Abstract
Sugarcane is a lignocellulosic crop and the juice extracted from its stalks provides the raw material for 86% of sugar production. Globally, sugarcane processing to obtain sugar and/or ethanol generates more than 279 million tons of solid and liquid waste annually, as well as by-products; namely, straws, bagasse, press mud, wastewater, ash from bagasse incineration, vinasse from ethanol distillation, and molasses. If not properly managed, this waste will pose risks to both environmental factors and human health. Lately, valorization of waste has gained momentum, having an important contribution to the fulfillment of policies and objectives related to sustainable development and circular bioeconomy. Various technologies are well-established and implemented for the valorization of waste and by-products from sugarcane processing, while other innovative technologies are still in the research and development stage, with encouraging prospects. We propose a sustainable sugarcane processing flow and present an analysis of the physico-chemical characteristics of generated wastes and by-products. We emphasize the available possibilities of valorizing each waste and by-product, considering that they are important biomass resources for obtaining biofuels and a wide range of other products with added value, which will contribute to the sustainability of the environment, agriculture, and human health worldwide.
Keywords:
biomass; pretreatment; cogeneration; bioethanol; bio-hydrogen; compost; fertilizer; wastewater 1. Introduction
Sugarcane, Saccharum officinarum L. (Poaceae), a perennial plant with thick and fibrous stems, is traditionally cultivated in more than 110 countries in the tropical and temperate regions of the world [1], occupying a production area of 27 million hectares [2]. In the period 2000–2019, the sugarcane crop accounted for 21% of global crop production [3]. Simultaneously, with the worldwide increase in the areas cultivated with sugarcane, there is also an increase in the demand for products derived from this sugar plant.
Sugarcane crops provide the raw material for 86% of the sugar produced globally (the remaining 14% being mostly obtained from sugar beet crops) [4]. In 2020, 1869.7 million tons of sugarcane were harvested worldwide, of which there were 757.1 million tons in Brazil, 370.5 million tons in India, 108.1 million tons in China, 81 million tons in Pakistan, 75 million tons in Thailand, 54 million tons in Mexico, 32.7 million tons in the USA, and 30.3 million tons in Australia [5]. The percentage distribution of sugarcane production by the top five major producing countries is as follows: 40% in Brazil, 20% in India, 7% in China, 6% in Thailand, and 5% in Africa [6].
From a physical point of view, sugarcane is made up of four major fractions (Figure 1); namely, fiber, insoluble solids, soluble solids, and water, and their relative size depends on the agro-industrial process of sugar extraction. The fibers are composed of the organic solid fractions originally found in the cane stem and are very heterogeneous. Insoluble solids (the fraction that cannot be dissolved in water) are mainly constituted by inorganic substances (rocks, soil, and other foreign materials) and depend on the agricultural conditions of cane processing, such as the type of cutting practiced and the type of harvesting. Soluble solids (the fraction that can be dissolved in water) are mainly composed of sucrose and may contain other chemical components in smaller proportions (such as wax).
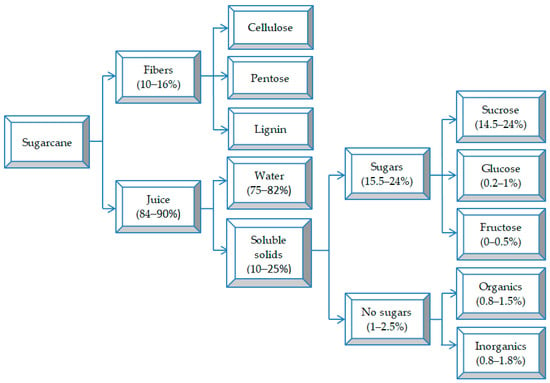
Figure 1.
Chemical composition of sugarcane; organics include starch, wax, amino acids, organic acids, phenolic compounds, etc.; inorganics include SiO2, K2O, P2O5, Fe2O3, etc. (adapted from [7]).
Sugarcane contains 53.6% juice (wet basis) and 26.7% fiber (dry basis) [8]. It is a plant rich in sugars (glucose, fructose, and sucrose), amino acids, and organic acids [9].
Sugarcane crop is harvested manually or mechanically every 6 months, then it is cut into pieces and transported to the processing plants, which are usually located in the vicinity of sugarcane fields, as the crop begins to deteriorate the next day after harvesting [8]. Sugarcane processing factories can be classified into three categories [10]: factories that only produce raw table sugar; plants that produce only ethanol; integrated plants, which produce both raw sugar and ethanol (these account for 80% of the plants). The juice extracted by pressing the stems is used in most cases to obtain table sugar and less to obtain ethanol [11]. In Brazil, the country with the largest sugarcane production, 90% of the harvested sugarcane is used to produce both sugar and ethanol, while only 7% of the crop is used to produce only ethanol and 3% to produce only sugar [12].
2. Characterization of Sugarcane Stems
At maturity, the sugarcane plant can reach 3–4 m in height, and its stem, with a diameter of about 5 cm, constitutes about 75% of the entire plant. The chemical composition of sugarcane stalks is variable, but typically a mature stalk is composed of 11–16% fibers, 12–16% soluble sugars, 2–3% non-sugar carbohydrates, and 63–73% water.
The cell wall of the stem is composed of 32.4–44.4% cellulose, 24.2–30.8% hemicellulose, and 12–36.1% lignin, and in smaller proportions it also contains ash (2.4–7.8%) and extractive substances (2.5–10.6%) such as minerals, sugars, proteins, and other compounds [13]. Other studies show similar values of the sugarcane cell wall components: 48.6% cellulose, 31.1% hemicellulose (mainly xylose and galactose), 19.1% lignin, and 1.2% ash [14]; respectively, 43.3% cellulose, 23.8% hemicellulose and 21.7% lignin, 0.8% ash, and 10.4% other extractives [8].
Cellulose is the most widespread polysaccharide in nature and is found in the primary and secondary cell walls of plants, giving them mechanical strength and elasticity. More than 65% of the cellulosic fraction of biomass is protected against degradation by a matrix of polymers that include lignin and hemicellulose [15] and is not accessible to water or other solvents. The cellulosic fraction can be transformed into glucose by enzymatic hydrolysis using cellulases, or chemically using acids (such as sulfuric acid), and the glucose can then be fermented to obtain ethanol [16].
Hemicellulose is a polysaccharide with a lower molecular weight than cellulose, and its highly branched structure differs substantially from the structure of cellulose in that it has a lower degree of polymerization [15], and that it is amorphous, which makes it easier to hydrolyze. The hemicellulosic fraction can be removed from lignocellulosic materials by acid hydrolysis [17] or by hydrothermal pretreatment [9], and the released sugars (mainly xylose) can be subsequently fermented to ethanol.
Lignin is a complex aromatic macromolecule, with heterogeneous and globular structure, with amorphous regions, insoluble in water. Lignin forms an impenetrable physical barrier around cellulose and hemicellulose [18], thus providing the plant with resistance against microbial attack. The content of lignin and its distribution in the cell wall of the plant determines the recalcitrance of lignocellulosic materials to enzymatic hydrolysis (limits the accessibility of enzymes), being necessary various delignification processes that have the role of improving enzymatic hydrolysis [19]. Sugarcane lignin is mainly used as a fuel, but it can be chemically modified for use as a chelating agent, for the removal of heavy metals from wastewater, or as a precursor material for the production of value-added products (activated carbon, surfactants, adhesives, etc.) [7].
3. Waste and By-Products Generated in Sugarcane Processing
3.1. Waste and By-Product Generation Flow in Sugarcane Processing Factories
In any sector of the food industry, food production is carried out with significant energy consumption, and relatively large amounts of waste result from the technological processes. Thus, major issues related to food technologies are energy and waste management.
In the sugar industry, approximately 279 million metric tons of sugarcane waste are generated annually worldwide [20]. From the global amount of sugarcane waste, South Africa has an annual share of over 1.353 million metric tons, of which more than 50% are recovered in cogeneration facilities [21]. The uncontrolled disposal of sugarcane waste can generate major problems on the environmental factors and, by consequence, on human health.
These wastes are of a solid, semi-solid, and liquid nature and can be classified into two categories: waste from the harvesting operation, represented by leaves and cane tips; and waste from the cane processing stream (Figure 2), including bagasse, ash from bagasse incineration, press mud (sludge from juice settling and residual cake from juice filtration), wastewater, vinasse, and molasses.
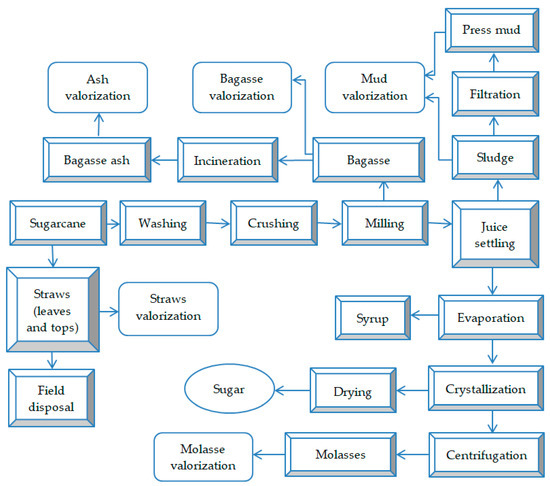
Figure 2.
Sustainable technological flow of cane sugar production, highlighting the generated waste and by-products.
Typically, crushing a ton of sugarcane yields about 280–300 kg of bagasse (wet basis) with 50% moisture content, 30 kg of press mud (wet basis), and 41 kg of molasses [22].
The energy content of one ton of sugarcane is 6560 MJ, distributed as follows: 2110 MJ in 280 kg of leaves and tops (50% moisture), 2110 MJ in 280 kg of bagasse (50% moisture), and 2340 MJ in 140 kg of sugar [23].
Currently, the global priority is not only to mitigate the environmental impact already caused by human and industrial activities, but also to respond to the need to produce more food and energy for a population estimated to exceed 10 billion people by 2050.
In this context, the valorization of waste and by-products from the sugar industry contributes to economic, social, and environmental sustainability. Waste valorization is also an opportunity to implement the principles of bioeconomy and circular economy, which aims to transform waste into resources [24] and contributes to the unification of production and consumption activities. In this way, the environmental footprint would be greatly reduced, but at the same time, what is considered waste today will be an important source of raw materials in the future.
Lately, in order to contribute to sustainable development, more and more factories are trying to find solutions to put into practice the principles of the circular bioeconomy concept, which refers to the production of energy, food, chemicals, and other biomaterials and compounds from biomass in a sustainable and integrated/cascaded way (biorefinery) while generating zero waste.
Currently, multiple technologies for the recovery of waste from the sugar industry are being implemented on a large scale, and others are currently in the research and development stage, but the prospects for the future are encouraging.
This work aims to analyze, both from the point of view of the quantities generated and the physico-chemical characteristics, each type of waste and by-product generated on the technological flow of sugarcane processing in sugar factories. Furthermore, we review recent reports in the literature and highlight some of the current methods by which these wastes and by-products are recycled and valorized for sustainability in countries that grow and process the sugarcane.
3.2. Sugarcane Leaves and Tops (Straws)
Sugarcane leaves and tops (also called straws or sugarcane litter) are lignocellulosic materials whose chemical composition varies depending on the stage of development and variety of the plant, place of collection, and climatic conditions. According to [25], these wastes have an approximate composition of 40% cellulose, 25% hemicellulose, and 18–20% lignin.
From one ton of sugarcane, between 270–280 kg of leaves and tops remain as harvesting waste [23,26]. Sugarcane straws can contain up to 5% m/m impurities (sand and other debris) due to transport and harvesting operations [27]. If these impurities would reach the cane processing plant, they would cause wear of the mill rolls [28].
Many times, these wastes are conventionally disposed by direct burning in the field [23,29]. Moreover, when traditional manual harvesting is used, farmers set fire to the sugarcane plantations before harvesting to burn off the sharp leaves and facilitate the manual cutting of the stalks. This practice contributes to severe air pollution with suspended particles, the occurrence of severe respiratory diseases in the affected area, and greenhouse gas emissions. In addition, polycyclic aromatic hydrocarbons formed during incineration will pollute both the soil and water.
The valorization of cane leaves and tips is practiced on some plantations. If the sugarcane crop is harvested while it is still green, the cane leaves and tips are left as such in the field as a vegetable mulch, to control weeds, reduce water evaporation from the soil, and return carbon and nutrients to the soil [6], thus contributing to the improvement of soil properties [30]. This waste can also be collected manually or mechanically and briquetted or pelletized for incineration for energy purposes [31]. In laboratory experiments, second-generation ethanol with a yield of 156 L/t was obtained from cellulose and hemicellulose mixtures extracted from sugarcane tops and leaves, thermochemically treated by alkaline catalysis, and subjected to simultaneous saccharification and fermentation [6].
3.3. Sugarcane Bagasse
3.3.1. Characterization of Sugarcane Bagasse
Bagasse is the primary industrial fibrous residue obtained after pressing (crushing) of sugarcane stalks in order to extract the juice. When processing one ton of sugarcane, between 0.25–0.30 tons of bagasse are obtained [32]. In factories in Brazil, 0.28 tons of bagasse are typically generated [33]. According to other studies, 0.14 tons of bagasse (dry mass) and 0.14 tons of straw (stems) are obtained from one ton of sugarcane [13,23].
It is also estimated that over 700 million tons of bagasse are produced annually worldwide [34], i.e., between 40–50% of the total weight of sugarcane produced annually in the world [35]. In only the top ten countries in terms of sugarcane production, more than 540 million tons of bagasse are generated annually [36].
The chemical composition of sugarcane bagasse varies with plant variety, cultivation conditions, harvesting practices, and processing methods. Sugarcane bagasse contains 45–50% water, 2–5% dissolved sugars and 40–45% fiber [37], and 60–80% carbohydrates [38]. Like any natural vegetable fiber, sugarcane bagasse has a fibrous structure consisting of cellulose, hemicellulose, and lignin [39], with different values of these components being presented in the literature (Table 1).

Table 1.
Chemical composition of sugarcane bagasse.
3.3.2. Uncontrolled Disposal of Sugarcane Bagasse
A significant amount of bagasse is disposed of uncontrollably in the form of waste piles directly on open land [53], which inevitably leads to environmental pollution due to the release of unpleasant odors that attract insects and other pests, and sometimes due to accidental occurrence of self-igniting fires.
Even the temporary storage of excess bagasse remaining on the technological flow, which can represent up to 50% of the bagasse produced in the sugar factory [54], has negative consequences on the environment. Uncontrolled disposal and landfills are the most unsustainable option for managing sugarcane wastes.
3.3.3. Valorization of Sugarcane Bagasse
In large sugarcane-growing countries, bagasse is an important type of waste that can be recovered [55], and there are currently more than 40 different uses for it [56]. Approximately 58–76% of the wet mass of bagasse is composed of polysaccharides that can be hydrolyzed into monosaccharides (glucose and xylose) and then transformed by microbial fermentation into biofuels, enzymes, proteins, lipids, feed, and other biochemical substances [57].
Incineration of Sugarcane Bagasse for Energy Recovery
Sugarcane bagasse with a moisture content of approximately 48% has a calorific value of 8021 kJ/kg [58] and is usually valorized as fuel in cogeneration plants to produce thermal energy (steam) and electricity [59,60]. These energy products can provide the necessary thermal and electrical energy for the operation of the sugar factory [61,62], and the eventual energy surplus is transferred to the national electrical network [63,64].
For example, India annually generates between 75–90 million tons of bagasse (wet mass) [65], which it mainly uses through incineration with cogeneration (electricity and heat generation) in medium and large sugar factories. In Brazil, the energy recovery of one ton of bagasse generates 12 kWh of electrical energy, 330 kWh of thermal energy (steam), and 16 kWh of mechanical energy [66].
In sugar mills, thermal energy and mechanical energy obtained by bagasse incineration are used to drive milling equipment, and electrical energy is used to drive rotating equipment in the factory during the harvest season [8].
Bagasse incineration is, however, associated with large volumes of CO2 emissions that contribute significantly to the global warming faced by all of humanity today [33,67].
Biofuels Obtained from Sugarcane Bagasse
Currently, the energy and transport systems, which are mainly based on fossil energy carriers, cannot be considered a sustainable system. The worldwide decrease of conventional energy resources, as well as the restrictive legislation regarding the level of environmental pollution, have created premises for the identification and exploitation of new, economic, and non-polluting sources of energy.
Thus, concerns have arisen for the manufacture of biofuels from renewable raw materials (biomass). The use of renewable energy sources can contribute to the reduction of conventional energy consumption, the reduction of greenhouse gas emissions and, therefore, the prevention of dangerous climate change.
Biomass is a valuable renewable resource that can be used on both a small and large scale [68] as an alternative to fossil fuels because its energy can be transformed into a variety of energy forms, such as heat, steam, electricity, and hydrogen, as well as biofuels, which are considered substitutes for fossil fuels. Biomass is transformed into bioenergy through mechanical (densification), biochemical (aerobic digestion, anaerobic digestion, fermentation), and thermochemical processes (pyrolysis, gasification, incineration, liquefaction).
Thus, biowastes from sugarcane processing are important ecological and cheap resources for obtaining densified biofuels (briquettes and pellets), biogas, ethanol, synthesis gas, and more recently, biohydrogen.
However, due to the high lignin content of sugarcane bagasse, various pretreatment methods are necessary to extract one of the hemicellulose or lignin fractions, or even both fractions [48].
Different methods are currently available for the pretreatment of lignocellulosic biomass, each method having its own specificity regarding the mechanism of action on the cell wall components of the biomass and the conditions of application. Among the pretreatment methods, there are [52,69]: physical pretreatment, which can be achieved by shredding, grinding, ultrasound, and microwaves; chemical pretreatment, which includes the use of acids and alkalis, ionic liquids, organic solvents, oxidizing agents, hot water hydrothermolysis, steam explosion, and fiber expansion with ammonia; biological pretreatment, which involves the use of a range of microorganisms and enzymes that degrade lignin; and different combinations of these methods.
Bioethanol from Sugarcane Bagasse
Ethanol is an excellent alternative fuel for use in modern internal combustion engines. Due to the huge amounts generated in the sugar industry and its high cellulose content, sugarcane bagasse has become an important raw material for bioethanol production [70,71].
The bioconversion of lignocellulosic biomass into ethanol consists of four stages (Figure 3). (1) The pretreatment of lignocellulosic biomass is applied to degrade lignin and/or hemicellulose by microbial enzymes in order to release the cellulose [72,73]. Bagasse quality and pretreatment processes decisively influence the final ethanol yield [74,75,76]. (2) Enzymatic hydrolysis of cellulose and hemicellulose is mainly carried out by cellulase and hemicellulase that splits the glycosidic bonds and converts them into glucose [73,77]. Bacteria such as Celulomonas, Bacillus, Thermonospora, Erwinia, etc., and the fungi Trichoderma, Fusarium, Phanerochaete, etc., produce enzymes useful in the production of cellulase [78]. Several biological and physico-chemical factors, including substrate concentration and type, temperature, pH, oxygen level, and types of microorganisms, affect the enzymatic conversion of sugarcane bagasse to value-added products. (3) Fermentation of saccharified biomass is carried out to obtain bioethanol using microorganisms such as Saccharomyces cerevisiae and Zymomonas mobilis, efficient in fermenting hexoses and pentoses into ethanol [79]. (4) Distillation of ethanol is the final stage, after which the ethanol will reach a purity of about 95% due to the formation of a low-boiling water-ethanol azeotrope [80,81].
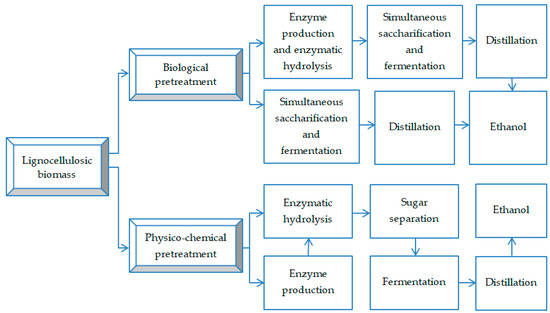
Figure 3.
Bioconversion of lignocellulosic waste into ethanol (adapted from [15]).
In the manufacturing process of first-generation ethanol, 50% of the sugarcane juice extracted from sugarcane is used in ethanol production and 50% in sugar production [81]. The main raw material for ethanol production in factories in Brazil is sugarcane juice [82], and in factories in Thailand, ethanol is obtained from sugarcane molasses [83].
Sugarcane can accumulate up to 42% of the dry weight of the plant in sucrose [84]. The theoretical yield of ethanol is 617 L/t sucrose, but the actual yield in ethanol distilleries is 510–530 L/t sucrose. The average yield of ethanol obtained in factories in Brazil is 82–85 L/t of freshly crushed sugarcane [85].
Various studies investigated the production of bioethanol from bagasse using different pretreatment methods and provided details of the process parameters to enable scale-up. The conversion methods, the microorganisms involved in the fermentation, and the second-generation bioethanol yields obtained are presented in Table 2.

Table 2.
Summary of experimental studies on obtaining bioethanol from sugarcane bagasse.
Bioethanol produced from sugarcane bagasse can be distributed through already existing infrastructures and used as a fuel for light commercial vehicles (ethanol hydrate) or as an additive in gasoline (anhydrous ethanol) [6], and the ethanol-gasoline mixture can be used in combustion engines. Furthermore, bioethanol from sugarcane waste is one of the most suitable alternatives for partial replacement of fossil fuels, as it provides renewable energy and is less carbon intensive than gasoline. In addition, the use of bioethanol as such or mixed with gasoline contributes to mitigating climate change [7] by reducing greenhouse gas emissions.
Biohydrogen from Sugarcane Bagasse
Biohydrogen is a colorless and odorless, clean-burning fuel with a calorific value of 122 kJ/kg, which is almost three times higher than the calorific value of other hydrocarbon-containing fuels [90].
Biohydrogen is obtained through ecological technologies and is the only fuel that does not emit CO2 as a by-product (its combustion produces water as a by-product) when used in fuel cells for the production of electricity and does not pollute the air. Thus, biohydrogen is increasingly promoted as an alternative energy carrier for a sustainable future.
Therefore, biohydrogen contributes to the achievement of the objective of climate neutrality provided for in the European Green Deal, established for the year 2050. By 2050, hydrogen could supply up to 24% of total energy demand, corresponding to 2251 TWh of energy in the EU + UK [91]. Although the current production of hydrogen is mainly based on fossil fuels, for ecological reasons, the use of biomass resources is preferred, which includes sugarcane bagasse.
Unlike bioethanol and biodiesel technologies, which are already well-developed, biohydrogen production is still at an early stage of development, but there are a variety of technologies available for converting sugarcane bagasse, as well as other types of biomass, for the production of biohydrogen (Figure 4).
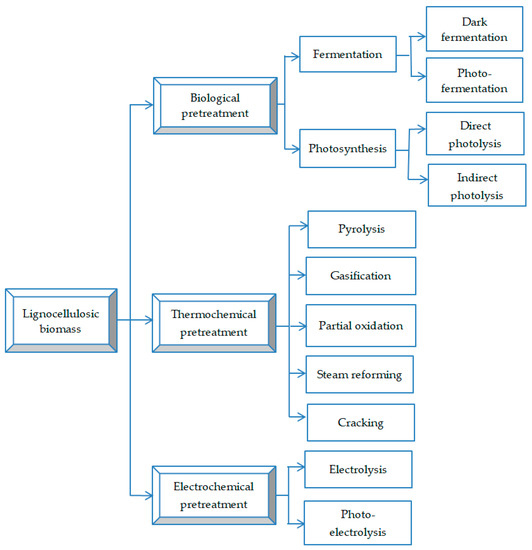
Figure 4.
Methods for biohydrogen production from biomass resources (adapted from [92]).
Although sugarcane bagasse is available in large quantities, its lower conversion efficiency, current deficiencies in bioreactor design, high costs for hydrogen purification, and storage systems are only some of the issues that need to be resolved before biohydrogen from bagasse can be obtained on a large scale.
Table 3 presents the conversion methods, the microorganisms involved in the fermentation, and the biohydrogen yields obtained in different experimental studies.

Table 3.
Summary of experimental studies on obtaining biohydrogen from sugarcane bagasse.
The results of the experimental research carried out in this field are encouraging, but the processes still need to be optimized before raising them to a large scale.
Other Options for Valorization of Sugarcane Bagasse
Bagasse is a valuable raw material in the field of biotechnologies. It can be used for synthesis of sugars and oligosaccharides such as glucose and prebiotic xylooligosaccharides [96,97]; a series of acids (citric, lactic, gluconic, succinic) [98,99]; industrial enzymes (xylanase, cellulase, α-amylase, lipase) [100,101]; furfural and xylitol [102,103], but here it should be noted that the processes of obtaining them generate some toxic compounds [104], and subsequent purification processes have high costs; and as a substrate for the fermentation of compounds with a coconut-like aroma [105,106].
Sugarcane bagasse is a beneficial growth medium for edible mushrooms; different studies proved that compared with other biomass substrates (rice straws, wheat straws, corn cobs, and saw dust), mushrooms grown on bagasse contained the highest amount of proteins because sugarcane bagasse has higher concentrations of nitrogen [107,108]. Furthermore, bagasse is an important carbon source for the cultivation of microalgae from which biomass and oils can be obtained and converted to biofuels [109].
Bagasse can be transformed into biochar, an ecological product that has many practical applications, including the removal of organic and inorganic compounds [110], the removal of ammonium and phosphate ions in aqueous solutions [111], and it can also be used against the leaching of nitrates from the soil [112]. Additionally, in environmental applications, bagasse can be transformed into cheap adsorbent materials for the removal of pesticides from the environment [113].
Some studies highlight the usefulness of bagasse in the field of nanotechnology. For example, they can be obtained for carbon-based nanomaterials [114], cheap cellulosic nanofibers [115], nanostructured films [116], or other types of bioadsorbents for the removal of heavy metals (copper, cadmium, lead, nickel, selenium, zinc etc.), and removal of phenols and dyes in wastewater [117,118,119,120,121]. The authors of study [117] reported that charred xanthated sugarcane bagasse has high adsorption efficiencies of heavy metals from aqueous solutions: 1.95 mol/kg for Cd(II), 2.91 mol/kg for Cu(II), 2.52 mol/kg for Ni (II), 1.58 mol/kg for Pb(II), and 2.40 mol/kg for Zn(II). Other experiments conducted in aqueous environments showed that bagasse treated with citric acid (C6H8O7) and sodium hydroxide (NaOH) had a maximum copper ion adsorption capacity of 31.53 mg/g, proving that it is a waste that can be successfully used in the treatment of wastewater [118], while the adsorption capacity of Cd2+ ions from an aqueous solution at a temperature of 25 °C and initial pH of 7 units was 96% [119].
Renewable hydrogel was obtained from the lignin contained in bagasse mixed with silane and/or acetyl groups. Compared with hydrogels obtained from other raw materials, the one obtained from this mixture showed superior mechanical characteristics and swelling capacity [122]. The hydrogels obtained from bagasse can be exploited in environmental applications but also in agriculture because they have a high capacity to store water, being especially useful in arid and semi-arid areas.
Bagasse fibers and ash are used as additives in products made of clay, glass, and ceramics [123,124]. In the construction materials industry, there are manufactured ecological products from bagasse, including agglomerated panels [125,126] and other composite materials obtained from fibers extracted from bagasse [34]; bagasse can be incorporated as reinforcement material in cement composites [127,128,129] and different materials for roofs of ecological buildings [130], and for ceramic tiles [131].
Bagasse is also used for obtaining recyclable and biodegradable composite materials from which some components in the automotive industry can be made, such as polypropylene reinforced with natural fibers extracted from sugarcane bagasse that can be used as a new substitute for polypropylene filled with talc [41] and as a substitute for glass fiber-reinforced polypropylene [132], but also as material in components for acoustic protection [133].
Fibers extracted from bagasse can be used in textile industry [134]. A very common field for the valorization of bagasse is that of manufacturing of paper pulp [135], hardboard, wrapping paper, and toilet paper [25,136,137], thus contributing to the reduction of deforestation. There is an emerging market for disposable cups, plates, glasses, and cutlery made from biodegradable materials like sugarcane bagasse [138,139]—a sector that started to develop especially since packaging and single-use plastic products were banned, as well as other value-added products.
Bagasse has a high content of nutrients and is used as fodder for cattle [52,140], but if it is not pretreated, due to its high lignin content [141], the ruminal digestion of hemicellulose and cellulose will be inhibited, which will lead to a lower nutritional value [52]. On the other hand, bagasse pretreated by mechanical pretreatment by shredding, chemical pretreatment with urea and ammonia [142], pretreatment with alkaline hydrogen peroxide [143], hydrolysis [144], biological pretreatment by fermentation with fungi [145], or co-digestion with poultry manure [146,147] has a higher nutritional value [148]. It does not interfere with the activity of cellulolytic bacteria in the rumen of cattle [144], and therefore it does not compromise the digestibility of fodder [149].
3.4. Residual Ash from Bagasse Incineration
3.4.1. Characterization of Sugarcane Bagasse Ash
Figure 5 shows a simplified production flow in the sugar factory. After extracting the sugary juice from the sugarcane, the sugarcane bagasse remains as waste. Bagasse represents approximately 50% of the composition of the sugarcane and is, in most factories, incinerated for energy purposes. After its incineration, bagasse ash (consisting of fly ash and bottom ash) will remain as the final waste from the sugar production stream [150].
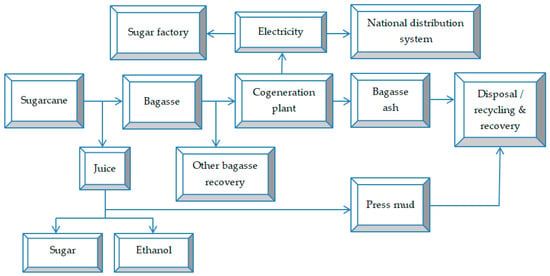
Figure 5.
Typical generation flow of bagasse ash in a cogeneration plant sugar factory.
Following the incineration of a ton of sugarcane bagasse, between 25–45 kg of ash is generated [151], of which fly ash represents only 5–6.6 kg [152]. In Brazil, approximately 148 million tons of bagasse are produced annually, generating 3.6 million tons of bagasse ash, considering that for each ton of processed sugarcane, around 250 kg of bagasse are obtained, whose combustion results in 6 kg of ash. In China, between 1.25–2 million tons of sugarcane bagasse ash are produced annually [153], and Australia generates over 230 thousand tons of bagasse ash [62].
Ash from the incineration of bagasse contains between 70–90% inorganic compounds, mainly silica [154], with different structures, including crystalline, glassy, and amorphous [155], with aluminum, iron, and calcium oxides, as well as unburnt carbon [156,157,158] (Table 4).

Table 4.
The chemical composition of bagasse ash (Reprinted with permission from Ref. [156]).
Bagasse ash is managed either by final disposal in landfills (the least desirable method, but very often practiced in India) [159], or by valorization with economic and environmental benefits (the ideal method, applies mostly in Brazil and China).
3.4.2. Valorization of Sugarcane Bagasse Ash
The utilization of bagasse ash can be achieved, at the present time, through different possibilities.
Bagasse ash can be applied as an amendment for agricultural land [25], although it does not contain a sufficient concentration of mineral nutrients and will therefore be a fertilizer with low nutritional value. To increase its nutrient content, ash can be mixed with sludge from cane juice filtration or vinasse from ethanol distillation. Another problem related to the utilization of ash as fertilizer in agriculture is its content of heavy metals (aluminum, chromium, lead) and total phenol, whose values exceed the levels allowed by many national standards [151,153]. These chemical pollutants accumulate in the soil (from where they are transferred to the food chain) and can also pollute groundwater, thus triggering potentially serious problems from an agronomic, environmental, and human health point of view.
Solid fuels were obtained by briquetting bagasse ash [150,160,161] in the presence of binders or additives. The additives also come from biomass waste or ecological materials, so the use of these solid biofuels contributes to reducing the carbon footprint and protecting the environment. The briquettes obtained in study [150] were starch, obtained from flour of tropical root cassava used as a binder, and presented good mechanical and thermal properties, having an average density of 1.12 g/cm3 and an average calorific value of 25.551 kJ/kg. By carbonizing sugarcane bagasse, ecological coal was obtained which was then added with clay and sugarcane molasses to obtain ecological briquettes for household use, whose combustion does not generate smoke and unpleasant odor, with the following characteristics: 36.4% ash content, 27.2% volatile matter, and 4.390 Kca/g calorific energy [160]. In a study [161], a mixture of bagasse with kraft lignin was briquetted at a temperature of 140 °C and a pressure of 17 MPa for 8 min. It was shown that the addition of kraft lignin improved the characteristics of the briquettes, such as resistance and durability. These characteristics are especially important during the storage and transport of operations because briquettes with low durability crumble easily, generating dust that affects human health; additionally, they can self-ignite if they are stored at high temperatures or they can absorb moisture, which will decrease their calorific value.
In the construction materials industry, bagasse is used as a substitute for sand [151], and additive in the manufacturing of bricks [162], cement [62,127,163], mortar, and concrete [123,164,165]. Cement-based materials in which bagasse ash have been incorporated have superior mechanical performance [166]. In addition, this type of ash utilization can help reduce greenhouse gases produced in cement factories, as it is known that the cement industry contributes 8% to the total CO2 emissions from anthropogenic sources [167]. In addition, utilization of bagasse ash in the cement industry decreases the costs of construction materials, alleviates the pressure of the final disposal of waste (storage costs are increasing), and prevents soil and air pollution.
In the rubber industry, bagasse ash and also raw bagasse and fibers extracted from bagasse are used as alternative reinforcements mixed with natural rubber, to obtain eco-friendly composite materials with improved mechanical properties (hardness and resistance to compression, deformation, and friction) [154].
Bagasse was also used as a raw material in the manufacture of ceramic membrane filters, with pore sizes in the range of 1–10 µm, and these filters can be used in gas-solid and solid-liquid separation operations [168], or for the separation of sludge from wastewater treatment in membrane bioreactors [169]. These filters have better physical stability at extremes of pH and temperature [156] and longer relative lifetime, but they are ten times more expensive than filters made of polymer membranes [25].
3.5. Vinasse from Ethanol Distillation
3.5.1. Characterization of Sugarcane Vinasse
From the ethanol distillation stage, vinasse results as waste, i.e., the fermented liquid medium without ethanol content, considered to be a severe environmental pollutant. Vinasse represents a flow of acidic wastewater (with pH = 3.5–5), dark brown in color, with an unpleasant odor [170], a high chemical oxygen demand (COD), and high salt content [171]; it also contains suspended organic solids, minerals, yeast, sugarcane proteins, and phenolic structures [172]; it has high concentrations of nutrients (nitrogen, phosphorus, potassium) [173,174], heavy metals (zinc, copper, barium, and chromium) [175], residual sugar, and some volatile compounds.
For each liter of ethanol produced from sugarcane, between 10–15 L of vinasse are generated [175]. It is estimated that by 2024 the production of vinasse will reach 1742 billion liters [171].
3.5.2. Valorization of Sugarcane Vinasse
Due to its large generation volumes and still limited fields of application, vinasse is successfully treated mainly by anaerobic digestion [173,176,177]. Anaerobic digestion of vinasse contributes to the reduction of pollution because its polluting load with organic compounds is considerably reduced by biological treatment, and the obtained biogas is a renewable biofuel. Due to the high concentration of sulfides in the vinasse, the biogas obtained will also have high concentrations of hydrogen sulfide, a highly corrosive gas that, if not removed quickly, will attack the pipes of the installations. Technologies for obtaining third-generation biodiesel and hydrogen from vinasse are currently in the development stage [178].
Because of the low concentration of fermentable carbon in vinasse, it can be mixed with carbohydrate sources (such as molasses) to allow the production of larger amounts of hydrogen.
The vinasse can also be temporarily stored in lagoons where treatment by oxidation takes place under natural conditions, to be later applied as liquid organo-mineral fertilizer [174,179,180], by sprinkling systems, on the sugarcane fields in the vicinity of the ethanol distilleries, thus contributing to higher agricultural productions. The phosphorus content of vinasse can fully cover the requirements of agricultural crops. Like any other type of fertilizer, it must be mentioned that the application of vinasse must be done at the times and rates required by the characteristics of the crop and the soil. If applied in excess, it will lead to reduced productivity and late maturation of the sugarcane crops, which will have a low sucrose content; additionally, excess vinasse causes environmental problems such as soil pollution with heavy metals, soil acidification and salinization, processes that disturb the soil biota, and salt leaching to groundwater.
On a laboratory scale, due to its high content of micronutrients, vinasse was used as a growth medium for filamentous fungus Neurospora intermedia, and as a substrate for obtaining unicellular products (proteins, lipids, enzymes, organic acids, alcohols). The obtained biomass contained 45% protein and important essential amino acids [181].
3.6. Press Mud (Cake)
3.6.1. Characterization of Sugarcane Press Mud
Press mud (cake) is the dark, spongy solid residue left after the juice extracted from sugarcane has been clarified and filtered. Processing one ton of cane yields 0.03 tons of press mud [182], which is rich in fiber, crude protein, crude wax, sugar, fat, and ash Therefore, press mud has a complex composition: 15–30% fibers, 5–15% crude proteins, 5–15% sugars, 5–14% crude wax, 9–10% total ash, 4–10% SiO, 1–4% CaO, 1–3% PO, and 0.5–1.5% MgO [183].
Press mud is an insoluble material, whose decomposition in natural conditions takes a long time, generates an acid leachate [184], emits unpleasant odors that attract insects and other pests, but also intense heat [185] and poses risks of self-ignition [184] when exposed to direct sunlight.
3.6.2. Valorization of Sugarcane Press Mud
Being a waste rich in nitrogen, phosphorus, calcium, and organic matter [186], press sludge can be used as soil fertilizer [187,188]. Its usefulness in reducing soil degradation through crusting, cracking, erosion, and compaction has been demonstrated [28].
Press sludge can be composted as such [189] or mixed with wastewater or residual vinasse from ethanol distillation [190], with animal manure and bagasse [25], or with other types of vegetable waste [191]. It can also be vermicomposted [53], in the presence of earthworm species such as Eisenia fetida or Eudrillus eugeniae. A drawback of composting is that it is a slow process that requires large spaces and infrastructure for turning, aeration, and watering the furrows or piles of organic matter. On the other hand, excessive application of press sludge to the field for long periods will result in soil contamination, due to accumulation of heavy metals (zinc, copper, lead) [22,192], and in negative effects on plant growth [193].
Other domains in which press sludge is used include aquaculture [194]; biosorbent for some metal ions and dyes from liquid solutions [195]; the production of hydrocarbons and chemicals [196]; the production of cement, paints, and foaming agents [182]; the production of biofuels, such as ethanol, biobutanol, and biogas [22] and of biofuel briquettes obtained from cane straws, bagasse, bagasse ash, and press sludge [197].
3.7. Wastewater from Sugarcane Processing
3.7.1. Characterization of Wastewater from Sugarcane Processing
Wastewater represents the most common waste of the food industry, and in most of the unitary operations of food product technologies taking place in an aqueous environment or requiring significant amounts of fresh water.
The sugar industry is one of the main users of fresh water at all stages of the technological stream, and consequently among the largest generators of wastewater.
In sugarcane factories (mills), wastewater is mainly generated from the washing operations of floors and equipment on the process stream, in evaporators (condensate water), by cane juice leaks at improperly attached taps of pipelines, from syrup and molasses generation stages, and from dewatering of press mud or of other solid waste [198] (Figure 6).
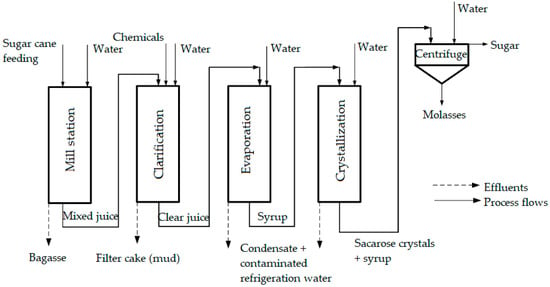
Figure 6.
Operations in sugarcane processing using fresh water and generation of wastewater (taken with permission from Elsevier, from [199]).
For example, processing one ton of sugar beet requires about 20 m3 of fresh water [200]. As a comparison, crushing a ton of sugarcane typically consumes between 1.5–2 m3 of fresh water and generates an average of 1 m3 of wastewater [190,201]. Some sugarcane processing plants in India produce between 0.2–1.8 m3 of wastewater per ton of sugar produced.
Wastewater from sugarcane factories has the following characteristics: pH between 4–7 units, chemical oxygen demand (COD) between 1800–3200 mg/L, biochemical oxygen demand (BOD5) between 720–1500 mg/L, solids total at 3500 mg/L, total nitrogen at 1700 mg/L, and total phosphorus at 100 mg/L [202]. According to other studies, these wastewaters have a COD between 2300–8000 mg/L, BOD5 between 1700–6600 mg/L, and total suspended solids at 5000 mg/L [203], and high ammonium content, respectively [198].
Wastewater from sugar factories also contains carbohydrates, nutrients, sulfates, chlorides, heavy metals [204], oils and fats from different equipment [205], pesticides, herbicides, and pathogens from contaminated surfaces or materials [206]. These wastewaters have high concentrations of organic and inorganic substances, including gaseous and solid pollutants.
If they are not properly treated, wastewaters from sugarcane processing have negative effects on the environment [207]. After only a week of storage in the raw state, they will turn black and emit unpleasant odors due to the generation of hydrogen sulfide [190].
In order to meet the principles of sustainable development, modern sugarcane mills aim to reduce their water consumption to zero, which involves water reuse, recycling, and regeneration. One of the ultimate goals of wastewater management is to eliminate any potential current and future threats of pollutants to living organisms, the environment, and human health. Therefore, the proper treatment of wastewater is very important.
To achieve different levels of pollutant reduction and removal, wastewater treatment processes must be combined in a variety of high-efficiency systems. Wastewater from sugar mills is generally subjected to conventional treatment, which consists of sieving, equalization, sedimentation, coagulation, oxidation in aerated ponds, and serial biofiltration [206], but there are also ideal situations where conventional purification is complemented by advanced purification processes such as precipitation, separation (filtration) through membranes, adsorption and biosorption [208], ion exchange, chemical coagulation, biochemical oxidation, and electrochemical oxidation [198,209].
In situ treatment of wastewater allows water to be reused and recycled within the sugarcane processing plant, and thus contributes to reducing the freshwater requirements and the amounts of generated wastewater.
3.7.2. Valorization of Wastewater from Sugarcane Processing
Wastewater from the manufacture of sugar and ethanol from sugarcane, as the vinasse (the liquid residue resulting from the distillation of ethanol) can be used for a variety of purposes.
Due to their content of organic matter and sugars, the wastewater from sugar industry quickly undergoes fermentation. Thus, it can be used in the generation of bioenergy: anaerobic digestion to obtain biogas, bioethanol, and biohydrogen production, etc. Additionally, sugarcane wastewater can be used as a culture medium for algae from which lipids can be extracted to obtain biodiesel [10].
These wastewaters can be used in other fermentation processes to obtain products with added value, such as organic acids (lactic, butyric, acetic) and enzymes (cellulase and laccase) [210].
Another possibility for valorizing wastewater consists of concentrating it through evaporation (incineration in boilers), a process from which the steam can be recovered to be used as a heating agent, and the condensate resulting from evaporation can be treated and utilized in the factory [10].
Wastewater, like vinasse, contains useful nutrients and therefore it can be applied as liquid fertilizer [175,180], which is an efficient way of valorization provided that the wastewater is properly treated and applied under the conditions specific to the culture and soil type. In addition to providing nutrients, applying wastewater to agricultural fields can support agriculture in non-irrigated and water-scarce areas where freshwater availability is very limited [211], on top of the fact that it can reduce the use of chemical fertilizers and the cost of irrigation water.
However, special attention should be paid to excessive fertigation, which can lead to adverse effects over time such as absorption of heavy metals into the soil, soil salinization, decreased yields of agricultural crops, as well as the emergence of risks for the health of farmers and people who consume crops from the lands irrigated with these waters.
3.8. Sugarcane Molasses
3.8.1. Characterization of Sugarcane Molasses
Molasses is the most important by-product of the sugar industry and is obtained from sugar crystallization. Molasses is a viscous substance similar to honey and contains 35% sucrose, 20% water, 9% fructose, 7% glucose, 3% reducing sugars, 4% carbohydrates, 4.5% nitrogenous compounds, 5% non-nitrogenous acids, 12% ash, and 5% other substances [212].
According to [213], sugarcane molasses has the following composition (values in g/kg): between 735–875 total solids, 447–587 total sugars, 157–469 sucrose, 97–300 sugars reducing agents, 0.25–1.5 nitrogen, 0.3–0.7 phosphorus, 19–54 potassium, 6–12 calcium, and 4–11 magnesium. It has an acidic pH (5–7 units), mineral and ash content between 8–13%, and its salt content between 2–8% contributes to the buffering capacity, flavor stabilization, and prevention of hydrolysis [214]. Molasses has a chemical oxygen demand of 862.842–935.62 g/L and a biological oxygen demand of 486.35–618.46 g/L [215].
3.8.2. Valorization of Sugarcane Molasses
Sugarcane molasses is usually exploited on industrial scale in the production of: biogas, with the mention that the anaerobic digestion of molasses is limited by high values of its chemical oxygen demand and ion concentration, as well as melanoidin content [215]; ethanol, with the mention that molasses no longer requires the pretreatment step before fermentation [216]; and bioethanol [217,218,219,220], but also ethyl alcohol, butanol, and acetone [221].
Molasses is also used as a raw material to obtain some food products. It is a major constituent of commercial fine brown sugar but is also used to sweeten and flavor foods [222], as well as in the alcoholic beverage industry such as rum [223], vodka, and sometimes beer [224]. Unlike highly refined sugars, molasses also contains significant amounts of vitamin B6 and minerals, including calcium, magnesium, iron, and manganese, so it is also an important dietary supplement.
Molasses is widely used as a supplement in animal feed, as a soil fertilizer, as a culture medium for the production of microbial transglutaminases [225], as a substrate for fermentation processes (production of acids: citric, lactic, succinic; alcohols; vitamins; monosodium glutamate; fructo-oligosaccharides) [60,226,227], and even as a raw material in cosmetic products (for example, moisturizing hair masks).
4. Conclusions
This paper reviewed the potential for sugarcane processing by-products and waste to contribute to environmental sustainability and bioeconomy. To meet the increasing demands of sugar for food and ethanol, the areas cultivated with sugarcane grow from year to year. Starting with the harvesting of sugarcane, and also on the processing flow, large volumes of waste and by-products are generated. If they are improperly managed, they quickly decompose, polluting the environment, and affecting human health. Due to the concerns about reducing greenhouse gas emissions and the dependence on fossil fuels, technologies have been developed for the utilization of biomass waste. Bioenergy is renewable and can be obtained by biochemical and thermochemical methods. Sugarcane waste contains different amounts of cellulose, hemicellulose, and lignocellulose and after pretreatment, biogas, bioethanol, biobutanol, synthesis gas, or bio-hydrogen can be obtained. The largest amount of solid waste from sugar factories is represented by bagasse. In addition to energy recovery, there are technological options for the valorization of sugarcane bagasse as a raw material or additive in obtaining a wide range of products, from growth media and substrate fermentation, nanomaterials, bioadsorbents, paper, biopolymers, biodegradable household products, construction materials, etc. Liquid waste, which includes the vinasse from ethanol distillation and wastewater from sugarcane processing stages, are used to obtain bioenergy, but also as organic fertilizers for agricultural land, with agricultural and environmental benefits, provided that the wastewater is properly treated to eliminate dangerous pollutants. Molasses can be used for biofuels without requiring pretreatment, as a raw material in the food industry (human and animal foods), or in the cosmetics industry. Although there is a wide range of value-added products that can be obtained from the by-products and waste of the sugarcane processing stream, not all technologies are commercially available yet. Thus, efforts are still needed to implement clean technologies and ecological alternatives for recycling and valorization of waste from the sugar industry and beyond. Research perspectives will focus on processes optimization to achieve technological maturity and to allow up-scaling, especially for the valorization of waste in the fields of biotechnologies and biofuels, so that both the used installations and the obtained value-added products become commercially available. This study can represent a starting point for young researchers who address the problem of waste from the sugar industry and who aim to contribute to mitigating the energy crisis and protecting the environment.
Author Contributions
Conceptualization, N.U.; methodology, N.U. and V.V.; validation, N.U. and S.-Ș.B.; formal analysis, S.-Ș.B.; investigation, N.U.; resources, V.V.; data curation, S.-Ș.B.; writing—original draft preparation, N.U.; writing—review and editing, N.U., V.V. and S.-Ș.B.; visualization, V.V.; supervision, V.V. and S.-Ș.B. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by University Politehnica of Bucharest, Romania, within the PubArt Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by a grant of the Romanian Research and Innovation Ministry, through Programme NUCLEU—PN 19 10 01 05 “Integrated management of works in agricultural, vineyard and fruit farms”, contract no. 5N/2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohamad, N.; Lakhiar, M.T.; Samad, A.A.A.; Mydin, A.O.; Jhatial, A.A.; Sofia, S.A.; Goh, W.I.; Ali, N. Innovative and sustainable green concrete—A potential review on utilization of agricultural waste. IOP Conf. Ser. Mater. Sci. Eng. 2019, 601, 012026. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C. Sugarcane industry waste recovery: A case study using thermochemical conversion technologies to increase sustainability. Appl. Sci. 2020, 10, 6481. [Google Scholar] [CrossRef]
- FAOSTAT Statistical Yearbook, World Food and Agriculture. 2021. Available online: https://www.fao.org/3/cb4477en/cb4477en.pdf (accessed on 4 July 2022).
- OECD-FAO Agricultural Outlook 2020–2029. Available online: https://www.oecd-ilibrary.org/sites/3736a600-en/index.html?itemId=/content/component/3736a600-en#section-d1e18381 (accessed on 4 July 2022).
- Faostat 2022. Sugarcane Production in 2020, Crops/Regions/World List/Production Quantity (Pick Lists). UN Food and Agriculture Organization, Corporate Statistical Database. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 4 July 2022).
- Amini, Z.; Self, R.; Strong, J.; Speight, R.; O’Hara, I.; Harrison, M.D. Valorization of sugarcane biorefinery residues using fungal biocatalysis. Biomass Convers. Biorefinery 2021, 12, 997–1011. [Google Scholar] [CrossRef]
- Canilha, L.; Chandel, A.K.; dos Santos Milessi, T.S.; Fernandes Antunes, F.A.; da Costa Freitas, W.L.; das Garcas Almeida, F.M.; da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Boussarsar, H.; Roge, B.; Mathlouthi, M. Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresour. Technol. 2009, 100, 6537–6542. [Google Scholar] [CrossRef] [PubMed]
- Fito, J.; Tefera, N.; Van Hulle, S.W.H. Sugarcane biorefineries wastewater: Bioremediation technologies for environmental sustainability. Chem. Biol. Technol. Agric. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Ometto, A.R.; Hauschild, M.Z.; Roma, W.N.L. Lifecycle assessment of fuel ethanol from sugarcane in Brazil. Int J. Life Cycle Assess 2009, 14, 236–247. [Google Scholar] [CrossRef]
- Martinelli, L.; Filoso, S.; Aranha, C.D.B.; Ferraz, S.F.B.; Andrade, T.M.B.; Ravagnani, E.D.C. Water use in sugar and ethanol industry in the State of Sao Paulo (Southeast Brazil). J. Sustain. Bioenergy Syst. 2013, 3, 135–142. [Google Scholar] [CrossRef]
- Costa, S.M.; Aguiar, A.; Luz, S.M.; Pessoa, A.; Costa, S.A. Sugarcane straw and its cellulosic fraction as raw materials for obtainment of textile fibers and other bioproducts. Polysaccharides 2014, 513–533. [Google Scholar] [CrossRef]
- Sanjuan, R.; Anzaldo, J.; Vargas, J.; Turrado, J.; Patt, R. Morphological and chemical composition of pith and fibers from Mexican sugarcane bagasse. Holz Als Roh-Und Werkst. 2011, 59, 447–450. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the valorization of lignocellulosic materials by biotechnology: An overview. BioResources 2013, 8, 3157–3176. [Google Scholar] [CrossRef]
- Rocha, G.J.M.; Martin, C.; Soares, I.B.; Souto Maior, A.M.; Baudel, H.M.; de Abreu, C.A.M. Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy 2011, 35, 663–670. [Google Scholar] [CrossRef]
- Canilha, L.; Santos, V.T.; Rocha, G.J.; Almeida e Silva, J.B.; Giulietti, M.; Silva, S.S.; Felipe, M.G.; Ferraz, A.; Milagres, A.M.; Carvalho, W. A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J. Ind. Microbiol. Biotechnol. 2011, 38, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable new material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Taherzsadeh, M.J.; Karimi, K. Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar] [CrossRef]
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorization of sugarcane bagasse for bioethanol production through simultaneous saccharification and fermentation: Optimization and kinetic studies. Fuel 2020, 262, 116552. [Google Scholar] [CrossRef]
- Smithers, J. Review of sugarcane trash recovery systems for energy cogeneration in South Africa. Renew. Sustain. Energy Rev. 2014, 32, 915–925. [Google Scholar] [CrossRef]
- Meghana, M.; Shastri, Y. Sustainable valorization of sugar industry waste: Status, opportunities, and challenges. Bioresour. Technol. 2020, 303, 122929. [Google Scholar] [CrossRef]
- Pierossi, M.; Bernhardt, H.W.; Funke, T. Sugarcane leaves and tops: Their current use for energy and hurdles to be overcome, particularly in South Africa, for greater utilisation. Proc. Annu. Congr. S. Afr. Sugar Technol. Assoc. 2016, 89, 350–360. [Google Scholar]
- Zuorro, A.; Lavecchia, R.; González-Delgado, Á.D.; García-Martinez, J.B.; L’Abbate, P. Optimization of enzyme-assisted extraction of flavonoids from corn husks. Processes 2019, 7, 804. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Batra, V.S. Valorization of solid waste in sugar factories with possible applications in India: A review. J. Environ. Manag. 2011, 92, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20. [Google Scholar] [CrossRef]
- Gómez, E.O.; Souza, R.T.G.; Rocha, G.J.M.; Almeida, E.; Cortez, L.A.B. A palha de cana-de-açúcar como matéria-prima para processos de segunda geração. In Bioetanol de Cana de Açúcar; Cortez, L.A.B., Ed.; Edgard Bleucher: São Paulo, Brazil, 2010; pp. 636–659. [Google Scholar]
- Nakhla, D.A.; El Haggar, S. A proposal to environmentally balanced sugarcane industry in Egypt. Int. J. Agric. Policy Res. 2014, 2, 321–328. [Google Scholar] [CrossRef]
- Singh, P.; Suman, A.; Tiwari, P.; Arya, N.; Gaur, A.; Shrivastava, A.K. Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J. Microbiol. Biotechnol. 2008, 24, 667–673. [Google Scholar] [CrossRef]
- Mahimairaja, S.; Dooraisamy, P.; Lakshmanan, A.; Rajannan, G.; Udayasoorian, C.; Natarajan, S. Composting Technology and Organic Waste Utilization in Agriculture; A.E. Publications: Coimbatore, India, 2008. [Google Scholar]
- Nunes, L.; Matias, J.; Catalão, J. A review on torrefied biomass pellets as a sustainable alternative to coal in power generation. Renew. Sustain. Energy Rev. 2014, 40, 153–160. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Small-sized biorefineries as strategy to add value to sugarcane bagasse. Chem. Eng. Res. Des. 2016, 107, 137–146. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Carrere, H.; Maciel Filho, R.; Costa, A.C. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour. Technol. 2011, 102, 7887–7895. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Candido, V.S.; Braga, F.O.; Bolzan, L.T.; Weber, R.P.; Drelich, J.W. Sugarcane bagasse waste in composites for multilayered armor. Eur. Polym. J. 2016, 78, 173–185. [Google Scholar] [CrossRef]
- Shafiq, N.; Hussein, A.A.E.; Nuruddin, M.F.; Al Mattarneh, H. Effects of sugarcane bagasse ash on the properties of concrete. Proc. Inst. Civ. Eng. 2018, 171, 123–132. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Chow, W.S.; Ismail, H. Sugarcane bagasse fiber and its cellulose nanocrystals for polymer reinforcement and heavy metal adsorbent: A review. Cellulose 2018, 25, 4303–4330. [Google Scholar] [CrossRef]
- Sahu, O. Assessment of sugarcane industry: Suitability for production, consumption, and utilization. Ann. Agrar. Sci. 2018, 16, 389–395. [Google Scholar] [CrossRef]
- Alves-Rezende, C.; de Lima, M.A.; Maziero, P.; Ribeiro de Azevedo, E.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.N.; Lopes, F.P.D.; Barbosa, A.P.; Bevitori, A.B.; Silva, I.L.A.; Costa, L.L. Natural lignocellulosic fibers as engineering materials—An overview. Metall. Mater. Trans. A 2011, 42, 2963–2974. [Google Scholar] [CrossRef]
- Dale, B.E. Biomass, Bioengineering of. In Encyclopedia of Physical Science and Technology, 3rd ed.; Robert, M., Ed.; Academic Press: Tarzana, CA, USA, 2003; pp. 141–157. ISBN 978-0-12-227410-7. [Google Scholar] [CrossRef]
- Luz, S.M.; Caldeira-Pires, A.; Ferrão, P.M.C. Environmental benefits of substituting talc by sugarcane bagasse fibers as reinforcement in polypropylene composites: Ecodesign and LCA as strategy for automotive components. Resour. Conserv. Recycl. 2010, 54, 1135–1144. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N.; Boussetta, N.; Pizzi, A. Isolation and characterization of lignin from Moroccan sugar cane bagasse: Production of lignin–phenolformaldehyde wood adhesive. Ind. Crops Prod. 2013, 45, 296–302. [Google Scholar] [CrossRef]
- Richards, D.; Yabar, H. Potential of renewable energy in Jamaica’s power sector: Feasibility analysis of biogas production for electricity generation. Sustainability 2022, 14, 6457. [Google Scholar] [CrossRef]
- Sidana, A.; Farooq, U. Sugarcane bagasse: A potential medium for fungal cultures. Chin. J. Biol. 2014, 2014, 840505. [Google Scholar] [CrossRef]
- De Moraes Rocha, G.J.; Nascimento, V.M.; Gonçalves, A.R.; Silva, V.F.N.; Martín, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Ind. Crop. Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Barrera, I.; Amezcua-Allieri, M.A.; Estupinan, L.; Martínez, T.; Aburto, J. Technical and economical evaluation ofbioethanol production from lignocellulosic residues in Mexico: Case of sugarcane and blue agave bagasses. Chem. Eng. Res. Des. 2016, 107, 91–101. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Datta, S.C.; Biswas, D.R.; Dotaniya, C.K.; Meena, B.L.; Rajendiran, S.; Refar, K.L.; Lata, M. Use of sugarcane industrial by-products for improving sugarcane productivity and soil health. Int. J. Recycl. Org. Waste Agric. 2016, 5, 185–194. [Google Scholar] [CrossRef]
- Gabov, K.; Hemming, J.; Fardim, P. Sugarcane bagasse valorization by fractionation using a water-based hydrotropic process. Ind. Crop. Prod. 2017, 108, 495–504. [Google Scholar] [CrossRef]
- Guida, M.Y.; Hannioui, A. Properties of bio-oil and bio-char produced by sugar cane bagasse pyrolysis in a stainless steel tubular reactor. Prog. Agric. Eng. Sci. 2017, 13, 13–33. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Zhang, X.K.; Zhang, W.W.; Lei, F.H.; Yang, S.; Jiang, J. Coproduction of xylooligosaccharides and fermentable sugars from sugarcane bagasse by seawater hydrothermal pretreatment. Bioresour. Technol. 2020, 309, 123385. [Google Scholar] [CrossRef]
- Anu, A.; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Management of sugar industrial wastes through vermitechnology. Int. Lett. Nat. Sci. 2016, 55, 35–43. [Google Scholar] [CrossRef]
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent advances in sugarcane industry solid by-products valorization. Waste Biomass Valorization 2017, 8, 241–266. [Google Scholar] [CrossRef]
- Munagala, M.; Shastri, Y.; Nalawade, K.; Konde, K.; Patil, S. Life cycle and economic assessment of sugarcane bagasse valorization to lactic acid. Waste Manag. 2021, 126, 52–64. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Amezcua-Allieri, M.A.; Sadhukhan, J.; Aburto, J. Sugarcane bagasse valorization strategies for bioethanol and energy production. In Sugarcane; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2018; Chapter 4. [Google Scholar] [CrossRef]
- Chandel, A.K.; Antunes, F.A.; Terán-Hilares, R.; Cota, J.; Ellilä, S.; Silveira, M.H.; dos Santos, J.C.; da Silva, S.S. Bioconversion of hemicellulose into ethanol and value-added products: Commercialization, trends, and future opportunities. In Advances in Sugarcane Biorefinery. Technologies, Commercialization, Policy Issues and Paradigm Shift for Bioethanol and By-Products; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 97–134. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef]
- Contreras-Lisperguer, R.; Batuecas, E.; Mayo, C.; Díaz, R.; Pérez, F.J.; Springer, C. Sustainability assessment of electricity cogeneration from sugarcane bagasse in Jamaica. J. Clean. Prod. 2018, 200, 390–401. [Google Scholar] [CrossRef]
- Iryani, D.; Hirajima, T.; Kumagai, S.; Nonaka, M.; Sasaki, K. Overview of Indonesian sugarcane industry and utilization of its solid waste. In Proceedings of the Annual Fall Meeting of the Mining and Materials Processing Institute of Japan (MMIJ), Akita, Japan, 14–16 October 2012. [Google Scholar]
- Hofsetz, K.; Silva, M.A. Brazilian sugarcane bagasse: Energy and non-energy consumption. Biomass Bioenergy 2012, 46, 564–573. [Google Scholar] [CrossRef]
- Arif, E.; Clark, M.W.; Lake, N. Sugar cane bagasse ash from a high efficiency co-generation boiler: Applications in cement and mortar production. Constr. Build. Mater. 2016, 128, 287–297. [Google Scholar] [CrossRef]
- Alves, M.; Ponce, G.H.; Silva, M.A.; Ensinas, A.V. Surplus electricity production in sugarcane mills using residual bagasse and straw as fuel. Energy 2015, 91, 751–757. [Google Scholar] [CrossRef]
- Carriel Schmitt, C.; Moreira, R.; Cruz Neves, R.; Richter, D.; Funke, A.; Raffelt, K.; Grunwaldt, J.D.; Dahmen, N. From agriculture residue to upgraded product: The thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process. Technol. 2020, 197, 106199. [Google Scholar] [CrossRef]
- Quereshi, S.; Naiya, T.K.; Mandal, A.; Dutta, S. Residual sugarcane bagasse conversion in India: Current status, technologies, and policies. In Biomass Conversion and Biorefinery; Springer Professional: Hong Kong, China, 2020. [Google Scholar] [CrossRef]
- Turdera, M.V. Energy balance, forecasting of bioelectricity generation and greenhouse gas emission balance in the ethanol production at sugarcane mills in the state of Mato Grosso do Sul. Renew. Sustain. Energy Rev. 2013, 19, 582–588. [Google Scholar] [CrossRef]
- Ravindranath, N.H.; Balachandra, P.; Dasappa, S.; Usha, R.K. Bioenergy technologies for carbon abatement. Biomass Bioenergy 2006, 30, 826–837. [Google Scholar] [CrossRef]
- Nenciu, F.; Paraschiv, M.; Kuncser, R.; Stan, C.; Cocârță, D.; Vlăduț, V. High-grade chemicals and biofuels produced from marginal lands using an integrated approach of alcoholic fermentation and pyrolysis of sweet sorghum biomass residues. Sustainability 2022, 14, 402. [Google Scholar] [CrossRef]
- Miskat, M.I.; Ahmed, A.; Chowdhury, H.; Chowdhury, T.; Chowdhury, P.; Sait, S.M.; Park, Y.-K. Assessing the theoretical prospects of bioethanol production as a biofuel from agricultural residues in Bangladesh: A review. Sustainability 2020, 12, 8583. [Google Scholar] [CrossRef]
- Krishnan, C.; da Costa Sousa, L.; Jin, M.; Chang, L.; Dale, B.E.; Balan, V. Alkali-based AFEX pretreatment for the conversion of sugarcane bagasse and cane leaf residues to ethanol. Biotechnol. Bioeng. 2010, 107, 441–450. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M. Delignification of sugarcane bagasse using pretreatment strategies for bioethanol production. Biocatal. Agric. Biotechnol. 2019, 20, 101263. [Google Scholar] [CrossRef]
- Kamzon, M.A.; Abderafi, S.; Bounahmidi, T. The efficient process for the conversion of bagasse and beet pulp to bioethanol. In Proceedings of the 3rd International Renewable and Sustainable Energy Conference (IRSEC), Marrakech, Morocco, 10–13 December 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Padella, M.; O’Connell, A.; Prussi, M. What is still limiting the deployment of cellulosic ethanol? Analysis of the current status of the sector. Appl. Sci. 2019, 9921, 4523. [Google Scholar] [CrossRef]
- Neves, P.V.; Pitarelo, A.P.; Ramos, L.P. Production of cellulosic ethanol from sugarcane bagasse by steam explosion: Effect of extractives content, acid catalysis and different fermentation technologies. Bioresour. Technol. 2016, 208, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Iram, M.; Asghar, U.; Irfan, M.; Huma, Z.; Jamil, S.; Nadeem, M.; Syed, Q. Production of bioethanol from sugarcane bagasse using yeast strains: A kinetic study. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 364–372. [Google Scholar] [CrossRef]
- Khonngam, T.; Salakkam, A. Bioconversion of sugarcane bagasse and dry spent yeast to ethanol through a sequential process consisting of solid-state fermentation, hydrolysis, and submerged fermentation. Biochem. Eng. J. 2019, 150, 107284. [Google Scholar] [CrossRef]
- Nava-Cruz, N.Y.; Contreras-Esquivel, J.C.; Aguilar-Gonzalez, M.A.; Nuncio, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Agave atrovirens fibers as substrate and support for solid-state fermentation for cellulase production by Trichoderma asperellum. BioTech 2016, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef]
- Pereira, L.G.; Chagas, M.F.; Dias, M.O.S.; Cavalett, O.; Bonomi, A. Life cycle assessment of butanol production in sugarcane biorefineries in Brazil. J. Clean. Prod. 2015, 96, 557–568. [Google Scholar] [CrossRef]
- Joglekar, S.N.; Dalwankar, G.; Qureshi, N.; Mandavgane, S.A. Sugarcane valorization: Selection of process routes based on sustainability index. Environ. Sci. Pollut. Res. 2022, 29, 10812–10825. [Google Scholar] [CrossRef]
- van den Wall Bake, J.D.; Junginger, M.; Faaij, A.; Poot, T.; Walter, A. Explaining the experience curve: Cost reductions of Brazilan ethanol from sugarcane. Biomass Bioenergy 2009, 33, 644–658. [Google Scholar] [CrossRef]
- Silalertruksa, T.; Gheewala, S.H.; Pongpat, P. Sustainability assessment of sugarcane biorefinery and molasses ethanol production in Thailand using eco-efficiency indicator. Appl. Energy 2015, 160, 603–609. [Google Scholar] [CrossRef]
- Moore, P. Temporal and spatial regulation of sucrose accumulation in the sugarcane stem. Aust. J. Plant Physiol. 1995, 22, 661–679. [Google Scholar] [CrossRef]
- Brito Cruz, C.H.; Mendes Souza, G.; Barbosa Cortez, L.A. Biofuels for transport. In Future Energy. Improved, Sustainable and Clean Options for our Planet, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Amore, I.; Ballesteros, I.; Manzanares, P.; Sáez, F.; Michelena, G.; Ballesteros, M. Ethanol production from sugarcane bagasse pretreated by steam explosion. Electron. J. Energy Environ. 2013, 1, 25–36. [Google Scholar] [CrossRef]
- Raj, K.; Krishnan, C. Improved co-production of ethanol and xylitol from low-temperature aqueous ammonia pretreated sugarcane bagasse using two-stage high solids enzymatic hydrolysis and Candida tropicalis. Renew. Energy 2020, 153, 392–403. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Chandel, A.K.; Brumano, L.P.; Teran Hilares, R.; Peres, G.F.D.; Ayabe, l.E.S.; Sorato, V.S.; Santos, J.R.; Santos, J.C.; Da Silva, S.S. A novel process intensification strategy for second-generation ethanol production from sugarcane bagasse in fluidized bed reactor. Renew. Energy 2018, 124, 189–196. [Google Scholar] [CrossRef]
- Sritrakul, N.; Nitisinprasert, S.; Keawsompong, S. Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric. Nat. Resour. 2017, 51, 512–519. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Clean Hydrogen Partnership. European Partnership for Hydrogen Technologies. Available online: https://www.clean-hydrogen.europa.eu/index_en (accessed on 29 July 2022).
- Singh, A.; Sevda, S.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen production from lignocellulosic biomass: Technology and sustainability. Energies 2015, 8, 13062–13080. [Google Scholar] [CrossRef]
- Lai, Z.; Zhu, X.; Yang, J.; Wang, S.; Li, S. Optimization of key factors affecting hydrogen production from sugarcane bagasse by a termophilic anaerobic pure culture. Biotechnol. Biofuels 2014, 7, 119. [Google Scholar] [CrossRef][Green Version]
- Ratti, R.P.; Delforno, T.P.; Sakamoto, I.K.; Varesche, M.B.A. Thermophilic hydrogen production from sugarcane bagasse pretreated by steam explosion and alkaline delignification. Int. J. Hydrogen Energy 2015, 40, 6296–6306. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P.; Ashtana, R.K.; Singh, S. Biohydrogen production from sugarcane bagasse by integrating dark- and photo-fermentation. Bioresour. Technol. 2014, 152, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Brienzo, M.; Carvalho, W.; Milagres, A.M.F. Xylooligosaccharides production from alkali-pretreated sugarcane bagasse using xylanases from Thermoascus aurantiacus. Appl. Biochem. Biotechnol. 2010, 162, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.L.; Zhao, S.; Liang, R.M.; Yin, X.; Jiang, S.X.; Su, L.H.; Yang, Q.; Duan, C.J.; Liu, J.L.; Feng, J.X. A biotechnological process efficiently co-produces two high value-added products, glucose and xylooligosaccharides, from sugarcane bagasse. Bioresour. Technol. 2016, 204, 130–138. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.K.; Shanker, G.; Srivastava, A. Citric acid production by solid state fermentation using sugarcane bagasse. Process Biochem. 2003, 38, 1731–1738. [Google Scholar] [CrossRef]
- Borges, E.R.; Pereira, N. Succinic acid production from sugarcane bagasse hemicellulose hydrolysate by Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1001–1011. [Google Scholar] [CrossRef]
- da Cunha, M.N.C.; dos Santos Nascimento, J.C.; Souza-Motta, M.C.; Albertini, A.V.P.; Lima, C.A.; Marques, D.D.A.V.; Porto, A.L.F. Production of enzymes by filamentous fungus using sugarcane and sugarcane bagasse as substrate. Rev. Bras. Biocienc. 2013, 11, 227–234. [Google Scholar]
- Sindhu, R.; Gnansounou, E.; Binod, P.; Pandey, A. Bioconversion of sugarcane crop residue for value added products—An overview. Renew. Energy 2016, 98, 203–215. [Google Scholar] [CrossRef]
- Prakash, G.; Varma, A.J.; Prabhune, A.; Shouche, Y.; Rao, M. Microbial production of xylitol from D-xylose and sugarcane bagasse hemicellulose using newly isolated thermotolerant yeast Debaryomyces hansenii. Bioresour. Technol. 2011, 102, 3304–3308. [Google Scholar] [CrossRef]
- Xu, L.; Liu, L.; Li, S.; Zheng, W.; Cui, Y.; Liu, R.; Sun, W. Xylitol production by Candida tropicalis 31949 from sugarcane bagasse hydrolysate. Sugar Tech. 2019, 21, 341–347. [Google Scholar] [CrossRef]
- Cueva-Orjuela, J.C.; Hormaza-Anaguano, A.; Merino-Restrepo, A. Sugarcane bagasse and its potential use for the textile effluent treatment. Dyna 2017, 84, 291–297. [Google Scholar] [CrossRef]
- Ladeira, N.; Peixoto, V.J.; Penha, M.P.; Barros, E.B.; Gomes Ferreira, S. Optimization of 6-pentyl-a-pyrone production by solid state fermentation using sugarcane bagasse as residue. Bioresources 2010, 5, 2297–2306. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef]
- Sardar, H.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Ali, S.; Haider, S.T. Effect of different agro-wastes, casing materials and supplements on the growth, yield and nutrition of milky mushroom (Calocybe indica). Folia Hortic. 2020, 32, 115–124. [Google Scholar] [CrossRef]
- Akter, M.; Halawani, R.F.; Aloufi, F.A.; Taleb, M.A.; Akter, S.; Mahmood, S. Utilization of agro-industrial wastes for the production of quality oyster mushrooms. Sustainability 2022, 14, 994. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Su, C.H.; Yu, Y.K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crop. Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Fonseca, G.C.d.; Oliveira, M.S.; Martins, C.V.C.; de Souza, J.C.P. How the carbonization time of sugarcane biomass affects the microstructure of biochar and the adsorption process? Sustainability 2022, 14, 1571. [Google Scholar] [CrossRef]
- Saleh, M.E.; Hedia, R. Mg-modified sugarcane bagasse biochar for dual removal of ammonium and phosphate ions from aqueous solutions. Alex. Sci. Exch. J. 2018, 39, 74–91. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Shiono, T.; Shinogi, Y. Influence of sugarcane bagasse derived biochar application on nitrate leaching in calcaric dark red soil. J. Environ. Qual. 2012, 41, 1131–1137. [Google Scholar] [CrossRef]
- Toledo-Jaldin, H.P.; Sanchez-Mendieta, V.; Blanco-Flores, A.; Lopez-Tellez, G.; Vilchis-Nestor, A.R.; Martin-Hernandez, O. Low-cost sugarcane bagasse and peanut shell magnetic composites applied in the removal of carbofuran and iprodione pesticides. Environ. Sci. Pollut. Res. 2020, 27, 7872–7885. [Google Scholar] [CrossRef]
- Oliveira Alves, J.; Soares Tenório, J.A.; Zhuo, C.; Levendis, Y.A. Characterization of nanomaterials produced from sugarcane bagasse. J. Mater. Res. Technol. 2012, 1, 31–34. [Google Scholar] [CrossRef]
- Shahi, N.; Min, B.; Sapkota, B.; Rangari, V.K. Eco-friendly cellulose nanofiber extraction from sugarcane bagasse and film fabrication. Sustainability 2020, 12, 6015. [Google Scholar] [CrossRef]
- Pereira, A.A.; Martins, G.F.; Antunes, P.A.; Conrrado, R.; Pasquini, D.; Job, A.E.; Curvelo, A.A.; Ferreira, M.; Riul, A., Jr.; Constantino, C.J. Lignin from sugar cane bagasse: Extraction, fabrication of nanostructured films, and application. Langmuir 2007, 23, 6652–6659. [Google Scholar] [CrossRef] [PubMed]
- Homagai, P.; Ghimire, K.N.; Inoue, K. Adsorption behavior of heavy metals onto chemically modified sugarcane bagasse. Bioresour. Technol. 2009, 101, 2067–2079. [Google Scholar] [CrossRef]
- Dos Santos, V.C.; De Souza, J.V.; Tarley, C.R.; Caetano, J.; Dragunski, D.C. Copper ions adsorption from aqueous medium using the biosorbent sugarcane bagasse in natura and chemically modified. Water Air Soil Pollut. 2011, 216, 351–359. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Al Arni, S. Extraction and isolation methods for lignin separation from sugarcane bagasse: A review. Ind. Crop. Prod. 2018, 115, 330–339. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Oyekunle, I.P.; Oladunjoye, I.O.; Ibitogbe, E.M.; Olorunfemi, T.S. A review on the mitigation of heavy metals from aqueous solution using sugarcane bagasse. Sugar Tech. 2022, 24, 1167–1185. [Google Scholar] [CrossRef]
- Akhramez, S.; Fatimi, A.; Okoro, O.V.; Hajiabbas, M.; Boussetta, A.; Moubarik, A.; Hafid, A.; Khouili, M.; Simińska-Stanny, J.; Brigode, C.; et al. The circular economy paradigm: Modification of bagasse-derived lignin as a precursor to sustainable hydrogel production. Sustainability 2022, 14, 8791. [Google Scholar] [CrossRef]
- Faria, K.C.P.; Gurgel, R.F.; Holanda, J.N.F. Recycling of sugarcane bagasse ash waste in the production of clay bricks. J. Environ. Manag. 2012, 101, 7–12. [Google Scholar] [CrossRef]
- Janbuala, S.; Eambua, M.; Satayavibul, A.; Nethan, W. Effect of bagasse and bagasse ash levels on properties of pottery products. Heliyon 2018, 4, e00814. [Google Scholar] [CrossRef] [PubMed]
- Pozzer, T.; Gauss, C.; Ament Barbirato, G.H.; Fiorelli, J. Trapezoidal core sandwich panel produced with sugarcane bagasse. Constr. Build. Mater. 2020, 264, 120718. [Google Scholar] [CrossRef]
- Cangussu, N.; Chaves, P.; da Rocha, W.; Maia, L. Particleboard panels made with sugarcane bagasse waste—An exploratory study. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, T.; Oyejobi, D.; Lawal, A. Evaluation of sugarcane bagasse ash as a replacement for cement in concrete works. Acta Tech. Corviniensis-Bull. Eng. 2014, 3, 71–76. [Google Scholar] [CrossRef]
- Hajiha, H.; Sain, M. The use of sugarcane bagasse fibres as reinforcements in composites. In Biofiber Reinforcements in Composite Materials; Faruk, O., Sain, M., Eds.; Woodhead Publishing: Sawston, UK, 2015; Chapter 17; pp. 525–549. [Google Scholar] [CrossRef]
- Kolawole, S.C.; Combrinck, R. State-of-the-art review on the use of sugarcane bagasse ash in cementitious materials. Cem. Concr. Compos. 2021, 118, 103975. [Google Scholar] [CrossRef]
- Araújo de Almeida, M.; Colombo, R. Construction of green roofs via using the substrates made from humus and green coconut fiber or sugarcane bagasse. Sustain. Chem. Pharm. 2021, 22, 100477. [Google Scholar] [CrossRef]
- Rainho Teixeira, S.; Arenales, A.; de Souza, A.E.; da Silva Magalhaes, R.; Vilche Pena, A.F.; Aquino, D.; Freire, R. Sugarcane bagasse: Applications for energy production and ceramic materials. J. Solid Waste Technol. Manag. 2015, 41, 229–238. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Julian, F.; Espinach, F.X.; Tarres, Q.; Ardanuy, M.; Mutje, P. Research on the use of lignocellulosic fibers reinforced bio-polyamide 11 with composites for automotive parts: Car door handle case study. J. Clean. Prod. 2019, 226, 64–73. [Google Scholar] [CrossRef]
- Dos Santos Pegoretti, T.; Mathieux, F.; Evrard, D.; Brissaud, D.; de Franca Arruda, J.R. Use of recycled natural fibres in industrial products: A comparative LCA case study on acoustic components in the Brazilian automotive sector. Resour Conserv. Recycl. 2014, 84, 1–14. [Google Scholar] [CrossRef]
- Samantra, K.K.; Basak, S.; Chattopadhyay, S.K. Recycled fibrous and nonfibrous biomass for value-added textile and nontextile applications. In Environmental Implications of Recycling and Recycled Products. Environmental Footprints and Eco-Design of Products and Processes; Muthu, S., Ed.; Springer: Singapore, 2015; pp. 167–212. [Google Scholar] [CrossRef]
- Poopack, S.; Reza, A.R. Environmental benefit of using bagasse in paper production—A case study of LCA in Iran. In Global Warming—Impacts and Future Perspective; Intech Open: London, UK, 2012; Chapter 8; pp. 205–222. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Wongsuchoto, P.; Pavasant, P. Life cycle assessment of bagasse waste management options. Waste Manag. 2009, 2, 1628–1633. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Ashori, A.; Khorasani, Z.; Abdulkhani, A.; Abyaz, A. Pre-extraction of hemicelluloses from bagasse fibers: Effects of dry-strength additives on paper properties. Ind. Crop. Prod. 2013, 43, 365–371. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Pandare, K.V.; Nair, G.; Varma, A.J. Utilization of sugarcane bagasse cellulose for producing cellulose acetates: Novel use of residual hemicellulose as plasticizer. Carbohydr. Polym. 2009, 76, 23–29. [Google Scholar] [CrossRef]
- Guna, V.; Ilangovan, M.; Hu, C.; Venkatesh, K.; Reddy, N. Valorization of sugarcane bagasse by developing completely biodegradable composites for industrial applications. Ind. Crop. Prod. 2019, 131, 25–31. [Google Scholar] [CrossRef]
- Okano, K.; Fukui, S.; Kitao, R.; Usagawa, T. Effects of culture length of Pleurotuseryngii grown on sugarcane bagasse on in vitro digestibility and chemical composition. Anim. Feed Sci. Technol. 2007, 136, 240–247. [Google Scholar] [CrossRef]
- Wanapat, M.; Anantasook, N.; Rowlinson, P.; Pilajun, R.; Gunun, P. Effect of carbohydrate sources and levels of cotton seedmeal in concentrate on feed intake, nutrient digestibility, rumen fermentation and microbial protein synthesis in young dairy bulls. Asian-Australas. J. Anim. Sci. 2013, 26, 529–536. [Google Scholar] [CrossRef]
- Balgees, A.; Elmnan, A.; Hemeedan, A.A.; Ahmed, R.I. Influence of different treatments on nutritive values of sugarcane bagasse. Glob. J. Anim. Sci. Res. 2016, 2, 295. [Google Scholar]
- Amjed, M.; Jung, H.G.; Donker, J.D. Effect of alkaline hydrogen peroxide treatment on cell wall composition and digestion kinetics of sugarcane residues and wheat straw. J. Anim. Sci. 1992, 70, 2877–2884. [Google Scholar] [CrossRef]
- Lee, S.; Scott, S.D.; Pekas, E.J.; Lee, J.; Park, S. Improvement of lipids and reduction of oxidative stress with octacosanol after taekwondo training. Int. J. Sports Physiol. Perform. 2019, 14, 1297–1303. [Google Scholar] [CrossRef]
- Olagunju, A.; Muhammad, A.; Aimola, I.A.; Abdullahi, S.A.; Danhassan, M.S. Effect of Lachnocladium spp. fermentation on nutritive value of pretreated sugarcane bagasse. Int. J. Mod. Biol. Med. 2014, 5, 24–32. [Google Scholar]
- Mahala, A.G.; Babiker, S.A.; Gutbi, N.E. Improvement of digestibility of sugarcane bagasse by fermentation with chicken manure. Res. J. Agric. Soil. Sci. 2007, 3, 115–118. [Google Scholar]
- Shahowna, E.M.; Mahala, A.G.; Mokhtar, A.M.; Amasaib, E.O.; Attaelmnan, B.A. Evaluation of nutritive value of sugar cane bagasse fermented with poultry litter as animal feed. J. Food Sci. Technol. 2013, 4, 106–109. [Google Scholar]
- Khan, M.F.; Ali, A.; Muller, Z.O. Nutritional evaluation of sugarcane bagasse based rations treated with urea and cattle manure. Anim. Feed Sci. Technol. 1992, 38, 135–141. [Google Scholar] [CrossRef]
- Rabelo, S.C.; da Costa, A.C.; Vaz Rossel, C.E. Industrial waste recovery. In Sugarcane: Agricultural Production, Bioenergy and Ethanol, 1st ed.; Santos, F., Borém, A., Caldas, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 365–381. [Google Scholar]
- Texteira, S.R.; Pena, A.F.V.; Miguel, A.G. Briquetting of charcoal from sugarcane bagasse fly ash (SCBFA) as an alternative fuel. Waste Manag. 2010, 30, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; Lima, S.A. Use of Brazilian sugarcane bagasse ash in concrete as sand replacement. Waste Manag. 2010, 30, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.V.R.; Rao, T.R.; Grover, P.D. Biomass Thermo-Chemical Characterization, 3rd ed.; Indian Institute of Technology: New Delhi, India, 2002. [Google Scholar]
- Xu, Q.; Ji, T.; Gao, S.-J.; Yang, Z.; Wu, N. Characteristics and applications of sugar cane bagasse ash waste in cementitious materials. Materials 2018, 12, 39. [Google Scholar] [CrossRef]
- Barrera Torres, G.; Dognani, G.; da Silva Agostini, D.L.; dos Santos, R.J.; Carmago Cabrera, F.; Gutierres Aguilar, C.M.; de Paiva, F.F.G.; Rainho Texteira, S.; Job, A.E. Potential eco-friendly application of sugarcane bagasse ash in the rubber industry. Waste Biomass Valori. 2021, 12, 4599–4613. [Google Scholar] [CrossRef]
- Santos, R.J.D.; Agostini, D.L.D.S.; Cabrera, F.C.; Ruiz, M.R.; Teixeira, S.R.; Job, A.E. Sugarcane bagasse ash: New filler to natural rubber composite. Polimeros 2014, 24, 646–653. [Google Scholar] [CrossRef]
- Umamaheswaran, K.; Batra, V.S. Physico-chemical characterization of Indian biomass ashes. Fuel 2008, 87, 628–638. [Google Scholar] [CrossRef]
- Mukundrao, G.S.; Changdeo, D.S.; Vikas, J.S. Experimental study of sugarcane bagasse ash blend & its application in M-30 grade of concrete for moderate exposure conditions. Int. J. Eng. Technol. 2017, 4, 1156–1159. [Google Scholar] [CrossRef]
- Paya, J.; Monzo, J.; Borrachero, M.V.; Tashima, M.M.; Soriano, L. Bagasse ash. In Waste and Supplementary Cementitious Materials in Concrete Characterisation, Properties and Applications; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2018; Chapter 17; pp. 559–598. [Google Scholar]
- Deepika, S.; Anand, G.; Bahurudeen, A.; Santhanam, M. Construction products with sugarcane bagasse ash binder. J. Mater. Civ. Eng. 2017, 29, 10. [Google Scholar] [CrossRef]
- Onchieku, J.; Chikamai, B.; Rao, M. Optimum parameters for the formulation of charcoal briquettes using bagasse and clay as binder. Eur. J. Sustain. Dev. 2012, 1, 477. [Google Scholar] [CrossRef]
- Setter, C.; Sanchez Costa, K.L.; Pires de Oliveira, T.J.; Farinassi Mendes, R. The effects of kraft lignin on the physicomechanical quality of briquettes produced with sugarcane bagasse and on the characteristics of the bio-oil obtained via slow pyrolysis. Fuel Process. Technol. 2020, 210, 106561. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Saleem, M.A.; Munir, M.J.; Khitab, A. Manufacturing of sustainable clay bricks: Utilization of waste sugarcane bagasse and rice husk ashes. Constr. Build. Mater. 2016, 120, 29–41. [Google Scholar] [CrossRef]
- de Siqueira, A.A.; Cordeiro, G.C. Sustainable cements containing sugarcane bagasse ash and limestone: Effects on compressive strength and acid attack of mortar. Sustainability 2022, 14, 5683. [Google Scholar] [CrossRef]
- Anjos, M.A.S.; Araújo, T.R.; Ferreira, R.L.S.; Farias, E.C.; Martinelli, A.E. Properties of self-leveling mortars incorporating a high-volume of sugar cane bagasse ash as partial Portland cement replacement. J. Build. Eng. 2020, 32, 101694. [Google Scholar] [CrossRef]
- Kulkarni, P.; Ravekar, V.; Rao, P.R.; Waigokar, S.; Hingankar, S. Recycling of waste HDPE and PP plastic in preparation of plastic brick and its mechanical properties. Clean Mat. 2022, 4, 100113. [Google Scholar] [CrossRef]
- Tripathy, A.; Acharya, P.K. Characterization of bagasse ash and its sustainable use in concrete as a supplementary binder—A review. Constr. Build. Mater. 2022, 322, 126391. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Batra, V.S.; Tewari, P.K. A Process for the Preparation of Inorganic Membrane Filters for Solid-Liquid and Solid-Gas Separations. Indian Patent 1326/Del/2006, 6 July 2006. [Google Scholar]
- Tewari, P.K.; Singh, R.K.; Batra, V.S.; Balakrishnan, M. Membrane bioreactor (MBR) for wastewater treatment: Filtration performance evaluation of low cost polymeric and ceramic membranes. Sep. Purif. Technol. 2010, 71, 200–204. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Hartanto, D.; Rohman, H.A.; Mitamaytawati; Qudus, N.; Daniyanto. Valorization of sugarcane-based bioethanol industry waste (vinasse) to organic fertilizer. In Valorisation of Agro-industrial Residues—Volume II: Non-Biological Approaches; Zakaria, Z., Aguilar, C., Kusumaningtyas, R., Binod, P., Eds.; Applied Environmental Science and Engineering for a Sustainable Future; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Hoarau, J.; Caro, Y.; Grondin, I.; Petit, T. Sugarcane vinasse processing: Toward a status shift from waste to valuable resources: A review. J. Water Process. Eng. 2018, 24, 11–25. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Bento, H.B.S.; Alves, T.M.; Carvalho, A.K.F.; De Castro, H.F. Vinasse treatment within the sugarcane-ethanol industry using ozone combined with anaerobic and aerobic microbial processes. Environments 2019, 6, 5. [Google Scholar] [CrossRef]
- Moraes, B.S.; Zaiat, M.; Bonomi, A. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives. Renew. Sustain. Energy Rev. 2015, 44, 888–903. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane vinasse as organo-mineral fertilizers feedstock: Opportunities and environmental risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef] [PubMed]
- Christofoletti, C.A.; Escher, J.P.; Correia, J.E.; Marinho, J.F.U.; Fontanetti, C.S. Sugarcane vinasse: Environmental implications of its use. Waste Manag. 2013, 33, 2752–2761. [Google Scholar] [CrossRef]
- Moreira, L.C.; Borges, P.O.; Cavalcante, R.M.; Young, A.F. Simulation and economic evaluation of process alternatives for biogas production and purification from sugarcane vinasse. Renew. Sustain. Energy Rev. 2022, 163, 112532. [Google Scholar] [CrossRef]
- Kiani, M.K.D.; Parsaee, M.; Ardebili, S.M.S.; Pereda Reyes, I.; Fuess, L.T.; Karimi, K. Different bioreactor configurations for biogas production from sugarcane vinasse: A comprehensive review. Biomass Biorenergy 2022, 161, 106446. [Google Scholar] [CrossRef]
- Ramos, L.R.; Lovato, G.; Domingues Rodrigues, J.A.; Silva, E.L. Scale-up and energy estimations of single- and two-stage vinasse anaerobic digestion systems for hydrogen and methane production. J. Clean. Prod. 2022, 349, 131459. [Google Scholar] [CrossRef]
- Tano, F.; Valenti, L.; Failla, O.; Beltrame, E. Effects of distillery vinasses on vineyard yield and quliaty in the D.O.C. “Oltrepo Pavese Pinot Nero”—Lombardy, Italy. Water Sci. Technol. 2005, 151, 199–204. [Google Scholar] [CrossRef][Green Version]
- Santos, F.; Eichler, P.; Machado, G.; De Mattia, J.; De Souza, G. By-products of the sugarcane industry. In Sugarcane Biorefinery, Technology and Perspectives; Santos, F., Rabelo, S., De Matos, M., Eichler, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 21–48. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Karimi, K.; Taherzadeh, M.J. Valorization of vinasse and whey to protein and biogas through an environmental fungi-based biorefinery. J. Environ. Manag. 2022, 303, 114138. [Google Scholar] [CrossRef]
- Yadav, R.L.; Solomon, S. Potential of developing sugarcane by-product based industries in India. Sugar Tech. 2006, 8, 104–111. [Google Scholar] [CrossRef]
- Partha, N.; Sivasubramanian, V. Recovery of chemicals from press mud—A sugar industry waste. Indian Chem. Eng. 2006, 48, 160–163. [Google Scholar]
- Ochoa-George, P.A.; Eras, J.J.; Gutierrez, A.S.; Hens, L.; Vandecasteele, C. Residue from sugarcane juice filtration (filter cake): Energy use at the sugar factory. Waste Biomass Valor. 2010, 1, 407–413. [Google Scholar] [CrossRef]
- Sen, B.; Chandra, T.S. Chemolytic and solid-state spectroscopic evaluation of organic matter transformation during vermicomposting of sugar industry wastes. Bioresour. Technol. 2007, 98, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Casas, L.; Hernández, Y.; Mantell, C.; Casdelo, N.; Ossa, E.M. Filter cake oil-wax as raw material for the production of biodiesel: Analysis of the extraction process and the transesterification reaction. J. Chem. 2015, 2015, 946462. [Google Scholar] [CrossRef]
- Jamil, M.; Qasim, M.; Zia, M.S. Utilization of press mud as organic amendment to improve physico-chemical characteristics of calcareous soil under two legume crops. J. Chem. Soc. Pak. 2008, 30, 577–583. [Google Scholar]
- Fantaye, A.; Fanta, A.; Melesse, A.M. Effect of filter press mud application on nutrient availability in aquert and fluvent soils of Wonji/Shoa sugarcane plantation of Ethiopia. In Landscape Dynamics, Soils and Hydrological Processes in Varied Climates; Melesse, A., Abtew, W., Eds.; Springer Geography: Berlin/Heidelberg, Germany, 2016; pp. 549–563. [Google Scholar] [CrossRef]
- Rakkiyappan, P.; Thangavelu, S.; Malathi, R.; Radhamani, R. Effect of biocompost and enriched pressmud on sugarcane yield and quality. Sugar Tech. 2001, 3, 92–96. [Google Scholar] [CrossRef]
- Sarangi, B.K.; Mudliar, S.N.; Bhatt, P.; Kalve, S.; Chakrabarti, T.; Pandey, R.A. Compost from sugar mill press mud and distillery spent wash for sustainable agriculture. Dyn. Soil Dyn. Plant 2009, 2, 35–49. [Google Scholar]
- Nenciu, F.; Stanciulescu, I.; Vlad, H.; Gabur, A.; Turcu, O.L.; Apostol, T.; Vlădut, V.N.; Cocârță, D.; Stan, C. Decentralized processing performance of fruit and vegetable waste discarded from retail, using an automated thermophilic composting technology. Sustainability 2022, 14, 2835. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K. Fertigation with agro-residue based paper mill effluent on a high yield spinach variety. Int. J. Veg. Sci. 2015, 21, 69–97. [Google Scholar] [CrossRef]
- Sarwar, G.; Schmeisky, H.; Hussain, N.; Muhammad, S.; Ibrahim, M.; Safdar, E. Improvement of soil physical and chemical properties with compost application in rice-wheat cropping system. Pak. J. Bot. 2008, 40, 275–282. [Google Scholar]
- Keshavanath, P.; Shivanna Gangadhara, B. Evaluation of sugarcane by-product press mud as a manure in carp culture. Bioresour. Technol. 2006, 97, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Tripathi, S.; Balomajumder, C. Characterization of press mud: A sugar industry waste. Fuel 2011, 90, 389–394. [Google Scholar] [CrossRef]
- Ansari, K.B.; Gaikar, V.G. Pressmud as an alternate resource for hydrocarbons of green engineering. Environ. Sci. Technol. 2014, 37, 94–101. [Google Scholar] [CrossRef]
- El Haggar, S.; El Gowini, M.M.; Nemerow, N.L.; Veziroglu, T.N. Environmentally balanced industrial complex for the cane sugar industry in Egypt. In Proceedings of the International Hydrogen Energy Congress and Exhibition IHEC, Istanbul, Turkey, 13–15 July 2005. [Google Scholar]
- Asaithambi, P.; Matheswaran, M. Electrochemical treatment of simulated sugar industrial effluent: Optimization and modeling using a response surface methodology. Arab. J. Chem. 2016, 9, S981–S987. [Google Scholar] [CrossRef]
- Ingaramo, A.; Heluane, H.; Colombo, M.; Mario Cesca, M. Water and wastewater eco-efficiency indicators for the sugar cane industry. J. Clean. Prod. 2009, 17, 487–495. [Google Scholar] [CrossRef]
- Zver, L.Ž.; Glavič, P. Water minimization in process industries: Case study in beet sugar plant. Resour Conserv Recycl. 2005, 43, 133–145. [Google Scholar] [CrossRef]
- Prakash, S. Sugar mill effluent induced histological changes in intestine of Channa punctatus. Ind. J. Biol. Stud. Res. 2011, 1, 32–35. [Google Scholar]
- Doble, M.; Kruthiventi, A.K. Industrial examples. In Green Chemistry and Engineering; Academic Press: Cambridge, MA, USA, 2007; Chapter 9; pp. 245–296. [Google Scholar] [CrossRef]
- Guven, G.; Perendeci, A.; Tanylac, A. Electrochemical treatment of simulated beet sugar factory wastewater. Chem. Eng. J. 2009, 151, 149–159. [Google Scholar] [CrossRef]
- Baraniya, C.; Jodhi, C. Performance evaluation of effluent treatment plant for sugar mill effluent. Int. J. Creat. Res. Thoughts IJCRT 2018, 6, 1492–1499. [Google Scholar]
- Memon, A.R.; Soomro, S.A.; Ansari, A.K. Sugar industry effluent—Characteristics and chemical analysis. J. App. Em. Sc. 2006, 1, 152–157. [Google Scholar]
- Sahu, O.P.; Chaudhari, P.K. Electrochemical treatment of sugar industry wastewater: COD and color removal. J. Electroanal. Chem. 2015, 739, 122–129. [Google Scholar] [CrossRef]
- Cavalett, O.; Junqueira, T.L.; Dias, M.O.; Jesus, C.D.; Mantelatto, P.E.; Cunha, M.P.; Franco, H.C.; Cardoso, T.F.; Maciel Filho, R.; Rossell, C.E.; et al. Environmental and economic assessment of sugarcane first generation biorefineries in Brazil. Clean. Technol. Environ. Policy 2012, 14, 399–410. [Google Scholar] [CrossRef]
- Martin-Lara, M.A.; Rico, I.L.R.; Aloma Vicente, I.C.; Garcia, G.B.; de Hoces, M.C. Modification of the sorptive characteristics of sugarcane bagasse for removing lead from aqueous solutions. Desalination 2010, 256, 58–63. [Google Scholar] [CrossRef]
- Khan, T.; Amin, M.; Sami, S.K.; Khan, M.N.; Ul Hassan, S.Z.; Sultan, S.H.; Asghar, A.; Ali, A.; Khan, M.Q.; Junaid, M. Valorization of sugarcane bagasse waste for fly ash production and application as an adsorbent. J. Appl. Emerg. Sci. 2020, 10, 93–97. [Google Scholar] [CrossRef]
- Lappa, K.; Kandylis, P.; Bekatorou, A.; Bastas, N.; Klaoudatos, S.; Athanasopoulos, N.; Kanellaki, M.; Koutinas, A.A. Continuous acidogenesis of sucrose, raffinose and vinasse using mineral kissiris as promoter. Bioresour. Technol. 2015, 188, 43–48. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water scarcity and wastewater reuse in crop irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Teclu, D.; Tivchev, G.; Laing, M.; Wallis, M. Determination of the elemental composition of molasses and its suitability as carbon source for growth of sulphate-reducing bacteria. J. Hazard. Mater. 2009, 161, 1157–1165. [Google Scholar] [CrossRef]
- Amorim, H.V.; Baso, L.C.; Lopes, M.L. Sugar cane juice and molasses, beet molasses and sweet sorghum: Composition and usage. In Alcohol Textbook; Nothingham University Press: Trumpton Nottingham, UK, 2003; pp. 39–46. [Google Scholar]
- Clarke, M.A. Syrups. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Luiz, T., Paul, F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Khan, S.; Lu, F.; Kashif, M.; Shen, P. Multiple effects of different nickel concentrations on the stability of anaerobic digestion of molasses. Sustainability 2021, 13, 4971. [Google Scholar] [CrossRef]
- Raharja, R.; Murdiyatmo, U.; Sutrisno, A.; Wardani, A.K. Bioethanol production from sugarcane molasses by instant dry yeast. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012076. [Google Scholar] [CrossRef]
- Basso, L.C.; Basso, T.O.; Rocha, S.N. Ethanol production in Brazil: The industrial process and its impact on yeast fermentation. Biofuel Prod. Recent Dev. Prospect. 2011, 1530, 85–100. [Google Scholar] [CrossRef]
- Kartini, A.M.; Dhokhikah, Y. Bioethanol production from sugarcane molasses with simultaneous saccharification and fermentation (SSF) Method using Saccaromyces cerevisiae-Pichia stipitis consortium. IOP Conf. Ser. Earth Environ. Sci. 2018, 207, 012061. [Google Scholar] [CrossRef]
- Jayanti, A.N.; Sutrisno, A.; Wardani, A.K.; Murdiyatmo, U. Bioethanol production from sugarcane molasses by instant dry yeast (effect of pretreatment and fermentation temperature). IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012102. [Google Scholar] [CrossRef]
- Parascanu, M.M.; Sanchez, N.; Sandoval-Salas, F.; Mendez Carreto, C.; Soreanu, G.; Sanchez-Silva, L. Environmental and economic analysis of bioethanol production from sugarcane molasses and agave juice. Environ. Sci Pollut. Res. 2021, 28, 64374–64393. [Google Scholar] [CrossRef]
- Afschar, A.S.; Vaz Rossell, C.E.; Schaller, K. Bacterial conversion of molasses to acetone and butanol. Appl. Microbiol. Biotechnol. 1990, 34, 168–171. [Google Scholar] [CrossRef]
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; O’Connor, K.E. Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Adv. Appl. Microbiol. 2013, 84, 139–200. [Google Scholar] [CrossRef]
- Nicol, D.A. Rum. In Fermented Beverage Production; Lea, A.G.H., Piggott, J.R., Eds.; Springer: Boston, MA, USA, 2003; pp. 263–287. [Google Scholar] [CrossRef]
- Gandiglio, M.; Lanzini, A. Biogas resource potential and technical exploitation. In Comprehensive Renewable Energy, 2nd ed.; Sayigh, A., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Portilla, O.M.; Espinosa, V.; Jarquin, L.; Salinas, A.; Velazquez, G.; Vazquez, M. Sugar cane molasses as culture media component for microbial transglutaminase production. Indian J. Biotechnol. 2017, 16, 419–425. [Google Scholar]
- Oliveira Lino, F.S.; Basso, T.O.; Sommer, M.O.A. A synthetic medium to simulate sugarcane molasses. Biotechnol. Biofuels 2018, 11, 221. [Google Scholar] [CrossRef]
- Vignesh Kumar, B.; Muthumari, B.; Kavitha, M.; John Praveen Kumar, J.K.; Thavamurugan, S.; Arun, A.; Jothi Basu, M. Studies on optimization of sustainable lactic acid production by Bacillus amyloliquefaciens from sugarcane molasses through microbial fermentation. Sustainability 2022, 14, 7400. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).