Hazard Identification of Hydrogen-Based Alternative Fuels Onboard Ships
Abstract
:1. Introduction
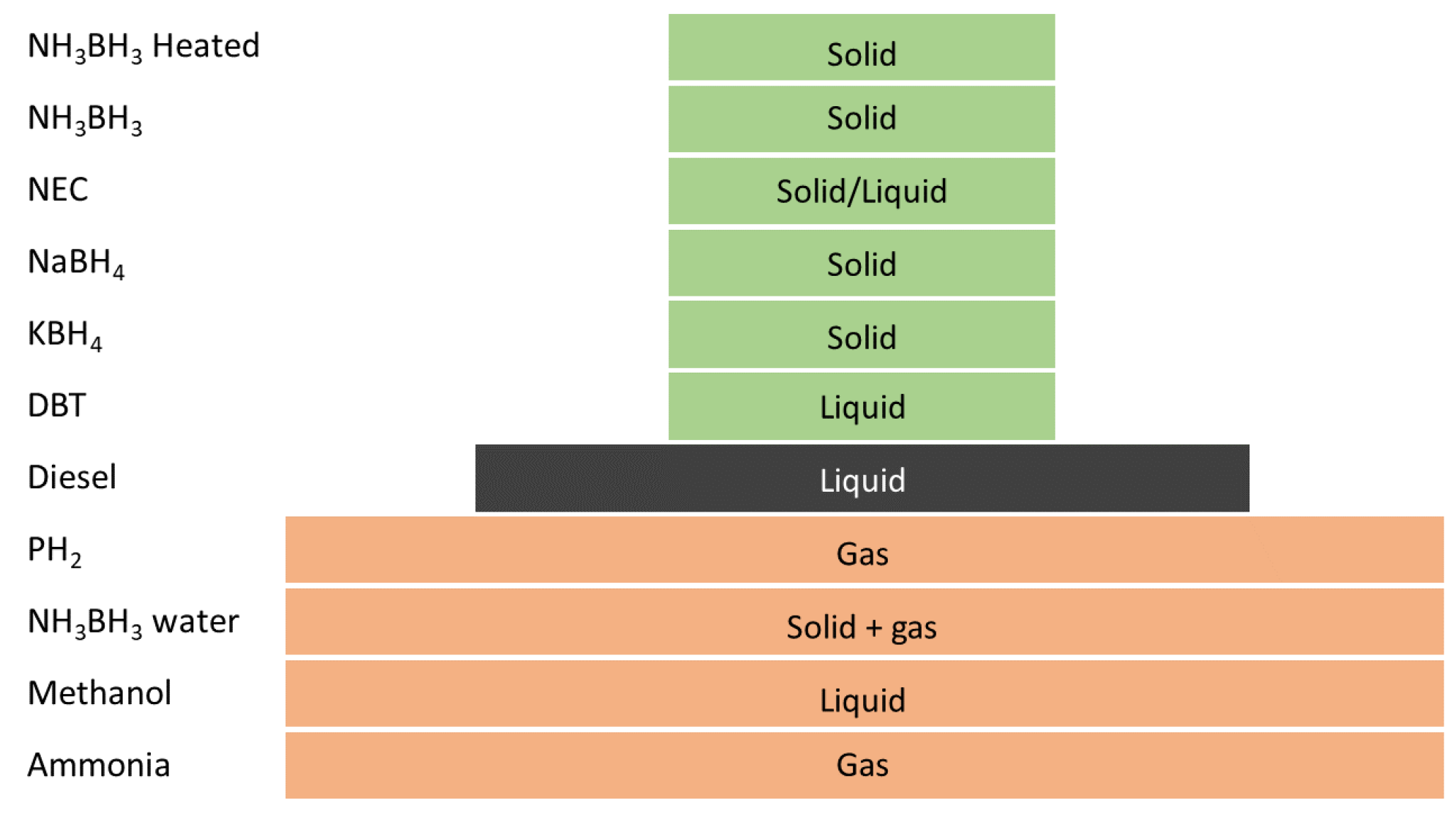
2. Hydrogen Carriers
| Carrier | MJ/kg | Source (MJ/L) | MJ/L | Source |
|---|---|---|---|---|
| AB | 23.52 | [8] | 14.4 | [18] |
| (hydrolysis) | 25.56 | [19] | 27.34 | [19] |
| (hydrolysis) | 17.76 | [20] | 20.78 | [20] |
| LOHC: NEC | 6.98 | [21] | 6.63 | [21] |
| LOHC: DBT | 7.44 | [11] | 7.0 | [22] |
| Ammonia | 21.12 | [23] | 11.5 | [23] |
| Methanol | 15.12 | [10] | 11.88 | [10] |
| MDO | 29 | [24] | 30 | [24] |
2.1. Borohydrides and AB
2.2. LOHCs
2.3. Ammonia and Methanol
3. Hazard Identification of Hydrogen Carriers and Selected Reference Fuels
3.1. Ghs Classification and Qualitative Research
3.2. Hazard Identification of LOHC: Dibenzyltoluene
3.3. Hazard Identification of LOHC: n-Ethylcarbazole
3.4. Hazard Identification of Borohydrides
3.4.1. Spent Fuel
3.4.2. Thermolysis
3.5. Hazard Identification of AB
- (Hydrolysis reaction, relatively stable reaction)
- (Hydrolysis reaction, see , relatively stable reaction)
- NH2BH2, aminoborane, extremely unstable, oligomerises easily (Thermolysis, 100 ℃)
- HNBH, iminoborane, extremely unstable, oligomerises easily (Thermolysis, 150 ℃)
- Borazine (Thermolysis, result of oligomerization)
3.5.1. Hydrolysis
3.5.2. Thermolysis
3.6. Hazard Identification of Ammonia and Methanol
3.7. Overview of Hazards of the Hydrogen Carriers and Reference Fuels Based on the GHS System
3.8. General Hydrogen Carrier Hazards
3.8.1. Hazard of Hydrogen Fire and Explosion
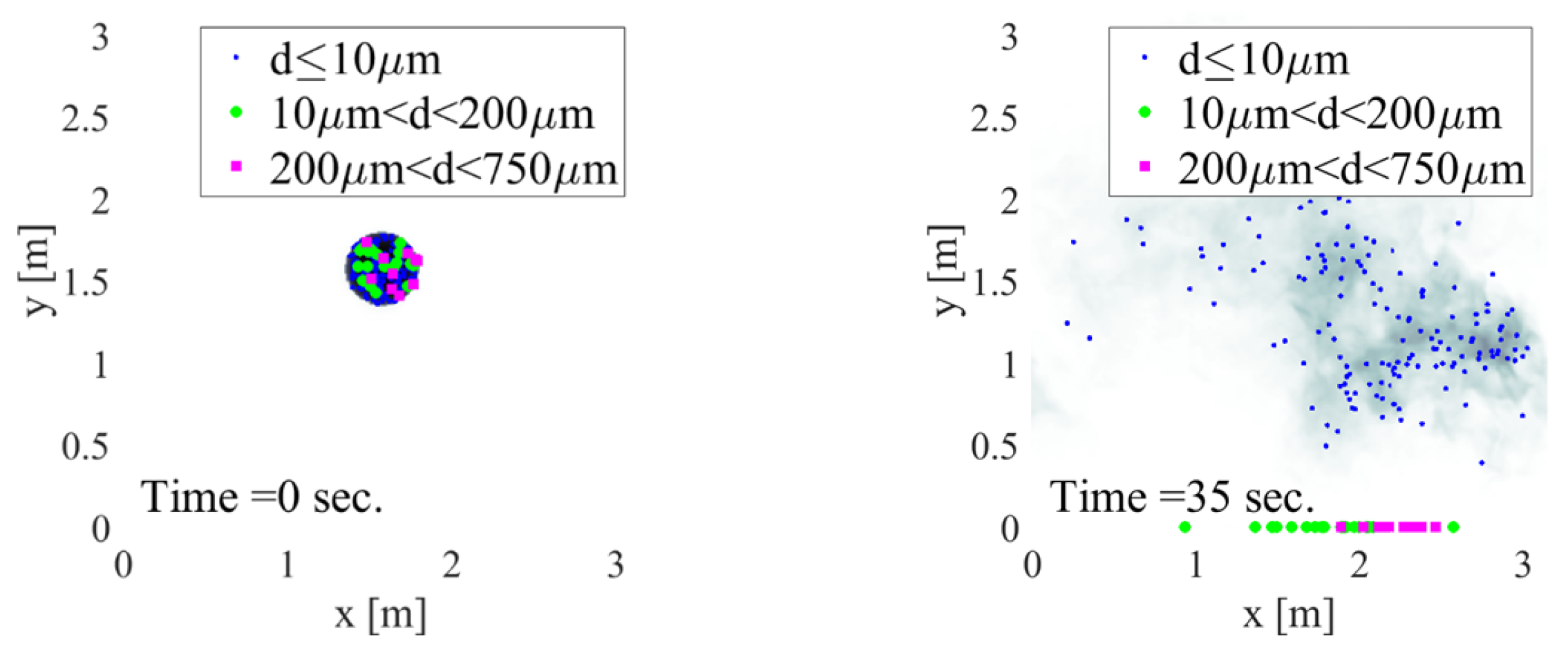
3.8.2. Aerosol Hazard Identification
4. Current Safety Approach in the Maritime Industry
4.1. IMO Safety Regulations
4.2. Goal-Based Standards (GBS)
- Safe and environmentally friendly means that the ship shall have adequate strength, integrity and stability to minimize the risk of loss of the ship or pollution to the marine environment due to structural failure, including collapse, resulting in flooding or loss of watertight integrity.
- Environmentally friendly also includes the ship being constructed of materials for environmentally acceptable dismantling and recycling.
- Safety also includes the ship’s structure being arranged to provide for safe access, escape, inspection and proper maintenance.
4.3. Implications of IMO Safety Regulations on Hydrogen Carriers
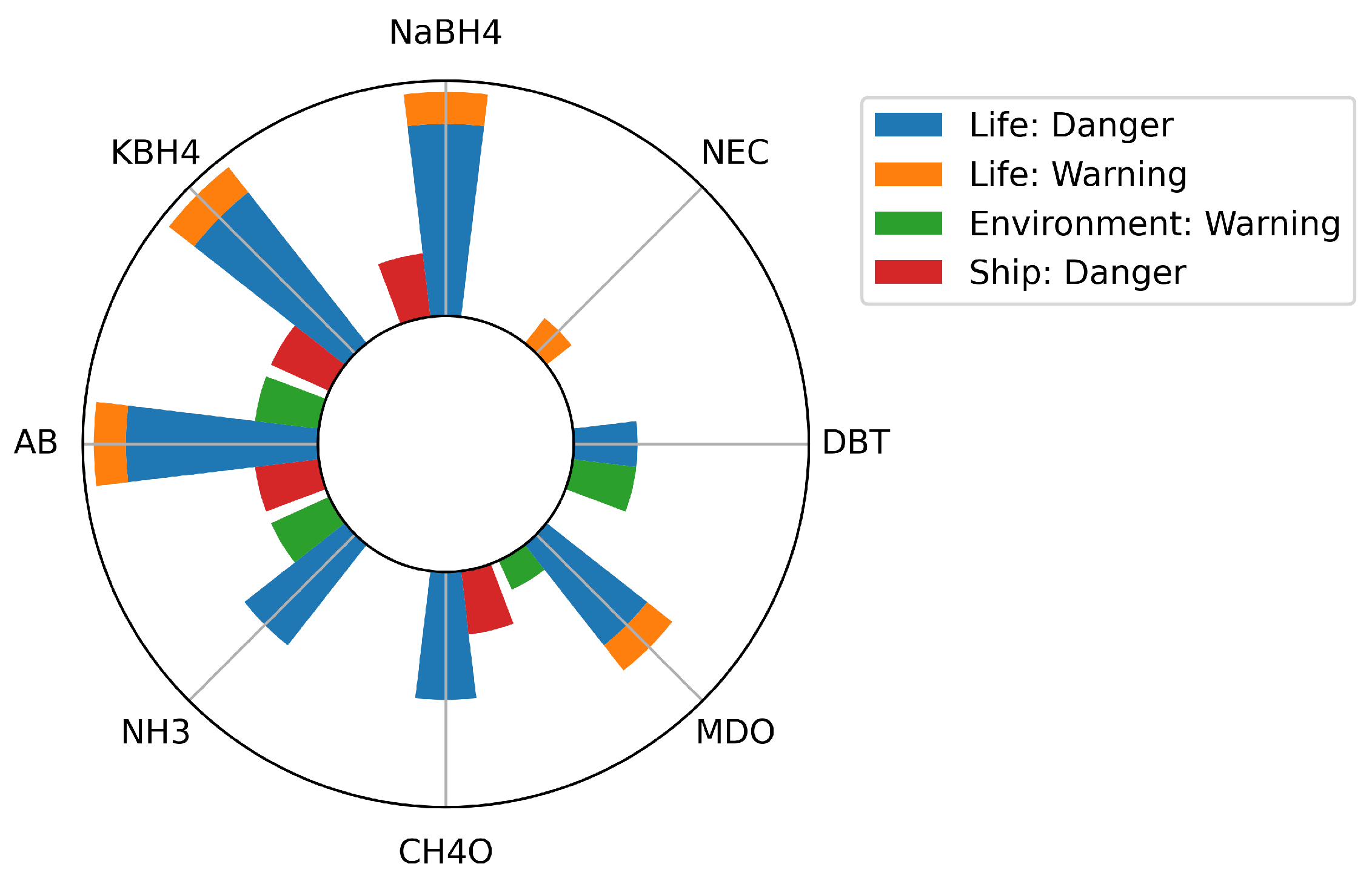
5. Influence of Hazards Accompanied by Alternative Fuels on The Approach to Safety on Ships
5.1. Possible Influence of Alternative Fuels on Life on Board
5.1.1. Methanol and LOHCs DBT and NEC
5.1.2. Borohydrides
5.1.3. AB
5.1.4. Ammonia
5.2. Possible Influence of Alternative Fuels on Ship Structural Integrity
5.3. Possible Influence of Alternative Fuels on the Environment
6. Conclusions
Discussion and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB | Ammoniaborane, |
| Tetrahydroxyboranuide | |
| Metaborate | |
| Carbon dioxide | |
| D | Danger |
| Diameter of a hydrogen fireball, conservative estimation | |
| DBT | 2,3-dibenzyltoluene |
| GBS | Goal-based standards |
| GHG | Greenhouse gas emissions |
| GHS | Globally Harmonized System of classification and labelling of chemicals |
| () | Borazine |
| HFO | Heavy fuel oil |
| IGF | International code of safety for ships using gases or other low-flashpoint fuels |
| ILO | International Labor Organization |
| IMO | International Maritime Organization |
| Potassium borohydride | |
| Potassium metaborate | |
| LNG | Liquid natural gas |
| LOHC | Liquid organic hydrogen carrier |
| UNCLOS | United Nations Convention on the Law of the Sea |
| MARPOL | International convention for the prevention of pollution from ships |
| MDO | Marine diesel oil |
| MEPC | Marine Environment Protection Committee |
| MLC | Maritime Labour Convention |
| MSC | Maritime Safety Committee |
| Sodium metaborate dihydrate | |
| Sodium borohydride | |
| Sodium metaborate | |
| NEC | N-ethylcarbazole |
| NH2BH2 | Aminoborane |
| Ammonia | |
| Ammonium | |
| NHBH | Iminoborane |
| Nitrogen oxides | |
| SOLAS | International Convention for the Safety of Life at Sea |
| Sulphur oxides | |
| TPI | Toxicity probability interval |
| TRL | Technology readiness level |
| W | Warning |
References
- IEA. International Shipping. Paris. 2021. Available online: https://www.iea.org/reports/international-shipping (accessed on 22 June 2022).
- Kass, M.D.; Sluder, C.S.; Kaul, B.C. Spill Behavior, Detection, and Mitigation for Emerging Nontraditional Marine Fuels; United States Department of Transportation, Maritime Administration: Washington, DC, USA, 2021. [Google Scholar]
- McKinlay, C.J.; Turnock, S.R.; Hudson, D.A. A Comparison of Hydrogen and Ammonia for Future Long Distance Shipping Fuels; Royal Institute of Naval Architects: London, UK, 2020. [Google Scholar]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.; Han, G.; Kang, S.; Yoo, Y.S.; Jeon, S.Y.; Bae, J. Comparative energetic studies on liquid organic hydrogen carrier: A net energy analysis. Renew. Sustain. Energy Rev. 2021, 150, 111447. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane: An extensively studied, though not yet implemented, hydrogen carrier. Energies 2020, 13, 3071. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Hoecke, L.V.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, H.; Wang, C.G.; Ye, E.; Xu, J.W.; Loh, X.J.; Li, Z. Current research progress and perspectives on liquid hydrogen rich molecules in sustainable hydrogen storage. Energy Storage Mater. 2021, 35, 695–722. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment based on chemical and economic properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Van Rheenen, E.S.; Padding, J.T.; Slootweg, J.C.; Visser, K. A review of the potential of hydrogen carriers for zero emission, low signature ship propulsion systems. In Proceedings of the International Naval Engineering Conference and Exhibition, Delft, The Netherlands, 8–10 November 2022. [Google Scholar] [CrossRef]
- Rothwell, D. Copyright Page. In The Oxford Handbook of the Law of the Sea; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Neste. Safety Data Sheet Marine Diesel Oil DMB Grade (MDODMB). 2017. Available online: https://www.neste.com/sites/neste.com/files/attachments/13999_marine_diesel_oileng.pdf (accessed on 12 November 2022).
- Neste. Safety Data Sheet Heavy Fuel Oil. 2019. Available online: https://www.neste.fi/static/ktt/14359_eng.pdf (accessed on 12 November 2022).
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Karkamkar, A.; Aardahl, C.; Autrey, T. Recent Developments on Hydrogen Release from Ammonia-Borane. Mater. Matters 2007, 2, 6–9. [Google Scholar]
- Rivarolo, M.; Improta, O.; Magistri, L.; Panizza, M.; Barbucci, A. Thermo-economic analysis of a hydrogen production system by sodium borohydride (NaBH4). Int. J. Hydrogen Energy 2018, 43, 1606–1614. [Google Scholar] [CrossRef]
- LaVersenne, L.; Bonnetot, B. Hydrogen storage using borohydrides. Ann. Chim. Sci. Mater. 2005, 30. [Google Scholar] [CrossRef]
- Brigljević, B.; Byun, M.; Lim, H. Design, economic evaluation, and market uncertainty analysis of LOHC-based, CO2 free, hydrogen delivery systems. Appl. Energy 2020, 274, 115314. [Google Scholar] [CrossRef]
- Wunsch, A.; Mohr, M.; Pfeifer, P. membranes Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes 2018, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Zwaginga, J.J.; Pruyn, J.F.J. An Evaluation of Suitable Methods to Deal with Deep Uncertainty Caused by the Energy Transition in Ship Design. In Proceedings of the SNAME 14th International Marine Design Conference, Vancouver, BC, Canada, 26–30 June 2022. [Google Scholar] [CrossRef]
- Interreg North-West Europe. H2SHIPS - System-Based Solutions for H2-Fuelled Water Transport in North-West Europe. 2022. Available online: https://www.nweurope.eu/projects/project-search/h2ships-system-based-solutions-for-h2-fuelled-water-transport-in-north-west-europe/ (accessed on 22 June 2022).
- Laversenne, L.; Goutaudier, C.; Chiriac, R.; Sigala, C.; Bonnetot, B. Hydrogen storage in borohydrides Comparison of hydrolysis conditions of LiBH4, NaBH4 and KBH4. J. Therm. Anal. Calorim. 2008, 94, 785–790. [Google Scholar] [CrossRef]
- Stephens, F.H.; Pons, V.; Baker, R.T. Ammonia–borane: The hydrogen source par excellence? J. Chem. Soc. Dalton Trans. 2007, 25, 2613–2626. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Hydrogenious. Novel Path Towards Safe Zero-Emission Shipping: Hydrogenious LOHC Technologies and Østensjø Group Join Forces with Tailwind from Enova Funding. 2021. Available online: https://www.hydrogenious.net/index.php/en/2021/07/02/lohc_maritime-2/ (accessed on 22 June 2022).
- Sekine, Y.; Higo, T. Recent Trends on the Dehydrogenation Catalysis of Liquid Organic Hydrogen Carrier (LOHC): A Review. Top. Catal. 2021, 64, 470–480. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Tanaka, H.; Tokoyoda, K.; Matsumoto, M.; Suzuki, Y.; Kiyobayashi, T.; Kuriyama, N. Hazard assessment of complex hydrides as hydrogen storage materials. Int. J. Hydrogen Energy 2009, 34, 3210–3218. [Google Scholar] [CrossRef]
- Makarov, D.; Shentsov, V.; Kuznetsov, M.; Molkov, V. Hydrogen Tank Rupture in Fire in the Open Atmosphere: Hazard Distance Defined by Fireball. Hydrogen 2021, 2, 134–146. [Google Scholar] [CrossRef]
- NCBI. GHS Classification. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/ghs/ (accessed on 12 November 2022).
- OSHA. Hazard Communication Standard: Labels and Pictograms. 2013. Available online: https://www.osha.gov/sites/default/files/publications/OSHA3636.pdf (accessed on 12 November 2022).
- Markiewicz, M.; Zhang, Y.Q.; Osmann, A.B.; Brückner, N.; Brückner, B.; Thöming, J.; Thöming, T.; Wasserscheid, P.; Stolte, S. Environmental and health impact assessment of Liquid Organic Hydrogen Carrier (LOHC) systems-challenges and preliminary results. Energy Environ. Sci. 2015, 8, 1035–1045. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound LCSS for CID 3034397, 2,3-Dibenzyltoluene. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2_3-Dibenzyltoluene#datasheet=LCSS (accessed on 12 November 2022).
- Caterbow, A.; Hausmann, J. The Impact of Hazardous Chemicals on Women, 2016. WECF . Available online: https://www.wecf.org/77912/ (accessed on 12 November 2022).
- Arkema. Dibenzyltoluene. 2013. Available online: https://www.arkema.com/files/live/sites/shared_arkema/files/downloads/socialresponsability/safety-summuries/hydrogen-peroxide-dibenzyltoluene-gps-2013-02-10-v0.pdf (accessed on 12 November 2022).
- ECHA. Dibenzylbenzene, Ar-Methyl Derivative. 2022. Available online: https://echa.europa.eu/nl/registration-dossier/-/registered-dossier/19786/6/2/1 (accessed on 22 November 2022).
- Markiewicz, M.; Zhang, Y.Q.; Empl, M.T.; Lykaki, M.; Thö, J.; Steinberg, P.; Stolte, S. Hazard assessment of quinaldine-, alkylcarbazole-, benzene-and toluene-based liquid organic hydrogen carrier (LOHCs) systems. Energy Environ. Sci. 2019, 12, 366. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound LCSS for CID 6836, 9-Ethylcarbazole. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9-Ethylcarbazole#datasheet=LCSS (accessed on 12 November 2022).
- ECHEMI. 9-Ethylcarbazole Safety Data Sheets. 2013. Available online: https://www.echemi.com/sds/n-ethylcarbazole-pid_Rock24510.html (accessed on 12 November 2022).
- NCBI. PubChem Compound Summary for CID 66624952, 9-Ethyldodecahydro-1H-carbazole. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9-Ethyldodecahydro-1H-carbazole (accessed on 12 November 2022).
- Amadis Chemical. 9-Ethyldodecahydro-1H-carbazole. 2022. Available online: https://www.amadischem.com/proen/591108/ (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 4311764, Sodium borohydride. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-borohydride#datasheet=LCSS (accessed on 12 November 2022).
- ROTH. Safety Data Sheet Sodium Borohydride 97%, Extra Pure. 2022. Available online: https://www.carlroth.com/medias/SDB-4051-GB-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wzMDMwOTB8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oNTkvaGFjLzkwNjEyODY0NDUwODYucGRmfDk5NjhmYjFiZTE2NzFlNmQxNzdjYjVlMWIxNzg3NWE3NTc5OTBmMTc1NTM2MzJkYTMwMGQ4OWUyNmU0ODM0ODc (accessed on 12 November 2022).
- CDH. Potassium Borohydride Cas No 13762-51-1 Material Safety Data Sheet SDS/MSDS. 2008. Available online: https://www.cdhfinechemical.com/images/product/msds/92_1897241378_POTASSIUMBOROHYDRIDECASNO13762-51-1MSDS.pdf (accessed on 12 November 2022).
- Zhou, Y.; Fang, C.; Fang, Y.; Zhu, F. Volumetric and Transport Properties of Aqueous NaB(OH)4 Solutions. Chin. J. Chem. Eng. 2013, 21, 1048–1056. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound LCSS for CID 145326, Sodium Metaborate. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-metaborate#datasheet=LCSS (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 23701135, Sodium Metaborate Dihydrate. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-metaborate-dihydrate#datasheet=LCSS (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 123325, Potassium Metaborate, 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-metaborate#datasheet=LCSS (accessed on 12 November 2022).
- Slootweg, J.C. (University of Amsterdam, Amsterdam, the Netherlands) Personal communication, 2022.
- NCBI. PubChem Compound LCSS for CID 16211214, Borax, 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Borax#datasheet=LCSS (accessed on 12 November 2022).
- Martelli, P.; Caputo, R.; Remhof, A.; Mauron, P.; Borgschulte, A.; Züttel, A. Stability and decomposition of NaBH4. J. Phys. Chem. C 2010, 114, 7173–7177. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound LCSS for CID 5360545, Sodium. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium#datasheet=LCSS (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 5462311, Boron. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Boron#datasheet=LCSS (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 24758, Sodium Hydride. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-hydride#datasheet=LCSS (accessed on 12 November 2022).
- LGC. SAFETY DATA SHEET Borol. 2022. Available online: https://static.cymitquimica.com/products/04/pdf/sds-A50000283MD.pdf (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 5462222, Potassium. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Potassium#datasheet=LCSS (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 82127, Potassium Hydride. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-hydride#datasheet=LCSS (accessed on 12 November 2022).
- NCBI. PubChem Compound LCSS for CID 132598553, Ammonia Borane. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia-borane#datasheet=LCSS (accessed on 12 November 2022).
- Sanyal, U.; Demirci, U.; Jagirdar, B.; Miele, P. Hydrolysis of Ammonia Borane as a Hydrogen Source: Fundamental Issues and Potential Solutions Towards Implementation. ChemSusChem 2011, 4, 1731–1739. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound LCSS for CID 223, Ammonium. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonium#datasheet=LCSS (accessed on 12 November 2022).
- Salama, Y.; Chennaoui, M.; Mountadar, M.; Rihani, M.; Assobhei, O. The Physicochemical and Bacteriological Quality and Environmental Risks of Raw Sewage Rejected in the Coast of the City of El Jadida (Morocco). Carpathian J. Earth Environ. Sci. 2013, 8, 39–48. [Google Scholar]
- Marion, G.M.; Millero, F.J.; Camões, M.F.; Spitzer, P.; Feistel, R.; Chen, C.T. PH of seawater. Mar. Chem. 2011, 126, 89–96. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound Summary for CID 16722522, Aminoborane. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aminoborane (accessed on 12 November 2022).
- NCBI. PubChem Compound Summary for CID 139816, Aminoboron. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aminoboron (accessed on 12 November 2022).
- Paetzold, P. Iminoboranes. Adv. Inorg. Chem. 1987, 31, 123–170. [Google Scholar] [CrossRef]
- Thompson, C.A.; Andrews, L. Reactions of B Atoms with NH3 To Produce HBNH, BNBH, and B2N. J. Am. Chem. Soc. 1995, 117, 6331. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound LCSS for CID 138768, Borazine. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Borazine#datasheet=LCSS (accessed on 28 March 2023).
- NCBI. PubChem Compound LCSS for CID 222, Ammonia. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia#datasheet=LCSS (accessed on 28 March 2023).
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Dolan, R.H.; Anderson, J.E.; Wallington, T.J. Outlook for ammonia as a sustainable transportation fuel. Sustain. Energy Fuels 2021, 5, 4830–4841. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound LCSS for CID 887, Methanol. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Methanol#datasheet=LCSS (accessed on 12 November 2022).
- Mjaavatten, A.; Bjerketvedt, D.K. A Hydrogen-Air Explosion in a Process Plant: A Case History. 2005. Available online: http://conference.ing.unipi.it/ichs2005/Papers/100096.pdf (accessed on 12 November 2023).
- Gerboni, R.; Salvador, E. Hydrogen transportation systems: Elements of risk analysis. Energy 2009, 34, 2223–2229. [Google Scholar] [CrossRef]
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Hydrogen Logistics: Safety and Risks Issues. In Hydrogen Infrastructure for Energy Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 127–148. [Google Scholar] [CrossRef]
- Molkov, V.; Saffers, J.B. Hydrogen jet flames. Int. J. Hydrogen Energy 2013, 38, 8141–8158. [Google Scholar] [CrossRef]
- Pasman, H.J.; Rogers, W.J. Safety challenges in view of the upcoming hydrogen economy: An overview. J. Loss Prev. Process Ind. 2010, 23, 697–704. [Google Scholar] [CrossRef]
- Baraldi, D.; Venetsanos, A.G.; Papanikolaou, E.; Heitsch, M.; Dallas, V. Numerical analysis of release, dispersion and combustion of liquid hydrogen in a mock-up hydrogen refuelling station. J. Loss Prev. Process Ind. 2009, 22, 303–315. [Google Scholar] [CrossRef]
- Ng, H.D.; Lee, J.H. Comments on explosion problems for hydrogen safety. J. Loss Prev. Process Ind. 2008, 21, 136–146. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q. Effect of vent size on vented hydrogen-air explosion. Int. J. Hydrogen Energy 2018, 43, 17788–17799. [Google Scholar] [CrossRef]
- Yanez, J.; Kuznetsov, M.; Souto-Iglesias, A. An analysis of the hydrogen explosion in the Fukushima-Daiichi accident. Int. J. Hydrogen Energy 2015, 40, 8261–8280. [Google Scholar] [CrossRef]
- Vuorinen, V.; Aarnio, M.; Alava, M.; Alopaeus, V.; Atanasova, N.; Auvinen, M.; Balasubramanian, N.; Bordbar, H.; Erästö, P.; Grande, R.; et al. Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Saf. Sci. 2020, 130, 104866. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, V.; Keskinen, K. DNSLab: A gateway to turbulent flow simulation in Matlab. Comput. Phys. Commun. 2016, 203, 278–289. [Google Scholar] [CrossRef]
- Li, K.X.; Wonham, J. Maritime legislation: New areas for safety of life at sea. Marit. Policy Manag. 2001, 28, 225–234. [Google Scholar] [CrossRef]
- IMO. International Convention for the Safety of Life at Sea (SOLAS), 1974, 2019. Available online: https://www.imo.org/en/About/Conventions/Pages/International-Convention-for-the-Safety-of-Life-at-Sea-(SOLAS),-1974.aspx (accessed on 12 November 2022).
- UNTERM. Safety, n.d. Available online: https://unterm.un.org/unterm/Display/record/IMO/NA?OriginalId=83476e19964244c08ce42b805157efcc (accessed on 12 November 2022).
- ILO. MLC, 2006: What It Is and What It Does, 2022. Available online: https://www.ilo.org/global/standards/maritime-labour-convention/what-it-does/lang–en/index.htm (accessed on 12 November 2022).
- MSC. MSC/Circ. 699 Revised Guidelines for Passenger Safety Instructions, Annex 1 Guidelines for Passenger Safety Instructions. 2022. Available online: https://puc.overheid.nl/nsi/doc/PUC_2593_14/1/ (accessed on 12 November 2022).
- IMO. Passenger Ships. 2019. Available online: https://www.imo.org/en/OurWork/Safety/Pages/PassengerShips.aspx (accessed on 12 November 2022).
- IMO. International Code of Safety for Ship Using Gases or Other Low-Flashpoint Fuels (IGF Code). 2019. Available online: https://www.imo.org/en/OurWork/Safety/Pages/IGF-Code.aspx (accessed on 12 November 2022).
- IMO. Interim Guidelines for the Safety of Ships Using Methyl/Ethyl Alcohol as Fuel. 2020. Available online: https://www.ics.org.ir/IFleet/CLDFiles/News/ae228117-cd22-45d0-9470-b75d890fca6d_MSC.1-Circ.1621%20-%20Interim%20Guidelines%20For%20The%20Safety%20Of%20ShipsUsing%20MethylEthyl%20Alcohol%20As%20Fuel%20(Secretariat).pdf (accessed on 25 November 2022).
- IMO. Development of Non-Mandatory Guidelines for Safety of Ships Using Ammonia as Fuel. 2021. Available online: https://www.ics-shipping.org/wp-content/uploads/2021/07/MSC-104-15-9-Development-of-non-mandatory-guidelines-for-safety-of-ships-using-ammonia-as-fuel-Japan-Singapore-ICS-and....pdf (accessed on 25 November 2022).
- IMO. International Convention for the Prevention of Pollution from Ships (MARPOL). 2019. Available online: https://www.imo.org/en/About/Conventions/Pages/International-Convention-for-the-Prevention-of-Pollution-from-Ships-(MARPOL).aspx (accessed on 12 November 2022).
- IBIA. IMO to Develop Guidelines for Safe Use of Ammonia. 2022. Available online: https://ibia.net/2022/05/04/imo-to-develop-guidelines-for-safe-use-of-ammonia/ (accessed on 12 November 2022).
- IMO. IMO Goal-Based Standards. 2019. Available online: https://www.imo.org/en/OurWork/Safety/Pages/Goal-BasedStandards.aspx (accessed on 12 November 2022).
- Hoppe, H. Goal-based Standards-A New Approach to the International Regulation of Ship Construction. WMU J. Marit. Aff. 2005, 4, 169–180. [Google Scholar] [CrossRef]
- IMO. Report of the Maritime Safety Committee on Its Eightieth Session. Technical Report, International Maritime Organization Maritime Safety Committee. 2005. Available online: https://www.dco.uscg.mil/Portals/9/DCO%20Documents/Marine%20Safety%20Center/Tonnage/Committee%20Docs/MSC_80-24_Report_of_the_MSC.pdf?ver=2017-06-20-121133-870 (accessed on 15 November 2022).
- Ha, S.m.; Lee, W.J.; Jeong, B.; Choi, J.H.; Kang, J. Regulatory gaps between LNG carriers and LNG fuelled ships. J. Mar. Eng. Technol. 2022, 21, 23–37. [Google Scholar] [CrossRef]
- IMO. Maritime Safety Committee (MSC). 2022. Available online: https://www.imo.org/en/MediaCentre/MeetingSummaries/Pages/MSC-Default.aspx (accessed on 12 November 2022).
- MSC. Interim Guidelines for the Safety of Ships Using Fuel Cell Power Installations. 2022. Available online: https://www.mardep.gov.hk/en/msnote/pdf/msin2235anx1.pdf (accessed on 12 November 2022).
- BV. NR671 Ammonia-Fuelled Ships—Tentative Rules. 2022. Available online: https://marine-offshore.bureauveritas.com/nr671-ammonia-fuelled-ships-tentative-rules (accessed on 25 November 2022).
- LR. Zero Ready Framework. 2022. Available online: https://www.lr.org/en/latest-news/lr-launches-zero-ready-framework/ (accessed on 25 November 2022).
- Brosché, S. Women, Chemicals and the Sdgs Gender Review Mapping with a Focus on Women and Chemicals: Impact of Emerging Policy Issues and the Relevance for the Sustainable Development Goals. 2020. Available online: https://ipen.org/sites/default/files/documents/ipen-gender-chemicals-report-v1_6dw-en.pdf (accessed on 13 November 2022).
- US EPA. Hazards of Ammonia Releases at Ammonia Refrigeration Facilities (Update). 2001. Available online: https://www.epa.gov/sites/default/files/2013-11/documents/ammonia.pdf (accessed on 22 June 2022).
- HNS-MS. Ammonia Anhydrous. 2017. Available online: https://www.hns-ms.eu/result/21 (accessed on 12 November 2022).
- Kozlovski, A. Parity and the Resolution of Value Conflicts in Design. Sci. Eng. Ethics 2022, 28, 22. [Google Scholar] [CrossRef]
- Gelder, P.V.; Taebi, B.; Ommen, R.V.; Poel, I.V.D.; Asveld, L.; Balkenende, R.; Hollmann, F.; Kampen, E.J.V.; Krebbers, R.; Lange, J.D.; et al. Safe-by-design in engineering: An overview and comparative analysis of engineering disciplines. Int. J. Environ. Res. Public Health 2021, 18, 6329. [Google Scholar] [CrossRef]


| Hazard Class | DBT | NEC | Methanol | MDO | ||||
|---|---|---|---|---|---|---|---|---|
| Flammable | D * | D * | D | D | ||||
| Acute Toxic | D | D | D * | D | D | D | ||
| Health Hazard | D | D | D * | D * | D | D | ||
| Corrosive to skin | D | D | W */D ⌃ | D | ||||
| Irritant | W | W | W * | W | W | |||
| Environmental Hazard | W | W * | W | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rheenen, E.; Scheffers, E.; Zwaginga, J.; Visser, K. Hazard Identification of Hydrogen-Based Alternative Fuels Onboard Ships. Sustainability 2023, 15, 16818. https://doi.org/10.3390/su152416818
van Rheenen E, Scheffers E, Zwaginga J, Visser K. Hazard Identification of Hydrogen-Based Alternative Fuels Onboard Ships. Sustainability. 2023; 15(24):16818. https://doi.org/10.3390/su152416818
Chicago/Turabian Stylevan Rheenen, Erin, Evelien Scheffers, Jesper Zwaginga, and Klaas Visser. 2023. "Hazard Identification of Hydrogen-Based Alternative Fuels Onboard Ships" Sustainability 15, no. 24: 16818. https://doi.org/10.3390/su152416818
APA Stylevan Rheenen, E., Scheffers, E., Zwaginga, J., & Visser, K. (2023). Hazard Identification of Hydrogen-Based Alternative Fuels Onboard Ships. Sustainability, 15(24), 16818. https://doi.org/10.3390/su152416818






