Abstract
In the preparation and response to the COVID-19 pandemic, a sufficient supply of personal protective equipment (PPE), particularly the face mask, is essential. Shortage of PPE due to growing demand leaves health workers at significant risk as they fight this pandemic on the frontline. As a mitigation measure to overcome potential mask shortages, these masks could be decontaminated and prepared for reuse. This review explored past scientific research on various methods of decontamination of the N95-type respirators and their efficiency against the SARS-CoV-2 virus. Ultraviolet germicidal irradiation (UVGI) and hydrogen peroxide vapor (HPV) show great potential as an effective decontamination system. In addition, UVGI and HPV exhibit excellent effectiveness against the SARS-CoV-2 virus on the N95 respirator surfaces.
1. Introduction
According to the WHO, COVID-19 human cases, which are caused by a novel coronavirus named SARS-CoV-2, were first reported in Wuhan City, China, in December 2019 [1]. Due to this unprecedented pandemic, the demand for face mask respirators has surged significantly. The WHO predicted that mask manufacturing industries need to increase manufacturing by 40 percent to meet the demand [2]. Frontline workers rely solely on PPE, especially N95 respirators, to protect themselves from being infected and infecting others. The N95 respirators should be disposed of after a sole patient visit, according to the Centers for Disease Control and Prevention.
Nevertheless, under acute PPE scarcity, it advises prolonged use of N95 respirators (using the same N95 respirator for many patient interactions) with limited reuse (keeping an N95 respirator during interactions for usage across several patients’ visits). During the COVID-19 pandemic, due to a shortage of N95 masks, several emergency services have implemented various N95 prolonged use strategies. However, there is insufficient scientific proof that they were successful. In one investigation, researchers examined how often duckbill N95s and dome-shaped N95s masks failed by using fit-tests when they were reused. They concluded that healthcare systems must closely monitor N95 fit throughout extended usage or reuse and avoid using duckbill masks if better options are available [3]. Among the available models of face masks, N95 respirators are designed and intended for healthcare usage [4].
Developing countries whose populations are mostly made up of people living in poverty, such as India, Pakistan, and Sri Lanka, face even greater challenges due to a shortage of masks. The slowed economies in these countries, coupled with a face mask price hike, made people prioritize daily necessities over face masks, promoting the risk of the COVID-19 pandemic still existing in the community [5]. Due to these shortages, health workers were forced to ration their face mask supply to one N95 mask per week with an additional surgical mask on top. In addition, healthcare facilities are restricted to performing some non-COVID-related medical care as these supply limitations are concentrated on COVID-related patients [6].
As a solution, extending the usage of N95 respirators can assist in overcoming the shortage of masks experienced worldwide. Decontamination procedures of face masks that reduce the pathogen burden show great potential to alleviate the shortage of mask issues. According to NIOSH, ultraviolet germicidal irradiation, vaporous hydrogen peroxide, and moist heat have shown the most potential procedures to decontaminate filtering facepiece respirators (FFR) [7].
In essence, the mask shortage problem during the pandemic needs to be addressed immediately. This review aimed to compare the decontamination procedures of the virus on the N95 respirator, particularly highlighting effective but economical methods.
2. Methods
Relevant studies were searched using the PubMed and Preprint platform (medRxiv) electronic databases using a combination of specified MeSH terms that were restricted from 2000 to 2021 (Table 1). Apart from the database searches, several studies were included based on the relevance to this review. In addition, regulatory documents related to the decontamination of N95 respirators were obtained from the official websites of the CDC, the FDA, the WHO, and 3M. Studies were selected for evaluation based on specified inclusion criteria: (a) studies reporting at least one of the selected N95 respirator decontamination procedures for this review (UVGI or HPV or heat or MGS or ethanol); (b) studies reporting at least one of the selected N95 respirator decontamination outcomes (reduction in pathogen load or mask performance or structural integrity of the mask).

Table 1.
Studies search strategies and outcomes.
3. SARS-CoV-2
The WHO named the pathogen that causes coronavirus disease (COVID-19) SARS-CoV-2 on 12 February 2020. CoVs is a single-stranded positive-sense RNA (+ssRNA) virus [8]. The schematic structure of the SARS-CoV-2 virus is illustrated in Figure 1. The SARS-CoV-2 virus was reported to possess 80% similarity in the aspect of the genome to previous human coronaviruses. Bats were deduced as the vital host and transmitting medium of the SARS-CoV-2 virus [9]. It was concluded that SARS-CoV-2 is transmitted mainly via respiratory droplets and direct contact [10]. Evaluation of the stability of SARS-CoV-2 on different environmental conditions demonstrated that after seven days, a detectable level of the virus still presents on the outer layer of the surgical mask [11]. The FDA calls for a policy where at least three log reductions must be achieved to sterilize devices intended for skin contact [12].
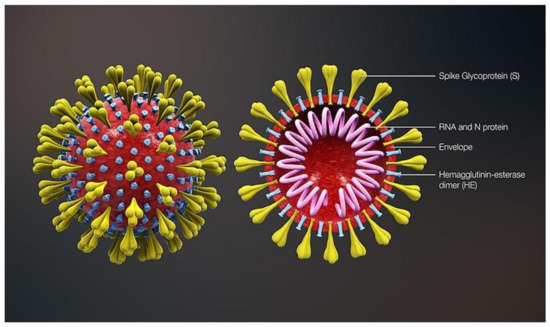
Figure 1.
Schematic structure of SARS-CoV-2 [13].
4. The N95 Respirator
The N95 respirator is a type of respiratory protective equipment with a specific design to tightly fit its user. This type of respirator undergoes a testing and evaluation process by NIOSH [14]. In comparison to other FFRs, the N95 respirator offers a minimum of 95% filtration efficiency against particulate aerosols [15]. Quantitative fit testing of FFRs proves the superior protection that the N95 respirator offers [16].
The N95 respirator is made up of four layers, namely, a coverweb, a shell, filter 1, and filter 2 as illustrated in Figure 2. The coverweb and the shell layers are made up of polyester; meanwhile, filter layers are made from polypropylene [4]. The filtration efficiency of the respirator is determined by the internal filtration layer, which is a high-efficiency melt-blown non-woven material [17].
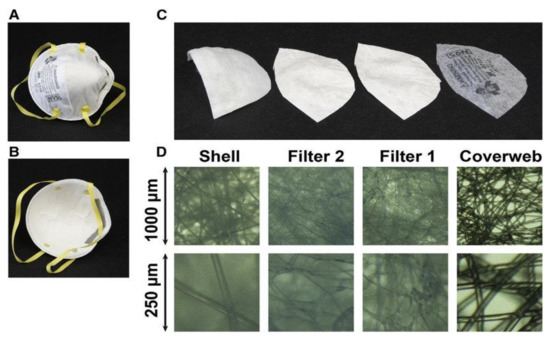
Figure 2.
Multilayer sandwich anatomy of N95 mask. (A) Environmental interface; (B) user interface; (C) from left to right: inner layer (shell), middle layers (filter 2 and filter 1), and outer layer (coverweb); (D) light microscope images of the four layers, with a lower row at four-fold higher magnification (3M model 8210). Adapted from [18] with permission.
5. Decontamination Treatment for N95 Respirators
5.1. Ultraviolet Germicidal Irradiation (UVGI)
UVGI is a scientifically proven decontamination method that can destroy the protein coating of the SARS-coronavirus, which possesses similar characteristics as the SARS-CoV-2 virus (COVID-19 virus) [19]. Ozog et al. [20] reported excellent decontamination results of the SARS-CoV-2 virus with a 1.5 J/cm2 UV dose, which was achieved using a 4 UVC lamp set-up. Vo et al. [21] produced the required decontamination levels up to a three-log reduction with a UV dose of 4.32 J/cm2 and complete decontamination with a ≥7.20 J/cm2 dosage against the MS2 virus. A relatively longer decontamination time was reported due to the low range of UV irradiation used in the research.
Lindsley et al. [22] tested a UV dose up to 950 J/cm2 on N95 respirators, which resulted in acceptable degradation on filtration performance and no effect in flow resistance. This study reported a perfect range for UVC-based decontamination treatment cycles. Ozog et al. [23] reported excellent fit testing results using N95 respirators with a total exposure of 60 J/cm2.
5.2. Hydrogen Peroxide Vapor (HPV)
HPV-based decontamination systems are regarded as some of the best decontamination systems due to their efficacy against various microorganisms and their rapid processing cycles [24]. Saini et al. [25] tested the N95 respirator’s decontamination against three biological indicators: Escherichia coli, Mycobacterium smegmatis, and spores of Bacillus stearothermophilus using an HPV machine. Excellent decontamination results were reported where decontamination up to a seven-log reduction was achieved using 11–12% HPV against E.coli. Jatta et al. [26] performed decontamination with a 59% HPV concentration using a VPRO maX low-temperature sterilization system. These research results exhibited no significant effect on the filtration performance and fit of the N95 mask after exposure to 59% HPV up to 10 cycles. The range of treatment time reported in this study provides a solid foundation for an HPV-based decontamination system design.
5.3. Heat
5.3.1. Moist Heat
Lore et al. [27] tested moist heat decontamination against the influenza virus applied on an N95 mask. In this study, a contaminated mask was heated to 65 + 5 °C for 3 h. The results show that the required decontamination level (>four-log reduction) was achieved. However, a relatively slow decontamination time can prove to be an inefficient decontamination procedure for everyday application. Rockey et al. [28] investigated the effect of humidity in virus heat inactivation against two bacteriophages (MS2 and phi6), a mouse coronavirus (murine hepatitis virus), and a recombinant human influenza A virus subtype H3N2 (IAV) using a humidity-controlled oven. Heat treatments illustrated greater decontamination results with increasing humidity, where six-log reductions were reported in humidity exceeding 50%.
Bopp et al. [29] tested multiple cycles of autoclaves on N95 respirators. Four different autoclave cycles (115 °C for one hour, 121.1 °C for 30 min, 130 °C for two minutes, and 130 °C for four minutes) were administered to N95 FFRs. N95 FFRs showed negligible differences in their functionality and integrity even after three cycles. Andregg et al. [30] applied heating decontamination to N95 respirators with moisture (85 °C, 60–85% humidity) in a polypropylene container and a convection oven setup. Post-decontamination N95 FFRs exhibited excellent results in both quantitative fit testing and filtration efficiency.
5.3.2. Dry Heat
Xiang et al. [31] implemented dry heat pasteurization for one hour at 70 °C for the N95 respirator’s decontamination. This study showed that this procedure can kill six species of respiratory bacteria and one fungi species and can inactivate the H1N1 indicator virus. In addition, neither the performance nor the integrity of N95 respirators showed significant degradation. This study shows that dry heat is capable of deactivating various pathogens but at a relatively slow rate. Pascoe et al. [32] successfully decontaminated pathogen (S. aureus) under dry heat of 70 °C by reducing log 4 in 90 min using a laboratory incubator. Despite strong decontamination results, the slow decontamination rate might prove to be the drawback of this method. Viscusi et al. [33] reported a slight increase in average penetration at N95 respirators when exposed to 80 °C after 60 min. These results can potentially act as a limitation for dry heat exposure to an N95 mask.
5.4. Microwave Generated Steam (MGS)
Fischer et al. [34] have proved up to a four-log reduction in bacteriophage MS2 pathogenic virus using sealed steam bags on a 1100-W-rated microwave for 90 s. In addition, tested N95 respirators also passed the minimum required filtration efficiency requirements of 95%. Zulauf et al. [35] reported a reduction greater than four logs measured in PFU on the N95 respirator. They tested MS2-phage-contaminated N95 respirators to microwave-generated steam for 3 min. Moreover, the respirators exhibited the required filtration performance and integrity even after 20 cycles of 3 min.
5.5. Ethanol
By using ethanol, decontamination of pathogens happens by protein denaturation. At a concentration of 60%–80%, ethanol proves to be effective against lipophilic viruses and many hydrophilic viruses [36]. Liao et al. [37] tested N95 respirators using a 75% ethanol treatment, which was immersed and dried. The filtration efficiency of the N95 respirators were affected considerably with treatment, which indicates that ethanol treatment could not retain the mask’s reusability properties.
5.6. Other Methods
N95 respirator decontamination procedures other than the methods selected for this review (UVGI or HPV or heat or MGS or ethanol) are highlighted based on their potential as a low-cost and accessible method. Lendvay et al. [38] tested SARS-CoV-2-inoculated N95 masks under methylene blue (MB) photochemical action for decontamination. They showed that MB activated by red or white light significantly inactivates SARS-CoV-2 on N95 mask surfaces without compromising the specimen’s integrity. Excellent virucidal activity of 99.8%–>99% was reported, and preservation of mask integrity proved up to five treatment cycles. Their findings suggested a strategy for decontaminating PPE and masks for reuse that is accessible and inexpensive and that can be used in high-resource and low-resource situations amid supply disruptions. This is due to the worldwide availability of MB light at an affordable cost without using specialized instruments. In addition, the New York City Department of Health and Mental Hygiene has released passive decontamination guidance to health workers to use a paper bag or other clean, breathable containers to store used N95 respirators to prolong their efficiency over multiple usages. The method is as follows. Each day, the healthcare workers would use one N95 respirator with a tagged name and the number of the day used and would place it in a paper bag or a ventilated container at the end of the shift. The mask should be disposed of after the seventh day of use. Healthcare workers must be aware that the N95 respirator could be contaminated albeit at a substantially lower rate. Limited storage periods may be considered, although they may raise the chance of contamination. As the more rigorous disinfecting techniques become accessible, this strategy could be integrated for higher efficiency [39]. Heimbuch et al. [40] evaluated the ability of wipe products available commercially to clean filtering facepiece respirators (FFRs) contaminated with pathogenic or non-pathogenic aerosols. They examined the decontamination effect of benzalkonium chloride, hypochlorite, and nonantimicrobial wipes on the N95 FFRs. The highest particle penetration capacity was observed in benzalkonium chloride wipes. They reported effective decontamination results of S aureus up to 99.72% (exterior of N95) and 98.60% (interior of N95) using benzalkonium chloride (BAC) wipes. Decontamination using wipes is readily available for public usage, but penetration of respirator due to wipe decontamination must be approached with caution.
5.7. Comparison of Decontamination Treatments for N95 Respirators
The reusability of a disinfected N95 respirator depends on several factors such as inactivation of the targeted organism, the safety of the user, and consistent filtration function and fit of the respirator. UVGI and HPV have demonstrated excellent results as an efficient decontamination method with effective elimination of SARS-CoV-2 virus while preserving the performance of the respirator. However, extensive studies are needed to incorporate HPV- and UVGI-based decontamination systems into a household-based portable commercial-ready product for commercial use. On the other hand, the MGS-based decontamination method exhibits great potential with rapid disinfection for household applications. Currently, there are still few studies about this method for decontamination application. Its rapid method enables a huge potential of applications. However, use in materials that are sensitive to steam could be a concern for material degradation. The other method includes the heat-based decontamination method, which has a major drawback for its time-consuming process and filtration performance degradation in extensive dosages. The conventional method of using ethanol has shown unavoidable degradation of the respirator by using this procedure. Table 2 demonstrates the effects of using a specified N95 decontamination treatment.

Table 2.
Advantages and disadvantages of decontamination treatments for N95 respirators.
6. Decontamination System Design for N95 Respirators
6.1. Ultraviolet Germicidal Irradiation
Several factors must be taken into account when designing a UVGI-based decontamination system, namely, the wavelength of the ultraviolet rays, the irradiance, and the exposure time. The effectiveness of a UVGI-based decontamination system depends on the dosage of UVC administered to the N95 mask. A safe dosage range must be estimated beforehand because excessive dosage can affect the integrity of the mask. On the other hand, an insufficient dosage can lead to incomplete deactivation of the virus. The UV dose for a specific system can be calculated using Equation (1) [41]. The system specifications and outcomes of studies related to UVGI-based N95 decontamination are listed in Table 3.

Table 3.
UVGI-based decontamination system specifications and outcomes.
6.2. Hydrogen Peroxide Vapor (HPV)
Most of the studies reviewed here used commercially available HPV-based decontamination machines. The efficiency of HPV-based decontamination systems depends on the concentration of the HPV used coupled with the time of exposure to the N95 respirator. HPV traces on mask surfaces might induce health hazards. Therefore, each HPV-based decontamination system must be able to produce residue-free N95 respirators upon the decontamination cycle. The system specifications and outcomes of studies related to HPV-based N95 decontamination are listed in Table 4.

Table 4.
HPV-based decontamination system specifications and outcomes.
6.3. Heat
Heat treatments can sterilize microbes by altering their membranes and denaturing proteins [68]. Heat-related decontaminations can be divided into two main classifications, namely, moist-heat and dry-heat decontamination. The efficiency of a heat-based decontamination system depends on the working temperature, the presence of humidity, and the exposure time. The existence of moisture in the heating procedure is proven to promote better decontamination results. The system specifications and outcomes of studies related to moist heat and dry heat-based N95 decontamination are listed in Table 5 and Table 6 respectively.

Table 5.
Moist-heat-based decontamination system specifications and outcomes.

Table 6.
Dry-heat-based decontamination system specifications and outcomes.
6.4. Microwave-Generated Steam (MGS)
MGS-based decontamination has enormous potential for wide application as it can be done with household items. It offers a rapid disinfection rate with minimal expertise needed to perform this treatment. The efficiency of MGS-based decontamination is affected by exposure time and is specific to the design of the selected face mask model for the treatment. However, many protocols use commercial steam bags or special materials that are available in laboratories. The system specifications and outcomes of studies related to MGS-based N95 decontamination are listed in Table 7.

Table 7.
Microwave-generated steam (MGS)-based decontamination system specifications and outcomes.
6.5. Ethanol
Ethanol-based disinfection is used widely around the world as an effective decontamination method. However, ethanol-based treatment does not produce an efficient result in the decontamination of N95 respirators. Ethanol is known to degrade the structure of the mask’s filtration and thus affects the integrity and performance of treated N95 respirators. The system specifications and outcomes of studies related to ethanol-based N95 decontamination are listed in Table 8.

Table 8.
Ethanol-based decontamination system specifications and outcomes.
7. Effectiveness of Decontamination Systems against SARS-CoV-2
The effectiveness of a specific decontamination system depends on critical parameters such as the exposure time. UVGI and HPV were investigated further in this review on their effectiveness against SARS-CoV-2, specifically from the surfaces of N95 respirators. The relationship between parameter control and effectiveness against the SARS-CoV-2 virus is illustrated in Figure 3 and Figure 4.
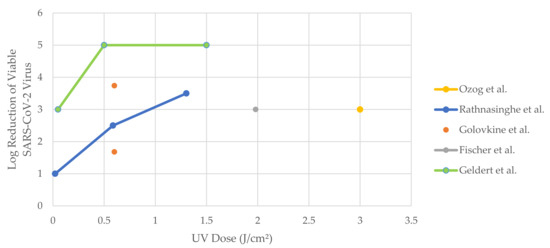
Figure 3.
Log reduction of viable SARS-CoV-2 virus with increasing UV dose (data represented in Figure 3 exhibit minimum log reduction achieved by specific dosage as upon reaching the limit of detection (LOD)—real data are not quantifiable).
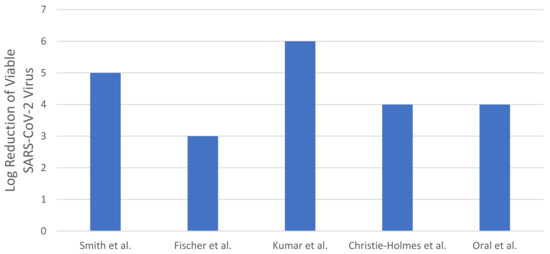
Figure 4.
Log reduction of viable SARS-CoV-2 virus with various HPV-based decontamination settings (data represented in Figure 4 exhibit minimum log reduction achieved by specific dosage as upon reaching the limit of detection (LOD)—real data are not quantifiable).
7.1. Ultraviolet Germicidal Irradiation
In a study, Ozog et al. [20] had demonstrated successful decontamination when an N95 mask was irradiated with 1.5 J/cm2 of UVC (254nm). It was concluded that the dose applied was sufficient. However, a concern on the disinfection of the strap arises due to its coverage by UVC on the strap surface. Rathnasinghe et al. [43] presented a simple UVC decontamination device without the mask’s strap decontamination. Golovkine et al. [44], Smith et al. [45], Fischer et al. [46], and Geldert et al. [47] investigated and compared the efficiency of UVC-based decontamination systems for N95 respirators with other decontamination methods such as ethanol, heat, UVA, ethylene oxide, hydrogen peroxide plasma and vapor, MGS, bleach, and liquid hydrogen peroxide. Comparing across the studies, a UVC-based N95 disinfection treatment with a dosage of greater than 0.5 J/cm2 can achieve the minimum pathogen load reduction required of three-log reduction against the SARS-CoV-2 virus. As Figure 3 illustrates, Geldert et al. [47] demonstrated notable disinfection of five-log reduction at a relatively low dosage of 0.5 J/cm2. Nevertheless, the reported sharp decline in the log reduction of SARS-CoV-2 [47] at lower UVC doses (0–0.5 J/cm2) must be addressed with caution.
7.2. Hydrogen Peroxide Vapor (HPV)
Smith et al. [45], Fischer et al. [46], Kumar et al. [58], Christie-Holmes et al. [59], and Oral et al. [60] have investigated the efficiency of HPV-based decontamination systems for N95 respirators against the SARS-CoV-2 virus. All the studies that reported HPV-based decontamination against the SARS-CoV-2 virus were designed using commercially available HPV generating machines. The comparison of the HPV-based N95 decontamination system efficiency across the studies is presented in Figure 4. The concentration of hydrogen peroxide exposed and the treatment time of a complete cycle comprised of four different processes are the variables that play a significant part in HPV-based decontamination systems to deliver the required decontamination efficiency. Notably, Kumar et al. [58] demonstrated a significant reduction in SARS-CoV-2 of six-log reduction while preserving the functional integrity of the N95 respirator post-treatment.
8. Conclusions
The COVID-19 pandemic shows the severity of the needed supply of PPE for healthcare workers to stay protected at all times. Decontamination of PPE could be an essential measure to mitigate the immediate risk of running out of PPE supply. UVGI- and HPV-based decontamination systems exhibit great potential as a good choice for N95 respirator decontamination. The study indicated that the UVGI and HPV methods could be used to deactivate the SARS-CoV-2 virus without affecting the integrity of the respirator. The excellent virucidal activity of UVGI- and HPV-based decontamination systems suggested that they are good candidates for N95 respirator decontamination.
Author Contributions
Conceptualization, M.R.M., N.S.M.N., N.A.H., J.J., and V.C.W.H.; methodology, M.R.M., T.G., and R.‘A.M.Y.; software, R.‘A.M.Y.; validation, M.R.M., N.S.M.N., N.A.H., J.J., and V.C.W.H.; formal analysis, T.G.; investigation, T.G.; resources, M.R.M., T.G., and R.‘A.M.Y.; data curation, T.G.; writing—original draft preparation, T.G.; writing—review and editing, T.G. and R.‘A.M.Y.; visualization, M.R.M. and T.G.; supervision, M.R.M.; project administration, M.R.M.; funding acquisition, M.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AUN/SEED-Net, grant number UMSPRAC 2101.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Acknowledgments
The authors would like to thank Mohd Fauzi Bakri Hashim for his assistance in this research project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report, 94; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Shortage of Personal Protective Equipment Endangering Health Workers Worldwide. Available online: https://www.who.int/news/item/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide (accessed on 16 July 2021).
- Degesys, N.F.; Wang, R.C.; Kwan, E.; Fahimi, J.; Noble, J.A.; Raven, M.C. Correlation Between N95 Extended Use and Reuse and Fit Failure in an Emergency Department. JAMA 2020, 324, 94–96. [Google Scholar] [CrossRef] [PubMed]
- 3M. 3M™ Particulate Respirator, 8210, N95 Technical Specifications. Available online: https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf (accessed on 16 July 2021).
- Gray, A. Exploring the Shortage of Affordable Masks in Developing Countries. Available online: https://www.borgenmagazine.com/affordable-masks/ (accessed on 6 September 2021).
- Parshley, L. The Mask Shortage Is Forcing Health Workers to Disregard Basic Coronavirus Infection Control. Available online: https://www.vox.com/2020/4/3/21206726/coronavirus-masks-n95-hospitals-health-care-doctors-ppe-shortage (accessed on 6 September 2021).
- Centers for Disease Control and Prevention. Implementing Filtering Facepiece Respirator (FFR) Reuse, Including Reuse after Decontamination, When There Are Known Shortages of N95 Respirators. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html (accessed on 16 July 2021).
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Turtil, S. Sterility Review of 510(k) Submissions: Common Microbiology Issues Found in 510(k) Submissions. Available online: https://www.fda.gov/media/141277/download (accessed on 16 July 2021).
- Scientific Animations Inc. 3D Medical Animation Corona Virus. Available online: https://commons.wikimedia.org/wiki/File:3D_medical_animation_corona_virus.jpg (accessed on 16 July 2021).
- Centers for Disease Control and Prevention (CDC); National Institute for Occupational Safety and Health. Understanding the Diffrence. Available online: https://www.cdc.gov/niosh/npptl/pdfs/understanddifferenceinfographic-508.pdf (accessed on 16 July 2021).
- Ji, D.; Fan, L.; Li, X.; Ramakrishna, S. Addressing the worldwide shortages of face masks. BMC Mater. 2020, 2, 9. [Google Scholar] [CrossRef]
- O’Kelly, E.; Arora, A.; Pirog, S.; Ward, J.; Clarkson, P.J. Comparing the fit of N95, KN95, surgical, and cloth face masks and assessing the accuracy of fit checking. PLoS ONE 2021, 16, e0245688. [Google Scholar] [CrossRef]
- Henneberry, B. How to Make N95 Masks. Available online: https://www.thomasnet.com/articles/plant-facility-equipment/how-to-make-n95-masks/#_How_are_N95 (accessed on 16 July 2021).
- Huber, T.; Goldman, O.; Epstein, A.; Stella, G.; Sakmar, T. Principles and practice of SARS-CoV-2 decontamination of N95 masks with UV-C. Biophys. J. 2020, 120, 2927–2942. [Google Scholar] [CrossRef]
- U.S. Food Drug Administration. UV Lights and Lamps: Ultraviolet-C Radiation, Disinfection, and Coronavirus. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/uv-lights-and-lamps-ultraviolet-c-radiation-disinfection-and-coronavirus (accessed on 16 July 2021).
- Ozog, D.M.; Sexton, J.Z.; Narla, S.; Pretto-Kernahan, C.D.; Mirabelli, C.; Lim, H.W.; Hamzavi, I.H.; Tibbetts, R.J.; Mi, Q.S. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int. J. Infect. Dis. 2020, 100, 224–229. [Google Scholar] [CrossRef]
- Vo, E.; Rengasamy, S.; Shaffer, R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl. Environ. Microbiol. 2009, 75, 7303–7309. [Google Scholar] [CrossRef][Green Version]
- Lindsley, W.G.; Martin, S.B., Jr.; Thewlis, R.E.; Sarkisian, K.; Nwoko, J.O.; Mead, K.R.; Noti, J.D. Effects of Ultraviolet Germicidal Irradiation (UVGI) on N95 Respirator Filtration Performance and Structural Integrity. J. Occup. Environ. Hyg. 2015, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ozog, D.; Parks-Miller, A.; Kohli, I.; Lyons, A.B.; Narla, S.; Torres, A.E.; Levesque, M.; Lim, H.W.; Hamzavi, I.H. The importance of fit testing in decontamination of N95 respirators: A cautionary note. J. Am. Acad. Dermatol. 2020, 83, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Sciences, S.L. The Advantages of Decontaminating with Vapor Phase Hydrogen Peroxide. Available online: https://www.sterislifesciences.com/resources/documents/technical-tips/the-advantages-of-decontaminating-with-vapor-phase-hydrogen-peroxide (accessed on 16 July 2021).
- Saini, V.; Sikri, K.; Batra, S.D.; Kalra, P.; Gautam, K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathog. 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Jatta, M.; Kiefer, C.; Patolia, H.; Pan, J.; Harb, C.; Marr, L.C.; Baffoe-Bonnie, A. N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 8–14. [Google Scholar] [CrossRef]
- Lore, M.B.; Heimbuch, B.K.; Brown, T.L.; Wander, J.D.; Hinrichs, S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann. Occup. Hyg. 2012, 56, 92–101. [Google Scholar] [CrossRef]
- Rockey, N.; Arts, P.J.; Li, L.; Harrison, K.R.; Langenfeld, K.; Fitzsimmons, W.J.; Lauring, A.S.; Love, N.G.; Kaye, K.S.; Raskin, L.; et al. Humidity and Deposition Solution Play a Critical Role in Virus Inactivation by Heat Treatment of N95 Respirators. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Bopp, N.; Bouyer, D.; Gibbs, C.; Nichols, J.; Ntiforo, C.; Grimaldo, M. Multicycle Autoclave Decontamination of N95 Filtering Facepiece Respirators. Appl. Biosaf. 2020, 25, 150–156. [Google Scholar] [CrossRef]
- Anderegg, L.; Meisenhelder, C.; Ngooi, C.O.; Liao, L.; Xiao, W.; Chu, S.; Cui, Y.; Doyle, J.M. A scalable method of applying heat and humidity for decontamination of N95 respirators during the COVID-19 crisis. PLoS ONE 2020, 15, e0234851. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, Q.; Gu, W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70 °C. Am. J. Infect. Control 2020, 48, 880–882. [Google Scholar] [CrossRef]
- Pascoe, M.J.; Robertson, A.; Crayford, A.; Durand, E.; Steer, J.; Castelli, A.; Wesgate, R.; Evans, S.L.; Porch, A.; Maillard, J.Y. Dry heat and microwave-generated steam protocols for the rapid decontamination of respiratory personal protective equipment in response to COVID-19-related shortages. J. Hosp. Infect. 2020, 106, 10–19. [Google Scholar] [CrossRef]
- Viscusi, D.; King, W.P.; Shaffer, R. Effect of Decontamination on the Filtration Efficiency of Two Filtering Facepiece Respirator Models. J. Int. Soc. Respir. Prot. 2007, 24, 93. [Google Scholar]
- Fisher, E.M.; Williams, J.L.; Shaffer, R.E. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS ONE 2011, 6, e18585. [Google Scholar] [CrossRef] [PubMed]
- Zulauf, K.E.; Green, A.B.; Nguyen Ba, A.N.; Jagdish, T.; Reif, D.; Seeley, R.; Dale, A.; Kirby, J.E. Microwave-Generated Steam Decontamination of N95 Respirators Utilizing Universally Accessible Materials. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Chemical Disinfectants. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html (accessed on 16 July 2021).
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 Respirators Be Reused after Disinfection? How Many Times? ACS Nano 2020, 14, 6348–6356. [Google Scholar] [CrossRef] [PubMed]
- Lendvay, T.S.; Chen, J.; Harcourt, B.H.; Scholte, F.E.M.; Lin, Y.L.; Kilinc-Balci, F.S.; Lamb, M.M.; Homdayjanakul, K.; Cui, Y.; Price, A.; et al. Addressing personal protective equipment (PPE) decontamination: Methylene blue and light inactivates severe acute respiratory coronavirus virus 2 (SARS-CoV-2) on N95 respirators and medical masks with maintenance of integrity and fit. Infect. Control. Hosp. Epidemiol. 2021, 1–10. [Google Scholar] [CrossRef]
- The NYC Health Department. COVID-19: Potential Decontamination Strategies for N95 Respirators. Available online: https://www1.nyc.gov/assets/doh/downloads/pdf/imm/n95-decontamination-strategies.pdf (accessed on 12 October 2021).
- Heimbuch, B.K.; Kinney, K.; Lumley, A.E.; Harnish, D.A.; Bergman, M.; Wander, J.D. Cleaning of filtering facepiece respirators contaminated with mucin and Staphylococcus aureus. Am. J. Infect. Control 2014, 42, 265–270. [Google Scholar] [CrossRef][Green Version]
- Mills, D.; Harnish, D.A.; Lawrence, C.; Sandoval-Powers, M.; Heimbuch, B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control 2018, 46, e49–e55. [Google Scholar] [CrossRef]
- Simmons, S.E.; Carrion, R.; Alfson, K.J.; Staples, H.M.; Jinadatha, C.; Jarvis, W.R.; Sampathkumar, P.; Chemaly, R.F.; Khawaja, F.; Povroznik, M.; et al. Deactivation of SARS-CoV-2 with pulsed-xenon ultraviolet light: Implications for environmental COVID-19 control. Infect. Control. Hosp. Epidemiol. 2021, 42, 127–130. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Karlicek, R.F.; Schotsaert, M.; Koffas, M.A.; Arduini, B.; Jangra, S.; Wang, B.; Davis, J.L.; Alnaggar, M.; Costa, A.; et al. Scalable, effective, and rapid decontamination of SARS-CoV-2 contaminated N95 respirators using germicidal ultra-violet C (UVC) irradiation device. medRxiv 2020. [Google Scholar] [CrossRef]
- Golovkine, G.R.; Roberts, A.W.; Cooper, C.; Riano, S.; DiCiccio, A.M.; Worthington, D.L.; Clarkson, J.P.; Krames, M.; Zhang, J.; Gao, Y.; et al. Practical considerations for Ultraviolet-C radiation mediated decontamination of N95 respirator against SARS-CoV-2 virus. PLoS ONE 2021, 16, e0258336. [Google Scholar] [CrossRef]
- Smith, J.S.; Hanseler, H.; Welle, J.; Rattray, R.; Campbell, M.; Brotherton, T.; Moudgil, T.; Pack, T.F.; Wegmann, K.; Jensen, S.; et al. Effect of various decontamination procedures on disposable N95 mask integrity and SARS-CoV-2 infectivity. J. Clin. Transl. Sci. 2020, 5, e10. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.J.; Morris, D.H.; van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- Geldert, A.; Su, A.; Roberts, A.W.; Golovkine, G.; Grist, S.M.; Stanley, S.A.; Herr, A.E. Nonuniform UV-C dose across N95 facepieces can cause 2.9-log variation in SARS-CoV-2 inactivation. medRxiv 2021. [Google Scholar] [CrossRef]
- Weaver, D.T.; McElvany, B.D.; Gopalakrishnan, V.; Card, K.J.; Crozier, D.; Dhawan, A.; Dinh, M.N.; Dolson, E.; Farrokhian, N.; Hitomi, M.; et al. UV decontamination of personal protective equipment with idle laboratory biosafety cabinets during the COVID-19 pandemic. PLoS ONE 2021, 16, e0241734. [Google Scholar] [CrossRef]
- Ludwig-Begall, L.F.; Wielick, C.; Jolois, O.; Dams, L.; Razafimahefa, R.M.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Thiry, E.; et al. “Don, doff, discard” to “don, doff, decontaminate”—FFR and mask integrity and inactivation of a SARS-CoV-2 surrogate and a norovirus following multiple vaporised hydrogen peroxide-, ultraviolet germicidal irradiation-, and dry heat decontaminations. PLoS ONE 2021, 16, e0251872. [Google Scholar] [CrossRef]
- Kayani, B.J.; Weaver, D.T.; Gopalakrishnan, V.; King, E.S.; Dolson, E.; Krishnan, N.; Pelesko, J.; Scott, M.J.; Hitomi, M.; Cadnum, J.L.; et al. UV-C tower for point-of-care decontamination of filtering facepiece respirators. Am. J. Infect. Control 2021, 49, 424–429. [Google Scholar] [CrossRef]
- Fisher, E.M.; Shaffer, R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J. Appl. Microbiol. 2011, 110, 287–295. [Google Scholar] [CrossRef]
- He, W.; Guo, Y.; Gao, H.; Liu, J.; Yue, Y.; Wang, J. Evaluation of Regeneration Processes for Filtering Facepiece Respirators in Terms of the Bacteria Inactivation Efficiency and Influences on Filtration Performance. ACS Nano 2020, 14, 13161–13171. [Google Scholar] [CrossRef]
- Wigginton, K.R.; Arts, P.J.; Clack, H.L.; Fitzsimmons, W.J.; Gamba, M.; Harrison, K.R.; LeBar, W.; Lauring, A.S.; Li, L.; Roberts, W.W.; et al. Validation of N95 Filtering Facepiece Respirator Decontamination Methods Available at a Large University Hospital. Open Forum Infect. Dis. 2021, 8, ofaa610. [Google Scholar] [CrossRef]
- Lin, T.H.; Tang, F.C.; Hung, P.C.; Hua, Z.C.; Lai, C.Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air 2018, 28, 754–762. [Google Scholar] [CrossRef]
- Chen, P.Z.; Ngan, A.; Manson, N.; Maynes, J.T.; Borschel, G.H.; Rotstein, O.D.; Gu, F.X. Transmission of aerosols through pristine and reprocessed N95 respirators. medRxiv 2020. [Google Scholar] [CrossRef]
- Ou, Q.; Pei, C.; Chan Kim, S.; Abell, E.; Pui, D.Y.H. Evaluation of decontamination methods for commercial and alternative respirator and mask materials—View from filtration aspect. J. Aerosol Sci. 2020, 150, 105609. [Google Scholar] [CrossRef]
- Ontiveros, C.C.; Sweeney, C.L.; Smith, C.; MacIsaac, S.; Bennett, J.L.; Munoz, S.; Stoddart, A.K.; Gagnon, G.A. Assessing the impact of multiple ultraviolet disinfection cycles on N95 filtering facepiece respirator integrity. Sci. Rep. 2021, 11, 12279. [Google Scholar] [CrossRef]
- Kumar, A.; Kasloff, S.B.; Leung, A.; Cutts, T.; Strong, J.E.; Hills, K.; Vazquez-Grande, G.; Rush, B.; Lother, S.; Zarychanski, R.; et al. N95 Mask Decontamination using Standard Hospital Sterilization Technologies. medRxiv 2020. [Google Scholar] [CrossRef]
- Christie-Holmes, N.; Tyli, R.; Budylowski, P.; Guvenc, F.; Weiner, A.; Poon, B.; Speck, M.; Naugler, S.; Rainville, A.; Ghalami, A.; et al. Vapourized hydrogen peroxide decontamination in a hospital setting inactivates SARS-CoV-2 and HCoV-229E without compromising filtration efficiency of unexpired N95 respirators. Am. J. Infect. Control 2021, 49, 1227–1231. [Google Scholar] [CrossRef]
- Oral, E.; Wannomae, K.K.; Connolly, R.; Gardecki, J.; Leung, H.M.; Muratoglu, O.; Griffiths, A.; Honko, A.N.; Avena, L.E.; McKay, L.G.A.; et al. Vapor H2O2 sterilization as a decontamination method for the reuse of N95 respirators in the COVID-19 emergency. medRxiv 2020. [Google Scholar] [CrossRef]
- Dave, N.; Pascavis, K.S.; Patterson, J.; Wallace, D.; Chowdhury, A.; Abbaszadegan, M.; Alum, A.; Herckes, P.; Zhang, Z.; Kozicki, M.; et al. Characterization of a novel, low-cost, scalable vaporized hydrogen peroxide system for sterilization of N95 respirators and other COVID-19 related personal protective equipment. medRxiv 2020. [Google Scholar] [CrossRef]
- Moschella, P.; Liao, W.; Litwin, A.; Foulk, J.; Anthony, J.; Player, M.; Chang, J.; Cole, C. Repeated vaporised hydrogen peroxide disinfection of 3M 1860 N95 mask respirators does not degrade quantitative fit performance. Br. J. Anaesth. 2021, 126, e125–e127. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Levine, C.; Grady, C.; Peixoto, B.; McCormick-Ell, J.; Block, T.; Gresko, A.; Delmas, G.; Chitale, P.; Frees, A.; et al. Decontaminating N95 respirators during the COVID-19 pandemic: Simple and practical approaches to increase decontamination capacity, speed, safety and ease of use. J. Hosp. Infect. 2021, 109, 52–57. [Google Scholar] [CrossRef]
- Al-Hadyan, K.; Alsbeih, G.; Nobah, A.; Lindstrom, J.; Falatah, S.; Faran, N.; Al-Ghamdi, S.; Moftah, B.; Alhmaid, R. In-House Filtration Efficiency Assessment of Vapor Hydrogen Peroxide Decontaminated Filtering Facepiece Respirators (FFRs). Int. J. Environ. Res. Public Health 2021, 18, 7169. [Google Scholar] [CrossRef] [PubMed]
- Levine, C.; Grady, C.; Block, T.; Hurley, H.; Russo, R.; Peixoto, B.; Frees, A.; Ruiz, A.; Alland, D. Use, re-use or discard? Quantitatively defined variance in the functional integrity of N95 respirators following vaporized hydrogen peroxide decontamination during the COVID-19 pandemic. J. Hosp. Infect. 2021, 107, 50–56. [Google Scholar] [CrossRef]
- Lieu, A.; Mah, J.; Zanichelli, V.; Exantus, R.C.; Longtin, Y. Impact of extended use and decontamination with vaporized hydrogen peroxide on N95 respirator fit. Am. J. Infect. Control 2020, 48, 1457–1461. [Google Scholar] [CrossRef]
- Beam, E.; Nesbitt, J.C.; Austin, M.D.; Ramar, K. Effect of vaporized hydrogen peroxide reprocessing on N95 respirators. Infect. Control. Hosp. Epidemiol. 2021, 42, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Veronique Greenwood; Quanta Magazine. Why Does Heat Kill Cells? Available online: https://www.theatlantic.com/science/archive/2017/05/heat-kills-cells/526377/ (accessed on 16 July 2021).
- Kumar, A.; Kasloff, S.B.; Cutts, T.; Leung, A.; Sharma, N.; Vazquez-Grande, G.; Drew, T.; Laframboise, D.; Orofino, O.; Tanelli, J.; et al. Standard hospital blanket warming cabinets can be utilized for complete moist heat SARS-CoV2 inactivation of contaminated N95 masks for re-use. Sci. Rep. 2021, 11, 18316. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.W.; Richardson, A.W.; Sunderman, M.; Mladineo, M.J.; Keyes, P.H.; Hofacre, K.C.; Middleton, J.K. Decontamination of SARS-CoV-2 contaminated N95 filtering facepiece respirators (FFRs) with moist heat generated by a multicooker. Lett. Appl. Microbiol. 2021, 72, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Wannomae, K.K.; Gil, D.; Connolly, R.; Gardecki, J.A.; Leung, H.M.; Muratoglu, O.K.; Tsurumi, A.; Rahme, L.; Jaber, T.; et al. Efficacy of moist heat decontamination against various pathogens for the reuse of N95 respirators in the COVID-19 emergency. medRxiv 2020. [Google Scholar] [CrossRef]
- Daeschler, S.C.; Manson, N.; Joachim, K.; Chin, A.W.H.; Chan, K.; Chen, P.Z.; Tajdaran, K.; Mirmoeini, K.; Zhang, J.J.; Maynes, J.T.; et al. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. CMAJ 2020, 192, E1189–E1197. [Google Scholar] [CrossRef]
- Harskamp, R.E.; van Straten, B.; Bouman, J.; van Maltha-van Santvoort, B.; van den Dobbelsteen, J.J.; van der Sijp, J.R.; Horeman, T. Reprocessing filtering facepiece respirators in primary care using medical autoclave: Prospective, bench-to-bedside, single-centre study. BMJ Open 2020, 10, e039454. [Google Scholar] [CrossRef]
- Czubryt, M.P.; Stecy, T.; Popke, E.; Aitken, R.; Jabusch, K.; Pound, R.; Lawes, P.; Ramjiawan, B.; Pierce, G.N. N95 mask reuse in a major urban hospital: COVID-19 response process and procedure. J. Hosp. Infect. 2020, 106, 277–282. [Google Scholar] [CrossRef]
- Benboubker, M.; Oumokhtar, B.; Hmami, F.; Mabrouk, K.E.; Alami, L.E.; Arhoune, B.; Belahsen, M.F.; Aboutajeddine, A. Covid-19 respiratory protection: The filtration efficiency assessment of decontaminated FFP2 masks responding to associated shortages. medRxiv 2021. [Google Scholar] [CrossRef]
- Doshi, S.; Banavar, S.P.; Flaum, E.; Kulkarni, S.; Kumar, S.; Chen, T.; Bhattacharya, A.; Prakash, M. Applying Heat and Humidity using Stove Boiled Water for Decontamination of N95 Respirators in Low Resource Settings. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.J.; Nofchissey, R.A.; Ye, C.; Donart, N.; Kell, A.; Foo-Hurwitz, I.; Muller, T.; Bradfute, S.B. COVID-19 global pandemic planning: Dry heat incubation and ambient temperature fail to consistently inactivate SARS-CoV-2 on N95 respirators. Exp. Biol. Med. 2021, 246, 952–959. [Google Scholar] [CrossRef]
- Price, A.; Cui, Y.; Liao, L.; Xiao, W.; Yu, X.; Wang, H.; Zhao, M.; Wang, Q.; Chu, S.; Chu, L. Is the fit of N95 facial masks effected by disinfection? A study of heat and UV disinfection methods using the OSHA protocol fit test. medRxiv 2020. [Google Scholar] [CrossRef]
- Loh, M.; Clark, R.; Cherrie, J. Heat treatment for reuse of disposable respirators during Covid-19 pandemic: Is filtration and fit adversely affected? medRxiv 2020. [Google Scholar] [CrossRef]
- Nazeeri, A.I.; Hilburn, I.A.; Wu, D.-A.; Mohammed, K.A.; Badal, D.Y.; Chan, M.H.W.; Kirschvink, J.L. Ethanol-Drying Regeneration of N95 Respirators. medRxiv 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).