Abstract
Plant-parasitic nematodes (PPNs) pose a serious threat to global agriculture by reducing both yield and quality in high-value crops. Although chemical nematicides provide rapid control, their application is increasingly restricted due to environmental pollution and toxicity to non-target organisms. These limitations have increased the search for sustainable and environmentally friendly alternatives. Plant-derived essential oils (EOs) have emerged as promising nematicides due to their sustainable nature and bioactivity. EOs of plant families such as Lamiaceae, Amaryllidaceae, Lauraceae, Apiaceae, and Zingiberaceae have been reported to exhibit nematicidal activity. Their major constituents include linalool, thymol, carvacrol, diallyl disulfide, cinnamaldehyde, γ-terpinene, cumin aldehydes, eucalyptol, and spathulenol. EOs suppress nematode populations through mechanisms including inhibition of egg development, increased larval mortality, and reduction in root gall formation. However, field efficacy can be limited by chemical composition variability, volatility, and phytotoxicity. Advanced formulation techniques, such as micro and nano-encapsulation, can improve EO stability, controlled release, and consistent efficacy. Future research should focus on clarifying synergistic and antagonistic interactions among EO constituents, optimizing field applications, and integrating EO-based products with other sustainable strategies. In addition, studies should prioritize standardizing extraction methods, conducting chemical profiling, and verifying their efficacy and safety through repeated field trials in various agricultural systems. In conclusion, plant-derived EOs represent promise as a sustainable method of managing nematodes and contribute to sustainable agriculture.
1. Introduction
Nematodes are unsegmented invertebrates that constitute the most abundant animal group on Earth, accounting for around 80% of all terrestrial animals [1,2]. Many soil-dwelling nematodes play important ecological roles, such as regulating the carbon cycle and enhancing the availability of nutrients to plants by recycling them, particularly nitrogen [3,4]. However, some nematode species are plant parasites that pose a serious threat to agriculture.
Plant-parasitic nematodes (PPNs) are multicellular obligate parasites and are the second most harmful group of plant pathogens after fungi [5]. Globally, PPNs cause over 100 billion US dollars in annual crop losses, negatively impacting food production and security [6]. The major PPN genera include root-knot nematodes (RKNs; Meloidogyne spp.), cyst nematodes (Heterodera and Globodera spp.), lesion nematodes (Pratylenchus spp.), and dagger nematodes (Xiphinema spp.). These nematodes cause an average yield loss of 12.6% in 20 economically important crop plants [7,8].
Among PPNs, the genus Meloidogyne is particularly destructive and poses a critical threat to global food security [9]. Species such as M. javanica, M. incognita, M. hapla, and M. arenaria can cause significant yield losses and reduce plants’ resistance to secondary infections. By attacking the root system, they block water and nutrient uptake, thereby halting plant growth and reducing crop yields [10].
During the 20th century, synthetic nematicides were the conventional method of nematode management in intensive production. However, due to growing concerns about environmental safety and human health, the use of several commercial nematicides has been restricted and their availability has decreased [11,12]. Consequently, environmentally friendly alternatives have begun to gain ground due to their biodegradable and low-toxic nature [13,14].
Of the existing options, volatile organic compounds (VOCs) produced by plants and microorganisms (e.g., alcohols, aldehydes, ketones, esters, phenols, and terpenoids) show promise in controlling nematodes [15,16]. Essential oils (EOs) are complex blends of volatile and aromatic molecules with a high content of terpenes and terpenoids. They are extracted from plants and belong to the category of secondary metabolites involved in plant defense mechanisms. These biochemical properties impart unique multiple biological activities to EOs, such as nematicidal activity [17].
Phytochemicals extracted from EOs act through various mechanisms, including targeting the nematode nervous system, disrupting cell membranes, penetrating protective egg structures, and disrupting cellular oxidative equilibrium [18,19]. This variety of mechanisms reduces the risk of resistance formation and makes EOs green nematode management choices.
To this end, this review aims to highlight the potential EOs have as an eco-friendly, effective and sustainable control measure for PPNs. It provides a comprehensive overview of their composition, biological activity and stability, nematicidal modes of action, action on different species of PPN, formulations and application strategies, as well as the limitations and challenges involved.
2. Essential Oils: Composition, Biological Activity, and Stability
EOs are mixtures of volatile substances composed of plant secondary metabolites and characterized by typical aromatic properties [20]. They are produced in various plant parts such as leaves, stems, flowers, fruits, buds, seeds, roots, or bark. They are stored in plant tissue in intercellular spaces, channels, secretory cells, epidermal cells, or secretory hairs [21]. EOs are typically localized in secretory parenchyma or secretory epithelium and structures such as glandular hairs (pisifera hairs) and secretory idioblasts. Though their ecological functions are unknown, it is considered that they are involved in protecting the plants against phytopathogens and parasites [22].
EOs production is widespread across many plant families, particularly Annonaceae, Apiaceae, Araceae, Asteraceae, Ericaceae, Lamiaceae, Lauraceae, Myrtaceae, and Rutaceae [23]. EOs can be obtained using traditional methods such as hydrodistillation, extraction using organic solvents, and cold pressing. In addition, new technologies such as microwave extraction and supercritical CO2 extraction are becoming increasingly popularity [24,25].
Chemically, EOs primarily comprise terpenes and terpenoids [26,27,28]. The nematicidal activity of EOs is determined by the structural characteristics of these bioactive compounds. Terpenes are hydrocarbons formed from the condensation of isoprene units (C5), while terpenoids are oxygenated derivatives of terpenes. The main classes of terpenes include monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) [27,28].
The bioactivity of EOs depends on the ratio and structure of their primary and secondary components [29]. These structures are influenced by intrinsic factors such as the plant’s genetic background (species, ecotype, chemotype), geographical origin, utilized organ, developmental stage, and harvesting time [30,31,32,33,34,35]. For instance, Thymus vulgaris at two years of age contained 51.15% thymol, whereas a five-year-old plant from the same cultivation cycle yielded only 19.38% [36]. Similar compositional shifts occur in Eucalyptus camaldulensis and Tagetes minuta, where monoterpene levels vary with growth phases [37,38]. The specific plant organ used also determines the oil profile; Salvia officinalis flowers are dominated by β-pinene, while the foliage primarily accumulates α- and β-thujones [39]. In Lantana camara, leaf-derived oils rich in bioactive constituents have been reported to exhibited stronger antifungal properties than those extracted from flowers [40]. Furthermore, intraspecific genetic differences can produce distinct chemotypes, as observed in T. vulgaris, where thymol-, carvacrol-, or geraniol-based types arise depending on genotype [41]. In addition, environmental conditions, soil type, fertilization, irrigation, cultivation and harvesting practices, and post-harvest treatments also affect the chemical composition of EOs [35,42,43]. For instance, increased light intensity enhances the biosynthesis of phenylpropanoids and monoterpenes in Ocimum basilicum and Satureja douglasii [44,45]. Similarly, mild water deficit stimulates EO production in Origanum vulgare and Satureja hortensis, whereas severe drought lowers oil content in Artemisia annua [46,47,48]. Soil chemistry also affects composition; Thymus spinulosus cultivated in calcareous soils produced monoterpene-rich oils, while siliceous soils favored sesquiterpene accumulation [49]. Variations in phosphorus and calcium availability influence the activity of enzymes involved in terpenoid biosynthesis [50,51]. Seasonal shifts are equally important as oxygenated monoterpenes predominate during winter in L. camara and S. libanotica, correlating with increased antimicrobial potential [52,53].
These physiological and environmental variables collectively modulate enzymatic activity and gene expression within the mevalonate and methylerythritol phosphate pathways, resulting in shifts in terpene and phenylpropanoid composition. Moreover, oxidative and polymerization reactions under stress may alter the molecular architecture of EO constituents, thereby affecting their biological efficacy and storage stability [54]. Figure 1 shows a schematic illustration of the principal factors governing EOs stability.
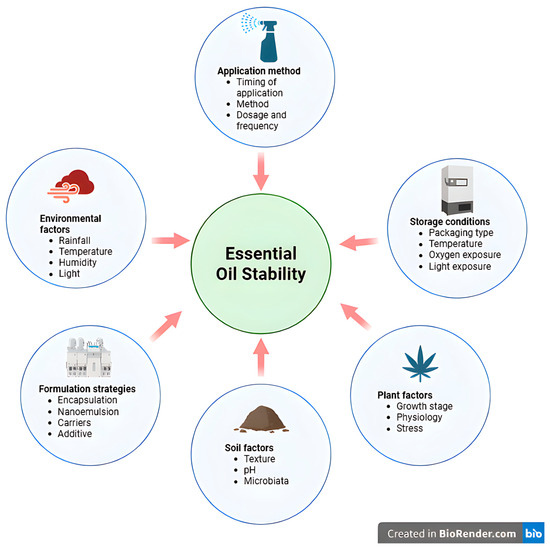
Figure 1.
Factors influencing essential oil stability and nematicidal efficacy.
EOs are easily degraded during storage and use due to their heat-sensitive and volatile constituents. The degradation reactions are through oxidation, isomerization, polymerization, and dehydrogenation [55]. Temperature, light, exposure to oxygen, and the presence of extraneous material significantly affect the stability of EOs [55]. In monoterpenes, in particular, light promotes oxidative reactions, leading to compositional changes [56,57].
Even at low temperatures, oxidation reactions may continue with the help of atmospheric oxygen to give rise to peroxides [58]. Thus, room temperature, absence of oxygen, and darkness are the best conditions for the storage of EOs [55,59]. The material of packaging, the contaminants, the moisture, and metal contamination are also the major factors affecting degradation [60].
In conclusion, storage and environmental conditions can affect the chemical constitution of EOs, which can reduce their biological activity, sensory properties and their nematicidal activity. Therefore, discovering adequate storage conditions and exhaustively establishing degradation processes are highly significant.
3. Nematicidal Mechanisms of Essential Oils
The nematicidal activity of EOs is determined by the structural characteristics of the bioactive chemical compounds they contain, particularly terpenes [27]. The lipophilic nature of terpenes disrupts the permeability of nematode plasma membranes, leading to dysfunctional cells. This membrane damage leads to leakage of intracellular macromolecules and disruption of cellular homeostasis, leading to cell death [18,61]. Certain monoterpenes also disrupt the polysaccharide, fatty acid, and phospholipid composition, leading to mitochondrial membrane depolarization and interference with energy production [21,62]. Such functions adversely interfere with the metabolic processes of the nematode.
Furthermore, certain monoterpene components of EOs destabilize nematode intracellular redox homeostasis, activating programmed cell death (apoptosis) mechanisms and causing DNA damage [18]. In addition to terpenes, organosulfur compounds also exhibit potent nematicidal activity. In particular, allyl isothiocyanate (AITC), diallyl disulfide (DADS), and diallyl trisulfide (DATS) inhibit nematode mobility and feeding by disrupting their nervous transmission and chemical perception systems [63,64]. Other sulfur-based metabolites, such as asparagusic acid, naturally occurring in Asparagus officinalis roots, act as preformed defense compounds (phytoanticipins) that suppress nematode activity by inhibiting egg hatching and juvenile motility [65]. This organosulfur compound is synthesized via pathways involving isobutyric and methacrylic acids, with cysteine serving as the sulfur donor [66,67] and contributes to nematode mortality by inducing oxidative stress and enzymatic dysfunction.
Phenylpropanoid members such as (E)-cinnamaldehyde, benzaldehyde, eugenol, and eugenol methyl ester are nematode-active via inhibition of the vacuolar-type proton-transporter ATPase (V-ATPase) enzyme. V-ATPase enables nematodes to maintain vital processes such as osmoregulation, nutrition, cuticle formation, and reproduction through the transport of protons by ATP hydrolysis. V-ATPase inhibition leads to the leading to nematode death [68]. Moreover, phenylpropanoid derivatives synthesized through the phenylpropanoid pathway (PPP), starting from L-phenylalanine via PAL, C4H, and 4CL enzymes, play an important role in nematode resistance by reinforcing plant cell walls and producing hydroxycinnamic acids, such as caffeic and chlorogenic acids, which are associated with reduced nematode infection [67,69,70,71].
The phenylpropanoid biosynthetic pathways are triggered by enzymes that are induced under conditions of stress such as injury or pathogen invasion in the case of plants and are known to induce nematode resistance [72]. In particular, volatile oils that consist of phenylpropanoid aldehydes work against these enzymes, hence inhibiting the survival of nematodes [68].
Also, phenolic, aldehyde, and alcoholic compounds cause oxidative damage to the nematode cytoplasmic membranes, leading to increased membrane permeability, disruption of cell processes, and subsequent death [18,21,68].
Overall, the potency of EOs against nematodes depends on the chemical constituent diversity, concentration, and molecular form. The combined effect of the constituents causes damage through a wide range of activities, from nematode cell membranes to metabolic and neural processes [18,61]. All these EO-induced nematicidal mechanisms are summarized in Figure 2.
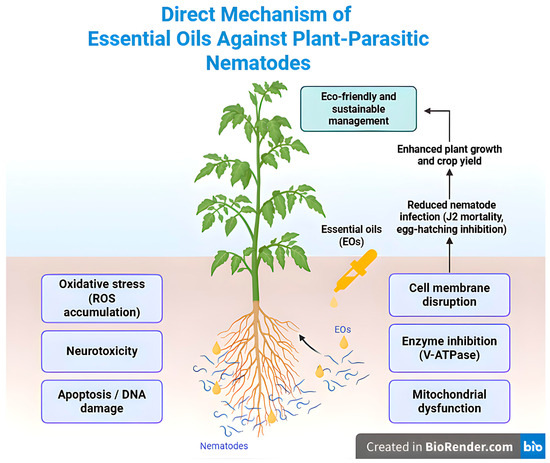
Figure 2.
Direct mechanism of essential oils against plant-parasitic nematodes: membrane disruption, V-ATPase inhibition, and ROS/apoptosis.
Table 1 summarizes the reported mechanisms of action of EOs components against PPNs. Various studies indicate that common mechanisms include inhibition of egg hatching, induction of juvenile (J2) death, suppression of bile formation, and reduction of egg mass production. Some EO components, such as thymol, carvacrol, linalool, cinnamaldehyde, and organosulfur compounds, are frequently reported to exert multiple effects simultaneously, impacting motility and reproduction.

Table 1.
Mode of action of essential oils components against plant-parasitic nematodes.
4. Effects of Essential Oils on Different Plant-Parasitic Nematode Species
4.1. Root-Knot Nematodes (Meloidogyne spp.)
RKNs of the genus Meloidogyne are the most frequently studied PPNs in EOs research. Numerous EOs from different plant families have demonstrated potent activity against M. incognita, M. javanica, and M. hapla under both in vitro and in vivo conditions.
Among Lamiaceae members, oils rich in monoterpenes such as thymol, carvacrol, and linalool consistently exhibited strong nematicidal effects. The linalool-dominant oil of Lavandula intermedia markedly decreased juvenile populations and root galling, while Monarda didyma and M. fistulosa EOs containing γ-terpinene and carvacrol induced rapid juvenile mortality and suppressed egg hatching [82,83]. Similarly, Mentha longifolia and M. piperita chemotypes showed high toxicity, and Thymus citriodorus, T. linearis, and T. vulgaris oils, particularly thymol-rich nanoemulsion formulations, resulted in complete mortality of M. javanica and a significant reduction in reproduction rates [73,74,75,76,93]. Species such as Nepeta cataria also demonstrated considerable nematicidal potential, while other plant species, including Warionia saharae (Asteraceae), Calendula officinalis (Asteraceae), and Cedrus atlantica (Pinaceae), achieved high juvenile mortality in Meloidogyne javanica under controlled conditions [94,95,96,97].
EOs from non-Lamiaceae families have shown comparable or even greater efficacy. Cinnamomum species (Lauraceae) containing cinnamaldehyde strongly inhibited egg hatching and gall formation in both M. incognita and M. javanica [80,98,99]. Organosulfur-rich oils from Allium sativum (Amaryllidaceae) exhibited exceptional potency, achieving complete inhibition of egg hatching and substantial reductions in nematode reproduction [64]. In addition, oils from Cuminum cyminum and Daucus carota (Apiaceae) were effective in inducing paralysis and suppressing hatching, while Schinus terebinthifolius (Anacardiaceae) and Piptadenia viridiflora (Fabaceae) reduced galling and egg production in tomato roots [92,100,101,102]. Tephrosia toxicaria EO, characterized by sesquiterpenes such as β-caryophyllene and germacrene D, further demonstrated activity against M. enterolobii and M. javanica [86]. Moreover, Hedychium coccineum (Zingiberaceae) and Brassica nigra (Brassicaceae) showed strong dose-dependent lethality, and Zanthoxylum alatum (Rutaceae) nanoemulsions induced significant juvenile mortality [63,89,91].
4.2. Cyst and Lesion Nematodes (Globodera spp. and Pratylenchus spp.)
EOs have also shown promising nematicidal potential against cyst-forming and migratory endoparasitic nematodes. Oils from A. sativum and Cinnamomum cassia significantly inhibited egg hatching and reproduction of Globodera rostochiensis, largely due to the synergistic effects of DADS, DATS, and cinnamaldehyde [80,103]. These volatile compounds are known to impair nematode respiration and induce cytoplasmic leakage through disruption of lipid bilayers. In the case of lesion nematodes, Lavandula × intermedia EO, rich in linalool and linalool acetate, caused 75.7% mortality of Pratylenchus vulnus J2s within hours of exposure [82]. Cinnamomum burmanni EO, also rich in cinnamaldehyde, exhibited moderate but consistent toxicity to P. penetrans [99]. Similarly, Myristica fragrans oil containing sabinene and α-pinene resulted in up to 75.3% mortality of P. thornei, demonstrating its strong potential in suppressing lesion nematodes [90].
4.3. Other Nematode Genera
Beyond Meloidogyne, Globodera, and Pratylenchus, several EOs have exhibited strong nematicidal activity against other economically important genera. Rosmarinus officinalis EO, containing 1,8-cineole, α-pinene, and camphor, completely killed Tylenchulus semipenetrans J2s at 15 µL mL−1 within 72 h and inhibited egg hatching [104]. Origanum vulgare and Pimpinella anisum EOs, rich in carvacrol, thymol, and trans-anethole, demonstrated potent nematicidal effects against Nacobbus aberrans, with up to 100% mortality in contact assays [105]. In addition, A. sativum and Cinnamomum burmanni oils produced significant mortality in Bursaphelenchus xylophilus [99,106], while Mentha longifolia EO significantly reduced Hoplolaimus spp. populations after 72 h of exposure [95].
In summary, EOs that are rich in monoterpenes, such as thymol, carvacrol and linalool, as well as oils that contain organosulfur and cinnamaldehyde, have been shown to consistently exhibit high nematicidal activity against various species of PPNs. Laboratory studies generally report higher mortality rates and stronger egg-hatching inhibition than greenhouse or field trials do, which highlights the need for further in vivo validation (Table 2).

Table 2.
Application of essential oils to combat various plant-parasitic nematodes.
5. Formulation and Delivery Strategies of Essential Oils
Promoting the use of EOs as “green pesticides” in agriculture requires increasing their effectiveness and persistence, especially in agroecological systems [107,108]. Limited stability and short-term effects are frequently reported in the field application of EO; Additionally, low yields and costly approval processes make EO use expensive [109]. To overcome these limitations, appropriate formulation and application strategies have been developed.
Product formulation creates a homogeneous and stable mixture of active and inactive components, thereby enhancing biological properties, product stability, and durability [110]. EOs can be toxic in their raw form, have low solubility, and are sensitive to environmental conditions; therefore, formulation techniques similar to those used in pesticides are applied [110,111]. The coating materials used are generally biologically sourced and biodegradable [112]. The choice of formulation depends on the intended use, application method, target pathogen, and environmental factors [107,113].
Emulsion techniques are widely used to enhance the stability and biological efficacy of EO. Emulsions are formed by stabilizing two immiscible phases in liquid form using surfactants [114]. Macroemulsions have large particle sizes and may destabilize over time, while nanoemulsions are less affected by gravity and aggregation forces due to the lower droplet size; they also enhance cell uptake, thereby potentiating the biological effect of EO [115,116]. Microemulsions are thermodynamically stable and share the same advantages [113,117]. Nanoemulsions require less surfactant to formulate and are also economically favorable [118]. A number of investigations indicated that the development of EOs as nanoemulsions increases the biological activity and stability of EOs [119,120,121].
Encapsulation is an important method that enhances the controlled release and stability of EOs [122,123,124]. Encapsulation refers to the coating of EO particles or the creation of a functional barrier between the core and wall material in order to preserve the biological, functional, and physicochemical properties of EO [125]. Spray drying and coacervation are the most common methods. Spray drying atomizes EO emulsions through high-temperature treatment, resulting in rapid evaporation of water and encapsulation of EO within the capsule [124,125]. Coacervation creates phase separation of biopolymers due to electrostatic attraction to produce micro- or nano-capsules [126,127].
EOs can also be encapsulated in various carrier systems such as cyclodextrins, biopolymers, and solid lipid nanoparticles (SLNs) [122,123,124,125]. For example, when lavandin EO is encapsulated in a biodegradable polymer, it provides a narrow particle size and controlled release [128], while M. piperita EO achieves controlled release by forming host-guest complexes with cyclodextrin [129]. A. arborescens EO encapsulated in SLN exhibited higher stability compared to raw EO [130].
In summary, emulsion and encapsulation techniques are among the promising methods for enhancing the efficacy and maintaining the stability of EO in agricultural applications. These formulation strategies, which provide controlled release, prevent the reduction of EO’s biological effect in the field and support the potential use of biopesticides.
6. Challenges and Limitations
6.1. Commercial and Technical Limitations
The commercial availability of EO-based nematicides remains restricted. The preponderance of products currently available on the market are derived from synthetic derivatives of a limited number of compounds, including thymol, geraniol, and eugenol [29]. This situation has the effect of limiting product diversity and hindering the development of new formulations suitable for different agricultural conditions. Moreover, the inherent properties of EOs, namely their low mobility in irrigation water and high volatility, constitute significant impediments to their effective and sustained utilization in field conditions. For instance, Borges et al. [100] demonstrated that although the EO from green fruits of S. terebinthifolius effectively inhibited M. javanica egg hatching and increased J2 mortality in vitro, it failed to control the nematode under field conditions. Consequently, advanced formulation methods, such as micro or nano-encapsulation, are imperative for enhancing the stability of active ingredients and ensuring controlled release [131,132,133]. However, the high cost of encapsulation technologies and the challenges encountered in scaling up production limit the widespread use of these solutions. Therefore, for EO-based nematicides to achieve commercial success, it is critical to develop new formulation strategies that are both low-cost and suitable for large-scale production.
6.2. Biological and Experimental Challenges
The biological activity of EOs is generally dose-dependent. High doses can cause phytotoxicity and may also have toxic effects on beneficial microorganisms and nematodes [134,135]. This highlights the importance of carefully balancing efficacy and toxicity. Furthermore, inconsistencies between laboratory and field trials make it difficult to transfer laboratory data directly to field applications [101,136]. These discrepancies suggest that environmental factors, nematode population fluctuations, and soil properties significantly impact the efficacy of EOs under field conditions.
6.3. Chemical Interactions and Standardization Issues
The nematicidal activity of EOs is not solely determined by a single major compound but is instead shaped by the complex mixture of constituents. Synergistic and antagonistic interactions among these compounds can significantly influence both the intensity and spectrum of the biological activity. Therefore, future research must focus on unraveling these interactions in a systematic manner [79,137]. In parallel, standardized extraction and formulation techniques are required to achieve reproducible and reliable products. Such standardization would ensure compositional uniformity, strengthen user confidence, and facilitate the consistent performance of EO-based nematicides in agricultural applications.
6.4. Regulatory and Policy Barriers
The volatility and high cost are the major factors limiting field application. Therefore, investment in new cost-efficient formulations as well as appropriate application technologies is necessary [12]. Standardization of plant growth conditions and nematicide extraction processes is also essential to facilitate commercial production with uniform composition and consistent nematicide activity. Volatile oil-based nematicide applications still face significant technical and regulatory barriers [17]. Therefore, it is crucial to develop flexible and adaptable regulatory mechanisms that consider the inherent properties of EOs [138]. Otherwise, even if technical and formulation advances are made, the use of EO-based nematicides on a large scale in agriculture will remain limited.
7. Conclusions and Future Perspectives
This review shows that plant-derived EOs have strong potential as sustainable and environmentally friendly tools for managing PPNs. Compared with synthetic nematicides, which act quickly but carry serious environmental and toxicological risks, EOs provide a safer and more compatible option for sustainable agriculture. Their biodegradability, multiple biological activities, and natural origin make them attractive candidates for future crop protection strategies, yet several obstacles continue to limit their practical use.
Variability in oil composition and fluctuations in nematode population dynamics reduce field consistency. Moreover, environmental factors further complicate EO performance. At the same time, volatility and phytotoxicity restrict direct application, while the complexity and expense of registration procedures create barriers, particularly for small and medium-sized producers. These factors explain why EO-based nematicides, despite promising laboratory results, have not yet achieved widespread field adoption.
Future research should focus on expanding field trials under different agroecological conditions, improving formulation technologies such as micro- and nano-encapsulation to stabilize active compounds and control their release, and examining the interactions among EO components to design mixtures that maximize efficacy while minimizing non-target effects. In parallel, regulatory systems need to adapt more closely to the biological nature of EOs, making approval processes faster and less costly and enabling broader participation in development and commercialization.
EOs should also be considered as part of a wider set of sustainable practices. When combined with approaches such as biofumigation, solarization, and crop rotation, EO-based products can contribute not only to nematode suppression but also to healthier soils and more resilient farming systems. If supported by innovation, regulation, and farmer adoption, EOs could move beyond experimental use and become reliable, environmentally responsible solutions for nematode management within sustainable agriculture.
Author Contributions
Conceptualization, A.D. and F.U.; methodology, A.D. and F.U.; investigation, A.D., F.U. and E.Y.; data curation, A.D., F.U. and E.Y.; visualization, A.D., F.U. and E.Y.; writing—original draft preparation, A.D., F.U. and E.Y.; writing—review and editing, M.S., M.A. and M.İ. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study did not involve the generation or analysis of new data; therefore, data sharing does not apply.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EOs | Essential oils |
| PPNs | Plant-parasitic nematodes |
| RKNs | Root-knot nematodes |
| J2 | Second-stage juvenile |
| LC50 | Lethal concentration 50 |
| AITC | Allyl isothiocyanate |
| DADS | Diallyl disulfide |
| DATS | Diallyl trisulfide |
References
- Lorenzen, S. The Phylogenetic Systematics of Freeliving Nematodes; Platt, H.M., Ed.; Ray Society: London, UK, 1994. [Google Scholar]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.; Scow, K. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralisation. Appl. Soil Ecol. 2004, 25, 19–35. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63–67. [Google Scholar] [PubMed]
- Quist, C.W.; Smant, G.; Helder, J. Evolution of plant parasitism in the phylum Nematoda. Annu. Rev. Phytopathol. 2015, 53, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, H.; Zhao, D.; Zhu, X.; Wang, Y.; Liu, X.; Liu, D.; Duan, Y.; Chen, L. Multifunctional efficacy of the nodule endophyte Pseudomonas fragi in stimulating tomato immune response against Meloidogyne incognita. Biol. Control 2021, 164, 104773. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Ning, J.; Zhou, J.; Wang, H.; Liu, Y.; Ahmad, F.; Feng, X.; Fu, Y.; Gu, X.; Zhao, L. Parallel evolution of C-type lectin domain gene family sizes in insect-vectored nematodes. Front. Plant Sci. 2022, 13, 856826. [Google Scholar] [CrossRef]
- Sorribas, F.J.; Djian-Caporalino, C.; Mateille, T. Nematodes. In Integrated Pest and Disease Management in Greenhouse Crops; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 147–174. [Google Scholar]
- Migunova, V.D.; Sasanelli, N. Bacteria as biocontrol tool against phytoparasitic nematodes. Plants 2021, 10, 389. [Google Scholar] [CrossRef]
- Oka, Y. From old-generation to next-generation nematicides. Agronomy 2020, 10, 1387. [Google Scholar] [CrossRef]
- Catani, L.; Manachini, B.; Grassi, E.; Guidi, L.; Semprucci, F. Essential oils as nematicides in plant protection—A review. Plants 2023, 12, 1418. [Google Scholar] [CrossRef]
- Lopes, E.A.; Dallemole-Giaretta, R.; dos Santos Neves, W.; Parreira, D.F.; Ferreira, P.A. Eco-friendly approaches to the management of plant-parasitic nematodes. In Plant Health Under Biotic Stress: Volume 1: Organic Strategies; Springer: Singapore, 2019; pp. 167–186. [Google Scholar]
- Pereira, G.; Barbosa, P.; Vicente, C.S.; Faria, J.M. Eco-Friendly Management of Root Lesion Nematodes Using Volatile Allelochemicals. Agronomy 2025, 15, 1605. [Google Scholar] [CrossRef]
- Campos, V.P.; Pinho, R.S.C.; Freire, E.S. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Cienc. Agrotec. 2010, 34, 525–535. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Lu, H.; Wang, X.; Zhang, K.Q.; Li, G.H. Effect of volatile organic compounds from bacteria on nematodes. Chem. Biodivers. 2015, 12, 1415–1421. [Google Scholar] [CrossRef]
- Sarri, K.; Mourouzidou, S.; Ntalli, N.; Monokrousos, N. Recent advances and developments in the nematicidal activity of essential oils and their components against root-knot nematodes. Agronomy 2024, 14, 213. [Google Scholar] [CrossRef]
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Thiruppathi, G.; Nivitha, S.; Sundararaj, P. Altitude-related changes in the phytochemical profile of essential oils extracted from Artemisia nilagirica and their nematicidal activity against Meloidogyne incognita. Ind. Crops Prod. 2019, 139, 111472. [Google Scholar] [CrossRef]
- Goyal, L.; Kaushal, S.; Dhillon, N.K.; Heena. Nematicidal potential of Citrus reticulata peel essential oil, isolated major compound and its derivatives against Meloidogyne incognita. Arch. Phytopathol. Plant Prot. 2021, 54, 449–467. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Lange, B.M. The evolution of plant secretory structures and emergence of terpenoid chemical diversity. Annu. Rev. Plant Biol. 2015, 66, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D. The Chemistry of Essential Oils Made Simple: God’s Love Manifest in Molecules; Care Publications: Marble Hill, MO, USA, 2005. [Google Scholar]
- Basile, A.; Jiménez-Carmona, M.M.; Clifford, A.A. Extraction of rosemary by superheated water. J. Agric. Food Chem. 1998, 46, 5205–5209. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, D.S. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography–mass spectrometry. J. Chromatogr. A 2002, 982, 31–47. [Google Scholar] [CrossRef]
- Sikkema, J.A.N.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Başer, K.H.C.; Demirci, F. Chemistry of essential oils. In Flavours and Fragrances; Springer: Berlin/Heidelberg, Germany, 2007; Volume 12. [Google Scholar]
- D’Addabbo, T.; Avato, P. Chemical composition and nematicidal properties of sixteen essential oils—A review. Plants 2021, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Mugao, L. Factors influencing yield, chemical composition and efficacy of essential oils. Int. J. Multidiscip. Res. Growth Eval. 2024, 5, 169–178. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar]
- Badi, H.N.; Yazdani, D.; Ali, S.M.; Nazari, F. Effects of spacing and harvesting time on herbage yield and quality/quantity of oil in thyme, Thymus vulgaris L. Ind. Crops Prod. 2004, 19, 231–236. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Vlachonasios, K.E. How biological and environmental factors affect the quality of lavender essential oils. Physiologia 2025, 5, 11. [Google Scholar] [CrossRef]
- Hosseinabadi, S.; Yavari, A.; Abdollahi, F. Essential oil variation in Melissa officinalis L. cultivated under industrial field conditions: Effects of different harvesting times and plant materials. J. Essent. Oil Bear. Plants 2024, 27, 285–299. [Google Scholar] [CrossRef]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications, 1st ed.; Baser, K.H.C., Buckbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 39–81. [Google Scholar]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variation during the vegetative cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Pagoula, F.; Baser, K.H.; Kurkcuoglu, M. Essential oil composition of Eucalyptus camaldulensis Dehnh from Mozambique. J. Essent. Oil Res. 2000, 12, 333–335. [Google Scholar] [CrossRef]
- Tiwari, A.; Goswami, P.; Bisht, B.S.; Chauhan, A.; Verma, R.S.; Padalia, R.C. Essential oil composition of African marigold (Tagetes minuta L.) harvested at different growth stages in foothill agroclimatic conditions of North India. Am. J. Essent. Oils Nat. Prod. 2016, 4, 4–7. [Google Scholar]
- Perry, N.B.; Andreson, R.E.; Brennan, N.J.; Douglas, M.H.; Heaney, A.J.; McGimpsey, J.A.; Smallfield, B.M. Essential oils from Dalmatian sage (Salvia officinalis L.): Variations among individuals, plant parts, season and sites. J. Agric. Food Chem. 1999, 47, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, S.; Rastogi, N.; Srivastava, M. Exploring the medicinal potential of Lantana camara: A comprehensive review of phytochemicals and therapeutic application. Phytochem. Rev. 2025, 1, 1–31. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Agostini, G.; Atti-Serfini, L.; Paroul, G.; Pauletti, G.F.; Atti dos Santos, A.C. Correlation between the chemical and genetic relationships among commercial thyme cultivars. J. Agric. Food Chem. 2001, 49, 4220–4223. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.; Rasool, S.; Shakeel-u-Rehman; Ganaie, M.; Qazi, P.H.; Shawl, A.S. Seasonal variation in chemical composition, antibacterial and antioxidant activities of the essential oil of leaves of Salvia officinalis (sage) from Kashmir, India. J. Essent. Oil Bear. Plants 2016, 19, 1129–1140. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Carrubba, A.; Lorigooini, Z. Organic manures enhance biomass and improve content, chemical compounds of essential oil and antioxidant capacity of medicinal plants: A review. Heliyon 2024, 10, e36693. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.; Bettio, L.E.; Oliveira, A.; Pazini, F.E.; Dalmarco, J.B.; Simionatto, E.L.; et al. Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 2013, 136, 999–1005. [Google Scholar] [CrossRef]
- Azizi, A.; Yan, F.; Honermeier, B. Herbage yield, essential oil content and composition of three oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crops Prod. 2009, 29, 554–561. [Google Scholar] [CrossRef]
- Baher, Z.F.; Mirza, M.; Ghorbanli, M.; Bagher Rezaii, M. The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortensis L. Flavour Fragr. J. 2002, 17, 275–277. [Google Scholar]
- Yadav, R.K.; Sangwan, R.S.; Sabir, F.; Srivastava, A.K.; Sangwan, N.S. Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol. Biochem. 2014, 74, 70–83. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V.; Bruno, M.; Tahiri, B.; Napolitano, F.; Senatore, F. Chemical composition and antibacterial activity of essential oils from Thymus spinulosus Ten. (Lamiaceae). J. Agric. Food Chem. 2003, 51, 3849–3853. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.; Vötsch, M.; Vierheilig, H.; Steinkellner, S.; Zitterl-Eglseer, K.; Franz, C.; Novak, J. Effects of soil composition on Salvia officinalis essential oil. J. Sci. Food Agric. 2009, 89, 1090–1098. [Google Scholar] [CrossRef]
- Dordas, C. Foliar application of calcium and magnesium improves growth, yield, and essential oil yield of oregano (Origanum vulgare ssp. hirtum). Ind. Crops Prod. 2009, 29, 599–608. [Google Scholar] [CrossRef]
- Kurade, N.P.; Jaitak, V.; Kaul, V.K.; Sharma, O.P. Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm. Biol. 2010, 48, 539–544. [Google Scholar] [CrossRef]
- Farhat, G.N.; Affara, N.L.; Gali-Muhtasib, H.U. Seasonal changes in the composition of the essential oil extract of East Mediterranean sage (Salvia libanotica) and its toxicity in mice. Toxicon 2001, 39, 1601–1605. [Google Scholar] [CrossRef]
- Das, S.; Prakash, B. Effect of environmental factors on essential oil biosynthesis, chemical stability, and yields. In Plant Essential Oils: From Traditional to Modern-Day Application; Springer Nature: Singapore, 2023; pp. 225–247. [Google Scholar]
- Xu, F.; Shi, Y.; Li, B.; Liu, C.; Zhang, Y.; Zhong, J. Characterization, stability and antioxidant activity of vanilla nano-emulsion and its complex essential oil. Foods 2024, 13, 801. [Google Scholar] [CrossRef]
- Misharina, T.A.; Polshkov, A.N. Antioxidant properties of essential oils: Autoxidation of essential oils from laurel and fennel and of their mixtures with essential oil from coriander. Appl. Biochem. Microbiol. 2005, 41, 610–618. [Google Scholar] [CrossRef]
- Castro, H.T.; Martínez, J.R.; Stashenko, E. Anethole isomerization and dimerization induced by acid sites or UV irradiation. Molecules 2010, 15, 5012–5030. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Odak, I.; Lukic, T.; Talic, S. Impact of storage conditions on alteration of juniper and immortelle essential oils. J. Essent. Oil Bear. Plants 2018, 21, 614–622. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef]
- Rasoul, M. Evaluation of nematicidal effects of monoterpenes against root-knot nematode, Meloidogyne incognita. J. Plant Prot. Pathol. 2013, 4, 445–456. [Google Scholar] [CrossRef]
- Dutta, A.; Mandal, A.; Kundu, A.; Malik, M.; Chaudhary, A.; Khan, M.R.; Shanmugam, V.; Rao, U.; Saha, S.; Patanjali, N.; et al. Deciphering the behavioral response of Meloidogyne incognita and Fusarium oxysporum toward mustard essential oil. Front. Plant Sci. 2021, 12, 714730. [Google Scholar] [CrossRef]
- Galisteo, A.; González-Coloma, A.; Castillo, P.; Andrés, M.F. Valorization of the hydrolate byproduct from the industrial extraction of purple Allium sativum essential oil as a source of nematicidal products. Life 2022, 12, 905. [Google Scholar] [CrossRef]
- Takasugi, M.; Yachida, Y.; Anetai, M.; Masamune, T.; Kegasawa, K. Identification of asparagusic acid as a nematicide occurring naturally in the roots of asparagus. Chem. Lett. 1975, 4, 43–44. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Waring, R.H. Asparagusic acid. Phytochemistry 2014, 97, 5–10. [Google Scholar] [CrossRef]
- Desmedt, W.; Mangelinckx, S.; Kyndt, T.; Vanholme, B. A phytochemical perspective on plant defense against nematodes. Front. Plant Sci. 2020, 11, 602079. [Google Scholar] [CrossRef]
- Jardim, I.N.; Oliveira, D.F.; Silva, G.H.; Campos, V.P.; De Souza, P.E. (E)-Cinnamaldehyde from the essential oil of Cinnamomum cassia controls Meloidogyne incognita in soybean plants. J. Pest Sci. 2018, 91, 479–487. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Mariama, K.; Elsen, A.; De Waele, D. Phenols and lignin are involved in the defence response of banana (Musa) plants to Radopholus similis infection. Nematology 2014, 16, 565–576. [Google Scholar] [CrossRef]
- Hölscher, D.; Dhakshinamoorthy, S.; Alexandrov, T.; Becker, M.; Bretschneider, T.; Buerkert, A.; Crecelius, A.C.; De Waele, D.; Elsen, A.; Heckel, D.G.; et al. Phenalenone-type phytoalexins mediate resistance of banana plants (Musa spp.) to the burrowing nematode Radopholus similis. Proc. Natl. Acad. Sci. USA 2014, 111, 105–110. [Google Scholar] [CrossRef]
- Ohri, P.; Kaur, S. Effect of phenolic compounds on nematodes: A review. J. Appl. Nat. Sci. 2010, 2, 344–350. [Google Scholar] [CrossRef]
- Gowda, A.P.; Shakil, N.A.; Rana, V.S.; Singh, A.K.; Bhatt, K.C.; Devaraja, K.P. Chemical composition and nematicidal activity of essential oil and piperitone oxide of Mentha longifolia L. against Meloidogyne incognita. Allelopath. J. 2023, 58, 165–181. [Google Scholar] [CrossRef]
- Felek, A.F.; Ozcan, M.M.; Akyazi, F. Effects of essential oils distilled from some medicinal and aromatic plants against root knot nematode (Meloidogyne hapla). J. Appl. Sci. Environ. Manag. 2019, 23, 1425. [Google Scholar] [CrossRef]
- Ntalli, N.; Bratidou Parlapani, A.; Tzani, K.; Samara, M.; Boutsis, G.; Dimou, M.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Thymus citriodorus (Schreb) botanical products as ecofriendly nematicides with bio-fertilizing properties. Plants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Kabdal, T.; Kumar, R.; Prakash, O.; Nagarkoti, K.; Rawat, D.S.; Srivastava, R.M.; Kumar, S.; Dubey, S.K. Seasonal variation in the essential oil composition and biological activities of Thymus linearis Benth. collected from the Kumaun region of Uttarakhand, India. Biochem. Syst. Ecol. 2022, 103, 104449. [Google Scholar] [CrossRef]
- Ajith, M.; Shakil, N.A.; Kaushik, P.; Rana, V.S. Chemical composition and nematicidal activity of essential oils and their major compounds against Meloidogyne graminicola (rice root-knot nematode). J. Essent. Oil Res. 2020, 32, 526–535. [Google Scholar] [CrossRef]
- Kundu, A.; Dutta, A.; Mandal, A.; Negi, L.; Malik, M.; Puramchatwad, R.; Antil, J.; Singh, A.; Rao, U.; Saha, S.; et al. A comprehensive in vitro and in silico analysis of nematicidal action of essential oils. Front. Plant Sci. 2021, 11, 614143. [Google Scholar] [CrossRef] [PubMed]
- Eloh, K.; Kpegba, K.; Sasanelli, N.; Koumaglo, H.K.; Caboni, P. Nematicidal activity of some essential plant oils from tropical West Africa. Int. J. Pest Manag. 2020, 66, 131–141. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Veronico, P.; Avato, P.; Argentieri, M.P. Nematicidal activity of the essential oil from Cinnamomum cassia and (E)-cinnamaldehyde against phytoparasitic nematodes. J. Pest Sci. 2025, 98, 521–533. [Google Scholar] [CrossRef]
- Basaid, K.; Chebli, B.; Bouharroud, R.; Elaini, R.; Alaoui, I.F.; Kaoui, S.; De Oliveira, A.L.; Furze, J.N.; Mayad, E.H. Biocontrol potential of essential oil from Moroccan Ridolfia segetum (L.) Moris. J. Plant Dis. Prot. 2021, 128, 1157–1166. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Argentieri, M.P.; Bellardi, M.G.; Avato, P. Nematicidal activity of essential oil from lavandin (Lavandula × intermedia Emeric ex Loisel.) as related to chemical profile. Molecules 2021, 26, 6448. [Google Scholar] [CrossRef]
- Laquale, S.; Avato, P.; Argentieri, M.P.; Bellardi, M.G.; D’Addabbo, T. Nematotoxic activity of essential oils from Monarda species. J. Pest Sci. 2018, 91, 1115–1125. [Google Scholar] [CrossRef]
- Keerthiraj, M.; Mandal, A.; Dutta, T.K.; Saha, S.; Dutta, A.; Singh, A.; Kundu, A. Nematicidal and molecular docking investigation of essential oils from Pogostemon cablin ecotypes against Meloidogyne incognita. Chem. Biodivers. 2021, 18, e2100320. [Google Scholar] [CrossRef]
- Ardakani, A.S.; Hosseininejad, S.A. Identification of chemical components from essential oils and aqueous extracts of some medicinal plants and their nematicidal effects on Meloidogyne incognita. J. Basic Appl. Zool. 2022, 83, 14. [Google Scholar] [CrossRef]
- Moreira, F.J.C.; de Abreu Araújo, B.; do Nascimento Lopes, F.G.; de Assis Lopes de Sousa, A.; Evami Cavalcante Sousa, A.; da Silva Andrade, L.B.; Ferreira Uchoa, A. Assessment of the Tephrosia toxicaria essential oil on hatching and mortality of eggs and second-stage juvenile (J2) root-knot nematode (Meloidogyne enterolobii and M. javanica). Aust. J. Crop Sci. 2018, 12, 1829–1836. [Google Scholar] [CrossRef]
- Hammad, E.A.; El-Sagheer, A.M. Comparative efficacy of essential oil nanoemulsions and bioproducts as alternative strategies against root-knot nematode, and its impact on the growth and yield of Capsicum annuum L. J. Saudi Soc. Agric. Sci. 2023, 22, 47–53. [Google Scholar] [CrossRef]
- Mamoci, E.; Andrés, M.F.; Olmeda, S.; González-Coloma, A. Chemical composition and activity of essential oils of Albanian coniferous plants on plant pests. Chem. Proc. 2022, 10, 15. [Google Scholar] [CrossRef]
- Arya, S.; Kumar, R.; Prakash, O.; Kumar, S.; Mahawer, S.K.; Chamoli, S.; Kumar, P.; Srivastava, R.M.; De Oliveira, M.S. Chemical composition and biological activities of Hedychium coccineum Buch.-Ham. ex Sm. essential oils from Kumaun Hills of Uttarakhand. Molecules 2022, 27, 4833. [Google Scholar] [CrossRef]
- Kamçılar, F.G.; Altinkoy, D.S. Pratylenchus thornei Sher et Allen (Tylenchida: Pratylenchidae)’a karşı Myristica fragrans Houtt (Magnoliales: Myristicaceae) bitki ekstraktının nematisidal etkinliğinin laboratuvar koşullarında belirlenmesi. Tekirdağ Ziraat Fak. Derg. 2024, 21, 1161–1169. [Google Scholar]
- Kumar, R.; Dutta, T.K.; Mandal, A.; Saha, S.; Dutta, A.; Kundu, A. Nanoemulsions of essential oil of Zanthoxylum alatum for protection against Meloidogyne incognita. J. Taibah Univ. Sci. 2025, 19, 2464461. [Google Scholar] [CrossRef]
- Barros, A.F.; Campos, V.P.; de Oliveira, D.F.; de Jesus Silva, F.; Jardim, I.N.; Costa, V.A.; Silva, G.H. Activities of essential oils from three Brazilian plants and benzaldehyde analogues against Meloidogyne incognita. Nematology 2019, 21, 1081–1089. [Google Scholar] [CrossRef]
- Hammad, E.A.; Hasanin, M.M.H. Antagonistic effect of nanoemulsions of some essential oils against Fusarium oxysporum and root-knot nematode Meloidogyne javanica on Coleus plants. Pak. J. Nematol. 2022, 40, 35–48. [Google Scholar] [CrossRef]
- Tamoor, K.; Maryam, H. Evaluation of Some Medicinal Plants for the Management of Root-Knot Diseases of Banana. Pak. J. Nematol. 2021, 39, 1. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.; Omer, E.A. Effect of essential oils of some medicinal plants on phytonematodes. Anz. Schädlingskd. Pflanzenschutz Umweltschutz 1995, 68, 82–84. [Google Scholar] [CrossRef]
- Mattei, D.; Dias-Arieira, C.R.; Puerari, H.H.; Dadazio, T.S.; Roldi, M.; Silva, T.R.B. Evaluation of Rosmarinus officinalis essential oil in inducing resistance to Meloidogyne javanica and Pratylenchus brachyurus in soybean. Food Agric. Environ. 2013, 11, 1171–1175. [Google Scholar]
- Krif, G.; El Aissami, A.; Zoubi, B.; Dababat, A.A.; Khfif, K.; Lahlali, R.; Mokrini, F. Evaluation of the nematicidal effect of some essential oils on Meloidogyne javanica under in vitro and in vivo conditions. J. Crop Health 2024, 76, 1519–1528. [Google Scholar] [CrossRef]
- Barros, A.; Campos, V.P.; Lopes De Paula, L.; Pedroso, L.A.; Silva, F.J.; Da Silva, J.C.P.; De Oliveira, D.F.; Silva, G.H. The role of Cinnamomum zeylanicum essential oil, (E)-cinnamaldehyde and (E)-cinnamaldehyde oxime in the control of Meloidogyne incognita. J. Phytopathol. 2021, 169, 229–238. [Google Scholar] [CrossRef]
- Ferreira, R.; Maleita, C.; Fonseca, L.; Esteves, I.; Sousa-Ferreira, I.; Cabrera, R.; Castilho, P. Chemical screening and nematicidal activity of essential oils from Macaronesian and Mediterranean plants for controlling plant-parasitic nematodes. Plants 2025, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.F.; Lopes, E.A.; Côrtes, F.R.; Visôtto, L.E.; Valente, V.M.M.; Souza, M.F. Nematicidal potential of essential oils of Ageratum fastigiatum, Callistemon viminalis and Schinus terebinthifolius. Biosci. J. 2018, 34, 90–96. [Google Scholar] [CrossRef]
- Pardavella, I.; Daferera, D.; Tselios, T.; Skiada, P.; Giannakou, I. The use of essential oil and hydrosol extracted from Cuminum cyminum seeds for the control of Meloidogyne incognita and Meloidogyne javanica. Plants 2020, 10, 46. [Google Scholar] [CrossRef]
- Kaur, A.; Chahal, K.K.; Kataria, D.; Urvashi, K.A. Assessment of carrot seed essential oil and its chemical constituents against Meloidogyne incognita. J. Pharmacogn. Phytochem. 2018, 7, 896–903. [Google Scholar]
- Faria, J.M.; Vicente, C. Essential oils and volatiles as nematodicides against the cyst nematodes Globodera and Heterodera. Biol. Life Sci. Forum 2021, 3, 1. [Google Scholar] [CrossRef]
- Zoubi, B.; Mokrini, F.; Amer, M.; Cherki, G.; Rafya, M.; Benkebboura, A.; Akachoud, O.; Laasli, S.-E.; Housseinia, A.-I.; Qaddoury, A.; et al. Eco-friendly management of the citrus nematode Tylenchulus semipenetrans using some aromatic and medicinal plants. Arch. Phytopathol. Plant Prot. 2023, 56, 66–86. [Google Scholar] [CrossRef]
- Sosa, A.L.; Girardi, N.S.; Rosso, L.C.; Salusso, F.; Etcheverry, M.G.; Passone, M.A. In vitro compatibility of Pimpinella anisum and Origanum vulgare essential oils with nematophagous fungi and their effects against Nacobbus aberrans. J. Pest Sci. 2020, 93, 1381–1395. [Google Scholar] [CrossRef]
- Park, I.K.; Park, J.Y.; Kim, K.H.; Choi, K.S.; Choi, I.H.; Kim, C.S.; Shin, S.C. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology 2005, 7, 767–774. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, L.H. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Jafari, S.M. Application of essential oils as natural biopesticides; recent advances. Crit. Rev. Food Sci. Nutr. 2024, 64, 6477–6497. [Google Scholar] [CrossRef]
- Alipour, M.; Saharkhiz, M.J.; Niakousari, M.; Damyeh, M.S. Phytotoxicity of encapsulated essential oil of rosemary on germination and morphophysiological features of amaranth and radish seedlings. Sci. Hortic. 2019, 243, 131–139. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; Guirao, P.; Díaz-Baños, F.G.; Cantó-Tejero, M.; Villora, G. Oil in water nanoemulsion formulations of botanical active substances. In Nano-Biopesticides Today and Future Perspectives; Rai, M.K., Singh, S.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–247. [Google Scholar]
- Libs, E.; Salim, E. Formulation of essential oil pesticides technology and their application. Agric. Res. Technol. 2017, 9, 555759. [Google Scholar]
- Kfoury, M. Préparation, Caractérisation Physicochimique et Évaluation des Propriétés Biologiques de Complexes D’inclusion à Base de Cyclodextrines: Applications à des Principes Actifs de Type Phénylpropanoïdes. Ph.D. Dissertation, Université du Littoral Côte d’Opale, Calais, France, 2015. [Google Scholar]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro- and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Yousef, N.; Niloufar, M.; Elena, P. Antipathogenic effects of emulsion and nanoemulsion of cinnamon essential oil against Rhizopus rot and grey mold on strawberry fruits. Foods Raw Mater. 2019, 7, 210–216. [Google Scholar]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- de Oca-Ávalos, J.M.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and physical properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Liang, D.; Feng, B.; Li, N.; Su, L.; Wang, Z.; Kong, F.; Bi, Y. Preparation, characterization, and biological activity of Cinnamomum cassia essential oil nano-emulsion. Ultrason. Sonochem. 2022, 86, 106009. [Google Scholar] [CrossRef]
- Xing, Z.; Xu, Y.; Feng, X.; Gao, C.; Wu, D.; Cheng, W.; Meng, L.; Wang, Z.; Xu, T.; Tang, X. Fabrication of cinnamon essential oil nanoemulsions with high antibacterial activities via microfluidization. Food Chem. 2024, 456, 139969. [Google Scholar] [CrossRef] [PubMed]
- Ayllón-Gutiérrez, R.; Díaz-Rubio, L.; Montaño-Soto, M.; Haro-Vázquez, M.D.P.; Córdova-Guerrero, I. Applications of plant essential oils in pest control and their encapsulation for controlled release: A review. Agriculture 2024, 14, 1766. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Altay, Ö.; Köprüalan, Ö.; İlter, I.; Koç, M.; Ertekin, F.K.; Jafari, S.M. Spray drying encapsulation of essential oils; process efficiency, formulation strategies, and applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 1139–1157. [Google Scholar] [CrossRef]
- Martins, M.; Henriques, M.; Azeredo, J.; Rocha, S.M.; Coimbra, M.A.; Oliveira, R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot. Cell 2007, 6, 2429–2436. [Google Scholar] [CrossRef]
- Ocak, B.; Gülümser, G.; Baloğlu, E. Microencapsulation of Melaleuca alternifolia (tea tree) oil by using simple coacervation method. J. Essent. Oil Res. 2011, 23, 58–65. [Google Scholar] [CrossRef]
- Varona, S.; Kareth, S.; Martín, Á.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluids 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Retention of aroma compounds from Mentha piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers. Food Chem. 2013, 138, 291–297. [Google Scholar] [CrossRef]
- Kusuma, R.; Manichandrika, P.; Yamini, M.; Venkata, K.; Varshitha, K.V.; Reddy, L.S.; Vaishnavi, T. Formulation and in vitro evaluation of Artemisia arborescens extract loaded solid lipid nanoparticles. J. Innov. Dev. Pharm. Technol. Sci. (JIDPTS) 2024, 7, 9. [Google Scholar]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Singh, M.; Chavan, A.; Wagh, N.S.; Lakkakula, J. Micro- and nanoencapsulation techniques in agriculture. In Agricultural Nanobiotechnology; Woodhead Publishing: Cambridge, UK, 2022; pp. 297–323. [Google Scholar]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; McDonald-Howard, K.L.; Mc Donnell, R.J.; Rae, R.; Williams, C.D. Toxicity of essential oils to slug parasitic and entomopathogenic nematodes. J. Pest Sci. 2020, 93, 1411–1419. [Google Scholar] [CrossRef]
- Greff, B.; Sáhó, A.; Lakatos, E.; Varga, L. Biocontrol activity of aromatic and medicinal plants and their bioactive components against soil-borne pathogens. Plants 2023, 12, 706. [Google Scholar] [CrossRef]
- Kotsinis, V.; Dritsoulas, A.; Ntinokas, D.; Giannakou, I.O. Nematicidal effects of four terpenes differ among entomopathogenic nematode species. Agriculture 2023, 13, 1143. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).