate modification, or using Ad-Blue are not applied to reduce NO, then in order to limit the increase in the NO level, limiting the energetic substitution ratio xE to 41.3% can be considered.
- -
- In-cylinder pressure increases to a maximum value which tends to approach the TDC, which may correspond to faster, more brutal combustion in the presence of air–hydrogen mixtures established in the cylinder at the moment of autoignition of the diesel dose. This trend is amplified by the increase in the cyclic hydrogen dose, when xE increases. In some individual combustion cycles, it can be observed that at maximum xE = 41.3%, the pressure oscillations produced by the brutal combustion are amplified.
- -
- The angle of maximum pressure appears sooner per cycle, with 1 CAD at xE = 8.4%, with 2 CAD at xE = 23.5%, and with 4 CAD at xE = 41.3%. The approach of the maximum pressure angle to the TDC can be correlated with the increase in the share of the premixed phase of combustion among the preformed mixtures involved in hydrogen fuelling.
- -
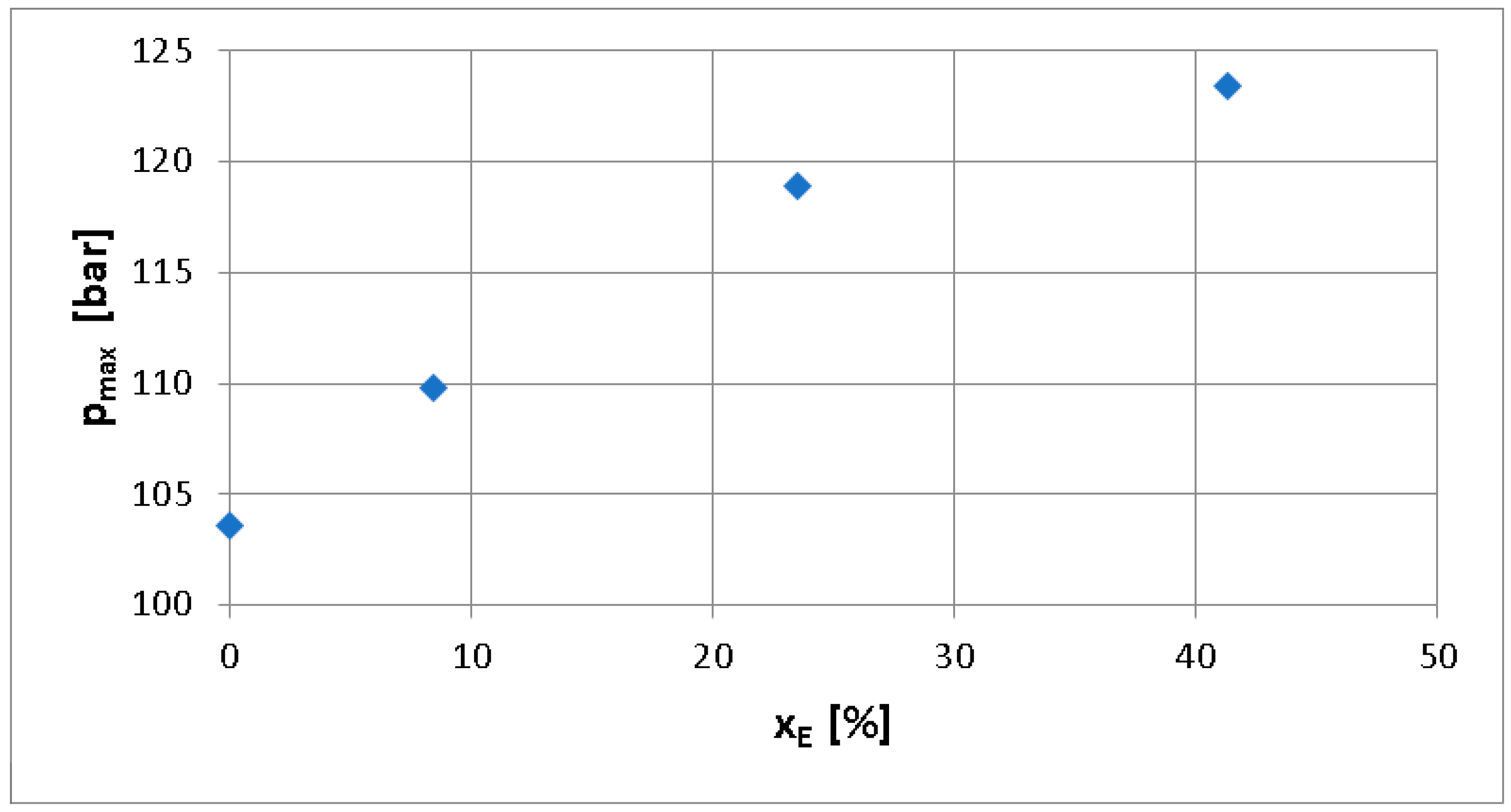
- The value of maximum pressure increases by 5.98% at xE = 8.4%, by 14.77% at xE = 23.5%, and by 19.11% for xE = 41.3% compared to xE = 0%. The higher combustion speed of air–hydrogen mixtures compared to that of air–diesel vapor mixtures at the same excess air coefficient can lead to a reduction in the duration of combustion, meaning that the use of hydrogen has an increasing influence on efficiency.
- -
- The maximum pressure rise rate increases by 6.51% at xE = 8.4%, by 50.93% at xE = 23.5%, and by 23.94% at xE = 41.3%, compared to xE = 0%. There is a correlation between the trends of decrease in the maximum pressure angle, increase in the maximum pressure, and increase in the maximum pressure rise rate. Although the recorded values do not exceed the range of usual values specific to diesel engines, the increasing trend recorded in the maximum pressure values and the pressure increase rate may be a criterion for limiting the cyclic hydrogen dose to xE = 41.3% in order to maintain engine reliability.
- -
- The peak of the increase in heat release rate was 45.77% at xE = 41.3%. For the energetic substitution ratios xE = 8.4% and xE = 23.5%, similar values were registered, but a tendency to accelerate the combustion process in the presence of hydrogen was observed for all energetic substitution ratios, with the peak of HRR occurring earlier in the cycle due to the higher value of the LHV of hydrogen, large inflammability limits, and a higher flame speed comparative to diesel fuel.
- -
- The angle at which the maximum value of the HRR occurs was reduced by 1 CAD at xE = 8.4%, by 5 CAD at xE = 23.5%, and by 9 CAD at xE = 41.3%. The reduction in the angle of maximum HRR correlates with the reduction in the angle at which the maximum pressure occurs.
- -
- The heat release law becomes more aggressive when the hydrogen cyclic dose is at its maximum, at xE = 41.3%, and the rapid phase of the combustion process increases its share at this value of xE = 41.3%.
- -
- The total duration of combustion is reduced when using hydrogen due to H2’s higher flame speed and wider inflammability limits. The combustion duration decreases by 2 CAD at xE = 8.4%, by 3 CAD at xE = 23.5%, and by 8 CAD for xE = 41.3% versus classic fuelling, xE = 0%. The combustion process reduces its duration by 5.7% at xE = 8.4%, by 8.5% at xE = 23.5%, and by 22.8% for xE = 41.3% compared to diesel fuelling. Reducing the duration of combustion leads to an increase in maximum pressure and maximum pressure rise rate when using H2; when using hydrogen, the reduction in the angle at which the maximum pressure appears per cycle indicates the approach of the rapid combustion phase to the TDC, which leads to an increase in the share of isochoric combustion, which itself leads to an increase in thermal efficiency. This increase correlates with the tendency to reduce the duration of combustion.
- -
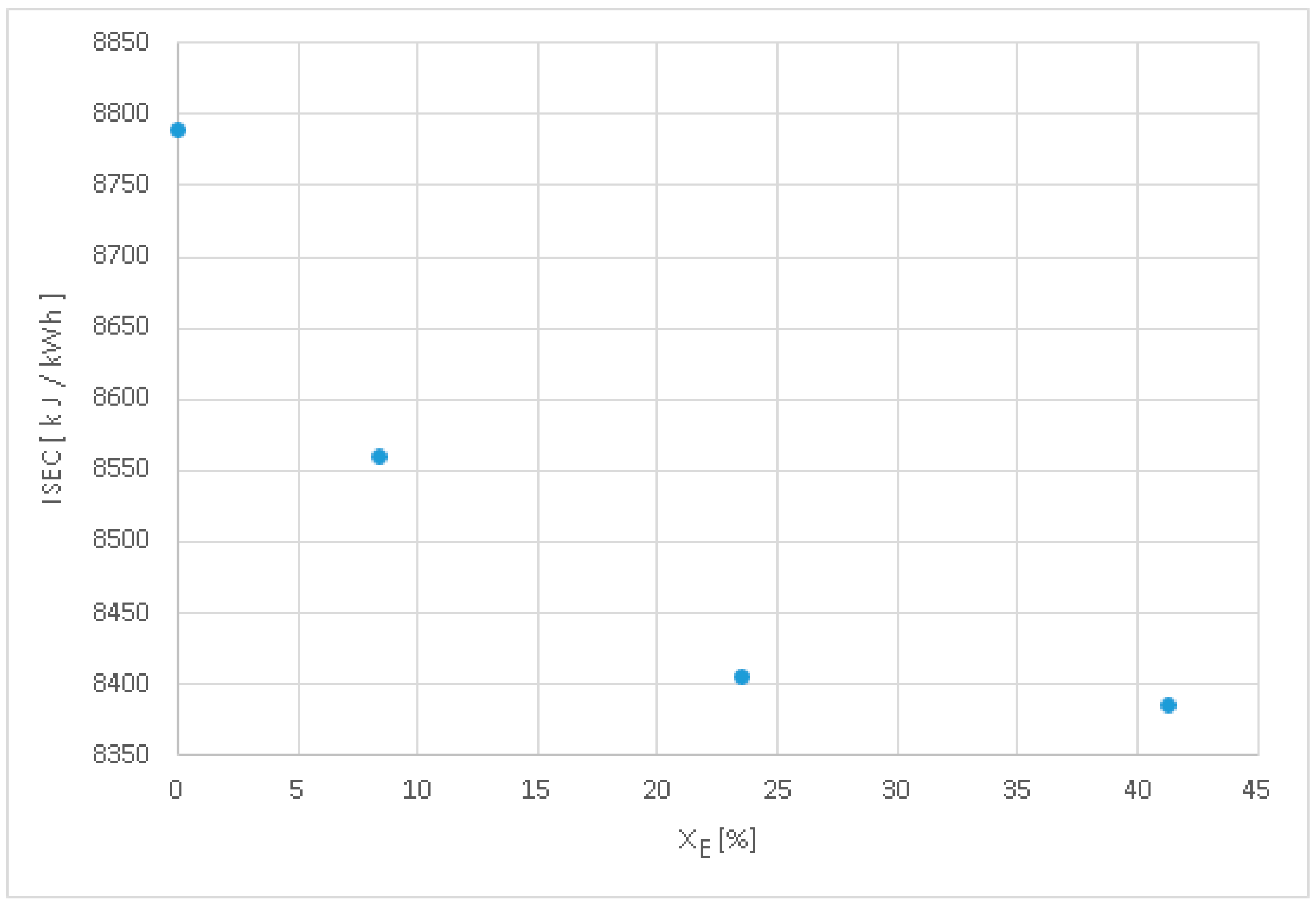
- The indicated specific energy consumption decreases by 2.58% to xE = 8.4%, by 4.35% at xE = 23.5%, and by 4.58% at xE = 41.3% compared to classic fuelling, xE = 0%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVL | Anstalt für Verbrennungskraftmaschinen List automotive research institute Graz Austria |
| BTE | Brake Thermal efficiencyEfficiency (or ηe) |
| BTDC | Before Top Dead Centre |
| BSFC | Brake-Specific Fuel Consumption |
| BSEC | Brake-Specific Energetic Consumption |
| CFR | Cooperative Fuel Research |
| CAD | Crank Angle Degree (or °CA) |
| Ch | Hourly Fuel Consumption |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| DI | Direct Injection |
| DME | Dimethyl Ether |
| EU | European Union |
| ECU | Engine Control Unit |
| EGR | Exhaust Gas Recirculation |
| G | Gasoline |
| H2 | Hydrogen |
| HC | Unburned Hydrocarbon |
| Hi | Lower Heating Value |
| HRR | Heat Release Rate |
| ICE | Internal Combustion Engine |
| IMEP | Indicate Mean Effective Pressure |
| J | Joule |
| K | Kelvin |
| L | Liter |
| LHV | Lower Heating Value |
| LPG | Liquid Petroleum Gas |
| lpm | Liter Per Minute |
| MTB | Maximum Torque Brake |
| m | Kinetic Parameter |
| m/s | Meter/Second |
| NOx | Nitrogen Oxides (Or NO) |
| PFI | Port Fuel Injection |
| pmax | Maximum Pressure |
| p | In-Cylinder Pressure |
| rpm | Revolutions Per Minute (Or Rev/Min) |
| SIE | Spark Ignition Engine (Or SI Engine) |
| SCR | Selective Catalytic Reduction |
| slpm | Standard Litre Per Minute |
| TDC | Top Dead Centre |
| T | In-Cylinder Temperature |
| λ | Coefficient Of Excess Air |
| xE | Energetic Substitution Ratio |
References
- Liu, X.; Seberry, G.; Kook, S.; Chan, Q.N.; Hawkes, E.R. Direct Injection of Hydrogen Main Fuel and Diesel Pilot Fuel in a Retrofitted Single-Cylinder Compression Ignition Engine. Int. J. Hydrogen Energy 2022, 47, 35864–35876. [Google Scholar] [CrossRef]
- Wright, M.; Lewis, A.C. Decarbonization of Heavy-Duty Diesel Engines Using Hydrogen Fuel: A Review of the Potential Impact on NOx Emissions. Environ. Sci. Atmos. 2022, 2, 852–866. [Google Scholar] [CrossRef]
- Akhtar, M.U.S.; Asfand, F.; Imran Khan, M.; Mishra, R.; Ball, A.D. Performance and Emission Characteristics of Hydrogen–Diesel Dual-Fuel Combustion for Heavy-Duty Engines. Int. J. Hydrogen Energy 2025, 143, 454–467. [Google Scholar] [CrossRef]
- Mukhtar, G.A.; Tange, K.; Nakatani, S.; Horibe, N.; Kawanabe, H.; Morita, G.; Hiraoka, K.; Koda, K. Performance and Emissions of a Hydrogen Dual-Fuel Engine Using Diesel and HVO as Pilot Fuels; SAE Technical Paper 2024-01-4286; SAE: Warrendale, PA, USA, 2024. [Google Scholar]
- Zhang, B.; Wang, H.; Wang, S. Computational Investigation of Combustion, Performance, and Emissions of a Diesel-Hydrogen Dual-Fuel Engine. Sustainability 2023, 15, 3610. [Google Scholar] [CrossRef]
- Alzohbi, G. An Overview of Hydrogen Energy Generation. ChemEngineering 2004, 8, 17. [Google Scholar] [CrossRef]
- Badea, N.I. Hydrogen as Energy Sources-Basic Concepts. Energies 2021, 14, 5783. [Google Scholar] [CrossRef]
- Han, W.; Dai, P.; Gou, X.; Chen, Z. A Review of Laminar Flame Speed of Hydrogen and Syngas Measured from Propagating Spherical Flames. Appl. Energy Combust. Sci. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Levikhin, A.A.; Boryaev, A.A. Physical Properties and Thermodynamic Characteristics of Hydrogen. Heliyon 2024, 10, e36414. [Google Scholar] [CrossRef] [PubMed]
- Mekonnin, A.S.; Waclawiak, K.; Humayum, M.; Zhang, S.; Ullah, H. Hydrogen Storage Technology, and Its Challenges: A Review. Catalysts 2025, 15, 260. [Google Scholar] [CrossRef]
- Mohamed, M.; Longo, K.; Zhao, H.; Hall, J.; Hamington, A. Hydrogen Engine Insights: A Comprehensive Experimental Examination of Port Fuel Injection and Direct Injection; SAE Technical Papers 2024-01-2611; SAE: Warrendale, PA, USA, 2024; pp. 1–16. [Google Scholar]
- Barik, D.; Bora, B.J.; Shanma, P.; Medhi, B.J.; Balasubramanian, D.; Krupakaran, R.L.; Ramegowda, R.; Kavalli, K.; Femilda Josephin, J.S.; Vikneswaran, M.; et al. Exploration of the Dual Fuel Combustion Mode on a Direct Injection Diesel Engine Powered with Hydrogen as gaseous Fuel in Port Injection and Diesel–Diethyl Ether Blend as Liquid Fuel. Int. J. Hydrogen Energy 2024, 52 Pt B, 827–840. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Wang, X.; Zhao, H. Hydrogen Crankcase Slip Phenomena: Experimental Study of Forced Ventilation Effects on Hydrogen Engine Performance and Emissions. SAE Int. J. Engine 2025, 18, 611–628. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, C.B.; Lata, D.B. Effect of hydrogen enrichment on exhaust gas temperature and emission of a dual fuel diesel engine. Mater. Today Proc. 2023, 72, 631–635. [Google Scholar] [CrossRef]
- Armbrustr, F.; Zepf, A.; Prager, M.; Hartl, M.; Jaensch, M. Fuel slip in hydrogen-powered port-fuel-injected large- bored stationary engines under stable operation and in the presence of combustion anomalies. Int. J. Hydrogen Energy 2025, 173, 151195. [Google Scholar] [CrossRef]
- Mohamed, M. Unveiling the Potential of Hydrogen in a Downsized Gasoline Direct Injection Engine Performance and Emissions Experimental Study. SAE Int. J. Fuel Lubr. 2024, 17, 225–242. [Google Scholar] [CrossRef]
- Zambelli, C. Exhaust System for Hydrogen Fuelled Combustion Engines: Effect of High-Water Content on the SCR Vanadium Catalyst. Master’s Thesis, KTH Royal Institute of Technology, Stocholm, Sweden, 2023; pp. 1–55. [Google Scholar]
- Mohamed, M.; Wang, X.; Zhao, H.; Peckham, M.; Hall, J.; Jiag, C. A Comprehensive Experimental Investigation of NOx Emission Characteristics in Hydrogen Engine Using an Ultra-Fast Crank Domain Measurement. Energies 2024, 17, 4141. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill, Inc.: New York, NY, USA, 1988; ISBN 0-07-028637-X. [Google Scholar]
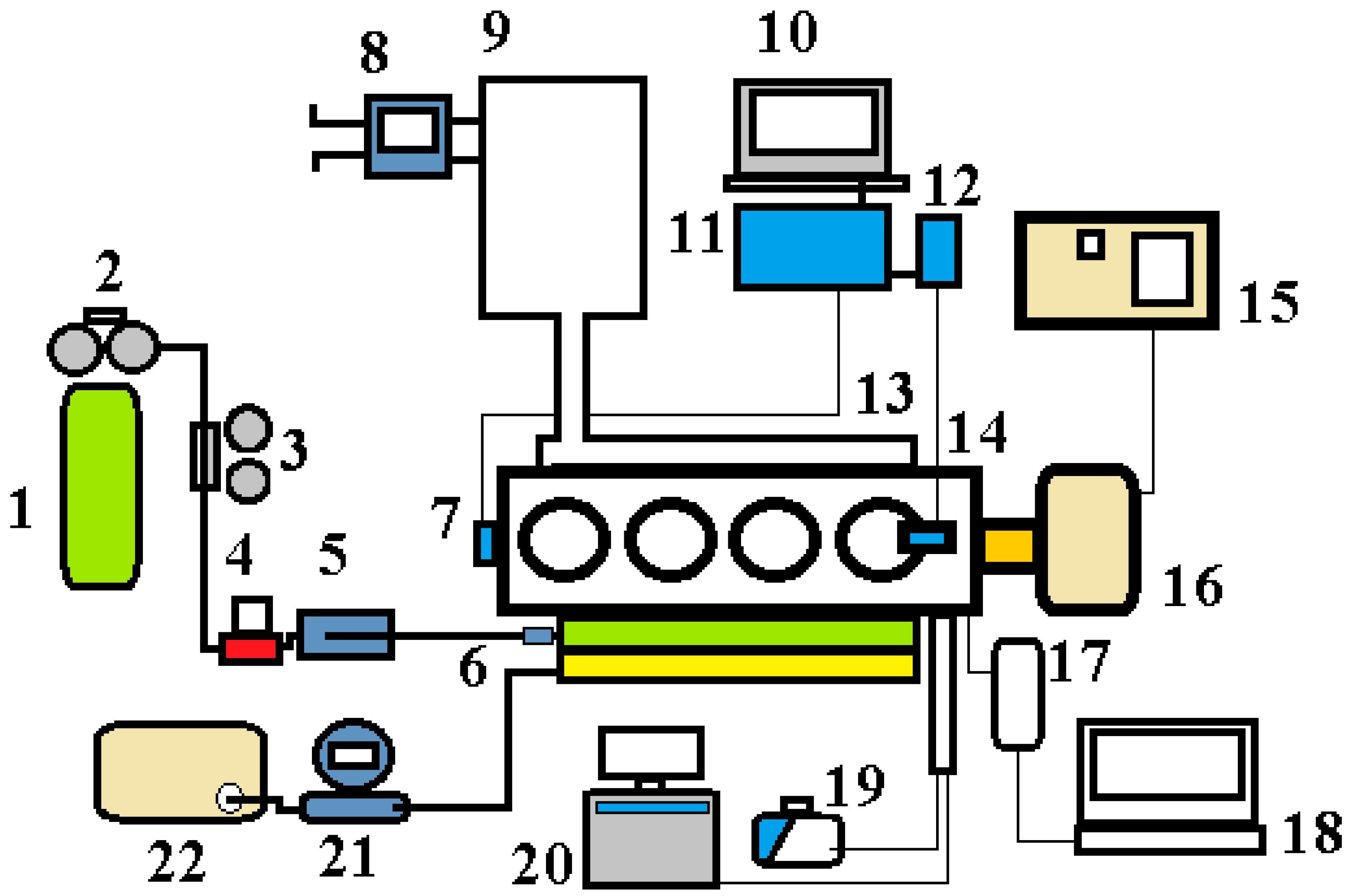







| Test Bed System | Operational Parameter | Errors |
|---|---|---|
| Power system | ||
| Horiba E90 | Torque [Nm] | ±0.2% |
| Speed [rpm] | ±1 rpm | |
| Data acquisition system | ||
| AVL GU 12P | Cylinder pressure [bar] | ±0.05% |
| AVL 365 CC | Crankshaft angular position [CAD] | ±0.1% |
| Fluid flowrates | ||
| Alicat Scientific MC50 | H2 flowrate [slpm] | ±0.2% |
| Krohne Optimass 3300 | Diesel fuel flowrate [kg/h] | ±0.1% |
| Krohne H 250 | Inlet air flow [m3/h] | ±0.3% |
| Exhaust Gases | ||
| AVL MDS 450 | Exhaust gas emission | ±0.65%vol for CO2 |
| ±0.10 ppm vol for HC | ||
| ±50 ppm vol for NO | ||
| ±0.1% for opacity | ||
| 0.001 for lambda | ||
| Temperatures of fluids | ||
| Thermocouple Cromel–Alumel TTC | Inlet air/cooling water | ±1 °C degree |
| Thermoresistance PT100 TTR | Exhaust gases | ±2 °C degree |
| Shimaden SR93 | Digital display | ±0.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panait, A.; Pana, C.; Cernat, A.; Negurescu, N.; Nutu, C.; Fuiorescu, D.; Nemoianu, L. The Use of Hydrogen in the Automotive Diesel Engine—An Efficient Solution to Control Its Operation with Reduced Carbon Emissions. Sustainability 2025, 17, 10369. https://doi.org/10.3390/su172210369
Panait A, Pana C, Cernat A, Negurescu N, Nutu C, Fuiorescu D, Nemoianu L. The Use of Hydrogen in the Automotive Diesel Engine—An Efficient Solution to Control Its Operation with Reduced Carbon Emissions. Sustainability. 2025; 17(22):10369. https://doi.org/10.3390/su172210369
Chicago/Turabian StylePanait, Andreea, Constantin Pana, Alexandru Cernat, Niculae Negurescu, Cristian Nutu, Dinu Fuiorescu, and Liviu Nemoianu. 2025. "The Use of Hydrogen in the Automotive Diesel Engine—An Efficient Solution to Control Its Operation with Reduced Carbon Emissions" Sustainability 17, no. 22: 10369. https://doi.org/10.3390/su172210369
APA StylePanait, A., Pana, C., Cernat, A., Negurescu, N., Nutu, C., Fuiorescu, D., & Nemoianu, L. (2025). The Use of Hydrogen in the Automotive Diesel Engine—An Efficient Solution to Control Its Operation with Reduced Carbon Emissions. Sustainability, 17(22), 10369. https://doi.org/10.3390/su172210369