Abstract
Dominant psychological models of wellbeing neglect the role that nature connection and other key factors, such as positive health behaviours and behaviour change, play in determining wellbeing. The present mixed-methods evaluation explores the impact of ”Surf-Ability”, an adapted surf therapy intervention delivered in collaboration with a UK neurorehabilitation service, on individuals with acquired brain injury (ABI) as part of an effort to design interventions based on advances in wellbeing science. Following five surf-therapy sessions, within-subjects analysis (n= 15) revealed significant improvements on the Warwick–Edinburgh mental wellbeing scale (t (15) = −2.164, p = 0.048), as well as in anxiety and happiness as measured via a brief visual analogue. No significant changes occurred in the Hospital Anxiety and Depression Scale (HADS) or resting heart rate variability (HRV). A ripple effects mapping (REM) session at 6–10 months follow-up (n = 6) revealed that the physical and psychological experience of a nature-based challenge initiated a mindset shift in participants, which ultimately led to them adopting wellbeing-promoting long-term behaviour changes. These changes occurred at the scale of (1) individual wellbeing—increased mindfulness and physical activity; (2) collective wellbeing—improved relationships, community participation and contribution to organisations; and (3) planetary wellbeing—connection to nature. These findings align with the GENIAL theoretical framework, which defines wellbeing from a biopsychosocial ecological perspective across multiple levels of scale. The findings support the need for healthcare providers—including neurorehabilitation services—to enhance interventions for patients by incorporating novel factors that improve wellbeing, such as nature-connection.
1. Introduction
Acquired brain injury (ABI) is the leading cause of death and disability in young people in the United Kingdom [1]. It is a chronic condition, commonly resulting in pervasive cognitive, psychological, physical and social difficulties, including, but not limited to, memory and executive functioning impairments [2], fatigue, pain [3], increased psychological distress, depression [4,5], loss of relationships [6], change in identity [7] and reduced social integration [8]. As a result, individuals with ABI frequently report having reduced quality of life and wellbeing following their injury [9,10]. One major challenge for the treatment of chronic conditions, including ABI, is that the “medical model” is still the predominant model of treatment in healthcare, despite being originally designed to treat acute conditions. The aim of the medical model is to diagnose deficits and reduce specific symptoms, as opposed to promoting whole health and wellbeing. Given that the impact of chronic conditions is pervasive and often cannot be “reversed”, the medical model’s reductionist approach is not equipped to manage the long-term consequences of chronic disease alone [11].
Within neurorehabilitation, the predominant healthcare model is now the holistic approach [12], which typically involves six steps: (1) psychoeducation, (2) cognitive assessment and intervention, (3) psychological intervention, (4) physiotherapy, (5) functional and daily living skills and (6) vocational training. The holistic approach proposes that ABI must be treated via integrated insights from a multi-disciplinary team [13], and such programs have been successful in improving employability outcomes [14,15], community integration, quality of life and self-efficacy [16] for ABI patients. However, it is argued that there are still improvements that might be made to the holistic approach via integration of advances in wellbeing research.
One criticism of the holistic approach to neurorehabilitation is that, of all the components, social contributions to recovery are most poorly defined [13]. Yet, evidence suggests that it is the difficulty maintaining a social life that makes the cognitive and physical impairments detrimental to lives. For example, one study found that cognitive symptoms in stroke are associated with reduced wellbeing (r = −0.36), but this relationship is mediated by one’s capacity to maintain group memberships (r = −0.30). This suggests that cognitive symptoms negatively impact wellbeing only to the extent to which they hamper one’s capacity to integrate socially [17]. According to social identity theory, belonging to multiple social groups protects wellbeing following ABI, as it provides individuals with more opportunity for social interaction and, thus, time to develop self-regulation skills (managing emotional distress, taking goal-oriented action and conforming to societal rules) [18]. It is essential that neurorehabilitation moves beyond individual social skills training and creates opportunities for patients to acquire new social groups in the community post-ABI, particularly given the common loss of social roles within the family, work and leisure participation [6,19,20,21].
Moreover, the holistic approach to neurorehabilitation does not specifically utilise nature connection as a strategy to promote wellbeing. Human beings have a complex and inter-connected relationship with nature [22], and it has been argued that high levels of wellbeing can only be achieved through the experiential realisation of nature connectedness [23]. Feeling connected to nature, including living animals, plants, geological processes and ”blue” and ”green” environments, such as parks, forests, mountain ranges, oceans, rivers or lakes, may reflect a basic human psychological need [24]. This argument is supported by the ever-growing body of evidence that spending time in natural environments promotes individual wellbeing. One example is from a representative survey of the English population (n = 7814) that found that having access to a domestic garden was associated with improved self-reported wellbeing [25]. Furthermore, a study on census data on 48.2 million people in England found that people who rated their health as ”good” were more likely to live in closer proximity to the coast [26]. Nature-based interventions such as Shinrin-yoku (“forest bathing”) have been found to improve physiological markers of health and wellbeing, such as increased parasympathetic activity (increased heart rate variability (HRV)) [27]. In addition, improving nature-connectedness may aid us in moving toward the goal of achieving ”planetary wellbeing”, which is defined as “the highest attainable standard of wellbeing for human and non-human beings and their social and natural systems” (Antó et al., 2021, p. 1). This is supported by recent evidence (n = 4960) that nature connectedness is positively related to eudemonic wellbeing and increased pro-environmental behaviour [28], leading the authors to propose that interventions that ”promote contact with and connection to nature are needed to make harmonious progress to both human and planetary health” [28].
Moreover, there is evidence to suggest that nature connection may be particularly beneficial for individuals with ABI. Firstly, according to attention restoration theory (ART) [29], being in natural environments triggers involuntary attentional processes, which allow top-down controlled mechanisms a chance to replenish [30]. Given that individuals with ABI commonly suffer from fatigue [31] and are more likely to suffer from heightened sensory sensitivity to light and noise [32], nature is likely to improve their ability to complete tasks that require such controlled processing. Moreover, individuals with ABI commonly face challenges with emotional regulation, particularly individuals with damage to the prefrontal cortex, given its role in dampening down emotionally charged signals from the amygdala [33,34,35]. According to the stress reduction theory of nature (SRT) [36], being in the presence of nature supports individuals to experience a parasympathetically dominated “relaxation response”, reducing cortisol, heart rate and blood pressure [37,38]. Both these hypotheses thus warrant further exploration regarding the potential positive role of nature connection in ABI neurorehabilitation.
This acknowledgement that nature plays a significant role in health is reflected in the evolution of wellbeing science across the last few decades. Early positive psychology initially defined wellbeing according to purely positive experiences [39]. Whilst this early work successfully highlighted the field’s biased attention on ill-being and paved the way for a new focus on wellbeing and flourishing, new waves of positive psychology have acknowledged a need to move beyond individualistic definitions of wellbeing to also include collective (or social) and planetary wellbeing [11,40,41,42]. For example, the Genomics—Environment—Vagus Nerve—Social Interaction—Allostatic Regulation—Longevity (GENIAL) theoretical framework of wellbeing is an evidence-based biopsychosocial ecological model integrating transdisciplinary research from across wellbeing science, including the role of nature and wellbeing [11,43,44]. It proposes that wellbeing both impacts and is impacted by: (1) self-connection (e.g., emotional regulation, meaning and purpose, as well as health behaviours, such as sleep, diet and exercise); (2) social connection (including personal relationships, social capital, social cohesion and social identity); and, finally, (3) nature connection. The framework proposes that, mechanistically, wellbeing is supported by the functioning of the vagus nerve.
The vagus nerve is the tenth cranial nerve, which connects the brain to most organs, including the heart, gut and lungs, and plays a key role supporting the parasympathetic nervous system, making it a worthy candidate for supporting wellbeing [45]. The functioning of the vagus nerve can be measured using heart rate variability (HRV) [46], whereby a higher HRV reflects greater vagal tone. The GENIAL model is supported by evidence that vagal function is promoted via interventions that increase self, social and nature connectedness; for example, via promoting a healthy lifestyle [47], mindfulness-based interventions [48], yoga and tai chi [49], loving kindness meditation [50] and forest bathing [27]. Across healthcare settings, there is still a focus on individual recovery that neglects the role of collective and planetary wellbeing, despite large epidemiology studies showing that social connections and nature connectedness reduce all-cause mortality and improve health/wellbeing outcomes [51,52,53,54]. To provide a truly “holistic” approach to neurorehabilitation, it is vital that services begin to implement the latest advances in wellbeing science and consider individual, collective and planetary wellbeing when designing interventions.
The healthcare service described herein (a National Health Service (NHS) community neurorehabilitation service in the UK) adapted its service model by forming collaborative partnerships between local community organisations and academics to create novel interventions for patients, underpinned by the GENIAL model of wellbeing [11,43,44]. Here, a pivotal intervention is presented—surf therapy, which was provided in collaboration with a third sector organisation (Surf-Ability) as part of neurorehabilitation treatment for individuals living with ABI. Surf therapy is a method of intervention that combines surf instruction/surfing and structured individual and/or group activities to promote wellbeing (International Surf Therapy Organization, 2019). Surf therapy is a relatively new intervention, with peer-reviewed evidence only emerging in the last 10 years—a systematic review in 2020 identified only 29 studies across six countries to be included. Initial data suggest that surf therapy has a beneficial impact on emotional regulation, personal growth, wellbeing and positive affect [55,56,57]; however, the majority of studies are solely qualitative designs and quantitative studies vary hugely in quality. The surf-therapy intervention was designed to facilitate the key determinants of wellbeing, as defined by the GENIAL model, whilst utilising the natural environment as a treatment opportunity [11,43,44].
A qualitative service evaluation was previously conducted using individual interviews from participants immediately after completing surf therapy [58]. The evaluation revealed seven central themes, including: (1) connection to nature, (2) facilitating trust and safety, (3) managing and accepting difficult emotions, (4) facilitating positive emotion, meaning and purpose, (5) building community through social connection, (6) positive change and (7) barriers and opportunities. Importantly, dynamic interactions between themes highlighted the importance of designing holistic interventions that consider the individual within the context of their community and natural environment, as well as socio-structural barriers.
The aim of the present evaluation was to assess whether surf-therapy intervention promoted wellbeing according to quantitative survey and physiological data. A second aim was to conduct an REM session with a sub-sample of participants at approximately one year follow-up to assess whether the impact of the intervention had longevity and, thus, determine whether it is an appropriate long-term use of service resources.
2. Materials and Methods
2.1. Study Design
A partially mixed sequential equal-status design was employed [59]. The term “sequential” describes the fact that the qualitative and quantitative phases of the study occurred separately; in this instance, the quantitative phase occurred at the time of the intervention and the qualitative phase occurred at 6–10 months follow-up. In partially mixed methods, both the quantitative and qualitative elements are conducted separately, only being mixed at the data interpretation stage. The design puts equal emphasis on both qualitative and quantitative components with respect to addressing the evaluation aims.
2.1.1. Quantitative Study Design
The quantitative component was a within-subjects repeated-measures design. Participants who attended the intervention each completed a routine series of survey measures related to wellbeing prior to and upon completion of the intervention. The aim was to compare participants’ scores using within-subjects analysis to establish whether there was a change in wellbeing-related measures following surf therapy. A within-subjects comparison was used because between-subjects comparison is not possible during a routine service evaluation.
2.1.2. Qualitative Study Design
Ripple effects mapping (REM) [60] was used to facilitate a follow-up focus group session 6–10 months prior to the intervention. REM is a qualitative tool to evaluate program outcomes using both participants and stakeholders, such as group facilitators or clinicians [60]. This method of evaluation was employed because it aligns with the goal of ”co-production”, given that it engages participants to reflect upon and visually map the effects of the program. REM has four key components: (1) appreciative inquiry, whereby participants in the session briefly share their experiences in pairs; (2) a participatory approach (participant involvement); (3) interactive group interviewing and reflection; and (4) “radiant thinking”. All of these components are incorporated in to one session that ends with a mind map of key themes and effects, as well as a recorded transcript of all discussions. REM is unique in its ability to capture a complex chain of events triggered by programs. There are three variations of REM, to be chosen depending on the context of the intervention. Variation two, ”In Depth Rippling”, was used in this instance, which involves mapping out rich and detailed narratives from each of the participants describing follow-up stories from the intervention. This approach was chosen because it allowed us to capture short-, medium- and long-term outcomes as resulting ”ripples”, which is important in ensuring interventions are helpful for patients long-term and, thus, that limited resources are used efficiently. In addition, it was felt that it would be beneficial for patients to share their personal stories in-depth, given this is an activity that is therapeutic in itself [61].
2.2. Participants
Participants were patients receiving neurorehabilitation under a Community Brain Injury Service or Community Neurorehabilitation Service in South Wales. Patients were invited to attend the surf-therapy intervention if (1) they met the eligibility criteria for the service and (2) their treating clinician felt they would benefit from attending the group because it had the potential to support them to achieve their rehabilitation goals and they would be able to engage in the intervention safely and meaningfully. Of 27 patients who were invited, 18 attended and 9 had to drop out before starting the intervention or very early on due to travel or health restrictions. Everyone who attended gave informed consent. See Figure 1 for the participant CONSORT flow diagram.
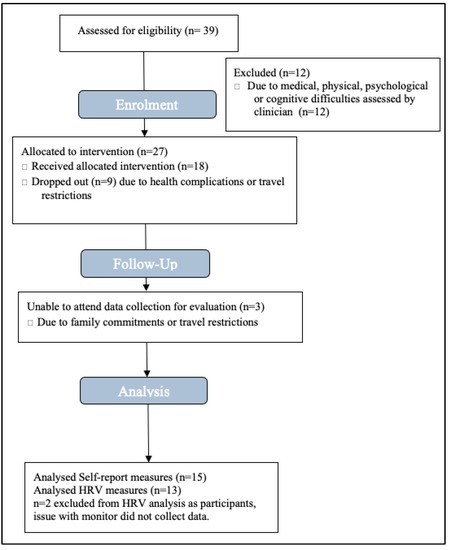
Figure 1.
Quantitative data CONSORT flow diagram.
Participants were not randomly assigned to the intervention because the data were collected as part of a service evaluation, and so it was not appropriate to assign patients randomly. This decision instead, was centred around whether Surf-Ability might help them achieve their individual rehabilitation goals. Common goals that participants’ involvement aimed to address included: creating new social connections, developing social skills, initiating the process of forming a post-injury identity, increasing independence, finding purpose in life, increasing physical activity and improving balance. Given that surf-therapy is a novel intervention for ABI, this individualised approach avoided engagement being negatively impacted due to patients’ expectations not matching their ‘assigned’ treatment [62].
All participants’ injuries were classed as moderate to severe, in line with the service’s eligibility criteria. Common challenges experienced by participants included cognitive difficulties, such as memory, attention and executive functioning, in addition to psychosocial challenges, such as increased psychological distress. Some participants had physical difficulties, including fatigue, dizziness and balance impairments, which were accommodated for using adaptive surf equipment and extra staff support.
Inclusion criteria: in accordance with the eligibility criteria for the service, participants were 18 years of age or older, had a confirmed diagnosis of an acquired brain injury and lived in the community and in the health board catchment areas. They were able to take part meaningfully and safely in the intervention.
Exclusion criteria: patients who had any medical, physical, cognitive or psychosocial difficulties that would prevent them engaging with neurorehabilitation or the surf therapy intervention (as assessed by their treating clinician) were excluded; for example, if a patient had uncontrollable epilepsy and was deemed not medically fit to take part. Risk assessments were carried out by clinicians, and all staff involved were made aware of individual participants’ needs and requirements. Patients who were unable to give informed consent were excluded from the evaluation. Patients who dropped out of the intervention due to ill health and travel difficulties (before completing at least four out of five sessions) were excluded.
2.3. Intervention
The intervention took place between 2019 and 2021 during the summer/autumn months, with a new cohort participating each year. The intervention was provided as part of a collaborative relationship between two NHS neurorehabilitation services and a local, community-based third sector organisation. Surf-Ability is a community interest group who provide adapted surf lessons for individuals with additional needs. Surf-Ability UK is located at Caswell Bay on the Gower Peninsula of South Wales and their mission is to make surfing accessible for everyone.
The intervention took place over 5 weeks and included a two-hour session once per week in a small group of no more than five participants. In each session, two members of the neurorehabilitation service were present to assist participants with their rehabilitation goals during the session. At least two qualified adaptive surf instructors were present, along with enough volunteers to support participants 1:1.
Surf-Ability staff assessed individual participants’ needs during the first session and provided appropriate equipment based on these needs; for example, if a participant had a physical disability and required a seated surfboard. A seated surfboard is an extra-large surfboard with a seat attached to the top (see Figure 2), which allows the surf instructor to paddle out to sea with the participant seated on the board, so they are still able to experience catching the waves. All participants were given a demonstration on shore on how to safely manage the board in the water and how to safely stand up, fall, call for assistance and recognise safety signals. In each session, staff helped participants guide or paddle their board out into the sea and ride the wave to shore. They chose to lie down or kneel or attempted to stand up on the board.

Figure 2.
Photographs of participants at Surf-Ability with an example of a seated surfboard.
Clinicians from the neurorehabilitation service encouraged participants to set goals for themselves at the start of every session based on their rehabilitation. Sometimes these were surfing-related, such as building confidence to stand on the board, sometimes psychosocial, such as engaging in more conversations with peers, or sometimes they were related to physical needs, such as managing fatigue. Clinicians also aimed to bring therapeutic concepts into the water; for example, by encouraging participants to practice mindfulness by staying present and aware during the session. They also encouraged them to savour their achievements and recognise their progress.
Written informed consent was obtained and permission granted from staff and patients to publish photographs. These were anonymised by the authors to reduce revelations of unnecessary information regarding patient identity.
2.4. Quantitative Data Collection
The lead author (LW) collected the data. LW is a part-time assistant psychologist within the neurorehabilitation service and a PhD candidate. Patients had met LW before because she works in the service and were therefore comfortable meeting her alone. LW arranged to meet participants at the neuropsychology department to collect physiological and questionnaire measures. Following the onset of COVID-19-related social distancing restrictions in the UK in March 2020, participants who attended the intervention during summer of 2020 were posted a HRV monitor through the mail and were asked to wear the device for 10 min at home whilst on a Zoom conferencing call to LW. At the start of the session, the participant was made aware of the purpose of the service evaluation, that their data would be used for service development purposes and that, if it were to be written up for publication in the future, data would be anonymised. Data collection only continued if the participant understood this, agreed to take part and signed the consent form. During HRV collection, participants were asked to wear the small device and were then left alone in the room for ten minutes. They were asked not to engage in any activities, such as using their phone, during this time and to simply sit in silence. Once ten minutes had passed, the device was removed and LW re-entered the room to administer the questionnaires. When HRV data were collected via Zoom, the exact same process occurred; however, LW gave instructions remotely and turned off their video and audio, retuning it after 10 min. If the participant was able to, they read through and answered the questionnaire measures themselves; if they required further support, LW read the questions aloud and marked the appropriate answer based on the participants’ responses. The data were managed by LW, who also transcribed scores on to a database on a secure NHS password-protected laptop.
2.5. Quantitative Outcome Measures
The following questionnaire measures are routinely administered in the service during patients’ first assessments and throughout their treatment because they provide important information about psychological functioning and whether it changes following different aspects of neurorehabilitation. They also allow us to follow changes in patients’ scores across their rehabilitation to track progress. These measures were chosen because they have robust psychometric properties and had been normed in the brain injury or clinical population. Since 2018, heart rate variability (HRV) has also become a routine method of evaluation in the service based on the emerging evidence supporting its link to wellbeing. HRV is potentially a more inclusive way to measure changes in patients’ health and wellbeing, given that many patients may face communication or visual impairments, which make surveys challenging. HRV has provided clinicians with invaluable insights into patients’ lifestyles, health behaviours and stress-activity. Physiological measures are also less vulnerable to social desirability, making them a less biased and more robust method for evaluation.
2.5.1. Primary Outcome Measure
Warwick–Edinburgh Mental Wellbeing Scale—Short Version (SWEMWBS): this survey was developed at the request of NHS Scotland in the UK to measure mental wellbeing and evaluate interventions designed to improve wellbeing [63]. It includes seven statements about the participant’s thoughts and feelings (mental wellbeing). Participants are asked to respond by choosing one of five responses, ranging from 1 = ”none of the time” to 5 = ”all of the time”. The total raw score is then transformed into metric scores using the conversion table. WEMWBS is reported to be valid, reliable and also responsive to meaningful change in clinical populations [64,65]. Spearman correlations between SWEMWBS and WEMWBS are above 0.95, demonstrating the effectiveness of the short version [66]. The WEMWBS also shows high test–retest reliability at one week (0.83) [63].
2.5.2. Secondary Outcome Measures
Hospital Anxiety and Depression Scale (HADS) [67]: HADS was designed to measure anxiety and depression in the patient population. It focuses on non-physical symptoms in order to diagnose patients who also have physically ill health. The HADS contains seven questions for anxiety and seven for depression. The HADS has been validated in studies on stroke populations, which found good internal consistency and sensitivity and adequate specificity when lowered stroke-specific cut-off scores were used [68,69]. The HADS is often sensitive to change over time and thus is helpful in tracking changes in mood [70].
Visual analogue scale: visual analogue scales are used to measure subjective characteristics or symptoms [71]. The VAS used in the present evaluation aimed to gather weekly data on participants’ anxiety, happiness and connection to others across the intervention period. The questionnaire consisted of three horizontal lines, marked at each end to indicate the extremes of the feeling. The three questions consisted of: “How anxious/happy/connected to others do you feel in this moment right now?” and participants were asked to mark down the number that best reflected their current state on a scale of 0–10. Participants were asked to complete the VAS at baseline, at the start of each weekly session (before the session took place) and once post-intervention. The aim was to track any fluctuations in relevant characteristics across the intervention period.
Resting heart rate variability (HRV): collecting HRV is a non-invasive approach; the devices used were commercial products (Firstbeat technologies monitors) available for public use. The data collected on the device contained no personal data that could identify the participants and was uploaded to a secure laptop only accessible to LW; all data was deleted from the device once saved on the laptop. Firstbeat monitors claim to detect the heartbeat with 1 ms accuracy (1000 HZ) and calculate RR intervals. Participants were asked to sit for ten minutes without moving or using a phone whilst their HRV was measured at baseline and post-intervention. HRV data were then analysed in Kubios Premium (version 3.5.0). Artefact correction threshold was adjusted individually; this is stated by Kubios to be best practice because inter-individual difference in HRV is significant, and therefore a fixed threshold (e.g., 5%) will not work for all participants [72]. The optimal threshold was identified by choosing the lowest correction level that identified all artefacts (RR intervals that fell outside of the 600–1200 ms range, but without identifying too many normal RR intervals as artefacts (<5% of all beats removed) [72]. On average, three beats were removed from each sample (range = 0–12 single beats, 0–4.23% of beats). Data were then extracted and entered in SPSS. Root mean square of successive differences (RMSSD) and normed high frequency were used as outcome measures because they are thought to reflect parasympathetic activity [73,74].
Relevant participant demographics and states: relevant lifestyle and demographic questions were asked [75]. To accurately interpret HRV data, it is important to know when the participant last exercised, ate, consumed caffeine and alcohol, slept and smoked cigarettes, as well as understanding any blood pressure, heart or respiratory conditions, to ensure these factors are not responsible for a change in HRV [75].
2.6. Statistical Analysis
All primary and secondary quantitative measures were subjected to tests of significant difference for HRV and survey scores between baseline and post-intervention. Significance was set at 0.05 and an effect size was calculated using Cohen’s guidelines (0.2 = small, 0.5 = medium, 0.8 = large). Normality was assessed by examining histograms and pp plots and conducting a Shapiro–Wilk test. Paired t-tests were used to compare the differences in measures that met parametric assumptions and the Wilcoxon signed ranks test was used as a non-parametric alternative if variables violated assumptions.
2.7. Qualitative Data Collection
All participants who attended surf therapy in the cohort for summer and autumn of 2021 were invited to attend an REM session in April 2022. Participants who attended in the cohort of 2019 and 2020 (whose quantitative measures were included in the evaluation) were not invited to the REM session because they had been discharged from the service by April 2022, when the session took place. Of eight potential participants, four accepted and attended the session, and four were unable to attend due to work, family or travel commitments. A three-hour session took place at the local hospital in which the neuropsychology service is based. Written consent was obtained at the start of the session. The session was facilitated by the lead author (LW), and the written whiteboard content and a voice recording of the entire session was captured. At the start of the session, participants interviewed each other as part of the appreciative inquiry process. They were provided with question prompts (see Supplementary Materials) and space to make notes from the conversation, which could later be used in group discussion. A facilitated group discussion then took place whereby participants shared what they had discussed with their partner in their interviews. Key details from each participant’s story were mapped out on a large whiteboard and the facilitator used probing questions to encourage rich reflections and narratives (What happened next? How did that impact you?). After each participant’s story was told, the group brainstormed possible theme names to capture the most significant impacts that arose during the discussion. Finally, a list of the key themes was written on the whiteboard, which everyone approved of.
2.8. Qualitative Data Analysis
The recording of the REM session (which totalled 78 min) was transcribed and uploaded to ATLAS.ti for Mac for data management. Data analysis followed the six steps for thematic analysis [76]. Stages one to four involved the lead author (LW) only. Stage one was familiarisation through listening to the audio recording and re-reading the transcript, and stage two involved reviewing the data with the initial generated themes in mind to ensure they captured distinct concepts and reflection on whether effects were short or long term. Steps three and four were an iterative process whereby the transcripts were coded and themes were modified to ensure all discussed ideas were reflected in the final themes. In stage five, all authors reviewed the themes until an agreement was made and, finally, the results were reported in stage six.
2.9. Ethical Considerations
Evaluations of service user experiences associated with the delivery of interventions in the healthcare sector are excluded from ethical review in the United Kingdom because they are considered to contain minimal risk. No randomisation, experimental or control condition was used, and the intervention was not withheld from any eligible participants. Patient care did not deviate from usual care and participants continued to receive usual healthcare treatments in addition to surf therapy. All participants were invited to take part in the evaluation. Only those who gave informed consent were included and participants understood their rights to withdraw. When the data were transcribed, any identifiable information was removed. All participants included valued the opportunity to participate in evaluation, provide feedback on their experiences and help support service development.
The service described in this evaluation is highly research-driven; interventions are designed based on strong theoretical foundations and are updated using advances in the wellbeing literature. It is acknowledged that service evaluations are often heterogeneous and are not generalisable beyond the patient group. However, this work is still vital, as it represents how all service evaluations should use rigorous methodology to assess outcomes in patients, yet this is seldom done. If service evaluations only use sub-par methods, remain unpublished and do not keep up to date with advances in science, then the research–treatment gap and lag will only continue to increase. If we want science and research to be translated to healthcare, and for healthcare to be grounded in a strong evidence base, then more effort needs to be made to design interventions based on emerging evidence and to share novel treatment opportunities through well-designed, rigorous evaluations that can inspire clinicians to trial these within their own services.
3. Results
3.1. Quantitative Findings
The participant demographics of the present sample (Table 1) are a good representative on average of the ABI patient population seen in this specific neurorehabilitation service.

Table 1.
Quantitative data participant characteristics.
All outcome variables were normally distributed except for the VAS anxiety variable. A Wilcoxon signed ranks test was therefore performed for the VAS anxiety variable as a non-parametric alternative to the T-test. The Wilcoxon signed ranks test indicated that the difference between VAS anxiety ratings at pre (M = 4.4 SD = 1.96) and post (M = 2.73 SD = 2.2) was statistically significant, z = −2.367, p = 0.018. t-test results (see Table 2) revealed that there was a significant increase in self-reported wellbeing upon completing the surf therapy intervention and a significant increase in VAS happiness score. Measures of HRV and HADS did not differ significantly. VAS changes compared baseline to post-intervention (Figure 3).

Table 2.
Paired t-test results.
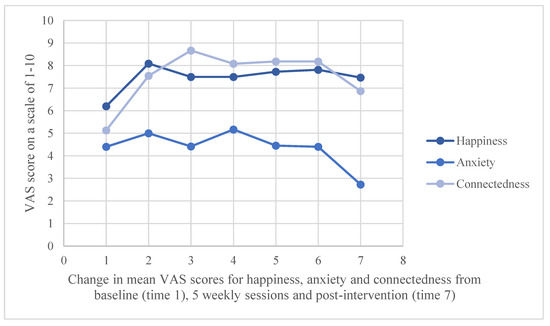
Figure 3.
Weekly self-reported scores from visual analogue scale: “On a scale of 1–10 how happy/anxious/connected to other people do you feel?”
3.2. Qualitative Findings
Four participants and two clinicians participated in the REM session. The age range of this sub-sample of participants was 21–60 years old, with 50% male, 50% TBI injury type and 75% unemployed. Time since injury range was 2–5 years. The two clinicians attended the intervention with participants but were not involved in the evaluation. In keeping with common practice in REM [60], the clinicians represented wider stakeholders’ views and reflections from the intervention.
During thematic analysis, a series of themes were identified as being significant consequences of attending the intervention (see Table 3), these themes could be clustered in to three key phases of change (see Figure 4). Firstly, participants noted that it was the unique physical and emotional experience of this nature-based challenge (phase 1) that initiated a powerful mindset shift towards empowerment and optimism for their future (phase 2). This mindset shift then led to long-term behaviour change (phase 3), which promoted participants’ wellbeing on an individual level, as well as contributing to improving collective and also planetary wellbeing.

Table 3.
Summary of qualitative themes that arose during ripple effects mapping session.
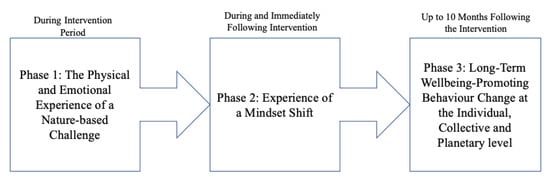
Figure 4.
A summary of the ”phases” in which significant changes took place for the participants.
3.3. Phase 1: The Physical and Emotional Experience of a Nature-Based Challenge
Participants identified four components of surf therapy that significantly contributed to shifting their mindset.
3.3.1. Intense Physical Challenge
The activity of surfing was very physically demanding for participants, especially given that fatigue is one of the most common consequences of ABI. Most reported that even walking down to the beach or getting changed into a wetsuit was tiring. This meant that they pushed themselves to tolerate much more activity than they had done since their injury and enabled them to recognise their limits and when they should rest.
“It gave me confidence that I could tolerate things that I thought, no way could I tolerate.”(P1)
3.3.2. Supported to Overcome Fear
The prospect of attending the intervention evoked a degree of fear and anxiety in all participants. Many felt unsure if they were capable of such a strenuous activity following their injury and many had negative preconceptions of the ocean, believing it was a dangerous environment. The intervention gave participants a safe and supported environment to challenge their negative perceptions and fully experience the beauty and benefits of nature and the ocean whilst trusting that they would not be in danger. This allowed them to confront a substantial fear and overcome it successfully.
“We hear via social media and the news, that the sea is so dangerous, and it’s always a negative thing to be, sort of, in a wave and riptides and whatnot. But when you’re actually in it, it’s, again, euphoric.”(P4)
“I remember the two people either side of me, when we were walking…the two helpers, you know. And the surf was really, really rough, and I was, like, going forward like this… But the sense of, actually, I’m battling through this, really big time, was actually huge.”(P1)
3.3.3. Invigoration and Achievement
The experience of surfing and being immersed in the ocean and nature was invigorating and energising for participants; several described this feeling as a “buzz”. They felt that being so immersed in nature was unique, one describing it as “magical” and another as “euphoric”. This was combined with a sense of pride in their achievement, after overcoming challenges and pushing themselves beyond their limits.
“[the days the ocean was rough] was more exhilarating and fulfilling than the days when it was really calm.”(P3)
3.3.4. Shared Experience
Participants reported feeling invested in each other’s progress. One described their group as being a “temporary family”, and they felt excitement and pride towards each other’s achievements. One reason for this was because they had empathy for how uniquely tough this challenge was following an ABI. It was this shared experience that subsequently led to feelings of inspiration and optimism described in phase 2.
“Because doing something extreme brings people together, but doing something extreme when you’re actually living…when you’ve survived something, and you’ve, you know, you’ve got those limitations anyway is…is much greater.”(P3)
3.4. Experience of a Mindset Shift
3.4.1. Inspiration and Optimism
Participants felt that observing fellow survivors of ABI endure this challenge and achieve their goals provided them with inspiration and enthusiasm for their own lives. It gave them a sense of hope and optimism for what they also might be able to achieve in their future. One participant described how they watched their fellow participant progress from sitting on the seated board to swimming completely independently by the final session and how this had a powerful impact on their own mindset.
“Just to see the progress…to see the progress that somebody can make, that makes you want to do the same? Makes you want to be a better person? If somebody can do…can be that brave, to make that sort of jump. You feel that you can…you can do anything as well.”(P3)
“I find that, with the community. I find that sense of inspiration.”(P2)
3.4.2. Increased Self-Confidence and Empowerment
This theme reflects a positive change that occurred in participants’ perceptions of themselves following the intervention. Participants felt that the achievements they had at surf therapy demonstrated that they were capable of being more independent than they previously thought. They reported a new zest for life and that their experience gave them a sense of “normality” post-ABI. Participants reported feeling empowered to take control of their life in some way.
“There’s no way you can get in that water’… Having got in there and survived it, I’m thinking, ‘yes I can.”(P1)
“[Surf-Ability] has helped me with other things. Because I know that I’m stronger than I thought, because I had that experience.”(P3)
3.5. Phase 3: Long-Term Wellbeing-Promoting Behaviour Change: Individual Scale
The long-term benefits of Surf-Ability were reflected in positive behaviour change which endured 6–10 months following the intervention (see Figure 5). It was evident that this positive behaviour change did not only improve individual wellbeing but, on a broader scale, also improved collective wellbeing; e.g., participants engaged in behaviour that improved the wellbeing of family, friends, their community and even local organisations. The rippling effects of Surf-Ability also improved planetary wellbeing, whereby participants felt more appreciative of nature and so began engaging with it more consciously and frequently.
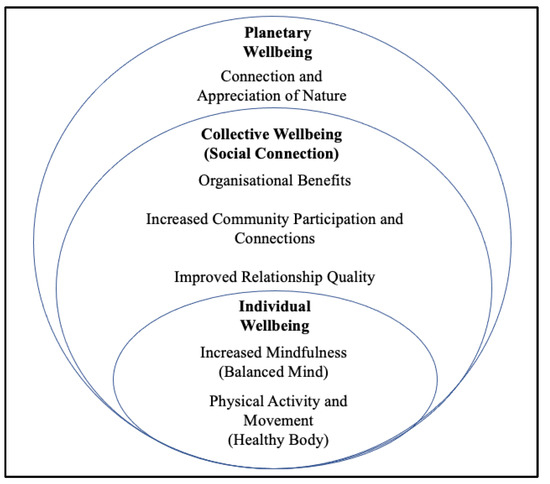
Figure 5.
A visual representation of the long-term wellbeing-promoting behaviour changes (phase 3), grouped according to level of scale (individual, collective or planetary).
3.5.1. Increased Mindfulness
Some participants reported that they felt Surf-Ability initiated an interest in mindfulness for them. Participants learned how to notice sensations and beauty in their surroundings, especially when spending more time in nature.
“It did give us time to be very mindful. And I realise more and more the importance of that to my wellbeing.”(P3)
3.5.2. Physical Activity and Movement
Some participants began participating in more physical activities following the intervention, including paddle boarding, surfing and cold-water swimming. One participant reported that they stopped taking anti-depressant medication because of attending the intervention. They felt this was because of the confidence they gained, which enabled them to continue cold-water swimming and begin exercising again, giving them a “natural high” (P3). Other participants felt that surf therapy empowered them to improve their mobility and their fatigue, which increased their capacity for activity and self-regulation.
“It leads me onto the cold water swimming. I just…I’m just one of those addicts now.”(P3)
3.6. Phase 3: Long-Term Wellbeing-Promoting Behaviour Change: Collective Scale
The behaviour changes that followed the intervention not only positively impacted the participants’ individual wellbeing but also improved wellbeing on a collective scale (see Figure 6).
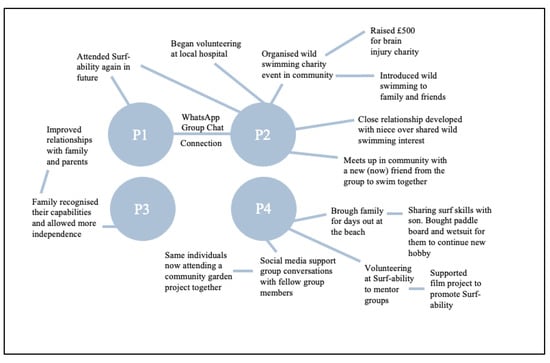
Figure 6.
Chain of events that occurred following Surf-Ability which improved collective wellbeing.
3.6.1. Improved Relationship Quality
One participant reported that the intervention empowered them to realise their capability for “independence”, which improved their mood long-term and made them more accepting of their family’s support instead of feeling resentful, thus improving their family dynamic. Other participants felt that they shared the skills and passions they learned at surf therapy with family and friends, which improved their connection and bond.
“Yeah I have got to say, again, turn a negative in to a positive, if I hadn’t have had the stroke, I wouldn’t have spent so much time on the beach with my boy [son].”(P4)
3.6.2. Improved Community Participation and Connections
Some participants made new connections from the intervention that still endured at follow-up; for example, meeting up in the community to go for cold swims together. Another participant created an online group chat following the intervention where they keep in contact with the group, and they now attend other community projects together such as a gardening group.
“I’ve been cold water swimming with my niece, friends, with all sorts of people! Even a friend who’s never done it before is going to come with me, because she loves the idea of it.”(P3)
3.6.3. Organisational Benefits
On an organisational level, one participant began to organise cold swim fund-raising events following surf therapy, and their first event raised £500 for an ABI charity. One participant began mentoring at Surf-Ability with other ABI participants and another has begun volunteering in a befriending scheme at a local hospice because of the confidence they gained from surf therapy. Some were involved in filming surfing and swimming content for (1) a documentary promoting the work of Surf-Ability and (2) a short educational video for a brain injury charity.
“When I was offered the chance to mentor within the Surf-Ability group, it gave me the chance to… help others.”(P4)
3.7. Phase 3: Long-Term Wellbeing-Promoting Behaviour Change: Planetary Scale
Increased Connection and Appreciation of Nature
Most participants reported that after attending surf therapy, they now spend significantly more time out in natural environments, such as the beach. Participants reported feeling an increased connection to nature and appreciation of its beauty, power and vastness.
“You can feel the joy of [the beach] that you didn’t see before.”(P3)
“I’ve never had a negative time on the beach, which has spurred me on to, every chance we get to go to the beach, even if its tipping down with rain.”(P4)
4. Discussion
The aim of the present evaluation was to assess whether a surf-therapy intervention promoted wellbeing in patients with acquired brain injury (ABI) according to a survey and physiological data. The secondary aim was to explore, using ripple effects mapping (REM) qualitative data, whether the impact of the intervention endured at approximately one year follow-up, thus ensuring it is an efficient use of service resources. Within-subjects analysis revealed significant increases in self-reported wellbeing between baseline and post-intervention (upon completing 4–5 weekly sessions). There were also significant increases in self-reported happiness and decreases in anxiety post-intervention versus baseline according to brief visual analogue scale ratings. There were no significant differences between baseline and post-intervention HADS depression and anxiety scores, nor connectedness (visual analogue scale rating), and no significant change in HRV measures. The improvement in participants’ self-reported wellbeing is coherent with previous qualitative findings on Surf-Ability, in which participants reported holistic improvements across a range of inter-connected wellbeing-promoting factors [58]. In addition, an REM session was conducted with a small sub-sample of participants at 6–10 months follow up. Thematic analysis identified four key components of the nature-based intervention that were unique in initiating a mindset shift in participants: the intense physical challenge, support to overcome fear, invigoration and achievement and a shared experience. These factors contributed to an increase in (1) inspiration and optimism and (2) self-confidence and empowerment and ultimately led to long-term wellbeing-promoting behaviour change. On an individual level, participants increased their mindfulness practice and their physical activity and movement, a change that persisted at the time of follow-up. On a collective level, the participants used the intervention to improve the quality of their relationships, increase community participation and engage in activities beneficial for local third-sector organisations in the months following the intervention. Finally, on a planetary level, participants felt more connected to and appreciative of nature, a trait associated with pro-environmentalism [28].
The present evaluation highlights the unique opportunity that blue spaces offer to improve wellbeing. Several previous theoretical frameworks have proposed explanations for the relationship between nature and health; the explanation of most relevance might be the attention restoration hypothesis [29], which claims that scenes of nature require only effortless attention, allowing cognitive resources to rest and recover. Given that cognitive fatigue and heightened sensory stimulation are common challenging symptoms following ABI [31,32,77,78], it is likely that spending time in nature might be particularly helpful for this population and, thus, neurorehabilitation services should consider ways to merge nature opportunities with clinical interventions. Moreover, given that surfing involves complete immersion into the (often tumultuous and cold) ocean, it is proposed that this experience offers a unique opportunity to be fully absorbed in nature beyond what one might experience from simply observing a nature scene. Whilst surfing might not be accessible to all neurorehabilitation services, there is a growing body of literature that promotes the more general use of water-based activities for improving wellbeing. For example a systematic review of 33 studies [79] proposed that psychosocial wellbeing can be improved via blue space interventions specifically designed with a therapeutic purpose (examples included: ocean therapy, surf therapy, kayaking, scuba diving, sailing and others). Theoretically, it is therefore possible that other water-based activities that it is possible to practice in other blue spaces, such as lakes or rivers (e.g., “wild” swimming, kayaking or paddle boarding), might also be beneficial for individuals with ABI; however, the evidence base for these is scarce and requires future investigation.
The present evaluation also illuminates the role that physical activity plays in promoting wellbeing. The mind–body dualism approach to health has been found to be inadequate, given the noteworthy role health behaviours, including exercise, have on psychological wellbeing. For example, one study found that individuals with ABI who exercised more than 90 min per week experienced a reduction in depression scores and higher perceived quality of life and mental health [80]. Moreover, a meta-analysis of 157 studies found a positive relationship (d = 0.360) between physical activity and subjective wellbeing [81]. Given that participants in the present evaluation reported that increasing their physical activity was an important contribution to their long-term wellbeing changes, it is proposed that neurorehabilitation services, where possible, provide opportunities for individuals with ABI to safely access group exercise, particularly within their own communities, so they are able to maintain the activity independently.
Both nature connection and positive health behaviours are factors that have commonly been neglected in previous models of wellbeing. The present findings support the need for a new biopsychosocial ecological model of wellbeing, such as the GENIAL model, which proposes that wellbeing is a product of (1) self-connection (e.g., emotional regulation, positive emotion, meaning and purpose and positive health behaviours, such as exercise, sleep and diet); (2) social connection (positive relationships, social capital, social cohesion and social identity); and (3) nature connection (spending time in and feeling connected to the natural environment) [11,43,44].
The present findings also suggest that it might be advantageous to incorporate more than one of these key determinants of wellbeing into neurorehabilitation interventions; e.g., social opportunity, natural environment, physical activity, mindfulness techniques, etc. It should be noted that improving wellbeing is not simply an effort to improve psychological functioning following ABI but that enhanced wellbeing has important benefits for all domains of the holistic model of neurorehabilitation, given the reciprocal interactions between psychological, cognitive, social and physical health. For example, evidence suggests that positive wellbeing improves performance in cognitive tasks including executive function, memory and processing speed [82]. In addition, higher levels of wellbeing are associated with physical health benefits, such as reduced inflammation, lower heart rate, lower blood pressure and less central obesity [83,84,85]. Future research may examine the role of wellbeing in promoting the components of the holistic model in individuals with ABI. It is predicted that providing opportunities for improved wellbeing will have simultaneous ”whole health” benefits for cognitive, functional, physical, psychological, social and vocational skills.
In addition, the evaluation illuminates the role of behaviour change in promoting health and wellbeing, a concept commonly neglected in previous models of wellbeing [39,86,87]. Whilst it might be difficult for participants to maintain the levels of wellbeing reached during an intervention, unless they learn how to make consistent changes to their behaviour, they risk regressing back to their baseline level of wellbeing. The findings suggest that participants required a change in their sense of identity and perception of their injury to change their behaviour long-term. This is in line with the ”Y-Shaped” model [7] that claims that the process of adaption following ABI requires individuals to resolve discrepancies within one’s social identity, interpersonal relationships and personal identity. Forming an updated, adapted and realistic identity is important for positive growth following an ABI, and thus neurorehabilitation services need to provide opportunities that facilitate this process of meaningful personal change. The findings suggest that nature-based interventions, particularly surf therapy, might offer a unique opportunity to facilitate this process of identity change following ABI by acting as a powerful behavioural experiment to realise their capabilities and subsequently change their behaviour. This change may also be explained by social identity theory, which proposes that experiencing a sense of collective and personal achievement will have positive consequences for one’s social and personal identity [13].
Barriers that may prevent individuals with ABI from integrating into social groups in the community include fear of stigmatisation [88], cognitive impairment (e.g., impaired executive functioning), behaviour challenges (e.g., disinhibition) and psychosocial challenges including depression and anxiety, lack of motivation and fatigue [89]. Whilst it is common for neurorehabilitation to offer group-based therapies and educational interventions within services, it is less common to actively support ABI patients to participate in community-based activities. Yet the present findings highlight that having trusted, skilled professionals present provides a “safety net”, allowing them to seek support and increasing their confidence that their needs will be adapted for. This finding is in line with polyvagal theory, which proposes that the ventral branch of the vagus nerve serves our social engagement system, which enables us to connect to others and navigate relationships [90,91]. When an environment is perceived to be safe, myelinated vagal motor pathways inhibit sympathetic fight or flight mechanisms via the ”vagal brake”. Alternatively, if a context is perceived to be dangerous, the body activates defence strategies (sympathetic ”fight or flight” or parasympathetic ”shutdown”), compromising social behaviours [90,91]. Given the psychosocial impacts of ABI, individuals are more likely to experience fear of rejection, lack of confidence and anxiety (activated ”defence” mode) prior to entering new social groups. However, if they are accompanied by a trusting professional with whom they feel safe, then they will be more able to socially participate. Moreover, according to broaden and build theory, increased vagal tone also predicts increases in positive emotions and social connectedness, which, in turn, further increase vagal tone in an upward spiral relationship [50,92]. This helps explain how the presence of a health professional might act as a gateway resource to enable ABI patients to connect socially and might possibly improve their individual capacity for future community participation when the “safety net” professional presence is removed.
There are an extensive number of third-sector and community organisations across the world providing opportunities for people to flourish, but it requires substantial confidence for patients to approach these spontaneously without support. Whilst it can be practically and financially challenging for neurorehabilitation services to attend community projects with patients, the present evaluation demonstrates that this goal can be achieved via collaborative partnerships between neurorehabilitation services and local community third-sector organisations [58,93]. Bridging the gap between healthcare and community projects can help overcome barriers for individuals with ABI who feel unable to access the community independently and thus are at risk of become marginalised [11].
Strengths, Limitations and Future Directions
One strength of the evaluation was the multi-method approach to measuring wellbeing. REM as a qualitative method aligns with the need for health research to be population-led and receive input from patients [94]. REM helps amplify the voices of people who may feel unheard. Adopting this approach may help ensure that wellbeing science evolves to reflect the needs of service users. This approach also helped to dissect the mechanism through which improvements in wellbeing occurred in participants, a discovery that would have been impossible through survey measures alone.
The use of HRV data also aligns with the need to understand wellbeing in a more holistic manner, integrating psychological states with physiological feedback. In this instance, coherence was not found between self-reported changes in wellbeing, and improvements in HRV; however, there are several potential explanations for this. One reason might be that any change in resting HRV may have occurred following the cascade of events that took place after the intervention. For example, the intervention alone may not have been enough to improve resting HRV, but the subsequent increase in physical activity, mindfulness, improved relationships, community participation and connection to nature that occurred in the months following the intervention may have led to improved vagal functioning in the long term. The present evaluation was limited by only two time-point measurements (pre and post). This could have been enhanced by measuring changes in resting HRV across all five weeks of the intervention, as well as in the months following. However, the evaluation took place as part of routine appraisal within an NHS public healthcare service and so lacked the required time and resources. Future studies with sufficient funding may utilise advances in smart devices, which can monitor HRV continuously for longer periods of time with greater ease, such as via a watch or ring. The monitors used in the present evaluation were not waterproof but, given the association between nature and parasympathetic activity [27,95,96,97], future research might consider tracking changes in HRV across the individual surf sessions and in the recovery period to assess whether surf therapy initiates moment-to-moment changes in nervous system activity.
In addition, participants in the present evaluation were not randomised into groups, as treatment-as-usual ensued. Therefore, those who chose to participate may have been more open to the experience and, thus, perhaps approached the intervention with more motivation or a more positive attitude than if participants were randomly assigned. Reasons why individuals were excluded were most commonly due to medical issues, such as uncontrollable epilepsy, because the individual was deemed at high risk of a second stroke or because they were employed and thus unable to take time off work. This might also explain why more participants in the present evaluation were unemployed or on sick leave from work than employed. Moreover, after discussions with their clinician, some felt their psychosocial challenges were too intense; e.g., they were clinically depressed or anxious and so were not yet ready to move beyond 1:1 therapy. This is common within the service, as patients who attend community projects are often further along in their rehabilitation journey. It is common for patients at the start of the patient pathway to have more 1:1 and within-service interventions, before gradually progressing to community projects later in the pathway, preparing them for an eventual discharge into the community. In addition, a common reason for dropping out of the intervention was because the neuropsychology service covers a large catchment area and, as individuals are commonly unable to drive following an ABI, several participants did not have access to a car, and public transport to the beach was not available for everyone. This barrier was acknowledged, and the team later began securing funding to provide patient transport to community projects.
A major constraint on the present evaluation was that it dealt with applied psychophysiological data outside of a laboratory setting. Participants’ lifestyle behaviours, which are known to influence HRV, were unable to be controlled, such as alcohol consumption, caffeine or exercise [75], which may have caused confounds. Moreover, during the intervention period, the UK faced COVID-19-related lockdown restrictions, meaning one cohort of participants were unable to attend appointments in person at the hospital clinic room, so data collection instead relied on participants fitting their own monitors whilst on a video call. This inevitably caused interference with HRV measurements, given that distractions in participants’ home environments were unable to be controlled for. Despite the constraints faced in the process of the evaluation, overall best efforts were made to utilise what resources were available to contribute towards the goal of progressing from single, self-reported measures toward utilising diverse methods, including physiological data, to understand wellbeing in a more holistic and multi-faceted way [98].
5. Conclusions
The present evaluation provides new quantitative data supporting the use of surf therapy as a nature-based intervention to promote wellbeing in individuals with ABI. It is proposed that being immersed in nature contributed to a positive change in participants’ self-perceptions and identity, which subsequently enabled some participants to maintain wellbeing-promoting behaviours at 6–10 months follow-up. This evaluation highlights the need to utilise diverse approaches to measure wellbeing in a holistic way, including physiological data, patient and stakeholder involvement and the capturing of wider collective and planetary impact of interventions. The need for wellbeing science and positive psychology to progress towards a transdisciplinary biopsychosocial ecological model of wellbeing, such as the GENIAL framework, which integrates known determinants of wellbeing, including nature connection and positive health behaviours, should be noted [43]. Findings support the need for healthcare providers—including neurorehabilitation services—to enhance interventions for patients by incorporating multiple factors that improve wellbeing, including nature connection. To do this, it is suggested that healthcare services consider forming collaborative partnerships with local third-sector organisations in addition to research academics in order to identify collaborative funding opportunities and co-deliver innovative interventions that exploit these advances in wellbeing science [99,100].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su14159605/s1, Ripple effects mapping topic guide.
Author Contributions
Conceptualization, Z.F., A.H.K. and L.W.; methodology, Z.F., A.H.K. and L.W.; data collection and analysis, L.W.; writing—original draft preparation, L.W.; writing—review and editing, Z.F., A.H.K. and L.W.; supervision, A.H.K. and Z.F. All authors have read and agreed to the published version of the manuscript.
Funding
£11,758.52 of funding was awarded to Surf-Ability by the Welsh Government—“Integrated Care Fund Wales” to support the surf therapy intervention reported in this paper. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Institutional Review Board Statement
Evaluations of service user experiences associated with the delivery of interventions in the healthcare sector are excluded from ethical review in the United Kingdom (GAfREC §2.3.12). This exemption was confirmed by the Research and Development Officer in Swansea Bay University Health Board. Service evaluations are characterised by minimal risk and therefore fall outside the remit of research ethics committees in the United Kingdom. There was no randomisation nor was the intervention withheld for any reason from eligible participants. Patient care did not deviate from the typical care provided by the service from which the data were generated. All participants were invited to participate in the service evaluation and all participants agreeing to participate provided consent. All participants valued the opportunity to provide feedback on our intervention to help inform future service development.
Informed Consent Statement
Informed consent was obtained from all participants involved in the service evaluation.
Data Availability Statement
The qualitative data that support the results of this study are ethically restricted and not openly available within the public domain. This is due to the majority of the transcripts containing potentially identifying contextual and sensitive patient information that could compromise research participant privacy if combined with local knowledge of the service and participants involved. These restrictions have been imposed by our Research and Development Department at Swansea Bay University Health Board (Room 104, Institute of Life Sciences 2, Swansea University, Singleton Campus, SA2 8PP; SBU.RandD@wales.nhs.uk). However, given that this local and contextual information would not be known to outside researchers, access to anonymised transcripts may be granted to researchers upon reasonable request and with the permission of the Research and Development Department. Please contact the Team Coordinator at the Regional Neuropsychology and Community Brain Injury Service, Swansea Bay University Health Board, Morriston Hospital, Morriston, Swansea, SA6 6NL. Data requests should be directed to sbu.communityneurorehabilitation@wales.nhs.uk and will be reviewed on a case-by-case basis. Anonymised quantitative data will be made available on request.
Acknowledgments
We are extremely grateful for the support and dedication of the Surf-Ability team and instructors, including Ben Clifford and Benedict Room, who enabled us to provide a unique opportunity for people living with acquired brain injury in South Wales. We would like to express our heartfelt thanks for the support of our service users for giving up their time to help support our service evaluation work. We would like to thank our colleagues at at Hywel Dda Health Board including Abigail Barker-Smith and Nia Wyn Davies in addition to our colleagues at Swansea Bay Health Board for their hard work organising and delivering the intervention, in addition to supporting data collection efforts. Finally, we would like to thank the Research and Development Department at Swansea Bay University Health Board for their on-going support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- NICE. National Institute for Health and Care Excellence (UK) Clinical Guidelines; NICE: London, UK, 2019; Volume 176. [Google Scholar]
- Rabinowitz, A.R.; Levin, H.S. Cognitive Sequelae of Traumatic Brain Injury. Psychiatr. Clin. N. Am. 2014, 37, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Baguley, I.J.; Cameron, I.D. 4: Rehabilitation after Traumatic Brain Injury. Med. J. Aust. 2003, 178, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Glenn, M.B.; O’Neil-Pirozzi, T.; Goldstein, R.; Burke, D.; Jacob, L. Depression amongst Outpatients with Traumatic Brain Injury. Brain Inj. 2009, 15, 811–818. [Google Scholar] [CrossRef]
- Jorge, R.E.; Robinson, R.G.; Moser, D.; Tateno, A.; Crespo-Facorro, B.; Arndt, S. Major Depression Following Traumatic Brain Injury. Arch. Gen. Psychiatry 2004, 61, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Douglas, J. Loss of Friendship Following Traumatic Brain Injury: A Model Grounded in the Experience of Adults with Severe Injury. Neuropsychol. Rehabil. 2019, 30, 1277–1302. [Google Scholar] [CrossRef] [Green Version]
- Gracey, F.; Evans, J.J.; Malley, D. Capturing Process and Outcome in Complex Rehabilitation Interventions: A “Y-Shaped” Model. Neuropsychol. Rehabil. 2009, 19, 867–890. [Google Scholar] [CrossRef]
- Lefebvre, H.; Cloutier, G.; Levert, M.J. Perspectives of Survivors of Traumatic Brain Injury and Their Caregivers on Long-Term Social Integration. Brain Inj. 2009, 22, 535–543. [Google Scholar] [CrossRef]
- Teasdale, T.W.; Engberg, A.W. Subjective Well-Being and Quality of Life Following Traumatic Brain Injury in Adults: A Long-Term Population-Based Follow-Up. Brain Inj. 2010, 19, 1041–1048. [Google Scholar] [CrossRef]
- Dijkers, M.P. Quality of Life after Traumatic Brain Injury: A Review of Research Approaches and Findings. Arch. Phys. Med. Rehabil. 2004, 85, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Fisher, Z. Wellbeing, Whole Health and Societal Transformation: Theoretical Insights and Practical Applications. Glob. Adv. Health Med. 2022, 11, 21649561211073076. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yishay, Y.; Daniels-Zide, E. Examined Lives: Outcomes After Holistic Rehabilitation. Rehabil. Psychol. 2000, 45, 112–129. [Google Scholar] [CrossRef]
- Haslam, C.; Jetten, J.; Cruwys, T.; Dingle, G.; Haslam, A. The New Psychology of Health; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Ben-Yishay, Y.; Silver, S.M.; Piasetsky, E.; Rattok, J. Relationship between Employability and Vocational Outcome after Intensive Holistic Cognitive Rehabilitation. J. Head Trauma Rehabil. 1987, 2, 35–48. [Google Scholar] [CrossRef]
- Klonoff, P.S.; Lamb, D.G.; Henderson, S.W. Outcomes from Milieu-Based Neurorehabilitation at up to 11 Years Post-Discharge. Brain Inj. 2009, 15, 413–428. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Mott, T.; Azulay, J.; Sharlow-Galella, M.A.; Ellmo, W.J.; Paradise, S.; Friel, J.C. A Randomized Controlled Trial of Holistic Neuropsychologic Rehabilitation After Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2008, 89, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Haslam, C.; Holme, A.; Haslam, S.A.; Iyer, A.; Jetten, J.; Williams, W.H. Maintaining Group Memberships: Social Identity Continuity Predicts Well-Being after Stroke. Neuropsychol. Rehabil. 2008, 18, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, E.L.; Muldoon, O.T.; Fortune, D.G.; Haslam, C. Collective Influences on Individual Functioning: Multiple Group Memberships, Self-Regulation, and Depression after Acquired Brain Injury. Neuropsychol. Rehabil. 2018, 30, 1059–1073. [Google Scholar] [CrossRef]
- Morton, M.V.; Wehman, P. Psychosocial and Emotional Sequelae of Individuals with Traumatic Brain Injury: A Literature Review and Recommendations. Brain Inj. 2009, 9, 81–92. [Google Scholar] [CrossRef]
- Wise, E.K.; Mathews-Dalton, C.; Dikmen, S.; Temkin, N.; Machamer, J.; Bell, K.; Powell, J.M. Impact of Traumatic Brain Injury on Participation in Leisure Activities. Arch. Phys. Med. Rehabil. 2010, 91, 1357–1362. [Google Scholar] [CrossRef]
- Roundhill, S.J.; Williams, W.H.; Hughes, J.M. The Experience of Loss Following Traumatic Brain Injury: Applying a Bereavement Model to the Process of Adjustment. Qual. Res. Psychol. 2007, 4, 241–257. [Google Scholar] [CrossRef]
- Seymour, V. The Human–Nature Relationship and Its Impact on Health: A Critical Review. Front. Public Health 2016, 4, 260. [Google Scholar] [CrossRef] [Green Version]
- Mayer, F.S.; Frantz, C.M. The Connectedness to Nature Scale: A Measure of Individuals’ Feeling in Community with Nature. J. Environ. Psychol. 2004, 24, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Baxter, D.E.; Pelletier, L.G. Is Nature Relatedness a Basic Human Psychological Need? A Critical Examination of the Extant Literature. Can. Psychol. 2019, 60, 21–34. [Google Scholar] [CrossRef]
- De Bell, S.; White, M.; Griffiths, A.; Darlow, A.; Taylor, T.; Wheeler, B.; Lovell, R. Spending Time in the Garden Is Positively Associated with Health and Wellbeing: Results from a National Survey in England. Landsc. Urban. Plan. 2020, 200, 103836. [Google Scholar] [CrossRef]
- Wheeler, B.W.; White, M.; Stahl-Timmins, W.; Depledge, M.H. Does Living by the Coast Improve Health and Wellbeing? Health Place 2012, 18, 1198–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (Taking in the Forest Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. 2009, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; White, M.P.; Hunt, A.; Richardson, M.; Pahl, S.; Burt, J. Nature Contact, Nature Connectedness and Associations with Health, Wellbeing and pro-Environmental Behaviours. J. Environ. Psychol. 2020, 68, 101389. [Google Scholar] [CrossRef]
- Kaplan, S. The Restorative Benefits of Nature: Toward an Integrative Framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Berman, M.G.; Jonides, J.; Kaplan, S. The Cognitive Benefits of Interacting with Nature. Psychol. Sci. 2008, 19, 1207–1212. [Google Scholar] [CrossRef]
- Ziino, C.; Ponsford., J. Selective Attention Deficits and Subjective Fatigue Following Traumatic Brain Injury. Available online: https://psycnet.apa.org/record/2006-06643-013 (accessed on 12 October 2020).
- Alwis, D.S.; Johnstone, V.; Yan, E.; Rajan, R. Diffuse Traumatic Brain Injury and the Sensory Brain. Clin. Exp. Pharmacol. Physiol. 2013, 40, 473–483. [Google Scholar] [CrossRef]
- Motzkin, J.C.; Philippi, C.L.; Wolf, R.C.; Baskaya, M.K.; Koenigs, M. Ventromedial Prefrontal Cortex Is Critical for the Regulation of Amygdala Activity in Humans. Biol. Psychiat. 2015, 77, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Banks, S.J.; Eddy, K.T.; Angstadt, M.; Nathan, P.J.; Phan, K.L. Amygdala–Frontal Connectivity during Emotion Regulation. Soc. Cogn. Affect. Neurosci. 2007, 2, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Van der Horn, H.J.; Liemburg, E.J.; Aleman, A.; Spikman, J.M.; van der Naalt, J. Brain Networks Subserving Emotion Regulation and Adaptation after Mild Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress Recovery during Exposure to Natural and Urban Environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Ewert, A.; Chang, Y. Levels of Nature and Stress Response. Behav. Sci. 2018, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Ikei, H.; Igarashi, M.; Takagaki, M.; Miyazaki, Y. Physiological and Psychological Effects of a Walk in Urban Parks in Fall. Int. J. Environ. Res. Public Health 2015, 12, 14216–14228. [Google Scholar] [CrossRef] [PubMed]
- Seligman, M. Flourish: A Visionary New Understanding of Happiness and Well-Being; Free Press: New York, NY, USA, 2011. [Google Scholar]
- Wong, P.T. Second Wave Positive Psychology’s (PP 2.0) Contribution to Counselling Psychology. Couns. Psychol. Q. 2019, 32, 275–284. [Google Scholar] [CrossRef]
- Lomas, T.; Waters, L.; Williams, P.; Oades, L.G.; Kern, M.L. Third Wave Positive Psychology: Broadening towards Complexity. J. Posit. Psychol. 2020, 16, 660–674. [Google Scholar] [CrossRef]
- Antó, J.M.; Martí, J.L.; Casals, J.; Bou-Habib, P.; Casal, P.; Fleurbaey, M.; Frumkin, H.; Jiménez-Morales, M.; Jordana, J.; Lancelotti, C.; et al. The Planetary Wellbeing Initiative: Pursuing the Sustainable Development Goals in Higher Education. Sustainability 2021, 13, 3372. [Google Scholar] [CrossRef]
- Mead, J.; Fisher, Z.; Kemp, A.H. Moving Beyond Disciplinary Silos Towards a Transdisciplinary Model of Wellbeing: An Invited Review. Front. Psychol. 2021, 12, 642093. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.; Arias, J.A.; Fisher, Z. Neuroscience and Social Science, The Missing Link; Springer International Publishing: Cham, Switzerland, 2017; pp. 397–427. [Google Scholar] [CrossRef] [Green Version]
- Kemp, A.H.; Koenig, J.; Thayer, J.F. From Psychological Moments to Mortality: A Multidisciplinary Synthesis on Heart Rate Variability Spanning the Continuum of Time. Neurosci. Biobehav. Rev. 2017, 83, 547–567. [Google Scholar] [CrossRef] [Green Version]
- Del Paso, G.A.R.; Langewitz, W.; Mulder, L.J.M.; Roon, A.; Duschek, S. The Utility of Low Frequency Heart Rate Variability as an Index of Sympathetic Cardiac Tone: A Review with Emphasis on a Reanalysis of Previous Studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef]
- Jandackova, V.K.; Scholes, S.; Britton, A.; Steptoe, A. Healthy Lifestyle and Cardiac Vagal Modulation over 10 Years: Whitehall II Cohort Study. J. Am. Heart Assoc. 2019, 8, e012420. [Google Scholar] [CrossRef]
- Heckenberg, R.A.; Eddy, P.; Kent, S.; Wright, B.J. Do Workplace-Based Mindfulness Meditation Programs Improve Physiological Indices of Stress? A Systematic Review and Meta-Analysis. J. Psychosom. Res. 2018, 114, 62–71. [Google Scholar] [CrossRef]
- Zou, L.; Sasaki, J.E.; Wei, G.-X.; Huang, T.; Yeung, A.S.; Neto, O.B.; Chen, K.W.; Hui, S.S. Effects of Mind–Body Exercises (Tai Chi/Yoga) on Heart Rate Variability Parameters and Perceived Stress: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2018, 7, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, B.E.; Coffey, K.A.; Cohn, M.A.; Catalino, L.I.; Vacharkulksemsuk, T.; Algoe, S.B.; Brantley, M.; Fredrickson, B.L. How Positive Emotions Build Physical Health. Psychol. Sci. 2012, 24, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Holt-Lunstad, J.; Smith, T.B.; Layton, J.B. Social Relationships and Mortality Risk: A Meta-Analytic Review. PLoS Med. 2010, 7, e1000316. [Google Scholar] [CrossRef]
- Wilker, E.H.; Wu, C.-D.; McNeely, E.; Mostofsky, E.; Spengler, J.; Wellenius, G.A.; Mittleman, M.A. Green Space and Mortality Following Ischemic Stroke. Environ. Res. 2014, 133, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Helbich, M.; Klein, N.; Roberts, H.; Hagedoorn, P.; Groenewegen, P.P. More Green Space Is Related to Less Antidepressant Prescription Rates in the Netherlands: A Bayesian Geoadditive Quantile Regression Approach. Environ. Res. 2018, 166, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.; Popham, F. Effect of Exposure to Natural Environment on Health Inequalities: An Observational Population Study. Lancet 2008, 372, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Benninger, E.; Curtis, C.; Sarkisian, G.V.; Rogers, C.M.; Bender, K.; Comer, M. Surf Therapy: A Scoping Review of the Qualitative and Quantitative Research Evidence. Community Psychol. Pract. 2020, 11, 1–26. [Google Scholar]
- Devine-Wright, H.; Godfrey, C. The Wave Project: Evidencing Surf Therapy for Young People in the UK. Community Psychol. Pract. 2020, 11, 1–15. [Google Scholar]
- De Matos, M.G.; Santos, A.; Fauvelet, C.; Marta, F.; Evangelista, E.S.; Ferreira, J.; Moita, M.; Conibear, T.; Mattila, M. Surfing for Social Integration: Mental Health and Well-Being Promotion through Surf Ther- Apy among Institutionalized Young People. HSOA J. Community Med. Public Health Care 2017, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, K.; Wilkie, L.; Jarman, J.; Barker-Smith, A.; Kemp, A.H.; Fisher, Z. Riding the Wave into Wellbeing: A Qualitative Evaluation of Surf Therapy for Individuals Living with Acquired Brain Injury. PLoS ONE 2022, 17, e0266388. [Google Scholar] [CrossRef] [PubMed]
- Leech, N.L.; Onwuegbuzie, A.J. A Typology of Mixed Methods Research Designs. Qual. Quant. 2009, 43, 265–275. [Google Scholar] [CrossRef]
- Chazdon, S.; Emery, M.; Hansen, D.; Higgins, L.; Sero, R. A Field Guide to Ripple Effects Mapping. 2017. Available online: https://www.researchgate.net/publication/321340208_A_Field_Guide_to_Ripple_Effects_Mapping (accessed on 1 June 2022).
- D’Cruz, K.; Douglas, J.; Serry, T. Narrative Storytelling as Both an Advocacy Tool and a Therapeutic Process: Perspectives of Adult Storytellers with Acquired Brain Injury. Neuropsychol. Rehabil. 2019, 30, 1409–1429. [Google Scholar] [CrossRef] [PubMed]
- Truzoli, R.; Reed, P.; Osborne, L.A. Patient Expectations of Assigned Treatments Impact Strength of Randomised Control Trials. Front. Med. 2021, 8, 648403. [Google Scholar] [CrossRef] [PubMed]
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh Mental Well-Being Scale (WEMWBS): Development and UK Validation. Health Qual. Life Outcomes 2007, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Cader, M.; Andrews, W.P.; Wijesekera, D.; Stewart-Brown, S.L. Responsiveness of the Short Warwick Edinburgh Mental Well-Being Scale (SWEMWBS): Evaluation a Clinical Sample. Health Qual. Life Outcomes 2018, 16, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trousselard, M.; Steiler, D.; Dutheil, F.; Claverie, D.; Canini, F.; Fenouillet, F.; Naughton, G.; Stewart-Brown, S.; Franck, N. Validation of the Warwick-Edinburgh Mental Well-Being Scale (WEMWBS) in French Psychiatric and General Populations. Psychiatry Res. 2016, 245, 282–290. [Google Scholar] [CrossRef]
- Fat, L.N.; Scholes, S.; Boniface, S.; Mindell, J.; Stewart-Brown, S. Evaluating and Establishing National Norms for Mental Wellbeing Using the Short Warwick–Edinburgh Mental Well-Being Scale (SWEMWBS): Findings from the Health Survey for England. Qual. Life Res. 2017, 26, 1129–1144. [Google Scholar] [CrossRef] [Green Version]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatry Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Sagen, U.; Vik, T.G.; Moum, T.; Mørland, T.; Finset, A.; Dammen, T. Screening for Anxiety and Depression after Stroke: Comparison of the Hospital Anxiety and Depression Scale and the Montgomery and Åsberg Depression Rating Scale. J. Psychosom. Res. 2009, 67, 325–332. [Google Scholar] [CrossRef]
- Turner, A.; Hambridge, J.; White, J.; Carter, G.; Clover, K.; Nelson, L.; Hackett, M. Depression Screening in Stroke. Stroke 2012, 43, 1000–1005. [Google Scholar] [CrossRef] [Green Version]
- Cameron, I.M.; Crawford, J.R.; Lawton, K.; Reid, I.C. Psychometric Comparison of PHQ-9 and HADS for Measuring Depression Severity in Primary Care. Br. J. Gen. Pract. 2008, 58, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimek, L.; Bergmann, K.-C.; Biedermann, T.; Bousquet, J.; Hellings, P.; Jung, K.; Merk, H.; Olze, H.; Schlenter, W.; Stock, P.; et al. Visual Analogue Scales (VAS): Measuring Instruments for the Documentation of Symptoms and Therapy Monitoring in Cases of Allergic Rhinitis in Everyday Health Care. Allergo J. Int. 2017, 26, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarvainen, M.; Lipponen, J.; Niskanen, J.-P.; Ranta-aho, P.O. Kubios HRV Software User’s Guide. 2021. Available online: https://www.kubios.com/downloads/Kubios_HRV_Users_Guide.pdf (accessed on 1 June 2022).
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Fatisson, J.; Oswald, V.; Lalonde, F. Influence Diagram of Physiological and Environmental Factors Affecting Heart Rate Variability: An Extended Literature Overview. Hear. Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Using Thematic Analysis in Psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef] [Green Version]
- Cantor, J.B.; Ashman, T.; Gordon, W.; Ginsberg, A.; Engmann, C.; Egan, M.; Spielman, L.; Dijkers, M.; Flanagan, S. Fatigue After Traumatic Brain Injury and Its Impact on Participation and Quality of Life. J. Head Trauma Rehabil. 2008, 23, 41–51. [Google Scholar] [CrossRef]
- Belmont, A.; Agar, N.; Hugeron, C.; Gallais, B.; Azouvi, P. Fatigue and Traumatic Brain Injury. Annal. Réadapt. Méd. Phys. 2006, 49, 370–374. [Google Scholar] [CrossRef]
- Britton, E.; Kindermann, G.; Domegan, C.; Carlin, C. Blue Care: A Systematic Review of Blue Space Interventions for Health and Wellbeing. Health Promot. Int. 2020, 35, 50–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, E.K.; Hoffman, J.M.; Powell, J.M.; Bombardier, C.H.; Bell, K.R. Benefits of Exercise Maintenance After Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2012, 93, 1319–1323. [Google Scholar] [CrossRef]
- Buecker, S.; Simacek, T.; Ingwersen, B.; Terwiel, S.; Simonsmeier, B.A. Physical Activity and Subjective Well-Being in Healthy Individuals: A Meta-Analytic Review. Health Psychol. Rev. 2020, 5, 574–592. [Google Scholar] [CrossRef]
- Allerhand, M.; Gale, C.R.; Deary, I.J. The Dynamic Relationship Between Cognitive Function and Positive Well-Being in Older People: A Prospective Study Using the English Longitudinal Study of Aging. Psychol. Aging 2014, 29, 306–318. [Google Scholar] [CrossRef]
- Steptoe, A.; Wardle, J.; Marmot, M. Positive Affect and Health-Related Neuroendocrine, Cardiovascular, and Inflammatory Processes. Proc. Natl. Acad. Sci. USA 2005, 102, 6508–6512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steptoe, A.; Demakakos, P.; de Oliveira, C.; Wardle, J. Distinctive Biological Correlates of Positive Psychological Well-Being in Older Men and Women. Psychosom. Med. 2012, 74, 501–508. [Google Scholar] [CrossRef]
- Dockray, S.; Steptoe, A. Positive Affect and Psychobiological Processes. Neurosci. Biobehav. Rev. 2010, 35, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Fredrickson, B.L. The Role of Positive Emotions in Positive Psychology. Am. Psychol. 2001, 56, 218–226. [Google Scholar] [CrossRef]
- Ryff, C.D.; Keyes, C.L.M. The Structure of Psychological Well-Being Revisited. J. Personal. Soc. Psychol. 1995, 69, 719–727. [Google Scholar] [CrossRef]
- McLellan, T.; Bishop, A.; McKinlay, A. Community Attitudes toward Individuals with Traumatic Brain Injury. J. Int. Neuropsych. Soc. 2010, 16, 705–710. [Google Scholar] [CrossRef]
- Mahar, C.; Fraser, K. Barriers to Successful Community Reintegration Following Acquired Brain Injury (ABI). Int. J. Disabil. Manag. 2011, 6, 49–67. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-Regulation; WW Norton & Company: New York, NY, USA, 2011. [Google Scholar]
- Kok, B.E.; Fredrickson, B.L. Upward Spirals of the Heart: Autonomic Flexibility, as Indexed by Vagal Tone, Reciprocally and Prospectively Predicts Positive Emotions and Social Connectedness. Biol. Psychol. 2010, 85, 432–436. [Google Scholar] [CrossRef] [Green Version]
- Wilkie, L.; Arroyo, P.; Conibeer, H.; Kemp, A.H.; Fisher, Z. The Impact of Psycho-Social Interventions on the Wellbeing of Individuals with Acquired Brain Injury During the COVID-19 Pandemic. Front. Psychol. 2021, 12, 648286. [Google Scholar] [CrossRef]
- Mader, L.B.; Harris, T.; Kläger, S.; Wilkinson, I.B.; Hiemstra, T.F. Inverting the Patient Involvement Paradigm: Defining Patient Led Research. Res. Involv. Engagem. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.-J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence of Forest Therapy on Cardiovascular Relaxation in Young Adults. Evid. Based Complementary Altern. Med. 2014, 2014, 834360. [Google Scholar] [CrossRef]
- Kobayashi, H.; Song, C.; Ikei, H.; Kagawa, T.; Miyazaki, Y. Analysis of Individual Variations in Autonomic Responses to Urban and Forest Environments. Evid. Based Complementary Altern. Med. 2015, 2015, 671094. [Google Scholar] [CrossRef]
- Kobayashi, H.; Song, C.; Ikei, H.; Park, B.-J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Forest Walking Affects Autonomic Nervous Activity: A Population-Based Study. Front. Public Health 2018, 6, 278. [Google Scholar] [CrossRef]
- Vella-Brodrick, D.A.; Gill, A.; Patrick, K. Seeing Is Believing: Making Wellbeing More Tangible. Front. Psychol. 2022, 13, 809108. [Google Scholar] [CrossRef]
- Fisher, Z.; Galloghly, E.; Vloglo, E.; Gracey, F.; Kemp, A.H. Emotion, wellbeing and the neurological disorders. In Encyclopedia of Behavioral Neuroscience; Della Sala, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 3, pp. 220–234. [Google Scholar]
- Gibbs, K.; Fisher, Z.; Kemp, A.H. Toward a Culture of Care for Societal Wellbeing: A Perspective from the Healthcare Sector. Kemp, A.H., Edwards, D.J., Eds.; to be Published in Broadening the Scope of Wellbeing Science: Multidisciplinary and Interdisciplinary Perspectives on Human Flourishing and Wellbeing; (Forthcoming); Palgrave Macmillan, Reproduced with Permission of Springer Nature Switzerland AG. Available online: https://www.researchgate.net/profile/Andrew-Kemp-6/publication/362060548_Toward_a_Culture_of_Care_for_Societal_Wellbeing_A_Perspective_from_the_Healthcare_Sector/links/62d41231fd347a451bc58ca0/Toward-a-Culture-of-Care-for-Societal-Wellbeing-A-Perspective-from-the-Healthcare-Sector.pdf (accessed on 1 June 2022). [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).