Abstract
The progress of dissimilatory iron(III) reduction is widespread in natural environments, particularly in anoxic habitats; in fact, wetland ecosystems are considered as “hotspots” of dissimilatory Fe(III) reduction. In this study, we conducted soil slurry and microbial inoculation anaerobic incubation with glucose, pyruvate, and soluble quinone anthraquinone-2,6-disulphonate (AQDS) additions in freshwater marsh and meadow wetlands in the Sanjiang Plain, to evaluate the role of carbon addition in the rates and dynamics of iron reduction. Dissimilatory Fe(III) reduction in marsh wetlands responded more quickly and showed twice the potential for Fe(III) reduction as that in meadow wetland. Fe(III) reduction rate in marsh and meadow wetlands was 76% and 30%, respectively. Glucose had a higher capacity to enhance Fe(III) reduction than pyruvate, which provides valuable information for the further isolation of Fe reduction bacteria in pure culture. AQDS could dramatically increase potential Fe(III) reduction as an electron shuttle in both wetlands. pH exhibited a negative relationship with Fe(III) reduction. In view of the significance of freshwater wetlands in the global carbon and iron cycle, further profound research is now essential and should explore the enzymatic mechanisms underlying iron reduction in freshwater wetlands.
1. Introduction
Iron is the most abundant redox-active element in the Earth’s crust, and iron oxides occur ubiquitously in natural environments [1]. Fe(III) reduction was previously regarded as the abiotic oxidation dominating the reaction progress till the late 1980’s, when it was discovered that microorganisms can enzymatically reduce Fe(III) to Fe(II) [2,3]. Under strict and facultative anaerobic conditions, dissimilatory iron reduction occurs when microorganisms conserve energy through redox reactions without assimilating iron into its biomass [4]. Iron oxide reduction and microorganisms exist in almost all anaerobic conditions. Given the abundance of iron, iron reduction-oxidation reactions have the potential of supporting substantial microbial populations in soil and sediments.
Wetlands soils are often dominated by fluctuating redox reactions and abundant organic carbon, which provide an ideal model with which to study the relationship between carbon and iron. While Fe(III) reduction likely occurs in the top few centimeters of submerged soils and freshwater sediments, this might represent an important aspect of carbon anaerobic oxidation because this zone typically contains the most labile fraction of organic matter and supports the highest rates of organic matter decomposition [2]. In fact, dissimilatory Fe(III) reduction has been proven to be the dominant pathway for the mineralization of anaerobic organic matter in many aquatic soils and sediment. For example, in a previous study, Fe(III) reduction explained 65% of total carbon metabolism in vegetated sediments, compared to 22% for methanogens [5]. Lipson et al. [6] estimated that Fe(III) reduction was an important pathway for respiration, and given an adequate supply of Fe(III), approximately 30% of heterotrophic respiration could be driven by Fe reduction in Arctic peat soils. Another study reported that microbial iron reduction along the inundation gradient in the Min River Estuary accounted for 20–89% of the mineralization of anaerobic organic matter [7].
As an important substrate for dissimilatory iron microbial bacteria, organic matter can also accept, donate, and transfer electrons [8,9]. Organic carbon, as an electron acceptor and electron shuttle, was previously reported as one of the dominant factors affecting Fe(III) reduction, with important implications for carbon and iron cycling [10,11,12]. The rate of Fe(III) reduction, and the metabolic pathways involved, is significantly affected by the availability and composition of soil organic carbon, which offers the potential for the translation and development of functional microbial communities. This also indicates the crucial role of organic carbon in microbial Fe(III) reduction.
Among various carbon sources, glucose exists widely in natural environments and is generally considered to be one of the most important intermediate products in the decomposition of polysaccharide. Pure culture studies also indicated that iron reduction bacteria can use a wide range of substrates for Fe(III) reduction [2]. Quinone moieties are implicated in the redox reactions observed in humus, and have been reported to act as electron shuttles and play an important role in the biochemical cycles of soils and sediments [11,13,14]. Therefore, iron reduction in wetland soils with fluctuating redox has the potential to directly influence carbon cycling due to the fact that it represents a source of CO2 as a terminal electron acceptor for microbes via heterotrophic respiration. Iron reduction can also cause indirect effects by altering the availability of nutrients, potentially causing limitation effects on plants and microbes [15].
However, there appears to be differences in the patterns of carbon use across different types of dissimilatory iron reduction bacteria and few studies have documented the process and patterns of substrate use in microbial iron reduction in freshwater wetlands in the high latitudes of China. In the present study, we incubated an anaerobic slurry and microbial inoculation incubation in which different carbon sources (glucose, pyruvate, and soluble quinone anthraquinone-2,6-disulphonate (AQDS)) were added to both marsh and meadow wetland soils to investigate the response of Fe(III) reduction in carbon addition. The goal of our study was to evaluate the effects of these factors on Fe(III) reduction by indigenous microorganisms and thus enhance current understanding of how the effects of carbon addition might affect the biogeochemical cycling of iron in freshwater wetlands. Furthermore, we hope that our study can provide useful information for carbon source usage in the cultivation and isolation of iron microbial.
2. Materials and Methods
2.1. Study Area
This study was conducted in a freshwater marsh and meadow wetland near the Sanjiang Experimental Station of Wetland Ecology, Chinese Academy of Sciences (47°35′ N, 133°31′ E, 56 m a.s.l.), which represents a seasonal frozen zone where the soil and water are completely frozen from late October to early next April. The mean annual temperature is approximately 2.52 °C and mean annual precipitation is approximately 588 mm with substantial inter-annual variations [16]. Carex lasiocarpa is the dominant species in the marsh while Deyeuxia angustifolia is the dominant species in the meadow. Glyceria spiculosa and Phragmites australis are the most important accompanying species. More detailed information about this study site has been provided previously by Song et al. [17] and Hou [18]. Detailed data on soil characteristics in these two wetlands is shown in Table 1.

Table 1.
Characteristics of the soil collected from the experimental site. TN-total nitrogen; TOC-total organic carbon; Feox: easily reducible iron oxides; Fef: free iron; and Fea: Amorphous iron. Values are presented by mean ± standard error, n = 3.
2.2. Sample Collection
Soil samples were collected from the upper 15 cm of soil using a spade on the 26 of July 2017. To prevent contamination from the spade, or substrate surface, subsamples of approximately 125 cm3 were taken by hand with an inverted zip-lock bag from within the bottom of the original samples. Soil samples were then transported to the laboratory on ice in coolers. The samples were then air-dried after removing visible plant root material and were sieved with a 1-mm mesh. The sieved samples were then stored in darkness for further analysis.
2.3. Anaerobic Slurry Incubation
Soil samples were incubated as a slurry in 30 mL sterile serum vials. Exactly 1.00 g of dried soil from two wetlands was mixed with 10 mL of sterile distilled water. The vials were then filled with nitrogen for 3–5 min, and sealed with aluminum covers and incubated at 30 °C in incubators in darkness for 30 days.
2.4. Soil Microbial Inoculation Incubation
Exactly 5.00 g of the dried soil from marsh and meadow wetlands, mixed with 50 mL sterile distilled water, were incubated for one week to recover the microbial community. Then, the soil slurries were mixed with 80 mL of distilled water in sterile centrifuge tubes. Microbial community extraction was then conducted at 30 °C for one hour in a shaking incubator (CHA-S, Jiangsu, China), after which the tube was centrifuged (Anke GL-20B, Shanghai, China) for 20 min. The supernatant was then collected as a soil microbial inoculant. There were five treatments in total, including the addition of glucose (0.25 mol/L), pyruvate (0.5 mol/L), glucose and AQDS (0.5 mol/L), pyruvate and AQDS (0.5 mol/L), and a control treatment. Synthetic ferrihydrite (FeOOH) was added to each treatment as a sole electron acceptor, along with phosphate buffer solution (1 mL, 0.025 mol L−1) and NH4Cl solution (1 mL, 5 g/L) as a nitrogen source.
2.5. Sample Analysis
For each experiment, two vials were taken randomly for each treatment on days 0, 1, 3, 5, 7, 10, 15, 20, 25, and 30 for analysis of Fe(II) concentration and pH during anaerobic incubation. The Fe(II) concentration was determined by the 1,10-orthophenanthroline method [19]. In brief, the soil slurry was well shaken and a 0.4 mL subsample was quickly transferred into 4.6 mL of 0.5 mol/L HCl solution and allowed to react under N2 atmosphere for 24 h. Then, the extracts were centrifuged for 20 min, and 1 mL of supernatant sample was mixed with 5 mL of 1 mol/L sodium acetate and 1 mg/mL phenanthroline to develop color. The concentration of Fe(II) was then measured based on absorbance at 510 nm. pH was measured using a glass electrode.
2.6. Data Analysis
The metric for Fe reduction typically uses the net change in Fe(II) concentrations with incubation time. A logistic model was used to fit the relationship between Fe(II) accumulation and incubation time in each treatment. This logistic model was expressed as:
where y was the Fe(II) concentration measured after incubation time for x (d); a referred to the maximum Fe(II) accumulation, b was the parameter, and k was the reaction rate constant.
The independent samples t-test was used to compare the differences in iron reduction between marsh and meadow wetlands. One-way analyses of variance (ANOVA) were performed to determine the differences among different treatments in soil microbial inoculation incubation. In all analyses, the factors and relationships tested were considered to be statistically significant at p < 0.05. All statistical analyses were performed using SPSS 16.0 and figures were prepared by Sigma Plot 11.0.
3. Results
3.1. Anaerobic Slurry Incubation
Ferrous iron (Fe(II)) accumulation exhibited an increasing trend during the anaerobic incubation period in the C. lasiocarpa dominated marsh and D. angustifolia dominated meadow (Figure 1). Furthermore, Fe(II) accumulation increased more quickly in marsh than that in meadow wetlands. Significant amounts of reduced iron increased from day one (0.65 mg/g) to day three (3.17 mg/g) and then moved to equilibrium in marsh wetland (Figure 1a). In meadow wetlands, reduced iron increased more slowly (Figure 1b). There was a significant difference in reduced Fe(II) accumulation when compared between marsh and meadow wetlands (p < 0.01). The reduced iron concentration was 3.14 ± 0.07 mg/g in marsh wetland, three times higher than that in meadow soil (0.9 ± 0.02 mg/g).
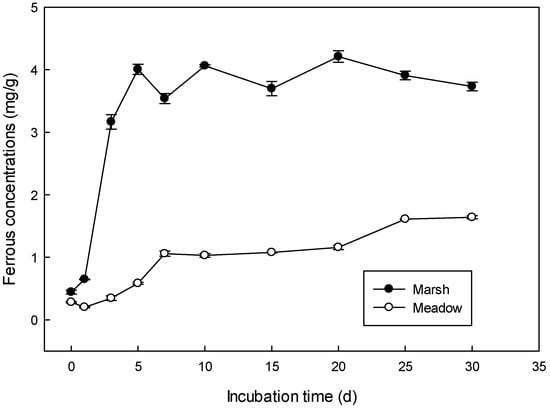
Figure 1.
Variations of Fe(II) concentration in the C. lasiocarpa dominated marsh and D. angustifolia dominated meadow soils during 30 days of anaerobic incubation. Error bars represent the standard error of the mean, n = 3.
The dynamics of Fe(II) accumulation with incubation time fitted well into a logistic model which reflected the potential for Fe(III) reduction, with a coefficient of determination in the marsh (0.97) and the meadow (0.86). As shown in Table 1, the Fe(III) reduction potential (a) in marsh wetland was twice that in the meadow (Table 2). The maximum reaction rate (Vmax) was much higher and the incubation time for Vmax was earlier in marsh wetland compared to that in meadow wetland (Table 2).

Table 2.
The parameters for logistic models in marsh and meadow soils during 30 days of anaerobic incubation. Vmax represented the maximum reaction rate. Tvmax represented the incubation time for Vmax.
3.2. Soil Microbial Inoculation Incubation
There were significant differences in the effect of carbon additions on ferrous iron accumulation in both wetlands. When glucose and pyruvate were added, Fe(II) concentrations increased slightly, both in marsh and meadow wetlands. The response of Fe(III) reduction in both wetlands was more sensitive to pyruvate (Figure 2). The Vmax with pyruvate and glucose addition was 9.6 and 13.6 times faster than that in controls in the marsh, and 6.8 and 11.9 times in the meadow, respectively. Furthermore, promotion effects were much more obvious after the electron shuttle AQDS was added, and Fe(II) concentrations were dramatically increased in both wetlands. Fe(III) reduction potential (a) was as high as 903.11 and 966.15 mg/g, respectively, with the addition of AQDS and glucose, and with AQDS and pyruvate in the marsh, and 966.15 and 814.22 mg/g, in the meadow (Table 3). In marsh wetland, ferrous iron concentrations increased linearly until day 20 after the addition of AQDS. The trend for Fe(II) accumulation was similar in both wetlands. The maximal reaction rate was higher in meadow soils than that in marsh wetland when pyruvate was added. There was no significant difference in the Fe(III) reduction potential, or time of maximal reaction rate when compared between marsh and meadow wetland.
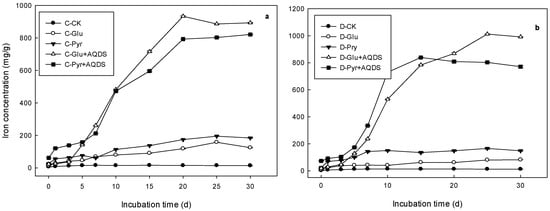
Figure 2.
Variations of Fe(II) concentration in microbial inoculation incubations of soils from marsh (a) and meadow (b) with different carbon sources (glucose and pyruvate) and AQDS. C is referred as Carex lasiocarpa marsh, and D as Deyeuxia angustifolia meadow. CK represented the control treatment, Glu for the glucose addition treatment, and Pyr for the pyruvate addition treatment.

Table 3.
Kinetic parameters of Fe(III) reduction in microbial inoculation incubations of the marsh and meadow following the addition of different carbon sources (glucose and pyruvate) and anthraquinone-2,6-disulphonate (AQDS).
3.3. pH Changes in Microbial Inoculation Incubations
As shown in Figure 3, pH in the control treatment was relatively stable in marsh (a) and meadow (b) wetlands, with values ranging from 6.2 to 6.4. However, with the addition of pyrite, glucose and electron shuttles, a marked reduction in pH was detected in both marsh and meadow over the first three-to-five days of anaerobic incubation, which was followed by a stable pH of six. With the addition of glucose, the pH in both marsh and meadow soil samples reached maximal levels of five. In addition, the pH of marsh soil samples decreased to a greater extent than meadow soils.
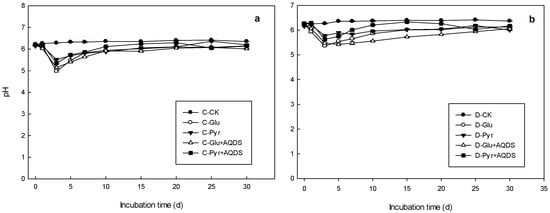
Figure 3.
Variations in the pH of microbial inoculation incubations of marsh (a) and meadow (b) soil samples with different carbon sources (glucose and pyruvic sodium) and AQDS.
4. Discussion
4.1. Soil Slurry Incubation
Our analyses detected significantly different dynamics and concentrations of Fe(III) reduction when compared between marsh and meadow wetlands. Using a soil slurry assay, we found that Fe(III) reduction in marsh wetlands responded more quickly and showed higher reductive activity (Figure 1). There may be several factors controlling the rates of iron reduction in soils. Firstly, these two kinds of wetlands differ in terms of soil structure. The C. lasiocarpa dominated marsh is characterized by a fibrous and brown root layer, a thin peat layer and a pale yellow and sticky gley soil layer in the soil profile. In contrast, in the D. angustifolia dominated meadow, the soil is a typical meadow marsh soil classified as Inceptisols in the US soil taxonomy classification system [20]. Therefore, soils particles, thermal properties, and the type and amount of substrates in soil are different (Table 1). These differences may lead to differences in their respective drivers and the microhabitat of iron reduction. Peat soils tend to be finely textured, have high thermal properties and can transfer more heat to iron reduction microorganisms [21]. Soil particle size and available surface area may also affect the bioavailability of Fe(III) oxides. Clay minerals could change the dissolved iron to a solid phase by absorbing charged Fe ions or colloids [22]. The proportion of clay in soils (<0.002 mm) at 0–20 cm was 2.52% in the marsh, and was 39.32% in the meadow; this would lead to less dissolved iron for reduction in meadow wetlands. Furthermore, more substrates, such as organic acids, phenolic compounds, carbohydrates, and labile carbon, are expected to be used by bacteria in peatland soil than iron reduction in the marsh soils. However, Todoroval et al. found that there was a negative correlation between iron reduction and organic matter contents [21]. The actual mechanisms underlying changes in organic matter in natural environments still need be determined.
Secondly, roots and rhizosphere microorganisms promote rapid enzymatic Fe(III) reduction in wetland soils [10]. A previous study found that root morphology and chemical compositions, such as length, biomass, surface area, root carbon, and nitrogen concentration, were different when compared between C. lasiocarpa and D. angustifolia [23]. Furthermore, it has been suggested that Fe(III) mineralogy, carbon availability, microbial community, and Fe plaques (root-associated Fe(III) mineral deposits) in the rhizosphere is different when compared between these two wetlands. Roots are likely to represent a relatively abundant source of organic chelators as electron shuttles maintaining Fe(III) in a soluble form or humic compounds that can be used as electron donors, which can influence the rates of Fe(III) reduction. Additionally, the leaf and culm mass decomposition of C. lasiocarpa and D. angustifolia was different [24], which may also affect Fe(III) reduction in these two wetlands.
Moreover, the concentration and phase of Fe(III) minerals are the main factors that control iron redox chemistry in soil environments. The frequent oxidation-reduction reactions in wetlands do not allow enough time for stable crystalline forms of Fe(III) oxides to form. Therefore, there is a tendency for amorphous and poorly crystalline forms of Fe(III) oxides to dominate wetlands [22]. Poorly crystalline minerals of Fe(III), such as ferrihydrite, are thought to be more reducible than a more crystalline phase. Based on soil characteristics, the relative proportions of easily reducible Fe and amorphous Fe in marsh wetlands are much higher than that in meadows (Table 1). The presence of more reducible Fe minerals leads to a greater content of ferrous iron. Therefore, a more significant microbial community of iron reduction, and a more suitable microhabitat, coupled with more available substrates in peatland, leads to a greater extent of Fe(III) reduction. To clarify the changes in soil microorganisms induced by organic carbon, research on microbial community abundance, and structures related to Fe(III) reduction should be further explored.
4.2. Microbial Inoculation Incubation
Dissimilatory Fe(III) reduction can be regulated by the addition of organic carbon. However, different compositions of organic carbon can affect degradation rates and related microbial communities, thus leading to differences in iron reduction activity. The addition of glucose and pyruvate can both promote the growth metabolism of iron reduction bacteria and rapidly accelerate the Fe(III) reduction process. In contrast, the lack of Fe(II) accumulation in our control treatment showed that an insufficient supply of electron donors could not stimulate Fe(III) reduction. Glucose had a higher capacity to transfer electrons to Fe(III) than pyruvate. While we were unable to find a similar study in wetlands, a similar conclusion was previously reported for paddy soils [25].
In contrast to the case of pyruvate, the microbial metabolism of glucose can yield actual electron donors, such as H2, pyruvate, acetate, and lactate. This can support Fe(III) reduction by providing preferential and abundant electron donors for Fe(III) reduction bacteria, and also for fermentative bacteria. Additionally, microbial dehydrogenation and hydrogen has been reported to couple with the fermentation of organic matter and play a significant role in microbial Fe(III) reduction [26,27]. With an increase in nutrient source after glucose additions, the abundance of fermentation reducers, such as Clostridium, Pseudomonas, and Bacillus also increased. Clostridium was the main form of bacterial species present after the consumption of O2 and the main representative of fermentative microbes [28]. Clostridium can produce a sufficient amount of H2 to act as an electron donor for the reduction of Fe(III).
Previous reports indicated that only a few dissimilatory iron reduction bacteria isolates were capable of using glucose directly [29]. Hydrogen-dependent iron reducing bacteria are most likely to represent the main microbes at the beginning of flooding and contribute predominantly to iron reduction [30]. Weber et al. [31] also found that the community composition of microbes changed between the early and late stages of flooding. In the early stage, fermentative microbes tend to be dominant; while in the late stage, syntrophic acetate and H2-utilizing methanogenic bacteria are more dominant. We should therefore pay more attention to separate and purify hydrogen-dependent iron reducing bacteria in the future.
Furthermore, the stable Fe(II) concentrations at the end of the incubation may indicate that intermediates transferred electrons to Fe(III) oxide, but not glucose directly. We also observed some bubbles in the glucose treatment, which may be a mixture of CO2 and H2 arising from the action of hydrogenase produced during glucose fermentation [32].
AQDS can influence soil biogeochemistry not only in an indirect manner by changing soil structure and chemistry, but also by directly mediating the electron transfer process by functioning as an electron shuttle [33]. AQDS as an electron shuttle between microbes and insoluble organic matter, can transfer more electrons to Fe(III), thus causing a distinct acceleration in the rate of Fe(III) reduction. In the presence of AQDS, complexation would render Fe(III) more accessible to micro-organisms. Our study showed that after the addition of AQDS, the Vmax was 30–50 times higher than in controls. Chen et al. [34] also found that Fe(III) reduction bacteria were enriched by the additional presence of AQDS.
Moreover, AQDS, which has the ability to form humic-metal complexes, can also influence the amount of reduction as a terminal electron acceptor [11,35]. However, the extremely high rates observed after the addition of AQDS in our study indicates that iron reduction was more limited by the availability of electron acceptors than by energy or mineral nutrients; this observation was also reported by Lipson et al. [6] in an Arctic peat soil.
When there was no exogenous carbon source, the physiological metabolism of iron reduction stagnated, so the pH in the control treatment remained stable. This also indicated that the process of Fe(III) reduction relies upon enzyme reactions. The increase in pH was largely due to the consumption of H+. Thereafter, pH tended to stable or increased by only a small amount. This may have been caused by the short-chain fatty acids released during mineralization in floods. The pattern of pH generally reflected a negative relationship between H+ and iron reduction.
5. Conclusions
Marsh wetlands exhibited higher Fe(III) reduction rates than meadow wetlands due to differences in soil structure, plant roots and the concentration, and phase of Fe(III) minerals. We suggest that glucose enrichments, characterized by the most predominant species, play an important role during iron reduction. Our study provides valuable information for the further isolation of iron reduction bacteria in pure culture. AQDS can dramatically increase potential Fe(III) reduction as an electron shuttle. Given the importance of wetland soils in the global carbon cycle, further research is now essential in exploring the enzymatic mechanisms of iron reduction.
Author Contributions
Conceptualization, X.Z. and M.J.; Methodology, X.Z. and M.J.; Software, Y.Y.; Validation, M.J.; Formal analysis, X.Z. and Y.Y.; Investigation, X.Z. and Y.Y.; Resources, X.Z.; Data curation, X.Z.; Writing—original draft preparation, X.Z.; Writing—review and editing, M.J.; Visualization, X.Z.; Supervision, M.J.; Project administration, M.J.; Funding acquisition, X.Z. and M.J.
Funding
This research was funded by “CPSF-CAS Joint Foundation for Excellent Postdoctoral Fellows, grant number 20150010”, “Science and Technology Development Project of Jilin Province, grant number 20170520081JH, 20180520100JH” and “National Natural Science Foundation of China, grant number 41601101, 41771120”.
Acknowledgments
We thank Xuehui Dong and Mingyu Sang assisting with the chemical analysis, and the editor and the anonymous reviewers for their constructive comments and time.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Martin, J.M.; Meybeck, M. Elemental mass-balance of material carried by major world rivers. Mar. Chem. 1979, 7, 173–206. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Fe(III) and Mn (IV) reduction. FEMS Microbiol. Rev. 1991, 55, 259–287. [Google Scholar]
- Myers, C.R.; Nealson, K.H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 1988, 240, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.A.; Urrutia, M.M.; Churchill, P.F.; Kukkadapu, R.K.; Roden, E.E. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ. Microbiol. 2006, 8, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Roden, E.E.; Wetzel, R.G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol. Oceanogr. 1996, 41, 1733–1748. [Google Scholar] [CrossRef]
- Lipson, D.A.; Jha, M.; Raab, T.K.; Oechel, W.C. Reduction of iron(III) and humic substances plays a major role in anaerobic respiration in an Arctic peat soil. Biogeosciences 2010, 115. [Google Scholar] [CrossRef]
- Luo, M.; Zeng, C.S.; Tong, C.; Huang, J.F.; Chen, K.; Liu, F.Q. Iron reduction along an inundation gradient in a tidal sedge (Cyperus malaccensis) marsh: The rates, pathways, and contributions to anaerobic organic matter mineralization. Estuar. Coast. 2015, 39, 1679–1693. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445. [Google Scholar] [CrossRef]
- Keller, J.K.; Weisenhorn, P.B.; Megonigal, J.P. Humic acids as electron acceptors in wetland decomposition. Soil Biol. Biochem. 2009, 41, 1518–1522. [Google Scholar] [CrossRef]
- Weiss, J.V.; Emerson, D.; Megonigal, J.P. Geochemical control of microbial Fe(III) reduction potential in wetlands: Comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiol. Ecol. 2004, 48, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Uchimiya, M.; Sposito, G. Iron(III) bioreduction in soil in the presence of added humic substances. Soil Sci. Soc. Am. J. 2009, 73, 65–71. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2009, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, A.; Behrens, S.; Kappler, A. Comparison of humic substance-and Fe(III)-reducing microbial communities in anoxic aquifers. Geomicrobiol. J. 2014, 31, 917–928. [Google Scholar] [CrossRef]
- Li, L.; Wenbing, T.; Guoan, W. Electron transfer mechanisms of humic substances and their environmental implications: A review. Environ. Chem. 2016, 35, 254–266. [Google Scholar]
- Liptzin, D.; Silver, W.L. Effects of carbon additions on iron reduction and phosphorus availability in a humid tropical forest soil. Soil Biol. Biochem. 2009, 41, 1696–1702. [Google Scholar] [CrossRef]
- Song, C.; Xu, X.; Tian, H.; Wang, Y. Ecosystem–atmosphere exchange of CH4 and N2O and ecosystem respiration in wetlands in the Sanjiang Plain, Northeastern China. Glob. Chang. Biol. 2009, 15, 692–705. [Google Scholar] [CrossRef]
- Song, C.; Liu, D.; Song, Y.; Yang, G.; Wan, Z.; Li, Y.; Xu, X. Effect of exogenous phosphorus addition on soil respiration in Calamagrostis angustifolia freshwater marshes of Northeast China. Atmos. Environ. 2011, 45, 1402–1406. [Google Scholar] [CrossRef]
- Hou, C. Effects of Hydrological Changes on Soil Carbon Sequestration of Marsh in the Sanjiang Plain. Ph.D. Thesis, Graduate School of Chinese Academy of Sciences, Changchun, China, 2012. [Google Scholar]
- Lovley, D.R.; Phillips, E.J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [PubMed]
- Mao, R.; Song, C.; Zhang, X.; Wang, X.; Zhang, Z. Response of leaf, sheath and stem nutrient resorption to 7 years of N addition in freshwater wetland of Northeast China. Plant Soil 2013, 364, 385–394. [Google Scholar] [CrossRef]
- Todorova, S.G.; Siegel, D.I.; Costello, A.M. Microbial Fe(III) reduction in a minerotrophic wetland–geochemical controls and involvement in organic matter decomposition. Appl. Geochem. 2005, 20, 1120–1130. [Google Scholar] [CrossRef]
- DeLaune, R.D.; Reddy, K.R. Biogeochemistry of Wetlands: Science and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 405–443. [Google Scholar]
- Shi, F.; Song, C.; Zhang, X.; Mao, R.; Guo, Y.; Gao, F. Plant zonation patterns reflected by the differences in plant growth, biomass partitioning and root traits along a water level gradient among four common vascular plants in freshwater marshes of the Sanjiang Plain, Northeast China. Ecol. Eng. 2015, 81, 158–164. [Google Scholar] [CrossRef]
- Zhang, X.; Song, C.; Mao, R.; Yang, G.; Tao, B.; Shi, F.; Zhu, X.; Hou, A. Litter mass loss and nutrient dynamics of four emergent macrophytes during aerial decomposition in freshwater marshes of the Sanjiang plain, Northeast China. Plant Soil 2014, 385, 139–147. [Google Scholar] [CrossRef]
- Yi, W.; Wang, B.; Qu, D. Diversity of isolates performing Fe(III) reduction from paddy soil fed by different organic carbon sources. Afr. J. Biotechnol. 2012, 11, 4407–4417. [Google Scholar]
- Balashova, V.V.; Zavarzin, G.A. Anaerobic reduction of ferric iron by hydrogen bacteria. Mikrobiologiia 1979, 48, 773–778. [Google Scholar] [PubMed]
- Li, L.; Qu, Z.; Wang, B.; Qu, D. Dynamics of the abundance and structure of metabolically active Clostridium community in response to glucose additions in flooded paddy soils: Closely correlated with hydrogen production and Fe(III) reduction. J. Soil Sediments 2017, 17, 1727–1740. [Google Scholar] [CrossRef]
- Lüdemann, H.; Arth, I.; Liesack, W. Spatial changes in the bacterial community structure along a vertical oxygen gradient in flooded paddy soil cores. Appl. Environ. Microbiol. 2000, 66, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Coates, J.D. Novel forms of anaerobic respiration of environmental relevance. Curr. Opin. Microbiol. 2000, 3, 252–256. [Google Scholar] [CrossRef]
- Jia, R.; Li, L.; Qu, D. pH shift-mediated dehydrogenation and hydrogen production are responsible for microbial iron(III) reduction in submerged paddy soils. J. Soil Sediments 2015, 15, 1178–1190. [Google Scholar] [CrossRef]
- Weber, S.; Stubner, S.; Conrad, R. Bacterial populations colonizing and degrading rice straw in anoxic paddy soil. Appl. Environ. Microbiol. 2001, 67, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qu, D. Dissimilatory Fe(III) reduction characteristics of paddy soil extract cultures treated with glucose or fatty acids. J. Environ. Sci. 2008, 20, 1103–1108. [Google Scholar] [CrossRef]
- Kappler, A.; Benz, M.; Schink, B.; Brune, A. Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol. Ecol. 2004, 47, 85–92. [Google Scholar] [CrossRef]
- Chen, M.; Shih, K.; Hu, M.; Li, F.; Liu, C.; Wu, W.; Tong, H. Biostimulation of indigenous microbial communities for anaerobic transformation of pentachlorophenol in paddy soils of southern China. J. Agric. Food Chem. 2012, 60, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.J.; Van Der Velde, S.; Lettinga, G.; Field, J.A. Quinones as terminal electron acceptors for anaerobic microbial oxidation of phenolic compounds. Biodegradation 2000, 11, 313–321. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).