Abstract
Even though acute heart failure (AHF) is one of the most common admission diagnoses globally, its pathogenesis is poorly understood, and there are few effective treatments available. Despite an heterogenous onset, congestion is the leading contributor to hospitalization, making it a crucial therapeutic target. Complete decongestion, nevertheless, may be hard to achieve, especially in patients with reduced end organ perfusion. In order to promote a personalised pathophysiological-based therapy for patients with AHF, we will address in this review the pathophysiological principles that underlie the clinical symptoms of AHF as well as examine how to assess them in clinical practice, suggesting that gaining a deeper understanding of pathophysiology might result in significant improvements in HF therapy.
1. Introduction
Acute heart failure (AHF) is a clinical syndrome characterized by the rapid or gradual onset of symptoms and/or signs related to heart failure [1]; these symptoms or signs should be significant enough to prompt the patient to seek urgent medical intervention, resulting in unplanned hospitalization or visit to the emergency department [2]. Despite substantial advances in pharmacologic and non-pharmacologic therapy in managing patients with chronic heart failure with marked improvements in long-term survival, rates of rehospitalization at 3 months and mortality at 12 months after an AHF episode remain respectively at 10–30% [3,4]. Although the clinical presentation of AHF is highly variable, the most common reason for hospitalization is significant volume overload and, subsequently, congestive symptoms. Fewer patients present with hypotension and symptoms of reduced organ perfusion [5]. As congestion and hypoperfusion play a central role in the management of AHF and in determining the prognosis, understanding the underlying pathophysiological mechanisms related to them is essential for the appropriate treatment of patients with AHF. Therefore, in this review, we will discuss practically the pathophysiological principles underlying the clinical syndrome of AHF and examine how to evaluate them in clinical practice to promote a tailored pathophysiological-based treatment of patients with AHF.
2. Pathophysiology of Congestion
In AHF, there are two main types of congestion [6]:
- Peripheral congestion: characterized by a progressive increase in body weight, peripheral edema, jugular distension, hepatomegaly, ascites, and renal venous stasis [7];
- Pulmonary congestion: featured by worsening dyspnea, pulmonary rales, and B-lines at lung ultrasound [8].
Peripheral congestion usually coexists with pulmonary congestion, but the reverse is not always true.
These two types of congestion recognize different pathophysiologic mechanisms, whereby peripheral congestion recognizes fluid retention as the primary mechanism (congestion related to cardiac failure) [9]. In contrast, fluid redistribution is the leading cause of pulmonary congestion (congestion related to vascular failure) [10].
2.1. Congestion Related to Cardiac Failure
The reduction in cardiac output secondary to myocardial dysfunction results in arterial underfilling that is sensed by mechanoreceptors present in the left ventricle, carotid sinus, aortic arch, and renal afferent arterioles resulting in an increased sympathetic outflow from the central nervous system, activation of the renin-angiotensin-aldosterone system (RAAS) and the nonosmotic release of arginine-vasopressin [11,12,13,14,15].
Activation of these systems, together with increased release of substances with vasoconstrictive activity (e.g., endothelin and vasopressin) and the development of resistance to the action of endogenous natriuretic peptides [16], contribute to the retention of sodium and water that tend to balance (through an increase in cardiac output) adverse effects of AHF on oxygen delivery to the peripheral tissues [17]. However, persistent activation of these systems results in impaired regulation of sodium excretion through the kidneys, which results in sodium and, secondarily, fluid accumulation and tissue edema [7]. Tissue edema develops when the amount of transudate fluid moving from the capillaries to the interstitium exceeds the maximum drainage capacity of the lymphatic system [18]. The transudate of plasma fluid into the interstitium depends on the relationship between oncotic and hydrostatic pressure in the capillaries and interstitium (Figure 1): increasing the transcapillary gradient of hydrostatic pressure and decreasing the transcapillary gradient of oncotic pressure promotes the formation of interstitial edema [18].
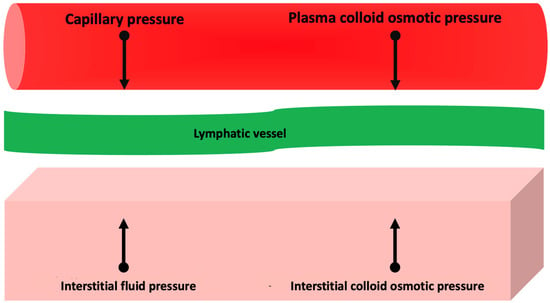
Figure 1.
Pathophysiology of interstitial edema. See text for further information.
Several studies have elucidated further mechanisms promoting interstitial edema. The impairment of the network of glycosaminoglycans (due to chronic sodium accumulation) of connective tissues, which in the healthy subject can buffer a high amount of reabsorbed sodium, thus preventing compensatory water retention, contributes to edema formation in the patient with AHF [19].
Venous congestion is linked to renal and hepatic dysfunction, which may play a role in edema formation as indicated by different research [20,21]. Historically, worsening renal function in AHF patients was hypothesized as a consequence of a reduced cardiac output resulting in renal hypoperfusion. In contrast, recent data indicate that venous congestion (assessed as increased central venous pressure) is the primary hemodynamic determinant for developing renal dysfunction [22], whereas reduced cardiac output has minor effects on renal function [23]. Moreover, visceral congestion can increase intra-abdominal pressure in AHF, with further adverse effects on renal function [24,25]. Recent data have indeed shown that reducing central and intra-abdominal venous pressure by decongestive therapy (diuretics, ultrafiltration, paracentesis) can improve glomerular filtrate [26,27,28,29,30].
Regardless of the mechanisms implicated in the onset of acute cardio-renal syndrome, renal dysfunction can exacerbate sodium and fluid retention with, consequently, further, increase in capillary hydrostatic pressure and promotion of interstitial edema formation [31].
Transient hepatic dysfunction is often present in patients with AHF and, in the overwhelming majority of cases, is cholestatic and related to right heart failure [32].
Furthermore, in patients with AHF (particularly in patients with advanced stage of the disease), hepatic dysfunction, together with intestinal congestion, may contribute to a reduction in protein synthesis [33] with a consequent decrease in oncotic capillary pressure that promotes the formation of interstitial edema. Finally, there are plenty of investigations suggesting that venous congestion is not simply an epiphenomenon secondary to cardiac dysfunction but instead plays an active and detrimental role in the pathophysiology of AHF by inducing pro-oxidant [34], pro-inflammatory [35], and hemodynamic stimuli that contribute to the progression of AHF [36]. Previous in vitro studies highlighted endothelium activity in diverse experimental models [37,38,39]. These observations were further investigated in animal [40] and human models [35,41]. Nitric oxide (NO), prostaglandins (PGs), reactive oxygen species (ROS), and cytokines are just a few of the molecules that endothelium produces. These factors are essential for maintaining a state of stable of chronic heart failure as well as promoting the shift to AHF [42]. Mechanistic insights by which these pathophysiological processes are induced remains poorly understood, but models indicate that biomechanical forces generated in early stages of congestion contribute significantly to endothelial and neurohumoral activation [43,44]. The endothelium works as a master regulator of vascular homeostasis continuously recording its surrounding environment [42]. Indeed, biomechanical stressors as congestion-derived wall stretch and biochemical triggers as increased RAAS activity, are sensed by endothelial cells [45]. Therefore, working as a control system, ECs undergo a phenotypic change toward a pro-oxidant and pro-inflammatory vasoconstriction state [41,46]. These pleiotropic effects have consequences on kidneys, affecting tubuloglomerular feedback [47], and on endothelium itself, increasing the permeability [48]. Thus, the vicious cycle of peripheral congestion is continued [42].
2.2. Congestion Related to Vascular Failure
Fluid accumulation alone cannot explain the entire pathophysiology of congestion in AHF; in fact, most patients with AHF have only a slight increase in body weight (<1 kg) before the onset of clinical symptoms [49].
In these patients, congestion is precipitated predominantly by fluid redistribution rather than fluid accumulation [50].
Indeed, it is well known that adrenergic stimulation results in a transient vasoconstriction that leads to a sudden displacement of fluids from the splanchnic and peripheral venous system to the pulmonary circulation in the absence of exogenous fluid retention [51,52]. However, the prerequisite for that mechanism to be realized is the pre-existence of some degree of peripheral and splanchnic congestion (albeit minimal).
Under physiological conditions, the capacitating veins contain about 25% of the circulating volume and, through a dampening of volume overload, induce stabilization of cardiac preload [53]. In hypertensive-based AHF, the mismatch in the ventricular-vascular coupling relationship due to an increase in afterload and an increase in preload by vasoconstriction of the capacitance veins results in the appearance of pulmonary edema [8].
Both fluid accumulation and redistribution are responsible for congestion during an AHF episode, but their significance depends on the patient profile. While fluid accumulation represents the primary mechanism of peripheral congestion in patients with worsening heart failure with reduced ejection fraction [17], fluid redistribution represents the predominant pathophysiologic mechanism in de novo vascular type AHF in patients with preserved ejection fraction [54]. Consequently, therapy aimed at resolving congestion should be individualized. While in patients with fluid accumulation, diuretics should be the drugs of choice [55], on the other hand vasodilators are the most appropriate drugs for restoring ventricular-vascular coupling in patients with fluid redistribution [8].
A detailed description of the clinical parameters, ultrasonographic data, and biomarkers used to identify congestion in patients with AHF is beyond the scope of this review. However, the main elements used in clinical practice for the identification of pulmonary and peripheral congestion are summarized in Table 1.

Table 1.
Clinical, echocardiographic, and laboratory parameters used for the assessment of congestion in clinical practice. JVP: Jugular Venous Pulsation. HF: Heart Failure NT-proBNP: N-terminal fragment pro B-type Natriuretic Peptide.
3. Clinical Pathophysiology of Hypoperfusion
AHF with a clinical presentation of low cardiac output and subsequent organ hypoperfusion is much less common than a congestion profile with normal perfusion [56]. Usually, this condition tends to manifest as overt cardiogenic shock and, therefore, with systolic arterial pressure values < 90 mmHg and mean arterial pressure < 65 mmHg, although in some cases, patients may present with a low output syndrome with more chronic and subacute manifestations related to cellular adaptation to this chronic hypoperfusion state. Once established, hypoperfusion due to low cardiac output (possibly amplified by venous congestion) can adversely affect the function of all organs bringing to a state of multiorgan failure [57]. (Figure 2) The heart can be damaged in AHF due to increased left ventricular pressure and, consequently, parietal stress, increased inotropic and chronotropic sympathetic stimulation [58], and increased afterload due to vasoconstriction, all of which can cause an imbalance between oxygen supply and demand, resulting in myocardial damage (documented by the rise of troponin).
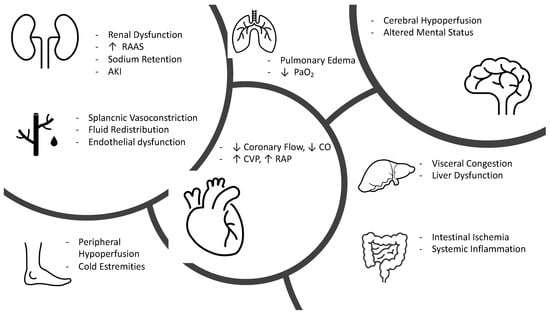
Figure 2.
Pathophysiology of hypoperfused AHF. RAAS, renin angiotensin aldosterone system; AKI, acute kidney injury; CO, cardiac output; CVP, central venous pressure; RAP, right atrial pressure.
Cerebral hypoperfusion represents one of the earliest manifestations of shock and presents clinically as altered mental status, drowsiness, and dullness [57]. New evidence documents that the intestine is one of the first organs to suffer damage as a result of systemic hypoperfusion with early onset of intestinal barrier ischemia resulting in increased bacterial translocation and release of lipopolysaccharide and endotoxins produced by gram-negative bacteria into the circulatory system resulting in the production of cytokines and increased of inflammation [59,60].
The course of AHF is characterized by normal or even increased vascular volume (in case of peripheral congestion) but with reduced effective arterial blood volume [61].
This initial state of hypoperfusion initially results in acute kidney injury (AKI) that is functional (reversible); however, if the state of hypoperfusion becomes prolonged, it can result in tubular epithelial cell damage with structural (irreversible) renal damage [62].
Hypoxic liver injury (HLI), due to an imbalance between hepatic oxygen supply and demand, can complicate AHF. Generally, this condition is manifested by a marked increase in liver enzymes in the absence of any other known cause of liver injury and, rarely, by severe upper abdominal pain due to liver congestion [63]. Both AKI and HLI represent negative prognostic factors in patients with AHF.
4. Pathophysiology-Based Management of AHF
-Congested and normoperfused patient: this is the most frequent combination. Supportive therapy is based on intravenous administration of loop diuretics and nitroderivatives. Loop diuretics are the cornerstone of therapy for patients with AHF with pulmonary and/or systemic congestion. In patients with AHF they should be administered intravenously at 1–2.5 times the home dose with an assessment of diuretic response at six hours [30]. In case of inadequate diuretic response (diuresis less than 100–150 mL/h), one can either double the dose of diuretic to be administered intravenously (up to a maximum of 400–600 mg furosemide or 200–300 mg torasemide) or combine metolazone (sequential nephron blockade) [64] to reach a daily diuresis target of 3–5 L [30]. This target should be maintained until euvolemia is reached. Several reports, indeed, established benefits of discharging patients after a full resolution of congestion [65,66,67]. However, this is a difficult task to achieve and even more to assess. Many patients are still dismissed from clinics with residual congestion [67,68] with significant higher rates of mortality and rehospitalization [4,69,70]. Clinical evaluation alone, has proven insufficient to examine volume status. Hence, experts recommend using multiparameter-based tools, comprehensive of imaging techniques and NT-proBNP measurements. (Table 2) [30] Even though proBNP-NT is the most studied [71,72] and the only biomarker included in this model, a bunch of novel molecules have been linked with AHF outcomes. Soluble ST2 receptor, expressed when myocardial fibrosis occurs, has been linked with worse prognosis in AHF [73,74,75,76]. Growth differentiation factor 15 (GDF15) belongs to TGF-B family has been linked with all cause death and HF hospitalizations (HHF) in secondary analysis of pivotal clinical trials [77,78,79]. Finally, Fibroblast Growth Factor-23 (FGF23) is a hormone, mostly produced in bones, promoting phosphate excretion managing mineral homeostasis [80]. FGF23 increases during transition of HF from a stable state to a decompensated status and is strictly related with disease severity [81]. However, additional studies are expected to further implement use of these biomarkers in clinical practice.

Table 2.
Assessment of residual congestion. Adapted from [30]. JVP, jugular venous pulsation, 6MWT: 6-min walk test. BNP: B-type Natriuretic Peptide. NT-proBNP: N-terminal fragment pro B-type Natriuretic Peptide.
Vasodilators improve left ventricular performance through venous vasodilatation and thus reduced preload (increased due to congestion) and arterial vasodilatation with reduced afterload [58].
They are used predominantly in the patient with acute vascular type HF who generally has blood pressure values above 140 mmHg [82].
The most widely used are nitroglycerin and nitroprusside, both of which are administered intravenously with low initial doses (10–20 µg/min for nitroglycerin, 0.3 µg/kg/min for nitroprusside) that are subsequently adjusted to the patient’s pressor response (up to a maximum dose of 200 µg/min for nitroglycerin and 5 µg/kg/min for nitroprusside) having as target pressors a systolic blood pressure between 90–120 mmHg and a mean blood pressure between 65–70 mmHg [83,84].
Such patients in the absence of high-risk criteria (troponin elevation, worsening renal function) can be managed in intensive brief observation and if responsive to drug therapy do not require hospitalization.
-Dry and normoperfused patient: These are generally patients with initial flare-up of chronic HF in whom hospitalization is not indicated but it is sufficient to increase oral therapy. Hospitalization for HF decompensation is often a good time for optimizing guideline-directed medical therapy (GDMT). Most of patients are admitted while on ACE inhibitors/ARBs and beta blockers therapy [56,85,86]. According to recent studies this approach could be safe and effective. In PIONEER-HF patients in sacubitril/valsartan group had reduced rate of HHF and lower levels of NT-proBNP [87]. Additionally, data from TRANSITION supported the feasibility of this approach [88]. Empagliflozin conferred significant net clinical benefit against placebo in EMPULSE study, whether ejection fraction and diabetes [89]. Miller et al. propose a phenotype-based approach, suggesting initiation of low dose mineralocorticoid receptor antagonists in normo-hypertensive patients [90]. Moreover, it is unlike that beta blocker are accountable for decompensation unless they were recently started or uptitrated. Indeed, a recent meta-analysis stated benefit of maintaining beta blocker therapy on death and hospitalizations [91]. Finally, aside prioritizing disease-modifying therapies, we suggest stopping or downtitrating drugs without proven cardiovascular benefit that could impair GDMT tolerance thus facilitating onset of adverse effects such as hypotension.
-Congested and hypoperfused patient: These are the most critical patients who need to be managed in the intensive care setting. They can be further divided into two categories according to systolic blood pressure (SBP) [92]:
- -
- SBP > 90 mmHg: the patient benefits from intravenous administration of diuretics and nitroderivatives. It is important to remember that in cases of hypoperfusion, the use of diuretics should be considered after perfusion is restored. If insufficient, the use of positive inotropic drugs such as levosimendan (particularly in patients treated with ß-blockers) or dobutamine should be considered [93].
- -
- SBP < 90 mmHg (cardiogenic shock): we recommend seeing specific readings [60,94].
-Dry and hypoperfused patient: hypovolemia should be suspected in these cases, so intravenous fluid administration is useful. A “fluid challenge” [95] can be performed, which is the administration of 250 mL of saline in 15 min and subsequent evaluation of the change in stroke-volume (calculated on echocardiogram) from the baseline value. In patients with an increase in stroke-volume > 10–15%, the reduction in stroke-volume is attributable to the reduction in preload (due to hypovolemia) and consequently adequate hydration therapy should be instituted (in the absence of specific need with an infusion of 25–30 mL/kg/day. saline). (Figure 3).
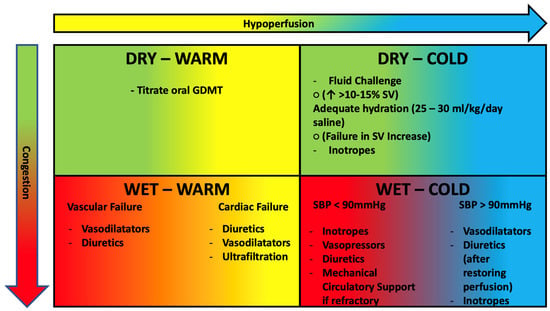
Figure 3.
Clinical profiles of AHF patients based on congestion and hypoperfusion. See text for further treatment information. GDMT, guideline directed medical therapy; SV, stroke volume; SBP, systolic blood pressure. DBP, diastolic blood pressure. Green: Dry. Red: Congested. Yellow: Warm. Blue: Cold.
In patients with failure to increase stroke volume after fluid challenge (in whom therefore the reduction in output is not preload dependent), the use of inotropes is necessary.
Finally, two aspects often overlooked in common clinical practice should be pointed out:
- -
- Oxygen therapy is not routinely indicated in patients with AHF but only in patients with documentation of hypoxemia (SPo2 < 90%, PaO2 < 60 mmHg); in such patients, the target to be achieved is a Pa02 between 60 and 90 mmHg [96] (generally corresponding to a SaO2 > 90% in chronic hypoxics and a SaO2 > 95 mmHg in other subjects), avoiding hyperoxia that could lead increase peripheral vascular resistance lowering cardiac output [97].
- -
- Disease-modifying drug therapy should be continued in cases of HF flare-ups, except in the patient with hemodynamic instability (symptomatic hypotension or bradycardia, cardiogenic shock), pre-renal acute renal failure, and severe hyperkalemia. In these cases, one should first try to reduce therapy without discontinuing it all together until the patient is stabilized.
5. Conclusions
Despite the increasing number of treatment choices for chronic heart failure, people with AHF have not seen the same advancements. AHF is a separate illness with a complex pathophysiology that is still not fully understood and is not being adequately controlled, therefore a large unmet need still weighs on AHF patients. There are significant differences between intravascular and tissue congestion. We suggest that each form of congestion should be treated differently addressing underlying pathophysiology. However, further research is needed to test this hypothesis on hard clinical outcomes.
Author Contributions
Conceptualization: D.M., G.P., F.V. and M.V.; writing—original draft preparation: D.M., L.F. and M.L.M.; writing—review and editing: L.F. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Endorsed by the European Society of Intensive Care Medicine; Nieminen, M.S.; Böhm, M.; Cowie, M.; Drexler, H.; Filippatos, G.S.; Jondeau, G.; Hasin, Y.; Lopez-Sendon, J.; Mebazaa, A.; et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: The Task Force on Acute Heart Failure of the European Society of Cardiology. Eur. Heart J. 2005, 26, 384–416. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Follath, F.; Harjola, V.-P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L.; et al. EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur. Heart J. 2006, 27, 2725–2736. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.-P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Warner Stevenson, L.; Ahmad, T.; Amin, V.J.; Bozkurt, B.; Butler, J.; Davis, L.L.; Drazner, M.H.; Kirkpatrick, J.N.; Peterson, P.N.; et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2019, 74, 1966–2011. [Google Scholar] [CrossRef]
- Sokolska, J.M.; Sokolski, M.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Swoboda, K.; Gajewski, P.; Jankowska, E.A.; Banasiak, W.; et al. Distinct clinical phenotypes of congestion in acute heart failure: Characteristics, treatment response, and outcomes. ESC Heart Fail. 2020, 7, 3830–3840. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Viau, D.M.; Sala-Mercado, J.A.; Spranger, M.D.; O’Leary, D.S.; Levy, P.D. The pathophysiology of hypertensive acute heart failure. Heart 2015, 101, 1861–1867. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Evangelista, I.; Nuti, R. Congestion occurrence and evaluation in acute heart failure scenario: Time to reconsider different pathways of volume overload. Heart Fail. Rev. 2019, 25, 119–131. [Google Scholar] [CrossRef]
- Fudim, M.; Hernandez, A.F.; Felker, G.M. Role of Volume Redistribution in the Congestion of Heart Failure. J. Am. Heart Assoc. 2017, 6, e006817. [Google Scholar] [CrossRef]
- Schrier, R.W.; Abraham, W.T. Hormones and Hemodynamics in Heart Failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Berl, T.; Anderson, R.J. Osmotic and nonosmotic control of vasopressin release. Am. J. Physiol. Physiol. 1979, 236, F321–F332. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Li, J. The role of the renin-angiotensin-aldosterone system in heart failure. J. Renin-Angiotensin-Aldosterone Syst. 2004, 5, S7–S10. [Google Scholar] [CrossRef]
- Weber, K.T. Aldosterone in congestive heart failure. N. Engl. J. Med. 2001, 345, 1689–1697. [Google Scholar] [CrossRef]
- Malpas, S.C. Sympathetic Nervous System Overactivity and Its Role in the Development of Cardiovascular Disease. Physiol. Rev. 2010, 90, 513–557. [Google Scholar] [CrossRef] [PubMed]
- Charloux, A.; Piquard, F.; Doutreleau, S.; Brandenberger, G.; Geny, B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur. J. Clin. Investig. 2003, 33, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, M.; Parissis, J.T.; Akiyama, E.; Mebazaa, A. Understanding acute heart failure: Pathophysiology and diagnosis. Eur. Heart J. Suppl. 2016, 18, G11–G18. [Google Scholar] [CrossRef]
- Clark, A.L.; Cleland, J.G.F. Causes and treatment of oedema in patients with heart failure. Nat. Rev. Cardiol. 2013, 10, 156–170. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.W.; Mullens, W. The Pathophysiological Role of Interstitial Sodium in Heart Failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef]
- Mullens, W.; Abrahams, Z.; Skouri, H.N.; Francis, G.S.; Taylor, D.O.; Starling, R.C.; Paganini, E.; Tang, W.W. Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function? J. Am. Coll. Cardiol. 2008, 51, 300–306. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Nijst, P. Cardiac Output and Renal Dysfunction: Definitely More Than Impaired Flow. J. Am. Coll. Cardiol. 2016, 67, 2209–2212. [Google Scholar] [CrossRef]
- Hanberg, J.S.; Sury, K.; Wilson, F.P.; Brisco, M.A.; Ahmad, T.; ter Maaten, J.M.; Broughton, J.S.; Assefa, M.; Tang, W.W.; Parikh, C.R.; et al. Reduced Cardiac Index Is Not the Dominant Driver of Renal Dysfunction in Heart Failure. J. Am. Coll. Cardiol. 2016, 67, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- èMullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Wilson Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Taylor, D.O.; Starling, R.C.; Tang, W.W. Prompt Reduction in Intra-Abdominal Pressure Following Large-Volume Mechanical Fluid Removal Improves Renal Insufficiency in Refractory Decompensated Heart Failure. J. Card. Fail. 2008, 14, 508–514. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Guglin, M.E.; Saltzberg, M.T.; Jessup, M.L.; Bart, B.A.; Teerlink, J.R.; Jaski, B.E.; Fang, J.C.; Feller, E.D.; Haas, G.J.; et al. Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure. J. Am. Coll. Cardiol. 2007, 49, 675–683. [Google Scholar] [CrossRef]
- Bart, B.A.; Goldsmith, S.R.; Lee, K.L.; Givertz, M.M.; O’Connor, C.M.; Bull, D.A.; Redfield, M.M.; Deswal, A.; Rouleau, J.L.; LeWinter, M.M.; et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N. Engl. J. Med. 2012, 367, 2296–2304. [Google Scholar] [CrossRef]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 64, 797–805. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.-P.; Mebazaa, A.; Rocca, H.-P.B.-L.; Martens, P.; Testani, J.M.; Tang, W.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Mullens, W.; Verbrugge, F.H.; Nijst, P.; Tang, W.H.W. Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. Eur. Heart J. 2017, 38, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Arques, S.; Ambrosi, P. Human Serum Albumin in the Clinical Syndrome of Heart Failure. J. Card. Fail. 2011, 17, 451–458. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Colombo, P.C.; Banchs, J.E.; Celaj, S.; Talreja, A.; Lachmann, J.; Malla, S.; DuBois, N.B.; Ashton, A.W.; Latif, F.; Jorde, U.P.; et al. Endothelial Cell Activation in Patients With Decompensated Heart Failure. Circulation 2005, 111, 58–62. [Google Scholar] [CrossRef]
- Colombo, P.C.; Onat, D.; Sabbah, H.N. Acute heart failure as “acute endothelitis”—Interaction of fluid overload and endothelial dysfunction. Eur. J. Heart Fail. 2008, 10, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, G.P.; Song, H.; Tressel, S.L.; Hwang, J.; Dikalov, S.; Smith, D.A.; Boyd, N.L.; Platt, M.O.; Lassègue, B.; Griendling, K.K.; et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ. Res. 2004, 95, 773–779. [Google Scholar] [CrossRef]
- Kawai, M.; Naruse, K.; Komatsu, S.; Kobayashi, S.; Nagino, M.; Nimura, Y.; Sokabe, M. Mechanical stress-dependent secretion of interleukin 6 by endothelial cells after portal vein embolization: Clinical and experimental studies. J. Hepatol. 2002, 37, 240–246. [Google Scholar] [CrossRef]
- Wang, B.-W.; Chang, H.; Lin, S.; Kuan, P.; Shyu, K.-G. Induction of matrix metalloproteinases-14 and -2 by cyclical mechanical stretch is mediated by tumor necrosis factor-alpha in cultured human umbilical vein endothelial cells. Cardiovasc. Res. 2003, 59, 460–469. [Google Scholar] [CrossRef]
- Colombo, P.C.; Rastogi, S.; Onat, D.; Zacà, V.; Gupta, R.C.; Jorde, U.P.; Sabbah, H.N. Activation of endothelial cells in conduit veins of dogs with heart failure and veins of normal dogs after vascular stretch by acute volume loading. J. Card. Fail. 2009, 15, 457–463. [Google Scholar] [CrossRef]
- Colombo, P.C.; Onat, D.; Harxhi, A.; Demmer, R.T.; Hayashi, Y.; Jelic, S.; LeJemtel, T.H.; Bucciarelli, L.; Kebschull, M.; Papapanou, P.N.; et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur. Heart J. 2013, 35, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ganda, A.; Onat, D.; Demmer, R.T.; Wan, E.; Vittorio, T.J.; Sabbah, H.N.; Colombo, P.C. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr. Heart Fail. Rep. 2010, 7, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.W. Are hemodynamic goals viable in tailoring heart failure therapy? Hemodynamic goals are relevant. Circulation 2006, 113, 1020–1027. [Google Scholar] [CrossRef]
- Drexler, H. Endothelium as a Therapeutic Target in Heart Failure. Circulation 1998, 98, 2652–2655. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Nagel, T.; Topper, J.N. Biomechanical activation: An emerging paradigm in endothelial adhesion biology. J. Clin. Investig. 1997, 99, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Luxán, G.; Dimmeler, S. The vasculature: A therapeutic target in heart failure? Cardiovasc. Res. 2022, 118, 53–64. [Google Scholar] [CrossRef]
- Whaley-Connell, A.T.; Chowdhury, N.A.; Hayden, M.R.; Stump, C.S.; Habibi, J.; Wiedmeyer, C.E.; Gallagher, P.E.; Tallant, E.A.; Cooper, S.A.; Link, C.D.; et al. Oxidative stress and glomerular filtration barrier injury: Role of the renin-angiotensin system in the Ren2 transgenic rat. Am. J. Physiol. Physiol. 2006, 291, F1308–F1314. [Google Scholar] [CrossRef]
- Scagliola, R.; Brunelli, C. Venous Congestion and Systemic Hypoperfusion in Cardiorenal Syndrome: Two Sides of the Same Coin. Rev. Cardiovasc. Med. 2022, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.I.; Wang, Y.; Concato, J.; Gill, T.; Krumholz, H.M. Patterns of Weight Change Preceding Hospitalization for Heart Failure. Circulation 2007, 116, 1549–1554. [Google Scholar] [CrossRef]
- Cotter, G.; Metra, M.; Milo-Cotter, O.; Dittrich, H.C.; Gheorghiade, M. Fluid overload in acute heart failure—Re-distribution and other mechanisms beyond fluid accumulation. Eur. J. Heart Fail. 2008, 10, 165–169. [Google Scholar] [CrossRef]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically mediated changes in capacitance: Redistribution of the venous reservoir as a cause of decompensation. Circ. Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef]
- Balmain, S.; Padmanabhan, N.; Ferrell, W.R.; Morton, J.J.; Mcmurray, J. Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. Eur. J. Heart Fail. 2007, 9, 865–871. [Google Scholar] [CrossRef]
- Gelman, S.; Mushlin, P.S. Catecholamine-induced Changes in the Splanchnic Circulation Affecting Systemic Hemodynamics. Anesthesiology 2004, 100, 434–439. [Google Scholar] [CrossRef]
- Bishu, K.; Redfield, M.M. Acute Heart Failure with Preserved Ejection Fraction: Unique Patient Characteristics and Targets for Therapy. Curr. Heart Fail. Rep. 2013, 10, 190–197. [Google Scholar] [CrossRef]
- Mentz, R.J.; Kjeldsen, K.; Rossi, G.P.; Voors, A.A.; Cleland, J.G.; Anker, S.D.; Gheorghiade, M.; Fiuzat, M.; Rossignol, P.; Zannad, F.; et al. Decongestion in acute heart failure. Eur. J. Heart Fail. 2014, 16, 471–482. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Maggioni, A.P.; Harjola, V.P.; Rosano, G.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Lainscak, M.; Ponikowski, P.; et al. Acute heart failure congestion and perfusion status—Impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2019, 21, 1338–1352. [Google Scholar] [CrossRef]
- Harjola, V.-P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef]
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Tang, W.H.W. Intersections between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef]
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.; Antohi, E.; Arrigo, M.; Ben Gal, T.; et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1315–1341. [Google Scholar] [CrossRef]
- Ghionzoli, N.; Sciaccaluga, C.; Mandoli, G.; Vergaro, G.; Gentile, F.; D’Ascenzi, F.; Mondillo, S.; Emdin, M.; Valente, S.; Cameli, M. Cardiogenic shock and acute kidney injury: The rule rather than the exception. Heart Fail. Rev. 2020, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A. Low-Flow Acute Kidney Injury: The Pathophysiology of Prerenal Azotemia, Abdominal Compartment Syndrome, and Obstructive Uropathy. Clin. J. Am. Soc. Nephrol. 2022, 17, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.C. Hypoxic liver injury. Mayo Clin. Proc. 2006, 81, 1232–1236. [Google Scholar] [CrossRef]
- Knauf, H.; Mutschler, E. Sequential Nephron Blockade Breaks Resistance to Diuretics in Edematous States. J. Cardiovasc. Pharmacol. 1997, 29, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gracia, J.; Demissei, B.G.; Ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef]
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart Fail. 2015, 17, 1172–1181. [Google Scholar] [CrossRef]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar]
- O’Connor, C.M.; Stough, W.G.; Gallup, D.S.; Hasselblad, V.; Gheorghiade, M. Demographics, Clinical Characteristics, and Outcomes of Patients Hospitalized for Decompensated Heart Failure: Observations from the IMPACT-HF Registry. J. Card. Fail. 2005, 11, 200–205. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Maestro, A.; Fernández-Martínez, J.; López-López, L.; Solé-González, E.; Vives-Borrás, M.; Montero, S.; Mesado, N.; Pirla, M.J.; Mirabet, S.; et al. Prevalence and prognostic impact of subclinical pulmonary congestion at discharge in patients with acute heart failure. ESC Heart Fail. 2020, 21, 2621–2628. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Greene, S.J.; Fonarow, G.; Voors, A.A.; Butler, J.; Gheorghiade, M. Hemoconcentration-guided Diuresis in Heart Failure. Am. J. Med. 2014, 127, 1154–1159. [Google Scholar] [CrossRef]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur. Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.; Kozhuharov, N.; Coats, A.J.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Mueller, T.; Januzzi, J.L., Jr. Characteristics of the Novel Interleukin Family Biomarker ST2 in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2008, 52, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Peacock, W.F.; Maisel, A.S.; Chae, C.U.; Jesse, R.L.; Baggish, A.L.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; van Kimmenade, R.R.; et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J. Am. Coll. Cardiol. 2007, 50, 607–613. [Google Scholar] [CrossRef]
- Mueller, T.; Dieplinger, B.; Gegenhuber, A.; Poelz, W.; Pacher, R.; Haltmayer, M. Increased Plasma Concentrations of Soluble ST2 are Predictive for 1-Year Mortality in Patients with Acute Destabilized Heart Failure. Clin. Chem. 2008, 54, 752–756. [Google Scholar] [CrossRef]
- Aimo, A.; Vergaro, G.; Ripoli, A.; Bayes-Genis, A.; Figal, D.A.P.; de Boer, R.A.; Lassus, J.; Mebazaa, A.; Gayat, E.; Breidthardt, T.; et al. Meta-Analysis of Soluble Suppression of Tumorigenicity-2 and Prognosis in Acute Heart Failure. JACC Heart Fail. 2017, 5, 287–296. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Demissei, B.G.; Cotter, G.; Prescott, M.F.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Severin, T.M.; Wang, Y.; et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: Results from the RELAX-AHF trial. Eur. J. Heart Fail. 2017, 19, 1001–1010. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Claggett, B.; Zile, M.; McMurray, J.J.; O’Meara, E.; Packer, M.; Prescott, M.F.; Swedberg, K.; Solomon, S.D.; Rouleau, J.L.; et al. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: The PARADIGM-HF trial. Eur. J. Heart Fail. 2018, 20, 1701–1709. [Google Scholar] [CrossRef]
- Cornelissen, A.; Florescu, R.; Kneizeh, K.; Cornelissen, C.; Brandenburg, V.; Liehn, E.; Schuh, A. Intact fibroblast growth factor 23 levels and outcome prediction in patients with acute heart failure. Sci. Rep. 2021, 11, 15507. [Google Scholar] [CrossRef]
- Pöss, J.; Mahfoud, F.; Seiler, S.; Heine, G.H.; Fliser, D.; Böhm, M.; Link, A. FGF-23 is associated with increased disease severity and early mortality in cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U.; Janmohamed, M.; Habib, M.; Hatamizadeh, P. Vasodilators in the management of acute heart failure. Crit. Care Med. 2008, 36, S95–S105. [Google Scholar] [CrossRef] [PubMed]
- Alzahri, M.S.; Rohra, A.; Peacock, W.F. Nitrates as a Treatment of Acute Heart Failure. Card. Fail. Rev. 2016, 2, 51–55. [Google Scholar] [CrossRef]
- Piper, S.; McDonagh, T. The Role of Intravenous Vasodilators in Acute Heart Failure Management. Eur. J. Heart Fail. 2014, 16, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Delgado Jimenez, J.F.; Metra, M.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B.; et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007, 50, 768–777. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E. Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef]
- Wachter, R.; Senni, M.; Belohlavek, J.; Straburzynska-Migaj, E.; Witte, K.K.; Kobalava, Z.; Fonseca, C.; Goncalvesova, E.; Cavusoglu, Y.; Fernandez, A.; et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: Primary results of the randomised transition study. Eur. J. Heart Fail. 2019, 21, 998–1007. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Miller, R.J.; Howlett, J.G.; Fine, N.M. A Novel Approach to Medical Management of Heart Failure with Reduced Ejection Fraction. Can. J. Cardiol. 2021, 37, 632–643. [Google Scholar] [CrossRef]
- Prins, K.; Neill, J.; Tyler, J.; Eckman, P.; Duval, S. Effects of Beta-Blocker Withdrawal in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2015, 3, 647–653. [Google Scholar] [CrossRef]
- Takagi, K.; Kimmoun, A.; Sato, N.; Mebazaa, A. Management of Acute Heart Failure during an Early Phase. Int. J. Heart Fail. 2020, 2, 91–110. [Google Scholar] [CrossRef]
- Mebazaa, A.; Nieminen, M.S.; Filippatos, G.S.; Cleland, J.G.; Salon, J.E.; Thakkar, R.; Padley, R.J.; Huang, B.; Cohen-Solal, A. Levosimendan vs. dobutamine: Outcomes for acute heart failure patients on beta-blockers in survive. Eur. J. Heart Fail. 2009, 11, 304–311. [Google Scholar] [CrossRef]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef]
- Cecconi, M.; Parsons, A.K.; Rhodes, A. What is a fluid challenge? Curr. Opin. Crit. Care 2011, 17, 290–295. [Google Scholar] [CrossRef]
- Girardis, M.; Busani, S.; Damiani, E.; Donati, A.; Rinaldi, L.; Marudi, A.; Morelli, A.; Antonelli, M.; Singer, M. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA 2016, 316, 1583–1589. [Google Scholar] [CrossRef]
- Reinhart, K.; Bloos, F.; König, F.; Bredle, D.; Hannemann, L. Reversible Decrease of Oxygen Consumption by Hyperoxia. Chest 1991, 99, 690–694. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).