Abstract
The use of rock powders is an agricultural practice that facilitates the agroecological transition and autonomy of many farmers. These inputs should be used in conjunction with management systems that enhance the weathering of the minerals contained in the rocks. This study aimed to assess the impact of incorporating gneiss powder on soil quality and coffee cultivation within agroecological and organic frameworks, encompassing agroforestry systems (AFSs) as well as areas fully exposed to sunlight (FS). Comprehensive analyses, including chemical, microbiological, and physical assessments, were carried out on the soil. The study involved evaluating various parameters such as electrical conductivity, grain density, total titratable acidity, and pH of the exudates to gauge the coffee quality. Following a 24-month application of rock powder, noteworthy observations included increased soil moisture in agroforestry systems (AFSs), presumably attributable to enhanced nutrient availability (potassium, calcium, magnesium, copper, and zinc) derived from the gneiss powder. In addition, a higher level of CO2 was derived from microbial respiration than from soil production. Similarly, coffee beans presented lower electrical conductivity, higher density, and fewer defects in AFSs than fully exposed sun systems (FS). The total titratable acidity values remain consistent with the limits indicated in the literature for quality coffees; the pH values, however, were lower. The results suggest that the use of gneiss powder enhances soil microorganism activity and accelerates the biological weathering of minerals for coffee plantations in AFSs.
1. Introduction
Soil quality is assessed using physical, chemical, and biological indicators, which indicate changes in ecological functions that result from use and management [1,2]. In tropical agroecosystems, soil quality often depends on the vegetation [3] that provides food for edaphic organisms and soil cover, especially where plant litter is found. Plant biomass also helps improve aggregation, water retention, and nutrient cycling [4,5]. Other agricultural practices, such as monoculture, fire, and the application of pesticides and soluble fertilizers, can cause the degradation of the biological quality of soil [6]. Conversely, agricultural systems based on agroecology with greater biodiversity, such as agroforestry systems (AFSs), increase or preserve soil quality.
Various advantages are associated with the adoption of AFSs, including enhancements in nutrient cycling [7]. Despite these benefits, the common practice of utilizing mineral fertilization to facilitate system development and sustain crop productivity raises sustainability concerns. The use of soluble fertilizers poses challenges as they derive from non-renewable sources, coupled with the additional expenses incurred in their production, transportation, and distribution [8]. In Brazil, around 84% of these soluble fertilizers are imported [9,10] and are prohibited for use in organic agriculture [11] due to the adverse effects they cause, such as salinization of the soil, soil structure breakdown, groundwater contamination, and emission of pollutant gases, among others [12,13].
As a result, finding alternative sources of mineral nutrients is important. Silicate rock powders are one option, especially after Law nº 12.890, 10 December 2013 and Normative Instruction (IN) nº 05, 10 March 2016 [14,15] were put in place, which outline the concept of remineralizer and its determinants and guarantees of use. These sources slowly release macro- and micronutrients and result in a minimal saline effect compared to conventional sources. They are less susceptible to nutrient loss through leaching and have a greater residual effect on the soil, reducing the need for fertilizers and time spent on management and labor [16,17]. What’s more, due to their wide spatial distribution, their energy costs are lower [18]. In addition to increased soil fertility, product quality, and productivity [19], rock powders are known for their carbon capture capabilities. Instead of emitting greenhouse gases, they capture and store atmospheric carbon in the soil [20].
The nutrients present in silicate rock powders typically exhibit slow-release characteristics due to their inherent low solubility, a process influenced by factors like composition, particle size, mineral alteration levels, and edaphoclimatic conditions [21,22,23]. However, various mechanisms exist to enhance nutrient accessibility for plants. Implementing management systems that incorporate organic matter and foster increased biological activity in the soil, such as AFSs, can facilitate these processes [17,24]. Numerous research findings already attest to the efficacy of rock powders in enhancing soil fertility and promoting the productivity of coffee plants cultivated in fully exposed environments [25,26,27]. These studies highlight a notable increase in nutrient availability through remineralization in AFSs, positively impacting tree growth, including coffee plants.
For organic coffee production, agroecological farmers have tried to add value to their products [28]. Cortez [29] believes sensory analysis to be an important indicator (albeit subjective and subject to criticism) since its parameters are defined by the International Coffee Organization (ICO). Regarding physical and chemical parameters, density and electrical conductivity serve as valuable indicators for evaluating coffee quality, especially concerning the structural integrity of grain cell walls [30]. This approach ensures a more objective and quantifiable determination of coffee quality [31]. The primary objective of this study was to evaluate the impact of gneiss powder application on measurable parameters related to soil fertility and coffee quality. The availability of nutrients derived from gneiss rock in soil cultivated with coffee in agroforestry systems was of particular importance when comparing them to fully exposed sun cultivation and its effect on the physicochemical quality of coffee beans.
2. Material and Methods
2.1. Outline of Experiment Locations
This research was carried out on the agricultural holdings of two families located in the municipality of Divino (−20.615981 latitude, −42.156477) longitude, situated in the state of Minas Gerais. The climate in this region is categorized as Cwa (Cwa: C = Mild temperate, w = Dry winter, a = Hot summer), as per the Köppen–Geiger classification system. The average annual temperature stands at 19.9 °C, accompanied by an average annual precipitation of 1282 mm, given the altitude of 950 m. The farming properties are in the Taquaraçu community and are referred to as GA (Gilvânia and Anacleto) and LA (Luís and Aparecida). The coffee plantations have been operating for about 12 years, but they have been using organic cultivation for the last five (Figure 1).
Figure 1.
Location of experimental areas. GA (Gilvânia and Anacleto) and LA (Luís and Aparecida).
Two areas of coffee plants (red and yellow Catuaí variety—Coffea arabica) were selected from each property on which to conduct our research: one using an agroforestry system (AFSs) and the other using full exposure to the sun (FS). The soils were identified and categorized as Red-Yellow Argisol (RYA) and Yellow Latosol (YL). It is noteworthy that the property with RYA soil exhibits a more extensive diversity of arboreal, herbaceous, and shrub plants in comparison to the property with YL soil, as outlined in Table 1.

Table 1.
Arboreal and herbaceous species identified at the beginning of the study in agroforestry systems on properties in this study.
Soil characterization was conducted on both farming properties before applying the gneiss powder. Composite samples were collected from the agroforestry system (AFSs) and the full exposure to sun (FS) areas in the analyzed properties (Table 2). These samples were classified as sandy and clay loam soils.

Table 2.
Chemical characteristics of soils cultivated with coffee in agroforestry (AFSs) and full exposure to sun (FS) systems on family-run agroecological farming properties (RYA and YL) at a depth of 0–20 cm, in the municipality of Divino, Minas Gerais.
2.2. Characterization of Rock Powder
Gneisses are metamorphic rocks of varied composition, from medium to high grade, and result from pre-existing materials such as granites and/or argillaceous quartz sedimentary rocks. The material used for our research purposes was obtained from a mine in the municipality of São João do Manhuaçu (Minas Gerais), where it is sold as a construction material. According to Figueiredo [32], the gneiss is an enderbitic migmatite, a lithotype common to the Juiz de Fora complex, and is composed of orthopyroxene, plagioclase, clinopyroxene, hornblende, biotite, and quartz, in addition to zircon, apatite, epidote, and opaque minerals. The finely granulated material was previously air-dried and then filtered through a 0.074 mm sieve for subsequent X-ray diffraction analysis. Lithochemical characterization involved X-ray fluorescence for the macroelements. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) was applied after a complete triacid opening for the remaining elements [33] (Table 3).

Table 3.
Chemical characterization of gneiss powder.
2.3. Experimental Design
A nested statistical model (mixed nested factorial) of 2 × 4 was used for this study, with 4 repetitions. The two main predictive factors were two family-owned farming properties and four types of management (coffee plantations in AFSs or FS, with the symbols (+) or (−) to indicate addition or not, respectively, of rock powder), based on the following treatments: AFSRYA+, AFSRYA−, FSRYA+, FSRYA− and AFSYL+, AFSYL−, FSYL+, FSYL−. Those plantations using rock powder received 2 kg plant−1 of gneiss powder, applied in January 2019, for the coffee plants. This was equally distributed on both sides of the rows and was not introduced to the soil. No additional fertilization was utilized. Each plantation had four homogeneous plots (7 × 9 m) containing 30 plants (3 rows, 10 plants in each). Only the six plants in the central row of each plot were sampled.
2.4. Analysis of Soil Quality
The soil analysis occurred two years after the application of the input. Twelve individual samples were collected from each plot (0–20 cm deep) using a Dutch auger. These samples were taken beside the coffee plant canopy, yielding a composite sample. After homogenization, the samples were divided into three parts: the first part was sieved (2 mm), air-dried, and then subjected to fertility analyses to assess F (phosphorus), K (potassium), Ca (calcium), Mg (magnesium), Mn (manganese), Fe (iron), Cu (copper), Zn (zinc), Ni (nickel), Cd (cadmium), Cr (chromium), and Pb (lead) available levels. These levels were determined after extraction in a KCl mil L−1 solution (for Ca2+ and Mg2+), while the remaining nutrients were determined after extraction using the Mehlich-1 method [34]. The second portion was stored refrigerated in a hermetically sealed bag for microbiological analysis, and the third portion was weighed on a precision scale and dried for 48 h in an oven at 105 °C for moisture analysis.
Microbial respiration in the soil was determined following the methodology adapted from Mendonça et al. [35], utilizing the incubation method for 21 days. The total CO2 produced was calculated by summing the values obtained in each sampling. The CO2 calculation (mg C g−1 soil) employed the following formula: CO2 = (B − V) × M × (V1/V2), where B represents the volume (mL) utilized in the blank titration test, V signifies the volume (mL) of acid used in the titration of each sample, M denotes the molar concentration of the acid used in titration (set at 6), and V1/V2 represents the ratio between the volume of NaOH used in CO2 capture and the volume used in titration (in this case, 1/6).
Microbial biomass carbon (Cmic) was calculated according to Ferreira et al. [36] using the irradiation-extraction method, where the Cmic was calculated from the difference in the C levels in the irradiated and non-irradiated samples, using the following formula: Cmic = (CI − CNI)/Kc = mg kg−1 of C in the soil, Cmic = microbial biomass carbon, CI = Carbon in an irradiated sample, CNI = Carbon in a non-irradiated sample, Kc = 0.33—the correction factor referring to the fraction of C extracted by K2SO4.
2.5. Coffee Quality Assessments
The coffee quality was assessed through physicochemical analyses of the beans, after harvesting and stripping them with a propylene cloth. Harvesting began with an estimated percentage of green coffee beans of less than 10%. They were suspended and dried in the sun until the beans reached an 11% moisture level and were subsequently peeled.
To determine the physical-chemical quality indicators, we conducted analyses of the electrical conductivity, bulk density, pH, and total titratable acidity. Electrical conductivity was evaluated using raw bean extract in a portable conductivity meter as per the methodology described in Borém et al. [37]. Apparent density was measured by calculating the mass/volume ratio using a 1 L beaker. The beans were weighed on a precision scale [38]. The pH was measured in a pH meter at room temperature, using a water extract of roasted grains, as per Instituto Adolfo Lutz [39]. To determine the total titratable acidity, the same extract used in the pH analysis was titrated with NaOH 0.1 mol L−1 using a pH meter, up to a pH level of 8.2. This was done because the color made it impossible to visualize the turning point using an indicator [39,40].
2.6. Data Analysis
The collected data underwent a thorough analysis of variance, adhering to the statistical model pre-established by the sampling plan (Nested). The two farming locations were associated with fixed effects, while the four types of coffee plantation management were linked to random effects. To ensure the reliability of the analysis, normality, and homoscedasticity were assessed for errors through the Jarque-Bera and Bartlett tests. The means between different management practices were subsequently compared utilizing the SNK test, with an error probability (α) set at 5%. All statistical analyses were performed using the SPEED Stat 2.4 software [41].
3. Results
3.1. Chemical Alterations in the Soil
The pH values varied within the range of 6.1 to 6.94. Al+3 levels were absent in all treatments, and the H+Al level was identified as low, displaying no significant differences between the various treatments. Key soil attributes such as soil organic matter (SOM), the sum of bases (SB), and the cation exchange capacity (CEC) exhibited variations based on the management practices employed (Table 4). SB can be classified as good or very good in all treatments [42], but AFS+ (RYA and YL) showed the highest values. Potential CEC was rated as good in all treatments, but the AFS+ systems (RYA and YL) had the highest (p < 0.05) CEC [42].

Table 4.
Soil chemical characteristics after 24 months of application (+) or non-application (−) of gneiss powder to coffee plantations under agroforestry management (AFSs) or full exposure to the sun (FS) for the two agroecological family-owned farming properties (RYA and YL).
The addition of gneiss powder stimulated and altered macronutrient contents such as P (phosphorus) and K (potassium), especially in coffee plantation soils in AFSs. The P level increased by 110% (AFSRYA+) and 171% (AFSYL+) compared to AFSs with no rock powder (Figure 2A). The P levels in FS, with or without rock powder, increased by at least 199% (FSRYA+) and 30% (FSYL+). K levels increased by at least 40% (AFSRYA+) compared to the other treatments (Figure 2B). The Ca+2 levels were 67.1% (AFSRYA+) and 89.3% (AFSYL+) higher (Figure 2C), and the Mg2+ levels were 103.3% (AFSRYA+) and 59.4% (AFSYL+) higher (Figure 2D). The macronutrient levels in FS+ and FS− did not differ.
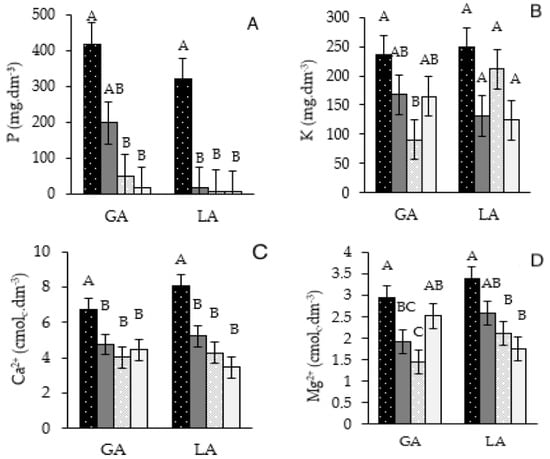
Figure 2.
Availability of P (A), K (B), Ca2+ (C) and Mg2+ (D) of macronutrients in the soil after 24 months of application (+) or non-application (−) of gneiss powder in coffee plantations in agroforestry management (AFSs) or full exposure to sun (FS) for two agroecological family-owned farming properties (RYA and YL) located in Divino, Minas Gerais. Means followed by different letters differ statistically from each other by the SNK test (p < 0.05).
In general, micronutrient availability was higher in AFSs+ than in FS (Table 4). Cu levels were 74.4% (AFSRYA+) and 234.2% (AFSYL+), Zn levels were 34.8% (AFSRYA+) and 227.9% (AFSYL+), and Ni levels were 288.1% (AFSRYA+) and 147.6% (AFSYL+) (Table 5). The Mn levels were 74.4% (AFSRYA+), and Fe levels did not differ between both treatments. Potentially toxic elements (PTEs) such as Cd, Cr, and Pb were unchanged by applying gneiss powder. YL did present a difference between treatments (p < 0.05) (Table 5), but all contents were below the reference values for soil quality in Minas Gerais [43].

Table 5.
Levels of micronutrients and heavy metals in soil after 24 months of application (+) or non-application (−) of gneiss powder in coffee plantations in agroforestry management (AFSs) or full exposure to sun (FS) for two agroecological family-owned farming properties (RYA and YL) located in Divino, Minas Gerais. Means followed by different letters differ statistically from each other by the SNK test (p < 0.05).
3.2. Microbiological Changes in Soil
The release of CO2 because of microbial respiration was higher in soils in AFSs than in FS, with or without the application of gneiss powder (Table 6). In RYA, Cmic was higher in soils in AFSs, while in YL there was no difference between treatments (Table 6).

Table 6.
Release of CO2 because of microbial respiration in soils under agroforestry management (AFSs) or full exposure to the sun (FS) after 24 months of application (+) or non-application (−) of gneiss powder in two agroecological family-owned farming properties (RYA and YL). Means followed by different letters differ statistically from each other by the SNK test (p < 0.05).
3.3. Physical Changes to Soil
Soil moisture differed between treatments as it was higher in AFSs soils than FS soils, with or without rock powder. In YL, the moisture in AFS+ was higher than in AFS−. In RYA, the moisture in FS+ was higher than in FS−. This demonstrates the effect rock powder has on maintaining moisture in the system (Figure 3).
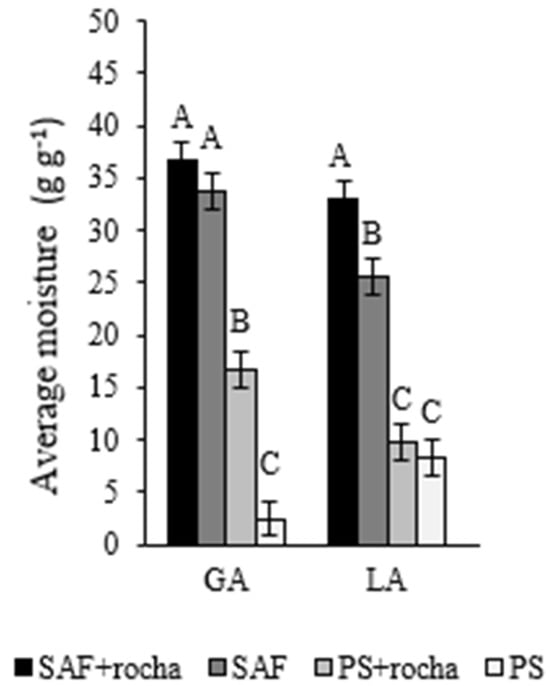
Figure 3.
Average moisture (g g−1) on a wet basis of soil samples after 24 months of application (+) or non-application (−) of gneiss powder in coffee plantations in agroforestry management (AFS) or full exposure to the sun (FS) for two agroecological family-owned farming properties (RYA and YL) located in Minas Gerais. Means followed by different letters differ statistically from each other by the SNK test (p < 0.05).
3.4. Assessment of Coffee Bean Attributes
Coffee beans grown on FS presented higher electrical conductivity (Figure 4A) than on AFSs (RYA: 50.3% and YL: 51.1%), demonstrating the effect that agroforestry systems have on coffee quality regardless of whether rock powder is used or not. Bean density differed between treatments (Figure 4B). Coffee beans grown under AFSs had a higher density (38.2% for RYA and 19.4% for YL) when compared to FS+ (Figure 4B).
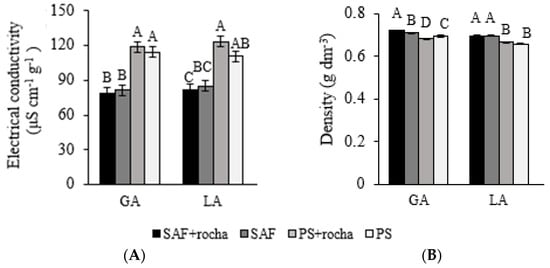
Figure 4.
Electrical conductivity (A) of coffee exudates and bean density (B) after 24 months of application (+) or non-application (−) of gneiss powder in coffee plantations in agroforestry management (AFSs) or full exposure to the sun (FS) for two agroecological family-owned farming properties (RYA and YL) located in Divino, Minas Gerais. Means followed by different letters differ statistically from each other by the SNK test (p < 0.05).
The titratable acidity of coffee exudates was lower in AFSRYA− and higher in AFSYL− but did not differ between the other treatments (Figure 5A). The pH level differed only in FSRYA− (average of 2.3% lower than in other treatments (Figure 5B)).
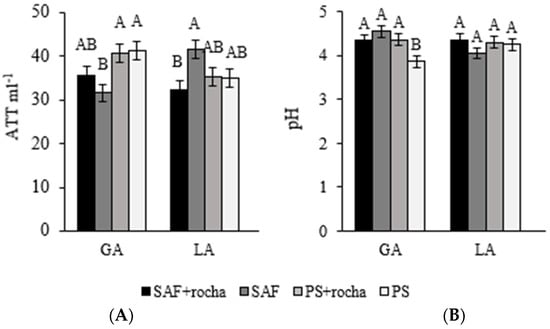
Figure 5.
Total titratable acidity (ATT ml−1) (A) and pH (B) of coffee exudates after 24 months of application (+) or non-application (−) of gneiss powder in coffee plantations in agroforestry management (AFSs) or full exposure to the sun (FS) for two agroecological family-owned farming properties (RYA and YL). Means followed by different letters differ statistically from each other by the SNK test (p < 0.05).
4. Discussion
4.1. Chemical Changes to the Soil
The improvement in soil quality observed in its chemical, physical, and biological attributes, mainly under cultivation in AFSs+, demonstrates the potential of gneiss powder, especially for processes that foster the life of the soil. In AFSs, the greater diversification of strata and the contribution of organic matter encourage greater activity of microorganisms in the soil, which can have several benefits, such as greater nutrient cycling and increased solubility of minerals in rocks. In general, trees with deep roots prefer arbuscular mycorrhizal fungi (AMF) and greater cycling and availability of nutrients [44,45].
As shown in Table 4, the application of rock powders in AFSs+ raises the soil pH, which is in line with the results from Soares [27], Carvalho [46], and Gillman et al. [47]. Some of the released nutrients (Figure 2) also make up the soil exchange complex, displacing Al+3, which precipitates and stops participating in the acid hydrolysis that releases H+ and reduces the pH [48].
Farmers can control the spontaneous vegetation by pruning and trimming it. The AFSs have a continual input of organic matter [49] that comes from the pruning and trimming, the leaves that fall from the aerial part, and the decomposition of roots. Hydrolysis, one of the main chemical reactions that occur during the dissolution of silicate minerals, uses free H+ from the soil and facilitates an increase in pH, an indicator of rock reactivity. Basic (Figure 2) and metallic cations are released during hydrolysis, as well as silicate anions (HnSiO4−) [50].
The large amount of organic matter because of the decomposition of leaves and branches, combined with the action of soil microorganisms [51], may have enhanced the release (weathering) of nutrients (F (phosphorus), K (potassium), Ca (calcium), and Mg (magnesium) in the gneiss powder in the AFS+ areas.
Due to the mineralogy of the rock, the greater availability of nutrients is likely derived from the andesine, amphiboles, and biotite minerals. These minerals are more susceptible to weathering, followed by orthoclase (feldspar-K), which is one of the main gneiss minerals [52,53]. The weathering of apatite, an accessory mineral of gneiss [54], may have contributed to the availability of P.
When P interacts with Si in the soil, it can increase the availability of P [55] due to its desorption in clay minerals [56]. Together with soil microbiota [57], this is one of the main mechanisms of P availability in weathered soils [58].
As a result, P levels in AFSs may be higher than in full exposure to sun areas [45,59]. Furthermore, according to Beenhouwer et al. [44], coffee plantations in AFSs have a greater number of mycorrhizal fungi roots and spores compared to coffee plantations in full exposure to the sun, which itself favors P cycling, yet does not favor microbial activity in less complex and deep root systems.
The higher K levels in AFS (Figure 2B) are favorable for building coffee plantations as they are especially demanding in terms of K. The loss of K+ from leaching is reduced in these AFS systems as they have better structuring and higher levels of organic matter in the soil [60]. In our research, we were able to verify that the biological conditions of the AFSs (Table 6) facilitate the availability of K derived from minerals (especially micas) in the gneiss.
The greater biological activity of the AFSs may have also contributed to the greater release of Cu, Zn, and Ni levels (Table 5) in some minerals such as tourmaline, which is rich in Zn, apatite, and P, but can lead to increased levels of Zn. The dissolution of hydroxyapatites can lead to increased levels of Zn, which can replace Ca2+ in the phosphate structure [61,62].
According to Carvalho [46], the greater diversity of plants in complex systems such as the AFSs (Table 1), or together with spontaneous plants, beans, and green manure, tends to add to the availability of Zn from the minerals that constitute gneiss (Table 5). The Cu levels in RYA and YL, before and after the experiment, were higher than expected due to a pH above 6.0 and adequate levels of organic matter in the soil. However, the Cu levels (including Zn, Ni, Cd, Cr, and Pb) are below the limits established by the soil quality reference values for Minas Gerais [43].
The pH above 6 and the average values of organic matter contributed to the low levels of Cd in the soils (Table 5). The factors that are most important for controlling the mobility of Cd ions in the soil are pH and oxidation potential (in acidic pH conditions, Cd has high mobility). Fe, Al, and Mn oxides, clay minerals, and organic matter are the main adsorbents of Cd in soil [63].
According to Linhares et al. [64], Pb has a similar dynamic to Cd in immobilization and availability in the soil, where the main ligands of Cd are also ligands of Pb. In tropical soils, Fe and Al oxides are usually retained through chemosorption [64], which is highly specific and high-energy adsorption of Pb, resulting in the formation of inner-sphere complexes [65]. These connections make Pb less mobile in soils because they are less reversible than ion exchange connections [66].
Lastly, gneiss powders, despite their demonstrated potential, are not considered remineralizers by IN 5/2016 due to their free silica content (in the form of quartz) being greater than 25%. However, the results obtained in our research indicate that its use should be encouraged. Any material that does not qualify as a remineralizer but presents positive agronomic results can be classified as a new product, according to the norms set forth by the Brazilian Ministry of Agriculture. Using these materials can contribute to the autonomy of family-owned farming and the general sustainability of agroecosystems.
4.1.1. Microbiological Changes in the Soil
The greater diversity of plants (Table 1) in the AFSs was a possible factor in the greater microbiological activity of the soils (Table 5) since microorganisms are responsible for nutrient cycling in addition to contributing to plant nutrition. Where there is a greater diversity of plants, there is a greater production of root exudates, leaf residues, etc., for organisms [51,67].
According to Carvalho [46], the bioavailability of nutrients derived from rocks affects the activity of arbuscular mycorrhizal fungi (AMF) and, as a result, plant growth. This is particularly true in AFSs due to the greater amount of organic matter, which favors the biological activity of the soil [68,69]. The release of organic acids and CO2 during microbial respiration increases the degradation of silicates, which leads to the release of nutrients [70]. Soil microorganisms also play a vital role in phosphorus mineralization and immobilization [71]. P availability was greater in the AFSs, especially when gneiss powder was added. This reaffirms the action of microorganisms in bioweathering with these inputs [72].
The FS areas in monoculture (less diversity of plants) showed less microorganism activity (Table 5), even though they currently have good yields and adequate levels of organic matter [73].
4.1.2. Physical Changes to the Soil
Rock powders can improve plant development in the short term and contribute to improving the physical quality of the soil, such as through better aggregation and greater soil porosity. Consequently, they can improve water infiltration into the soil [74], as occurred in AFS+RYA. Theodoro et al. [17] also observed greater water retention capacity in soils cultivated in agroforestry systems that were fertilized with remineralizers.
In addition, the conditions of permanent soil cover in AFSs and the microclimate were both factors in maintaining the humidity in these areas (Figure 3). Compare this to FS areas, which either have lower runoff or loss due to evaporation, as observed by Franco et al. [75]. The extensive coverage of the tree canopy diminishes direct solar radiation and minimizes the emission of indirect radiation from the air that reaches the ground [76]. It also increases water infiltration, reduces water stress in the AFSs [77,78], and helps maintain the water supply for soil organisms, including during the dry period [75].
4.2. Electrical Conductivity, pH, Total Titratable Acidity, and Coffee Quality
Shaded coffee plantations in AFSs not only provide greater sustainability in cultivation but are also capable of generating a higher-quality beverage when compared to areas of conventional cultivation in full exposure to the sun [79,80]. Our research confirmed this capacity through the electrical conductivity of coffee exudates grown in AFSs and through coffee bean density.
According to Romero et al. [81], coffees grown in AFSs produce a lower percentage of bean defects, which results in lower electrical conductivity values and higher beverage quality. Beans can be damaged by organisms such as the coffee borer beetle, one of the main causes of defects in coffee beans, which leads to further loss of quality [82].
Electrical conductivity also affects coffee density [56]. Our results show that lower electrical conductivity produces denser coffee beans, especially when fertilized with gneiss powder, like in AFS+(RYA). This finding corresponds with results from DaMatta [82], (Figure 4). Denser fruits produce better quality beverages as they have a lower percentage of defects, and the beans contain greater physical integrity [56], possibly due to better plant nutrition from the minerals in gneiss. The presence of K in the soil is essential for producing a higher-quality coffee, especially when N (nitrogen) is present for the formation and physical integrity of the beans, chemical composition, and final quality of the beverage [83].
The levels of titratable acidity (Figure 5A) were within the acidity limit values (from 10.95 to 40.04 mL NaOH 100 g−1) discovered by authors of Arabica coffees in conventional cultivation systems [83,84], however, no titratable acidity data were found for quality coffees grown in AFSs. In general, low levels of total titratable acidity indicate quality loss due to the fermentation process that occurs during the drying and harvesting of beans before they have matured [37,84,85]. Even so, there are few studies that relate the management system to the titratable acidity levels of coffee [37,86].
The lower pH of the coffee exudate obtained in FSRRYA− (varying between 3.9 and 4.5) indicates that the management of AFS and the addition of gneiss powder led to increased pH levels in the exudates (Figure 5B), even though they remained below 5. According to Sivetz and Desrosier [87], for coffee beans to produce quality beverages, their pH values must be between 5.31 and 5.61. Other variables could affect the pH values here, such as the efficiency and roasting point of the beans [35]. However, some researchers suggest that total acidity, not pH, is the factor that best determines coffee acidity [88].
5. Conclusions
The use of rock powder tends to contribute to more sustainable agriculture, and this material is widely available in Brazil. For it to be more efficient, it should be used with ecological management that enhances the activity of soil microorganisms and accelerates the bioweathering of minerals.
The application of gneiss powder improved the quality of soil cultivated with coffee, mainly in AFSs, and favored greater biodiversity and higher microbial activity, which made it possible to increase the availability of macro- and micronutrients for plants. The increase in soil quality indicators was reflected in coffee plants, which produced beans with better attributes, especially those produced in AFSs, verified by electrical conductivity, density, and titratable acidity.
Author Contributions
F.d.P.M.: Investigation, Original draft preparation, Data curation; A.M.X.d.C.: Methodology, Data curation; C.G.R.: Writing and correction; S.H.T.: Original draft preparation and correction; G.L.D.: Writing Reviewing; I.M.C.: Supervision and support in analyses. All authors have read and agreed to the published version of the manuscript.
Funding
Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for the PhD grant of Medeiros, FP.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for the PhD grant of Medeiros, FP. We also thank the farmers participating in the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jackson, L.E.; Calderon, F.J.; Steenwerth, K.L.; Scow, K.M.; Rolston, D.E. Responses of soil microbial processes and community structure to tillage events and implications for soil quality. Geoderma 2003, 114, 305–317. [Google Scholar] [CrossRef]
- Araújo, A.S.F.; Melo, W.J. Soil microbial biomass in organic farming system. Ciência Rural 2010, 40, 2419–2426. [Google Scholar] [CrossRef]
- Jandl, R.; Rodeghiero, M.; Martinez, C.; Cotrufo, M.F.; Bampa, F.; Van Wesemael, B.; Harrison, R.B.; Guerrini, I.A.; Richter, D.d., Jr.; Rustad, L.; et al. Current status, uncertainty and future needs in soil organic carbon monitoring. Sci. Total Environ. 2014, 468–469, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems: Philosophical Transactions of the Royal Society. Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Rigueiro-Rodríguez, A.; Fernández-Núñez, E.; Gonzalez, M.C.A.; Mosquera-Losada, M.R. Agroforestry Systems in Europe: Productive, Ecological and Social Perspectives. In Agroforestry in Europe—Current Status and Future Prospects; Advances in Agroforestry; Springer: Dordrecht, The Netherlands, 2008; Volume 6, pp. 43–65. [Google Scholar] [CrossRef]
- Vallejo, V.E.; Roldan, F.; Dick, R.P. Soil enzymatic activities and microbial biomass in an integrated agroforestry chronosequence compared to monoculture and a native forest of Colombia. Biol. Fertil. Soils 2010, 46, 577–587. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Buresh, R.J.; Mugendi, D.N.; Latt, C.R. Nutrient cycling in tropical agroforestry systems: Myths and science. In Agroforestry in Sustainable Agricultural Systems; Buck, L.E., Lassoie, J.P., Fernandes, E.C.M., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–31. [Google Scholar]
- Fixen, P.E.; Johnston, A.M. World fertilizer nutrient reserves: A view to the future. J. Sci. Food Agric. 2012, 92, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Martins, É.S.; Resende, Á.V.; Oliveira, C.G.; Furtini Neto, A.E. Materiais silicáticos como fontes regionais de nutrientes e condicionadores de solos. In Agrominerais Para o Brasil; Fernandes, F.R., Luz, A.B., Castilhos, Z.C., Eds.; CETEM/MCT. 89-104; Centro de Tecnologia Mineral: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Manning, D.C.; Theodoro, S.H. Enabling food security through use of local rocks and minerals. Extr. Ind. Soc. 2018, 7, 480–487. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa n° 27, de 5 June 2006. 2006. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-sda-27-de-05-06-2006-alterada-pela-in-sda-07-de-12-4-16-republicada-em-2-5-16.pdf (accessed on 1 September 2022).
- Vieira-Megda, M.X.; Mariano, E.; Leite, J.M.; Megda, M.M.; Ocheuze Trivelin, P.C. Chloride ion as nitrification inhibitor and its biocidal potential in soils. Soil Biol. Biochem. 2014, 72, 84–87. [Google Scholar] [CrossRef]
- Pereira, D.G.C.; Santana, I.A.; Megda, M.M.; Megda, M.X.V. Potassium chloride: Impacts on soil microbial activity and nitrogen mineralization. Ciencia Rural 2019, 49, e20180556. [Google Scholar] [CrossRef]
- Brasil. Lei n° 12.890 10th December, de 2013. Available online: http://www.planalto.gov.br/ccivil_03/Ato20112014/2013/Lei/L12890.htm/ (accessed on 1 September 2022).
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa nº 5, de 10th March, 2016. Diário Oficial da União. 2016. Available online: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/21393222/do1-2016-03-14-instrucao-normativa-n-6-de-10-de-marco-de-2016-21393092 (accessed on 1 September 2022).
- Caner, L.; Radtke, L.M.; Vignol-Lelarge, M.L.; Inda, A.V.; Bortoluzzi, E.C.; Mexias, A.S. Basalt and rhyo-dacite weathering and soil clay formation under subtropical climate in southern Brazil. Geoderma 2014, 235, 100–112. [Google Scholar] [CrossRef]
- Theodoro, S.H.; Medeiros, F.P.; Ianniruberto, M.; Jacobson, T.K.B. Soil remineralization and recovery of degraded areas: An experience in the tropical region. J. S. Am. Earth Sci. 2021, 107, 103014. [Google Scholar] [CrossRef]
- Fyfe, W.S.; Leonardos, O.H.; Theodoro, S.H. Sustainable farming with native rocks: The transition without Revolution. An. Acad. Bras. Ciências 2006, 78, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Burbano, D.F.M.; Theodoro, S.H.; Carvalho, A.X.M.; Ramos, C.G. Crushed vulcanic rock as soil remineralizer: A strategy to overcome the global fertilizer crisis. Nat. Resour. Res. 2022, 31, 2197–2210. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kronberg, B.I.; Leonardos, O.H.; Fyfe, W.S. The use of ground rocks in laterite systems—An improvement to the use of conventional soluble fertilizers. Chem. Geol. 1987, 60, 361–370. [Google Scholar] [CrossRef]
- Manning, D.A. Innovation in resourcing geological materials as crop nutrients. Nat. Resour. Res. 2018, 27, 217–227. [Google Scholar] [CrossRef]
- Van Straaten, P. Distribution of agromineral resources in space and time—A global geological perspective. Pesqui. Agropecuária Bras. 2022, 57, e01453. [Google Scholar] [CrossRef]
- Theodoro, S.H.; Manning, D.A.C.; Carvalho, A.X.M.; Ferrão, F.R.; Almeida, G.R. Soil remineralizer: A new rote to sustentability for Brazil, a giant exporting agro-mineral commoditites. In Routledge Handbook of the Extractive Industries and Sustainable Development, 1st ed.; Yakovleva, N., Nickless, E., Eds.; Taylor & Francis Ltd.: London, UK, 2022; pp. 261–281. [Google Scholar] [CrossRef]
- Silva, N.M.R.M. Diversidade Microbiana e Microbiota Solubilizadora de Fosfato em Solos de Cafezais Orgânicos em Sistemas Agroflorestais e a Pleno Sol. Master’s thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2017. [Google Scholar]
- Dias, K.G.L.; Guimarães, P.T.G.; Carmo, D.L.; Reis, T.H.P.; Lacerda, J.J. Fontes alternativas de potássio em cafeeiros para melhoria da fertilidade do solo, da produtividade e da qualidade de bebida. Pesqui. Agropecuária Bras. 2018, 53, 1355–1362. [Google Scholar] [CrossRef]
- Soares, G.J. Influência da Rochagem no Desenvolvimento de Sistemas Agroflorestais e na Captura de Dióxido de Carbono Atmosférico. Master’s Thesis, University of Brasília, Brasília, Brazil, 2018; 99p. Available online: https://repositorio.unb.br/handle/10482/33088?locale=en (accessed on 27 July 2023).
- Malta, M.R.; Pereira, R.G.A.; Chagas, S.J.R. Condutividade elétrica e lixiviação do potássio em exsudados de grãos de café: Alguns fatores que podem influenciar nas avaliações. Ciênc. Agrotec. Lavras 2005, 29, 1015–1020. [Google Scholar] [CrossRef]
- Cortez, J.G. Aptidão Climática para Qualidade da Bebida nas Principais Regiões Cafeeiras de Minas Gerais; Informe Agropecuário: Belo Horizonte, Brazil, 1997. [Google Scholar]
- Pimenta, C.J. Qualidade de Café. Lavras: UFLA, 2003. 304 p. PIMENTA, C.J. Qualidade do Café (Coffea arabica. L.) Colhido em Diferentes Estádios de Maturação. Master’s Thesis, Universidade Federal de Lavras, Lavras, Brazil, 1995; 93p. [Google Scholar]
- Prete, C.E.C. Condutividade Elétrica do Exsudato de Grãos de Café (Coffea arabica L.) e Sua Relação com a Qualidade da Bebida. Ph.D Thesis, ESALQ/USP, Piracicaba, Brazil, 1992. [Google Scholar]
- Figueiredo, C.M. O Arco Magmático Brasiliano na Conexão Entre os Orógenos Araçuaí e Ribeira. Master’s Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2009; p. 104. Available online: https://repositorio.ufmg.br/handle/1843/MPBB-7ULNPK (accessed on 3 March 2023).
- USEPA. Method 3052, Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- Silva, F.C. Manual de Análises Químicas de Solos Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; 627p. [Google Scholar]
- Mendonça, L.M.L.; Pereira, R.F.A.; Mendes, A.G. Parâmetro bromatológicos de grãos crus e torrados de cultivares de café (Coffea arabica L.). Food Sci. Technol. 2005, 25, 239–243. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Camargo, F.A.O.; Vidor, C. Utilização de micro-ondas na avaliação da biomassa microbiana do solo. Rev. Bras. Ciência Solo 1999, 23, 991–996. [Google Scholar] [CrossRef]
- Borém, F.M.; Coradi, P.C.; Saath, R.; Oliveira, J.A. Qualidade do café natural e despolpado após secagem em terreiro e com altas temperaturas. Ciência Agrotecnologia 2008, 32, 1609–1615. [Google Scholar] [CrossRef]
- Clarke, R.J.; Macrea, R. Coffee: Techonoly; Elsevier Applied Science: Amsterdam, The Netherlands, 1987; 321p. [Google Scholar]
- IAL—Instituto Adolfo Lutz. Métodos Físicoquímicos Para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. [Google Scholar]
- Filho, T.L.; Lucia, S.M.D.; Saraiva, S.H.; Sartori, M.A. Composição físico-química e qualidade sensorial de café conilon produzido no Estado do Espírito Santo e submetido a diferentes formas de processamento. Semin. Ciências Agrárias 2013, 34, 1723–1730. [Google Scholar] [CrossRef][Green Version]
- Carvalho, A.M.X.; Mendes, F.Q.; Mendes, F.Q.; Tavares, L.F. SPEED Stat: A free, intuitive, and minimalist spreadsheet program for statistical analyses of experiments. Crop Breed. Appl. Biotechnol. 2020, 20, e327420312. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.H. Recomendação para o Uso de Corretivos e Fertilizantes em Minas Gerais—5ª Aproximação; Ed. Universidade de Viçosa: Viçosa, Brazil, 1999; 359p. [Google Scholar]
- COPAM—Conselho Estadual de Política Ambiental. Resolução nº 166, de 29 de junho de 2011. Diário do Executivo—Minas Gerais. 2011. Available online: http://www.siam.mg.gov.br/sla/download.pdf?idNorma=14670 (accessed on 22 February 2023).
- De Beenhouwer, M.; Muleta, D.; Peeters, B.; Van Geel, M.; Lievens, B.; Honnay, O. DNA pyrosequencing evidence for large diversity differences between natural and managed coffee mycorrhizal fungal communities. Agron. Sustain. Dev. 2014, 35, 241–249. [Google Scholar] [CrossRef]
- Jakobsen, I.; Hammer, E.C. Nutrient dynamics in arbuscular mycorrhizal networks. In Mycorrhizal Networks; Horton, T.R., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 91–131. [Google Scholar] [CrossRef]
- Carvalho, A.M.X. Rochagem e Suas Interações no Ambiente Solo: Contribuições para Aplicação em Agroecossistemas sob Manejo Agroecológico. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2012; 116p. Available online: http://locus.ufv.br/handle/123456789/1631 (accessed on 22 February 2023).
- Gillman, G.P.; Burkett, D.C.; Coventry, R.J. A laboratory study of application of basalt dust to highly weathered soils: Effect on soil cation chemistry. Aust. J. Soil Res. 2001, 39, 799–811. [Google Scholar] [CrossRef]
- Boniao, R.D.; Shamshuddin, J.; Van Ranst, E.; Zauyah, S.; Omar, S.R.S. Changes in chemical properties and growth of corn in volcanic soils treated with peat, ground basalt pyroclastics, and calcium silicate. Commun. Soil Sci. Plant Anal. 2002, 33, 1219–1233. [Google Scholar] [CrossRef]
- Maia, S.M.F.; Xavier, F.A.S.; Oliveira, T.S.; Sá Mendonça, E.; Filho, J.A.A. Impactos de sistemas agroflorestais e convencional sobre a qualidade do solo no semiárido cearense. Rev. Árvore 2006, 30, 837–848. [Google Scholar] [CrossRef]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2022, 807, 150976. [Google Scholar] [CrossRef]
- Dafydd EM, O.; Samuel, R.; Both, S.; Goodall, T.; Majalap-Lee, N.; Ostle, N.J.; McNamara, N.P. Soil Microbial Community and Litter Quality Controls on Decomposition Across a Tropical Forest Disturbance Gradient. Front. For. Glob. Change 2020, 3, 81. Available online: https://www.frontiersin.org/articles/10.3389/ffgc.2020.00081 (accessed on 20 March 2023).
- Formoso, M.L.L. Some topics on geochemistry of weathering: A review. Acad. Bras. Ciências 2006, 78, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Dalmora, A.C.; Müller Kautzmann, R.; Staub, J.; Homrich Schneider, I.A. Crushed amygdaloidal basalt rock and its effects on tomato production. LADEE 2022, 3, 1–10. [Google Scholar] [CrossRef]
- Melo, V.F.; Castilho, R.M.V.; Pinto, L.S. Reserva mineral do solo. In Química e mineralogia do solo. Parte I; Melo, V.F., Alleoni, R.F.S., Eds.; SBCS: Viçosa, Brazil, 2009; 695p. [Google Scholar]
- Tavakkoli, E.; English, P.; Guppy, C.N. Interaction of Silicon and Phosphorus Mitigate Manganese Toxicity in Rice in a Highly Weathered Soil. Soil Sci. Plant Anal. 2011, 42, 503–513. [Google Scholar] [CrossRef]
- Santos, L.F.d.; Sodré, F.F.; Martins, É.d.S.; Figueiredo, C.C.d.; Busato, J.G. Efeitos de biotita sienito sobre os níveis de nutrientes e cargas elétricas em Latossolo de Cerrado: Effects of finely ground biotite syenite. Pesqui. Agropecuária Trop. 2021, 51, e66691. Available online: https://www.revistas.ufg.br/pat/article/view/66691 (accessed on 5 February 2023). [CrossRef]
- Owino-Gerroh, C.; Gascho, G.L. Effect of silicon on low pH soil phosphorus sorption and uptake and growth of maize. Commun. Soil Sci. Plant Anal. 2004, 35, 2369–2379. [Google Scholar] [CrossRef]
- Pozza, A.A.; Costa, E.S.; Guilherme, L.G.; Marques, J.J.M.; Motta, P.F. Retenção e dessorção competitivas de ânions inorgânicos em gibbsite natural de solo. Pesqui. Agropecuária Bras. 2007, 42, 1627–1633. [Google Scholar] [CrossRef]
- Notaro, K.A.; Medeiros, E.V.; Duda, G.P.; Silva, A.O.; Moura, P.M. Agroforestry systems, nutrients in litter and microbial activity in soils cultivated with coffee at high altitude. Sci. Agric. 2014, 71, 87–95. [Google Scholar] [CrossRef]
- Nigussi, A.; Kissi, E. The contribution of coffee agroecosystem to soil fertility in Southwestern Ethiopia. Environmental Science. Afr. J. Agric. Res. 2012, 7, 74–81. [Google Scholar] [CrossRef]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mater. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie Academic & Professional: London, UK, 2013. [Google Scholar]
- Linhares, L.A.; Egreja Filho, F.B.; Oliveira, C.V.; Bellis, V.M. Adsorção de cádmio e chumbo em solos tropicais altamente intemperizados. Pesqui. Agropecuária Bras. 2009, 44, 291–299. [Google Scholar] [CrossRef]
- Novais, R.F.; Mello, J.W.V. Relação Solo-Planta. In Fertilidade do Solo; Novais, R.F., de Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Lima, J.C., Eds.; Universidade Viçosa: Viçosa, Brazil, 2007; pp. 133–205. [Google Scholar]
- Silva, M.L.S.; Vitti, G.C. Fracionamento de metais pesados em solo contaminado antes e após cultivo de arroz. Química Nova 2008, 31, 1385–1391. [Google Scholar] [CrossRef][Green Version]
- Menezes, J.M.T.; Van Leeuwen, J.; Valeri, S.V.; Cruz, M.P.; Leandro, R.C. Comparison of soils used for agroforestry and of remaining forests, in northern Rondônia State, Brazil. Rev. Bras. Ciência Solo 2008, 32, 893–898. [Google Scholar] [CrossRef]
- Chander, K.; Goyal, S.; Nandal, D. Soil organic matter, microbial biomass and enzyme activities in a tropical agroforestry system. Biol. Fertil. Soils 1998, 27, 168–172. [Google Scholar] [CrossRef]
- Prasad, R.; Arunachalam, A.; Shukla, A.; Singh, P.; Gupta, A.; Saroj, N.K.; Tripathi, V.D. Field management practices in agroforestry systems influence organic carbon and biological properties of soil. Agrofor. Syst. 2023, 97, 1375–1390. [Google Scholar] [CrossRef]
- Satter, M.A.; Hanafi, M.M.; Mahmud, T.M.; Azizah, H. Influence of arbuscular mycorrhiza and phosphate rock on uptake of major nutrients by Acacia mangium seedlings on degraded soil. Biol. Fertil. Soils 2006, 42, 345–349. [Google Scholar] [CrossRef]
- Wang, J.; Ren, C.; Cheng, H.; Zou, Y.; Bughio, A.M.; Li, Q. Conversion of rainforest into agroforestry and monoculture plantation in China: Consequences for soil phosphorus forms and microbial Community. Sci. Total Environ. 2017, 595, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Quirk, J.; Beerling, D.J.; Banwart, S.A.; Kakonyi, G.; Romero-Gonzalez, M.E.; Leake, J.R. Evolution of trees and mycorrhizal fungi intensifies silicate mineral weathering. Biol. Lett. 2012, 8, 1006–1011. [Google Scholar] [CrossRef]
- Nunes, L.P.L.; Dias, L.E.; Jucksch, I.; Barros, N.F.; Kasuya, M.C.M.; Correia, M.E.F. Impacto do monocultivo de café sobre os indicadores biológicos do solo na zona da mata mineira. Ciência Rural 2009, 39, 2467–2474. [Google Scholar] [CrossRef]
- Campos, B.C.; Reinert, D.J.; Nicolodi, R.; Ruedell, J.; Petrere, C. Estabilidade estrutural de um Latossolo vermelho-escuro distrófico após sete anos de rotação de culturas e sistemas de manejo de solo. Rev. Bras. Ciência Solo 1995, 19, 121–126. [Google Scholar]
- Franco, F.S.; Couto, L.; Carvalho, A.F.; Jucksch, I.; Filho, E.I.F.; Silva, E.; Neto, J.A.M. Quantificação de erosão em sistemas agroflorestais e convencionais na zona da mata de Minas Gerais. Rev. Árvore 2002, 26, 751–760. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes-Filho, E.I.; Daher, M.; Gomes, L.C.; Cardoso, I.M.; Fernandes, R.B.A.; Schaefer, C.E.G.R. Microclimate and soil and water loss in shaded and unshaded agroforestry coffee systems. Agroforest Syst. 2021, 95, 119–134. [Google Scholar] [CrossRef]
- Young, A. Agroforestry takes root in Ethiopia. Agrofor. Today 1989, 1, 13–16. [Google Scholar]
- Narain, P.; Singh, R.K.; Sindhwal, N.S.; Joshie, P. Water balance and water efficiency of different land uses in western Himalayan valley region. Agric. For. Meteorol. 1998, 37, 225–240. [Google Scholar] [CrossRef]
- Toledo, V.M.; Moguel, P. Coffee and Sustainability: The Multiple Values of Traditional Shaded Coffee. J. Sustain. Agric. 2012, 36, 353–377. [Google Scholar] [CrossRef]
- Bote, A.D.; Vos, J. Tree management and environmental conditions affect coffee (Coffea arabica L.) bean quality. NJAS Wagening. J. Life Sci. 2017, 83, 39–46. [Google Scholar] [CrossRef]
- Romero, J.C.P.; Romero, J.P.; Gomes, F.P. Condutividade elétrica (CE) do exsudato de grãos de Coffea arabica em 18 cultivares analisados no período de 1993 a 2002. Rev. Agric. Piracicaba 2003, 78, 293–302. [Google Scholar] [CrossRef]
- DaMatta, F.M. Restrições ecofisiológicas na produção de café com e sem sombra: Uma revisão. Field Crops Res. 2004, 86, 99–114. [Google Scholar] [CrossRef]
- Clemente, J.M.; Cirillo, M.A.; Malta, M.R.; Caixeta, F.; Pereira, C.C.; Rosa, S.F. Effects of nitrogen and potassium on the chemical composition of coffee beans and on beverage quality. Acta Scientiarum. Agronomy 2015, 37, 297–305. [Google Scholar] [CrossRef]
- Silva, P.A.; Oliveira, M.G.; Coelho, P.O.; Silva, J.A.C. Quality of coffee cultivated in Campos Gerais, Minas Gerais. Acta Sci. Technol. 2016, 38, 1–5. [Google Scholar] [CrossRef]
- Filho, T.L.; Lucia, S.M.D.; Saraiva, S.H.; Lima, R.M. Características físico-químicas de bebidas de café tipo expresso preparadas a partir de blends de café arábica e conilon. Rev. Ceres 2015, 62, 333–339. [Google Scholar] [CrossRef][Green Version]
- Silva, C.F.; Pereira, M.G.; Gomes, J.H.G.; Fontes, M.A.; Silva, E.M.R. Enzyme Activity, Glomalin, and Soil Organic Carbon in Agroforestry Systems. Floresta Ambiente 2020, 27, e20170716. [Google Scholar] [CrossRef]
- Sivetz, M.; Desrosier, N.W. Physical and chemical aspects of coffee. In Coffee Techonology; AVI Publishing Company: Westport, DC, USA, 1979; pp. 527–575. [Google Scholar]
- Voilley, A.; Sauvageot, F.; Simatos, D.; Wojcik, G. Influence of some processing conditions on the quality of coffee brew. J. Food Process. Preserv. 2007, 5, 135–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).