Evaluation of the Quality and Nutritional Value of Modified Corn Wet Distillers’ Grains Plus Solubles (mcWDGS) Preserved in Aerobic and Anaerobic Conditions
Abstract
1. Introduction
2. Materials and Methods
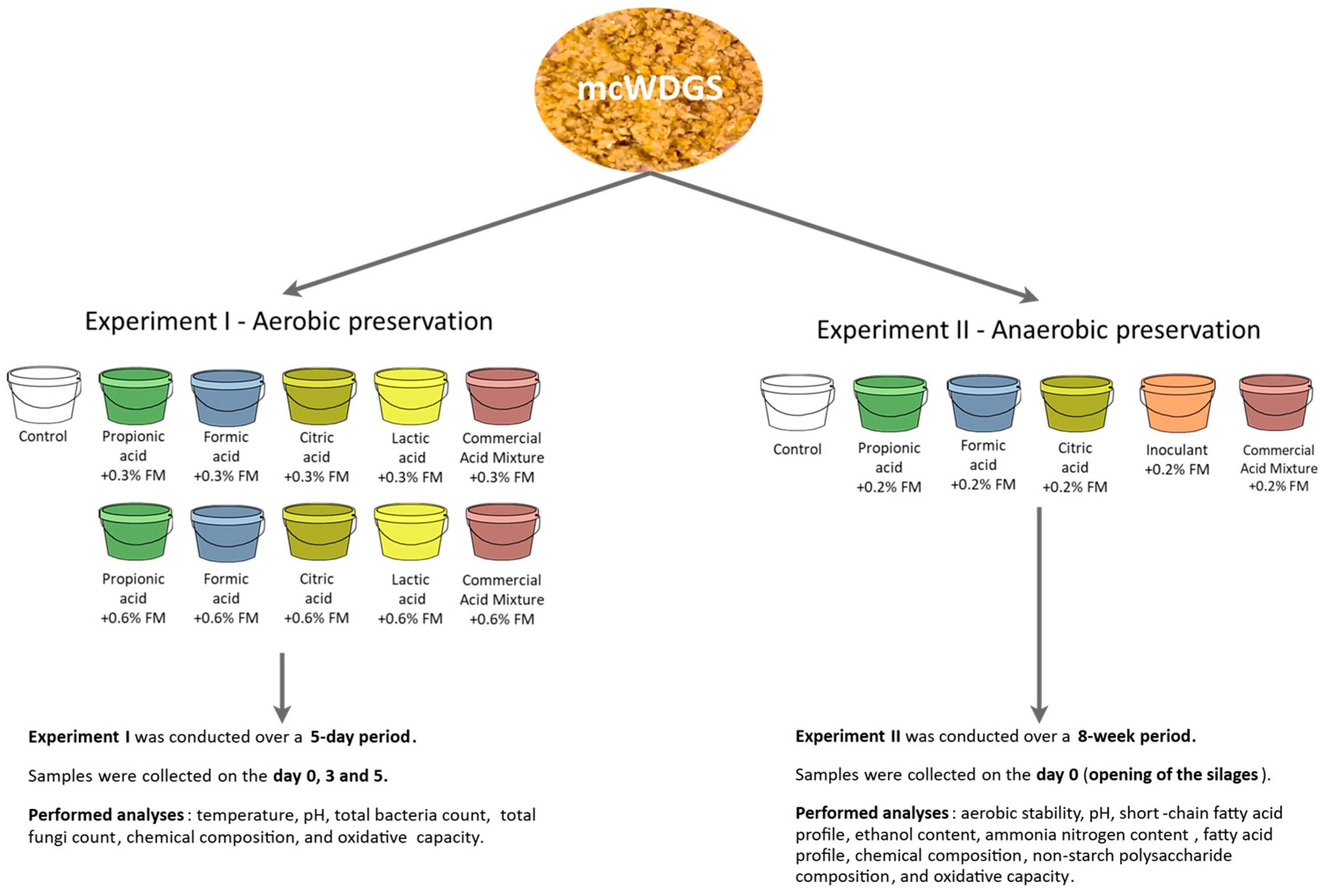
2.1. Experimental Design and Research Procedures
2.2. Chemical and Physical Analyses
2.2.1. Temperature Measurements and Aerobic Stability
2.2.2. pH Level
2.2.3. Microbial Analyses
2.2.4. Short-Chain Fatty Acid Composition and Lactic Acid, Ethanol, and Ammonia Nitrogen Content
2.2.5. Chemical Composition
2.2.6. Non-Starch Polysaccharide Composition
2.2.7. Fatty Acid Composition
2.2.8. Oxidative Capacity
2.3. Statistical Analysis
3. Results
3.1. The First Experiment—Aerobic Storage of mcWDGS
3.1.1. Temperature Measurements
3.1.2. pH Measurements
3.1.3. Microbial Analyses
3.1.4. Chemical Composition
3.1.5. Oxidative Capacity
3.2. The Second Experiment—Anaerobic Storage (Ensiling) of mcWDGS
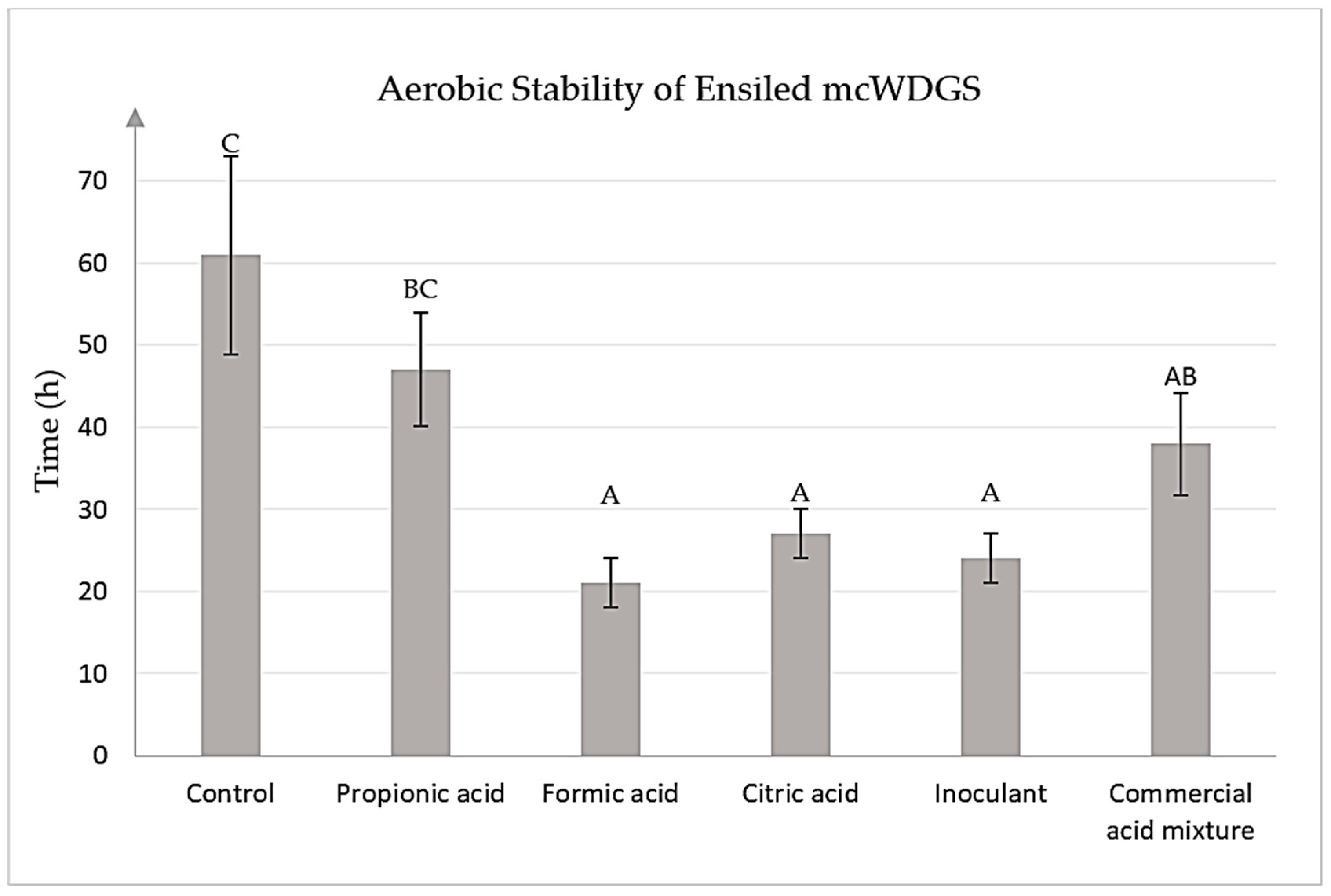
3.2.1. Aerobic Stability
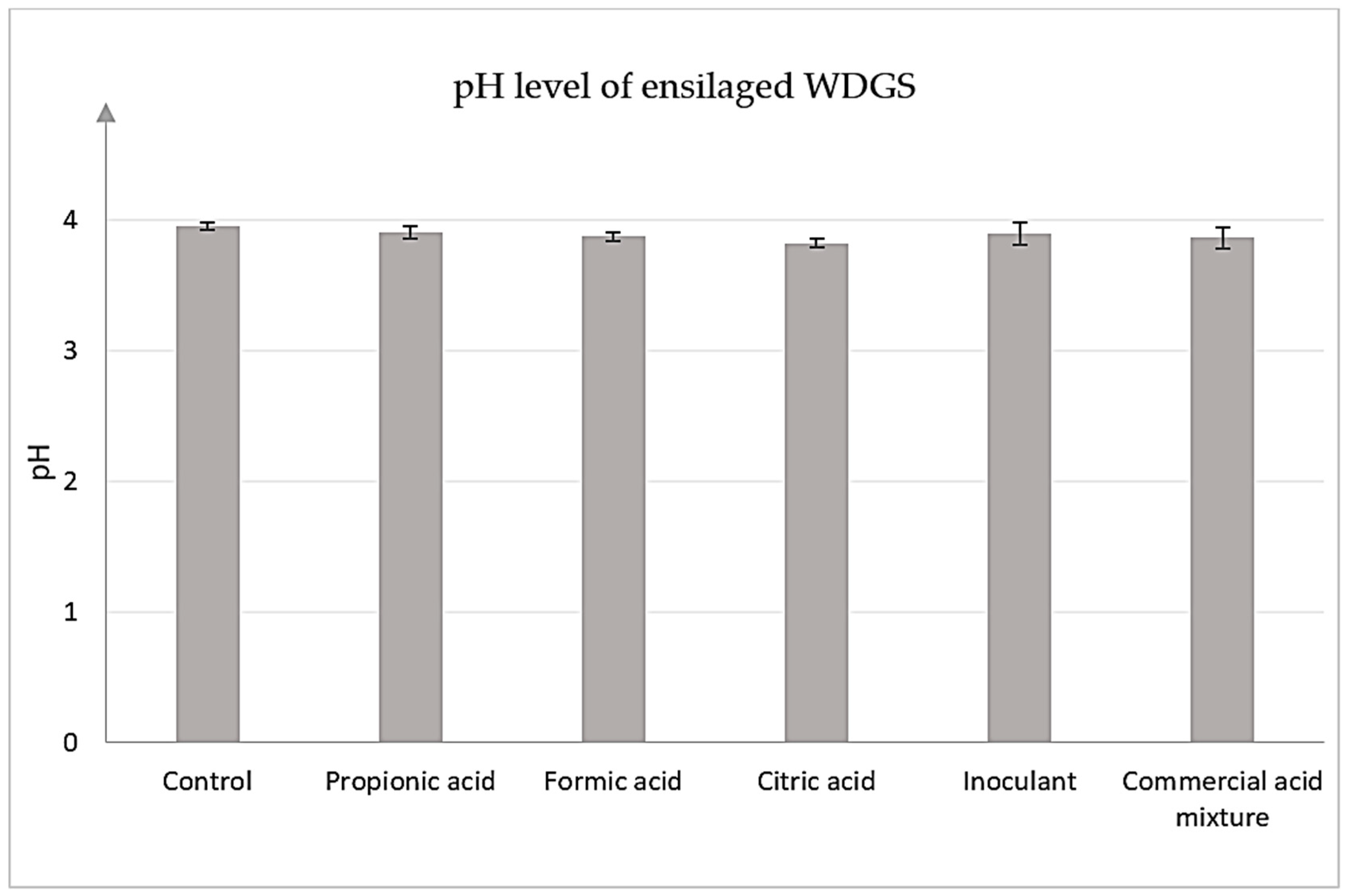
3.2.2. pH Measurement
3.2.3. Short-Chain Fatty Acid Composition and Lactic Acid and Ethanol Content
3.2.4. Chemical Composition
3.2.5. Non-Starch Polysaccharide Composition
3.2.6. Fatty Acid Profile
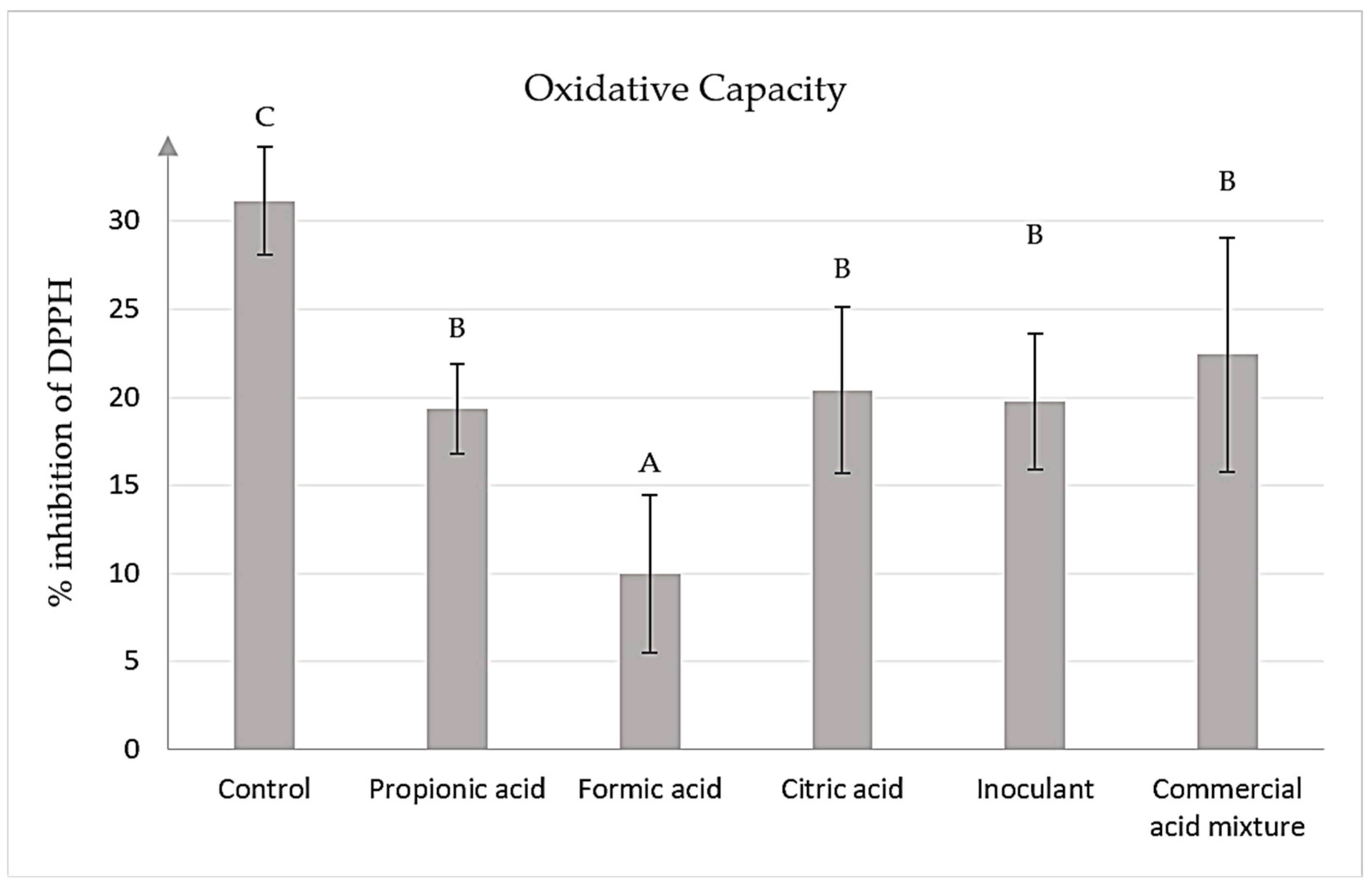
3.2.7. Oxidative Capacity
4. Discussion
4.1. Aerobic Preservation of mcWDGS
4.2. Anaerobic Preservation of mcWDGS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NNCF | non-normative fraction of corn |
| CCDS | condensed corn distillers solubles |
| WDG | wet distillers’ grains |
| WDGS | wet distillers’ grains plus solubles |
| mcWDGS | wet distillers’ grains + condensed corn distillers solubles + non-normative fraction of corn mixture |
| DDG | dried distillers’ grains |
| DDGS | dried distillers’ grains plus solubles |
| N/A | not applicable |
| SEM | standard error of the mean |
| TBC | total bacteria count |
| TFC | total fungal count |
| CFU | colony-forming unit |
| FM | fresh matter |
| DM | dry matter |
| NDF | neutral detergent fiber |
| ADF | acid detergent fiber |
| WSC | water-soluble carbohydrate |
| LA/AA | lactic acid-to-acetic acid ratio |
| NH3-N | ammonia nitrogen |
| SFA | saturated fatty acid |
| UFA | unsaturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
References
- Yang, K.; Qing, Y.; Yu, Q.; Tang, X.; Chen, G.; Fang, R.; Liu, H. By-Product Feeds: Current Understanding and Future Perspectives. Agriculture 2021, 11, 207. [Google Scholar] [CrossRef]
- EL-Mesery, H.S.; El-khawaga, S.E. Drying process on biomass: Evaluation of the drying performance and energy analysis of different dryers. Case Stud. Therm. Eng. 2022, 33, 101953. [Google Scholar] [CrossRef]
- Urbano, D.G.; Aquino, A.; Scrucca, F. Energy Performance, Environmental Impacts and Costs of a Drying System: Life Cycle Analysis of Conventional and Heat Recovery Scenarios. Energies 2023, 16, 1523. [Google Scholar] [CrossRef]
- Amândio, M.S.T.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Bioethanol from Wastes for Mobility: Europe on the Road to Sustainability. In Clean Fuels for Mobility; Energy, Environment, and Sustainability; Di Blasio, G., Agarwal, A.K., Belgiorno, G., Shukla, P.C., Eds.; Springer: Singapore, 2022; pp. 97–123. [Google Scholar] [CrossRef]
- Shad, Z.M.; Venkitasamy, C.; Wen, Z. Corn distillers dried grains with solubles: Production, properties, and potential uses. Cereal Chem. 2021, 98, 999–1019. [Google Scholar] [CrossRef]
- Troyer, B.C.; Dennis, E.J.; DiCostanzo, A.; Erickson, G.E. Pooled analysis on the effects of inclusion, moisture and oil removal from distillers grains on cattle performance and economic returns in diets with different corn processing. J. Anim. Sci. 2023, 101, skac358. [Google Scholar] [CrossRef] [PubMed]
- Probst, K.V.; Ileleji, K.E.; Ambrose, R.P.K.; Clementson, C.L.; Garcia, A.A.; Ogden, C.A. The effect of condensed distillers solubles on the physical and chemical properties of maize distillers dried grains with solubles (DDGS) using bench scale experiments. Biosyst. Eng. 2013, 115, 221–229. [Google Scholar] [CrossRef]
- Cihacek, L.; Alghamdi, R. Some Thoughts on Nutrient Mineraliztion and Cycling in No-Till Systems. North Cent. Ext.-Ind. Soil Fertil. Conf. 2021, 51, 33–37. Available online: https://northcentralfertility.com/files/NCSFC_2021_Proceedings.pdf (accessed on 25 July 2025).
- Majoni, S.; Wang, T. Characterization of Oil Precipitate and Oil Extracted from Condensed Corn Distillers Solubles. J. Am. Oil Chem. Soc. 2010, 87, 205–213. [Google Scholar] [CrossRef]
- Costa, S.I.F.R.; Stringhini, J.H.; Ribeiro, A.M.L.; Pontalti, G.; MacManus, C. Utilization of Different Corn Fractions by Broilers. Rev. Bras. De Ciência Avícola 2015, 17, 307–312. [Google Scholar] [CrossRef]
- Gawande, S.B.; Patil, I.D. Experimental Investigation and Optimization for Production of Bioethanol from Damaged Corn Grains. Mater. Today Proc. 2018, 5, 1509–1517. [Google Scholar] [CrossRef]
- Dennis, E.; Gertner, D.; Erickson, G. Economic Research on Ethanol Feed-Use Coproducts: A Review, Synthesis, and Path Forward. Animals 2024, 14, 1551. [Google Scholar] [CrossRef]
- Malvandi, A.; Coleman, D.; Loor, J.J.; Feng, H. A novel sub-pilot-scale direct-contact ultrasonic dehydration technology for sustainable production of distillers dried grains (DDG). Ultrason. Sonochem. 2022, 85, 105982. [Google Scholar] [CrossRef]
- Lehman, R.M.; Rosentrater, K.A. Aerobic stability of distillers wet grains as influenced by temperature. J. Sci. Food Agric. 2012, 93, 498–503. [Google Scholar] [CrossRef]
- Rosentrater, K.A.; Yang, L. Toward an Understanding of Physical and Biological Properties of Corn-Based Whole Stillage, Thin Stillage, and Condensed Distillers Solubles and Changes Thereof During Storage. Front. Energy Res. 2021, 9, 722950. [Google Scholar] [CrossRef]
- Jaworski, N.W.; Lærke, H.N.; Bach Knudsen, K.E.; Stein, H.H. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J. Anim. Sci. 2015, 93, 1103–1113. [Google Scholar] [CrossRef]
- Salami, S.A.; O’Grady, M.N.; Luciano, G.; Priolo, A.; McGee, M.; Moloney, A.P.; Kerry, J.P. Fatty acid composition, shelf-life and eating quality of beef from steers fed corn or wheat dried distillers’ grains with solubles in a concentrate supplement to grass silage. Meat Sci. 2021, 173, 108381. [Google Scholar] [CrossRef]
- Lehman, R.M.; Rosentrater, K.A. Microbial development in distillers wet grains produced during fuel ethanol production from corn (Zea mays). Can. J. Microbiol. 2007, 53, 1046–1052. [Google Scholar] [CrossRef]
- Bhadra, R.; Rosentrater, K.; Muthukumarappan, K.; Kannadhason, S. Drying Characteristics of Distillers Wet Grains Under Varying Condensed Distillers Solubles and Drying Temperature Levels. Appl. Eng. Agric. 2011, 27, 777–786. [Google Scholar] [CrossRef]
- Roguski, M.; Zielińska-Górska, M.; Bendowski, W.; Niemiec, T.; Piotrowska, K.; Żyłowski, D.; Łozicki, A. Impact of substituting corn silage with ensiled modified corn WDGS on milk yield, composition, cow health, and production economics. J. Anim. Feed. Sci. 2025, 34, 430–441. [Google Scholar] [CrossRef]
- Roguski, M.; Łozicki, A.; Sońta, M.; Bendowski, W.; Niemiec, T.; Zglińska, K.; Zielińska-Górska, M. Effect of Using Ensilaged Corn Wet Distillers’ Grains Plus Solubles (WDGS) as a Partial Replacement for Concentrated Feed for Wet Lot Fed Fatteners during Fattening on Growth Performance, Carcass Characteristics and Pork Quality. Agriculture 2023, 13, 2017. [Google Scholar] [CrossRef]
- Alvarez, E.; Cardoso, M.; Depetris, G.; Castellari, C.; Cristos, D.; Montiel, M.D.; Bartosik, R. Storage of WDGS under hermetic and non-hermetic conditions: Effect on sensory properties, microorganisms, mycotoxins and nutritional value. J. Stored Prod. Res. 2019, 80, 65–70. [Google Scholar] [CrossRef]
- Gunn, P.J.; Buckmaster, D.R.; Lemenager, R.P.; Van Emon, M.L.; Claeys, M.C.; Lake, S.L. Preservation characteristics of modified wet distillers grains with solubles stored with marginal-quality feedstuffs in laboratory-scale mini silos. Prof. Anim. Sci. 2013, 29, 671–676. [Google Scholar] [CrossRef]
- Erickson, G.; Klopfenstein, T.; Rasby, R.; Stalker, A.; Plugge, B.; Bauer, D.; Mark, D.; Adams, D.; Benton, J.; Greenquist, M.; et al. Storage of Wet Corn Co-Products. Fac. Pap. Publ. Anim. Sci. 2008, 506, 1–17. Available online: https://digitalcommons.unl.edu/animalscifacpub/506/ (accessed on 15 May 2025).
- Moriel, P.; Artioli, L.F.A.; Poore, M.H.; Ferraretto, L.F. Dry matter loss and nutritional composition of wet brewers grains ensiled with or without covering and with or without soybean hulls and propionic acid. Prof. Anim. Sci. 2015, 31, 559–567. [Google Scholar] [CrossRef]
- Moriel, P.; Piccolo, M.B.; Artioli, L.F.A.; Santos, G.S.; Poore, M.H.; Ferraretto, L.F. Method of propionic acid–based preservative addition and its effects on nutritive value and fermentation characteristics of wet brewers grains ensiled in the summertime. Prof. Anim. Sci. 2016, 32, 591–597. [Google Scholar] [CrossRef]
- da Silva Dias, M.S.; Ghizzi, L.G.; Marques, J.A.; Nunes, A.T.; Grigoletto, N.T.S.; Gheller, L.S.; Silva, T.B.P.; Silva, G.G.; Lobato, D.N.; e Silva, L.F.C.; et al. Effects of organic acids in total mixed ration and feeding frequency on productive performance of dairy cows. J. Dairy Sci. 2021, 104, 5405–5416. [Google Scholar] [CrossRef]
- Fomina, N.S.; Kovalchuk, V.P.; Vovk, I.M.; Fomin, O.O.; Kovalenko, I.M. Antimicrobial activity assessment of food preservatives containing organic carboxylic acids. Clin. Prev. Med. 2024, 5, 80–86. [Google Scholar] [CrossRef]
- Marques, C.; Sotiles, A.R.; Farias, F.O.; Oliveira, G.; Mitterer-Daltoé, M.L.; Masson, M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arab. J. Chem. 2020, 13, 9118–9129. [Google Scholar] [CrossRef]
- Harding, J.L.; Cornelius, J.E.; Rolfe, K.M.; Shreck, A.L.; Erickson, G.E.; Klopfenstein, T.J. Effect of Storage Method on Nutrient Composition and Dry Matter Loss of Wet Distillers Grains. Neb. Beef Cattle Rep. 2012, 670, 58–60. Available online: https://digitalcommons.unl.edu/animalscinbcr/670 (accessed on 8 July 2025).
- Commission Regulation (EC). No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364, 5–24. Available online: https://eur-lex.europa.eu/eli/reg/2006/1881/oj/eng (accessed on 19 June 2025).
- Kung, L., Jr.; Sheperd, A.C.; Smagala, A.M.; Endres, K.M.; Bessett, C.A.; Ranjit, N.K.; Glancey, J.L. The Effect of Preservatives Based on Propionic Acid on the Fermentation and Aerobic Stability of Corn Silage and a Total Mixed Ration. J. Dairy Sci. 1998, 81, 1322–1330. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.; Li, J.; Wang, S.; Dong, Z.; Shao, T. Effect of lactic acid bacteria and propionic acid on conservation characteristics, aerobic stability and in vitro gas production kinetics and digestibility of whole-crop corn based total mixed ration silage. J. Integr. Agric. 2017, 15, 1592–1600. [Google Scholar] [CrossRef]
- Cunniff, P.; Washington, D. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg/Rockville, MD, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1997, 74, 3583–3597. [Google Scholar] [CrossRef]
- European Commission. Fifth Commission Directive (74/203/EEC) of 25 March 1974 establishing Community methods of analysis for the official control of feedingstuffs. Off. J. Eur. Communities 1974, L 102, 7–24. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31974L0203 (accessed on 15 July 2025).
- Englyst, H.N.; Wiggins, H.S.; Cummings, J.H. Determination of nonstarch polysaccharides in plant food by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Simplified method for the measurement of total nonstarch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984, 109, 937–942. [Google Scholar] [CrossRef]
- PN-EN ISO 12966-1:2015-01; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. British Standards Institution: London, UK, 2014; Technical Committee ISO/TC 34/SC 11.
- PN-EN ISO 12966-2:2017-05 pkt. 5.2; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. British Standards Institution: London, UK, 2017; Technical Committee ISO/TC 34/SC 11.
- PN-EN ISO 12966-4:2015-07; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography. British Standards Institution: London, UK, 2015; Technical Committee ISO/TC 34/SC 11.
- Shahbandeh, M. European Union-27: Distiller’s Dry Grains Production Volume Forecast 2019–2033; Statista: Hamburg, Germany, 2024; Available online: https://www.statista.com/statistics/545737/distillers-dry-grains-production-volume-european-union-28/ (accessed on 10 September 2024).
- Garcia, A.; Kalscheur, K. Ensiling Wet Distillers Grains with Other Feeds. SDSU Ext. Extra 2006, 127, 4029. Available online: http://openprairie.sdstate.edu/extension_extra/127 (accessed on 8 July 2025).
- Kudra, T.; Mujumdar, A. Special Drying Techniques and Novel Dryers. In Handbook of Industrial Drying, 4th ed.; Mujumdar, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 433–489. [Google Scholar] [CrossRef]
- Strohbehn, D.R.; Loy, D.D.; Morrical, D.G.; Seller, J.H.; Maxwell, D. Evaluation of bagging to extend storage life of wet and modified distillers’ grains—A demonstration project. In Iowa State University Animal Industry Report; A.S. Leaflet R2289; Iowa State University: Ames, IA, USA, 2008. [Google Scholar] [CrossRef][Green Version]
- Latham, K.G.; Simone, M.I.; Dose, W.M.; Allen, J.A.; Donne, S.W. Synchrotron based NEXAFS study on nitrogen doped hydrothermal carbon: Insights into surface functionalities and formation mechanisms. Carbon 2017, 114, 566–578. [Google Scholar] [CrossRef]
- Martinez, A.; Vargas, R.; Galano, A. Citric Acid: A Promising Copper Scavenger. Comput. Theor. Chem. 2018, 1133, 47–50. [Google Scholar] [CrossRef]
- Selwet, M. Effect of propionic and formic acid mixtures on the fermentation, fungi development and aerobic stability of maize silage. Pol. J. Agron. 2009, 1, 37–42. [Google Scholar]
- Reed, C.; Doyungan, S.; Ioerger, B.; Getchel, A. Response of storage molds to different initial moisture contents of maize (corn) stored at 25 °C, and effect on respiration rate and nutrient composition. J. Stored Prod. Res. 2007, 43, 443–458. [Google Scholar] [CrossRef]
- Schizodimou, A.; Kotoulas, I.; Kyriacou, G. Electrochemical reduction of formic acid through its decarbonylation in phosphoric acid solution. Electrochim. Acta. 2016, 210, 236–239. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Li, D.-X.; Wang, X.-K.; Lin, Y.-L.; Zhang, Q.; Chen, X.-Y.; Yang, F.-Y. Fermentation quality and aerobic stability of mulberry silage prepared with lactic acid bacteria and propionic acid. Anim. Sci. J. 2019, 90, 513–522. [Google Scholar] [CrossRef]
- Rodrigues, P.H.M.; Pinedo, L.A.; Meyer, P.M.; da Silva, T.H.; da Silva Brandão Guimarães, I.C. Sorghum silage quality as determined by chemical–nutritional factors. Grass Forage Sci. 2020, 75, 462–473. [Google Scholar] [CrossRef]
- da Silva, É.B.; Kung, L., Jr. A Meta-Analysis of the Effects of a Chemical Additive on the Fermentation and Aerobic Stability of Whole-Plant Maize Silage. Agriculture 2022, 12, 132. [Google Scholar] [CrossRef]
- Lyberg, K.; Borling, J.; Lindberg, J.E. Characterization and nutrient evaluation of wet and dried wheat distillers’ grain for growing pigs. Anim. Feed. Sci. Technol. 2013, 186, 45–52. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal Capacity of Poolish-Type Sourdough Supplemented with Lactiplantibacillus plantarum and Its Aqueous Extracts In Vitro and Bread. Antibiotics 2022, 11, 1813. [Google Scholar] [CrossRef]
- Axelsson, L. Lactic Acid Bacteria: Classification and Physiology. In Lactic Acid Bacteria Microbiological and Functional Aspects, 3rd ed.; Revised and Expanded; Salminen, S., von Wright, A., Ouwehand, A., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–66. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Ralton, I. Energy changes during ensilage. J. Sci. Food Agric. 1973, 24, 827–834. [Google Scholar] [CrossRef]
- Auerbach, H.; Theobald, P.; Theobald, B.; Weiss, K. Effects of Various Additives on Fermentation, Aerobic Stability and Volatile Organic Compounds in Whole-Crop Rye Silage. Agronomy 2020, 10, 1873. [Google Scholar] [CrossRef]
- You, S.; Du, S.; Ge, G.; Wan, T.; Jia, Y. Selection of lactic acid bacteria from native grass silage and its effects as inoculant on silage fermentation. Agron. J. 2021, 113, 3169–3177. [Google Scholar] [CrossRef]
- Mao, K.; Franco, M.; Xu, Y.; Chai, H.; Wang, J.; Huang, S.; Wang, Z.; Xun, W.; Liang, Z.; Yu, Z.; et al. Fermentation Parameters, Amino Acids Profile, Biogenic Amines Formation, and Bacterial Community of Ensiled Stylo Treated with Formic Acid or Sugar. Animals 2024, 14, 2397. [Google Scholar] [CrossRef]
- Wei, S.N.; Li, Y.F.; Jeong, E.C.; Kim, H.J.; Kim, J.G. Effects of formic acid and lactic acid bacteria inoculant on main summer crop silages in Korea. J. Anim. Sci. Technol. 2021, 63, 91–103. [Google Scholar] [CrossRef]
- Duncan, Z.M.; DeBord, Z.L.; Pfughoeft, M.G.; Hollenbeck, W.R.; Titgemeyer, E.C.; Olson, K.C.; Blasi, D.A. Effect of wheat middling incorporation into wet corn distillers grains with solubles on apparent diet digestibility and ruminal fermentation characteristics in growing and fnishing diets. Transl. Anim. Sci. 2024, 8, txae083. [Google Scholar] [CrossRef]
- Barnharst, T.; Sun, X.; Rajendran, A.; Urriola, P.; Shurson, G.; Hu, B. Enhanced protein and amino acids of corn–ethanol co-product by Mucor indicus and Rhizopus oryzae. Bioprocess Biosyst. Eng. 2021, 44, 1989–2000. [Google Scholar] [CrossRef]
- Mammi, L.M.E.; Buonaiuto, G.; Ghiaccio, F.; Cavallini, D.; Palmonari, A.; Fusaro, I.; Massa, V.; Giorgino, A.; Formigoni, A. Combined Inclusion of Former Foodstuff and Distiller Grains in Dairy Cows Ration: Effect on Milk Production, Rumen Environment, and Fiber Digestibility. Animals 2022, 12, 3519. [Google Scholar] [CrossRef]
- Gheller, L.S.; Ghizzi, L.G.; Takiya, C.S.; Grigoletto, N.T.S.; Silva, T.B.P.; Marques, J.A.; Dias, M.S.S.; Freu, G.; Rennó, F.P. Different organic acid preparations on fermentation and microbiological profile, chemical composition, and aerobic stability of whole-plant corn silage. Anim. Feed. Sci. Technol. 2021, 281, 115083. [Google Scholar] [CrossRef]
- Kienberger, M.; Weinzettl, C.; Leitner, V.; Egermeier, M.; Demmelmayer, P. (Selective) Isolation of acetic acid and lactic acid from heterogeneous fermentation of xylose and glucose. Chem. Eng. J. Adv. 2023, 16, 100552. [Google Scholar] [CrossRef]
- Veeravalli, S.S.; Mathews, A.P. Continuous fermentation of xylose to short chain fatty acids by Lactobacillus buchneri under low pH conditions. Chem. Eng. J. 2018, 337, 764–771. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Dalsgaard, S.; Bach Knudsen, K.E.; Yu, S.; Lærke, H.N. Compositional profile and variation of Distillers Dried Grains with Solubles from various origins with focus on non-starchpolysaccharides. Anim. Feed Sci. Technol. 2014, 197, 130–141. [Google Scholar] [CrossRef]
- Liu, W.; Si, Q.; Sun, L.; Wang, Z.; Liu, M.; Du, S.; Ge, G.; Jia, Y. Effects of Cellulase and Xylanase Addition on Fermentation Quality, Aerobic Stability, and Bacteria Composition of Low Water Soluble Carbohydrates Oat Silage. Fermentation 2023, 9, 638. [Google Scholar] [CrossRef]
- Lei, Y.; Li, M.; Liu, Y.; Wang, J.; He, X.; Zhao, Y.; Chen, Y.; Cheng, Q.; Chen, C. Lactic Acid Bacteria and Formic Acid Improve Fermentation Quality and Beneficial Predicted Functional Characteristics in Mixed Silage Consisting of Alfalfa and Perennial Ryegrass. Fermentation 2024, 10, 43. [Google Scholar] [CrossRef]
- Jalč, D.; Laukova, A.; Simonova, M.P.; Varadyova, Z.; and Homolka, P. Bacterial inoculant effects on corn silage fermentation and nutrient composition. Asian-Australas. J. Anim. Sci. 2009, 22, 977–983. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; Khattab, M.S.A.; Abd El Tawab, A.M.; Elnahr, M.; Cieślak, A.; Szumacher-Strabel, M. Oil-in-Water Nanoemulsion Can Modulate the Fermentation, Fatty Acid Accumulation, and the Microbial Population in Rumen Batch Cultures. Molecules 2023, 28, 358. [Google Scholar] [CrossRef] [PubMed]
- Garron, A.; Epron, F. Use of formic acid as reducing agent for application in catalytic reduction of nitrate in water. Water Res. 2005, 39, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Omole, M.A.; K’Owino, I.O.; Sadik, O.A. Palladium nanoparticles for catalytic reduction of Cr(VI) using formic acid. Appl. Catal. B Environ. 2007, 76, 158–167. [Google Scholar] [CrossRef]
| Item | Bioethanol By-Products | |||
|---|---|---|---|---|
| WDG | NNCF | CCDS | mcWDGS | |
| Dry Matter (FM) | 412.5 | 906.6 | 792.7 | 435.0 |
| Crude Protein | 238.5 | 68.9 | 242.4 | 247.2 |
| Ether Extract | 79.8 | 34.6 | 132.0 | 69.8 |
| Crude Fiber | 204.2 | 36.4 | 18.0 | 158.0 |
| Starch | 56.1 | 743.0 | 16.2 | 275.5 |
| Crude Ash | 29.6 | 19.0 | 24.7 | 29.1 |
| Treatment Group | Experimental Groups | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | PA0.3% | PA0.6% | FA0.3% | FA0.6% | CA0.3% | CA0.6% | LA0.3% | LA0.6% | CAM0.3% | CAM0.6% | |||
| day 0 | 24.0 | 23.7 | 23.1 | 23.8 | 23.6 | 23.4 | 23.8 | 23.3 | 23.6 | 23.7 | 24.0 | 0.061 | 0.052 |
| 3rd day | 47.8 DE | 31.7 BC | 22.7 A | 22.4 A | 22.7 A | 47.9 DE | 49.7 E | 46.1 D | 50.9 E | 34.2 C | 29.3 B | 1.153 | <0.001 |
| 5th day | 39.8 CD | 26.1 A | 24.1 A | 44.0 D | 24.5 A | 37.8 BC | 42.5 D | 36.1 BC | 39.8 CD | 48.6 E | 33.8 B | 0.832 | <0.001 |
| Treatment Group | Experimental Groups | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | PA0.3% | PA0.6% | FA0.3% | FA0.6% | CA0.3% | CA0.6% | LA0.3% | LA0.6% | CAM0.3% | CAM0.6% | |||
| day 0 | 4.69 F | 4.29 DE | 4.26 DE | 3.75 B | 3.34 A | 4.20 CDE | 4.10 CD | 4.33 E | 4.13 CD | 4.26 DE | 4.00 C | 0.036 | <0.001 |
| 3rd day | 7.2 EF | 5.01 C | 4.50 B | 4.20 AB | 3.79 A | 6.77 DE | 6.89 DE | 7.54 F | 6.98 E | 6.47 D | 4.50 B | 0.138 | <0.001 |
| 5th day | 4.74 AB | 4.74 AB | 4.75 AB | 4.79 B | 4.7 A | 4.71 A | 4.70 A | 4.72 A | 4.68 A | 4.74 AB | 4.71 A | 0.005 | <0.001 |
| Treatment Group | Experimental Groups | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | PA0.3% | PA0.6% | FA0.3% | FA0.6% | CA0.3% | CA0.6% | LA0.3% | LA0.6% | CAM0.3% | CAM0.6% | |||
| day 0 | 4.8 × 102 | N/A | N/A | ||||||||||
| 3rd day | 2 × 104 C | 5 × 103 AB | 3 × 102 A | 2.9 × 102 A | 4 × 101 A | 1.3 × 104 ABC | 1.7 × 104 BC | 2 × 104 C | 8.2 × 103 ABC | 3.4 × 103 AB | 6.7 × 102 A | 1538 | <0.001 |
| 5th day | N/A | 1.3 × 104 b | 5.4 × 103 ab | 1.3 × 103 ab | 2 × 101 a | N/A | N/A | N/A | N/A | 4.8 × 103 ab | 4.2 × 103 ab | 1340 | 0.042 |
| Treatment Group | Experimental Groups | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | PA0.3% | PA0.6% | FA0.3% | FA0.6% | CA0.3% | CA0.6% | LA0.3% | LA0.6% | CAM0.3% | CAM0.6% | |||
| day 0 | 1 × 102 | N/A | N/A | ||||||||||
| 3rd day | 7.7 × 103 BC | 2.5 × 103 AB | 8.5 × 102 A | 3.3 × 101 A | 0 A | 4.8 × 103 AB | 1.4 × 104 C | 3.2 × 103 AB | 4.7 × 103 AB | 5.1 × 103 AB | 2.4 × 102 A | 740 | 0.002 |
| 5th day | N/A | 5.7 × 103 ab | 3.6 × 102 a | 1.9 × 102 a | 2.8 × 102 a | N/A | N/A | N/A | N/A | 7.4 × 103 b | 6.8 × 103 b | 881 | 0.023 |
| Treatment Group | Item | Experimental Groups | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | PA0.3% | PA0.6% | FA0.3% | FA0.6% | CA0.3% | CA0.6% | LA0.3% | LA0.6% | CAM0.3% | CAM0.6% | ||||
| 3rd day | Dry Matter (FM) | 514.6 | 448.2 | 460.3 | 443.7 | 453.7 | 438.0 | 381.0 | 414.8 | 435.3 | 453.6 | 443.0 | 7.260 | 0.051 |
| Crude Protein | 281.7 C | 246.7 AB | 224 A | 235.2 A | 238.2 A | 272.7 BC | 297.7 C | 282.3 C | 274.7 BC | 238.7 A | 233.8 A | 4.515 | 0.002 | |
| Ether Extract | 50.2 | 66.9 | 65.2 | 67.7 | 69.1 | 65.5 | 64.5 | 55.2 | 62.1 | 58.1 | 65.4 | 1.520 | 0.273 | |
| Crude Fiber | 123.7 | 125.0 | 124.1 | 123.8 | 123.1 | 106.1 | 113.7 | 104.4 | 104.6 | 98.9 | 116.7 | 3.184 | 0.372 | |
| Crude Ash | 34.4 C | 28.7 AB | 27.4 A | 27.6 AB | 26.7 A | 31 BC | 29.9 AB | 33.6 C | 33.9 C | 30.0 ABC | 29.4 AB | 0.486 | 0.002 | |
| 5th day | Dry Matter (FM) | N/A | 460.3 | 465.3 | 446 | 465.4 | N/A | N/A | N/A | N/A | 490 | 466.3 | 5.218 | 0.620 |
| Crude Protein | N/A | 245.6 ab | 222.5 a | 238.4 a | 224.8 a | N/A | N/A | N/A | N/A | 269.7 b | 238.7 a | 4.183 | 0.013 | |
| Ether Extract | N/A | 64.4 | 64.6 | 58.2 | 57.5 | N/A | N/A | N/A | N/A | 45 | 65.6 | 2.209 | 0.172 | |
| Crude Fiber | N/A | 95.0 ab | 99.4 b | 92.5 ab | 107.1 b | N/A | N/A | N/A | N/A | 80.7 a | 106.7 b | 2.444 | 0.014 | |
| Crude Ash | N/A | 28.8 B | 26.5 A | 31.6 C | 26.5 A | N/A | N/A | N/A | N/A | 32.3 C | 30 B | 0.555 | 0.008 | |
| Treatment Group | Experimental Groups | SEM | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | PA0.3% | PA0.6% | FA0.3% | FA0.6% | CA0.3% | CA0.6% | LA0.3% | LA0.6% | CAM0.3% | CAM0.6% | |||
| day 0 | 31.09 | N/A | N/A | ||||||||||
| 3rd day | 82.14 CDE | 70.99 B | 65.03 A | 83.61 DE | 86.9 E | 80.18 CD | 79.64 CD | 77.47 C | 79.98 CD | 78.84 CD | 77.75 C | 0.760 | <0.001 |
| 5th day | N/A | 51.62 AB | 50.9 AB | 55.98 B | 70.76 C | N/A | N/A | N/A | N/A | 64.92 C | 46.49 A | 1.584 | <0.001 |
| Treatment Group | Experimental Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| C2 | PA0.2% | FA0.2% | CA0.2% | I0.2% | CAM0.2% | |||
| L-lactic acid | 4.65 BC | 4.19 AB | 4.02 A | 5.81 D | 5.18 C | 5.18 D | 0.122 | <0.001 |
| D-lactic acid | 6.28 C | 5.61 BC | 3.56 A | 6.17 C | 4.83 B | 6.17 C | 0.175 | <0.001 |
| total lactic acid | 10.92 bcd | 9.80 b | 7.57 a | 11.99 d | 10.01 c | 11.95 d | 0.257 | 0.013 |
| acetic acid | 4.77 B | 4.14 AB | 4.19 AB | 4.13 A | 4.12 A | 4.55 AB | 0.067 | 0.003 |
| butyric acid | ND | ND | ND | ND | ND | ND | N/A | N/A |
| ethanol | 0.27 A | 0.06 A | 0.07 A | 0.09 A | 0.06 A | 0.59 B | 0.039 | <0.001 |
| LA/AA | 2.3 AB | 2.4 B | 1.8 A | 2.9 C | 2.4 BC | 2.6 BC | 0.074 | <0.001 |
| NH3-N | 0.037 BC | 0.035 ABC | 0.031 A | 0.034 AB | 0.04 C | 0.037 BC | 0.001 | <0.001 |
| Treatment Group | Experimental Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| C2 | PA0.2% | FA0.2% | CA0.2% | I0.2% | CAM0.2% | |||
| Dry Matter (FM) | 413.1 AB | 416.5 AB | 411.5 A | 425.0 B | 405.2 A | 415.3 AB | 8.098 | 0.004 |
| Crude Protein | 252.0 C | 246.3 BC | 234.0 AB | 235.3 AB | 233.3 A | 244.3 ABC | 1.933 | <0.001 |
| Ether Extract | 83.4 | 87.4 | 82.4 | 84.4 | 84.5 | 86.3 | 0.762 | 0.485 |
| Crude Fiber | 118.0 | 117.0 | 145.0 | 122.5 | 120.3 | 115.3 | 3.752 | 0.192 |
| Crude Ash | 27.9 A | 28.1 A | 30.5 AB | 29.0 A | 32.8 B | 29.8 AB | 0.477 | 0.007 |
| NDF | 337.2 A | 350.8 AB | 378.3 B | 362.1 AB | 339.0 A | 353.9 AB | 3.745 | 0.007 |
| ADF | 184.4 | 192.7 | 180.3 | 192.5 | 184.5 | 188.0 | 1.446 | 0.068 |
| WSC | 6.7 a | 7.6 a | 24.4 b | 6.6 a | 22.6 b | 7.3 a | 2.004 | 0.040 |
| Starch | 252.0 | 253.4 | 212.1 | 229.1 | 262.7 | 216.8 | 6.172 | 0.106 |
| Treatment Group | Experimental Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| C2 | PA0.2% | FA0.2% | CA0.2% | I0.2% | CAM0.2% | |||
| S-NSP | ||||||||
| arabinose | 0.44 | 0.32 | 0.34 | 0.36 | 0.31 | 0.39 | 0.017 | 0.186 |
| xylose | 0.32 A | 0.49 B | 0.47 B | 0.51 B | 0.31 A | 0.37 A | 0.020 | <0.001 |
| mannose | 0.98 | 0.99 | 0.99 | 0.88 | 0.89 | 0.85 | 0.024 | 0.334 |
| galactose | 0.17 | 0.15 | 0.16 | 0.16 | 0.14 | 0.16 | 0.003 | 0.343 |
| glucose | 0.51 b | 0.38 a | 0.39 a | 0.39 a | 0.40 ab | 0.45 ab | 0.014 | 0.014 |
| I-NSP | ||||||||
| arabinose | 4.85 | 4.98 | 4.73 | 4.68 | 4.73 | 4.74 | 0.042 | 0.364 |
| xylose | 7.31 | 7.95 | 7.56 | 7.41 | 7.35 | 7.39 | 0.082 | 0.202 |
| mannose | 0.82 | 0.79 | 0.72 | 0.72 | 0.74 | 0.81 | 0.017 | 0.374 |
| galactose | 1.54 | 1.56 | 1.48 | 1.49 | 1.51 | 1.50 | 0.020 | 0.884 |
| glucose | 9.10 | 9.53 | 8.66 | 8.72 | 8.59 | 9.08 | 0.139 | 0.386 |
| Treatment Group | Experimental Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| C2 | PA0.2% | FA0.2% | CA0.2% | I0.2% | CAM0.2% | |||
| SFA | 12.95 abc | 12.74 a | 13.08 bc | 12.86 abc | 13.14 c | 12.80 ab | 0.041 | 0.043 |
| C16:0 | 10.39 ab | 10.25 a | 10.54 b | 10.37 ab | 10.58 b | 10.30 a | 0.033 | 0.035 |
| C18:0 | 1.76 | 1.70 | 1.74 | 1.70 | 1.76 | 1.71 | 0.009 | 0.091 |
| C20:0 | 0.40 ab | 0.39 a | 0.41 b | 0.40 ab | 0.40 ab | 0.39 a | 0.001 | 0.033 |
| C21:0 | 0.13 | 0.12 | 0.12 | 0.13 | 0.13 | 0.14 | 0.002 | 0.073 |
| C22:0 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.001 | 0.416 |
| C24:0 | 0.15 | 0.15 | 0.15 | 0.15 | 0.16 | 0.15 | 0.001 | 0.149 |
| UFA | 82.25 ab | 82.48 b | 82.16 ab | 82.35 ab | 82.07 a | 82.41 b | 0.041 | 0.045 |
| MUFA | 26.56 | 26.58 | 26.50 | 26.6 | 26.45 | 26.69 | 0.034 | 0.262 |
| C16:1 | 0.13 | 0.12 | 0.12 | 0.12 | 0.13 | 0.13 | 0.002 | 0.556 |
| C18:1n9c | 25.57 | 25.61 | 25.51 | 25.42 | 25.41 | 25.64 | 0.026 | 0.051 |
| C20:1n9 | 0.25 | 0.25 | 0.24 | 0.25 | 0.25 | 0.26 | 0.003 | 0.384 |
| PUFA | 55.68 | 55.9 | 55.66 | 55.75 | 55.63 | 55.72 | 0.035 | 0.312 |
| ω-3 | 1.60 CD | 1.51 AB | 1.49 A | 1.57 BC | 1.59 CD | 1.65 D | 0.013 | 0.009 |
| C18:1n7c | 0.61 | 0.61 | 0.61 | 0.62 | 0.66 | 0.6 | 0.005 | 0.077 |
| C18:3n3 | 1.49 | 1.51 | 1.49 | 1.57 | 1.59 | 1.65 | 0.022 | 0.051 |
| ω-6 | 54.2 | 54.38 | 54.17 | 54.18 | 54.03 | 54.08 | 0.042 | 0.162 |
| C18:2n6c | 54.2 | 54.38 | 54.17 | 54.18 | 54.03 | 54.08 | 0.042 | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roguski, M.; Zielińska-Górska, M.; Radomski, A.; Zawadzki, J.; Gzowska, M.; Rygało-Galewska, A.; Łozicki, A. Evaluation of the Quality and Nutritional Value of Modified Corn Wet Distillers’ Grains Plus Solubles (mcWDGS) Preserved in Aerobic and Anaerobic Conditions. Sustainability 2025, 17, 7097. https://doi.org/10.3390/su17157097
Roguski M, Zielińska-Górska M, Radomski A, Zawadzki J, Gzowska M, Rygało-Galewska A, Łozicki A. Evaluation of the Quality and Nutritional Value of Modified Corn Wet Distillers’ Grains Plus Solubles (mcWDGS) Preserved in Aerobic and Anaerobic Conditions. Sustainability. 2025; 17(15):7097. https://doi.org/10.3390/su17157097
Chicago/Turabian StyleRoguski, Mateusz, Marlena Zielińska-Górska, Andrzej Radomski, Janusz Zawadzki, Marlena Gzowska, Anna Rygało-Galewska, and Andrzej Łozicki. 2025. "Evaluation of the Quality and Nutritional Value of Modified Corn Wet Distillers’ Grains Plus Solubles (mcWDGS) Preserved in Aerobic and Anaerobic Conditions" Sustainability 17, no. 15: 7097. https://doi.org/10.3390/su17157097
APA StyleRoguski, M., Zielińska-Górska, M., Radomski, A., Zawadzki, J., Gzowska, M., Rygało-Galewska, A., & Łozicki, A. (2025). Evaluation of the Quality and Nutritional Value of Modified Corn Wet Distillers’ Grains Plus Solubles (mcWDGS) Preserved in Aerobic and Anaerobic Conditions. Sustainability, 17(15), 7097. https://doi.org/10.3390/su17157097







