Abstract
Cationic polyelectrolytes, characterized by positively charged functional groups, play an essential role in industries ranging from food solutions, water treatment, medical, cosmetic, textiles and agriculture due to their electrostatic interactions, biocompatibility, and functional versatility. This paper critically examines the transition from petroleum-based synthetic polymers such as poly(diallyldimethylammonium chloride) and cationic polyacrylamides to sustainable natural alternatives derived from agri-forestry resources like starch derivatives and cellulose. Through a cradle-to-gate life cycle assessment, we highlight the superior renewability, biodegradability, and lower carbon footprint of bio-based polycations, despite challenges in agricultural sourcing and processing. This study examines cationization processes by comparing the environmental limitations of traditional chemical methods, such as significant waste production and limited scalability, with those of second-generation reactive extrusion (REX), which enables solvent-free and rapid modification. REX also allows for adjustable degrees of substitution and ensures uniform charge distribution, thereby enhancing overall functional performance. Groundbreaking research and optimization achieved through the integration of artificial intelligence and machine learning for parameter regulation and targeted mechanical energy management underscore REX’s strengths in precision engineering. By methodically addressing current limitations and articulating future advancements, this work advances sustainable innovation that contributes to a circular economy in materials science.
1. Introduction
Cationic polymers, or polyelectrolytes, are macromolecules defined by positively charged functional groups, typically quaternary ammonium or amine moieties, distributed along their molecular backbone [1]. This charge imparts a unique capacity for strong electrostatic interaction with anionic surfaces and molecules, underpinning their functional utility in a vast array of functionalities, including flocculation, emulsification, antimicrobial activity, and adhesion [2]. Consequently, these polymers are integral to addressing global challenges in environmental engineering, materials science, and medicine, from water purification, to food solutions, to advanced drug delivery systems and resource management [3,4,5].
Traditionally, the field has been dominated by synthetic cationic polymers derived from petroleum-based feedstocks [6]. However, a global necessity for sustainability has catalyzed a decisive shift toward natural polyelectrolytes sourced from renewable agri-forestry biomass. The term “agri-forestry biomass” refers to lignocellulosic feedstocks, including crop residues (e.g., corn stover, wheat straw, sugarcane), wood processing waste (e.g., bark), crops (e.g., corn, switchgrass), all rich in starch, cellulose or hemicellulose suitable for cationization. These biopolymers offer inherent advantages such as biocompatibility, biodegradability, and renewability. However, their transition from raw biomass to functional material presents significant process engineering challenges. The chemical modification required to introduce cationic character is often energy- and solvent-intensive, potentially offsetting the environmental benefits of the feedstock. This paper suggests that the future of sustainable cationic polyelectrolytes lies not only in the selection of natural raw materials but, critically, in the adoption of advanced manufacturing paradigms. It will first contrast the utility and limitations of synthetic polymers with their natural counterparts. Subsequently, it will focus on the process-related bottlenecks in producing cationized biopolymers and, finally, explore how the convergence of reactive extrusion (REX) with artificial intelligence (AI) offers a transformative pathway toward efficient, sustainable, and optimized manufacturing.
1.1. The Synthetic Benchmark
Petroleum-based synthetic cationic polymers, such as poly(diallyldimethylammonium chloride) (PDADMAC), cationic polyacrylamides (PAMs), and polyethyleneimine (PEI), have long served as the industrial standard (Figure 1). Their widespread adoption is attributable to precise control over molecular architecture, including molecular weight, charge density, and branching, which is achievable through established polymerization techniques. This tunability, combined with cost-effective production scaled by a mature petrochemical infrastructure, has allowed for the creation of highly specialized polymers for applications ranging from water treatment and papermaking to food solutions [7,8,9].
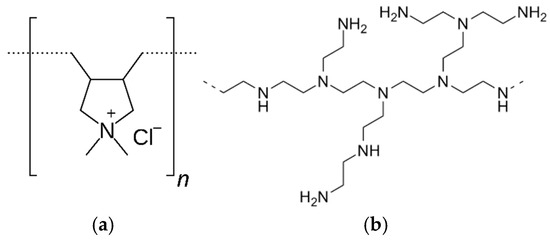
Figure 1.
Synthetic Benchmark (a) PolyDADMAC and (b) Polyethylenimine.
Despite their functional efficacy, the long-term viability of these synthetic polymers is undermined by significant environmental and health-related deficiencies [10]. Their petroleum origin leads them to a non-renewable resource base with a substantial carbon footprint. Furthermore, their robust chemical structures render them resistant to biodegradation, leading to persistence and accumulation in terrestrial and aquatic ecosystems [11]. This concern is magnified by the potential toxicity of certain monomers and polymer residuals, which can pose risks to aquatic organisms and limit their application in sensitive biomedical contexts. This growing awareness of their environmental liabilities has accelerated the scientific and industrial pivot toward bio-based alternatives.
1.2. Cationic Polysaccharides from Agri-Forestry Biomass
Cationic polysaccharides derived from agri-forestry biomass represent a compelling sustainable alternative [12]. Materials such as starch derivatives and cellulosic products offer a renewable and biodegradable polymer backbone that aligns with the principles of a circular economy. Through chemical modification, typically quaternization with agents like glycidyltrimethylammonium chloride (GTAC), these biopolymers are given with the positive charges necessary for high-performance applications [13]. The resulting materials retain the fundamental benefits of their natural origin while gaining enhanced aqueous solubility, thermal stability, and the physicochemical properties required for effective performance.
These cationized biopolymers have demonstrated excellence across numerous sectors. For instance, cationic starch is a cornerstone of the paper industry, improving filler retention and mechanical strength by binding to anionic cellulose fibers [14]. In environmental applications, cationic polysaccharides act as effective flocculants for removing pollutants from wastewater. In biomedical, their mucoadhesive properties are leveraged in advanced drug delivery systems, while their biocompatibility makes them ideal for tissue engineering scaffolds and wound dressings [15]. A detailed summary of their applications across these and other key sectors is provided in Table 1.

Table 1.
A detailed summary of cationic biobased industrial applications.
While the use of renewable feedstock is a critical first step, the overall sustainability of polycation biopolymers is contingent upon the methods used for their production. Conventional cationization processes are often conducted in batch reactors using aqueous or organic solvents, which can be inefficient, energy-intensive, and generate significant waste streams. These procedural drawbacks represent a major bottleneck, hindering the scalability and cost-effectiveness of these otherwise eco-friendly materials. To truly realize their potential, a paradigm shift in process engineering is required.
This paper will critically assess the limitations of current cationization methods and explore emerging strategies for sustainable process intensification. We aim to address the following key questions:
- What are the primary environmental and economic drawbacks of conventional cationization processes?
- How can process intensification, specifically using reactive extrusion (REX), overcome these limitations by enabling solvent-free, continuous, and highly efficient modification of biopolymers?
- How can Artificial Intelligence (AI) and Machine Learning (ML) be integrated with REX to navigate its complex, multi-parameter space, thereby optimizing reaction conditions to maximize throughput, minimize waste, and ensure consistent product quality?
By focusing on the synergistic integration of sustainable raw materials from agri-forestry resources with intelligent manufacturing processes, this paper aims to outline a pathway for the next generation of environmentally and economically viable cationic polyelectrolytes for industrial markets.
2. Sustainability Assessment
A comparison between cationic polysaccharides from agri-forestry biomass and their petroleum-derived synthetic counterparts requires a holistic analytical framework. Life Cycle Assessment (LCA) stands out as the most comprehensive methodology for this purpose, providing a standardized, quantitative evaluation of environmental impacts across the entire value chain, from raw material extraction to manufacturing (cradle-to-gate in this study). Unlike narrower approaches, LCA identifies hotspots, quantifies trade-offs (e.g., between renewability and processing energy), and supports decision-making aligned with global sustainability frameworks like the UN Sustainable Development Goals (SDGs). For instance, while Carbon Footprint Analysis (CFA) excels at isolating greenhouse gas emissions (e.g., 500–1500 kg CO2 eq/ton for bio-based polycations), it overlooks multifaceted impacts such as water scarcity or toxicity. Environmental Impact Assessment (EIA) offers qualitative, site-specific insights but lacks LCA’s systemic lifecycle view, making it less suitable for material comparisons. Material Flow Analysis (MFA) tracks resource inputs/outputs effectively but does not integrate environmental burden quantification, and Eco-Efficiency Analysis (EEA) adds economic dimensions, yet often suffers from subjectivity and incomplete scope. By adopting LCA, this study ensures a balanced, evidence-based assessment that highlights the superior renewability, biodegradability, and lower carbon footprint of bio-based polycations, while acknowledging challenges in agricultural sourcing and processing. To clarify LCA’s advantages, Table 2 compares it with other common environmental evaluation methodologies, such as Carbon Footprint Analysis (CFA), Environmental Impact Assessment (EIA), Material Flow Analysis (MFA), and Eco-Efficiency Analysis (EEA). As shown, CFA focuses solely on greenhouse gas emissions, missing broader impacts like biodegradability, while EIA’s project-specific scope lacks LCA’s systemic perspective. MFA tracks resource flows but does not quantify environmental burdens, and EEA’s economic-environmental integration often suffers from subjectivity. Table 2 underscores why LCA is optimal for this study, providing a robust foundation for comparing bio-based and synthetic polycations. Synthesized insights from peer-reviewed literature reveal clear trends in key sustainability metrics such as renewability, biodegradability, carbon footprint, and socio-environmental risks. These comparisons, summarized in Table 3, generally underscore the superior sustainability profile of biopolymer-derived polycations while simultaneously illuminating pathways for the strategic redesign of their synthetic analogs.

Table 2.
Comparison of Life Cycle Analysis (LCA) with alternative Environmental Evaluation Methodologies.

Table 3.
Comparative Sustainability Metrics of Cationic Polyelectrolytes.
For synthetic polycations, environmental hotspots are predominantly concentrated in upstream monomer production and energy-intensive manufacturing. The synthesis of PDADMAC, for example, depends on hazardous precursors like diallyldimethylammonium chloride, which is derived from allyl chloride, a toxic compound that poses significant health and ecological risks throughout the supply chain [16]. The chemical industry’s shift to efficient, low-waste enzymatic bioprocesses for acrylamide monomer production represents a landmark achievement in green chemistry. However, this upstream gain is often counterbalanced by downstream polymerization techniques (e.g., emulsion or dispersion) that carry burdens from hydrocarbon solvents, chemical additives, and high energy demands for emulsification and distillation. These processes elevate carbon emissions and create nuanced trade-offs, where an apparently “eco-friendly” component is embedded within a resource-intensive system.
In contrast, cationic starch exemplifies a lower-impact alternative, with a carbon footprint often ranging from 500–1500 kg CO2 equivalents per ton [17]. This advantage is rooted in its bio-based origin from abundant crops like maize or wheat. The primary environmental hotspots for these biopolymers are associated with agricultural inputs such as fertilizer use, leading to potential acidification and the cationization step itself, which typically employs petrochemical reagents like 2,3-epoxypropyltrimethylammonium chloride (EPTMAC) [18]. This dependency introduces fossil-fuel inputs and can generate chemical effluents. Nonetheless, the inherent renewability and biodegradability of the starch feedstock (with decomposition rates, k ≈ 0.01–0.1 day−1) confer a decisive advantage over synthetic polymers [19]. However, to ensure sustainability, these benefits must be contextualized with regional considerations, such as responsible land use and water management.
The LCA framework not only diagnoses challenges but also illuminates pathways for advancement. For synthetics, the success of enzymatic acrylamide production demonstrates a transformative potential that could be expanded through the development of bio-based monomers or the incorporation of biodegradable segments to mitigate persistence and toxicity. For polysaccharides, the focus must be on responsible sourcing, prioritizing agricultural residues over primary food crops, and adopting more efficient modification processes. The shift toward polysaccharide-based polycations fosters a circular economy by allocating fossil fuels to other markets and reducing environmental pressures. Interdisciplinary innovations, from agronomic science to advanced polymer engineering, are imperative to establish these bio-based materials as foundational elements of a sustainable industrial ecosystem, in alignment with frameworks like the UN Sustainable Development Goals (e.g., SDG 12). Finally, the LCA serves as a strategic catalyst for innovation, enhancing both environmental stewardship and long-term economic resilience in a resource-constrained world.
3. Conventional Methods for Cationization of Biopolymers
The cationization of biopolymers, encompassing starch and other polysaccharides sourced from agri-forestry resources, represents a cornerstone chemical modification that introduces positive charges, thereby elevating their functionalities such as solubility, adhesion, emulsification, and flocculation efficacy. This transformation has catalyzed their integration into diverse business sectors, including sustainable packaging, cosmetics, construction, textiles, water treatment, and biomedicine, where electrostatic interactions strengthen enhanced performance. Yet, the historical evolution of cationization techniques underscores a persistent contrast between achieving functional versatility and adhering to sustainability directives. Conventional approaches, rooted in conventional chemistry, have forged the foundation of this field but are overloaded with carbon footprints, operational inefficiencies, and scalability constraints. This section undertakes a critical examination of these traditional paradigms, outlining their mechanistic basics, procedural details, and inherent limitations. It progresses to explore complementary alternatives, such as dry processes and ionic liquid-mediated systems, which mitigate some drawbacks while introducing novel avenues for manufacturing optimization. By synthesizing empirical insights, technical analyses, and forward-oriented perspectives, this discourse illuminates the progressive refinement of cationization methods, ultimately highlighting their collective challenges to advocate for paradigm-shifting innovations like reactive extrusion as a sustainable trajectory for future biopolymer manufacturing and engineering.
3.1. Wet Chemistry
Wet chemistry remains the dominant conventional platform for biopolymer cationization, leveraging aqueous or solvent-enhanced environments to arrange nucleophilic reactions that graft cationic moieties onto polysaccharide backbones [20,21,22]. These methodologies, while versatile and well-established, primarily manifest through etherification and/or esterification, each tailored by reagent selection, catalytic conditions, and reaction parameters to modulate substitution efficiency and product attributes.
Etherification constitutes the most rooted technique, involving the nucleophilic displacement of biopolymer hydroxyl groups on epoxide-functionalized reagents like glycidyltrimethylammonium chloride (GTMAC) or 3-chloro-2-hydroxypropyltrimethylammonium chloride (QUAB 188 ©) under alkaline catalysis (Figure 2). Sodium hydroxide (0.5–2 M) deprotonates hydroxyls to generate alkoxide nucleophiles, concurrently activating QUAB 188 © to its epoxide counterpart (QUAB 151 ©), concluding in stable ether bonds with DS values typically reaching 0.01–0.06, though constrained by steric factors to 0.08 in many cases [23,24,25]. Regioselectivity preferentially targets primary hydroxyls (C6 in starch’s anhydroglucose (AUG) units), with secondary sites (C2, C3) engaging under extended durations, as corroborated by 1H NMR studies showing shifts at δ = 3.1–3.3 ppm for quaternary ammonium protons [26]. Solvents such as isopropanol or dimethyl sulfoxide may augment reagent solubility, albeit exacerbating volatile organic compound (VOC) emissions. Procedurally, starch slurries (20–40% solids) are prepared for rheological stability, followed by sequential reagent (5–20% w/w) and alkali addition, agitation at 35–50 °C for 8–24 h to avert gelatinization, pH neutralization (6–7) with hydrochloric acid, filtration, ethanol washing, and drying at 60–80 °C [27].
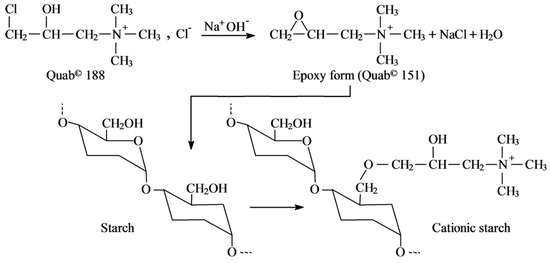
Figure 2.
Etherification of Cationic Starch.
Esterification offers a milder balance, forging ester bonds between hydroxyls and cationic entities from betaine derivatives, operating at 20–60 °C and pH 4–7 to maintain polymer integrity. As demonstrated by Grano et al. (2000) [28], betainyl chloride derived from anhydrous betaine and thionyl chloride reacts with native potato starch in refluxing 1,4-dioxane with pyridine catalysis, achieving DS 0.02–0.06 via acyl substitution, predominantly at C6 hydroxyls [28]. The process initiates with starch suspension in dioxane, dropwise reagent addition (1–3 equiv per AGU), reflux at 100 °C for 4–6 h, precipitation with diethyl ether, washing, and vacuum drying (Figure 3).
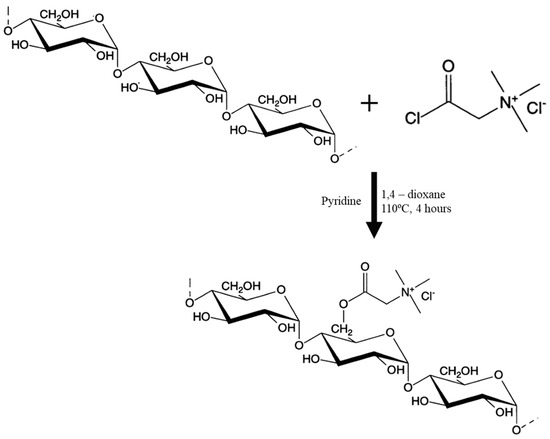
Figure 3.
Esterification of Cationic Starch Betainate.
3.2. Dry Process
The dry process for starch cationization utilizes a semi-aqueous or anhydrous system that substantially minimizes water consumption and effluent production [29]. This method is performed in solid-phase conditions with mechanical agitation to achieve degrees of substitution (DS) exceeding 0.08, surpassing wet counterparts. Caustic soda facilitates high reaction efficiencies (>80%) but requires uniform mixing to prevent gelatinization. Optimal moisture levels (17–22%) are essential for reactivity but necessitate anti-agglomerants like silica to mitigate clumping, while neutralization with solid acids (e.g., adipic or fumaric) maintains pH stability without introducing excess water. Equipment design is based on high-shear mixers supplemented by choppers for turbulence and nozzle injections for precise liquid distribution to achieve yields above 75% (Figure 4). Process conditions yielding 15–25% cost savings through reduced resource use, albeit requiring stringent controls to address salt accumulation and viscosity fluctuations.
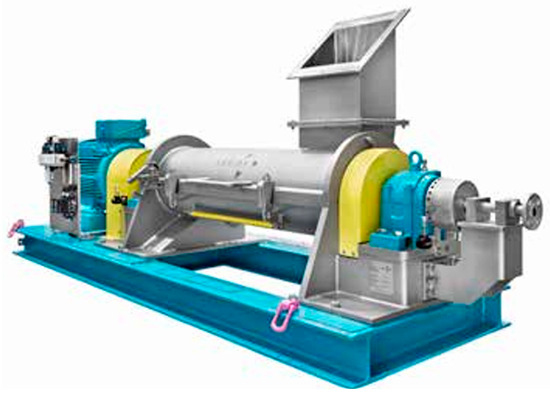
Figure 4.
Cationic Dry Process: Lodige Ringlayer CoriMix® for continuous operation CM 80.
3.3. Ionic Liquid
Ionic liquids (ILs) have revolutionized cationization by providing recyclable solvents that enable homogeneous reactions, yielding high-DS cationic starch with superior uniformity. Wang and Xie (2010) [30] dissolved corn starch in BMIMCl (15 wt.%), reacting with GTAC at 80 °C for 2 h, achieving DS 0.99 exceeding heterogeneous limits. ILs disrupt crystallinity by exposing hydroxyls for efficient etherification at C2/C6. The protocol involves starch dissolution at 80–100 °C, GTAC/NaOH addition, ethanol precipitation, IL recycling (>90% over 5 cycles), and drying. The cost of IL ($5–10/kg) and dissolution energy (10–20 kJ/mol) challenge scale-up.
3.4. Challenges
The operational limitations commonly associated with conventional cationization methods, such as excessive solvent consumption, elevated energy requirements and substantial waste generation, are not just superficial phenomena. Instead, they reflect underlying kinetic and mass transport constraints that are inherent to batch and phase-separated reaction systems. From a reaction engineering perspective, these conventional processes are overwhelmed by critical bottlenecks. First, mass transfer is constrained in wet chemistry, where batch reactors operate in a diffusion-limited regime. The low diffusivity of cationic reagents (D~10−9 m2/s) through viscous aqueous biopolymer slurries (up to 100 mPa·s) creates steep concentration gradients, which limits reaction efficiency to 50–70% and necessitates extended residence time of 8–24 h to achieve low degrees of substitutions (DS < 0.06). Second, reagent accessibility is limited by the heterogeneous nature of the reaction; in both aqueous slurries and dry blends, the native crystalline structure of biopolymer macromolecules restricts reagent penetration, resulting in cationic agents remaining predominantly surface bound. Finally, heat transfer is limited by the low thermal conductivity of hydrated biopolymer gels, resulting in temperature non-uniformity and localized overheating. These conditions promote undesirable side reactions, such as hydrolysis or gelatinization, which further diminish process efficiency and product quality. Ultimately, these limitations arise from the inherent nature of discontinuous operation, low shear (<50 s−1), and phase-separated environments.
Beyond these fundamental reaction engineering limitations, current methodologies meet significant operational and economic challenges. Wet chemistry approaches, reliant on solvent-intensive processes, generate substantial environmental concerns and waste streams that require costly treatment. Dry processes, while reducing water consumption, face challenges related to the formation of sodium chloride byproducts, which can cause both viscosity reduction and product discoloration, alongside significantly higher equipment costs (20–50% greater than wet conventional systems). Furthermore, highly effective methods like ionic liquid-based systems, while capable of achieving high degrees of substitution, sustain substantial material costs ($5–10/kg) and require significant energy for the necessary dissolution steps (10–20 kJ/mol). These material and energy costs, coupled with inherent scalability constraints and rigorous regulatory requirements, drive up compliance and feedstock expenses. To provide a comprehensive evaluation of these methodologies, Table 4 synthesizes their key attributes, mechanisms, procedural steps, achievable DS, advantages, challenges, and sustainability implications.

Table 4.
Comparative Standard Operating Procedures (SOPs) for Conventional Cationization Methods. This table outlines key procedural elements, conditions, optimizations, and challenges for wet (etherification and esterification), dry, and ionic liquid approaches, facilitating direct comparisons and highlighting areas for sustainable innovation.
4. Transition to Reactive Extrusion: A Sustainable Paradigm Shift
The necessary conditions for sustainable processing technologies have positioned reactive extrusion (REX) as a leading second-generation approach for the functionalization and cationization of biopolymers. In contrast to the conventional methods, REX employs a continuous, solvent-free system that integrates mechanical shear, heat, and pressure to facilitate rapid reactions (in the order of minutes rather than hours) while minimizing waste generation. This shift not only mitigates environmental impacts but also enhances precision in controlling reaction efficiency, degree of substitution (DS), molecular weight distribution, and product morphology. Consequently, REX supports the advancement of second-generation biomanufacturing, delivering superior performance and aligning with the principles of sustainable agri-forestry biomass processing.
4.1. Historical Evolution and Technical Foundations
The development of extrusion technology, originating in the early 19th century with lead pipe manufacturing, has evolved significantly through its application in food processing (e.g., macaroni presses before 1850) and subsequently in polymeric materials. By the 1880s, the extrusion of cellulose nitrate was achieved using hydraulic “stuffer presses,” marking an early milestone in polymer processing. The introduction of screw extruders in the 1930s transformed the field by enabling continuous processing, with solvent-free thermoplastic extrusion emerging by 1938, driven by innovations from companies such as the Detroit Macoid Corporation [31].
Contemporary reactive extrusion (REX) leverages advanced configurations, including single-, twin-, or multi-screw extruders, each tailored to specific processing needs. Single-screw extruders, characterized by zoned barrels (feeding, compression, and metering), facilitate efficient material transport and plasticization, making them well-suited for thermoplastic applications [26]. Twin-screw extruders, equipped with intermeshing co- or counter-rotating screws, offer superior mixing and shear control, rendering them ideal for reactive modifications, such as cationic biopolymer processing [32]. Critical operational parameters such as screw speed, barrel temperature, and residence time are precisely controlled using sensors and programmable logic controllers (PLCs), ensuring consistent and high-quality outputs (Figure 5).
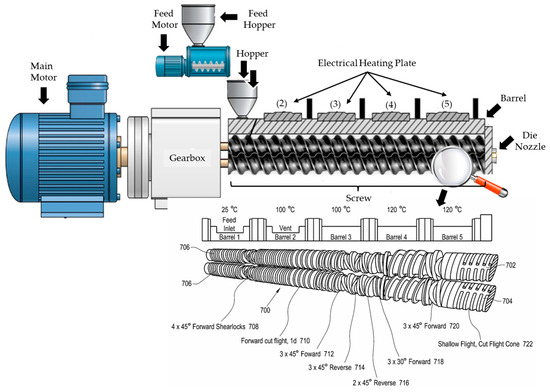
Figure 5.
This schematic illustrates a twin-screw extruder equipped with a main motor, feed motor, and heated barrel sections, alongside various screw configurations (including low shear conveying, high shear mixing, triple flight cone screws). The design enables precise modulation of Specific Mechanical Energy (SME), which defines low, medium, and high shear regimes, critical for controlling the physical and chemical transformation of biopolymer feedstocks. Such systems are foundational to drive innovations for producing highly functionalized agri-forestry biomass, supporting sustainable packaging, building material and other BioSolutions business markets.
Industry leaders, including Clextral (specialists in twin-screw technology), Coperion (experts in compounding), and Bühler (focused on bio-based processing), continue to advance extrusion technology by prioritizing energy efficiency and circular economy principles. These innovations underscore extrusion’s role as a sustainable and versatile processing method. Table 5 provides a comparative analysis of single- and twin-screw extruders, elucidating their applicability to cationic biopolymer processing and highlighting their respective strengths in fostering sustainable manufacturing practices.

Table 5.
Comparative analysis of single screw and twin-screw extruders.
4.2. REX Biopolymer Cationization
The historical trajectory of reactive extrusion (REX) for biopolymer cationization represents a compelling narrative of innovation, driven by the need to overcome the environmental, operational, and scalability limitations of traditional wet chemistry methods. This evolution began in the late 20th century, as researchers sought to harness continuous, solvent-free processing to enhance efficiency, reduce waste, and enable industrial viability. By integrating mechanical and chemical principles in extruders, pioneers transformed starch modification from labor-intensive batch operations into streamlined, adaptable technology. This progression not only addressed persistent challenges such as low degrees of substitution (DS), product degradation, and environmental impacts but also paved the way for customizable, sustainable biopolymers suited to diverse applications. Below, we trace this journey from foundational empirical studies to advanced modeling, highlighting key contributions from Della Valle et al. [33], Carr et al. [34], Ayoub et al. [35], and Berzin et al. [36], whose work on scaling through predictive models marked a critical turning point.
The story commences with early exploration efforts that demonstrated REX’s potential as a chemical reactor. In 1991, Della Valle et al. [33] pioneered the cationization of wheat starch using 3-chloro-2-hydroxypropyl-trimethylammonium chloride (CHPTAC) in a twin-screw extruder. Their research focused on optimizing reaction temperature and the sequence of reagent and catalyst addition, achieving a reaction efficiency of 82% after a 48 h maturation period. Although the resulting DS was modest at 0.045, and starch degradation produced low-viscosity products, this work illuminated REX’s ability to accelerate processes and minimize maturation times, offering advantages for applications like papermaking aids where reduced viscosity enhances performance.
Building on this foundation, Carr et al. in 1992 [34] advanced the field by examining corn starch cationization in a Werner & Pfleiderer ZSK-30 extruder, employing CHPTAC and sodium hydroxide (NaOH). Through systematic optimization, they identified elevated temperatures and higher reagent-to-starch ratios as primary drivers of efficiency, with screw speed exerting a more limited influence. This underscored the predominance of chemical kinetics over mechanical factors in REX, yielding DS values of 0.03–0.06. While challenges such as limited DS and degradation persisted, these studies established empirical benchmarks and highlighted pathways toward scalable industrial processes. A notable advancement that took place in 2004 and 2021 demonstrated the feasibility of reactive extrusion for the cationization of thermoplastic wheat and corn starch under molten conditions [35,37]. The degree of substitution achieved was in the range of 0.01–0.1 with 5 min as the residence time compared to hours in batch methods. This approach delivered exceptional reproducibility, scalability, and parameter tunability. By adjusting temperature and shear, researchers could precisely control viscosity, solubility, and molecular weight distribution, decoupling these properties from the constraints of wet methods (typically limited to DS 0.01–0.05). This innovation facilitated tailored biopolymers for sectors including packaging, personal care, food, and bio-composites, marking a paradigm shift toward versatile, second-generation biomass manufacturing.
To capture this progression, the following timeline table synthesizes key milestones in starch cationization via REX, emphasizing innovations, achievements, and their contributions to sustainability (Table 6).

Table 6.
This table uniquely illustrates REX’s maturation from exploratory empiricism to precision-engineered sustainability, fostering a future where biopolymer cationization aligns seamlessly with economic imperatives.
4.3. Homogeneous Charge Distribution and Enhance Functionality
A defining feature of second-generation reactive extrusion (REX) lies in its capacity to achieve a consistent distribution of cationic charges throughout the biopolymer matrix, effectively addressing the shortcomings of wet chemistry. In applications such as papermaking, where negatively charged cellulosic fibers require consistent electrostatic interactions to facilitate flocculation and bonding, this homogeneity translates into significant performance enhancements (Figure 6). The underlying mechanism involves REX’s capacity to melt starch granules under intense shear and elevated temperatures (typically 100–200 °C), disrupting their crystalline structure and enabling reagents like 3-chloro-2-hydroxypropyl-trimethylammonium chloride (CHPTAC) to penetrate deeply into the polymer matrix. Microstructural evidence underscores this advantage. Scanning electron microscopy (SEM) analyses reveal that wet-chemistry-modified starches retain intact granular structures with cationic charges predominantly localized on the surface, limiting their interaction depth. In contrast, REX-processed starches exhibit complete granule disruption, indicative of comprehensive internal modification that ensures charges are distributed throughout the polymer. Further validation comes from energy-dispersive X-ray spectroscopy (EDS) mapping, which highlights stark differences in cationic group distribution (Figure 7). Wet-chemistry samples display patchy chlorine distributions, a marker for cationic groups, reflecting uneven modification, whereas REX samples exhibit smooth, gradient-free contrasts, confirming homogeneous charge incorporation [37].
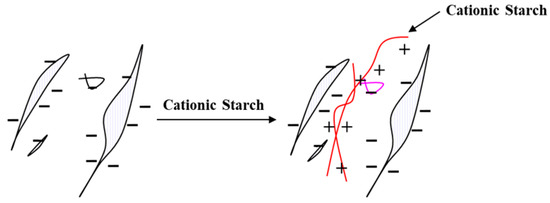
Figure 6.
Interaction between cationic starch and anionic cellulosic materials.
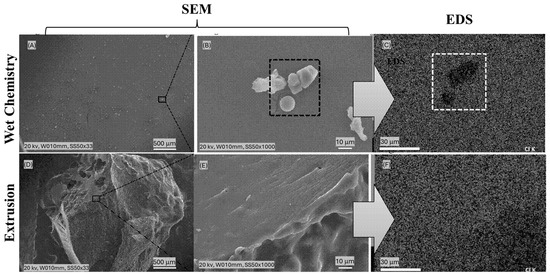
Figure 7.
SEM Micrographs and EDS Elemental Mapping of Chlorine Distribution in Cationic Starch: Comparative Analysis of Wet Chemistry (A–C) and REX-Processed (D–F) Samples Using JEOL JSM-6010LA (20 kV). Caption: Panels (A–C) show SEM images (A,B) and EDS chlorine mapping (C) of cationic starch produced via conventional wet chemistry. Panels (D–F) display SEM images (D,E) and EDS mapping (F) of cationic starch obtained through reactive extrusion (REX) with fully disrupted granules. All samples were mounted with double-sided carbon tape and analyzed at 20 kV using a JEOL JSM-6010LA SEM equipped with a solid-state EDS detector. The images illustrate that REX-processed starches exhibit deep and uniform cationization, as evidenced by homogeneous chlorine distribution, while wet chemistry samples show regions with lower chlorine content. This highlights the effectiveness of REX in overcoming mass transfer limitations and achieving uniform modification.
The molecular uniformity achieved through reactive extrusion (REX) offers a spectrum of functional advantages, notably within papermaking and extending to broader applications such as adhesives, coatings, and flocculants, as demonstrated in studies conducted in 2004 and 2024 [38,39]. This uniformity facilitates enhanced fiber flocculation by enabling efficient bridging of anionic cellulosic fibers, which accelerates pulp drainage and improves sheet formation rates, thereby boosting process efficiency by approximately 3%. Furthermore, the consistent electrostatic interactions contribute to strengthened bonding, augmenting tensile, burst, and internal bond strengths by around 5%, resulting in more robust and durable paper products. Additionally, the optimized charge distribution reduces material usage by 10–20% while enhancing fines retention, thereby minimizing waste and fostering cost-effectiveness.
To further validate the link between microstructural charge modification and macroscopic performance, quantitative data from biopolymer derivative processing illustrate a strong correlation between the average surface charge modification and subsequent strength improvements. While specific metrics such as Zeta potential distribution or adsorption isotherms are not detailed, the incorporation of cationic carbohydrate-based derivatives measurably alters the electrostatic environment of the final pulp. For instance, a 100% virgin pulp system, which is highly anionic with an average Zeta potential of −33.9 mV, shifts significantly toward neutral to −23.5 mV when 10% of the fiber is replaced with REX-produced polycation biomass. A similar neutralizing effect is observed in recycled fiber systems, which shift from −33 mV to −19.8 mV. Enhancing the system’s electrostatic interactions leads to improved strength. In recycled fiber systems, adding 10% cationic polycations biomass boosts Burts strength by 15% and Internal Scott Bond by 19%. This evidence provides a direct quantitative link, illustrating how the enhanced charge modification achieved via REX leads to more efficient and stable electrostatic interactions, concluding significant improvements to the physico-chemical properties. By using advanced techniques to study surface properties and adsorption, researchers can directly and quantitatively connect uniform charge distribution at the microscopic level to overall performance on a larger scale. For instance, Zeta potential distribution measurements reveal that cationic starch (DS 0.058) exhibits a narrow, symmetric distribution (+32 ±−3.1 mV) compared to the broader, less uniform distribution of wet-chemistry counterparts (+28 ± 8.7 mV), reflecting uniform cationic grafting. This homogeneity translates into superior performance, as seen in Langmuir adsorption isotherms showing 30–48% higher maximum binding capacity and stronger affinity constants when it bonds to anionic cellulose. In flocculation tests, the REX–starch polycations achieve 92–96% turbidity removal within 2 min (vs. 78–84% for wet chemistry) and form denser flocs. This enhanced efficiency correlates directly to end-use properties where REX polycations starch to yield 15–22% higher internal bond strength and 10–18% improved drainage rates [39]. These metrics quantitatively validate that the microstructural homogeneity from REX polycations drives more efficient, stable, and predictable electrostatic performance across industrial applications.
4.4. Comparison of Efficacy of Reactive Extrusion Versus Wet Chemistry
Comparative analyses between reactive extrusion (REX) and wet-chemistry-derived cationic starches highlight the manufacturing efficiency and functionality improvement, particularly within the context of papermaking. For starches exhibiting an equivalent degree of substitution (DS), such as 0.058, REX-modified variants exhibit distinctly higher ash and overall retention rates (80–90% compared to 60–70% for wet methods), attributable to an optimized charge distribution that enhances electrostatic interactions with anionic fibers. This improvement fosters enhanced flocculation, increased bonding strength, and reduced starch consumption, collectively contributing to diminished production costs and reduced environmental footprints.
Furthermore, REX’s adjustable processing parameters encompassing temperature (100–200 °C), pressure, screw speed (50–500 rpm), and residence time (1–5 min), facilitate precise customization of biopolymer properties, a flexibility absent in the rigid, solvent-reliant framework of wet chemistry. For instance, modulation of shear rates enables fine-tuning of molecular weight distribution, while temperature adjustments influence reaction kinetics, permitting tailored outputs for diverse applications. In papermaking, REX-processed starches achieve notable enhancements of 15–25% in tensile and burst strengths relative to their wet-chemistry counterparts, thereby improving paper durability while optimizing material utilization.
The following table consolidates the comparative advantages of REX over wet chemistry in cationic biopolymer functionalization, underscoring its influence on critical performance metrics (Table 7).

Table 7.
Comparative advantages of REX over wet chemistry in cationic biopolymer functionalization.
4.5. Economic Analysis of Reactive Extrusion (REX)
Reactive extrusion (REX) offers transformative environmental and functional benefits, yet its industrial adoption requires a rigorous evaluation of capital investment, operational economics, and scale-up challenges to ensure long-term viability. The core equipment, high-shear, co-rotating twin-screw extruders optimized for biopolymer cationization, carries an initial capital cost ranging from $500,000 to $2.5 million, depending on throughput capacity (50–1000 kg/h), screw modularity, and integration of advanced process analytical technology (PAT) such as in-line NIR spectroscopy and torque monitoring systems. This represents a 30–55% premium over traditional batch reactors ($350,000–$1.2 million), driven by the need for precision-engineered barrels, corrosion-resistant alloys, and modular screw elements capable of withstanding high shear (100–500 s−1) and temperature (150–200 °C) under continuous operation. Operational costs depend on energy, maintenance, and labor factors. REX uses 10–20 kWh/kg of biomass, more than wet chemistry’s 5–10 kWh/kg alone, but offers 15–25% net energy savings by eliminating solvent recovery, wastewater treatment, and drying. Annual maintenance typically represents 6–9% of capital expenditures ($30,000–$180,000), mainly because of screw configuration and the need to recalibrate sensors under harsh molten-state conditions. Automated REX lines reduce labor demands to 0.5 operator hours, compared to 2–3 operator hours in batch systems. However, specialized training in extrusion rheology, AI-based control, and biomass variability management adds an additional $15,000–$25,000 per facility each year. However, the return on investment can be achieved within 3–4 years at 500 kg/h scale, driven by a 15–25% reduction in chemical reagent use through solvent-free processing, 20–35% lower waste disposal costs ($0.8–$1.2/kg saved) and 15–25% higher product consistency that reduces off spec losses from 7% to under 2%, with regional variations showing faster payback in Southeast Asia (2.7 years) using low-cost bagasse ($25–40/ton) and high papermaking demand, compared to 3.1 years in North America with corn stover and biomass incentives. Strategic pathways to accelerate adoption include developing open source AI control frameworks to eliminate proprietary software licensing and forming industry-academic consortia for shared training and validation protocols. In conclusion, while REX demands higher upfront investment and technical sophistication, its continuous, solvent-free, AI-optimized operation delivers long-term economic resilience, regulatory compliance, and sustainable scalability, establishing it as the cornerstone of next-generation biomanufacturing in a circular bioeconomy. In addition, REX-modified cationic polycations have been thoroughly tested to meet the environmental and safety standards. They show high biodegradability and low toxicity, outperform petroleum-based alternatives, and are certified for use in food packaging and sustainable paper products.
5. Precision Control in Reactive Extrusion for Biopolymer Cationization: Leveraging Artificial Intelligence and Machine Learning
Reactive extrusion (REX) has emerged as a highly efficient, solvent-free platform for the chemical modification of biopolymers, such as starch and cellulose cationization, offering significant advantages over traditional wet chemistry methods. However, its efficacy hinges on achieving precise control over a complex interplay of process parameters, including screw speed, feed rate, barrel temperature, and material properties [40]. The central challenge is Specific Mechanical Energy (SME (kJ/kg)), a critical metric defined as:
where (P) is the motor power (W), (τ) is the torque (N·m), (γ) is the mass flow rate (kg/s), and (N) is the screw speed (rpm). SME captures the mechanical work conveyed per unit mass, integrating the effects of screw speed, flow rate, and material viscosity (reflected in torque) into a single, physically meaningful parameter. This metric directly influences macromolecular transformations, including distributive and dispersive mixing, shear-induced heating, and the activation energy for chemical reactions. The operational window for SME in biopolymer cationization is narrow. An insufficient SME results in heterogeneous mixing and incomplete reactions, yielding low and non-uniform degrees of substitution (DS). Excessive SME causes thermal and mechanical degradation, leading to polymer chain scission, reduced molecular weight, and undesirable side reactions that compromise functional properties [41,42].
The integration of machine learning (ML) with traditional industrial technologies like reactive extrusion (REX) shows a powerful synergy that regenerates established processes for modern sustainability challenges. REX, a cornerstone of industrial polymer processing since the 1930s, relies on mechanical principles such as shear, heat, and pressure in screw-based systems to functionalize biopolymers. However, its traditional operation often involves trial-and-error adjustments, leading to inefficiencies in energy use and product consistency. By combining ML algorithms, such as Artificial Neural Networks (ANNs) or Model Predictive Control (MPC), with these conventional setups, we can achieve real-time optimization of key parameters like Specific Mechanical Energy (SME), screw speed (50–500 rpm), and residence time (1–5 min). For instance, ML models trained on historical extrusion data can predict and adjust torque to maintain optimal SME (200–250 kJ/kg), preventing degradation while enhancing cationization uniformity. This combination not only improves reaction efficiency by 20–30% but also extends the lifespan of the equipment and reduces the need for costly upgrades. Nonetheless, this approach encourages broader adoption, for example, applying ML to traditional dry processes (Section 3.2) could minimize clumping via predictive moisture control, or to wet chemistry batch reactors by optimizing reagent addition sequences. Future initiatives should focus on developing open-source machine learning frameworks specifically designed for industrial production, while promoting collaborative efforts between materials engineers and data scientists. Ultimately, combining ML with traditional technologies like REX accelerates the transition to sustainable biomanufacturing, minimizing waste, enhancing scalability, and valorizing agri-forestry biomass in alignment with circular principles. Traditional optimization strategies, such as proportional-integral-derivative (PID) controllers and mathematical programming (e.g., linear programming [LP] or nonlinear programming [NLP]), often fall short in addressing REX’s complexity. These methods rely on simplified, steady-state models that fail to capture the non-linear, dynamic interactions among parameters like temperature, moisture content, and reaction extent, which alter the rheological behavior of biopolymers in real time. This necessitates a paradigm shift toward data-driven approaches, where artificial intelligence (AI) and machine learning (ML) offer transformative solutions for predictive modeling, autonomous control, and process insight.
To effectively integrate AI and ML into REX for biopolymer cationization, a structured approach is essential. The following table outlines key steps, tailored to incorporate SME as a critical metric and emphasize traditional systems (Table 8).

Table 8.
Key steps for integrating Artificial Intelligence and Machine Learning with Traditional reactive Extrusion for biopolymer cationization.
A case study explored the application of artificial intelligence (AI)-driven Model Predictive Control (MPC) to facilitate this transition, leveraging dynamic process models to forecast behavior and optimize control actions in real time, thereby ensuring consistent product quality while adhering to operational constraints [43,44]. A key challenge in implementing MPC lies in reconciling model accuracy with the computational demands of real-time decision-making, as predictive models must deliver results within seconds to guide process adjustments effectively. However, their computational intensity renders them impractical for real-time applications, restricting their utility to offline process design and optimization. In contrast, Artificial Neural Network (ANN)-based surrogate models, trained on experimental data or high-fidelity simulations, provide a viable alternative by generating rapid predictions in milliseconds with exceptional accuracy, evidenced by correlation coefficients (R2) exceeding 0.99 [45]. This approach has been successfully validated in twin-screw wet granulation, where ANNs have accurately modeled granule size distributions based on variables such as screw speed and liquid-to-solid ratio, enabling seamless integration with MPC frameworks.
For biopolymer cationization via REX, ANN models could offer a robust solution by predicting critical quality attributes, including degree of substitution (DS) and viscosity, as functions of Specific Mechanical Energy (SME) and other process parameters. By embedding these models within an MPC system, REX operations can dynamically adjust key variables such as screw speed or feed rate to maintain an optimal SME range (e.g., 200–250 kJ/kg), ensuring consistent cationization outcomes. This adaptive control minimizes energy consumption and reduces material waste, aligning with sustainability goals. This approach underscores the transformative potential in enabling continuous manufacturing, providing a scalable framework that enhances efficiency and product reliability in the production of cationic biopolymers.
6. Limitations, Future Directions, and Sustainability
Despite the promising advancements in cationic polyelectrolytes from agri-forestry resources, several limitations persist that warrant attention. Conventional wet chemistry methods suffer from high waste generation, solvent dependency, and limited scalability, while reactive extrusion (REX), though superior, faces challenges such as equipment fouling from cationic residues, thermal degradation of sensitive biopolymers, and variability in raw material quality from agricultural sources, which can affect regioselectivity and charge homogeneity. Although REX alone removes the need for solvents and speeds up reaction times (reducing them to 1–5 min compared to 8–24 h with batch methods), it creates a complex and non-linear set of variables. Factors like screw speed (50–500 rpm), barrel temperature (100–200 °C), feed rate, and variations in biomass (such as moisture levels from 8–25% and amylose content from 15–70%) all interact dynamically to affect Specific Mechanical Energy (SME), degree of substitution (DS), and the uniformity of changes. Traditional control strategies (manual tuning) fail to capture these interactions, resulting in 5–12% off-spec product, 15–20% energy inefficiency and inconsistent charge distribution despite REX’s mechanical advantages. Artificial Intelligence (AI) and Machine Learning (ML) are not optional enhancements but essential enablers that transform REX from a powerful platform into a precision-engineered manufacturing system. By integrating real-time sensor data (torque, pressure, NIR) with predictive models, AI/ML dynamically optimizes SME (200–250 kJ/kg), ensures homogenous cationization, and adapts to raw material fluctuations, achieving <2% off spec, 18–25% energy savings, and uniform DS across biomass types. In the absence of AI, REX may not fulfill its full theoretical potential; however, with the integration of AI, it evolves into a technology that is scalable, sustainable, and resilient for industrial applications.
Future directions should focus on hybrid enzymatic-REX systems to enhance selectivity and reduce energy inputs, nanotechnology for improved functionality (e.g., nanoparticle-embedded polycations for targeted applications), and advanced spectroscopic techniques to deepen understanding of structure-property relationships. Moreover, interdisciplinary collaborations could explore bio-based reagents to eliminate petrochemical dependencies in cationization, alongside scalable pilot studies for real-world validation.
From a sustainability perspective, these innovations align with circular economy principles by valorizing agri-forestry wastes, minimizing environmental footprints through lower carbon emissions (e.g., 500–1500 kg CO2 eq/ton for bio-based vs. higher for synthetics), and promoting biodegradability to mitigate pollution. By prioritizing renewable sourcing, waste reduction, and lifecycle assessments, cationic polyelectrolytes can evolve into cornerstones of biomanufacturing, supporting global goals like SDG 6 (clean water) and SDG 12 (responsible consumption), ultimately fostering resilient and eco-efficient industrial markets.
7. Conclusions
Cationic polyelectrolytes from agri-forestry resources represent a paradigm shift toward sustainable materials, bridging the gap between synthetic efficacy and natural renewability. Through comprehensive analyses of synthesis methods from the inefficiencies of wet chemistry to the precision of reactive extrusion enhanced by AI and machine learning, this paper underscores the transformative potential of bio-based polycations in papermaking, packaging, agriculture, and water treatment. By overcoming environmental hotspots identified in lifecycle assessments and leveraging innovations like homogeneous charge distribution and regioselective modification, these materials not only enhance performance metrics such as retention, strength, and efficiency but also align with pressing demands for biodegradability. As the BioSolutions sector advances, the adoption of second-generation processes promises to catalyze a circular economy, minimizing ecological impacts while driving economic viability. Ultimately, the integration of AI and ML into REX heralds a new era of precision control for biopolymer cationization, overcoming the limitations of traditional empirical and deterministic approaches. AI-driven REX promises tailored functional properties, reduced off-spec production, and seamless transitions to continuous manufacturing. Future research should focus on hybrid models combining ML with mechanistic insights, developing explainable AI to enhance trust in autonomous systems, and leveraging multi-source data while protecting proprietary information. By synthesizing domain expertise with advanced data science, REX is poised to redefine biomanufacturing, delivering high-value, sustainable biopolymers for diverse applications.
Author Contributions
Conceptualization, A.A. and L.A.L.; methodology, A.A. and L.A.L.; formal analysis, A.A. and L.A.L.; resources, A.A. and L.A.L.; writing—original draft preparation, A.A.; writing—review and editing, A.A. and L.A.L.; visualization, A.A. and L.A.L.; supervision, A.A. and L.A.L.; project administration, A.A. and L.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gonçalves, R.A.; Holmberg, K.; Lindman, B. Cationic surfactants: A review. J. Mol. Liq. 2023, 375, 121335. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and synthetic polymers for biomedical and environmental applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Duis, K.; Junker, T.; Coors, A. Environmental fate and effects of water-soluble synthetic organic polymers used in cosmetic products. Environ. Sci. Eur. 2021, 33, 21. [Google Scholar] [CrossRef]
- Yan, C.; Li, W.; Liu, Z.; Zheng, S.; Hu, Y.; Zhou, Y.; Guo, J.; Ou, X.; Li, Q.; Yu, J.; et al. Ionogels: Preparation, properties and applications. Adv. Funct. Mater. 2024, 34, 2314408. [Google Scholar] [CrossRef]
- Wei, T.; Yangcui, Q.; Yi, Z.; Yanxia, Z.; Qian, Y. Exploration of smart antibacterial coatings for practical applications. Curr. Opin. Chem. Eng. 2021, 34, 100727. [Google Scholar] [CrossRef]
- Koczoń, P.; Bartyzel, B.; Iuliano, A.; Klensporf-Pawlik, D.; Kowalska, D.; Majewska, E.; Tarnowska, K.; Zieniuk, B.; Gruczyńska-Sękowska, E. Chemical structures, properties, and applications of selected crude oil-based and bio-based polymers. Polymers 2022, 14, 5551. [Google Scholar] [CrossRef]
- Yuan, M.; Bustamante, H.; Mahmoudi, N.; Gradzielski, M. Colloidal chemistry in water treatment: The effect of Ca2+ on the interaction between humic acid and poly (diallyldimethylammonium chloride) (PDADMAC). Langmuir 2024, 40, 4108–4121. [Google Scholar] [CrossRef]
- Mohseni, A.; Fan, L.; Roddick, F.; Li, H.; Gao, Y.; Liu, Z. Cationic starch: An effective flocculant for separating algal biomass from wastewater RO concentrate treated by microalgae. J. Appl. Phycol. 2021, 33, 917–928. [Google Scholar] [CrossRef]
- Tajbakhsh, S.F.; Mohmmadipour, R.; Janani, H. One-pot production of a graft copolymer of cationic starch and cationic polyacrylamide applicable as flocculant for wastewater treatment. J. Macromol. Sci. Part A 2022, 59, 698–710. [Google Scholar] [CrossRef]
- Mori, R. Replacing all petroleum-based chemical products with natural biomass-based chemical products: A tutorial review. RSC Sustain. 2023, 1, 179–212. [Google Scholar] [CrossRef]
- Gowthaman, N.; Lim, H.; Sreeraj, T.; Amalraj, A.; Gopi, S. Advantages of Biopolymers Over Synthetic Polymers: Social, Economic, and Environmental Aspects. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar]
- Diab, M.; Curtil, D.; El-Shinnawy, N.; Hassan, M.L.; Zeid, I.F.; Mauret, E. Zeid, and Evelyne Mauret. Biobased polymers and cationic microfibrillated cellulose as retention and drainage aids in papermaking: Comparison between softwood and bagasse pulps. Ind. Crops Prod. 2015, 72, 34–45. [Google Scholar] [CrossRef]
- Shen, J.; Singh, R.; Konduri, M.; Fatehi, P. Cationic hemicellulose as a product of dissolving pulp based biorefinery. Ind. Eng. Chem. Res. 2015, 54, 1426–1432. [Google Scholar] [CrossRef]
- Bendoraitiene, J.; Kavaliauskaite, R.; Klimaviciute, R.; Zemaitaitis, A. Peculiarities of starch cationization with glycidyltrimethylammonium chloride. Starch-Stärke 2006, 58, 623–631. [Google Scholar] [CrossRef]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.S.; Alves, L.; Medronho, B.; Ferreira, P.J.T.; Rasteiro, M.d.G. Eco-friendly methods for extraction and modification of cellulose: An overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, Y.; Jia, X.; Wang, Y.; Chen, T. Research progress on typical quaternary ammonium salt polymers. Molecules 2022, 27, 1267. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Chauhan, D.S.; Aslam, R.; Banerjee, P.; Aslam, J.; Quadri, T.W.; Zehra, S.; Verma, D.K.; Quraishi, M.A.; Dubey, S.; et al. Principles and theories of green chemistry for corrosion science and engineering: Design and application. Green Chem. 2024, 26, 4270–4357. [Google Scholar] [CrossRef]
- Kończak, B.; Białowąs, M. The Perspectives on the Application of Biopolymers for a Sustainable Agriculture. J. Ecol. Eng. 2024, 25, 203–217. [Google Scholar] [CrossRef]
- Tyagi, P.; Agate, S.; Velev, O.D.; Lucia, L.; Pal, L. A critical review of the performance and soil biodegradability profiles of biobased natural and chemically synthesized polymers in industrial applications. Environ. Sci. Technol. 2022, 56, 2071–2095. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Chen, T.; Ce, S.; Xiuzhi, T.; Xue, J.; Meiyun, Z. Natural polysaccharide: Modification and application. Pap. Biomater 2021, 6, 43–58. [Google Scholar]
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible double-membrane hydrogels from cationic cellulose nanocrystals and anionic alginate as complexing drugs codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mal, D.; Singh, R. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, F.; Zheng, H. Synthesis and flocculating properties of cationic starch derivatives. Carbohydr. Polym. 2008, 74, 673–679. [Google Scholar] [CrossRef]
- Chiu, C.-w.; Daniel, S. Modification of starches. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 629–655. [Google Scholar]
- Radosta, S.; Vorwerg, W.; Ebert, A.; Begli, A.H.; Grülc, D.; Wastyn, M. Properties of low--substituted cationic starch derivatives prepared by different derivatisation processes. Starch-Stärke 2004, 56, 277–287. [Google Scholar] [CrossRef]
- Kavaliauskaite, R.; Klimaviciute, R.; Zemaitaitis, A. Factors influencing production of cationic starches. Carbohydr. Polym. 2008, 73, 665–675. [Google Scholar] [CrossRef]
- Granö, H.; Yli-Kauhaluoma, J.; Suortti, T.; Käki, J.; Nurmi, K. Preparation of starch betainate: A novel cationic starch derivative. Carbohydr. Polym. 2000, 41, 277–283. [Google Scholar] [CrossRef]
- Zhang, M.; Ju, B.-Z.; Zhang, S.-F.; Ma, W.; Yang, J.-Z. Synthesis of cationic hydrolyzed starch with high DS by dry process and use in salt-free dyeing. Carbohydr. Polym. 2007, 69, 123–129. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohydr. Polym. 2010, 80, 1172–1177. [Google Scholar] [CrossRef]
- Bouvier, J.-M.; Osvaldo, H.C. Extrusion Processing Technology: Food and Non-Food Biomaterials; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ek, P.; Girish, M.G. Basics of Extrusion Processing. In Extrusion Cooking; Woodhead Publishing: Sawston, UK, 2020; pp. 1–28. [Google Scholar]
- Della Valle, G.; Colonna, P.; Tayeb, J. Use of a twin--screw extruder as a chemical reactor for starch cationization. Starch-Stärke 1991, 43, 300–307. [Google Scholar] [CrossRef]
- Carr, M.E. Preparation of cationic starch containing quaternary ammonium substituents by reactive twin--screw extrusion processing. J. Appl. Polym. Sci. 1994, 54, 1855–1861. [Google Scholar] [CrossRef]
- Ayoub, A.; Bliard, C. Cationisation of glycerol plasticised wheat starch under microhydric molten conditions. Starch-Stärke 2003, 55, 297–303. [Google Scholar] [CrossRef]
- Berzin, F.; Tara, A.; Tighzert, L.; Vergnes, B. Computation of starch cationization performances by twin--screw extrusion. Polym. Eng. Sci. 2007, 47, 112–119. [Google Scholar] [CrossRef]
- Ayoub, A.; James, C.B.; Ryan, N.C.U.S. Modified Biopolymers and Methods of Producing and Using the Same. Patent 10,982,012, 2021. [Google Scholar]
- Ayoub, A. Synthèse, Structure et Propriétés des Amidons Modifiés en Milieu Fondu Peu Hydraté. Ph.D. Thesis, University of Reims, Reims, France, 2004. [Google Scholar]
- Malm, M.; Ali, A.; Matt, K. Novel Wheat Milling Derivative Products, Methods of Making and Uses of the Same. WO Patent Application 202412679A1, 13 June 2024. [Google Scholar]
- Berzin, F.; Bruno, V. Preparation of Cationic Starches by Reactive Extrusion: Experiments and Modelling. In Biomass Extrusion and Reaction Technologies: Principles to Practices and Future Potential; American Chemical Society: Washington, DC, USA, 2018; pp. 67–88. [Google Scholar]
- Giménez, M.A.; Gremasqui, I.d.L.A.; Segundo, C.N.; Dominguez, N.E.; Sammán, N.C.; Lobo, M.O. Changes in starch and protein organization with positive impact on the quality of extruded Andean corn-pasta. J. Food Meas. Charact. 2025, 19, 4234–4243. [Google Scholar] [CrossRef]
- Wagner, C.E.; Richter, J.K.; Dey, D.; Finnie, S.; Ganjyal, G.M. Impact of tamarind seed gum on the viscosity behavior, thermal properties, and extrusion characteristics of native corn starch. J. Food Sci. 2023, 88, 1595–1609. [Google Scholar] [CrossRef]
- Jelsch, M.; Roggo, Y.; Brewer, M.; Géczi, Z.-A.; Heger, P.; Kleinebudde, P.; Krumme, M. Advanced process automation of a pharmaceutical continuous wet granulation line: Perspectives on the application of a model predictive control from solid feeders to dryer. Powder Technol. 2023, 429, 118936. [Google Scholar] [CrossRef]
- Sareminia, S.; Najafi, A. Enhancing Extrusion-Spheronization Pharmaceutical Production Efficiency: An AI-Driven Approach to Pellet Formulation and Manufacturing. J. Pharm. Innov. 2025, 20, 113. [Google Scholar] [CrossRef]
- Akram, M.B.; Gilarno, C.; McCoy, K.; Osten, S.; Rumps, M.; Thota, U.; Campbell, D.; Balasubramanian, G. Machine learned and explainable prediction of melt pressure and temperature for polypropylene extrusion. J. Intell. Manuf. 2025, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).