Abstract
The Indonesian pharmaceutical industry faces increasing pressure to achieve sustainable performance amid regulatory constraints and evolving healthcare demands. This study aims to identify and prioritize strategic dimensions of sustainable marketing innovation using the Fuzzy Analytic Hierarchy Process (Fuzzy AHP). Expert judgments were collected from eight senior professionals across academia, industry, and government through structured online questionnaires and digital interviews. Six innovation dimensions (product, process, organization, price, promotion, and distribution) were evaluated. The analysis produced normalized priority weights, revealing that promotion (0.2384) and process (0.2253) innovations ranked highest, followed by product (0.1790) and price (0.1699), while distribution (0.1202) and organization (0.0672) held lower importance. These results highlight the critical role of ethical promotion, digital engagement, and operational excellence in strengthening competitive and sustainable market performance. A sensitivity analysis confirmed the stability of these rankings across varying fuzzification scales. By integrating the Resource-Based View (RBV) with Fuzzy AHP, this study contributes a reproducible framework for transforming expert knowledge into strategic priorities under uncertainty. The findings offer practical guidance for pharmaceutical firms to allocate innovation resources more effectively and enhance long-term sustainability.
1. Introduction
Innovation has long been recognized as a key engine of industrial transformation and sustainable competitiveness, particularly in highly regulated and research-intensive sectors such as pharmaceuticals. In Indonesia, the pharmaceutical market continues to grow, driven by demographic expansion, the implementation of the National Health Insurance Program (Jaminan Kesehatan Nasional—JKN), and increasing public awareness of health and wellness. However, this growth also brings structural challenges, including pricing constraints under the JKN reimbursement system, disparities in medicine distribution, and intensified regulatory oversight [1,2,3]. These dynamics require pharmaceutical firms to adopt sustainable marketing innovation strategies to maintain competitiveness while ensuring equitable access to healthcare [4].
Sustainable marketing innovation is increasingly viewed as a fundamental determinant of long-term corporate resilience and value creation [5]. According to the Oslo Manual, marketing innovation encompasses significant changes in product design, pricing, promotion, and distribution to better satisfy customer needs [6]. Within the pharmaceutical context, this extends to process and organizational innovations that enable ethical promotion, data-driven engagement with healthcare professionals, and responsible supply chain practices [7,8]. Empirical evidence highlights that firms adopting sustainability-oriented innovation strategies achieve superior performance when marketing and technological dimensions are aligned. Studies have shown that marketing-led innovation enhances adaptive capacity and market responsiveness [9], while digital communication and stakeholder co-creation play pivotal roles in embedding sustainability within marketing systems [10]. Moreover, competition intensity and product life-cycle management significantly influence innovation performance and strategic decision-making in the pharmaceutical industry [8,11].
Despite these advances, the current literature remains fragmented, often addressing single innovation dimensions such as product or promotion without providing an integrated model for prioritization. In emerging markets like Indonesia, few studies have systematically evaluated how different marketing innovation dimensions contribute to sustainability outcomes or how resource allocation decisions can be optimized under uncertainty [12,13,14]. This research addresses these gaps by employing the Fuzzy Analytic Hierarchy Process (Fuzzy AHP) to prioritize six innovation dimensions—product, process, organization, price, promotion, and distribution—based on expert judgments from diverse clinical, managerial, and policy backgrounds.
Beyond methodological application, this study advances previous research by integrating the Resource-Based View (RBV) with Fuzzy AHP to establish a robust theoretical foundation for decision modeling in sustainable marketing. Prior studies applying Fuzzy AHP have primarily focused on technical weighting of criteria without linking the approach to strategic management theory [15,16,17]. By contrast, this study operationalizes the RBV by interpreting expert knowledge, marketing capabilities, and stakeholder engagement as valuable, rare, inimitable, and non-substitutable (VRIN) resources that shape innovation priorities. Through this integration, the model bridges the gap between sustainable marketing theory and operational prioritization tools, offering a reproducible framework for strategic decision-making in regulated industries.
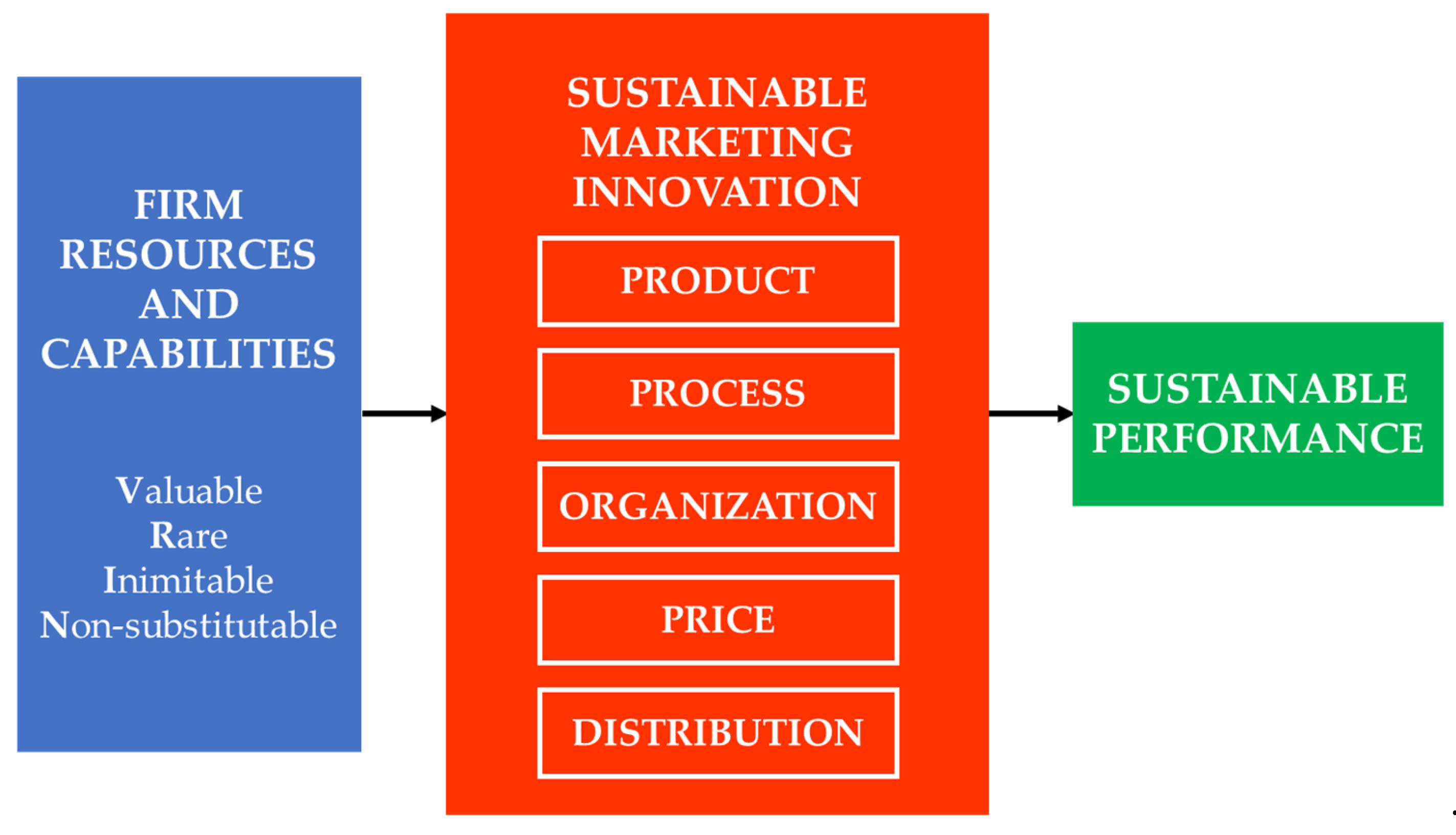
To reinforce theoretical transparency, a conceptual framework figure (Figure 1) is proposed to visualize how RBV constructs interact with the six marketing innovation dimensions. The framework depicts how firm resources and capabilities act as antecedents that influence product, process, organization, price, promotion, and distribution innovations, which collectively drive sustainable competitive advantage. This visualization clarifies the theoretical pathway linking internal resources to measurable innovation outcomes and positions marketing innovation as both a sustainability enabler and a strategic decision domain.
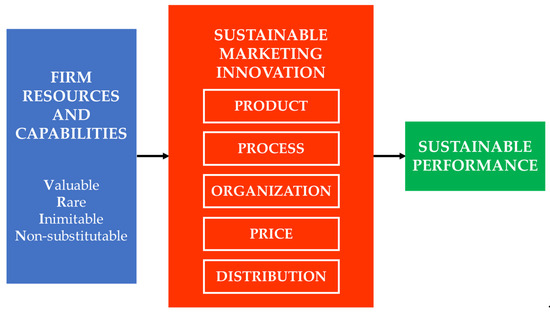
Figure 1.
Conceptual framework linking RBV constructs with sustainable marketing innovation dimensions and sustainable performance.
Accordingly, this study aims to identify and prioritize the marketing innovation dimensions most critical to enhancing sustainable performance in Indonesia’s pharmaceutical sector. By embedding RBV into a Fuzzy AHP-based decision framework, the research contributes both theoretically by extending sustainability and innovation theory into a resource-based context, and methodologically by refining multi-criteria decision modeling to better reflect uncertainty and expert-driven judgment.
2. Materials and Methods
2.1. Research Design
A mixed methods approach was adopted, integrating qualitative and quantitative techniques to achieve a more comprehensive understanding of the research problem [18]. The process followed three stages: (1) identification of innovation dimensions from literature, (2) expert elicitation through semi-structured interviews with pairwise comparisons, and (3) prioritization of innovation dimensions using Fuzzy AHP [19,20]. The Fuzzy AHP analysis was conducted using Python version 3.13.5. This design is appropriate for decision-making problems requiring expert knowledge under uncertainty [21].
2.2. Identification of Marketing Innovation Dimensions
Six innovation dimensions were identified based on the Oslo Manual and prior research: product, process, organization, price, promotion, and distribution [6,12]. These cover both internal aspects (product, process, organization) and external aspects (price, promotion, distribution) relevant to pharmaceutical marketing (Table 1).

Table 1.
Presents the operational definitions for each dimension, adapted from the literature.
2.3. Expert Panel and Data Collection
Purposive sampling was used to recruit eight experts from academia, pharmaceutical companies, multinational firms, and the Ministry of Health. Table 2 summarizes the expert panel composition. Each expert participated in a semi-structured interview, provided pairwise comparisons of the six innovation dimensions, and consented to the use of anonymized responses.

Table 2.
Summarizes the expert panel composition.
2.3.1. Expert Selection and Qualifications
Eight experts participated in this study. The number was determined according to methodological guidance for the Fuzzy AHP approach, which prioritizes expertise and judgmental consistency over sample size. A panel of eight was considered sufficient to ensure methodological rigor, analytical reliability, and data saturation while maintaining manageable cognitive complexity during pairwise comparisons [17]. Experts were selected purposively based on three inclusion criteria: (1) at least ten years of professional experience in the pharmaceutical or health sector; (2) specialized knowledge in marketing strategy, innovation management, clinical or regulatory affairs, or public health policy; and (3) active involvement in strategic decision-making or academic research related to pharmaceutical innovation and sustainability.
The panel represented a diverse mix of academic, industrial, and governmental professionals, providing complementary expertise:
- A professor of pulmonology and respiratory medicine from Universitas Indonesia and Universitas YARSI, also Adjunct Professor at Griffith University (Australia), specializing in environmental health, respiratory epidemiology, and public health policy;
- A senior marketing consultant and former marketing director at major pharmaceutical companies (Pharos Indonesia, Fresenius Kabi, and Merck), with expertise in marketing strategy, leadership, and organizational development;
- A professor of oncology surgery at Universitas Udayana, experienced in multidisciplinary cancer management and clinical research;
- A regional medical affairs leader at Pfizer responsible for Malaysia, Indonesia, Singapore, and the Philippines, specializing in evidence generation, market access, and real-world data analysis;
- A country group head at Wellesta Indonesia, focusing on strategic management, business development, and corporate governance in healthcare;
- A medical and market-access head at PT Anvita Pharma Indonesia, specializing in health economics, regulatory affairs, and policy advocacy;
- A director general of pharmacy and medical devices at the Indonesian Ministry of Health, formerly acting head of the National Agency for Drug and Food Control (BPOM), with expertise in national pharmaceutical policy and regulation;
- A director of pharmaceutical management and services at the Ministry of Health, specializing in supply chain management, pharmaceutical quality control, and hospital pharmacy optimization.
All expert qualifications were rigorously verified through curriculum vitae, professional records, and institutional credentials. This comprehensive verification process ensured the inclusion of balanced, credible, and authoritative perspectives on Indonesia’s pharmaceutical innovation ecosystem.
2.3.2. Data Collection Procedure
Data were collected through a structured online questionnaire supported by short digital interviews. This hybrid approach facilitated expert participation while accommodating their schedules and geographic dispersion. The Fuzzy AHP questionnaire employed linguistic pairwise comparisons distributed via secure email links with concise instructions and illustrative examples. A pilot test involving two external professionals (not part of the final panel) verified the clarity and logical flow of the questionnaire, which is presented in Appendix A. Minor wording adjustments improved readability and consistency in interpreting the nine-point fuzzy comparison scale. The data collection process lasted approximately five weeks. Experts completed digital forms in Microsoft Excel, which automatically converted linguistic judgments into triangular fuzzy numbers (TFNs). Responses were reviewed for accuracy and internal consistency, and brief follow-up interviews clarified any ambiguous entries. A survey-based approach was chosen because it enables structured quantification of expert judgment while minimizing interviewer bias. It also allows transparent aggregation of evaluations and aligns with previous Fuzzy AHP studies that emphasize controlled expert elicitation in complex decision-making environments.
2.3.3. Consistency and Validation of Expert Judgments
To ensure reliability, all experts joined a short orientation session explaining the principles of the Fuzzy AHP method, the linguistic evaluation scale, and an example of pairwise comparison. This session ensured a consistent understanding of the methodology before data collection. Each expert’s comparison matrix underwent a fuzzy consistency check. Matrices exceeding the acceptable threshold (CR > 0.10) were returned for revision. After validation, individual matrices were aggregated using the geometric-mean method to generate a single group matrix. The Converting Fuzzy Data into Crisp Scores (CFCS) method was then applied for defuzzification and weight derivation [24]. Finally, a sensitivity analysis tested the stability of results across different fuzzification scales, confirming consistent ranking outcomes. These procedures ensured logical coherence and robustness of expert judgments, thereby enhancing transparency and reproducibility in evaluating sustainable marketing-innovation strategies.
2.4. Fuzzy Analytic Hierarchy Process (Fuzzy AHP)
The Fuzzy AHP method combines AHP with fuzzy set theory to better model human judgment under uncertainty [15,19]. The analysis followed these steps:
- Pairwise comparison matrix construction: Experts compared criteria using a linguistic scale;
- Linguistic–TFN conversion: Each linguistic judgment was converted into a triangular fuzzy number (TFN) (Table 3);
 Table 3. Linguistic scale and TFNs.
Table 3. Linguistic scale and TFNs. - Aggregation of fuzzy matrices: Expert matrices were aggregated using the geometric mean [19];
- Synthetic extent analysis and defuzzification: Chang’s extent method was used to compute fuzzy synthetic extents and defuzzify into crisp weights [15];
- Normalization and consistency check: Defuzzified weights were normalized to sum to 1, and a consistency ratio (CR < 0.1) was verified [19].
2.5. Data Handling and Reproducibility
All pairwise data, aggregated matrices, and defuzzified results are available in Figure 1 and Figure 2. The methodology aligns with recommendations for multi-criteria decision-making in sustainability research [25].
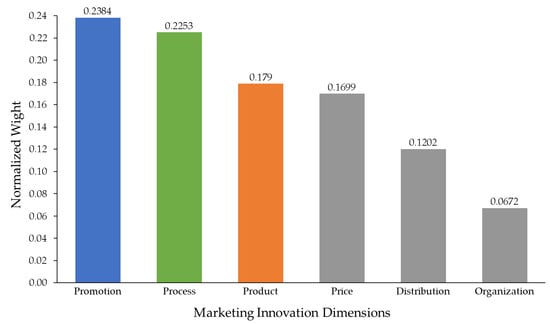
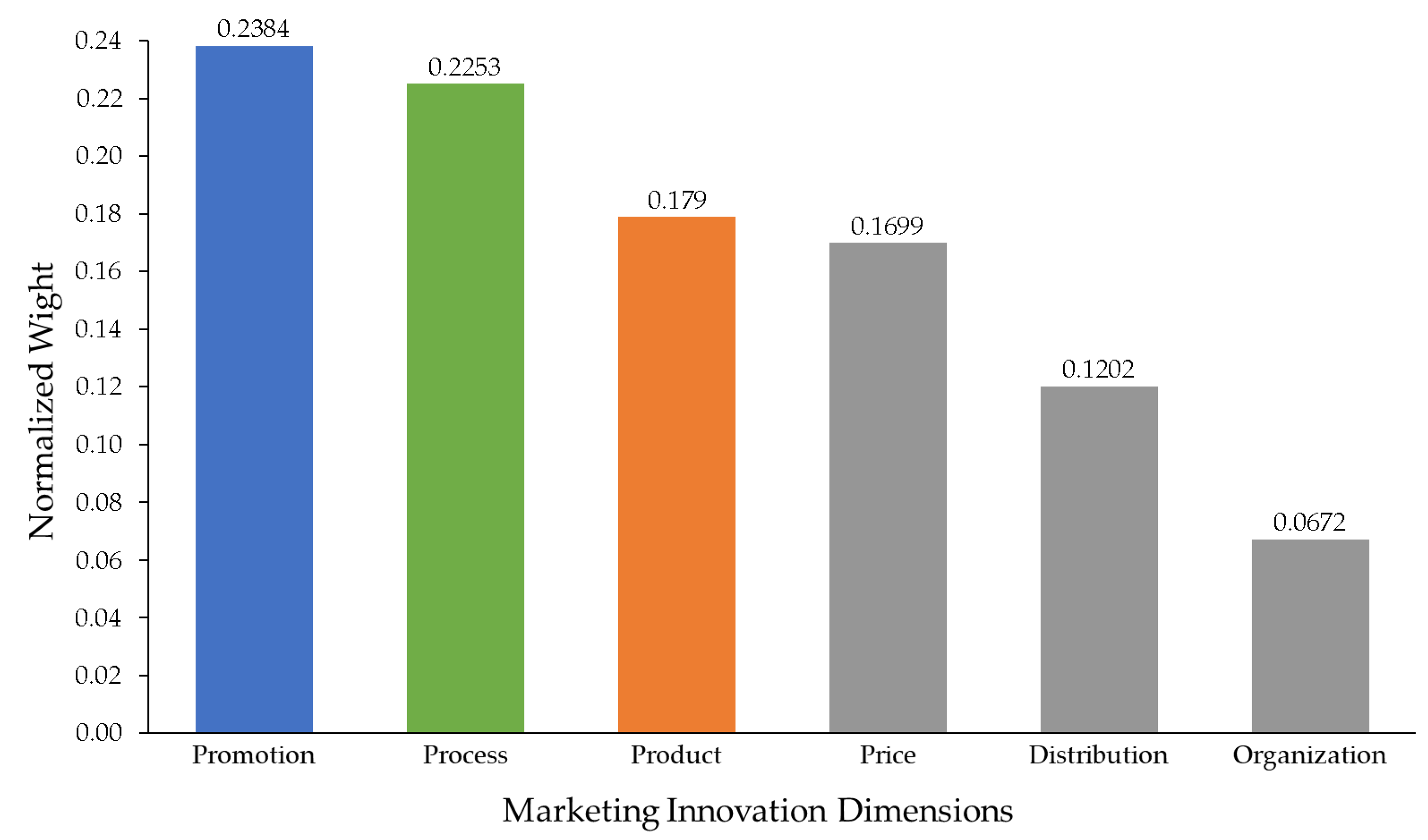
Figure 2.
Normalized priority weights of marketing innovation dimensions.
2.6. Literature Review and Method Justification
2.6.1. Applications of Multi-Criteria Decision-Making (MCDM) Methods
Multi-criteria decision-making (MCDM) methods have become essential for addressing complex sustainability and management problems involving multiple interdependent factors. These approaches enable structured evaluation by integrating qualitative and quantitative dimensions, supporting decisions that require trade-offs among economic, environmental, and strategic criteria. Widely used techniques include the Analytic Hierarchy Process (AHP), Analytic Network Process (ANP), Technique for Order Preference by Similarity to Ideal Solution (TOPSIS), and VIKOR, as well as their fuzzy and hybrid extensions. In recent years, MCDM frameworks have been applied to diverse sustainability challenges, including pharmaceutical supply chains, manufacturing optimization, and innovation management [26,27,28]. The introduction of fuzzy logic has further improved their flexibility, allowing for the handling of linguistic or uncertain inputs common in expert-based decision-making [17,24,29,30,31,32,33]. Within pharmaceutical innovation, MCDM methods have helped identify critical drivers of R&D performance, assess market risks, and prioritize competitive strategies [16,25,34]. These findings affirm that MCDM techniques are particularly effective when expert judgment, uncertainty, and multiple interrelated criteria must be evaluated simultaneously, conditions that align closely with this study’s focus on sustainable marketing innovation.
2.6.2. Rationale for Selecting the Fuzzy AHP Method
The Fuzzy Analytic Hierarchy Process (Fuzzy AHP) was chosen because it combines the hierarchical structure and logical clarity of AHP [35,36] with the ability of fuzzy set theory to represent uncertainty [26,37]. Traditional AHP relies on precise pairwise comparisons and assumes that decision-makers can express their preferences with full confidence. In real-world contexts, especially in strategic marketing and innovation, experts often face ambiguity, incomplete information, and subjectivity. Fuzzy AHP addresses these challenges by translating linguistic judgments such as “moderately important” into fuzzy numbers. This conversion allows for the incorporation of uncertainty directly into the evaluation process, reducing bias and increasing reliability. The method’s strength lies in its capacity to produce robust priority weights even when expert assessments differ slightly. Compared to other methods, Fuzzy AHP offers a balanced combination of transparency, simplicity, and analytical rigor. Fuzzy ANP captures interdependencies among criteria but is computationally complex and data-intensive [30], while Fuzzy TOPSIS and VIKOR are efficient for ranking alternatives yet lack hierarchical weighting mechanisms [20,31]. Considering that this study’s six innovation elements, namely product, process, organization, price, promotion, and distribution, form a hierarchical structure, Fuzzy AHP represents the most suitable analytical choice.
2.6.3. Comparative Overview of MCDM Approaches
A concise summary of major MCDM techniques is provided in Table 4. Each method differs in its analytical focus, treatment of uncertainty, and computational requirements.

Table 4.
Comparison of major multi-criteria decision-making (MCDM) methods.
This comparison confirms that Fuzzy AHP achieves an optimal balance between interpretability, robustness, and the capacity to incorporate linguistic expert judgments, making it the most effective tool for prioritizing strategic innovation criteria in this research.
2.6.4. Methodological Innovations in This Study
Several methodological refinements were introduced to strengthen the conventional Fuzzy AHP. First, expert evaluations were expressed as triangular fuzzy numbers (TFNs), ensuring consistent conversion of qualitative judgments into measurable values [17]. Second, a fuzzy consistency check was applied before defuzzification to validate pairwise comparisons and maintain logical coherence. Third, the Converting Fuzzy Data into Crisp Scores (CFCS) approach was implemented to normalize and aggregate expert assessments, facilitating reliable weight derivation among innovation dimensions [17,24]. Finally, a sensitivity analysis was performed to test the robustness of ranking results under different fuzzification scales, confirming the method’s stability [20,26]. These enhancements improve the transparency, reproducibility, and precision of the Fuzzy AHP application, providing a solid methodological foundation for evaluating sustainable marketing innovation strategies in Indonesia’s pharmaceutical sector. By refining the decision-making framework, the study contributes a more nuanced, evidence-based approach consistent with the Resource-Based View (RBV) and sustainability-driven competitiveness. In addition, the level of agreement among the experts was qualitatively assessed to ensure consistency and reliability of their collective judgments, following standard practices in expert-based MCDM applications [20]. The comparison matrices demonstrated a high degree of directional alignment across all eight experts, indicating that their evaluations were coherent and mutually reinforcing. This consensus supports the robustness of the aggregated fuzzy pairwise comparisons and strengthens the credibility of the resulting priority structure.
2.6.5. Sensitivity Analysis Procedure
To ensure the robustness of the priority rankings derived from the Fuzzy AHP model, a sensitivity analysis was conducted. The analysis tested how variations in the fuzzification scale (±10%) affected the final ranking of innovation criteria. The same set of expert judgments was reprocessed under different triangular fuzzy number (TFN) configurations to observe any changes in weight distribution. The results of this procedure confirmed the stability of the model’s output, as no significant reordering occurred among the six innovation dimensions. This validation step enhanced the reliability and reproducibility of the findings.
3. Results
This section reports the empirical outcomes of the Fuzzy Analytic Hierarchy Process (Fuzzy AHP) applied to prioritize six marketing innovation dimensions for pharmaceutical firms in Indonesia. Expert judgments from eight specialists were aggregated and processed following the procedure described in Section 2. The Results are presented in four parts: aggregated pairwise judgments, fuzzy synthetic extents and defuzzified values, final priority ranking and visualization, and interpretation with strategic implications. Consistency checks show the aggregated judgments are acceptable and reproducible.
3.1. Aggregated Expert Judgments (Consensus Pairwise Comparisons)
All expert pairwise comparisons were converted into triangular fuzzy numbers (TFNs) and aggregated using the geometric mean rule to form a consensus assessment. The resulting aggregated pairwise comparison matrix is shown in Table 5, where each cell represents the relative importance of the criterion in the corresponding row compared with the criterion in the corresponding column. Diagonal values equal 1, and the lower triangular values are reciprocals of the upper triangular values. This consensus matrix served as the input for the fuzzy synthetic extent analysis and subsequent defuzzification.

Table 5.
Aggregated pairwise comparison matrix (geometric mean ratios).
3.2. Fuzzy Synthetic Extent, Defuzzification, and Normalized Weights
From the aggregated pairwise comparisons shown in Table 5, fuzzy synthetic extent values Si were computed for each innovation dimension using Chang’s extent analysis method [38]. The sum of row values (crisp aggregation) and the resulting synthetic extent for each dimension are shown in Table 6.

Table 6.
Row sums and fuzzy synthetic extent (Si).
From these synthetic extents, defuzzified values were derived by the centroid/defuzzification routine used in the processing workbook. These defuzzified scores were normalized so that their sum equals 1. Final normalized weights and ranking are shown in Table 7.

Table 7.
Defuzzified weights, normalized priority weights, and ranking.
The aggregated pairwise comparisons exhibit acceptable internal consistency, with all consistency ratios (CR) below the conventional threshold of 0.10 [39].
3.3. Priority Ranking and Visualization
The normalized weights obtained from Table 6 were used to rank the six innovation dimensions. Promotion emerged as the top priority (0.2384), followed by process (0.2253) and product (0.1790). Price, distribution, and organization received lower relative weights, reflecting their comparatively smaller contribution to sustainable marketing performance.
Figure 2 presents a bar chart of normalized weights, allowing quick visual comparison across all six dimensions. Each bar represents a criterion, and its height corresponds to the normalized weight. The values are shown above each bar to highlight the relative differences among criteria.
Interpretation of the ranking in relation to the research objective:
- The objective is to prioritize the marketing innovation dimensions that most strongly contribute to sustainable performance. The Result shows that resource allocation emphasizing promotion, process, and product innovation will likely yield the highest sustainable impact, according to the expert panel;
- Promotion ranks first, indicating that effective, ethical, and targeted stakeholder engagement strategies are perceived by experts as the most leverageable area for immediate sustainability gains. Promotion here includes activities such as educational outreach to healthcare professionals, digital engagement, and value communication;
- Process ranking second reflects the experts’ view that operational improvements, supply chain resilience, and compliance mechanisms are foundational to sustainable marketing outcomes;
- Product ranking third underscores the continuing importance of product-level innovation such as formulation, packaging, and service enhancements.
3.4. Key Findings Linked to the Study Objective
- Promotion is the primary area to prioritize for sustainable marketing innovation in Indonesia. Investing in promotion yields strategic advantages in stakeholder engagement, uptake, and responsible market behavior. This aligns with literature emphasizing the role of communication and market engagement in realizing innovation value [38];
- Process improvements are essential to operationalize sustainable marketing. Strengthening manufacturing practices, logistics, and regulatory alignment improves product availability and reduces risks, thereby complementing promotional efforts [39];
- Product innovation remains crucial for differentiation and value creation, particularly when paired with promotion and process enhancements [34];
- Price, distribution, and organization are important support dimensions, but with lower immediate priority; these should be managed after the top three priorities, or used selectively based on firm size and capability.
Managerial recommendations derived from results:
- Reallocate a larger proportion of innovation budgets to promotion initiatives that are evidence-based, ethically framed, and digitally enabled;
- Launch process-improvement programs targeting regulatory compliance, quality assurance, and supply chain visibility to complement product and promotion strategies;
- Maintain a strong product innovation pipeline focused on affordability, patient-centric features, and sustainable production practices.
Academic contribution:
This study demonstrates how Fuzzy AHP can be used to generate expert-informed, reproducible priority rankings for innovation strategy in regulated sectors such as pharmaceuticals. The approach contributes to the literature on strategic innovation prioritization by integrating expert judgment under uncertainty with quantitative weighting methods.
4. Discussion
The prioritization results provide clear guidance on which innovation strategies pharmaceutical firms should emphasize to support sustainable growth in Indonesia. Promotion innovation is identified as the highest-priority dimension, consistent with previous literature emphasizing the central role of stakeholder engagement and communication in realizing the value of innovation [38]. Promotion activities, particularly those focusing on ethical education for healthcare professionals, digital engagement, and transparent value communication, are essential for generating demand and encouraging responsible prescribing practices [39].
Process innovation ranks second, aligning with studies that highlight operational efficiency, regulatory compliance, and supply chain resilience as critical foundations of competitive advantage [34]. Strengthening internal processes reduces risk, ensures product availability, and reinforces promotional efforts by enabling reliable and consistent delivery to the market.
Product innovation, ranked third, confirms the continuing need for differentiation through formulation improvements, packaging innovation, and patient-centric solutions [40]. While promotion and process improvements facilitate market access, product-level innovations provide the tangible value that drives adoption and loyalty.
Price, distribution, and organization received lower weights than the top three dimensions. This pattern suggests that, although these areas remain important, they are perceived as less urgent in the Indonesian market context. This finding contrasts with evidence from developed markets, where pricing strategies and organizational innovation often serve as primary competitive levers [41]. The difference likely reflects Indonesia’s regulatory constraints and universal health coverage framework, which limit pricing flexibility and emphasize equitable access and operational reliability.
The empirical findings can be interpreted through the Resource-Based View (RBV), suggesting that firms in highly regulated sectors achieve sustainable competitive advantage by leveraging distinctive internal resources such as marketing knowledge, stakeholder engagement, and operational agility [8,9]. These findings are consistent with broader evidence that firms in emerging economies develop context-adaptive innovation capabilities to overcome institutional and market constraints [42]. Within Indonesia’s regulatory and cultural environment, these priorities reflect the critical importance of ethical promotion practices, compliance-oriented communication, and adaptive operational processes that align with national health policy objectives [42,43]. Compared with other emerging markets, where institutional maturity and digital transformation progress vary, the relative emphasis on promotion and process innovation may differ in both scope and strategic focus [44]. This underscores the context-specific nature of sustainable marketing innovation in Indonesia’s pharmaceutical sector.
Overall, these findings not only clarify the strategic priorities for sustainable marketing innovation but also demonstrate how Fuzzy AHP can serve as a methodological bridge linking empirical prioritization with theoretical constructs under the Resource-Based View (RBV). This conceptual connection is further elaborated in the following section.
4.1. Implications for Theory
This study advances theoretical understanding of strategic innovation prioritization by explicitly linking expert judgment and fuzzy multi-criteria decision-making to the Resource-Based View (RBV) of the firm. The RBV argues that firms achieve competitive advantage through the development and deployment of valuable, rare, inimitable, and non-substitutable (VRIN) resources [35,36]. By applying Fuzzy AHP, the study demonstrates how expert knowledge, an intangible but strategic resource, can be systematically captured and transformed into reproducible priority rankings, thereby operationalizing RBV principles in innovation strategy research. This result is consistent with the literature that emphasizes collaborative alliances among drug discovery firms, biotechnology partners, and academic institutions as crucial mechanisms for building VRIN resources and accelerating sustainable innovation outcomes [37].
The use of Fuzzy AHP also addresses a key gap in the literature by incorporating uncertainty in expert preferences, which conventional crisp AHP does not capture [15,19]. This advantage is particularly relevant in the pharmaceutical industry, where decision contexts involve high uncertainty and multiple stakeholders.
The prioritization outcome, which places promotion and process as the top-ranked dimensions, provides empirical support for the Oslo Manual’s framework that market-facing innovations (product design, promotion, distribution, and pricing) are critical drivers of competitive performance, especially in highly regulated and price-constrained markets [6,41]. Similar findings appear in the sustainability literature, where marketing and process innovations accelerate progress toward environmental and social performance goals [1,22,23,45].
Overall, these findings reinforce the theoretical proposition that, in emerging pharmaceutical markets, prioritizing innovations that directly engage stakeholders and enhance operational resilience generates superior short-term impact and lays the groundwork for long-term sustainable advantage [34,38,39,40].
4.2. Implications for Practice
The findings offer actionable guidance for pharmaceutical managers and policymakers designing innovation strategies in emerging markets. First, the prioritization results indicate that resources should be allocated preferentially to promotion initiatives that strengthen stakeholder engagement, provide ethical education for healthcare professionals, expand digital marketing channels, and communicate product value transparently [38,39]. These activities are critical for generating responsible demand, ensuring appropriate medicine use, and building trust between industry and the medical community.
Second, process improvement programs should be implemented to reinforce supply chain resilience, ensure regulatory compliance, and strengthen quality assurance systems [34]. Reliable product availability supports promotional efforts and reduces the risk of shortages or compliance breaches that can damage reputation and sales.
Third, investment in product innovation must remain an ongoing priority. Improvements in formulation, packaging, and patient-centric features create differentiation and sustained value in competitive markets [22,23,46,47]. By simultaneously reinforcing promotion, process, and product innovation, firms can develop an integrated innovation strategy that delivers superior market and social outcomes. In addition, leveraging outbound open-innovation approaches such as out-licensing deals can improve commercialization efficiency and accelerate market access for novel therapies, supporting both business growth and public health goals [11].
Finally, managers should monitor price, distribution, and organizational innovation as supporting dimensions, applying targeted interventions only when these directly complement the top three priorities. This staged approach promotes efficient use of innovation budgets and aligns with Indonesia’s regulatory and reimbursement environment, where pricing flexibility is limited [6,40].
4.3. Limitations and Future Research
This study is subject to several limitations that merit acknowledgment. First, the findings are derived from the judgments of a selected expert panel, which may not fully represent the breadth of perspectives within Indonesia’s pharmaceutical ecosystem. Broadening the respondent base to include policymakers, hospital procurement officers, and patient advocacy groups would enhance both representativeness and analytical robustness. Second, the cross-sectional research design constrains the ability to observe temporal shifts in innovation priorities. Future studies could employ a longitudinal approach that links Fuzzy AHP-derived priorities with empirical indicators such as sales performance, prescription behavior, and market outcomes to validate predictive capability. Subsequent investigations might also explore how outbound open-innovation strategies and desorptive capabilities mediate the relationship between prioritized innovation dimensions and firm performance, drawing on frameworks established for biopharmaceutical outbound strategies [11].
Third, although this study applies Fuzzy AHP to generate priority rankings, complementary analytical techniques are needed to uncover causal pathways and hierarchical dependencies among innovation factors. Fuzzy Interpretive Structural Modeling (ISM) can be employed to map inter-factor linkages [48], whereas Fuzzy ISM–MICMAC and DEMATEL analyses help identify driver and dependent variables that most strongly influence innovation-strategy formulation [45,49,50]. Integrating these perspectives would yield a more holistic decision-support framework for both managers and policymakers. Comparative studies across different emerging markets would further clarify whether the prioritization pattern observed in Indonesia is context-specific or generalizable.
Finally, future research could incorporate additional multi-criteria decision-making techniques—such as Fuzzy DEMATEL or Structural Equation Modeling (SEM-PLS)—to investigate directional influences and causal interdependencies among innovation dimensions, thereby enriching causal inference and strengthening the managerial relevance of the findings [48,51]. Comparative analyses across emerging markets would further clarify whether the prioritization pattern observed in Indonesia is context-specific or broadly generalizable.
5. Conclusions
This study developed and validated a decision-support model for prioritizing marketing innovation strategies in the Indonesian pharmaceutical industry. Using Fuzzy AHP, expert judgments were systematically captured, defuzzified, and normalized to derive reproducible priority weights for six innovation dimensions: promotion, process, product, price, distribution, and organization. The results indicate that promotion holds the highest priority weight, followed by process and product innovation, suggesting that market-facing and operational improvements are the most impactful levers for short-term performance gains in highly regulated and price-constrained environments [6,23,38,39,46]. The robustness of these findings was further verified through a sensitivity analysis. When the fuzzification scale was adjusted by ±10%, the resulting priority weights and ranking order of the six innovation dimensions remained unchanged. This stability confirms the internal consistency and reliability of the Fuzzy AHP model, ensuring that the conclusions drawn are not sensitive to minor variations in expert input or parameter assumptions.
By linking these findings to the Resource-Based View (RBV) [35,36], the study provides empirical evidence that intangible resources such as expert knowledge and stakeholder engagement capabilities constitute valuable, rare, inimitable, and non-substitutable (VRIN) assets that drive competitive advantage. These results reinforce the Oslo Manual’s proposition that marketing-related innovations and process efficiency are critical drivers of competitiveness, particularly in emerging economies [6].
From a practical perspective, the study recommends that pharmaceutical firms allocate a larger share of innovation budgets to promotion initiatives, including ethical education for healthcare professionals, digital engagement channels, and stakeholder communication campaigns, while also implementing process improvement programs to strengthen supply chain resilience and regulatory compliance [34,38,39]. Empirical evidence from the drug discovery sector suggests that strategic alliances and in-licensing partnerships accelerate the translation of research into sustainable commercial innovations, making external collaboration a complementary means of implementing promotion and process innovations in pharmaceutical firms [37]. At the same time, firms should maintain a continuous product innovation pipeline to ensure differentiation and long-term competitiveness [22,23].
5.1. Theoretical and Managerial Contributions
Theoretically, this research advances the literature by integrating fuzzy multi-criteria decision-making techniques with the Resource-Based View (RBV), demonstrating how uncertainty in expert preferences can be incorporated into strategic innovation prioritization models [15,19,36]. It also contributes to sustainability research by illustrating that marketing and process innovations can accelerate responsible market development and enhance health outcomes [3,22,23].
From a managerial perspective, the findings provide an evidence-based framework for decision-makers to allocate resources across innovation dimensions in a manner consistent with Indonesia’s regulatory and reimbursement context. This structured approach supports sustainable business growth by improving patient access, strengthening trust among healthcare professionals, and reducing operational risks.
5.2. Limitations and Future Directions
This study is not without limitations. The expert panel sample size, while adequate for Fuzzy AHP analysis, may not fully represent the diversity of stakeholder perspectives across Indonesia’s pharmaceutical ecosystem. Future research should broaden the respondent pool to include policymakers, hospital procurement officers, and patient advocates to enhance representativeness. In addition, the cross-sectional design limits the ability to observe changes in innovation priorities over time. Longitudinal studies that link Fuzzy AHP rankings to actual sales and market performance would strengthen causal inference. Future research could also integrate Fuzzy ISM, MICMAC, and DEMATEL techniques to model causal relationships and interdependencies among innovation dimensions [52]. Comparative studies across ASEAN markets would further clarify whether the prioritization pattern identified in this study is generalizable or context-specific.
Author Contributions
Conceptualization: Z.; methodology: Z., R.H., Z.A. and T.N.; validation: R.H. and Z.A.; formal analysis: Z.; investigation: Z.; resources: Z.; data curation: R.H., Z.A. and T.N.; writing—original draft preparation: Z.; writing—review and editing: Z., R.H., Z.A. and T.N.; visualization: Z.; supervision: R.H., Z.A. and T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study is waived for ethical review as according to the institutional policies of the School of Business, IPB University, this type of anonymous, minimal-risk survey research does not involve sensitive ethical issues by Institution Committee.
Informed Consent Statement
Informed consent for participation was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Part 1: Instructions for Completing the Questionnaire
You will be asked to compare two marketing innovation dimensions at a time (e.g., Process Innovation vs. Product Innovation) based on their relative importance in improving pharmaceutical marketing performance.
Please use the nine-point scale below to indicate your judgment:
| Scale | Description |
| 1 | Both dimensions are equally important |
| 3 | Dimension A is slightly more important than Dimension B |
| 5 | Dimension A is more important than Dimension B |
| 7 | Dimension A is much more important than Dimension B |
| 9 | Dimension A is extremely more important than Dimension B |
| 1/3, 1/5, 1/7, 1/9 | Used when Dimension B is more important than Dimension A |
Guidelines:
- Select only one value per comparison;
- Answer all questions;
- There are 15 comparison items in total;
- Please base your answers on your professional knowledge, experience, and judgment.
Part 2: Expert Questionnaire Form
The following table presents the pairwise comparison items between six dimensions of marketing innovation based on the Fuzzy AHP method. Please indicate your judgment by marking the appropriate box corresponding to the relative importance between each pair of dimensions.
| No. | Dimension A | Dimension B | 1/9 | 1/7 | 1/5 | 1/3 | 1 | 3 | 5 | 7 | 9 |
| 1 | Process Innovation | Product Innovation | □ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 2 | Process Innovation | Organizational Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 3 | Process Innovation | Price Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 4 | Process Innovation | Promotion Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 5 | Process Innovation | Distribution Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 6 | Product Innovation | Organizational Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 7 | Product Innovation | Price Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 8 | Product Innovation | Promotion Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 9 | Product Innovation | Distribution Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 10 | Organizational Innovation | Price Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 11 | Organizational Innovation | Promotion Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 12 | Organizational Innovation | Distribution Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 13 | Price Innovation | Promotion Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 14 | Price Innovation | Distribution Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
| 15 | Promotion Innovation | Distribution Innovation | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ | ☐ |
References
- Chomać-Pierzecka, E. Innovation as an Attribute of the Sustainable Development of Pharmaceutical Companies. Sustainability 2025, 17, 2417. [Google Scholar] [CrossRef]
- BPOM. Laporan Tahunan Badan POM 2023; BPOM RI: Jakarta, Indonesia, 2023; Available online: https://pusakom.pom.go.id/report/download/laporan-tahunan-2023 (accessed on 4 October 2025).
- Kementerian Kesehatan RI. Profil Kesehatan Indonesia Tahun 2023; Kementerian Kesehatan RI: Jakarta, Indonesia, 2023; Available online: https://repository.kemkes.go.id/book/1276 (accessed on 4 October 2025).
- Kotler, P.; Keller, K.L. Marketing Management, 16th ed.; Pearson: London, UK, 2022. [Google Scholar]
- Kayan, F.; Bilişli, Y.; Kayakuş, M.; Yiğit Açıkgöz, F.; Başdeğirmen, A.; Güler, M. Analysing Sustainability and Green Energy with Artificial Intelligence: A Turkish English Social Media Perspective. Sustainability 2025, 17, 1882. [Google Scholar] [CrossRef]
- OECD/Eurostat. Oslo Manual 2018: Guidelines for Collecting, Reporting and Using Data on Innovation, 4th ed.; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Kikuchi, S.; Kodama, K.; Sengoku, S. Characteristics and Classification of Technology Sector Companies in Digital Health for Diabetes. Sustainability 2021, 13, 4839. [Google Scholar] [CrossRef]
- Shakouhi, F.; Tavakkoli-Moghaddam, R.; Baboli, A.; Bozorgi-Amiri, A. A competitive pharmaceutical supply chain under the marketing mix strategies and product life cycle with a fuzzy stochastic demand. Ann. Oper. Res. 2023, 324, 1369–1397. [Google Scholar] [CrossRef]
- Garcia, L.J.; Botura Junior, G.; Plácido da Silva, J.C.R. Innovation and Marketing Strategy: A Systematic Review. Sustainability 2023, 15, e23150. [Google Scholar]
- Barbosa, B. Contemporary Trends in Innovative Marketing Strategies; IGI Global: Hershey, PA, USA, 2024. [Google Scholar]
- Kim, E.; Lee, I.; Kim, H.; Shin, K. Factors Affecting Outbound Open Innovation Performance in Bio-Pharmaceutical Industry—Focus on Out-Licensing Deals. Sustainability 2021, 13, 4122. [Google Scholar] [CrossRef]
- Schumpeter, J.A. The Theory of Economic Development; Harvard University Press: Cambridge, MA, USA, 1934. [Google Scholar]
- Schumpeter, J.A. Capitalism, Socialism and Democracy; Harper & Brothers: New York, NY, USA, 1942. [Google Scholar]
- Cankurtaran, P.; Beverland, M.B. Using Design Thinking to Respond to Crises: B2B Lessons from the 2020 COVID-19 Pandemic. Ind. Mark. Manag. 2020, 88, 255–260. [Google Scholar] [CrossRef]
- Buckley, J.J. Fuzzy Hierarchical Analysis. Fuzzy Sets Syst. 1985, 17, 233–247. [Google Scholar] [CrossRef]
- Chang, D.Y. Applications of the Extent Analysis Method on Fuzzy AHP. Eur. J. Oper. Res. 1996, 95, 649–655. [Google Scholar] [CrossRef]
- Hosseinzadeh Lotfi, F.; Allahviranloo, T.; Pedrycz, W.; Shahriari, M.; Sharafi, H.; Razipour GhalehJough, S. Fuzzy Decision Analysis: Multi Attribute Decision Making Approach; Studies in Computational Intelligence; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Ahmed, A.; Pereira, L.; Jane, K. Mixed Methods Research: Combining Both Qualitative and Quantitative Approaches. Mixed Methods Res. 2024. Available online: https://www.researchgate.net/publication/384402328 (accessed on 5 October 2025).
- Saaty, T.L. Decision Making with the Analytic Hierarchy Process. Int. J. Serv. Sci. 2008, 1, 83–98. [Google Scholar] [CrossRef]
- Mardani, A.; Nilashi, M.; Zavadskas, E.K.; Awang, S.R.; Zakuan, N.; Ikram, M.; Tan, K.S. Fuzzy AHP and Fuzzy Methods for Supplier Selection: A Case Study in a Manufacturing Company. J. Manuf. Technol. Manag. 2017, 28, 292–308. [Google Scholar]
- Liu, H.C.; You, J.X.; Fan, X.J.; Lin, Q.L. Failure Mode and Effect Analysis Using D Numbers and Grey Relational Projection Method. Expert Syst. Appl. 2014, 41, 4670–4679. [Google Scholar] [CrossRef]
- Anzules-Falcones, W.; Novillo-Villegas, S. Innovation Capacity, Entrepreneurial Orientation, and Flexibility: An Analysis from Industrial SMEs in Ecuador. Sustainability 2023, 15, 10321. [Google Scholar] [CrossRef]
- Ievseitseva, O.; Mihalatii, O. The Importance of Marketing Innovations as the Basis of Management of the Enterprise’s Competitiveness. Management 2023, 38, 85–95. [Google Scholar] [CrossRef]
- Zadeh, L.A. Fuzzy Sets. Inf. Control 1965, 8, 338–353. [Google Scholar] [CrossRef]
- Vinodh, S.; Ramiya, R.A.; Gautham, S.G. Application of Fuzzy Analytic Network Process for Supplier Selection in a Manufacturing Organization. Expert Syst. Appl. 2011, 38, 272–280. [Google Scholar] [CrossRef]
- Taherdoost, H.; Madanchian, M. Multi-Criteria Decision Making (MCDM) Methods and Concepts. Encyclopedia 2023, 3, 77–87. [Google Scholar] [CrossRef]
- Trzaskalik, T.; Wachowicz, T. (Eds.) Multiple Criteria Decision Making ’10–11; The University of Economics in Katowice: Katowice, Poland, 2011; ISBN 978-83-7246-722-5. [Google Scholar]
- Ashek-Al-Aziz, M.; Mahmud, S.; Islam, M.A.; Al Mahmud, J.; Khan, M.H. A Comparative Study of AHP and Fuzzy AHP Method for Inconsistent Data. Int. J. Sci. Basic Appl. Res. 2020, 54, 16–37. [Google Scholar]
- Rane, N.L.; Choudhary, S.P. Fuzzy AHP and Fuzzy TOPSIS as an Effective and Powerful Multi-Criteria Decision-Making (MCDM) Method for Subjective Judgements in Selection Process. Int. Res. J. Mod. Eng. Technol. Sci. 2023, 5, 3786–3799. [Google Scholar] [CrossRef]
- Okfalisa, O.; Rusnedy, H.; Iswavigra, D.U.; Pranggono, B.; Haerani, E.H.; Saktioto, S. Decision Support System for Smartphone Recommendation: The Comparison of Fuzzy AHP and Fuzzy ANP in Multi-Attribute Decision Making. SINERGI 2021, 25, 101–110. [Google Scholar] [CrossRef]
- Nazim, M.; Chaudhary Wali Mohammad, C.W.; Sadiq, M. A comparison between fuzzy AHP and fuzzy TOPSIS methods to software requirements selection. Alex. Eng. J. 2022, 61, 10851–10870. [Google Scholar] [CrossRef]
- Van Laarhoven, P.J.M.; Pedrycz, W. A Fuzzy Extension of Saaty’s Priority Theory. Fuzzy Sets Syst. 1983, 11, 229–241. [Google Scholar] [CrossRef]
- Liu, Y.; Eckert, C.M.; Earl, C. A Review of Fuzzy AHP Methods for Decision-Making with Subjective Judgements. Expert Syst. Appl. 2020, 161, 113738. [Google Scholar] [CrossRef]
- Damle, M.; Krishnamoorthy, B. Identifying Critical Drivers of Innovation in the Pharmaceutical Industry Using TOPSIS Method. MethodsX 2022, 9, 101677. [Google Scholar] [CrossRef]
- Wernerfelt, B. A Resource-Based View of the Firm. Strateg. Manag. J. 1984, 5, 171–180. [Google Scholar] [CrossRef]
- Barney, J.B. Firm Resources and Sustained Competitive Advantage. J. Manag. 1991, 17, 99–121. [Google Scholar] [CrossRef]
- Harada, Y.; Wang, H.; Kodama, K.; Sengoku, S. Drug Discovery Firms and Business Alliances for Sustainable Innovation. Sustainability 2021, 13, 3599. [Google Scholar] [CrossRef]
- Afuah, A. Strategic Innovation: New Game Strategies for Competitive Advantage; Routledge: New York, NY, USA, 2009. [Google Scholar]
- Baker, W.E.; Sinkula, J.M. Market Orientation, Learning Orientation and Product Innovation: Delving into the Organization’s Black Box. J. Mark.-Focus. Manag. 2002, 5, 5–23. [Google Scholar] [CrossRef]
- Jung, Y.L.; Yoo, H.S. Competition and Pharmaceutical Innovation: The Moderating Role of Size and Age of Leading Companies in the Market. IEEE Trans. Eng. Manag. 2022, 71, 3088–3097. [Google Scholar] [CrossRef]
- Moreno, S.G.; Epstein, D. The Price of Innovation—The Role of Drug Pricing in Financing Pharmaceutical Innovation: A Conceptual Framework. J. Market Access Health Policy 2019, 7, 1583536. [Google Scholar] [CrossRef]
- Ahmed, P.; Shepherd, C. Innovation Management: Context, Strategies, Systems and Processes, 1st ed.; FT Publishing International: Harlow, UK, 2013; Available online: https://www.pearson.com/en-gb/subject-catalog/p/Shepherd-Innovation-Management-Context-strategies-systems-and-processes/P200000003567 (accessed on 29 October 2025).
- Na, K.; Kang, Y.-H. Relations between Innovation and Firm Performance of Manufacturing Firms in Southeast Asian Emerging Markets: Empirical Evidence from Indonesia, Malaysia, and Vietnam. J. Open Innov. Technol. Mark. Complex. 2019, 5, 98. [Google Scholar] [CrossRef]
- Boso, N.; Story, V.M.; Cadogan, J.W. Entrepreneurial Orientation, Market Orientation, Network Ties, and Performance: Study of Entrepreneurial Firms in a Developing Economy. J. Bus. Ventur. 2013, 28, 708–727. [Google Scholar] [CrossRef]
- Yang, G.; Liu, F.; Singhdong, P. Exploring the Impacts of Green Supply Chain Integration and Ambidextrous Green Innovation on the Financial Performance of China’s Pharmaceutical Manufacturing Enterprises. Sustainability 2024, 16, 6501. [Google Scholar] [CrossRef]
- Chomać-Pierzecka, E. Pharmaceutical Companies in the Light of the Idea of Sustainable Development—An Analysis of Selected Aspects of Sustainable Management. Sustainability 2023, 15, 8889. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Velidandi, A. An Overview on the Role of Government Initiatives in Nanotechnology Innovation for Sustainable Economic Development and Research Progress. Sustainability 2025, 17, 1250. [Google Scholar] [CrossRef]
- Attri, R.; Dev, N.; Sharma, V. Interpretive Structural Modelling (ISM) Approach: An Overview. Res. J. Manag. Sci. 2013, 2, 3–8. [Google Scholar]
- Shanker, S.; Barve, A. Analysing Sustainable Concerns in Diamond Supply Chain: A Fuzzy ISM–MICMAC and DEMATEL Approach. Int. J. Sustain. Eng. 2021, 14, 1269–1285. [Google Scholar] [CrossRef]
- Sharma, A.; Abbas, H.; Siddiqui, M.Q. Modelling the Inhibitors of Cold Supply Chain Using Fuzzy Interpretive Structural Modeling and Fuzzy MICMAC Analysis. PLoS ONE 2021, 16, e0249046. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.I.; Chen, W.C.; Chang, C.J. A Fuzzy AHP and BSC Approach for Evaluating Performance of IT Department in the Manufacturing Industry in Taiwan. Expert Syst. Appl. 2008, 34, 96–107. [Google Scholar] [CrossRef]
- Ajmera, P.; Jain, V. A Fuzzy Interpretive Structural Modeling Approach for Evaluating the Factors Affecting Lean Implementation in Indian Healthcare Industry. Int. J. Lean Six Sigma 2020, 11, 376–397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).