Abstract
This study aims to investigate if high-resolution dissolved organic matter (DOM) data, obtained from water by chromatographic analyses, enable us to assess whether seasonal climate change and anthropogenic activities in the catchment area have an impact on the ecosystem’s sustainability. More specifically, the changes in the molecular properties of heterogeneous DOM from the hypertrophic River Vääna, Estonia, during the winter and spring seasons have been examined. The chromatographic HPLC method (HPLC-SEC), combined with UV-Vis spectroscopic detection, was used to characterize and fractionate DOM. Changes in several chromatographic/molecular parameters were investigated. The microbial-derived low-molecular-mass aromatic and heterocyclic compounds, humic substances (HS), and protein-like (PL) components were identified in the DOM. The HS to PL fractions ratio has been found to reflect the seasonal climatic change and can be applied as a potential environmental indicator. The River Vääna’s water was evaluated as sustainable, and even an anthropogenic impact was revealed. The results point out the usefulness of HPLC-SEC together with UV-Vis spectroscopy detection for climate change-related DOM studies in real environmental conditions.
1. Introduction
To protect and use natural waters and to ensure the sustainability of valuable water resources, it is important to know the ecological status of rivers and the processes occurring there. Most of the rivers in Estonia are classified as clean a by the European standard, that is, the waters are assessed as average or good quality. However, their high content of nutrients is one of the main environmental concerns. The total phosphorus (P) concentrations exceed 0.06 mg P L−1 in one fourth of Estonian rivers, caused mainly by industrial wastewater inputs and agricultural activity. The long-time monitoring data indicate improvement in the chemical and biological status of river waters because of advanced wastewater treatment procedures and new water purification plants. There have been improvements in agricultural activity, and less fertilisers have been used as well [1].
Dissolved organic matter (DOM) has an important ecological role in aquatic environments as it influences water’s properties and quality, but its chemical composition is variable, depending on the water source, and complex. It contains humic substances (HS) that are complex aromatic macromolecules, amino acids, amino sugars, peptides, and aliphatic compounds [2,3]. The HS, containing humic and fulvic acids, are reported to indicate the terrestrial input to water systems. Amino acids and other protein-like (PL) compounds are used as a universal marker for bacterial matter [4,5]. The DOM components are actually not considered as organic pollutants which are hazardous for the environment and, thus, are not measured in water monitoring stations. However, HS can interact with both inorganic and organic pollutants, like heavy metals and polycyclic hydrocarbons, and thus regulate their bioavailability and transport [6,7]. The organic pollution in Estonian rivers is not evaluated directly by analysing distinct chemicals but by measuring the biochemical oxygen demand over 7 days (BOD7) for easily degradable organic compounds, and for total organic compounds—the permanganate consumption. The latter also measures the content of HS indirectly. Hence, high permanganate consumption values might not only indicate organic pollutants but also a high HS content [8]. These methods provide indirect data on organic matter content, but very little information about its composition.
Important DOM distribution and transformation occurs in rivers and lakes, and these processes depend on local or global environmental conditions [9]. Increases in DOM concentrations in water have been observed in many countries but the reasons are unclear. Usually, factors like climate, land use, and anthropogenic input have been outlined. High concentrations of DOM were reported to reflect a browning process that increases the water colour and is caused by terrestrial and wetland DOM. Its increase has been explained by climate change, weather conditions, and altered land-use [10]. Factors influencing DOM mobility in water systems are, according to [11], acid deposition; climate change increasing temperature, precipitation, and thawing; land, forest, and wetland cover; land use, agricultural activities; site-specific practices such as drainage; and combinations of all of those. Climate change has been stated to enhance terrestrial productivity. Increases in precipitation increase the amount of DOM leaching from soils. Even a 30% increase in DOM in freshwater originating from soils as a result of a 10% precipitation increase has been reported [11]. A comprehensive review that addresses the increasing trends in DOM in connection to browning, with an emphasis on the Nordic country Sweden, has been published [10]. The authors point out the effect of snowmelt, as it exports the highest amounts of DOM to surface waters. Another extensive study covers the increase processes of DOM in Finnish forest lakes during a time period from 1987 to 2003 [12]. The authors attempted to identify causes and found catchment size to be one of the important factors to consider as increasing DOM trends are recorded. The other factor that they reported was the change in runoff both annually and seasonally. As in the case of Finland, Estonia also has large seasonal temperature fluctuations and, in winter season, lakes and rivers are covered with ice and land with snow; thus, the reasoning reported in [12] can be reckoned. In wintertime, air and water temperatures are low and DOM is expected not to leach from soils to river water. During springtime, the increases in temperature and in the amount of precipitation (rain) or snowmelt cause elevated runoff from soils and increasing discharge [13,14]. The DOM concentrations in some streams have been found to increase seasonally from spring to autumn and exhibit the lowest concentrations in winter [12].
The DOM components’ contents and their changes can reflect water quality and could be useful for tracking the man-made influence on aquatic systems and evaluating their sustainability. Relatively few studies have focused on environmental parameters and their effects on DOM constituents in Estonia [15]. This is the second study on Estonian rivers. The first study was conducted with water samples from River Pirita. The present study focuses on River Vääna’s DOM components and composition changes. This river is classified in Estonia based on its water type: light water with a low content of organic matter. The river has nature conservation restrictions and nearly all of it is within the Natura 2000 area. A comprehensive review of the River Vääna, including its hydromorphological description, water properties, and quality overview, key risks, and impact factors can be found in [16]. As this information is largely unavailable, the most important information is briefly reported below. According to the available data, the natural bed of Vääna River has been preserved relatively well, and despite some revised passages, the river has acquired a close-to-natural look. Regarding its DOM contents, only BOD7 data are available and its water quality was assessed based on those values. The reported BOD7 varied between 2 and 6 mg L−1 and showed a decreasing trend from the winter months (October to April) to spring and summer [16].
Since DOM is naturally a heterogeneous mixture of molecules, the chemical characterisation of it is very complicated. The chromatographic methods are useful in the case of such complex matrices. High-performance size-exclusion chromatography (HPLC-SEC) has been one of the most used among them because of several previously reported advantages [17,18,19,20,21]. As a part of DOM is optically active, containing chromophores, the spectroscopic methods based on UV-absorption can be used for detection; among them is the more widely applied diode array detection (DAD) [22]. The HPLC data output (chromatogram) thus represents a fingerprint of chromophores in DOM, separated into size fractions in the order of decreasing molecular masses. The fingerprinting approach can provide qualitative information on organic compounds without the need for calibrating the separation column for molecular mass determination. Peak identification in chromatograms is carried out by extracting the UV-Vis spectra of the peak from the DAD data and comparing them with the computer database records of suitable standards (proteins, amino acids, humic and fulvic acids). Because of its higher sensitivity and selectivity, fluorescence spectroscopy has become more widely used to characterize DOM in natural waters. Fluorescence excitation-emission spectroscopy combined with parallel factor analysis has revealed the protein-like and humic matter like fluorophores in DOM. An even more detailed division of DOM fluorophores has been described in [23]: from protein-like to tyrosine; tryptophan- and phenylalanine-like; from humic-like to visible; and humic-like.
HPSEC combined with organic carbon and UV detection has been used to characterise DOM and separate it into five types, based on molecular mass: humic substances, biopolymers, building blocks that are breakdown products of humic substances, LMM acids, and LMM neutrals [24]. Fluorescence analysis was conducted separately from HPSEC and additional information was obtained as five fluorophores were detected, two aromatic protein-like, fulvic- and humic-like, and soluble microbial products [24].
HPSEC connected directly to UV and fluorescence detection has been applied in DOM transformation studies during water treatment processes and water quality monitoring [25,26]. The advantages and disadvantages of both detection methods were pointed out. The advantages of UV detection are the detection of aromatic components of DOM and the detection of substances including conjugated double bonds in their structure. The disadvantages, when using only one specific detection wavelength of 254 nm, are low sensitivity and the impossible differentiation of humic compounds from aromatic proteins. Fluorescence detection enables detection at a much lower concentration level than UV and has made DOM fractions’ characterisation possible at different excitation-emission wavelengths [25,26]. Based on these studies, it can be concluded that these methods could also be successfully used in the study of the quality of natural waters and in the study of changes in organic matter in freshwater.
The aim of the present study was to investigate the changes in the chemical properties of DOM components separated by HPLC from the hypertrophic River Vääna during the winter and spring seasons (from March to May), when it is known to have high organic pollutant records. Since the water temperature in Estonia in winter is near zero, a self-purification process of the river might not work at all, and the organic pollutants are not degraded but carried with water up to the Baltic Sea [16]. However, at low temperatures, the dissolved oxygen concentrations in the water should be permanent and, despite missing biological activity, very slow self-purification could be possible. Generally, the self-purification methods of water can be divided into physical, chemical, and biological methods (dilution, oxidation, reduction, sedimentation, bio- and photodegradation). Dilution under aerobic conditions decreases the concentration of DOM. Parts of DOM can be oxidized by aerobic bacteria using dissolved oxygen in the water, while the opposite process of DOM reduction by anaerobic bacteria can also occur [27]. The virtually non-existent process of self-purification might enable us to assess the water quality by studying the DOM and its constituents and their dynamics in relation to seasonal climate change.
A secondary aim was to clarify the role of a potential man-made pollution source on the river water quality. Effluents from the nearby factory may contain organic matter, although the wastewater is treated on-site. The purified wastewater is directly discharged to River Vääna. The water quality is assessed by studying the DOM fingerprints registered by HPLC-SEC before and after the factory (1 km distance between the sampling points). Conclusions from these studies make it possible to assess the sustainability of river water.
2. Materials and Methods
2.1. Study Site and Sampling
The Vääna River, Harju County, Estonia, is a 64 km-long river and its catchment area is 316 km2. Its catchment land types are agricultural (44%), forest (35%), arable (25%), and wetlands (10%). The Vääna River starts from the sources of Paekna in Kiili municipality and flows into the Gulf of Lohusalu in Harku municipality (Gulf of Finland). The variations in flow are seasonally large, for example the average flow rate (m3s−1) is 1.92, with minimum values in winter of 0.40 and in summer of 0.27. The condition of the river is poor, with characteristic brown muddy water colour. The floodwaters flow into the river from ditches, bringing pollution that is caused by domestic and industrial effluents, as well as agricultural activities. In midsummer, the water stagnates and the shallow river is thickly grassy. The Vääna River water has one of the worst qualities in Estonia, being hypertrophic and slightly alkaline with a pH of 8.0–8.2 [28,29].
The locations of the sampling points were chosen to identify possible anthropogenic pollution in the Vääna River. From the 1960s to the 1990s, the Vääna River was heavily polluted. Under the influence of the wastewater of the Saku brewery, the fish disappeared completely from the middle course of the river. When wastewater purification was launched in Saku in the 1970s, the situation improved. A brewery is located between the test points and is the largest potential source of pollution throughout the Vääna River. Water samples were taken nine times weekly during the winter and spring seasons, from March to May 2013. Sampling point locations were 59°17′46.7″ N 24°40′17″ E and 59°18′7.8″ N 24°40′0.15″ E, to investigate river water quality before and after a small pond nearby the potential pollution source—Saku Brewery (Figure 1). The factory uses, annually, 370,700 m3 of groundwater (2015) that is purified and lead to the common drainage system [29]. The factory is stated to cause no hazard to the surrounding environment. However, the exact data of compounds in the purified wastewater are unavailable. River water hydrology data obtained from Estonian Weather Service are presented in Figure 2. Water and air temperatures and water level measurements were carried out directly on the monitoring station [30]; water discharge values are available in [31]. The water samples were stored at 4 °C in the dark and analysed on the next day.
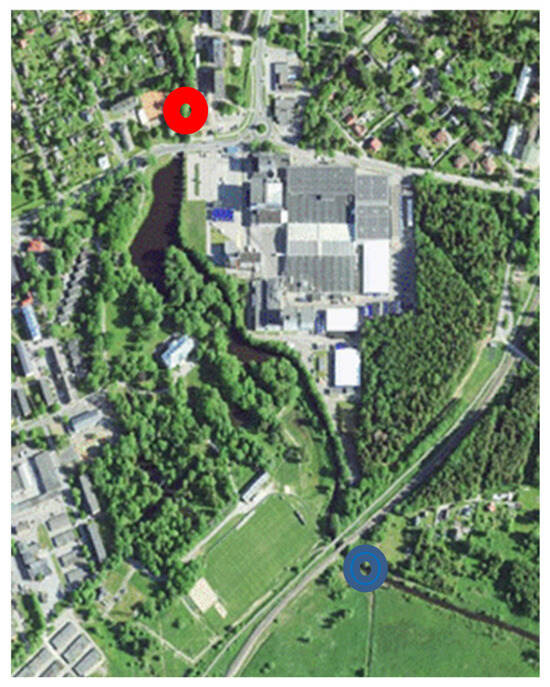
Figure 1.
Map of sampling locations indicated by rings: before (blue) and after (red) the factory. Base map data: Estonian Land Board 2023 (https://maaamet.ee/en/spatial-data-and-maps/, accessed on 14 June 2023).
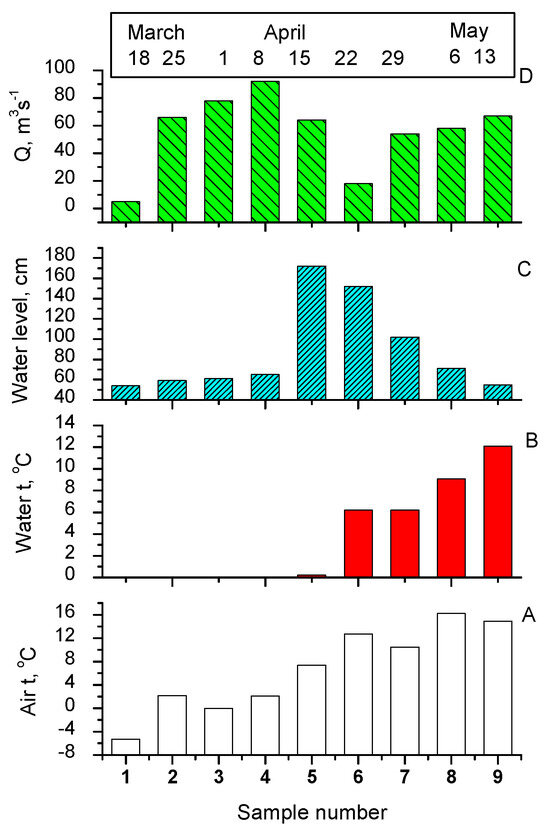
Figure 2.
Hydrological parameters, sampling dates, and numbering for River Vääna water samples: (A) air temperature, (B) water temperature, (C) water level, (D) discharge (Q).
2.2. Chromatographic Analysis
Chromatographic analysis procedure was described previously in [15,32]. Briefly, HPLC system with DAD (Agilent 1200 Series, Agilent Technologies, Stockport, UK) was used to obtain chromatograms and UV-Vis spectra of DOM. Separation was carried out on size-exclusion silica-based separation column BioSep-SEC-S2000 (analytical, length 300 mm, diameter 7.8 mm, Phenomenex, Torrance, CA, USA), preceded by a suitable security guard cartridge (Phenomenex). A 20 mM phosphate buffer with 10% methanol (pH 6.8) was used as eluent. Isocratic elution at flow rate of 0.5 mL min−1 was applied. All the injection volumes of sample solutions and standards were 20 μL. From three to six replicates were carried out for each sample. All solutions for HPLC measurements were prepared using distilled water passed through a MilliQ water purification system, filtered through 0.45 µm pore-size filters (Millipore, Burlington, MA, USA), and degassed. The chromatograms were recorded and processed by Agilent ChemStation software for LC 3D Systems Rev 8.02.01. In principle, spectral data were collected after every 2 s by scanning wavelengths from 200 nm to 600 nm. From the dataset, chromatograms recorded at three wavelengths 210, 254, and 280 nm were extracted and more closely monitored. The data obtained at the selected wavelength of 210 nm were used for sample comparison and assessment in the present paper. DOM dynamics were quantitatively examined by comparison of peak areas at selected wavelengths during three months. Because of the usage of spectroscopic detection, the obtained fractions reflect only chromophoric UV-absorbing DOM fractions. The quantitative characteristic—peak area, detected at 210 nm—corresponds to relative concentration of respective DOM fraction. This parameter was used to examine the temporal changes of DOM fractions and, thus, the possible effect of seasonal climate change.
The qualitative analysis of separated DOM fractions (high molecular mass (HMM), HS, PL, low molecular mass (LMM)) was accomplished by comparing the absorption spectra with existing records. The library was lab-made from different compounds and standards were run under similar conditions to the present study. As reference compounds, from the International Humic Substances Society (IHSS), natural organic matter (IHSS Nordic Reservoir NOM 1R108N), humic acid (IHSS Nordic Aquatic HA 1R105H), and fulvic acid (IHSS Nordic Aquatic FA 1R105F); the protein standards and tryptophan (bovine thyroglobulin, human gamma globulin, ovalbumin, myoglobin, uridine, Aqueous SEC1, (all from Phenomenex), peptide (tripeptide), nitrate; and LMM compounds (cytosine, creatinine, orotic acid, and uracil, all from Sigma-Aldrich, St. Louis, MO, USA) were analysed. In the chromatogram of DOM, recorded at wavelength 210 nm, peaks were assigned to (in the order of increasing retention time and decreasing molecular mass) HMM, HS, PL, and LMM fractions, and the total peak area was calculated as the sum of the separated fractions’ peak areas. Water quality was assessed by comparison of DOM chromatograms before and after the factory.
2.3. Statistical Analysis
The statistical data analyses were carried out using WinSTAT for Excel Version 2009.1 software (R. Fitch Software, Bad Krozingen, Germany). Basic statistics and confidence intervals at 95% level were calculated and used to discriminate between samples.
3. Results and Discussion
3.1. DOM Molecular Fractions and Water Quality
In order to evaluate the quality of the water of River Vääna and to reveal the possible site-specific effects, water was sampled at two locations 1 km apart, separated by a small pond and named before and after. There is an inflow of purified wastewater from a nearby factory to the pond. In the case of natural changes in the DOM caused by seasonal climate conditions, the chromatograms should look similar. Figure 3a shows two overlayed chromatograms of sample No. 1 from March before and after the factory. Generally, the DOM was separated into HMM, HS, PL, and LMM fractions. However, HMM and LMM fractions were missing from the early spring samples.
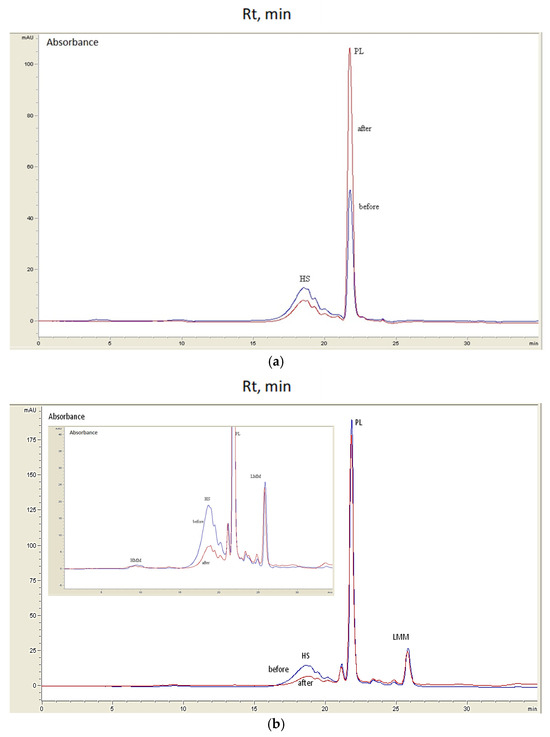
Figure 3.
Examples of River Vääna water DOM chromatograms before (blue line) and after (red line) the pond (factory) at detection wavelength of 210 nm; samples: (a) No. 1, 18 March; (b) No. 8, 6 May. Note: Rt, retention time.
The HMM fraction (1–2% of total HPLC peak) was not present in all of the samples that were analysed (not detected in March samples, Nos. 1 and 2), and contained aggregated humic molecules. The HS fraction (broad peak eluting between 16 and 21 min) was clearly separated from the following PL fraction (sharp peak at 22 min, identified as tripeptide) [15]. The chromatograms of Figure 3b from sample No. 8 (6 May) show differences in the HS, PL, and LMM fractions. Sample 1 (18 March) and both May (6 and 13, Nos. 8 and 9) samples are characterized by a higher HS content before the pond that decreased by 50% after. The opposite was noticed for the PL fraction. The decrease in HS content after the pond can be explained by purified effluent water input from the nearby factory that is defined as a dilution effect. The increase in the PL fraction might indicate wastewater inflow that is not sufficiently treated. Alternatively, springtime enhances microbial activity in natural water systems. The bacteria living in river water can oxidize organic matter, giving rise to PL compounds [33]. Thus, the current study indicated the possible decay of refractory humic compounds. The increase in the LMM fraction content (elution at 23–26 min) was following the PL fraction behaviour. The LMM fraction consisted of several small peaks having a UV-absorbance maximum at around 280 nm, characteristic of aromatic and heterocyclic compounds, but the exact identification by spectral library data was not possible. The rest of the data did not indicate substantial differences between chromatograms and thus reflected a similar and unchanged water quality. The HMM fraction composition was proposed by [25] to contain organic colloidal matter and biopolymers such as protein-like compounds, aminosugars, and polysaccharides. According to the results of the previous study, large molecules of humic substances can form aggregates together with protein-like molecules. Large protein-like structures can be derived from bacteria and other biological activity. These HMM compounds are considered to be weakly bonded because of van der Waals forces and a change in hydrological conditions can possibly degrade the aggregates and produce LMM compounds of an aromatic character [15]. The HMM compounds can possibly be transformed by microbial action into bioavailable forms. The absence of an HMM fraction can be explained by decomposition processes prevailing in the water environment. The LMM compounds can also be produced in a water environment by microorganisms and plants. The LMM compounds can form structures that are found in HS by condensation or polymerization reactions and, in this process, amino acids and carbohydrates are released [2].
3.2. Temporal Changes
The changes in DOM components of water from River Vääna were examined by the comparison of HPLC-SEC chromatogram peak areas as quantitative characteristics throughout three months. In Figure 4, only the results obtained at 210 nm are presented, as this wavelength enables the detection of, besides HS, amino acids/peptides and simple carboxylic acids. As shown in Figure 4B,D, the total DOM and PL fraction contents changed similarly during the studied time. The PL fraction maximum was detected on 15 April (sample No. 5) in the high-water period when snowmelt enters the river (water level is 172 cm, see Figure 2C) and brings fresh organic material to the river water. The HS fraction (Figure 3a) reached its maximum value on 29 April (sample No. 7), at the end of the high-water period (water level is 102 cm).
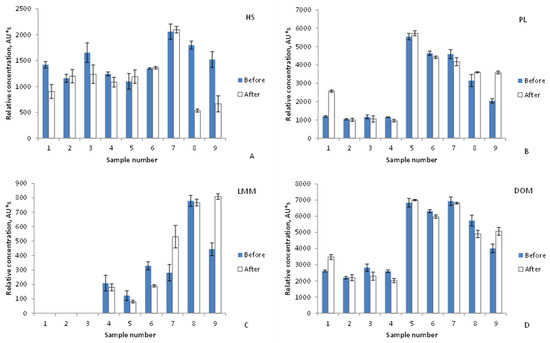
Figure 4.
Changes in River Vääna water DOM and its components: (A) HS, (B) PL, (C) LMM, (D) DOM. Relative concentration = peak area of respective fraction, detection at 210 nm. For sample numbers and dates, see Figure 2. Vertical bars indicate confidence interval (±95%). Note: DOM, dissolved organic matter; HS, humic substances; PL, protein-like compounds, LMM, low molecular mass compounds.
The high-water period usually lasts from 30 to 45 days [29]. The water level in this river is normally around 55–65 cm. Water level increases result in the decreasing of the HS and the increasing of the PL fractions, as observed in a previous study of River Pirita [15]. The possible anthropogenic sources of PL substances in river water could be wastewater and sewage inputs, and agricultural activity. Similar findings have been reported in an earlier study [34]. A natural source of PL compounds can be the bacterial degradation of organic matter or phytoplankton growth [35]. Microbial components have also been found to be present in water samples due to soil characteristics [36]. A decrease in HS content can be the result of the dilution of HS originating from the soil. High discharge values for River Vääna in March–April (Figure 2D) can also indicate the inflow of soil water and thus the transportation of DOM into the river from the surrounding catchment area.
3.3. Seasonal Climate Induced Changes
Besides the high flow conditions, other environmental parameters like water and air temperatures can also have an influence on DOM changes in flow water systems. Temperature rises increase DOM concentrations in waters [37]. Water temperature is an excellent proxy for air temperature. Water temperatures in River Vääna were around 0 °C until 15 April and thereafter rose up to 12.1 °C (13 May) (Figure 2B). The air temperatures varied between −5.3 and 16.2 °C (March to May) (Figure 2A). According to environmental monitoring data, the coldest month of winter 2012–2013, with an average air temperature of −6.3 °C, (long–time average is −1.3 °C) was March. Rain and snowfall were also exceptionally low in March (only 13 mm, long-time average 33 mm) [29].
The present study indicates that the LMM fraction is the most influenced by water temperature increases. Figure 4C shows different values for the LMM fraction concentration. In chromatograms of samples Nos. 1–3, this fraction is non-existent. Thereafter, samples Nos. 4, 5, and 8 show statistically similar concentrations before and after the factory. Thus, the results refer to the natural origin of the LMM compounds found in the river water and not man-made pollution, except for samples 7 and 9. Figure 5 shows the effect of the air temperature on the relative contents of the DOM components HS (a), PL (b), and LMM (c). Air and water temperatures from under 0 to +7 °C, and from 0 to +6 °C, respectively, had no influence on DOM component concentrations. However, at higher temperatures, the PL components contents decreased (dependence on water temperature is strong, correlation coefficient R2 0.998). Figure 5B shows the change (decreasing PL fraction contents are surrounded by ellipse). The decrease in the PL fraction can be attributed to rising microbial activity, as in the case of River Pirita [15]. Possibly, the purified wastewater inflow caused decreased concentrations of HS components after the factory (circled), since the opposite trend is evident for samples before it (Figure 5A). The LMM fraction content continuously increased with rises in air temperature (Figure 5C), similarly to water temperature. The obtained results indicate that temperature changes might not be the only reason for changes in DOM components, and that anthropogenic factors must be considered as well.
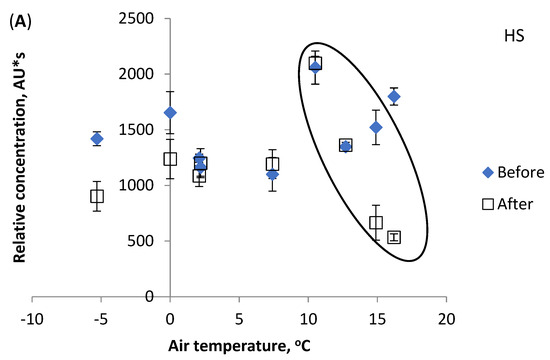
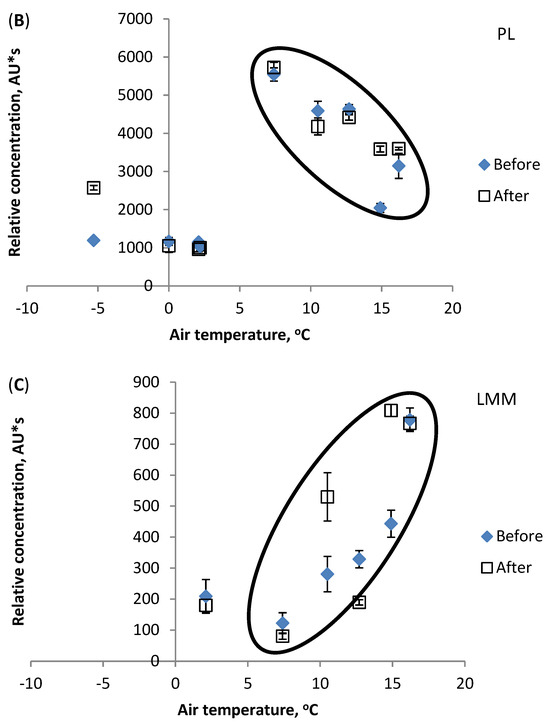
Figure 5.
Effect of air temperature on relative concentration (peak area) of DOM components: (A) HS, (B) PL, (C) LMM. Vertical bars indicate confidence interval at ±95% level.
3.4. Environmental Indicators and Water Sustainability
The river water DOM was separated into four fractions. Only HS and PL were present in every sample studied. The ratio of relative concentrations of the HS to the PL change is shown in Figure 6. The seasonal climate change effect on DOM can be observed from the central part of the figure, where the ratio of the HS to the PL sharply decreases from 1.2 to 0.2. Anthropogenic or man-made effects on DOM can be seen in the beginning and at the end of the studied time period, where the difference in the ratio for water before and after the pond is the largest. Before the pond, it was 1.2, and it then decreased to 0.4 (sample No. 1, 18 March). A similar trend was observed for both May samples, No. 8 and 9 (decrease from 0.6–0.8 to 0.2). Considering the results, the usage of the HS to PL fractions ratio as an indicator for monitoring of the natural as well as anthropogenic effects on river water systems could be largely applicable.
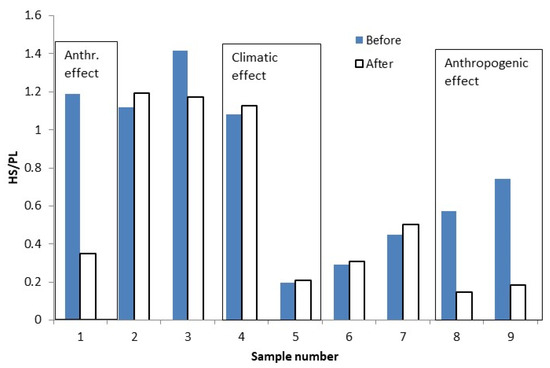
Figure 6.
Changes in DOM components ratio HS/PL in water before and after the factory.
For water system sustainability evaluation, the investigation of DOM separated molecular fractions data could find a larger application. The present study indicated the changes in freshwater during the winter and spring seasons: the absence of an aggregated HMM fraction and LMM fraction at low temperatures (Figure 4 and Figure 5C). The detection of all DOM fractions indicated sustainable conditions for biological activity in the river. HS are carried into the river from the catchment area as can be seen from Figure 4A and Figure 5A (see data before the factory). The decrease in this fraction after sample No. 7 (Figure 4A) could be explained by increasing water discharge (Figure 2D) or by utilization by algae, bacteria, and aquatic plants. Temperature rises could also support this phenomenon. The organic matter has been found to be sensitive to climate changes, water discharge from watersheds and from the catchment area, and to temperature, biological productivity, and oxygen content [38].
The sustainability of the water was assessed by analyzing DOM fractions after the factory (pond) in comparison with the obtained data from the natural undisturbed river water (see in Figures: marked as before). The HS data (see Figure 4) indicate differences and decreasing contents (early March and both May samples) that may be explained by an additional purified water input. At the same time, the PL and LMM DOM fractions concentrations increased in the water after the factory, suggesting either non-efficient water purification or enhanced biological activity. Figure 6 shows the anthropogenic effect on water as evaluated by the HS to the PL ratio. Thus, the chromatographic method can detect man-made changes in river water. Despite obvious anthropogenic influence, the water system can be evaluated as sustainable in the immediate vicinity of the brewery.
4. Conclusions
The water quality of River Vääna was monitored and assessed and changes in DOM and its components were studied by the liquid chromatographic method. Based on the results, the following conclusions could be made:
- The HPLC–SEC method enables DOM analysis with excellent reproducibility and efficiency;
- River Vääna water DOM is separated into HMM, HS, PL, and LMM fractions;
- Relative HS fraction content can serve as an indicator for the anthropogenic effect on river water systems (dilution effect by factory effluent water input);
- Air and water temperatures from under 0 to +7 °C, and from 0 to +6 °C, respectively, have no influence on DOM components concentrations; at higher temperatures (>7 °C), the PL concentration strongly depends on the water temperature, and the LMM concentration depends on both temperatures;
- The HS to PL ratio reflects the seasonal climatic change and also the anthropogenic factors, and can serve as a potential environmental indicator;
- The water of River Vääna can be evaluated as sustainable, based only on DOM results of the present study, since no sustainability standards exist, in the immediate vicinity of the brewery, although an anthropogenic impact was revealed.
The results showed that analysing organic compounds in flow-waters can give significant information about the natural or man-made processes in the surrounding environment. More knowledge can be obtained on how DOM changes correlate with seasonal climate change and anthropogenic impacts.
Generally, the results point out the usefulness of HPLC for climate change-related DOM studies in real environmental conditions. The used method enables detailed monitoring of the HS as well as PL components of DOM. The new data obtained in the present study may help to clarify the role of the HS and PL components of DOM on bioactivity changes due to temperature and water level effects.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The assistance during sampling and chromatographic analysis by K. Suursööt and constructive comments of the four anonymous reviewers are thankfully acknowledged.
Conflicts of Interest
The author declares no conflict of interest.
References
- Iital, A.; Pachel, K.; Loigu, E.; Pihlak, M.; Leisk, Ü. Recent trends in nutrient concentrations in Estonian rivers as a response to large-scale changes in land-use intensity and life-styles. J. Environ. Monit. 2010, 12, 178–188. [Google Scholar] [CrossRef]
- Klavins, M. Aquatic Humic Substances: Characterization, Structure and Genesis; Riga University Press: Riga, Latvia, 1997; ISBN 9-98-45165-20. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Hudson, N.; Bake, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Terashima, M.; Tanaka, S.; Fukushima, M. Distribution behavior of pyrene to adsorbed humic acids on kaolin. J. Environ. Qual. 2003, 32, 591–598. [Google Scholar] [CrossRef]
- Xu, X.; Thomson, N.R. A long-term bench-scale investigation of permanganate consumption by aquifer materials. J. Contam. Hydrol. 2009, 110, 73–86. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G.; Haumaier, L.; Zech, W. The composition of dissolved organic matter in forest soil solutions: Changes induced by seasons and passage through the mineral soil. Org. Geochem. 2002, 33, 307–318. [Google Scholar] [CrossRef]
- Kritzberg, E.S.; Maher Hasselquist, E.; Skerlep, M.; Löfgren, S.; Olsson, O.; Stadmark, J.; Valinia, S.; Hansson, L.-A.; Laudon, H. Browning of freshwaters: Consequences to ecosystem services, underlying drivers, and potential mitigation measures. Ambio 2020, 49, 375–390. [Google Scholar] [CrossRef]
- Blanchet, C.C.; Arzel, C.; Davranche, A.; Kahilainen, K.K.; Secondi, J.; Taipale, S.; Lindberg, H.; Loehr, J.; Manninen-Johansen, S.; Sundell, J.; et al. Ecology and extent of freshwater browning—What we know and what should be studied next in the context of global change. Sci. Total Environ. 2022, 812, 152420. [Google Scholar] [CrossRef]
- Vuorenmaa, J.; Forsius, M.; Mannio, J. Increasing trends of total organic carbon concentrations in small forest lakes in Finland from 1987 to 2003. Sci. Total Environ. 2006, 365, 47–65. [Google Scholar] [CrossRef]
- Laudon, H.; Köhler, S.; Buffam, I. Seasonal TOC export from seven boreal catchments in northern Sweden. Aquat. Sci. 2004, 66, 223–230. [Google Scholar] [CrossRef]
- Kortelainen, P.; Saukkonen, S.; Mattsson, T. Leaching of nitrogen from forested catchments in Finland. Glob. Biogeochem. Cycles 1997, 11, 627–638. [Google Scholar] [CrossRef]
- Lepane, V.; Depret, L.; Väli, A.-L.; Suursööt, K. Impact of seasonal climate change on optical and molecular properties of river water dissolved organic matter by HPLC-SEC and UV-vis spectroscopy. Chem. Biol. Technol. Agric. 2015, 2, 14. [Google Scholar] [CrossRef]
- Järvekülg, R.; Lauringson, E. Vääna Jõe Seisund, Probleemid Ja Võimalikud Rehabilitatsioonimeetmed (Harku Valla Piires); Thymallus OÜ: Tallinn, Estonia, 2010. (In Estonian) [Google Scholar]
- Yan, M.; Korshin, G.; Wang, D.; Cai, Z. Characterization of dissolved organic matter using high-performance liquid chromatography (HPLC)—Size exclusion chromatography (SEC) with a multiple wavelength absorbance detector. Chemosphere 2012, 87, 879–885. [Google Scholar] [CrossRef]
- Chin, Y.P.; Aiken, G.; O’Loughlin, E. Molecular Weight, Polydispersity, and Spectroscopic Properties of Aquatic Humic Substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Asakawa, D.; Kiyota, T.; Yanagi, Y.; Fujitake, N. Optimization of conditions of high-performance size-exclusion chromatography of different soil humic acids. Anal. Sci. 2008, 24, 607–613. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Foss, D.; Cho, J.; Yoon, Y.; Kosenka, P. Optimization of method for detecting and characterizing NMO by HPLC-size exclusion chromatography with UV and on-line DOC detection. Environ. Sci. Technol. 2002, 36, 1069–1076. [Google Scholar] [CrossRef]
- Nagao, S.; Matsunaga, T.; Suzuki, Y.; Ueno, T.; Amano, H. Characteristics of humic substances in the Kuji River waters as determined by high-performance size exclusion chromatography with fluorescence detection. Water Res. 2003, 37, 4159–4170. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.W.K.; Drikas, M.; Korshin, G.; Amal, R. Multi-wavelength spectroscopic and chromatography study on the photocatalytic oxidation of natural organic matter. Water Res. 2010, 44, 2525–2532. [Google Scholar] [CrossRef]
- Gullian-Klanian, M.; Gold-Bouchot, G.; Sanchez-Solis, M.J. Characteristics of Chromophoric Dissolved Organic Matter (CDOM) Produced by Heterotrophic Bacteria Isolated from Aquaculture Systems. J. Mar. Sci. Eng. 2022, 10, 672. [Google Scholar] [CrossRef]
- Hidayah, E.N.; Lai, W.L.; Cahyonugroho, O.H.; Rizqa, F. Organic matter from biofilter nitrification by high performance size exclusion chromatography and fluorescence excitation-emission matrix. Glob. J. Environ. Sci. Manag. 2020, 6, 133–144. [Google Scholar] [CrossRef]
- Ignatev, A.; Tuhkanen, T. Step-by-step analysis of drinking water treatment trains using size-exclusion chromatography to fingerprint and track protein-like and humic/fulvic-like fractions of dissolved organic matter. Environ. Sci. Water Res. Technol. 2019, 5, 1568. [Google Scholar] [CrossRef]
- Pettersson, S.; Ignatev, A.; Lindholm-Lehto, P.; Tuhkanen, T. Monitoring of water quality with HPLSEC and fluorescence method in the ozonated recirculating aquaculture system. Environ. Monit. Assess. 2023, 195, 1497. [Google Scholar] [CrossRef]
- Nugraha, W.D.; Sarminingsih, A.; Alfisya, B. The Study of Self Purification Capacity Based on Biological Oxygen Demand (BOD) and Dissolved Oxygen (DO) Parameters. IOP Conf. Ser. Earth Environ. Sci. 2020, 448, 012105. [Google Scholar] [CrossRef]
- Järvekülg, A. Eesti Jõed; Tartu Ülikooli Kirjastus: Tartu, Estonia, 2001; ISBN 9-98-59293-06. (In Estonian) [Google Scholar]
- Kaukver, K. Estonian Environmental Monitoring 2013; Estonian Environmental Agency: Tallinn, Estonia, 2015. [Google Scholar]
- Hydrology Data. Available online: http://ilmateenistus.ee/siseveed/vaatlusandmed/tabel/ (accessed on 25 November 2014).
- Discharge Data. Available online: http://www.ilmateenistus.ee/siseveed/ajaloolised-vaatlusandmed/ (accessed on 17 December 2014).
- Lepane, V.; Tõnno, I.; Alliksaar, T. HPLC approach for revealing age-related changes of aquatic dissolved organic matter in sediment core. Procedia Chem. 2010, 2, 101–108. [Google Scholar] [CrossRef][Green Version]
- Simate, G.S.; Cluett, J.; Iyuke, S.E.; Musapatika, E.T.; Ndlovu, S.; Walubita, L.F.; Alvarez, A.E. The treatment of brewery wastewater for reuse: State of the art. Desalination 2011, 273, 235–247. [Google Scholar] [CrossRef]
- Goldman, J.H.; Rounds, S.A.; Needoba, J.A. Applications of fluorescence spectroscopy for predicting percent wastewater in an urban stream. Environ. Sci. Technol. 2012, 46, 4374–4381. [Google Scholar] [CrossRef]
- Reader, H.E.; Stedmon, C.A.; Nielsen, N.J.; Kritzberg, E.S. Mass and UV-visible spectral fingerprints of dissolved organic matter: Sources and reactivity. Front. Mar. Sci. 2015, 2, 88. [Google Scholar] [CrossRef]
- Pavelescu, G.; Ghervase, L.; Ioja, C.; Dontu, S.; Spiridon, R. Spectral fingerprints of groundwater organic matter in rural areas. Rom. Rep. Phys. 2013, 65, 1105–1113. [Google Scholar]
- Rodríguez-Murillo, J.C.; Zobrist, J.; Filella, M. Temporal trends in organic carbon content in the main Swiss rivers, 1974–2010. Sci. Total Environ. 2015, 502, 206–2017. [Google Scholar] [CrossRef]
- Street, J.H.; Scott Anderson, R.; Paytan, A. An organic geochemical record of Sierra Nevada climate since the LGM from Swamp Lake, Yosemite. Quat. Sci. Rev. 2012, 40, 89–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).