Abstract
Introduction: Total hip replacements and total knee replacements are among the most frequently performed operations worldwide, and the demand for such procedures is ever-growing. It is essential to focus on preventable medical complications that can arise from these procedures, specifically postoperative hyponatraemia. Postoperative hyponatraemia has an incidence of 20–40% in total hip and knee replacement patient cohorts. Even mild postoperative hyponatraemia is clinically relevant, as it is associated with cognitive impairment and gait disturbance and may undermine the aims of enhanced recovery protocols. Severe postoperative hyponatraemia can lead to seizures, coma, intensive care admission, and death. Although uncommon, the high volume of patients treated in busy orthopaedic centres means such cases will inevitably be encountered. This narrative review summarises the current evidence on incidence, risk factors and consequences of postoperative hyponatraemia in total hip and knee replacement populations. Methods: A literature review was performed through the EBSCO and PubMed databases to identify relevant studies. Key search terms included were “hyponatraemia”, “total hip replacement”, and “total knee replacement”. Results: The incidence of postoperative hyponatraemia is largely between 20% and 40%; however, there are some outliers to this. Multiple risk factors have been identified through observational studies, including age, preoperative hyponatraemia, female sex and certain medications, which signal a need for a risk stratification strategy that can assist in preoperative assessment and the early identification of patients at higher risk of developing postoperative hyponatraemia. Evidence is scarce regarding interventional studies for the prevention and management of postoperative hyponatraemia, despite multiple studies highlighting the issue. Conclusion: Future work should focus on testable, quality improvement interventions, such as automatic sodium checks on postoperative day one, weight-based oral fluid protocols, oral salt supplementation, and escalation pathways for high-risk patients. Incorporating these into enhanced recovery frameworks has the potential not only to optimise safe early discharge for the majority but also to prevent rare but significant complications.
1. Introduction
Total hip replacements (THRs) and total knee replacements (TKRs) are amongst the most frequently performed surgeries worldwide, and their numbers are steadily rising. In the United Kingdom (UK) alone, it is projected that both procedures will increase by 40% by 2060, as compared to 2018 [1]. This is primarily driven by an ageing population and growing prevalence of osteoarthritis, and this trend is seen globally, with increasing demands for THR and TKR seen within the United States of America [2,3,4]. Given this ever-growing demand for THR and TKR, attention should continue to focus on the preventable medical and surgical complications that occur. Jørgensen et al. [5] concluded that a relatively small group of patients, identifiable by specific risk factors, account for the majority of potentially preventable medical complications following THR and TKR, highlighting the importance of targeted perioperative strategies.
Within the evolving field of enhanced recovery, same-day discharge has become a key goal. Whilst this is not yet standard practice for all in the UK, it has been successfully adopted for suitable patients in some centres and multiple centres internationally [6]. As the number of day-case procedures is ever growing, there are concerns regarding the incidence of complications compared to inpatient procedures and the delay in which these complications will be recognised. Wainwright et al. [7] highlighted that while day-case procedures are attainable for lower-risk patients, there is room for further research regarding risk stratification models to identify “high-risk” patients for whom a slightly longer hospital stay may be appropriate. Although THR and TKR are highly effective in reducing pain and restoring patient quality of life, the increasing number of patients undergoing these procedures emphasises the importance of safe clinical practice perioperatively to minimise the risk of complications, which may undermine patient recovery.
One critical aspect of perioperative safety is the management of fluid balance and electrolytes, as inappropriate interventions can result in electrolyte disturbances, such as hyponatraemia. Factors such as surgical stress, anaesthesia, and inadequate management of postoperative pain and nausea [8,9,10] can contribute to the development of Syndrome of Inappropriate Antidiuretic Hormone (SIADH), a condition in which the release of antidiuretic hormone is stimulated despite no osmotic trigger, resulting in water retention and dilutional hyponatraemia [9].
Hyponatraemia is defined as a sodium level less than 135mmol/L [11] and is one of the most common electrolyte abnormalities noted in clinical practice [12,13]. The current literature demonstrates that hyponatraemia is a factor for increased mortality risk within general patient populations, with ongoing debate as to whether this is a direct effect or an indicator of illness severity [13,14,15]. Importantly, regarding surgical patients, it has been noted that postoperative hyponatraemia (POH) plays a role in increased mortality and morbidity risk [15,16,17], increased length of stay (LOS) [16,18,19,20,21], increased cost [18] and increased readmission rates [22]. Severe hyponatraemia can manifest as confusion, lethargy and seizures; however, even mild hyponatraemia can result in patients having cognitive impairment, gait disturbance and increased fall risk [11]. Once hyponatraemia is identified in a patient, discharge can be delayed until this stabilises or improves to a safe level. Consequently, it can be appreciated that even mild POH can undermine the aims of enhanced recovery pathways, which are targeted at optimising patient recovery to accelerate the achievement of discharge criteria.
The incidence of hyponatraemia postoperatively in THR and TKR patients is generally between 20 and 40% [19,20]; however, despite this, there has been little attention focused on the prevention and management of postoperative hyponatraemia in orthopaedic patients, particularly within the context of enhanced recovery pathways. It has been reported that patients undergoing THR and TKR are becoming increasingly frail [23] and given the combination of an elderly, comorbid patient population, widespread use of medications known to be predisposed to hyponatraemia, and the evolution of enhanced recovery pathways to day-case procedures, this topic is particularly relevant to current-day practice. We anticipated that POH is common in elective THR and TKR populations and that existing studies would identify consistent risk factors and clinical consequences, along with highlighting gaps in management within enhanced recovery pathways.
2. Materials and Methods
An initial scoping review of the available literature established that specific research in this area was limited and too methodologically heterogeneous to perform a formal systematic review. Therefore, a narrative review approach was undertaken for the analysis, underpinned by a rigorous literature search. Studies included in this review were identified through the EBSCO database (including MEDLINE/CINAHL) and PubMed. Key search terms included “hyponatraemia”, “total hip replacement”, and “total knee replacement” to ensure a broad search reach (Table 1).

Table 1.
Search strategy concepts for narrative review of postoperative hyponatraemia in total hip and knee replacements. * is a wildcard to find variations of words starting with hypon.
Initial screening of the study titles and abstracts was conducted, followed by an in-depth review of the full text by two separate authors. Studies included were those published between 1 January 2000 and 15 September 2025, with the aim of reviewing data collected from perioperative pathways in keeping with contemporary surgical practice and enhanced recovery pathways. The reference lists from articles found in the literature search were then reviewed to identify any further relevant studies (Figure 1).
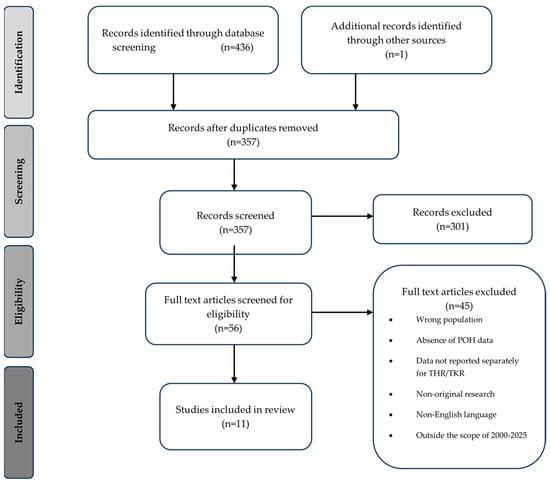
Figure 1.
Flow diagram demonstrating the identification and selection of studies included in the narrative review of postoperative hyponatraemia in total hip and knee replacement.
Given the small and heterogenous evidence base, all available original research was included, as restricting study types would have excluded clinically relevant information. Studies had to be in English and published between 2000 and 2025. Exclusion criteria are listed in Figure 1. As this was a narrative review PRISMA, full search strings, dual screening, or formal risk-of-bias assessment are not required. This is due to narrative reviews differing fundamentally from systematic reviews in purpose, structure, and methodological requirements and are used when the available evidence is limited or methodologically heterogeneous, which is the case in this topic area [24].
3. Discussion
A total of 11 studies met the inclusion criteria (Table 2 and Table 3). The available evidence is limited in quantity and quality, as most studies are retrospective, single-centre cohorts with heterogenous definitions of POH and variable timings and frequency of sodium monitoring. One interventional study was identified, and this was a service evaluation study rather than a controlled trial. Sample sizes ranged from 189 to 3071 patients, and most studies were focused on mixed THR and TKR populations. Biochemical definitions of POH varied, but the majority used a serum sodium of <135 mmol/L. To ensure clarity and that the aims of the narrative review were achieved, the findings were categorised into five themes:

Table 2.
Summary of studies demonstrating the incidence and severity of postoperative hyponatraemia in total hip and knee replacement.

Table 3.
Summary of studies examining risk factors and outcomes of postoperative hyponatraemia in total hip and knee replacement.
- Definition and classification of hyponatraemia.
- Incidence of postoperative hyponatraemia.
- Risk factors.
- Clinical consequences.
- Prevention and management strategies.
3.1. Definition and Classification of Hyponatraemia
Hyponatraemia is classed as a sodium (Na) level <135 mmol/L [11]. It is one of the most common electrolyte imbalances encountered in hospitalised patients and can be classified by biochemical severity, fluid status and duration (Table 4):

Table 4.
Classification of hyponatraemia by biochemical severity, fluid status and duration—associated clinical features and causes are described where appropriate [11,33].
3.2. Incidence of Hyponatraemia in THR and TKR
Whilst hyponatraemia is a common postoperative complication for elective THR and TKR patients with, its reported incidence is variable, and this may be attributed to the methodological heterogeneity across the available studies, including differing thresholds for defining hyponatraemia, variability in the timing of when sodium measurements were taken, and the decision to take sodium measurements routinely or selectively.
In the broader surgical context, POH has been described across a range of surgical specialities, with incidence figures generally reported to be between 15 and 40% [34,35,36]. One study focused on spinal surgery in the elderly population reported an incidence of POH of 15.8%, resulting in an average two-day longer inpatient stay [36]. Neurosurgical and cardiothoracic patients have reported vastly higher rates of 56.6% [35] and 59% [16], respectively. In the neurosurgical cohort, this is secondary to SIADH and cerebral salt-wasting syndrome [37]; meanwhile, in cardiothoracic patients, this contributed to significant perioperative fluid shifts, longer inpatient stays and critical illness [16]. This highlights that POH is a frequent postoperative complication across multiple specialities; however, the patient demographic experienced in elective TKR and THR justifies further evaluation in this review.
Cunningham et al. [20] found an incidence of 21.7% (mild 81.6%, moderate 17.1%, severe 1.4%) in a large retrospective study of 1000 patients, in which serum sodium levels were measured on postoperative day (POD) 1 and POD 2. The standardised measurement of sodium levels optimised the detection of early POH; however, event recognition post-POD 2 may be incomplete and late-onset POH may be underestimated. Furthermore, the retrospective design does not allow for control over confounding factors, such as perioperative medication management and fluid protocols. Cunningham et al. [20] have acknowledged that the missing data for certain variables was a limitation of their study, which may impact the significance of the identified risk factors. Despite these reservations, the incidence rate and severity profile of these cases are consistent with other available literature in the field, supporting the clinical relevance of the studies.
Sah et al. [30] found a higher incidence of 40% (mild: 81.93%, moderate: 14.19%, severe: 3.87%) in a large prospective study, and most commonly identified POH on day 1. The prospective design minimised missing data and allowed for improved control over the exposure definitions. Serum sodium levels were checked daily for inpatients; therefore, the higher incidence may be attributed to this, as late-onset POH may have been captured for those with delayed discharge.
An outlier to these findings is the study by Murkartihal et al. [31], who found an incidence of 84.9%. Several features of this patient cohort should be taken into consideration when understanding this unusually high rate of incidence. This was a single tertiary centre retrospective study based in India, in which patients were admitted to an intensive treatment unit (ITU) postoperatively. This practice does not align with multimodal enhanced recovery pathways, which aim to minimise the surgical stress response and accelerate the achievement of discharge criteria. To illustrate this, discharge was planned for POD 12 once the staples were removed, which is in contrast to the earlier discharges that enhanced recovery pathways facilitate. Prolonged admission suggests recovery for patients was slower, and it may also have resulted in prolonged monitoring of electrolytes and allowed for recognition of cases that would go undetected in patients discharged on the enhanced recovery pathways. For these reasons, the findings of Murkartihal et al. [31] may not be directly comparable to patient cohorts who are managed under enhanced recovery strategies, and their results should be interpreted cautiously within the context of this narrative review.
Despite multiple studies highlighting the impact and consequences of POH, there is only one published study that has attempted to develop an intervention to assist in the management of these patients. Waller et al. [32] performed a closed-loop audit, which demonstrated that the introduction of a direct endocrine referral pathway reduced the incidence of POH after THR and TKR. Furthermore, it was noted that the severity of POH cases that arose from the use of the referral pathway was reduced. Within the first cycle of the audit, 11% of POH patients required ITU admission; however, with the implementation of the referral pathway, no ITU admissions were required.
Although the majority of POH cases following THR and TKR are mild, a small but important proportion progress to severe hyponatraemia (Table 3). Severe cases carry a markedly higher risk of serious outcomes, including the need for intensive care admission, the development of osmotic demyelination syndrome during correction, and death [15,17,32]. While the absolute percentage of patients who develop severe POH is relatively low, the very large numbers of THR and TKR procedures performed each year mean that these complications are encountered in busy orthopaedic units. Therefore, prevention not only focuses on improving recovery and supporting early discharge for the majority of patients but also prevents potentially fatal severe hyponatraemia.
3.3. Risk Factors for Postoperative Hyponatraemia
Several risk factors have been identified that can contribute to the development of POH in THR and TKR patients, including patient-related characteristics, medication use, surgical and anaesthetic factors, perioperative fluid management, and enhanced recovery-specific practices (Table 4).
3.3.1. Patient Demographics
Studies have consistently demonstrated that older age is an independent risk factor for developing POH [19,20,21,25,26,29,30,31,32]. It has been observed that there is an age-related reduction in glomerular filtration rate, resulting in the impaired ability to excrete water and increased susceptibility to hypoosmolality and hyponatraemia [38]. It has been repeatedly confirmed that osmoreceptor sensitivity is enhanced in the elderly, triggering increased secretion of antidiuretic hormone (ADH) [39,40]. There is an age-related decrease in the percentage of total body water, as muscle mass reduces with age. This, in turn, can produce greater fluctuations in serum sodium levels, due to the inversely proportional relationship between serum sodium and total body water [38,41]. This mechanism may also explain why patients with a lower body weight are at an increased risk of POH. Female sex has also been identified as a risk factor [30,31], possibly secondary to increased sensitivity to water retention and sodium dilution secondary to increased receptors to ADH in the renal collecting ducts [42]. However, it should be noted that there are studies that have not found sex to be a risk factor [19,20,21,25,27,28,29,32].
3.3.2. Comorbidities and Medications
Several commonly prescribed medications have been linked to increased risk of POH. Most notedly so are thiazide diuretics [19,30,31,32], which impair renal sodium reabsorption and have been linked with hyponatraemia in the older population outside of surgical studies [43,44]. Angiotensin-converting-enzyme inhibitors have been identified as a risk factor [30,31]; it was highlighted that whilst these medications may not present with a hyponatraemia in the preoperative period, they can predispose patients to developing a POH in combination with surgical stress and fluid balance abnormalities. Beta blockers have been associated with POH in elective orthopaedic cohorts [28]; however, the mechanism for this is undefined. It should be highlighted that in general populations, certain beta blockers have been recognised to cause hyponatraemia in the initiation phase of treatment, whilst others do so regardless of their treatment duration [45]. Furthermore, the associations between these cardiovascular medications and the development of hyponatraemia may not be solely due to the medications but also the conditions for which they treat, primarily hypertension and heart failure.
Selective serotonin reuptake inhibitors (SSRIs) and proton pump inhibitors (PPIs) have also been implicated as risk factors for developing hyponatraemia; however, this has not been reflected in an elective orthopaedic cohort. Despite this, one study in traumatic hip fracture patients demonstrated an association between SSRIs and POH [46]; the risk of developing hyponatraemia with SSRIs is associated with initiation of treatment [47]. Cunningham et al. [20] reported that nearly half of the patients who developed POH were taking a PPI in the perioperative period; however, this relationship was statistically insignificant in multivariate analysis. It has been well established in the literature that PPIs can result in patients developing hyponatraemia [46,48,49]. Therefore, despite the statistical insignificance of the relationship noted in the Cunningham et al. [20] study, this is a possible association worth monitoring.
Preoperative hyponatraemia and lower sodium levels have been identified as a risk factor for developing POH in multiple studies [19,20,26,30,31]. The underlying mechanism is not fully defined; therefore, it is hypothesised that increasingly frail or comorbid patients, combined with perioperative factors such as SIADH and fluid shifts, make them more vulnerable to sodium fluctuations and may accelerate the onset and severity of POH [38,41].
3.3.3. Surgical and Anaesthesia
It has been well-established in the literature that the physiological stress of surgery stimulates a secretion of ADH, which may persist for three to five days depending on the amplitude of surgical stress experienced [50]. Anaesthetic procedures may also play a role, as demonstrated by Baker et al. [21], identifying that general anaesthesia was a risk factor for POH. Spinal anaesthesia can trigger sympathetic blockade and vasodilation [51], often requiring additional fluid boluses to maintain blood pressure. In contrast, general anaesthesia amplifies the surgical stress response, with direct effects on ADH secretion and perioperative fluid balance [52]. Volume of blood loss and the requirement for blood transfusion have been identified as risk factors for POH [28,29], likely secondary to haemodilution and stimulation of ADH to replace lost volume.
Waller et al. [32] showed that POH was more common in patients who underwent TKR than THR. The available literature is divided on whether there is a procedure-related risk associated with POH, as certain studies [26,30,31] have demonstrated similar results to those of Waller et al, whereas other studies have found no significant difference between the two procedures [20,21,25,28]. This discrepancy illustrates the importance of further large-scale prospective studies to clarify incidence rates and the risk between THR and TKR. Furthermore, bilateral knee arthroplasty was identified as a risk factor for POH [29,31]. This may be secondary to increased operative time, fluid administration and surgical stress.
3.3.4. Fluid Management
Careful selection of perioperative fluids and monitoring of fluid balance is imperative in mitigating the risk of developing POH. Hypotonic intravenous fluids, such as those containing dextrose, can potentiate hyponatraemia initially triggered by the stress response. Dextrose is rapidly metabolised on infusion and acts as water [53,54]. Therefore, it can be appreciated that in elderly, fragile patients, small changes in total body water can result in significant changes in serum sodium, potentially precipitating or propagating POH.
In contrast, isotonic crystalloids, including 0.9% sodium chloride and balanced solutions, such as Hartmann’s solution, have osmolarities parallel to that of plasma and are designed to maintain intravascular volume without the risk of dilutional hyponatraemia associated with hypotonic solutions [55]. Balanced solutions also contain buffers, which act to maintain normal acid-base balance. 0.9% sodium chloride has similar benefits to Hartmann’s solution; however, it contains an excess of chloride compared to human plasma. As a result, there is a risk of hyperchloraemic metabolic acidosis when administered in large volumes [55].
Perioperative fluid strategy is another priority. Goal-directed fluid therapy is a perioperative strategy in which intravenous fluids are titrated according to haemodynamic parameters to achieve euvolaemia [56,57]. It has been shown in abdominal surgery to reduce complications and accelerate recovery [56]; however, evidence in orthopaedic surgery is limited. Best practice is ill-defined and is generally on a surgeon-by-surgeon basis; however, there is growing evidence that urinary catheters are not required for those undergoing THR and TKR with spinal anaesthetic. This limits the risks of developing a urinary tract infection and optimises postoperative recovery and engagement with rehabilitation. Secondary to this, intraoperative intravenous fluids are minimised to avoid postoperative urinary retention [58]. In conjunction with the enhanced recovery pillar of early oral intake postoperatively, these patients return to drinking early postoperatively, intending to achieve euvolaemia [59].
3.4. Clinical Consequences of Hyponatraemia
POH can impact recovery and rehabilitation, neurological function, hospital resource use, and overall patient outcomes. In THR and TKR patients, who are often elderly, frail, and taking multiple medications, even mild hyponatraemia may carry significant clinical implications.
Rehabilitation is particularly affected. Early mobilisation is central to successful recovery after THR and TKR, helping to reduce complications and achieve fast-track discharge. Patients who develop POH may present with weakness, dizziness, or confusion [11], resulting in limited ability to engage in physiotherapy. This delays progress towards discharge and may have knock-on effects on long-term functional outcomes.
An increase in LOS has been consistently demonstrated in patients with POH [21,25,30]. Haider et al. [25] reported a median LOS increase of two days compared with normonatraemic patients, and similar findings have been observed in other retrospective analyses.
The relationship between hyponatraemia, morbidity, and mortality has been reported across surgical and medical populations [15,16,17], although it remains uncertain whether this reflects a causal role or marks frailty and comorbidity. Studies have demonstrated that most cases are mild to moderate (Table 3), yet even these can be clinically significant, especially in older, frailer patients.
Neurological manifestations are perhaps the most immediate concern. Acute hyponatraemia may cause confusion, agitation, impaired concentration, or delirium; in severe cases, seizures, coma, or death can occur [11]. Even mild cases can affect cognition and balance, increasing fall risk during the early mobilisation phase [11]. As early mobilisation is a cornerstone of enhanced recovery protocols, any neurological compromise undermines progress and delays discharge readiness.
3.5. Prevention and Management Strategies
The prevention and management of POH in THR and TKR patients requires a collective approach, combining preoperative risk recognition, perioperative fluid optimisation, and alignment with enhanced recovery strategies. Within THR and TKR cohorts, there is a paucity of interventional studies regarding POH. A closed-loop audit in which a direct endocrinology referral pathway was implemented, alongside predefined sodium monitoring, demonstrated a reduction in both the incidence and severity of POH, whilst also eliminating the need to escalate patients to critical care [32]. This demonstrates the potential impacts of interventional studies and highlights the requirement for further prospective studies.
Multiple papers have highlighted the need for a risk stratification model to identify the patients who would be considered at high risk of POH [24,60,61,62]. Currently, there is no validated model available for use in orthopaedic patients; however, use of risk stratification could identify high-risk patients with the potential for preoptimisation before surgery. Preoptimisation could potentially include the involvement of endocrinology to address pre-existing hyponatraemia, reviewing medication and educating at-risk patients on oral fluid intake targets in the immediate postoperative period.
The role of risk stratification needs to be considered further, because the predictive value of identified risk factors is limited, owing to the patient cohort for THR and TKR. These patients are generally older, more likely to be frail and taking multiple medications, so it could be considered that many of these patients would likely be classed as “high-risk”. Thus, standard care for arthroplasty patients should treat all patients as if they are at risk of POH. The only benefit of risk stratification in terms of pathway design is identifying patients who are low-risk, who might not need to adhere to all aspects of standard care, or who might be more suitable for a day case.
3.6. Relevance to Enhanced Recovery Pathways
Enhanced recovery pathways are designed to optimise patient recovery, reduce the risk of complications and allow early identification of delayed recovery via evidence-based recommendations. Several key components of enhanced recovery directly intersect with the risk of developing POH and its consequences. In reality, many aspects of orthopaedic enhanced recovery have developed from trial and error, or adopted from other types of surgery, rather than from research. Several key components of enhanced recovery directly relate to the risk of developing POH, and yet there is minimal evidence to support these practices in orthopaedics. For example, the role of intravenous fluid therapy and, in particular, the interplay between the use of intravenous fluids and the need for urinary catheterisation are likely to have a significant bearing on the genesis of POH but lack any evidence base. Whilst early oral intake has been demonstrated to be of benefit following gastrointestinal surgery, the volume of oral fluid intake and the nature of that fluid have not been studied for arthroplasty patients, despite a clear relevance with respect to POH.
Optimised fluid therapy is a pillar of enhanced recovery, ensuring patients achieve euvolaemia; however, inappropriate fluid strategies or administration of hypotonic fluids can precipitate the development of hyponatraemia [55,63]. Multimodal analgesia protocols are aimed at using a combination of analgesics to manage postoperative pain, commonly involving non-steroidal anti-inflammatory drugs, opiates and other adjunctive medications, some of which can promote an SIADH response [64,65]. Postoperative management of nausea and vomiting is another cornerstone of enhanced recovery strategies, as it can improve patients’ ability to return to adequate nutrition, hydration and mobilisation early. Furthermore, it has been associated with patient morbidity and prolonged LOS. Excessive vomiting would result in excess water and sodium loss, possibly potentiating POH. Early oral intake is aimed at restarting oral intake within 24 h postoperatively and may reduce postoperative complications and LOS, whilst aiding in bowel recovery postoperatively [66]. Early patient mobilisation is a critical component of the enhanced recovery pathway and has been shown to reduce the risk of postoperative complications and patient deconditioning [67]. Wainwright et al. [58] advise patients undergoing THR and TKR should “mobilise as early as they are able to in order to facilitate early achievement of discharge criteria”. POH can hinder the aims of the enhanced recovery protocol, as physical manifestations of such a condition include confusion, weakness and increased risk of falls.
Most available studies were conducted in traditional inpatient settings with longer hospital stays, routine postoperative blood tests, and fluid practices that differ from modern enhanced recovery protocols. This may impact the reported incidence of POH, as longer admission and routine daily labs in older cohorts likely increased detection of POH. In contrast, enhanced recovery pathways, with earlier discharge and less routine testing, may underestimate its actual incidence. Furthermore, identified contributors to POH observed in historical inpatient studies, such as prolonged intravenous fluids and delayed oral intake, may be less applicable within enhanced recovery pathways, which emphasise early mobilisation, early drinking, and minimal intravenous fluids.
3.7. Gaps in Knowledge and Future Research Directions
Current evidence on POH in THR and TKR cohorts remains limited. Most studies are retrospective and single-centre, with variability in monitoring practices, leading to inconsistent incidence rates and uncertainty around when and how to intervene. Large, multi-centred prospective studies are lacking and would allow further clarification of incidence rates, risk factors and patient outcomes.
There is also no agreed pathway for screening or management. Questions remain over which patients should be monitored, appropriate monitoring protocols, and thresholds for escalation. Preventive measures such as balanced crystalloids, avoidance of hypotonic fluids, and medication review are logical and low cost, but have not been tested together as a structured bundle in orthopaedics. The development of consensus guidelines integrated into enhanced recovery protocols relevant to this population would be highly valuable (Table 5).

Table 5.
Practical recommendations for prevention and management of POH in THR and TKR.
Looking forward, digital tools and risk-prediction models may help identify high-risk patients and prompt timely monitoring or referral. This would support consistent practice in a busy and variable ward environment, though there is not yet a validated model within orthopaedics. Finally, there is clear potential for quality improvement projects, for example, testing sequential changes in weight-based fluid and electrolyte management and embedding them into routine enhanced recovery care. These initiatives may aid in standardising the monitoring and management of POH and align with the enhanced recovery principles by reducing preventable complications.
3.8. Strengths and Limitations
This review has several strengths, as it focuses on a clinically important but relatively under-valued post-surgical complication in a high-volume surgical population and considers this within the context of modern enhanced recovery pathways. A structured literature search was performed and the data was grouped across incidence, risk factors, consequences and management to ensure all aspects of the topic were covered.
However, it is important to note the limitations of the review. Most of the included studies were retrospective observational cohorts, often single-centre, and primarily conducted within inpatient settings without clearly defined enhanced recovery pathways; therefore, their applicability to the modern concept of fast-track recovery is uncertain. Definitions of POH, timing and frequency of sodium level measurements varied widely between studies, which limited direct comparison and did not allow for meta-analysis. Finally, methodological heterogeneity of the included studies precluded a systematic review being performed. Secondary to this, a formal risk assessment bias was not performed.
4. Conclusions
Hyponatraemia is a common and clinically relevant postoperative complication following total hip and knee replacement, yet it remains largely preventable. The prevention and management of POH may be best managed in a multi-disciplinary approach, as it would likely improve consistency in recognition and management; however, implementation may be challenging in busy hospital environments, due to frequent changes in ward staffing and the knock-on effect on adherence to protocols. Given the consistently high incidence reported across elective THR and TKR cohorts, it is reasonable to assume that many patients are inherently at risk of developing postoperative hyponatraemia. Therefore, further evaluation of perioperative pathways needs to take place. One potential approach worth exploring is the design that all patients warrant routine monitoring and prevention strategies, with risk stratification used to identify those who may safely require less intensive surveillance. Despite growing awareness of the risks and consequences of POH, interventional studies in this cohort remain scarce. There is a clear rationale for further quality improvement initiatives focused on structured, standardised fluid management protocols, embedded within enhanced recovery frameworks, to support early discharge while prioritising patient safety. However, these require testing, and definitive recommendations cannot be made until higher-quality evidence exists.
Author Contributions
Conceptualization, J.C. and R.G.M.; methodology, L.T., J.C., T.W.W. and R.G.M.; formal analysis, L.T. and J.C.; investigation, J.C. and R.G.M.; data curation, J.C. and R.G.M.; project administration, J.C., T.W.W. and R.G.M.; visualisation, L.T.; supervision, T.W.W. and R.G.M.; writing—original draft preparation, L.T.; writing—review and editing, L.T., J.C., T.W.W. and R.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| POH | Postoperative hyponatraemia |
| THR | Total hip replacement |
| TKR | Total knee replacement |
| SIADH | Syndrome of inappropriate antidiuretic hormone |
| Na | Sodium |
| POD | Postoperative day |
| SSRI | Selective serotonin reuptake inhibitors |
| PPI | Proton pump inhibitors |
| LOS | Length of stay |
| ITU | Intensive treatment unit |
References
- Matharu, G.S.; Culliford, D.J.; Blom, A.W.; Judge, A. Projections for primary hip and knee replacement surgery up to the year 2060: An analysis based on data from The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Ann. R. Coll. Surg. Engl. 2022, 104, 443–448. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Jørgensen, C.C.; Petersen, M.A.; Kehlet, H. Preoperative prediction of potentially preventable morbidity after fast-track hip and knee arthroplasty: A detailed descriptive cohort study. BMJ Open 2016, 6, e009813. [Google Scholar] [CrossRef]
- Vehmeijer, S.B.W.; Husted, H.; Kehlet, H. Outpatient total hip and knee arthroplasty. Acta. Orthop. 2018, 89, 141–144. [Google Scholar] [CrossRef]
- Wainwright, T.W.; Memtsoudis, S.G.; Kehlet, H. Fast-track hip and knee arthroplasty...how fast? Br. J. Anaesth. 2021, 126, 348–349. [Google Scholar] [CrossRef]
- Oh, G.J.; Sutherland, S.M. Perioperative fluid management and postoperative hyponatremia in children. Pediatr. Nephrol. 2016, 31, 53–60. [Google Scholar] [CrossRef]
- Mentrasti, G.; Scortichini, L.; Torniai, M.; Giampieri, R.; Morgese, F.; Rinaldi, S.; Berardi, R. Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH): Optimal Management. Ther. Clin. Risk Manag. 2020, 16, 663–672. [Google Scholar] [CrossRef]
- Pillai, B.P.; Unnikrishnan, A.G.; Pavithran, P.V. Syndrome of inappropriate antidiuretic hormone secretion: Revisiting a classical endocrine disorder. Indian J. Endocrinol. Metab. 2011, 15, S208–S215. [Google Scholar] [CrossRef]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol. Dial. Transplant. 2014, 29, i1–i39. [Google Scholar] [CrossRef]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Incidence and prevalence of hyponatremia. Am. J. Med. 2006, 119, S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.; Huda, B.; Boyd, A.; Skagen, K.; Wile, D.; Watson, I.; van Heyningen, C. Characteristics and mortality of severe hyponatraemia—A hospital-based study. Clin. Endocrinol. 2006, 65, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Mount, D.B.; Curhan, G.C. Mortality after Hospitalization with Mild, Moderate, and Severe Hyponatremia. Am. J. Med. 2009, 122, 857–865. [Google Scholar] [CrossRef]
- Asadollahi, K.; Beeching, N.; Gill, G. Hyponatraemia as a risk factor for hospital mortality. QJM 2006, 99, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Crestanello, J.A.; Phillips, G.; Firstenberg, M.S.; Sai-Sudhakar, C.; Sirak, J.; Higgins, R.; Abraham, W.T. Postoperative Hyponatremia Predicts an Increase in Mortality and In-Hospital Complications after Cardiac Surgery. J. Am. Coll. Surg. 2013, 216, 1135–1143. [Google Scholar] [CrossRef]
- Upadhyay, U.M.; Gormley, W.B. Etiology and Management of Hyponatremia in Neurosurgical Patients. J. Intensive Care Med. 2012, 27, 139–144. [Google Scholar]
- Hennrikus, E.; Ou, G.; Kinney, B.; Lehman, E.; Grunfeld, R.; Wieler, J.; Damluji, A.; Davis, C., III; Mets, B. Prevalence, Timing, Causes, and Outcomes of Hyponatremia in Hospitalized Orthopaedic Surgery Patients. J. Bone Jt. Surg. Am. 2015, 97, 1824–1832. [Google Scholar] [CrossRef]
- Singh, N.; Tai, J.Y.; Dimech, J.; Gormack, N.J.; Cameron, A.J.D.; Lightfoot, N.J. Predictors of hyponatremia following elective primary unilateral knee arthroplasty at a tertiary centre: A retrospective observational cohort and predictive model. J. Orthop. 2020, 21, 491–495. [Google Scholar] [CrossRef]
- Cunningham, E.; Gallagher, N.; Hamilton, P.; Bryce, L.; Beverland, D. Prevalence, risk factors, and complications associated with hyponatraemia following elective primary hip and knee arthroplasty. Perioper. Med. 2021, 10, 25. [Google Scholar] [CrossRef]
- Baker, C.M.; Goh, G.S.; Tarabichi, S.; Sherman, M.B.; Khan, I.A.; Parvizi, J. Hyponatremia Is an Overlooked Sign of Trouble Following Total Joint Arthroplasty. J. Bone Jt. Surg. Am. 2023, 105, 744–754. [Google Scholar] [CrossRef]
- Wester, C.; Zhang, T.S.; Harrington, M.A.; Halawi, M.J. Sodium Abnormalities Are an Independent Predictor of Complications in Total Joint Arthroplasty: A Cautionary Tale! J. Arthroplast. 2021, 36, 3859–3863. [Google Scholar] [CrossRef]
- National Joint Registry. NJR 22nd Annual Report 2025. 2025. Available online: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2022nd%20Annual%20Report%202025.pdf (accessed on 1 December 2025).
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Tareen, H.U.; Syed, F.O. Frequency and Risk Factors of Postoperative Hyponatremia in Primary Hip and Knee Arthroplasties Patients at a Tertiary Care Hospital, Karachi, Pakistan. J. Postgrad. Med. Inst. 2024, 38, 203–207. [Google Scholar] [CrossRef]
- Macdonald, J.; Cunningham, E.; Gallagher, N.; Hamilton, P.; Cassidy, R.; Bryce, L.; Beverland, D. Can patients with mild post-operative hyponatraemia following elective arthroplasty be discharged safely? A large-scale service evaluation suggests they can. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2022, 59, 116–124. [Google Scholar] [CrossRef]
- Orfanos, G.; Kumar, N.N.; Redfern, D.; Burston, B.; Banerjee, R.; Thomas, G. The incidence and risk factors for abnormal postoperative blood tests following primary total joint replacement. Bone Jt. Open 2023, 4, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Sinno, E.; De Meo, D.; Cavallo, A.U.; Petriello, L.; Ferraro, D.; Fornara, G.; Persiani, P.; Villani, C. Is postoperative hyponatremia a real threat for total hip and knee arthroplasty surgery? Medicine 2020, 99, e20365. [Google Scholar] [CrossRef]
- Sarkar, S.; Gautam, D.; Anand, R.K.; Goyal, D.; Batra, S.; Malhotra, R.; Khanna, P.; Baidya, D.K. Clinicoepidemiological profile of acute postoperative hyponatraemia in patients undergoing joint replacement surgery: A prospective observational study. J. Perioper. Pract. 2025. [Google Scholar] [CrossRef]
- Sah, A.P. Hyponatremia after primary hip and knee arthroplasty: Incidence and associated risk factors. Am. J. Orthop. 2014, 43, E69–E73. [Google Scholar]
- Mukartihal, R.; Puranik, H.G.; Patil, S.S.; Dhanasekaran, S.R.; Menon, V.K. Electrolyte imbalance after total joint arthroplasty: Risk factors and impact on length of hospital stay. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 1467–1472. [Google Scholar] [CrossRef]
- Waller, M.; Barkley, S.; Harrison, T. Can introducing a direct endocrine pathway reduce hyponatraemia in elective knee and hip replacements? A closed-loop audit and service evaluation study. Ann. R. Coll. Surg. Engl. 2025, 107, 469–472. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Hyponatraemia: NICE Guideline. July 2025. Available online: https://cks.nice.org.uk/topics/hyponatraemia/background-information/causes/ (accessed on 15 September 2025).
- Ruccia, F.; Savage, J.A.; Sorooshian, P.; Lees, M.; Fesatidou, V.; Zoccali, G. Hyponatremia after Autologous Breast Reconstruction: A Cohort Study Comparing Two Fluid Management Protocols. J. Reconstr. Microsurg. 2023, 39, 35–42. [Google Scholar] [PubMed]
- Park, S.; An, J.N.; Lee, J.P.; Oh, Y.K.; Kim, D.K.; Joo, K.W.; Kim, Y.S.; Lim, C.S. Association between postoperative hyponatremia and renal prognosis in major urologic surgery. Oncotarget 2017, 8, 79935–79947. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Tamai, K.; Oka, M.; Habibi, H.; Terai, H.; Hoshino, M.; Toyoda, H.; Suzuki, A.; Takahashi, S.; Nakamura, H. Prevalence, risk factors, and potential symptoms of hyponatremia after spinal surgery in elderly patients. Sci. Rep. 2022, 12, 18622. [Google Scholar] [CrossRef]
- Sherlock, M.; O’Sullivan, E.; Agha, A.; Behan, L.A.; Rawluk, D.; Brennan, P.; Tormey, W.; Thompson, C.J. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin. Endocrinol. 2006, 64, 250–254. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Makri, A.; Elisaf, M.S.; Liamis, G. Hyponatremia in the elderly: Challenges and solutions. Clin. Interv. Aging 2017, 12, 1957–1965. [Google Scholar] [CrossRef]
- Davies, I.; O’Neill, P.A.; Mclean, K.A.; Catania, J.; Bennett, D. Age-associated Alterations in Thirst and Arginine Vasopressin in Response to a Water or Sodium Load. Age Ageing 1995, 24, 151–159. [Google Scholar] [CrossRef]
- Phillips, P.A.; Rolls, B.J.; Ledingham, J.G.; Forsling, M.L.; Morton, J.J.; Crowe, M.J.; Wollner, L. Reduced Thirst after Water Deprivation in Healthy Elderly Men. N. Engl. J. Med. 1984, 311, 753–759. [Google Scholar] [CrossRef]
- Cowen, L.E.; Hodak, S.P.; Verbalis, J.G. Age-Associated Abnormalities of Water Homeostasis. Endocrinol. Metab. Clin. North Am. 2023, 52, 277–293. [Google Scholar] [CrossRef]
- Hendriksen, L.C.; Mouissie, M.S.; Herings, R.M.C.; van der Linden, P.D.; Visser, L.E. Women have a higher risk of hospital admission associated with hyponatremia than men while using diuretics. Front. Pharmacol. 2024, 15, 1409271. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Ruzieh, M.; Foy, A. Thiazide-Associated Hyponatremia: Clinical Manifestations and Pathophysiology. Am. J. Kidney Dis. 2020, 75, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.J. The Silent Epidemic of Thiazide-Induced Hyponatremia. J. Clin. Hypertens. 2008, 10, 477–484. [Google Scholar] [CrossRef]
- Falhammar, H.; Skov, J.; Calissendorff, J.; Nathanson, D.; Lindh, J.D.; Mannheimer, B. Associations Between Antihypertensive Medications and Severe Hyponatremia: A Swedish Population–Based Case–Control Study. J. Clin. Endocrinol. Metab. 2020, 105, e3696–e3705. [Google Scholar] [CrossRef] [PubMed]
- Rudge, J.E.; Kim, D. New-onset hyponatraemia after surgery for traumatic hip fracture. Age Ageing 2014, 43, 821–826. [Google Scholar] [CrossRef]
- Mannheimer, B.; Lindh, J.D.; Fahlén, C.B.; Issa, I.; Falhammar, H.; Skov, J. Drug-induced hyponatremia in clinical care. Eur. J. Intern. Med. 2025, 137, 11–20. [Google Scholar] [CrossRef]
- Falhammar, H.; Lindh, J.D.; Calissendorff, J.; Skov, J.; Nathanson, D.; Mannheimer, B. Associations of proton pump inhibitors and hospitalization due to hyponatremia: A population–based case–control study. Eur. J. Intern. Med. 2019, 59, 65–69. [Google Scholar] [CrossRef]
- El-Alali, E.; Al Jaber, E. Association of proton pump inhibitor use and significant hyponatremia-a US population-based case-control study. Proc. (Bayl. Univ. Med. Cent.) 2022, 35, 434–436. [Google Scholar] [CrossRef]
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef]
- Ferré, F.; Martin, C.; Bosch, L.; Kurrek, M.; Lairez, O.; Minville, V. Control of Spinal Anesthesia-Induced Hypotension in Adults. Local Reg. Anesth. 2020, 13, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cusack, B.; Buggy, D.J. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef]
- Lane, N.; Allen, K. Hyponatraemia after orthopaedic surgery. BMJ 1999, 318, 1363–1364. [Google Scholar] [CrossRef]
- Tambe, A.A.; Hill, R.; Livesley, P.J. Post-operative hyponatraemia in orthopaedic injury. Injury 2003, 34, 253–255. [Google Scholar] [CrossRef]
- Semler, M.W.; Kellum, J.A. Balanced Crystalloid Solutions. Am. J. Respir. Crit. Care Med. 2019, 199, 952–960. [Google Scholar] [CrossRef]
- Gao, M.; Chen, M.; Dai, G.; Zhu, D.; Cai, Y. Clinical study: The impact of goal-directed fluid therapy on volume management during enhanced recovery after surgery in gastrointestinal procedures. Acta Biochim. Pol. 2024, 71, 12377. [Google Scholar] [CrossRef]
- Kendrick, J.B.; Kaye, A.D.; Tong, Y.; Belani, K.; Urman, R.D.; Hoffman, C.; Liu, H. Goal-directed fluid therapy in the perioperative setting. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S29–S34. [Google Scholar] [CrossRef]
- Carlock, K.D.; Mills, Z.D.; Geiger, K.W.; Manner, P.A.; Fernando, N.D. Routine Indwelling Urinary Catheterization Is Not Necessary During Total Hip Arthroplasty Performed Under Spinal Anesthesia. Arthroplast. Today 2022, 16, 68–72. [Google Scholar] [CrossRef]
- Wainwright, T.W.; Gill, M.; McDonald, D.A.; Middleton, R.G.; Reed, M.; Sahota, O.; Yates, P.; Ljungqvist, O. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop. 2020, 91, 3–19. [Google Scholar] [CrossRef]
- Halawi, M.J.; Plourde, J.M.; Cote, M.P. Routine Postoperative Laboratory Tests Are Not Necessary After Primary Total Hip Arthroplasty. J. Arthroplast. 2019, 34, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Tischler, E.H.; Restrepo, C.; Ponzio, D.Y.; Austin, M.S. Routine Postoperative Chemistry Panels Are Not Necessary for Most Total Joint Arthroplasty Patients. J. Bone Jt. Surg. Am. 2021, 103, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Byrom, I.; Agnew, N.; Starks, I.; Phillips, S.; Malek, I.A. Routine postoperative blood tests in all patients undergoing Total Hip Arthroplasty as part of an enhanced recovery pathway: Are they necessary? J. Clin. Orthop. Trauma 2021, 16, 114–118. [Google Scholar] [CrossRef]
- Zhu, A.C.; Agarwala, A.; Bao, X. Perioperative Fluid Management in the Enhanced Recovery after Surgery (ERAS) Pathway. Clin. Colon Rectal Surg. 2019, 32, 114–120. [Google Scholar] [CrossRef]
- Kim, S.; Joo, K.W. Electrolyte and Acid-Base Disturbances Associated with Non-Steroidal Anti-Inflammatory Drugs. Electrolytes Blood Press. 2007, 5, 116–125. [Google Scholar] [CrossRef]
- Falhammar, H.; Calissendorff, J.; Skov, J.; Nathanson, D.; Lindh, J.D.; Mannheimer, B. Tramadol- and codeine-induced severe hyponatremia: A Swedish population-based case-control study. Eur. J. Intern. Med. 2019, 69, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Canzan, F.; Longhini, J.; Caliaro, A.; Cavada, M.L.; Mezzalira, E.; Paiella, S.; Ambrosi, E. The effect of early oral postoperative feeding on the recovery of intestinal motility after gastrointestinal surgery: A systematic review and meta-analysis of randomized clinical trials. Front. Nutr. 2024, 11, 1369141. [Google Scholar] [CrossRef] [PubMed]
- Tazreean, R.; Nelson, G.; Twomey, R. Early mobilization in enhanced recovery after surgery pathways: Current evidence and recent advancements. J. Comp. Eff. Res. 2022, 11, 121–129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).