A Digital Twin Strategy Combined with a Monte Carlo Simulation Framework to Predict Outcomes in Patients with Unusual-Site Venous Thrombosis Treated with Direct Oral Anticoagulants Versus Vitamin K Antagonists Using Data from Real-World Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Setting
2.2. Baseline Variables
2.3. Outcomes
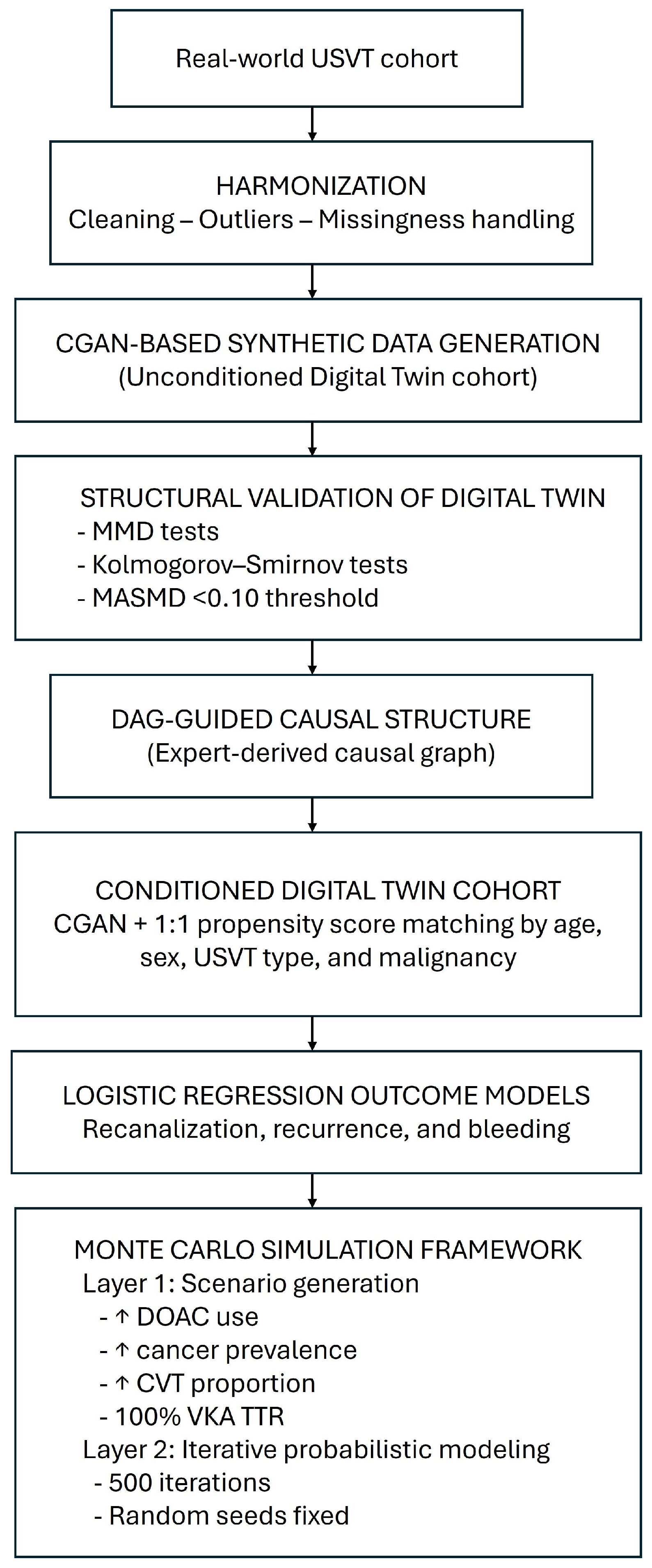
2.4. Digital Twin Generation and Validation
2.4.1. Synthetic Cohort Generation
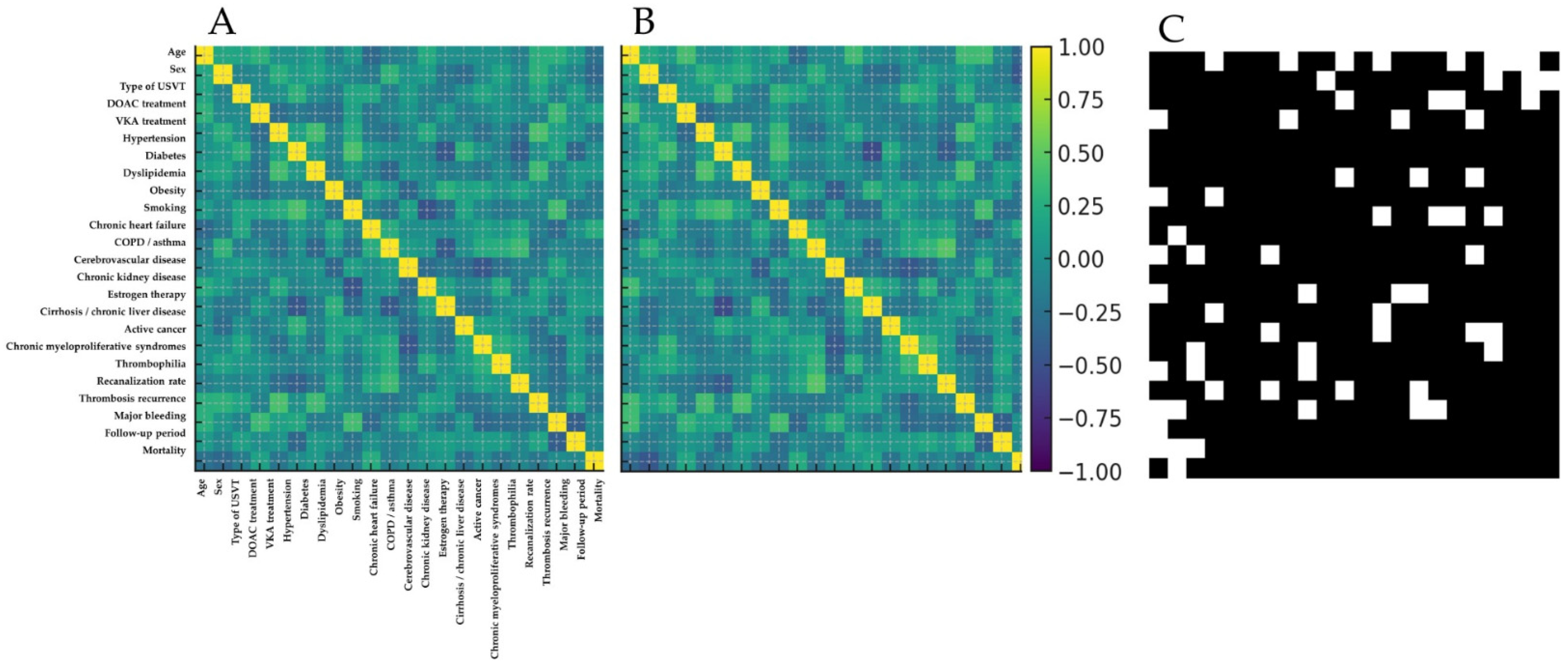
2.4.2. Structural Validation and Causal Coherence
2.4.3. Conditional Cohort Creation and Model Evaluation
2.5. Outcome Modeling
2.6. Monte Carlo Simulation Framework
2.6.1. Scenario Generation (Layer 1)
2.6.2. Outcome Simulation (Layer 2)
2.6.3. Validation and Sensitivity Analyses
2.7. Statistical Analysis in the Original Cohort
3. Results
3.1. Characteristics and Outcomes in Real-World Patients
3.2. Digital Twin
3.2.1. Non-Conditioned Digital Twin Cohort and Internal Structure Validation
3.2.2. Conditioned Twins and Treatment Effect Estimation
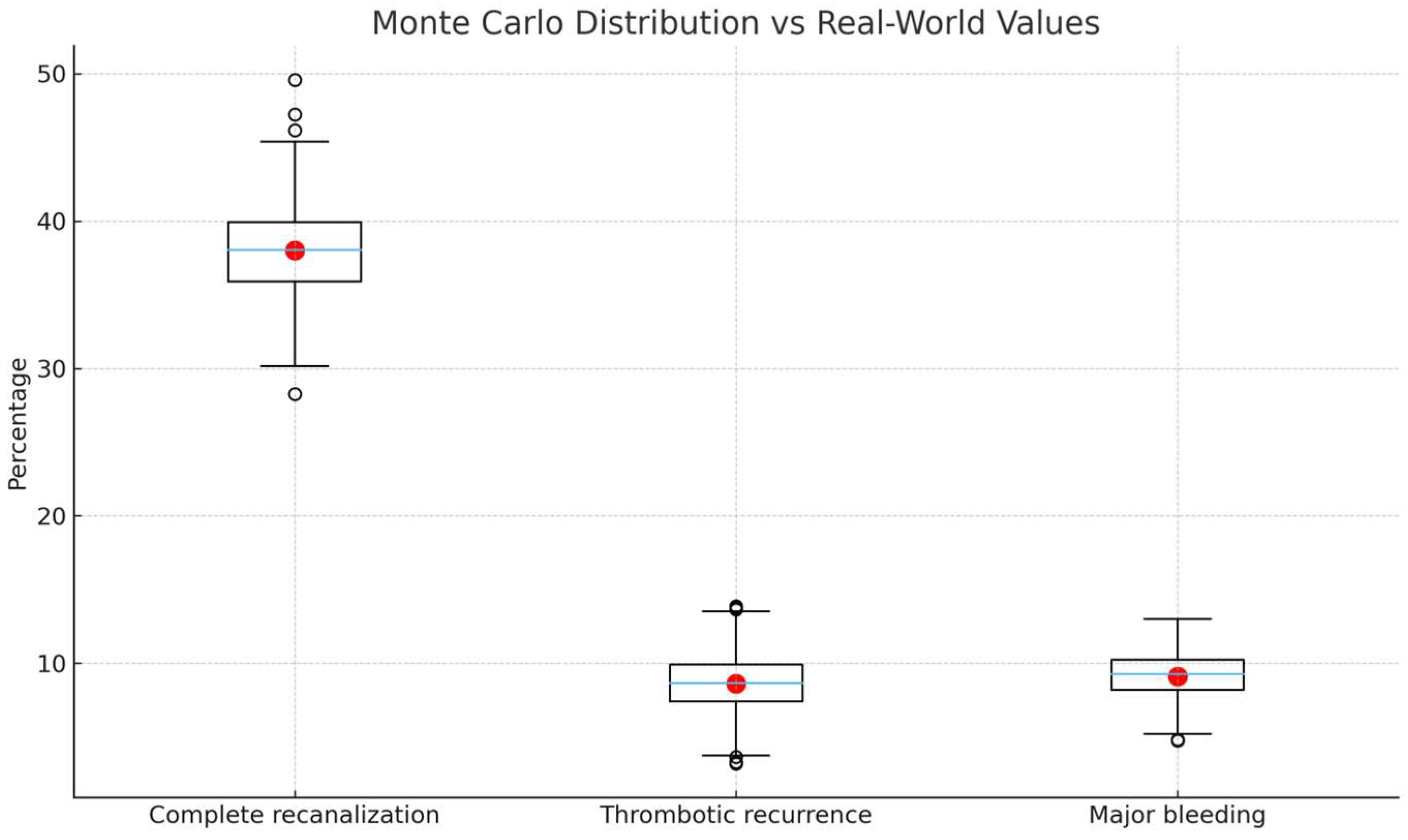
3.2.3. Sensitivity and Uncertainty Analysis
3.3. Monte Carlo Simulation Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CGAN | Conditional Generative Adversarial Network |
| CI | Confidence Interval |
| CT | Computed tomography |
| CVT | Cerebral Venous Thrombosis |
| DAG | Directed Acyclic Graph |
| DOAC | Direct Oral Anticoagulant |
| DT | Digital Twin |
| GAN | Generative Adversarial Network |
| INR | International Normalized Ratio |
| LMWH | Low-molecular-weight heparin |
| MRI | Magnetic resonance imaging |
| PVT | Portal Vein Thrombosis |
| RCT | Randomized controlled trial |
| SD | Standard Deviation |
| SVT | Splanchnic Vein Thrombosis |
| TTR | Time in Therapeutic Range |
| UEDVT | Upper Extremity Deep Vein Thrombosis |
| USVT | Unusual-Site Venous Thrombosis |
| VKA | Vitamin K Antagonist |
References
- Bejjani, A.; Khairani, C.D.; Assi, A.; Piazza, G.; Sadeghipour, P.; Talasaz, A.H.; Fanikos, J.; Connors, J.M.; Siegal, D.M.; Barnes, G.D.; et al. When Direct Oral Anticoagulants Should Not Be Standard Treatment. J. Am. Coll. Cardiol. 2024, 83, 444–465. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Coutinho, J.M.; Dentali, F.; Kobayashi, A.; Alasheev, A.; Canhão, P.; Karpov, D.; Nagel, S.; Posthuma, L.; Roriz, J.M.; et al. Safety and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients with Cerebral Venous Thrombosis. JAMA Neurol. 2019, 76, 1457–1465. [Google Scholar] [CrossRef]
- Ma, H.; Gu, Y.; Bian, T.; Song, H.; Liu, Z.; Ji, X.; Duan, J. Dabigatran etexilate versus warfarin in cerebral venous thrombosis in Chinese patients (CHOICE-CVT): An open-label, randomized controlled trial. Int. J. Stroke 2024, 19, 635–644. [Google Scholar] [CrossRef]
- Field, T.S.; Dizonno, V.; Almekhlafi, M.A.; Bala, F.; Alhabli, I.; Wong, H.; Norena, M.; Villaluna, M.K.; King-Azote, P.; Ratnaweera, N.; et al. Study of Rivaroxaban for Cerebral Venous Thrombosis: A Randomized Controlled Feasibility Trial Comparing Anticoagulation with Rivaroxaban to Standard-of-Care in Symptomatic Cerebral Venous Thrombosis. Stroke 2023, 54, 2724–2736. [Google Scholar] [CrossRef]
- Maqsood, M.; Khan, M.I.H.; Yameen, M.; Ahmed, K.A.; Hussain, N.; Hussain, S. Use of oral rivaroxaban in cerebral venous thrombosis. J. Drug Assess. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Connor, P.; van Kammen, M.S.; Lensing, A.W.A.; Chalmers, E.; Kállay, K.; Hege, K.; Simioni, P.; Biss, T.; Bajolle, F.; Bonnet, D.; et al. Safety and efficacy of rivaroxaban in pediatric cerebral venous thrombosis (EINSTEIN-Jr CVT). Blood Adv. 2020, 4, 6250–6258. [Google Scholar] [CrossRef]
- Woller, S.C.; Stevens, S.M.; Johnson, S.A.; Bledsoe, J.R.; Galovic, B.; Lloyd, J.F.; Wilson, E.L.; Armbruster, B.; Evans, R.S. Apixaban for Routine Management of Upper Extremity Deep Venous Thrombosis (ARM-DVT): Methods of a prospective single-arm management study. Res. Pr. Thromb. Haemost. 2019, 3, 340–348. [Google Scholar] [CrossRef]
- Plessier, A.; Goria, O.; Cervoni, J.P.; Ollivier, I.; Bureau, C.; Poujol-Robert, A.; Minello, A.; Houssel-Debry, P.; Rautou, P.E.; Payancé, A.; et al. Rivaroxaban Prophylaxis in Noncirrhotic Portal Vein Thrombosis. NEJM Évid. 2022, 1, EVIDoa2200104. [Google Scholar] [CrossRef]
- Ageno, W.; Westendorf, J.B.; Contino, L.; Bucherini, E.; Sartori, M.T.; Senzolo, M.; Grandone, E.; Santoro, R.; Carrier, M.; Delluc, A.; et al. Rivaroxaban for the treatment of noncirrhotic splanchnic vein thrombosis: An interventional prospective cohort study. Blood Adv. 2022, 6, 3569–3578. [Google Scholar] [CrossRef] [PubMed]
- Barnum, K.J.; Patell, R.; Berry, J.; Bauer, K.A. Splanchnic vein thrombosis: Management for the thrombosis specialist. J. Thromb. Haemost. 2024, 23, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Riat, R.; Gomez, K.; Taskforce, T.B.S.F.H.H.A.T. Addendum to guidelines on the investigation and management of venous thrombosis at unusual sites (Br. J. Haematol. 2012;159:28–38): Use of Direct Oral Anticoagulants. Br. J. Haematol. 2022, 198, 46–49. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2021, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Bruynseels, K.; Santoni de Sio, F.; van den Hoven, J. Digital Twins in Health Care: Ethical Implications of an Emerging Engineering Paradigm. Front. Genet. 2018, 9, 31. [Google Scholar] [CrossRef]
- Björnsson, B.; Borrebaeck, C.; Elander, N.; Gasslander, T.; Gawel, D.R.; Gustafsson, M.; Jörnsten, R.; Lee, E.J.; Li, X.; Lilja, S.; et al. Digital twins to personalize medicine. Genome Med. 2019, 12, 4. [Google Scholar] [CrossRef]
- Velikova, T.; Mileva, N.; Naseva, E. Method “Monte Carlo” in healthcare. World J. Methodol. 2024, 14, 93930. [Google Scholar] [CrossRef]
- Franco-Moreno, A.; Madroñal-Cerezo, E.; Martínez-Casa-Muñoz, A.; Ortiz-Sánchez, J.; Ancos-Aracil, C.L. Direct Oral Anticoagulants for the Treatment of Unusual-Site Venous Thrombosis: An Update. Pharmaceutics 2025, 17, 342. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C. The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Leuk. Res. 2017, 45, 1887–1894. [Google Scholar] [CrossRef]
- Janczak, D.T.; Mimier, M.K.; McBane, R.D.; Kamath, P.S.; Simmons, B.S.; Bott-Kitslaar, D.M.; Lenz, C.J.; Vargas, E.R.; Hodge, D.O.; Wysokinski, W.E. Rivaroxaban and Apixaban for Initial Treatment of Acute Venous Thromboembolism of Atypical Location. Mayo Clin. Proc. 2018, 93, 40–47. [Google Scholar] [CrossRef]
- Li, A.; Zhang, M.C.; Li, P.; Eshaghpour, A.; Li, K.; Carrier, M.; Wells, P.; Crowther, M.A. Direct oral anticoagulants for the treatment of splanchnic vein thrombosis—A systematic review and meta-analysis. Thromb. Res. 2023, 229, 209–218. [Google Scholar] [CrossRef]
- Riva, N.; Ageno, W. Direct oral anticoagulants for unusual-site venous thromboembolism. Res. Pr. Thromb. Haemost. 2021, 5, 265–277. [Google Scholar] [CrossRef]
- Ferro, J.M.; Bousser, M.-G.; Canhão, P.; Coutinho, J.M.; Crassard, I.; Dentali, F.; di Minno, M.; Maino, A.; Martinelli, I.; Masuhr, F.; et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—Endorsed by the European Academy of Neurology. Eur. Stroke J. 2017, 2, 195–221. [Google Scholar] [CrossRef]
- Casado-Suela, M.Á.; Torres-Macho, J.; Izquierdo-Martínez, A.; Ancos-Aracil, C.L.; Ferreira-Burguillos, L.; Madroñal-Cerezo, E.; Talaván-Zañón, T.; Castañeda-Mata, A.; Escobar-Curbelo, L.; de la Casa-Muñoz, A.M.; et al. A Digital Twin Strategy to Predict Thrombotic Recurrence in Antiphospholipid Syndrome Patients Treated with Direct Oral Anticoagulants vs. Vitamin K Antagonists Using Data from Real-World Populations. J. Clin. Med. 2025, 14, 5716. [Google Scholar] [CrossRef] [PubMed]
- Canestaro, W.J.; Patrick, A.R.; Avorn, J.; Ito, K.; Matlin, O.S.; Brennan, T.A.; Shrank, W.H.; Choudhry, N.K. Cost-Effectiveness of Oral Anticoagulants for Treatment of Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Shan, G. Monte Carlo cross-validation for a study with binary outcome and limited sample size. BMC Med. Inform. Decis. Mak. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Total (n = 90) | VKAs Group (n = 65) | DOACs Group (n = 25) | p-Value |
|---|---|---|---|---|
| Demographic data | ||||
| Age (mean ± SD) | 67.5 (±17.7) | 68.9 (±17.2) | 63.9 (±18.6) | 0.448 |
| Female sex, n (%) | 49 (54.4) | 33 (50.8) | 16 (64.0) | 0.259 |
| Type of USVT, n (%) | ||||
| SVT | 55 (61.1) | 45 (69.2) | 10 (40.0) | 0.016 |
| UEDVT | 33 (36.7) | 20 (30.8) | 13 (52.0) | 0.087 |
| CVT | 2 (2.2) | 0 (0) | 2 (8.0) | 0.075 |
| Previous conditions, n (%) | ||||
| Hypertension | 48 (53.3) | 39 (60.0) | 9 (36.0) | 0.041 |
| Diabetes | 26 (28.9) | 21 (32.3) | 5 (20.0) | 0.249 |
| Dyslipidemia | 40 (44.4) | 29 (44.6) | 11 (44.0) | 0.958 |
| Obesity (BMI ≥ 30 kg/m2) | 18 (20.0) | 10 (15.4) | 8 (32.0) | 0.078 |
| Current smoker | 31 (34.4) | 23 (35.4) | 8 (32.0) | 0.810 |
| Chronic heart failure | 9 (10.0) | 7 (10.8) | 2 (8.0) | 0.695 |
| COPD/asthma | 23 (25.6) | 18 (27.7) | 5 (20.0) | 0.454 |
| Cerebrovascular disease | 11 (12.2) | 8 (12.3) | 3 (12.0) | 0.986 |
| Chronic kidney disease | 13 (14.4) | 8 (12.3) | 5 (20.0) | 0.352 |
| Estrogen therapy | 1 (1.1) | 1 (1.5) | 0 | 0.533 |
| Cirrhosis/chronic liver disease | 25 (27.8) | 22 (33.8) | 3 (12.0) | 0.038 |
| Active cancer | 29 (32.2) | 25 (38.5) | 4 (16.0) | 0.041 |
| Paroxysmal nocturnal hemoglobinuria | 0 (0) | 0 (0) | 0 (0) | — |
| Chronic myeloproliferative syndromes | 1 (1.1) | 0 (0) | 1 (4.0) | 0.279 |
| Laboratory parameters (median, IQR) † | ||||
| Hemoglobin (g/dL) | 13.1 (11.8–14.4) | 12.9 (11.6–14.2) | 13.6 (12.3–14.9) | 0.181 |
| Platelets (×109/L) | 238 (182–296) | 231 (178–282) | 251 (190–315) | 0.279 |
| D-dimer (ng/mL) | 2982 (2143–3921) | 3123 (2256–4050) | 2743 (2080–3615) | 0.295 |
| Concomitant antiplatelet therapy | 7 (7.8) | 6 (9.2) | 1 (4.0) | 0.407 |
| Any thrombophilia, n (%) | 19 (21.1) | 14 (21.5) | 5 (20.0) | 0.873 |
| Complete recanalization during follow-up, n (%) * | 15/40 (37.5) | 9/25 (36.0) | 6/15 (40.0) | 0.827 |
| Thrombosis recurrence during follow-up, n (%) | 7 (7.8) | 5 (7.7) | 2 (8.0) | 0.961 |
| Major bleeding during follow-up, n (%) | 9 (10.0) | 7 (10.8) | 2 (8.0) | 0.695 |
| Follow-up period (median, IQR) | 29.5 (20.1–43.7) | 30.1 (20.3–45.2) | 27.9 (19.8–42.1) | 0.386 |
| Mortality, n (%) | 44 (48.9) | 39 (60.0) | 5 (20.0) | <0.001 |
| Outcome | VKAs, % (95% CI) | DOACs, % (95% CI) | Absolute Difference * |
|---|---|---|---|
| Complete recanalization | 38.0 (30.8–45.6) | 40.3 (33.5–47.3) | +2.3 |
| Thrombotic recurrence | 8.6 (4.8–13.6) | 10.9 (6.7–16.5) | +2.3 |
| Major bleeding | 9.1 (5.1–14.5) | 7.6 (4.1–12.8) | −1.5 |
| Scenario | Outcome | VKAs %, 95% CI | DOACs %, 95% CI | Δ* (Percentage Points) |
|---|---|---|---|---|
| Baseline § | Complete recanalization | 38.1 [36.0–40.3] | 40.4 [38.3–42.6] | +2.3 |
| Thrombotic recurrence | 8.7 [7.9–9.6] | 10.9 [10.0–11.9] | +2.2 | |
| Major bleeding | 9.1 [8.2–10.1] | 7.6 [6.8–8.5] | −1.5 | |
| 70% DOACs | Complete recanalization | 38.0 [35.9–40.1] | 40.2 [38.1–42.4] | +2.2 |
| Thrombotic recurrence | 8.6 [7.7–9.5] | 11.1 [10.1–12.2] | +2.5 | |
| Major bleeding | 9.2 [8.3–10.2] | 7.6 [6.8–8.6] | −1.6 | |
| +50% Cancer | Complete recanalization | 36.0 [34.0–38.2] | 38.2 [36.1–40.4] | +2.2 |
| Thrombotic recurrence | 10.8 [9.8–11.9] | 13.0 [12.0–14.2] | +2.2 | |
| Major bleeding | 10.4 [9.4–11.5] | 9.0 [8.1–10.0] | −1.4 | |
| 40% CVT | Complete recanalization | 31.5 [29.4–33.7] | 33.8 [31.6–36.0] | +2.3 |
| Thrombotic recurrence | 12.1 [11.0–13.2] | 14.4 [13.2–15.6] | +2.3 | |
| Major bleeding | 11.7 [10.6–12.9] | 10.2 [9.2–11.3] | −1.5 | |
| 100% TTR for VKAs | Complete recanalization | 40.0 [37.9–42.2] | 40.3 [38.2–42.5] | +0.3 |
| Thrombotic recurrence | 6.9 [6.1–7.8] | 10.9 [10.0–11.9] | +4.0 | |
| Major bleeding | 8.0 [7.1–8.9] | 7.6 [6.8–8.5] | −0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Moreno, A.; Escobar-Curbelo, L.; Torres-Macho, J.; Muñoz-Rivas, N.; Ancos-Aracil, C.L.; Martínez de la Casa-Muñoz, A.; Bustamante- Fermosel, A.; Arranz-García, P.; Casado-Suela, M.Á. A Digital Twin Strategy Combined with a Monte Carlo Simulation Framework to Predict Outcomes in Patients with Unusual-Site Venous Thrombosis Treated with Direct Oral Anticoagulants Versus Vitamin K Antagonists Using Data from Real-World Populations. Clin. Pract. 2025, 15, 237. https://doi.org/10.3390/clinpract15120237
Franco-Moreno A, Escobar-Curbelo L, Torres-Macho J, Muñoz-Rivas N, Ancos-Aracil CL, Martínez de la Casa-Muñoz A, Bustamante- Fermosel A, Arranz-García P, Casado-Suela MÁ. A Digital Twin Strategy Combined with a Monte Carlo Simulation Framework to Predict Outcomes in Patients with Unusual-Site Venous Thrombosis Treated with Direct Oral Anticoagulants Versus Vitamin K Antagonists Using Data from Real-World Populations. Clinics and Practice. 2025; 15(12):237. https://doi.org/10.3390/clinpract15120237
Chicago/Turabian StyleFranco-Moreno, Anabel, Luis Escobar-Curbelo, Juan Torres-Macho, Nuria Muñoz-Rivas, Cristina Lucía Ancos-Aracil, Ana Martínez de la Casa-Muñoz, Ana Bustamante- Fermosel, Paz Arranz-García, and Miguel Ángel Casado-Suela. 2025. "A Digital Twin Strategy Combined with a Monte Carlo Simulation Framework to Predict Outcomes in Patients with Unusual-Site Venous Thrombosis Treated with Direct Oral Anticoagulants Versus Vitamin K Antagonists Using Data from Real-World Populations" Clinics and Practice 15, no. 12: 237. https://doi.org/10.3390/clinpract15120237
APA StyleFranco-Moreno, A., Escobar-Curbelo, L., Torres-Macho, J., Muñoz-Rivas, N., Ancos-Aracil, C. L., Martínez de la Casa-Muñoz, A., Bustamante- Fermosel, A., Arranz-García, P., & Casado-Suela, M. Á. (2025). A Digital Twin Strategy Combined with a Monte Carlo Simulation Framework to Predict Outcomes in Patients with Unusual-Site Venous Thrombosis Treated with Direct Oral Anticoagulants Versus Vitamin K Antagonists Using Data from Real-World Populations. Clinics and Practice, 15(12), 237. https://doi.org/10.3390/clinpract15120237






